Abstract
Previous studies have demonstrated that modest, physiologically relevant increases in maternal cortisol in late gestation result in enlargement of the fetal heart. In this study, we investigated the role of mineralocorticoid (MR) or glucocorticoid receptor (GR) in this enlargement. Ewes with single fetuses were randomly assigned at ~ 120d gestation to one of four groups: maternal cortisol infusion (1mg kg−1 day−1, cortisol); maternal cortisol infusion with fetal intrapericardial infusion of the MR antagonist potassium canrenoate (600µg day−1; cortisol + MRa); maternal cortisol infusion with fetal intrapericardial infusion of the GR antagonist mifepristone (50µg day−1, cortisol + GRa); and maternal saline infusion (control). At ~130 days gestation, fetal heart to body weight ratio and right (RV) and left ventricular (LV) free wall thickness were increased in the cortisol group compared to control group. Fetal hearts from the cortisol +MRa group weighed significantly less, with thinner LV, RV and interventricular septum walls, compared to the cortisol group. Fetal hearts from the cortisol + GRa group had significantly thinner RV walls than the cortisol group. Fetal arterial pressure and heart rate were not different among groups at 130 days. Picrosirius red staining of fetal hearts indicated that the increased size was not accompanied by cardiac fibrosis. These results suggest that physiologic increases in maternal cortisol late in gestation induce fetal cardiac enlargement via MR and, to a lesser extent, by GR, and indicate the enlargement is not secondary to an increase in fetal blood pressure or an increase in fibrosis within the fetal heart.
Keywords: corticosteroid, fetus, cardiac muscle, glucocorticoid receptor, mineralocorticoidreceptor
Introduction
In late gestation, normal fetal growth and fetal cardiovascular homeostasis is dependent on the proper regulation of maternal cortisol levels. Although reductions in maternal cortisol prevent the normal increases in maternal plasma volume and uteroplacental blood flow and reduce fetal growth (Jensen et al. 2002a; Jensen et al. 2005), increases in maternal cortisol also alter fetal growth. Chronically increased maternal cortisol levels, within the range that occurs with maternal stress, reduce fetal growth rates while increasing heart growth in fetal sheep (Jensen et al. 2002b; Jensen et al. 2005).
The mechanisms by which chronically elevated maternal cortisol levels increase the size of the fetal heart are not known. Giraud et al. have shown that cortisol chronically infused directly into the coronary artery increased cell cycle activity in myocytes of late gestation sheep fetuses, suggesting a direct induction by cortisol of hyperplastic growth rather than hypertrophic growth (Giraud et al. 2006). Conversely, it has been demonstrated that large doses of cortisol infused directly into the fetus in late gestation causes left ventricular (LV) hypertrophy along with an increase in fetal arterial pressure and cardiac expression of angiotensinogen mRNA (Lumbers et al. 2005). We have shown that maternal cortisol infusion in sheep during late gestation caused an increase in fetal heart size and wall thickness without increasing fetal arterial pressure or cardiac angiotensinogen; we found an increase in the ratio of angiotensin type 2 receptor (AT2 receptor) to angiotensin type 1 receptor (AT1 receptor) mRNA in the fetal heart, suggesting that the renin-angiotensin system (RAS) may play a key role in the enlargement process. Furthermore, in the same study it was observed that left ventricular expression of 11β-HSD2 mRNA, the enzyme that converts cortisol into cortisone, decreased in the fetal hearts in response to the elevated cortisol, suggesting that cortisol can act directly on mineralocorticoid (MR) or glucocorticoid (GR) receptors to induce the cardiac enlargement (Reini et al. 2006).
In adult hearts, both MR and the RAS have been implicated in cardiac fibrosis and hypertrophy after injury (Fraccarollo et al. 2003; Fraccarollo et al. 2005; Xiao et al. 2004). We propose that corticosteroid receptors also play a role in cardiac enlargement in the fetal heart, although by mechanisms independent of cardiac injury and fibrosis. The purpose of this study was to test the hypothesis that increase in fetal heart weight and wall thickness in response to increased maternal cortisol is mediated by cardiac corticosteroid receptors, MR and/or GR, and to determine if cardiac fibrosis accompanies the cardiac enlargement in response to cortisol. We hypothesized that cortisol acts within the myocardium on MR receptors, and to a lesser degree GR receptors, to induce enlargement of the fetal heart. We also hypothesized that cardiac fibrosis is not involved in the enlargement of the heart observed in the fetuses of cortisol-infused ewes.
Materials and Methods
Experimental Design
Ewes (Ovis aries) pregnant with single fetuses were studied. All animal use was approved by the University of Florida Institutional Animal Care and Use Committee and conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Ewes and their fetuses were operated on between 118 and 123 days of gestation (term approximately 148 days). Animals were randomly assigned to one of four groups at the time of surgery. The first group consisted of six control animals; the second group consisted of five ewes to which cortisol (hydrocortisone hemisuccinate; Sigma, St Louis, MO) was administered by continuous intravenous infusion (1 mg kg−1 day−1; cortisol); the third group consisted of six ewes to which cortisol was infused, with infusion of the MR antagonist potassium canrenoate (Sigma; 600 µg day−1; cortisol + MRa) directly into the pericardial space of the fetus; and the fourth group consisted of four ewes to which cortisol was infused, with infusion of the GR antagonist mifipristone (Sigma; 50 µg day−1; cortisol + GRa) directly into the pericardial space of the fetus. For the control and cortisol groups, there were no infusions into the pericardial space. The intrapericardial infusions were performed by use of Alzet minipumps (2ML2; 5 µl·hour−1; Cupertino CA) in order to achieve continuous infusion of the antagonists into the pericardial space without any appreciable increase in pericardial fluid volume (0.12 ml/day). The doses of MR and GR antagonists were calculated based on their effective systemic doses, and scaled to reflect the smaller distribution volume of the fetal heart (20g). Because these drugs are steroid (mifepristone) or lactone (canrenoate) derivatives, they are able to distribute throughout tissue over the 10 days of study after mixing in the pericardial fluid. Effects of the MR and GR antagonists were confirmed using immunohistochemistry to confirm the expected cellular redistribution of receptors with antagonist administration (see below)
The cortisol dose and the duration of cortisol infusion (10 days) were determined based on a previous study in this laboratory (Jensen et al. 2005) showing that infusion at this rate and duration produces levels similar to mild maternal stressors and results in enlargement of the fetal heart.
Surgical Procedures
Halothane (1.5–2.5%) in oxygen was used to anesthetize ewes during surgery. Fetal femoral tibial artery catheters and an amniotic fluid catheter were placed as previously described (Jensen et al. 2002a; Wood & Rudolph 1983). Catheters were also placed in the fetal pericardial space for the delivery of drug as previously described (Wood 2002). In each case, an incision was made in the uterus over the left side of the fetal chest and an incison was made between the third and fourth fetal ribs. The fetal skin was marsupialized to the uterus to prevent leakage of amniotic fluid. The fetal heart was exposed and a small incision was made in the pericardium, through which a silastic catheter (0.76 mm id, 1.65 od; Dow Corning, Midland, Michigan) was placed and held in place with use of a purse-string suture (4-0 Tevdek; Teleflex Medical, Mansfield, MA). For infusion of potassium canrenoate, the silastic catheter was connected to a Tygon tubing connector (1.27 mm od; St Gobain Performance plastics; Akron , OH) which was connected at its other end to the the Alzet pump containing the drug (50 mg ml−1 in 0.9% saline). Because mifepristone is not soluble in aqueous solution and therefore cannot be directly loaded into the pump reservoir, mifepristone was dissolved in 47.5% ethanol in saline (0.42 mg ml−1 ethanol-saline); this solution was placed in a polyethylene tubing (1.40 mm id, 1.90 mm od) which was then connected to the silastic pericardial catheter on one end and to the Alzet minipump on the other end using smaller gauge polyethylene tubing. The Alzet pump, filled with saline, provided the flow to pump the mifepristone solution from the tubing into the pericardium. The pump was placed under the skin of the fetus near the scapula. In the control group, 5 of the 6 fetuses also had pericardial catheters placed, but no infusion was delivered; in the cortisol group, 3 of 5 fetuses had pericardial catheters placed, but no infusion was delivered.
After closure of the uterus, catheters were placed in the maternal femoral artery and vein and routed to the maternal flank. All ewes were treated with flunixamine (1 mg kg−1 im; Fort Dodge Animal Health, Fort Dodge, IA) at the end of the surgical procedure, before recovery from anesthesia.
Ewes were returned to their pen after recovery from anesthesia. At this time, the intravenous infusion of cortisol (1mg kg−1 day−1 cortisol as cortisol hemisuccinate in normal saline; Sigma) or infusion of saline to the ewe was initiated. Maternal infusions were delivered through a 0.22 µm filter (Fisher Scientific) via a syringe pump at the rate of 1.17 ml hour−1. Animals were housed in individual pens with access to water, food, and salt blocks ad libitum. Ampicillin (500mg im bid; Webster Veterinary) was administered for 3 days postoperatively. Flunixamine was administered on the morning after surgery.
Experimental Protocol
Fetuses were studied from the day of surgery until death on 129–132 days gestation. All cortisol infused ewes and their fetuses were sacrificed on day 10 of infusion. Fetal and maternal blood samples were withdrawn on day 5 (124–126 days gestation) and day 10 (129–132 days gestation) after the start of the infusion for determination of blood gases, plasma cortisol and plasma ACTH concentrations. All blood samples were taken immediately after entering the room in which the ewes were housed in order to minimize the effect of handling on plasma ACTH and cortisol. On day 10 of infusion, maternal and fetal blood pressure and heart rate were recorded over a 40 minute interval using LabView software (National Instruments, Austin, TX) and disposable pressure transducers (Transpac; Hospira, Lake Forest, IL) Amniotic fluid pressures were subtracted from fetal intra-arterial pressures in order to calculate fetal arterial pressure. In two animals, one in the cortisol group and one in the cortisol+MRa group, we were unable to reliably measure fetal heart rate; data from those two fetuses are excluded from analysis.
The ewe was euthanized on day 10 using an overdose of euthanasia solution containing pentobarbital, and the fetus was removed and weighed. The fetal heart was also immediately dissected, blotted to remove blood from the chambers, and weighed. Ventricular and septal wall thicknesses were measured using a micrometer at a standardized site on the heart, taking care to exclude measurement at the level of the papillary muscles or valves.
Analysis
Blood gases and pH were measured with a blood gas/electrolyte analyzer (ABL77; Radiometer America, Westlake, OH). Electrolytes (sodium and potassium) were measured using an electrolyte analyzer (Roche 9180, Basel, Switzerland). For measurement of packed cell volume (PCV), blood was spun in microcapillary tubes for 3 minutes at 12,000 rpm (Damon Division, International Equipment, Needham Heights, MA). Plasma protein was determined using a refractometer.
Plasma ACTH was measured by radioimmunoassay, using an antibody to 1–39 ACTH (Bell et al. 1991) and plasma cortisol concentration was measured using a commercially available enzyme immunoassay kit (EA 65, Oxford Biomedical, Oxford, MI) which has minimal cross-reactivity with cortisone (2.08%).
Immunohistochemical localization of MR and GR
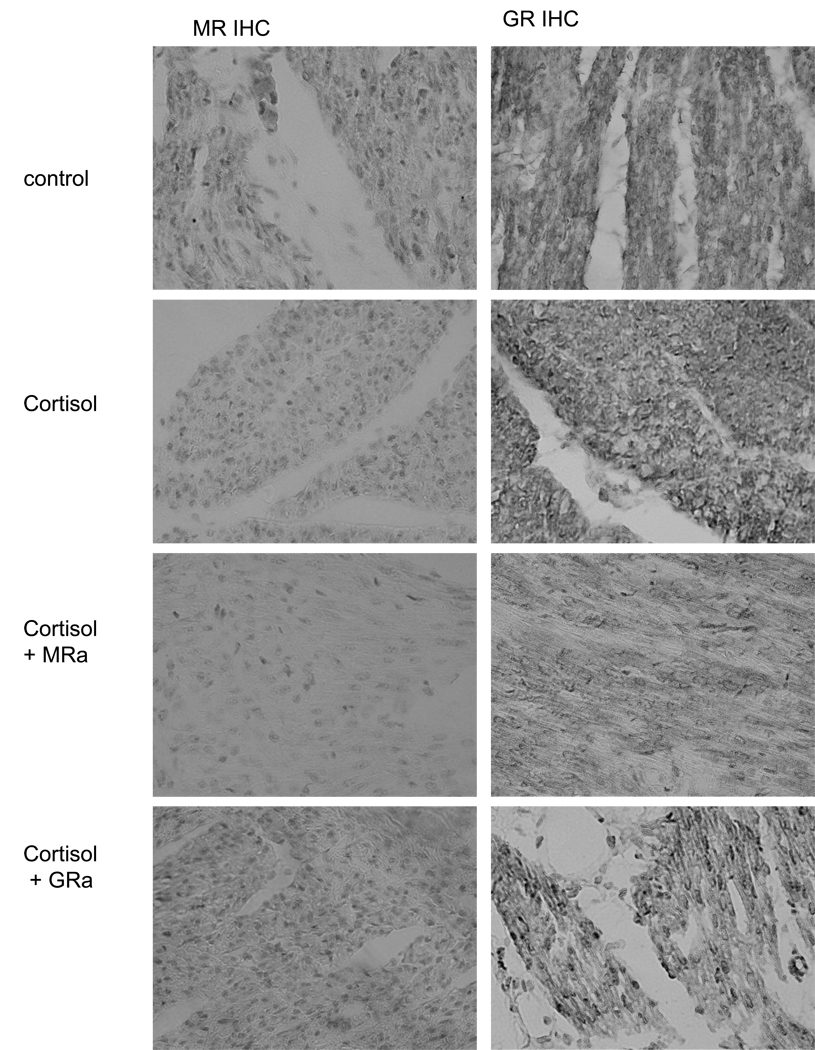
At the time of sacrifice, a section of the fetal heart was fixed in 4% buffered paraformaldehyde overnight. Hearts were dehydrated with increasing concentrations of reagent alcohol followed by xylene, embedded in paraffin wax, cut into 10-µm-thick sections on a Zeiss rotary microtome, and placed on poly-l-lysine coated slides. The sections were stained with anti-GR (Santa Cruz Bioreagents, M-20, ) or anti-MR ( M1-18, 6G1, gift of E. Gomez-Sanchez; (Gomez-Sanchez et al. 2006)) as previously described (Reini et al. 2006) This analysis was performed to assess the ability of the drugs to act in the heart and cause the expected changes in cytonuclear localization of the receptors. The MR antagonist canrenoate acts in a similar manner to spironolactone and would therefore be expected to prevent nuclear localization of MR (Fejes-Toth et al. 1998; Lombes et al. 1994); conversely the GR antagonist mifepristone (also known as RU486) causes nuclear localization even in the absence of agonist (Jewell et al. 1995; Scheuer et al. 2004). The localization observed (Figure 1) is consistent with these effects. In the control fetuses GR were primarily located in the cytosol, whereas MR were apparent in cytosol and nucleus. A dramatic increase in MR localization to the nucleus was apparent in the cortisol-treated fetuses, indicating cortisol activation of MR. We did not find as dramatic an increase in nuclear GR with cortisol, indicating fewer GR are activated. In the case of MR antagonist administration, fewer MR were apparent in the nucleus than with cortisol alone, whereas with GR antagonist, equivalent MR localization to the nucleus occurred as with cortisol alone. Consistent with the known effect of mefipristone, in GR antagonist -treated ewes, there was more nuclear GR than in the case of cortisol alone or cortisol+ MRa.
Figure 1.
Immunohistochemical localization of MR (left column) and GR (right column) in representative hearts from fetuses of control , cortisol , cortisol +MRa and cortisol+GRa groups. All photos at 40x power.
Collagen Staining
Sections from each group (n = 4–6) were stained with picrosirius red (Sigma) in order to determine collagen content. Sections were hydrated and immersed in sirius red (0.1% in saturated picric acid). The sections were then washed in acidified water (0.5% glacial acetic acid), dehydrated, and mounted in permount. All images were visualized using an Olympus DP71 microscope and Olympus software. Ten pictures of LV, five of RV, and five of septum were taken from each heart in areas without large blood vessels so that primarily interstititial, rather than perivascular, collagen deposition could be quantified. Picrosirius red staining was quantified using Image J software (NIH, Bethesda, MD) by three different people who were blinded as to the experimental group. The average value of the percentage of the image that stained red from these three observations was calculated.
Data Analysis
Fetal heart weight was normalized to body weight. The heart weight to body weight ratio, LV, RV and septal thickness, fetal and maternal blood pressure and heart rate, as well as fetal and maternal plasma ACTH and cortisol, sodium and potassium, and PCVs were analyzed by one way analysis of variance (ANOVA) with multiple comparison’s using Duncan’s method (Zar 1984). Plasma hormone (cortisol and ACTH) and protein concentrations were also analyzed by one-tailed t-test, comparing the data from all 3 groups of cortisol- treated ewes to the data from the control group (Zar 1984). Average cortisol values were calculated from the 5 day and 10 day values and were log transformed before analysis. The Mann-Whitney Rank Sum Test was used for maternal plasma protein analysis at 10d (Zar 1984).
Values for the picrosirius red staining were analyzed by two-way ANOVA in order to determine significance across the cortisol treatment groups and areas of the heart (LV, RV, and septum); the percent stained area data was transformed using arc sine prior to analysis to correct for heterscedascity (Winer 1971).
For all analyses, p< 0.05 was used as the criterion for statistical significance.
Results
Maternal Physiology
Maternal cortisol concentrations were significantly increased in the ewes treated with cortisol when compared to the non-treated ewes (5d and 10d day average, 9.0 ± 0.9 vs. 5.9 ± 1.4 ng ml−1). When the four groups were compared individually, there was a trend for each cortisol treated group to have increased cortisol concentrations as compared to the control ewes (Table 1), but there were no differences among groups. ACTH levels were not significantly altered in response to cortisol treatment, although there was a trend for cortisol treated ewes to have lower ACTH concentrations than the control group (Table 1).
Table 1.
Fetal and Maternal Cortisol concentrations (average of days 5 and 10) and ACTH concentration on day 10
| Maternal | Fetal | Maternal | Fetal | |
|---|---|---|---|---|
| Cortisol | Cortisol | ACTH | ACTH | |
| (ng/ml) | (ng/ml | (pg/ml) | (pg/ml) | |
| Control | 5.9 ± 1.4 | 1.5 ± 0.6 | 37 ± 8 | 36 ± 8 |
| Cortisol | 9.6 ± 2.3 | 2.7 ± 0.5 | 20 ± 1 | 27 ± 5 |
| Cortisol+MR antagonist | 8.7 ± 0.4 | 3.6 ± 1.0 | 31 ± 11 | 38 ± 6 |
| Cortisol+GR antagonist | 8.3 ± 1.5 | 3.9 ± 1.9 | 21 ± 1 | 57 ± 33 |
Data are expressed as mean ± SEM.
Maternal sodium, potassium, and packed cell volume values were not different between the groups at day 5 or at day 10 (data not shown). Maternal plasma protein concentrations were significantly elevated in the cortisol treated ewes on days 5 (8.2 ± 0.2 vs. 7.6 ± 0.2 g 100ml−1) and 10 (7.9 ± 0.1 vs. 7.4 ± 0.1g 100ml−1) days as compared to the control ewes.
Maternal arterial pressures and heart rates were not different between the four groups (data not shown).
Fetal Physiology
The average plasma cortisol concentrations (5 and 10 days) were significantly elevated in the fetuses whose mothers were infused with cortisol compared to control (3.4 ± 0.6 vs.1.5 ± 0.6 ng ml−1). There was a trend for each cortisol treated group to have increased cortisol concentrations as compared to the control fetuses when the four groups were compared individually (Table 1). ACTH levels were not significantly altered in response to cortisol treatment (Table 1).
There were no significant differences among the groups in the blood gas values or packed cell volume (Table 2), nor were there effects on fetal electrolytes (data not shown). There were also no effects of treatment on fetal heart rate and blood pressures (Table 3).
Table 2.
Fetal blood gas and packed cell volume
| Fetal PO2 (mmHg) | Fetal PCO2 (mmHg) | Fetal pH | Fetal Packed cell Volume (fractional volume of red blood cells) | |
|---|---|---|---|---|
| Control | 21.7 ± 1.0 | 56 ± 1 | 7.34 ± 0.01 | 0.313 ± 0.007 |
| Cortisol | 21.5 ± 1.0 | 53 ± 2 | 7.35 ± 0.01 | 0.326 ± 0.013 |
| Cortisol+MR antagonist | 20.7 ± 1.1 | 54 ± 2 | 7.30 ± 0.03 | 0.348 ± 0.007 |
| Cortisol+GR antagonist | 21.9 ± 0.4 | 55 ± 1 | 7.32 ± 0.02 | 0.325 ± 0.009 |
Data are expressed as mean ± SEM.
Table 3.
Fetal arterial pressure and fetal heart rate on day 10.
| Fetal Arterial pressure (mmHg) | Fetal Heart Rate (beats per minute) | |
|---|---|---|
| Control | 47.5 ± 2.7 | 170 ± 6 |
| Cortisol | 46.9 ± 2.7 | 168 ± 11 |
| Cortisol +MR antagonist | 43.0.± 0.9 | 172 ± 4 |
| Cortisol +GR antagonist | 46.0 ± 1.0 | 164 ± 7 |
Data are expressed as mean ± SEM
Fetal Heart Measurements
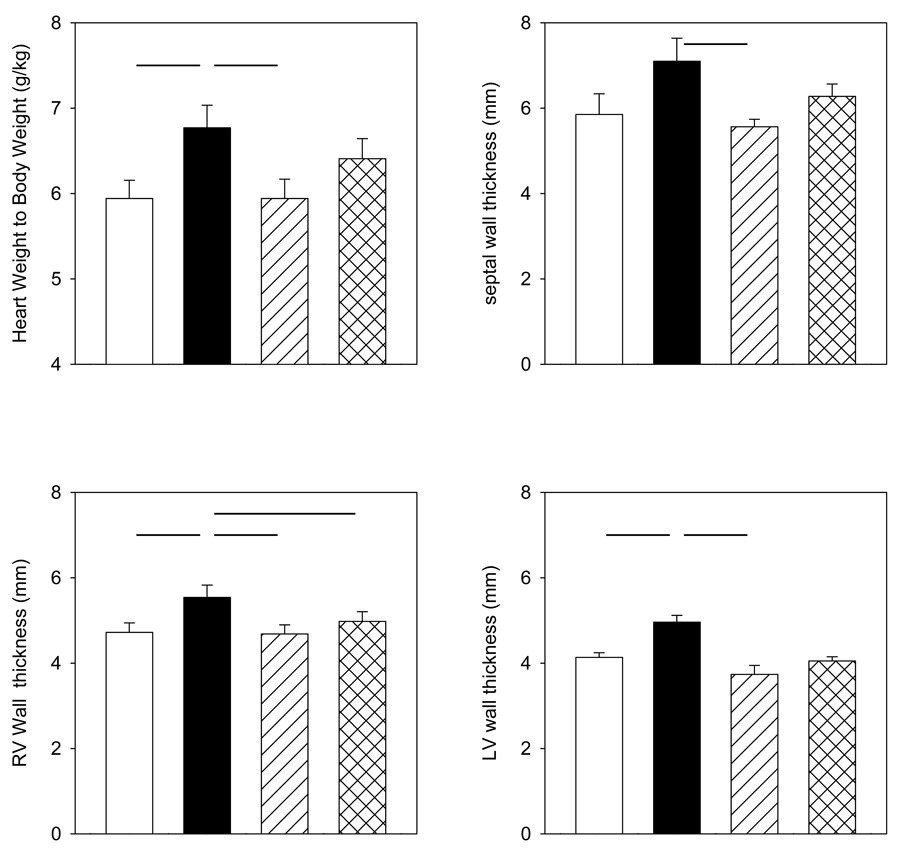
Heart weight was significantly greater in the cortisol group compared to the control group and cortisol + MRa group, but not the cortisol + GRa group (Figure 1). Left ventricular and right ventricular free wall thicknesses were significantly greater in the fetal hearts of the cortisol treated group as compared to the control group. Left and right ventricular free wall, as well as septum, thicknesses were greater in the fetal hearts of the cortisol group as compared to the cortisol + MRa group (Figure 1). Left ventricular free wall thickness and septum thickness were not different in the cortisol group as compared to the cortisol + GRa group (Figure 1). However, right ventricular wall thickness was greater in the cortisol group as compared to the cortisol+GRa group.
Collagen Staining
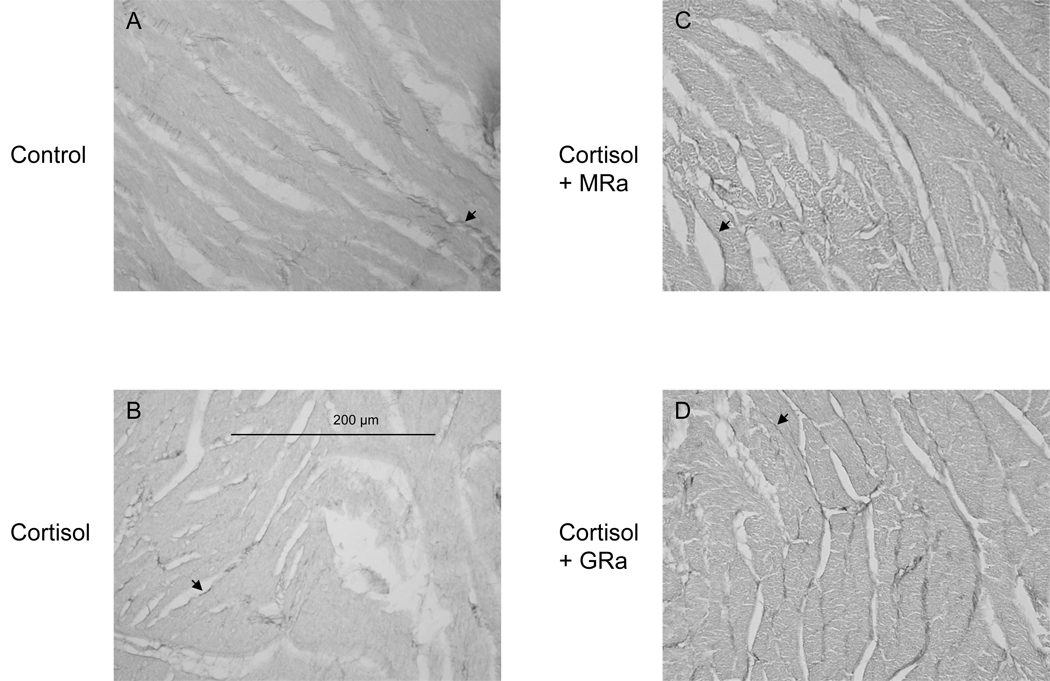
Fetal heart sections were stained with picrosirius red in order to measure the amount of interstitial collagen deposition (Figure 2). The percentage of collagen staining in the left ventricle, right ventricle, septum, and whole heart was not significantly altered among the groups (Table 4, Figure 2).
Figure 2.
Mean fetal heart measurements from control (open bars), cortisol (solid bars), cortisol+MRa (diagonal striped bars) or cortisol+GRa (cross-hatched bars) groups taken at time of sacrifice: heart to body mass ratio (upper left), left ventricular (LV) wall thickness (lower right), septal wall thickness (upper right), and right ventricular (RV) wall thickness (lower left). Data are expressed as mean ± SEM. Horizontal lines between groups indicate differences are statistically significant, p< 0.05.
Table 4.
Collagen content determined by picrosirius red staining (fraction of total area) in left ventricle (LV), right ventricle (LV) and septum
| LV | RV | Septum | |
|---|---|---|---|
| Control | 0.037 ± 0.005 | 0.039 ± 0.004 | 0.037 ± 0.006 |
| Cortisol | 0.050 ± 0.009 | 0.057 ± 0.009 | 0.052 ± 0.010 |
| Cortisol+MR antagonist | 0.049 ± 0.011 | 0.037 ± 0.007 | 0.043 ± 0.008 |
| Cortisol+GR antagonist | 0.060 ± 0.012 | 0.058 ± 0.013 | 0.059 ± 0.013 |
Data are expressed as mean ± SEM.
Discussion
We conclude that blockade of corticosteroid receptors in the fetal heart prevents the enlargement of the heart observed when maternal cortisol concentrations are chronically increased. We found that blockade of the mineralocorticoid receptors blocked the increase in heart weight, as well as in wall thickness. Blockade of glucocorticoid receptors significantly reduced right ventricular enlargement, and produced smaller, insignificant effects on thickness of the left ventricular free wall and septum and on heart weight. Neither administration of MR or GR blocker into the pericardium resulted in increases in fetal ACTH or fetal blood pressure, suggesting that the infusions of antagonist did not produce systemic effects. The results indicate that small increases in cortisol increase fetal heart size via an intracardiac action at the MR and, to a lesser extent, GR receptors within the fetal heart. We also conclude that the increase in fetal heart weight in response to elevated cortisol occurs without an increase in collagen deposition.
Role of MR and GR in the heart
Our laboratory has previously shown that both MR and GR are abundantly expressed in the heart in the late gestation ovine fetus (Reini et al. 2006), suggesting a role for these receptors in fetal heart development in vivo. Other investigators have found that aldosterone directly stimulates myocyte surface area (Okoshi et al. 2004) and remodeling of myocyte membrane (Kliche et al. 2006) in cultures of neonatal myocytes, and effect presumed to be mediated by MR in the myocytes. Cortisol also increases expression of atrial natriuretic peptide in cultured neonatal myocytes, and both cortisol and aldosterone potentiate the effect of phenylephrine on hypertrophy in these cultures (Lister et al. 2006), also indicating an intracardiac action at MR in these cultures.
One of the major factors influencing the ability of cortisol to activate MR and/or GR is the local activity of the 11hydroxysteroid dehydrogenase enzymes, 11β-HSD1 and 11β-HSD2. 11β-HSD1 primarily converts cortisone into cortisol, while 11β-HSD2 converts cortisol into cortisone, which is inactive at MR and GR (Krozowski et al. 1999). We had previously shown that mRNA expression of 11β-HSD2 mRNA is relatively low compared to 11β-HSD1 within the fetal heart (Reini et al. 2006). Using immunohistochemistry, we also found that although MR, GR, and 11β-HSD1 are abundantly expressed in both myocytes and blood vessels within the fetal heart, 11β-HSD2 seemed to be localized in blood vessels more abundantly than in myocytes. This suggested that cortisol has access to both MR and GR within the fetal heart, and that when plasma cortisol levels are increased, as in the present study, action of cortisol at MR and GR in the heart would also increase. Our present study demonstrates that the effect of cortisol is blocked by antagonists of the MR and/or GR, suggesting a role of intracardiac corticosteroid receptors. This is consistent with the ability of cortisol to alter myocyte growth in cultured myocytes. We also hypothesized that blockade of MR would have a greater effect in inhibiting the effect of cortisol than would blockade of GR, because MR has been shown to have greater affinity for cortisol than GR (Reul & DeKloet 1985; Richards et al. 2003). Indeed this is what we observed in the present study: in the cortisol group, there was a 14% increase in heart weight relative to body weight as compared to the control group; this enlargement was completely blocked when MR antagonist was administered to the heart, whereas there was only 44% blockade of the increase in weight after administration of the GR antagonist. Similarly, in the cortisol group, LV, RV, and septum thicknesses were approximately 20% thicker than control fetuses and the MR antagonist produced a 95%, 149%, and 114% reduction of this increase in thickness of the LV, RV, and septum respectively, whereas the GR antagonist group produced 63%, 110%, and 65% reductions of thickness. Overall, GR blockade was approximately half as effective as MR blockade in inhibiting the increase in heart weight or wall thickness.
The relative differences in effectiveness of MR and GR blockade are consistent with the expected relative binding of fetal cortisol at these receptors. The MR are higher affinity receptors with greater occupancy at low cortisol concentrations (Reul & DeKloet 1985), and therefore a greater effect would be expected after blockade of these receptors. Based on the expected free fraction of cortisol in the fetuses, we calculate that the free cortisol concentrations would be approximately 0.8 nM in the control fetuses and 1.9 nM in the fetuses of the cortisol-infused ewes. Based on previous studies of cortisol binding at ovine MR and GR (Richards et al. 2003), we would predict that these free concentrations would result in approximately 65% occupancy of MR and 35% occupancy of GR in the control fetuses, and 85% occupancy of MR and 60% occupancy of GR in the cortisol-infused fetuses. Thus, these levels would be expected to exert more effects via MR than via GR activation if both act at GRE to induce genes responsible for cardiac growth.
Role of MR in hypertrophy in the adult heart
In adult rats the mineralocorticoid receptor is thought to induce cardiac hypertrophy and fibrosis occurring in response to ischemia; systemic administration of MR blockers have been shown to reduce markers of inflammation and fibrosis in hearts of adult rats (Brilla et al. 1993; Fraccarollo et al. 2005; Sun et al. 2002). It has been established that in adult humans with severe heart failure, there is a reduction in the severity of cardiac hypertrophy and an increase in survival rate after treatment with the MR receptor antagonists eplenerone or spironolactone (Pitt et al. 1999; Pitt et al. 2001). The effect of MR blockers on survival rate appears to be the result of a decrease in cardiac fibrosis (Fraccarollo et al. 2004); increases in interstitial collagen content are a feature of adult cardiac hypertrophy (Pearlman et al. 1981), particularly in the case of hypertension or myocardial infarction (Young et al. 2007). The mechanism for the in vivo effect of MR in contributing to inflammation and subsequent fibrosis is not clear. It has been suggested that the effect is through a nongenomic action, and that the effect in ischemic tissue is predominately on vascular cells expressing MR, rather than on fibroblasts or on myocytes (Young et al. 2007; Mihailidou & Funder 2005). It is generally assumed that the protective effect of the MR antagonists results from blocking the action of aldosterone at MR. It has been suggested, however, that many heart failure patients without elevated plasma aldosterone levels still benefit from MR blockade, indicating aldosterone may not be the only relevant MR ligand (Young et al. 2007). Since plasma cortisol concentrations are much higher than aldosterone, and since there is not a significant amount of 11β-HSD2 expressed within the heart, it is reasonable to propose that cortisol may be playing a role in the fibrosis that is observed in heart failure patients.
In our studies the effects of cortisol do not appear to involve increase in fibrosis, as there was no increase in collagen content with maternal infusion of cortisol, nor were there any effects of either MR or GR blockade. This suggests that the mechanism of the enlargement of the fetal heart in the current study may be fundamentally different from what is observed in adult rat models or human pathology, in which ischemia is a contributing component.
Mechanisms of Enlargement of the Fetal Heart
Due to the unique ability of the fetal heart to grow through both hyperplasia and hypertrophy, either mechanism could account for the cortisol-induced increases in fetal heart weight and wall thickness in our model. In early gestation, cardiac growth is mostly a result of the production of new myocytes originating through cell division and proliferation (Smolich 1995). After approximately day 115 of gestation in sheep, however, cardiac growth results primarily from increases in myocyte size (Jonker et al. 2007). Myocytes lose their ability to divide and proliferate shortly after birth in an event in which there is nuclear division without subsequent cell division (Oparil et al. 1984). In fetal sheep the number of terminally differentiated or binucleate myocytes increases from ~115 days of gestation through term, and heart growth during this period is due to both increases in myocyte size and myocyte proliferation (Jonker et al. 2007). Theoretically, cortisol could be stimulating growth through either hypertrophy or hyperplasia, or possibly even both.
Rudolph et al. showed that cortisol (1.2 µg min−1) infusion for 72–80 hours directly into the left coronary artery of the ovine fetus (124–131d) decreased left ventricular DNA content (Rudolph et al. 1999). This was interpreted as cortisol-induced inhibition of myocyte proliferation in preparation for life after birth. The fetal blood pressures from that study were not reported. In a study by Lumbers et al., high dose infusion of cortisol (72.1mg d−1 for ~60h) increased left ventricular myocyte size and increase cardiac angiotensinogen mRNA (Lumbers et al. 2005), suggesting an induction of hypertrophy. However, there was also a significant increase in blood pressure in these fetuses, suggesting that the cardiac hypertrophy may have resulted from elevated blood pressure.
Conversely, maternal dexamethasone administration (48 µg d−1 from E17) increased relative heart weight and increased myocyte proliferation in the fetal and newborn rat heart (Torres et al. 1997). In agreement with this, Giraud et al. (Giraud et al. 2006) showed that subpressor doses of cortisol (0.5 µg kg−1min−1 for 7 days) infused directly into the circumflex coronary artery of the fetus led to an increase in Ki-67 stained myocytes in both the left and right ventricles; as Ki-67 is expressed only in cells in the proliferative phase, this suggested that cortisol stimulated proliferation in these hearts. Hearts infused with cortisol weighed more than control hearts in this study, but there were no changes in myocyte size or percent binucleation. Interestingly, there were also no differences in aortic, right atrial, systolic, and diastolic pressures between the groups. These studies suggest that elevated fetal cortisol concentrations directly stimulate cardiomyocyte proliferation in the late-term fetus.
The current study does not provide direct evidence for cardiomyocyte proliferation as a means of cardiac enlargement in response to cortisol. It is important to note that in this study a subpressor dose of cortisol was used, as in the study by Giraud et al.(Giraud et al. 2006). We did not observe an increase arterial pressure in response to the moderately elevated cortisol levels indicating that the fetal hearts in this study were not subjected to chronically increased systolic load, a possible trigger to myocyte hypertrophy seen in some other studies. Although in the present study blood pressure was only measured at 10 days of cortisol infusion, in our previous study (Jensen et al. 2005) fetal arterial blood pressure was not elevated at either 5 or 10 days of maternal cortisol infusion. The doses of cortisol administered in our study resulted in relatively small increases in feal cortisol, well below those that have been shown to increase fetal blood pressure in other studies (Unno et al. 1999; Tangalakis et al. 1992; Wood et al. 1987). Furthermore, in this study we observed no evidence within the fetal heart in support of interstitial collagen deposition, a symptom of cardiac hypertrophy in response to hypertension within the adult human heart (Diez 2007).
Conclusions
The data suggest that the enlargement of the fetal heart in response to a modest and chronic rise in maternal cortisol levels is mediated by MR receptors, and to a lesser extent, GR receptors within the fetal heart. Intrapericardial infusion of an MR antagonist completely prevented the increase in wall thickness and heart weight. GR blockade was less effective, although GR blockade prevented the increase in RV free wall thickness, and tended to attenuate the increase in left ventricular wall thickness and whole heart weight. The cortisol-induced enlargement is not accompanied by an increase in interstitial collagen deposition within the fetal heart. This indicates the possibility of a different mechanism for the enlargement observed in the fetal heart than that observed in adult cardiac hypertrophy and fibrosis.
Figure 3.
Representative pictures showing picrosirus red staining of collagen in left ventricular wall of fetal hearts from the A) control, B) cortisol, C) cortisol + MRa, and D) cortisol + GRa groups; 40x power. Bar indicates 200um. Arrows indicate the dark staining corresponding to positive Sirius red staining.
Acknowledgements
This work was supported by NIH grant DK62080 to M Keller-Wood and American Heart Association Florida-Puerto Rico Affiliate pre-doctoral fellowship grant 0615236B to S. Reini. We acknowledge the technical assistance of Jarret McCartney for assistance in the post-operative care of the sheep and for assistance with necropsies. We also acknowledge the assistance of Kavi Patel and Sasha Gidwani for analysis of the collagen content.
Footnotes
Publisher's Disclaimer: “Disclaimer. This is not the definitive version of record of this article. This manuscript has been accepted for publication in Journal of Endocrinology, but the version presented here has not yet been copy edited, formatted or proofed. Consequently, the Society for Endocrinology accepts no responsibility for any errors or omissions it may contain. The definitive version is now freely available at DOI: 10.1677/JOE-08-0022. © 2008 Society for Endocrinology.”
Reference List
- Bell ME, Wood CE, Keller-Wood M. Influence of reproductive state on pituitary-adrenal activity in the ewe. Domestic Animal Endocrinology. 1991;8:245–254. doi: 10.1016/0739-7240(91)90060-w. [DOI] [PubMed] [Google Scholar]
- Brilla CG, Matsubara LS, Weber KT. Antifibrotic effects of spironolactone in preventing myocardial fibrosis in systemic arterial hypertension. Am.J.Cardiol. 1993;71:12A–16A. doi: 10.1016/0002-9149(93)90239-9. [DOI] [PubMed] [Google Scholar]
- Diez J. Mechanisms of cardiac fibrosis in hypertension. J.Clin.Hypertens.(Greenwich.) 2007;9:546–550. doi: 10.1111/j.1524-6175.2007.06626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fejes-Toth G, Pearce D, Naray-Fejes-Toth A. Subcellular localization of mineralocorticoid receptors in living cells: effects of receptor agonists and antagonists. Proc.Natl.Acad.Sci.U.S.A. 1998;95:2973–2978. doi: 10.1073/pnas.95.6.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraccarollo D, Galuppo P, Bauersachs J. Mineralocorticoid receptor antagonism and cardiac remodeling in ischemic heart failure. Curr.Med.Chem.Cardiovasc.Hematol.Agents. 2004;2:287–294. doi: 10.2174/1568016043356219. [DOI] [PubMed] [Google Scholar]
- Fraccarollo D, Galuppo P, Hildemann S, Christ M, Ertl G, Bauersachs J. Additive improvement of left ventricular remodeling and neurohormonal activation by aldosterone receptor blockade with eplerenone and ACE inhibition in rats with myocardial infarction. J.Am.Coll.Cardiol. 2003;42:1666–1673. doi: 10.1016/j.jacc.2003.05.003. [DOI] [PubMed] [Google Scholar]
- Fraccarollo D, Galuppo P, Schmidt I, Ertl G, Bauersachs J. Additive amelioration of left ventricular remodeling and molecular alterations by combined aldosterone and angiotensin receptor blockade after myocardial infarction. Cardiovasc.Res. 2005;67:97–105. doi: 10.1016/j.cardiores.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Giraud GD, Louey S, Jonker S, Schultz J, Thornburg KL. Cortisol Stimulates Cell Cycle Activity in the Cardiomyocyte of the Sheep Fetus. Endocrinology. 2006 doi: 10.1210/en.2006-0061. [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, Gomez-Sanchez EP. Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology. 2006;147:1343–1348. doi: 10.1210/en.2005-0860. [DOI] [PubMed] [Google Scholar]
- Jensen E, Wood C, Keller-Wood M. The normal increase in adrenal secretion during pregnancy contributes to maternal volume expansion and fetal homeostasis. J.Soc.Gynecol.Investig. 2002a;9:362–371. [PubMed] [Google Scholar]
- Jensen E, Wood CE, Keller-Wood M. Chronic alterations in ovine maternal corticosteroid levels influence uterine blood flow and placental and fetal growth. Am.J.Physiol Regul.Integr.Comp Physiol. 2005;288:R54–R61. doi: 10.1152/ajpregu.00149.2004. [DOI] [PubMed] [Google Scholar]
- Jensen EC, Gallaher BW, Breier BH, Harding JE. The effect of a chronic maternal cortisol infusion on the late-gestation fetal sheep. J.Endocrinol. 2002b;174:27–36. doi: 10.1677/joe.0.1740027. [DOI] [PubMed] [Google Scholar]
- Jewell CM, Webster JC, Burnstein KL, Sar M, Bodwell JE, Cidlowski JA. Immunocytochemical analysis of hormone mediated nuclear translocation of wild type and mutant glucocorticoid receptors. J.Steroid Biochem.Mol.Biol. 1995;55:135–146. doi: 10.1016/0960-0760(95)00174-x. [DOI] [PubMed] [Google Scholar]
- Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J.Appl.Physiol. 2007;102:1130–1142. doi: 10.1152/japplphysiol.00937.2006. [DOI] [PubMed] [Google Scholar]
- Kliche K, Kuhn M, Hillebrand U, Ludwig Y, Stock C, Oberleithner H. Direct aldosterone action on mouse cardiomyocytes detected with atomic force microscopy. Cell Physiol Biochem. 2006;18:265–274. doi: 10.1159/000097673. [DOI] [PubMed] [Google Scholar]
- Krozowski Z, Li KX, Koyama K, Smith RE, Obeyesekere VR, Stein-Oakley A, Sasano H, Coulter C, Cole T, Sheppard KE. The type I and type II 11beta-hydroxysteroid dehydrogenase enzymes. J Steroid Biochem.Mol.Biol. 1999;69:391–401. doi: 10.1016/s0960-0760(99)00074-6. [DOI] [PubMed] [Google Scholar]
- Lister K, Autelitano DJ, Jenkins A, Hannan RD, Sheppard KE. Cross talk between corticosteroids and alpha-adrenergic signalling augments cardiomyocyte hypertrophy: a possible role for SGK1. Cardiovasc.Res. 2006;70:555–565. doi: 10.1016/j.cardiores.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Lombes M, Binart N, Delahaye F, Baulieu EE, Rafestin-Oblin ME. Differential intracellular localization of human mineralocorticosteroid receptor on binding of agonists and antagonists. Biochem.J. 1994;302(Pt 1):191–197. doi: 10.1042/bj3020191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumbers ER, Boyce AC, Joulianos G, Kumarasamy V, Barner E, Segar JL, Burrell JH. Effects of cortisol on cardiac myocytes and on expression of cardiac genes in fetal sheep. Am.J.Physiol Regul.Integr.Comp Physiol. 2005;288:R567–R574. doi: 10.1152/ajpregu.00556.2004. [DOI] [PubMed] [Google Scholar]
- Mihailidou AS, Funder JW. Nongenomic effects of mineralocorticoid receptor activation in the cardiovascular system. Steroids. 2005;70:347–351. doi: 10.1016/j.steroids.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Okoshi MP, Yan X, Okoshi K, Nakayama M, Schuldt AJ, O'Connell TD, Simpson PC, Lorell BH. Aldosterone directly stimulates cardiac myocyte hypertrophy. J.Card Fail. 2004;10:511–518. doi: 10.1016/j.cardfail.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Oparil S, Bishop SP, Clubb FJ., Jr Myocardial cell hypertrophy or hyperplasia. Hypertension. 1984;6:III38–III43. doi: 10.1161/01.hyp.6.6_pt_2.iii38. [DOI] [PubMed] [Google Scholar]
- Pearlman ES, Weber KT, Janicki JS. Quantitative histology of the hypertrophied human heart. Federation Proceedings. 1981;40:2042–2047. [PubMed] [Google Scholar]
- Pitt B, Williams G, Remme W, Martinez F, Lopez-Sendon J, Zannad F, Neaton J, Roniker B, Hurley S, Burns D, Bittman R, Kleiman J. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc.Drugs Ther. 2001;15:79–87. doi: 10.1023/a:1011119003788. [DOI] [PubMed] [Google Scholar]
- Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N.Engl.J.Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- Reini SA, Wood CE, Jensen E, Keller-Wood M. Increased maternal cortisol in late gestation ewes decreases fetal cardiac expression of 11{beta}-HSD2 mRNA and the ratio of AT1 to AT2 receptor mRNA. Am.J.Physiol Regul.Integr.Comp Physiol. 2006 doi: 10.1152/ajpregu.00294.2006. [DOI] [PubMed] [Google Scholar]
- Reul JMHM, DeKloet ER. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Richards EM, Hua Y, Keller-Wood M. Pharmacology and physiology of ovine corticosteroid receptors. Neuroendocrinology. 2003;77:2–14. doi: 10.1159/000068335. [DOI] [PubMed] [Google Scholar]
- Rudolph AM, Roman C, Gournay V. Perinatal myocardial DNA and protein changes in the lamb: effect of cortisol in the fetus. Pediatr.Res. 1999;46:141–146. doi: 10.1203/00006450-199908000-00002. [DOI] [PubMed] [Google Scholar]
- Scheuer DA, Bechtold AG, Shank SS, Akana SF. Glucocorticoids act in the dorsal hindbrain to increase arterial pressure. Am.J.Physiol Heart Circ.Physiol. 2004;286:H458–H467. doi: 10.1152/ajpheart.00824.2003. [DOI] [PubMed] [Google Scholar]
- Smolich JJ. Ultrastructural and functional features of the developing mammalian heart: a brief overview. Reprod.Fertil.Dev. 1995;7:451–461. doi: 10.1071/rd9950451. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang J, Lu L, Chen SS, Quinn MT, Weber KT. Aldosterone-induced inflammation in the rat heart : role of oxidative stress. Am.J.Pathol. 2002;161:1773–1781. doi: 10.1016/S0002-9440(10)64454-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tangalakis K, Lumbers ER, Moritz KM, Towstoless MK, Wintour EM. Effect of cortisol on blood pressure and vascular reactivity in the ovine fetus. Exp.Physiol. 1992;77:709–717. doi: 10.1113/expphysiol.1992.sp003637. [DOI] [PubMed] [Google Scholar]
- Torres A, Belser WW, III, Umeda PK, Tucker D. Indicators of delayed maturation of rat heart treated prenatally with dexamethasone. Pediatr.Res. 1997;42:139–144. doi: 10.1203/00006450-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Unno N, Wong CH, Jenkins SL, Wentworth RA, Ding XY, Li C, Robertson SS, Smotherman WP, Nathanielsz PW. Blood pressure and heart rate in the ovine fetus: ontogenic changes and effects of fetal adrenalectomy. Am.J.Physiol. 1999;276:H228–H256. doi: 10.1152/ajpheart.1999.276.1.H248. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. New York: McGraw-Hill; 1971. [Google Scholar]
- Wood CE. The ovine fetal endocrine reflex responses to haemorrhage are not mediated by cardiac nerves. J.Physiol. 2002;541:613–622. doi: 10.1113/jphysiol.2001.015461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CE, Cheung CY, Brace RA. Fetal heart rate, arterial pressure, and blood volume responses to cortisol infusion. American Journal of Physiology. 1987;253:R904–R909. doi: 10.1152/ajpregu.1987.253.6.R904. [DOI] [PubMed] [Google Scholar]
- Wood CE, Rudolph AM. Negative feedback regulation of adrenocorticotropin secretion by cortisol. Endocrinology. 1983;112:1930–1936. doi: 10.1210/endo-112-6-1930. [DOI] [PubMed] [Google Scholar]
- Xiao HD, Fuchs S, Campbell DJ, Lewis W, Dudley SC, Jr, Kasi VS, Hoit BD, Keshelava G, Zhao H, Capecchi MR, Bernstein KE. Mice with cardiac-restricted angiotensin-converting enzyme (ACE) have atrial enlargement, cardiac arrhythmia, and sudden death. Am.J.Pathol. 2004;165:1019–1032. doi: 10.1016/S0002-9440(10)63363-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MJ, Lam EY, Rickard AJ. Mineralocorticoid receptor activation and cardiac fibrosis. Clin.Sci.(Lond) 2007;112:467–475. doi: 10.1042/CS20060275. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. Englewood Cliffs, NJ: Prentice-Hall; 1984. [Google Scholar]