Abstract
The visinin-like protein (VSNL) subfamily, including the founder protein VILIP-1, VILIP-2, VILIP-3, hippocalcin and neurocalcin δ, constitute a highly homologous subfamily of neuronal calcium sensor (NCS) proteins. Comparative studies have shown that VSNLs are expressed predominantly in the brain with restricted expression patterns in different subsets of neurons, but are also found in peripheral organs. In addition, the proteins display differences in their calcium affinities, their membrane binding kinetics and in the intracellular targets to which they associate after calcium binding. Even though the proteins use a similar calcium-myristoyl switch mechanism to translocate to cellular membranes, they show calcium-dependent localization to different subcellular compartments when expressed in the same neuron. These distinct calcium-myristoyl switch properties might be explained by specificity for defined phospholipids and membrane-bound targets, which enable VSNLs to modulate various cellular signal transduction pathways, including cyclic nucleotide and MAPK signaling. An emerging theme is the direct or indirect effect of VSNLs on gene expression and the interaction with components of membrane trafficking complexes which may determine a role in membrane trafficking of different receptors and ion channels, such as glutamate receptors of the kainate and AMPA subtype, nicotinic ACh receptors and Ca2+-channels. One hypothesis is that the highly homologous VSNLs have evolved to fulfill specialized functions in membrane trafficking and thereby affect neuronal signaling and differentiation in defined subsets of neurons. VSNLs are involved in differentiation processes showing a tumor invasion suppressor function in peripheral organs. Finally, VSNLs play neuroprotective and neurotoxic roles and have been implicated in neurodegenerative diseases.
Keywords: AMPAR, calcium-myristoyl switch, cAMP/cGMP, endocytosis, exocytosis, natriuretic peptide receptor, Kainate receptor, MAPK pathways, neurodegeneration, neuronal calcium sensors, nicotinic acetylcholine receptor, membrane trafficking, receptor recycling
The visinin-like proteins (VSNLs)
During the early 90ies of last century a variety of Ca2+-sensing proteins have been identified in the nervous system, in addition to the ubiquitous Ca2+ sensor protein calmodulin, reflecting the importance of the regulative function of Ca2+ in neurons. The founding members were visinin and recoverin, expressed in the retina of different species (Yamagata et al., 1990; Dizhoor et al., 1991). By the end of that decade these proteins have been grouped together and termed neuronal Ca2+ sensor (NCS) proteins (Nef, 1996; Braunewell and Gundelfinger, 1999). 14 NCS protein genes are known to exist to date in various species and have been subdivided into five subfamilies. Pervious reviews have nicely given an overview on the whole family of NCS proteins (Braunewell and Gundelfinger, 1999; Burgoyne and Weiss, 2001; Burgoyne, 2007). To give some more detailed description on the current knowledge, we have focused here mainly on the subfamily of VSNLs.
Five proteins VILIP-1 (visinin-like protein 1, gene name VSNL1), VILIP-2 (visinin-like protein 2, gene name hippocalcin-like 4, HPCAL4), VILIP-3 (visinin-like protein 3, VILIP-3, gene name HPCAL1), hippocalcin (gene name HPCA) and neurocalcin δ (gene name:NCALD) show a high degree of amino acid identity between 67 to 94% (Fig. 1A, B) and form the subfamily of visinin-like proteins (VSNLs) (Braunewell and Gundelfinger, 1999; Burgoyne and Weiss, 2001; Spilker et al., 2002a; Burgoyne et al., 2004). The founder member of the subfamily, VILIP-1, was initially cloned as neural visinin-like protein 1 (NVP-1) from rat (Kuno et al., 1992) and visinin-like protein from chicken (Lenz et al., 1992). A variety of different names have been given to these proteins in different species over time (Fig. 1A), which makes it often difficult to obtain a comprehensive picture for a given protein. Thus, it might be most beneficial for the scientific community to add the official gene names and thereby complying with the recommendation of the HUGO Gene Nomenclature Committee (http://www.genenames.org). An overview of the nomenclature with the official gene names is also provided in Fig. 1A. Within the VSNL subfamily there are two branches which show 67–69% homology (Fig. 1A). Branch 1 with VILIP-1 and VILIP-2 are 89% homologous, whereas branch 2 with hippocalcin and VILIP-3 share 94% identity, and are 91% identical to neurocalcin δ (Spilker et al., 2002a). VSNLs are 191-193 amino acid residues long and have a consensus sequence (M-G-(X)3-S) for N-terminal myristoylation (Fig. 1B). Like all other members of the superfamily of EF-hand Ca2+-binding proteins, VSNLs possess the sequence motif D-X-D/N-X-D/N-X-Y-(X)4-E as EF-hand Ca2+ binding motif, where amino acids in bold are involved in the coordinative binding of Ca2+ and X can be any amino acid (Fig. 1B, EF1 EF4). Besides the EF-hand Ca2+-binding motifs no other functional domains have been identified yet. Due to changes in this amino acid sequence, e.g. removal of Asp and Asn involved in coordinative binding and addition of positively charged residues, EF-hand 1 is dysfunctional in all VSNLs. EF1 also forms the most variable part in the sequence of NCS proteins and therefore comprises a possible interaction site with target proteins.
Fig. 1.
A. Dendrogram of the subfamily of VSNLs. Based on amino acid similarity the relationships in the VSNL-subfamily are shown (identity of amino acids in % is indicated). Human sequences from the UniProtKB database (www.uniprot.org, data base accession numbers are: VILIP-1 (VSNL1, P62760), VILIP-2 (HPCAL4, P35332), VILIP-3 (HPCAL1, P62749), neurocalcin δ (NCALD, P61601), hippocalcin (HPCA, P84074)) and the clustalX and NJplot programs were used to generate the dendrogram. Calmodulin, the prototypical Ca2+ sensor, was used as reference. B. VSNL subfamily sequence alignment. UniProtKB database sequences showing the VSNL subfamily: VILIP-1 (gene name VSNL1, alternative names: VSNL-1, NVP-1, neurocalcin α, VLP-1), VILIP-2 (gene name HPCAL4, alternative names: NVP-2, neurocalcin β), VILIP-3 (gene name HPCAL1, alternative names: BDR-1, NVP-3, REM-1), hippocalcin (gene name HPCA, alternative name: BDR-2) and neurocalcin δ (gene name NCALD, alternative name: neurocalcin). The location of the four EF-hand Ca2+-binding motifs is indicated.
The distribution of VSNLs in the central nervous system
The expression patterns of NCS proteins and mRNAs have been previously reviewed indicating that VSNLs show a widespread but distinct expression pattern primarily in nerve cells (Braunewell and Gundelfinger, 1999). A detailed mRNA expression study of the VSNL subfamily describes the expression of VILIP-1, VILIP-2, VILIP-3 and hippocalcin in the rat brain (Paterlini et al., 2000). VILIP-1 mRNA shows a widespread distribution in most brain areas except the caudate-putamen. Hippocalcin and VILIP-3 exhibit overlapping expression patterns in the forebrain including neocortex, hippocampus and caudate-putamen. It seems that VILIP-3 has a quite restricted expression pattern in the cerebellum where it localizes to Purkinje and granule cells (Paterlini et al., 2000). A novel and very powerful tool to study and compare expression of the mRNAs for VSNLs, e.g with potential interaction partners, in the mouse brain and spinal cord is the Allen Brain Atlas (http:/www.brain-map.org).
On the protein level several comparative expression studies for members of the VSNL subfamily have been performed. These studies include the analysis of protein expression of VILIP-1 and VILIP-3 in the rat cerebellum and hippocampus (Spilker et al., 2000), of neurocalcin isoforms α and δ in the rat cerebellum (Kato et al., 1998); and of VILIP-1 and VILIP-3 in the human brain (Saitoh et al., 1995). Detailed studies on the distribution of VSNLs in the brain have focussed on hippocampus and cerebellum. Early studies in the hippocampus of rat and gerbil (Saitoh et al., 1995; Lenz et al., 1996) indicated that the highly homologous VILIP-1 and VILIP-2 (Fig. 1B) are differentially distributed. VILIP-2 expression is most prominent in rat CA1/CA2 regions and the dentate gyrus, whereas VILIP-1 immunoreactivity is strongest in gerbil CA3 region. However, in rat hippocampus VILIP-1 shows expression in all hippocampal subregions and the dentate gyrus (Zhao and Braunewell, 2008), which again differs from the expression profile observed in human hippocampus, where VILIP-1 immunoreactive neurons were found strongly expressed in CA1, CA4 and hilus, but very weak in the CA2 and CA3 subfields (Bernstein et al., 1999). Thus, in addition to regional differences of VSNL expression, species differences have to be taken into account. Hippocalcin is expressed in different parts of the rat hippocampus and is also detected in brain regions such as cerebellum (Saitoh et al., 1994; Grant et al., 1996). Hippocalcin mRNA and immunoreactivity was strongly expressed in the pyramidal cells of the hippocampus, intensely in cerebellar Purkinje cells, but only moderately in the dentate granule cells and pyramidal cells of cerebral cortex layers II-VI and weakly in the large neuronal cells of the caudate-putamen (Saitoh et al., 2003). VILIP-3 expression, which is highly homologous to hippocalcin, has been predominantly localized in the cerebellum but there is also weak expression in other brain regions including the hippocampus (Spilker et al., 2000; Spilker and Braunewell, 2003). At the mRNA level strong regional differences in the expression levels of hippocalcin and VILIP-3 are detected in rat hippocampus. Compared to other hippocampal subregions where hippocalcin clearly dominates, a relatively high expression level of VILIP-3 and a relatively low expression level of hippocalcin are detectable in the dentate gyrus (Spilker et al., 2000). VILIP-1 and VILIP-3 co-localize in hippocampal neurons, showing a strong expression for VILIP-1 in many neurons and weaker expression of VILIP-3 in a subset of neurons (Spilker and Braunewell, 2003). Co-immunolocalization studies of neurocalcin in the rat hippocampus have suggested that all neurocalcin immunoreactive neurons are GABAergic. Neurocalcin-positive neurons comprise about 19% of the GABAergic neurons. These data suggested that neurocalcin is a marker for a subpopulation of interneurons. However, its has to be taken into account that the neurocalcin antibody used in the study reacted with neurocalcins α, β, γ, δ, which are equivalent to VILIP-1, VILIP-2, VILIP-3, and neurocalcin δ, (Martinez-Guijarro et al., 1998). Recent studies with VILIP-1-specific antibodies show expression in principal and non-principal neurons, with very strong expression levels in a subpopulation of calbindin-D28K and calretinin-positive interneurons in different hippocampal regions (Zhao and Braunewell, 2008).
The cerebellum is the second brain structure which has been studied in detail. Northern blots reveal that VILIP-2 is not expressed, whereas VILIP-3 is prominently expressed in the rat cerebellum (Kajimoto et al., 1993). Immunohistochemical and in situ-hybridization data indicate that at the cellular level, VILIP-3 is mainly expressed in Purkinje cells, with weak VILIP-3 immunoreactivity in granule cells (Spilker et al., 2000; Hamashima et al., 2001). VILIP-1 transcripts are primarily found in the granule cell layer, but are virtually absent from Purkinje cells in chicken and rat cerebellum (Lenz et al., 1992; Kato et al., 1998; Spilker et al., 2000). Chicken and rat VILIP-1 protein and its bovine ortholog neurocalcin α are consistently absent from Purkinje cells. Immunoreactivity was found to be restricted to granule cells and to the molecular layer of the chicken and rat cerebellum (Lenz et al., 1996; Kato et al., 1998; Spilker et al., 2000). In the human brain studies with antibodies against VILIP-1 and VILIP-3 have been performed. Both proteins have been found to be expressed in subsets of neurons in virtually all brain regions. However, VILIP-1 displays a much more intense immunoreactivity than VILIP-3 (Bernstein et al., 1999). VSNLs are also expressed in neurons of sensory pathways including retina and olfactory system. In the retina of various species, VILIP-1 and neurocalcin δ are absent from photoreceptor cells but are expressed in subsets of bipolar, amacrine and retinal ganglion cells (Lenz et al., 1992; Nakano et al., 1992; DeRaad et al., 1995; Krishnan et al., 2004). Various studies show that in rat olfactory epithelium a subset of olfactory receptor neurons express VILIP-1 (Boekhoff et al., 1997; Bastianelli et al., 1995; Braunewell and Gundelfinger, 1999), hippocalcin (Mammen et al., 2004) and neurocalcin δ (Duda et al., 2002, 2004)
The distribution of visinin-like proteins (VSNLs) in the periphery
NCS proteins have been assumed to be a nervous system-specific gene family, however expression outside the nervous system receives increasing attention. VILIP-3, which is also described as REM-1, has been detected in cells of the haematopoietic system and in the gut (Kraut et al., 1995), but also in kidney, spleen and testis (Spilker et al., 2000). Substantial expression of VILIP-1 has been shown for heart, liver, lung and testis in human and rat and also in stomach and skin of rat (Gierke et al., 2004). VILIP-1 mRNA expression has been detected in Northern blots of heart and lung (Kajimoto et al., 1993), by RT-PCR in heart and colon (Ohya and Horowitz, 2002), and its presence in skin was shown at the protein and mRNA level (Mahloogi et al., 2003). In contrast, hippocalcin displays a restricted expression pattern exclusively in brain tissues. No protein expression of hippocalcin could be detected in the periphery, although EST sequences were found in a few additional peripheral tissues (Gierke et al., 2004). The peripheral distribution of VSNLs in relation to brain expression was much higher in embryonic tissue compared to adult rat tissues, indicating a developmental restriction of expression. A developmental decrease of VSNL expression in liver, lung, kidney, spleen, pancreas and colon occurs, which might hint to a specific function of VSNLs during organ development (Gierke et al., 2004). Thus, hippocalcin may have adapted to specific brain functions particularly in the hippocampus, whereas VILIP-1, neurocalcin δ and particularly VILIP-3 fulfil specific functions in peripheral organs. In addition, EST clones containing VSNL sequences were found in cancer tissue such as nervous tumor, hepatocellular carcinoma, adenocarcinoma, oligodendroglioma, lung squamous cell carcinoma and head/neck tumors. Thus, it is conceivable that VSNLs are also involved in the pathophysiology of cancer. Indeed, expression of VILIP-1 is lost in mouse squamous cell carcinoma of the skin (Mahloogi et al., 2003; Gonzalez-Guerrico et al., 2005), in human squamous cell carcinoma of the esophagus (Wickborn et al., 2006) and in non-small cell lung carcinomas (Fu et al., 2008). VILIP-1 plays a role as invasion suppressor gene negatively influencing invasiveness of squamous cell carcinoma (Mahloogi et al., 2003; Gonzalez-Guerrico et al., 2005). In contrast VILIP-1 is overexpressed in colon cancer cell lines (Fu et al., 2008) and in neuroblastoma, where it enhances neuroblastoma invasiveness (Xie et al., 2007). The precise signaling mechanisms leading to the opposite effects in different cell types are currently under investigation.
Ca2+-affinities and Ca2+-myristoyl switch of VSNLs
VSNLs possess a functional M-G-X3-S consensus sequence for N-terminal myristoylation and are able to translocate to subcellular membrane compartments by a molecular mechanism termed Ca2+-myristoyl switch, which is dependent on Ca2+-binding and the myristoyl modification of the proteins (Zozulya and Stryer, 1992). The molecular mechanism of the switch has been analyzed in detail from tertiary structure data for recoverin, a founding member of the NCS protein family, which was analyzed in its Ca2+-bound and Ca2+-free myristoylated forms (Ames et al., 1996, 1997). Without Ca2+ the four EF-hand motifs in recoverin are arranged in a compact tandem array, with the myristoyl side chain burried in a hydrophobic pocket (Tanaka et al., 1995). Binding of Ca2+ to recoverin induces a conformational change leading to surface exposure of hydrophobic protein parts and exposure of the myristoyl side chain, thereby making these structures available for interaction with cellular membranes and/or target proteins. Biochemically, the existence of the Ca2+-myristoyl switch mechanism has been shown for all members of the VSNL-subfamily (Kobayashi et al., 1993; Ladant, 1995; Lenz et al., 1996; Spilker et al., 2002b). Accordingly, after increasing the intracellular Ca2+ concentration VSNLs can translocate to subcellular membrane compartments in living cells (Ivings et al., 2002; Spilker et al., 2002b; O’Callaghan et al., 2002; O’Callaghan et al., 2003; Spilker and Braunewell, 2003). Ca2+-affinities of some VSNLs have been determined and are in the range of 200 nM to 10 μM (Cox et al., 1994; Ladant, 1995, O’Callaghan et al., 2005, Jheng et al., 2006). Unmyristoylated VILIP-1 was initially shown to have uncooperative Ca2+-binding with an affinity of 1 μM (Cox et al., 1994). Unmyristoylated neurocalcin δ showed cooperative binding with an affinity of 0.6μM (Ladant, 1995). When unmyristoylated VILIP-1/neurocalcin α and neurocalcin δ were compared they showed half-maximum changes in tryptophan fluorescence signals at 1.5 and 6.1 μM free Ca2+, respectively (Kato et al., 1998). Another recent comparison of unmyristoylated VILIP-1 and VILIP-3 revealed slightly higher and cooperative Ca2+-affinities of 5.8 μM for VILIP-1 and 13.9μM for VILIP-3 (Jheng et al., 2006). The reason for these lower Ca2+-affinities is currently unclear, but might be partially related to the use of intact His- and GST-fusion proteins compared to recombinant proteins with cleaved off fusion part. Large GST fusion proteins might influence affinities adversely. In living HeLa cells the Ca2+-concentration range necessary for Ca2+-myristoyl switch and membrane association of myristoylated hippocalcin has been evaluated to be in the range of 200–800 nM free Ca 2+ (O’Callaghan et al., 2003). Thus, it appears that the precise Ca 2+-affinities for VSNLs still have to be determined, but that comparative studies of myristoylated native proteins in living cells most likely will reflect more precisely the physiological calcium affinities, which are known to be strongly influenced by myristoylation (Ladant, 1995).
Possible impact of the Ca2+ myristoyl switch of VSNLs
The reversible localization of signaling proteins and Ca2+ sensors to distinct membrane compartments and signalling scaffolds in living neurons via the molecular mechanism of the Ca2+-myristoyl switch, has been postulated to be a signal transduction mechanism for the selective activation of downstream signaling cascades, such as receptors, receptor signaling complexes and signal effector molecules (Teruel and Meyer, 2000; Spilker et al., 2002b; Spilker and Braunewell, 2003). VSNLs have been shown to translocate from the cytosolic to the particulate fraction in a Ca2+-dependent manner in biochemical fractionation experiments (Kobayashi et al., 1993; Ladant, 1995; Lenz et al., 1996; Kato et al., 1998; Spilker et al., 2000), and when co-expressed in neural cell lines VSNLs exhibit similar Ca2+-myristoyl switch properties in living cells (Ivings et al., 2002; Spilker et al., 2002b; O’Callaghan et al., 2002, 2003). However, differences in the degree and/or mechanism of Ca2+-dependent membrane association became evident in biochemical experiments with VILIP-1 and VILIP-3 following repeated EGTA extraction of the membrane fraction. Under these conditions VILIP-1 is less extractable from the membrane fraction compared to VILIP-3 (Spilker et al., 2002a). Moreover, neurocalcin α and δ the cow counterparts of VSNLs, have different Ca2+ affinities and the degree of the Ca2+-induced conformational change differs as shown by Ca2+ overlay, gel migration assay and tryptophan fluorescence assay (Kato et al., 1998). VILIP-1 and -3 appear to differ in their Ca 2+-affinities (Jheng et al., 2006). In line with these biochemical data, the membrane association of VILIP-1 and VILIP-3 also appears to vary. VILIP-1 displays tight membrane association in granule cells, whereas VILIP-3 is evenly distributed in Purkinje cells (Spilker et al., 2002a). An equivalent subcellular distribution has been observed in hippocampal neurons co-expressing VILIP-1 and VILIP-3, which strongly indicates that these differences reflect the intrinsic properties of the proteins (Spilker and Braunewell, 2003). The Ca2+-dependent subcellular membrane localization of endogenously expressed VILIP-1 and VILIP-3 differed substantially in the same hippocampal neuron. VILIP-1 shows cell surface membrane association, including membranes of axons and dendrites, which is in line with the described function of VILIP-1 as modulator of cell surface associated proteins (Braunewell et al., 1997, 2001; Lin et al., 2002a, b). In addition, VILIP-1 only affiliates with trans-Golgi membranes following a Ca2+ stimulus in hippocampal neurons (Spilker and Braunewell, 2003), while VILIP-3 showed a weak Ca2+-independent Golgi localization that was only gradually enhanced following stimulation of hippocampal neurons (Spilker et al., 2002b). Furthermore, VILIP-3 interacts with intracellular juxtanuclear membranes and granular structures in the whole cytosol (Spilker and Braunewell, 2003), which fits to a possible function as a modulator of MAP kinases (Spilker et al., 2002a). Interestingly in this context, under conditions of disturbed Ca2+ homeostasis in Alzheimer’s disease an enhanced juxtanuclear localization of VSNLs exists (Braunewell et al., 2002; Blandini et al., 2004). In case of VILIP-1 and VILIP-3 the observed in vitro differences in Ca2+ affinities have been found to be paralleled by differences in subcellular localization and activity-dependent translocation to cellular signaling compartments, when co-expressed (Fig. 2, Kato et al., 1998; Spilker et al., 2002a, b). Thus, each NCS protein might respond to different Ca2+ concentrations, and thus, might have specificity for certain cell-types and specificity towards distinct receptors and signaling pathways (Burgoyne, 2007). The in vitro differences in Ca2+ affinities for NCS proteins might increase the dynamic range over which Ca2+ and NCS proteins can regulate neuronal activities (Burgoyne and Weiss, 2001; Burgoyne, 2007). Further comparative analysis of the functions of VSNLs will be crucial to understand the precise functional impact of the switch.
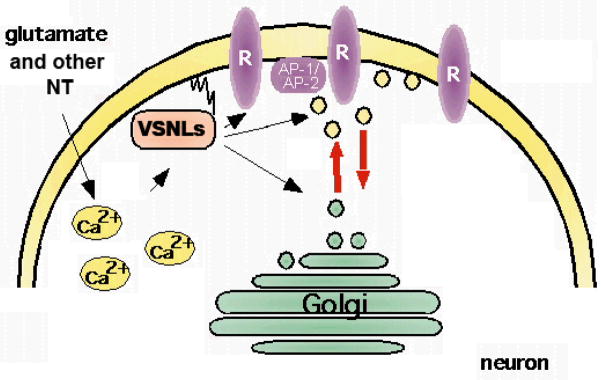
Fig. 2. The Ca2+-myristoyl switch of VSNLs and their localization to cellular membranes and target receptors.
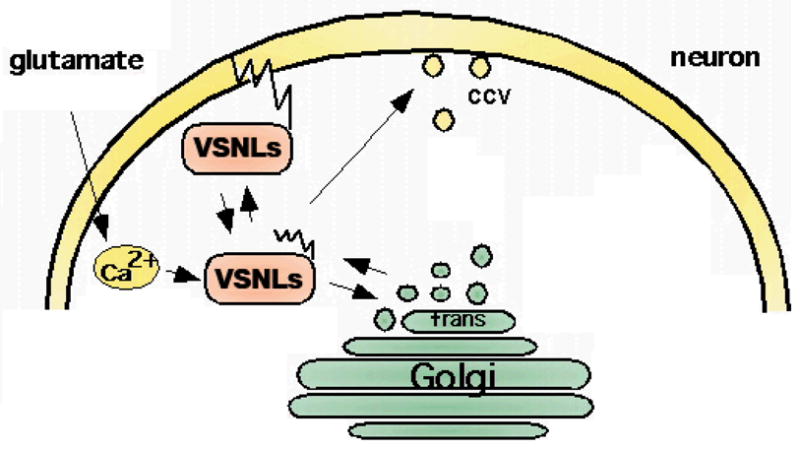
Following binding of Ca2+ a Ca2+-induced conformational change of cytosolic VSNLs occurs leading to the exposure of the hydrophobic myristoyl side chain. The Ca2+ sensors are able to shuttle from the cytosol to cellular membranes and can interact with membrane lipids. VSNLs are shown to associate with clathrin-coated vesicles (CCV) involved in endocytotic processes, with the cell surface membrane, and with trans-Golgi membranes. The Ca2+- and myristoylation-dependent interaction of VSNLs with cell membranes and phospholipids may be a pre-requisite for the interaction with membrane-bound target receptors.
The effect of VSNLs on signal transduction cascades
Spatially and temporally highly regulated increases of intracellular Ca2+ levels are evoked by a variety of extracellular signals including light, odorants, hormones, growth factors, electrical activity and neurotransmitters release. Neuronal Ca2+ sensors, such as VSNLs, serve as effectors to transduce these cellular Ca2+ signals. Similar to the prototypical Ca2+ sensor calmodulin, the VSNLs appear to be modulators of multiple intracellular targets showing a ‘pleiotropy’ of actions. There are several new observations shedding some light on the modulation of various Ca2+-dependent cellular signaling components and pathways by VSNLs.
VILIP-1 affects cAMP signaling
Initially, regulation of cAMP-levels by VILIP-1 has been detected in stably transfected rat C6 glioma cells (Braunewell et al., 1997; Braunewell and Gundelfinger, 1997). The myristoylation-deficient mutant of VILIP-1, which lacks the myristoylation consensus motif and therefore does not exhibit the Ca2+-myristoyl switch, showed a dominant-negative effect on cAMP-levels in C6 cells. Already basic cAMP levels appeared to be elevated in VILIP-1-transfected C6 cells, which is the cause for induction of differentiation of those glioma cells (Braunewell and Gundelfinger, 1997). Since the effect of VILIP-1 on adenylyl cyclase was not observed in other neural cell lines tested, such as PC12 or Neuro2A cells, the results pointed to an isoform-specific effect of VILIP-1 (Braunewell et al., 1997). Further support by more recent results indicate that VILIP-1 influences adenylyl cyclase activity in selected cell types including human embryonic kidney cells (Lin et al., 2002b), the pancreatic β cell line MIN6 (Dai et al., 2006), and various skin tumor cell lines (Mahloogi et al., 2003). High-level expression of VILIP-1 occurs in less aggressive squamous cell carcinoma (SCC) lines, but the expression is lost in more aggressive tumor cells. The reduced expression correlates with decreased cAMP-levels, which leads to increased expression of the metalloproteinase MMP-9 and enhanced rhoA activity. The loss of VILIP-1 and its effect on cAMP-dependent signaling accelerates invasiveness of carcinoma cells, which can be reversed by forcing the expression of VILIP-1 and thus, increasing VILIP-1-induced cAMP-levels (Mahloogi et al., 2003). Moreover, VILIP-1 is expressed in murine pancreatic islets and β cells. Overexpression of VILIP-1 in the MIN6 β cell line or isolated mouse islets increased cAMP-levels and at the same time increased glucose-stimulated insulin secretion, whereas knockdown of VILIP-1 decreased cAMP levels significantly. Similarly, the protein kinase A inhibitor H-89 attenuated increased glucose-stimulated insulin secretion. Thus, the effect of VILIP-1 on adenylyl cyclase in β cells was accompanied by enhanced exocytosis (Dai et al., 2006). Finally, VILIP-1 directly inhibits olfactory adenylyl cyclase type III activity in a Ca2+-dependent manner in olfactory membrane assays following odor stimulation, although at fairly high μM protein concentrations. These findings fit to the strong VILIP-1 immunoreactivity found in the olfactory epithelium especially in the olfactory knobs enriched in olfactory signaling cascades (Braunewell and Gundelfinger, 1999). Although the effect of VILIP-1 on olfactory guanylyl cyclase has not been tested yet, the protein is discussed as an additional modulator of olfactory adaptation processes (Boekhoff et al., 1997). Thus, the data obtained in very different cellular systems suggest a modulatory role for VILIP-1 on the activity of distinct adenylyl cyclase isoforms. No direct interaction of VILIP-1 with adenylyl cyclase isoforms have been detected, thus, an indirect modulation, for instance by regulating the trafficking and surface expression of distinct adenylyl cyclase isoforms might be possible. In peripheral organs, the VILIP-1 effect on cAMP-signaling appears to have important pathophysiological implications and identifies the protein as a putative tumor metastasis suppressor gene for human squamous cell carcinoma and non-small cell lung carcinoma (Wickborn et al., 2006; Fu et al., 2008).
VILIP-1 affects cGMP signaling
In stably transfected neural cell lines including C6 glioma, PC12 pheochromocytoma and Neuro2A neuroblastoma cells, VILIP-1 affects cGMP-levels. Following the stimulation of receptor GCs with natriuretic peptides an enhanced cGMP-accumulation was observed in the three cell lines stably expressing VILIP-1. The effect of VILIP-1 on the particulate receptor cyclases GC-A (NPR-A) and GC-B (NPR-B) depends on myristoylation and membrane-localization of wild type VILIP-1 and was strongly reduced with a myristoylation mutant (Braunewell et al., 2001). Thus, besides adenylyl cyclases VILIP-1 influences non-retinal receptor guanylyl cyclases, namely the receptor GC-A and -B in vitro. Moreover, the effect of VILIP-1 on cGMP-signaling was also found in primary cerebellar cultures, where the protein specifically affected neuronal GC-B, but not GC-A which is expressed in glial cells. The relevance of the effect of VILIP-1 on GC-B activity was further investigated in primary hippocampal neurons. In hippocampal neurons heterologous VILIP-1-expression led to an increase in the surface expression of GC-B and thereby enhances basal, as well as CNP-stimulated cGMP-accumulation. VILIP-1 attenuated the internalization of GC-B either by affecting endocytosis or recycling of the receptor. Thus, VILIP-1 might influence cGMP-dependent neuronal processes, including neuronal differentiation, neurite outgrowth, different forms of synaptic plasticity and learning and memory (Schuman and Madison, 1991; Teldegy, 1994; Monfort et al., 2002). VILIP-1 also interferes with recycling of the unrelated transferrin receptor and with clathrin-distribution in cells, indicating that the calcium sensor might be a general modulator of membrane trafficking (Brackmann et al., 2005).
VILIP-1 modulates α4β2 nicotinic acetylcholine receptors (nAChR)
VILIP-1 interacts with α4β2 nicotinic acetylcholine receptors (nAChR), and is able to modulate nAChR function (Lin et al., 2002a; Gierke et al., 2008). Co-expression of VILIP-1 with recombinant α4β2 nAChR up-regulated the surface expression levels by two-fold and increased the agonist-sensitivity to acetylcholine by three-fold. The VILIP-1 myristoylation mutant or mutants not able to bind Ca2+ are found to attenuate the modulation of α4β2 nAChR (Lin et al., 2002a). In hippocampal neurons, VILIP-1 was found to enhance surface expression and ligand sensitivity by affecting membrane trafficking of the receptor. VILIP-1 and α4β2 nAChR were found in a complex with the trans-Golgi SNARE syntaxin 6, involved in Golgi to surface membrane trafficking and constitutive exocytosis. Furthermore, VILIP-1 and α4β2 nAChR are both co-localized in a subpopulation of interneurons in the hippocampus (Zhao et al., 2008). Enhanced expression of VILIP-1 in interneurons in hippocampal cultures leads to an enhancement of ACh-evoked IPSCs (inhibitory postsynaptic currents) of nearby pyramidal neurons (Gierke et al., 2008). These results suggest that VILIP-1 represents a novel modulator of α4β2 nAChR, which increases the surface expression level and agonist sensitivity of the receptor in response to changes in the intracellular levels of Ca2+. Through the co-localization of VILIP-1 with α4β2 nAChR, particularly in interneurons, an enhancement of GABAergic neurotransmission is achieved, leading to increased IPSC activity in pyramidal neurons, thereby, changing the activity of the whole hippocampal network (Gierke et al., 2008).
VILIP-2 modulates P/Q-type Ca2+ channels
Branch one of the VSNLs also includes VILIP-2, which is 89% homologous to VILIP-1. Only few functional data are available for VILIP-2, which seems to binds to the presynaptic P/Q-type Ca2+ channel (CaV2.1). When co-expressing VSNL-2 with CaV2.1, an effect on the CaV2.1 inactivation rate was observed, which differed from the effect of calmodulin and CaBP1, and which was dependent on N-terminal myristoylation (Few et al., 2005; Lautermilch et al., 2005). Ca2+-activated calmodulin causes facilitation and enhances inactivation of CaV2.1 channels and Ca2+-binding protein 1 (CaBP1), a neurospecific CaM-like Ca2+ binding protein, accelerates inactivation and prevents facilitation. In contrast, myristoylated VILIP-2 did not alter CaV2.1 channel facilitation, but slowed the rate of inactivation and reduced inactivation of Ca2+-currents of the CaV2.1 channel during trains of repetitive depolarizations (Lautermilch et al., 2005). These data suggested that by regulating presynaptic CaV2.1 channels, VILIP-2, and other calcium sensors, can shape presynaptic Ca2+-transients, thereby affecting the time course and amount of neurotransmitter release. VILIP-1 might act as a potent regulator of synaptic transmission (Lautermilch et al., 2005). Although it has not been reported whether VILIP-2 influences the signaling pathways affected by VILIP-1, and vice versa, from the differential distribution of the two proteins in the cerebellum, where VILIP-1 but not VILIP-2 is present (Kajimoto et al., 1993), and the hippocampus where they show differential distribution (Saitoh et al., 1995), it can be assumed that despite their high homology the two proteins possess different cellular functions.
Neurocalcin δ affects receptor guanylyl cyclases
The second branch of VSNLs comprise hippocalcin, VILIP-3 and neurocalcin δ with homologies between 91% and 94%. Similar to VILIP-1 and VILIP-2, they show differential expression patterns, e.g. hippocalcin showing high abundance in the hippocampus and VILIP-3 in the cerebellum. VILIP-3 is least well examined compared to hippocalcin and neurocalcin δ and with respect to its function. Neurocalcin δ, like VILIP-1 and hippocalcin, influences cGMP-signaling by acting as a Ca2+-dependent regulator of the retinal guanylyl cyclase retGC1 (GC-E) (Kumar et al., 1999; Duda et al., 2001; Duda et al., 2004; Krishnan et al., 2004). RetGC1 and neurocalcin δ colocalize in neurons in the inner plexiform layer of the retina, e.g. in amacrine and ganglion cells, where they might influence synaptic signaling processes (Krishnan et al., 2004). This effect is specific for retGC1, since no influence on retGC2 or GC-A was observed (Kumar et al., 1999), the latter one being a target for VILIP-1 (Braunewell et al., 2001). Neurocalcin δ interacts with retGC1 and influences its function at 1μM Ca2+ concentration. The halfmaximal activity of neurocalcin δ was calculated at 0,8 μM. Neurocalcin δ is expressed in the olfactory epithelium and can interact and activate the olfactory guanylyl cyclase (GC-D) with similar sensitivity (Duda et al., 2004). Ca2+-bound myristoylated Neurocalcin δ binds to the catalytic domain of the olfactory GC-D and stimulated the cyclase with halfmaximal activity of approximately 0.8 μM neurocalcin δ (Duda and Sharma, 2008). Thus, neurocalcinδ is the second member of the VSNL subfamily to influence guanylyl cyclase family members.
Hippocalcin and adenylyl and guanylyl cyclase signaling
Hippocalcin, similar to VILIP-1 and neurocalcin δ, is the third VSNL to influence cyclic nucleotide signaling. At high Ca2+ concentrations, the activation of the olfactory adenylyl cyclase, but inhibition of the olfactory guanylyl cyclase ONE-GC (GC-D) was observed in membrane assays (Mammen et al., 2004). This effect is the opposite to what was found for VILIP-1, which inhibits olfactory adenylyl cyclase (Boekhoff et al., 1997), but is able to activate the natriuretic peptide receptor GC-B, which is abundantly expressed in the brain including the hippocampus and the olfactory epithelium (Braunewell et al., 2001; Brackmann et al., 2005). Thus, VILIP-1, neurocalcin δ and hippocalcin might be involved in fine-tuning of stimulus detection and odor adaptation mediated by cyclic nucleotide signaling in the olfactory epithelium. Moreover, using a GST fusion protein pull-down approach coupled with MALDI-MS and Western blotting, hippocalcin as well as neurocalcin δ were shown to interact with CaM-dependent cyclic nucleotide 3′,5′-phosphodiesterase (PDE) and CAPS1, a Ca2+-dependent activator protein for secretion which is involved in dense-core vesicle exocytosis (Haynes et al., 2005). Influence of the cAMP-PDE would provide yet an additional opportunity to influence cyclic nucleotide signaling, in addition to influencing cyclases directly. However, functional consequences of these interactions have not been determined yet.
Hippocalcin and ERK-signaling
Several lines of evidence indicate that hippocalcin influences the MAPK and related pathways. In a yeast two-hybrid interaction screen hippocalcin was identified as a possible interaction partner of MLK2/3 (mixed-lineage Ser/Thr kinases) (Nagata et al., 1998). MLKs are closely related to the mitogen-activated protein kinase kinase kinase (MAPKKK) family (Tibbles and Woodgett, 1999). MLK2 and 3 interact with the small GTPases Rac and Cdc42, with motor proteins of the kinesin superfamily and co-localize with the tubulin cytoskeleton, suggesting that they are involved in regulation of cellular cytoskeleton dynamics. MLK2/3 activates JNK (c-Jun N-terminal kinase), ERK (extracellular signal-regulated kinase) and p38 (Tibbles and Woodgett, 1999). However, to date no influence on kinase activity of MLKs has been observed. Therefore, the biological significance of the MLK-hippocalcin interaction remains unclear. In transgenic hippocalcin −/− mice an impaired CREB activation was observed which might be caused by a malfunctioning ERK cascade, since an ERK cascade inhibitor blocked NMDA- and KCl-stimulated CREB phosphorylation in control hippocampal slices but not in the knock-out mice (Kobayashi et al., 2005). When the Ca2+-dependent Ras/Raf/MEK/ERK signaling cascade was further tested, no direct effect of Ca2+-bound hippocalcin on Raf-1 kinase or MEK was observed. Hippocalcin did also not have an effect on the activation of ras, however, the −/− mice still displayed a defect in NMDA- and KCl-induced activation of Raf-1 kinase and ERK activation. This is explained as a possible effect of hippocalcin on alternative Raf-1 kinase activation pathways, such as protein kinase B/Akt and 14-3-3 protein (Noguchi et al., 2007). Noteworthy, hippocalcin in conjunction with the small rho-like GTPase cdc42 leads to a 1.8 fold increase in the Ca2+-dependent, but PKC-independent PLD activation. Hippocalcin alone did not activate PLD activity. This was postulated to be related to MAPK-dependent activation of PLD (Hyun et al., 2000). One testable possibility would be that hippocalcin affects MAPK signaling via regulating gene expression.
Similar effect of VILIP-3 and Hippocalcin on ERK-signaling and microsomal monooxygenase
VILIP-3 is mainly expressed in cerebellar Purkinje cells and might therefore be specialized to fulfill the signaling requirements of this neuronal cell type (Spilker et al., 2000, 2002a). However, expression in hippocampal neurons (Spilker and Braunewell, 2003) and in many peripheral organs (Pribanic et al., 2003; Gierke et al., 2004) also point to more general functions. VILIP-3 in contrast to VILIP-1, does not influence cGMP-signaling (Spilker et al., 2002a). Differences in the membrane association properties of VILIP-1 and VILIP-3 (Spilker and Braunewell, 2003), together with the fact that both proteins interact with a different set of putative binding partners (Spilker et al., 2002a), make it quite likely that VILIP-3 activates a distinct set of signaling cascades. Since VILIP-3 is highly homologous to hippocalcin, and hippocalcin was found initially to interact with MAP kinases (Nagata et al., 1998), and later on was found to influence phosphorylation of the MAP-kinase ERK2 (Kobayashi et al., 2005), an effect of VILIP-3 on MAPK activation seemed likely. Theoretically, since in hippocalcin-deficient mice reduced ERK2-activation was demonstrated (Kobayashi et al., 2005), the overexpression of VILIP-3 should then lead to increased ERK activation. This was investigated in transfected PC12 cells following VILIP-3-transfection, where a difference in the degree of ERK1/2-phosphorylation was detected in comparison to non-transfected cells (Spilker et al., 2002a). The fact that VILIP-3 associates with intracellular juxtanuclear membranes and granular structures in neurons (Spilker and Braunewell, 2003) fits to its possible function as a putative modulator of ERK1/2 MAP kinases. Moreover, VILIP-3 as well as hippocalcin, were shown to interact with the microsomal cytochrome b5, located in the endoplasmic reticulum-perinuclear region (Oikawa et al., 2004). The observed Ca2+-dependent translocation to the trans-Golgi and ER-rich perinuclear region, indicate that VILIP-3 may influence the microsomal monooxygenase complex composed of Cyb5-reductase, cytochrome P450 and other reductases of the ER (Oikawa et al., 2004). To date the functional implications of these interactions are still unknown. Additionally, VILIP-3-expressing cells also show an effect on ERK2 protein expression, indicating that VILIP-3 might affect gene expression of these downstream signaling proteins.
The effects of VSNLs on gene expression, intracellular trafficking, synaptic plasticity and neurodegeneration
VSNLs directly or indirectly affect gene expression
In hippocalcin −/− mice NMDA-stimulation- and depolarization-induced phosphorylation of cAMP-response element-binding protein (CREB) was shown to be attenuated, suggesting an impairment in an activity-dependent gene expression cascade (Kobayashi et al., 2005). Similar to these reports on hippocalcin-dependent CREB phosphorylation, hippocalcin appears to be involved in ERK-mediated transcriptional regulation of PLD2 expression (Oh et al., 2006). Thus, hippocalcin might influence gene expression (Kobayashi et al., 2005, Oh et al., 2006). Moreover, it was shown that bFGF induces hippocalcin expression in the immortalized hippocampal cell line H19-7 cells through PLC-gamma activation, which then leads to neurite outgrowth. Overexpression of hippocalcin dramatically elongated neurites and increased the expression of basic helix-loop-helix transcription factor, NeuroD. Treatment of H19-7 cells with hippocalcin siRNA completely blocked the bFGF-induced neurite outgrowth as well as NeuroD expression (Oh et al., 2008). These results indicate that hippocalcin affects neurite outgrowth via regulating NeuroD. NeuroD is a neuronal differentiation factor implicated in neuronal differentiation. The precise mechanism of the hippocalcin effect on NeuroD activity is unclear yet. Similarly, overexpression of VILIP-1 in mouse pancreatic β cells, which increased cAMP levels, was accompanied by increased transcription of preproinsulin mRNA. VILIP-1 also influenced expression of genes involves in cell cycle regulation, such as cyclin D2, Gsk3b, and transcription factors, such as cAMP-responsive element-binding protein (CREB) gene expression as well as the homeodomain transcription factor pdx-1 (Dai et al., 2006). VILIP-1 has also been shown to bind in a calcium-dependent manner to the double-stranded RNA of the neurotrophin receptor, trkB, localized to hippocampal dendrites (Mathisen et al., 1999), implying that VILIP-1 might play a role in regulation of gene expression as well. Moreover, VILIP-1 was found to be upregulated in highly-invasive human neuroblastoma cells, where it potentiated adhesion dependent apoptosis-resistance (anoikis-resistance) of neuroblastoma cells (Xie et al., 2008). Increased VILIP-1 expression led the upregulation of anoikis inhibitor trkB, but to the downregulation of intracellular adhesion molecule 1 (ICAM-1), major histocompatibility complex class I (MHC-1), and cell adhesion molecules CD44 and CD44v6. Thereby, VILIP-1 was shown to regulate cell migration of neuroblastoma cells, and hence influencing invasive properties of brain tumor cells. Taken together, these results suggest that VSNLs plays a role in gene expression either indirectly via increasing cAMP levels, ERK or raf-kinase signaling acting on transcription factors such as CREB, or directly acting as a modulator of transcription factors such as NeuroD. Interestingly in this context, KChIPs (potassium channel interacting proteins), a related subfamily of NCS proteins, act as Ca2+-dependent transcriptional repressors through direct binding to the downstream regulatory element (DRE) sequence present in a variety of genes (Carrion et al., 1999, Link et al., 2004). Particularly KChIP3, also known as DREAM (downstream regulatory element antagonistic modulator), has been shown to control expression of genes such as prodynorphin, the apoptotic hrk gene and the thyroblobulin gene (Carrion et al., 1999; Sanz et al., 2001; Cheng et al., 2002; Rivas et al., 2004; Zaidi et al., 2006). Dream has also been shown to directly interact with transcription factors such as CREM and CREB (Ledo et al., 2000, 2002; Melstrom et al., 2008). To date it is not clear whether VSNLs may also directly bind to transcription factors, and thereby influence gene expression or whether the effects are mostly indirect via affecting MAPK and cAMP-signaling pathways.
Hippocalcin affects AMPAR endocytosis, potassium channels and synaptic plasticity
Increasing evidence shows that NMDA receptor-dependent activation of the MAPK pathway and the associated CREB-translational activation is critical for neuronal plasticity and is an important molecular mechanism for learning and memory mechanisms (Wang et al., 2007). In line with this notion, hippocalcin −/− mice display deficits in spatial and associative memory (Kobayashi et al., 2005). Moreover, hippocalcin has been shown to function as a Ca2+ sensor in hippocampal long-term depression (LTD) (Palmer et al., 2005). Hippocalcin has been implicated in the Ca2+-dependent endocytosis of the GluR1 subunit of glutamate receptors of the AMPA-type relevant for NMDAR-dependent LTD. AMPAR endocytosis leads to depression of synaptic plasticity in the hippocampus (Palmer et al., 2005). More recently, hippocalcin was shown to gate the, yet unidentified, potassium channel that mediates the slow afterhyperpolarization current (IsAHP) in the hippocampus of hippocalcin −/− mice (Tzingounis et al., 2007). Thus, via Ca2+-dependent modulation of IsAHP hippocalcin may also affect NMDA receptor-mediated plasticity and memory formation. These results show once again the usefulness of the transgenic approach for unraveling physiological functions of NCS proteins.
VSNLs as mediators of Ca2+-regulated neurotoxicity: neuroprotective versus neurotoxic roles
For VILIP-1 and VILIP-3 it was reported that the number and staining intensity of immunoreactive neurons is reduced in the temporal cortex and in parts of the limbic system, the entorhinal cortex, in Alzheimer disease (AD) brains (Braunewell et al., 2001). These findings pointed to an involvement of the two NCS proteins in pathology and possibly pathophysiology of changed calcium homeostasis and hint towards a high vulnerability of VILIP-1-expressing neurons in AD. In calcium-induced cytotoxicity assays in PC12 cells transfected with VILIP-1 and/or the calcium buffer protein calbindin-D28K, VILIP-1 expression enhanced the neurotoxic effect of the calcium ionophor ionomycin, whereas calbindin-D28K protected against ionomycin-induced cytotoxicity (Schnurra et al., 2001). VILIP-1 expression also enhanced hyperphosphorylation of tau protein compared to non-transfected cells. Again, this VILIP-1 effect was attenuated via co-expression with the calcium buffer protein calbindin-D28K. Interestingly, VILIP-1 was also found to be associated with fibrillary tangles in AD brains (Braunewell et al., 2001). In contrast, hippocalcin was shown to be neuroprotective in various different neurotoxicity assays. Hippocalcin interacts with NAIP, the neuronal apoptosis inhibitory protein, and co-expression of both proteins synergistically facilitated neuronal survival against calcium-induced death stimuli such as ionomycin and thapsigargin (Mercer et al., 2000). Whereas, NAIP-hippocalcin interaction rescued neuroblastoma cells from cell death induced by high levels of calcium, in sympathetic neurons no significant effect on neuronal death induced by nerve growth factor (NGF) withdrawal was observed (Lindholm et al., 2002). These findings were supported by data from hippocalcin −/− mice which were more sensitive to thapsigargin induced cell death and excitotoxicity caused by kainic acid and quinolinic acid (Korhonen et al., 2005; Masuo et al., 2007). Moreover, these mice displayed increased caspase-12 activation and age-dependent increase in neurodegeneration (Korhonen et al., 2005). Thus, VSNL1 might play neurotoxic as well as neuroprotective roles in different types of neurons in the CNS and PNS. It will be interesting to further investigate the possible implications of VSNLs in different neurodegenerative disorders in the future.
An emerging theme: VSNLs affecting membrane trafficking
Besides the effect of NCS proteins on gene expression, which currently gets increasing attention, an emerging theme is their effect on trafficking of ion channels and surface receptors. NCS proteins, such as KChIPs and NCS-1, have been shown to influence surface expression of Ca2+- and potassium channels (An et al., 2000; Weiss et al., 2000). KChIP1 influences post ER trafficking of Kv4 potassium channels (An et al., 2000; O’Callaghan et al., 2003b). NCS-1 interacts with ARF1 to control trans-Golgi network-plasma membrane trafficking of the Kv4 potassium channel (Zhao et al., 2001; Haynes et al., 2005). In an approach to find proteins that are linked to clathrin-mediated trafficking, VILIP-1 and VILIP-3 were identified to interact with clathrin-coated vesicles from rat brain (Fig. 2, Blondeau et al., 2004). Vesicles which were enriched in clathrin heavy and light chain, the α, β, γ, μ–adaptin subunits of the AP-2 complex, which functions at the plasma membrane, and the AP-1 complex, which functions at the trans-Golgi network (TGN), where also associated with VILIP-1 and VILIP-3 (Fig. 2). Thus, VSNLs might be implicated in clathrin-dependent membrane trafficking processes, which is in line with a proposed role of VILIP-1 as regulator of post-Golgi membrane trafficking. VILIP-1 is able to increase cell surface expression of α4β2 nAChRs (Lin et al., 2002), but also influences receptor recycling of transferrin and GC-B receptor in clathrin-dependent manner (Brackmann et al., 2005). Neurocalcin δ is able to interact with α and β-clathrin and β-2-adaptin, important adapter proteins for receptor endocytosis (Ivings et al., 2002). Similarly, hippocalcin binds the β-2-adaptin subunit of the AP2 adaptor complex, which along with GluR2 can co-immunoprecipitate in a Ca2+-sensitive manner. Since infusion of a truncated mutant of hippocalcin that lacked the Ca2+-binding domains prevents synaptically evoked LTD, with no effect on LTP, it has been debated that the AP2-hippocalcin complex acts as a Ca2+-sensor that couples NMDAR-dependent activation to regulated endocytosis of AMPARs during LTD (Palmer et al., 2005). However, it should be noted that GST-hippocalcin and GST-neurocalcin δ binding to α-adaptin or γ-adaptin from brain extracts could not be confirmed in a large-scale protein pull-down approach (Haynes et al., 2006). More recently, neurocalcin δ has been implicated in the trafficking and membrane delivery of glutamate receptors of the kainate type, which have been identified as key players in the modulation of neuronal network activity (Coussen et al., 2006). Neurocalcin δ binds to the C-terminal domain of the GluR6b kainate receptor isoform and, thereby, might regulate kainate receptor trafficking and function during synapse formation and synaptic plasticity, similar to the effect of AMPA and NMDA receptor regulation (Coussen and Mulle, 2006). Thus, VSNLs appear to interact with molecules connected to membrane trafficking and to affect trafficking of multiple receptors, however, the mechanism of the VSNL-effect on the transport machinery yet needs to be discovered.
Critical remarks: artifacts, pleiotropy and in vivo functions
A variety of effects of VSNLs on signaling pathways have been identified, indicating that VSNLs similar to the prototypical calcium sensor calmodulin, have pleiotropic functions in the brain and peripheral organs. However, only a few signaling components from these pathways have been identified as interaction partners. The search for specific interaction partners for VSNLs turned out to be difficult (Spilker et al. 2002b; Haynes et al., 2006), and the signaling and physiological significance of the interactions that have been found are often unclear yet (Haynes et al., 2006). Only for hippocalcin an interaction partner has been characterized in respect to physiological function on the in vitro as well as in vivo level in knockout animals (Mercer et al., 2000; Lindholm et al., 2002; Korhonen et al., 2005). In other cases interaction with signaling pathways, e.g. guanylyl cyclase, has been shown (Braunewell et al., 2001; Duda et al., 2001, 2004), however, the precise interaction domains of VSNLs with these interaction partners have not been mapped yet and physiological functions are not known so far (Brackmann et al., 2005; Venkataraman et al., 2008; Duda and Sharma., 2008). The effect of VILIP-1 on adenylyl cyclase is most likely indirect, since no interaction has been found and no influence in membrane cyclase assays was detected (unpublished data). It is conceivable that some of the signaling effects are dependent on indirect effects, e.g. by affecting cross talk between signaling pathways, but effects on trafficking of receptors and membrane-localized signaling compounds are also conceivable, particularly since this is one of the novel emerging signaling mechanisms of VSNLs. There is definitely a need for more and comparative yeast 2-hybrid and biochemical studies, even though the outcome might be limited and functional significance might be uncertain. In some cases functional effects e.g. on cAMP signaling have been initially only described in overexpression studies (Braunewell et al., 1997). Nevertheless, in the case of cAMP signaling the results have been consistently repeated by different labs (Lin et al., 2002b; Mahloogi et al., 2003) and finally were also supported by siRNA knock down studies (Dai et al., 2006). In the future, use of siRNA and other knockout technologies will have to prove the correctness of many of the so far identified signaling functions. In terms of physiological functions only few knockouts, e.g. hippocalcin −/− mutants, have been generated and partially analyzed, e.g. towards neuroprotective function (Korhonen et al., 2005; Masuo et al., 2007) and functions in learning and memory processes (Kobayashi et al., 2005). Noticeably, these proteins have been implicated in a series of pathophysiological functions in diseases ranging from hypertension to diabetic nephropathy, ALS, stroke, Alzheimer’s disease and schizophrenia (Kamide et al., 2005; Kamiyama et al., 2007; Lederer et al., 2007; Laterza et al., 2006; Schnurra et al., 2001; Gierke et al., 2008). The molecular and cellular mechanisms of some of these pathophysiological roles have just started to be elucidated (Gierke et al., 2008). Thus, future analysis of knockout animals and the combination of these knockouts will greatly help to understand the physiological as well as the pathophysiological functions of this important family of Ca2+-sensor proteins. This area of research will largely benefit from concerted research action in different labs in order to confirm data and current working hypothesis of effects on signaling, gene expression and membrane trafficking of these different but closely related members of VSNLs.
Concluding remarks
The VSNL subfamily of NCS proteins defines a novel set of Ca2+-dependent signaling cascades in nerve and cancer cells. The Ca2+-myristoyl switch mechanism of VSNLs can shuttle information from the cytoplasm to the cell membrane and vice versa enabling the different VSNLs to confer Ca2+-dependence to intracellular signaling pathways, such cyclic nucleotide and MAPK signaling. VSNLs might regulate membrane trafficking processes via different vesicular pathways from the ER and Golgi to cell surface and the recycling pathway for receptors (Fig. 3). Finally, VSNLs might regulate gene expression. Through these global mechanisms VSNLs may contribute to processes of cell death, migration, differentiation and neuronal plasticity under physiological and pathological conditions.
Fig. 3. The VSNL-subfamily of NCS proteins may serve as Ca2+-dependent modulators of membrane receptor trafficking.
Following neurotransmitter activation and neuronal activity VSNLs Ca2+-dependently translocate to intracellular and cell surface membranes. VILIP-1 is a modulator of membrane-localized natriuretic peptide receptor (GCs) and nicotinic acetylcholine receptor (nAChRs) function and trafficking (Lin et al., 2002; Brackmann et al., 2005; Gierke et al., 2008). Similar to VILIP-1, neurocalcin δ and hippocalcin have been identified as modulators of guanylyl cyclase (olfactory and retinal guanylyl cyclase receptors) (Mammen et al., 2004). Moreover, VILIP-1 and neurocalcinδ have been implicated in modulating the trafficking of glutamate receptors of the kainate subtype (Coussen et al., 2006; Coussen and Mulle, 2006). Hippocalcin acts as a Ca2+ sensor for AMPA receptor endocytotic trafficking (Palmer et al., 2005). Thus, VSNLs might influence membrane trafficking of receptors at different check points, such as endo- or exocytosis or receptor recycling.
Acknowledgments
Work in KHB’s laboratories has been supported in the past by grants from DFG (Br1579/8-1 and Br1579/9-1, Priority Program of the German Research Foundation SPP1226), Deutsche Krebshilfe, Charite Berlin and Kultusministerium des Landes Sachsen-Anhalt. Work in AJK’s laboratory was supported by grants from the National Institutes of Health CA107257, CA06927, by an appropriation from the Commonwealth of Pennsylvania, and by a grant from the Pennsylvania Department of Health.
References
- Ames JB, Tanaka T, Stryer L, Ikura M. Portrait of a myristoyl switch protein. Curr Opin Struct Biol. 1996;6:432–438. doi: 10.1016/s0959-440x(96)80106-0. [DOI] [PubMed] [Google Scholar]
- Ames JB, Ishima R, Tanaka T, Gordon JI, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. doi: 10.1038/38310. [DOI] [PubMed] [Google Scholar]
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Bastianelli E, Polans AS, Hidaka H, Pochet R. Differential distribution of six calcium-binding proteins in the rat olfactory epithelium during postnatal development and adulthood. J Comp Neurol. 1995;354:395–409. doi: 10.1002/cne.903540308. [DOI] [PubMed] [Google Scholar]
- Bernstein H-G, Baumann B, Danos P, Diekmann S, Bogerts B, Gundelfinger ED, Braunewell K-H. Regional and cellular distribution of neural visinin-like protein immunoreactivities (VILIP-1 and VILIP-3) in human brain. J Neurocytol. 1999;28:655–662. doi: 10.1023/a:1007056731551. [DOI] [PubMed] [Google Scholar]
- Blandini F, Braunewell K-H, Manahan-Vaughan D, Orzi F, Sarti P. Neurodegeneration and energy metabolism: from chemistry and clinics. Cell Death Differ. 2004;11:479–484. doi: 10.1038/sj.cdd.4401323. [DOI] [PubMed] [Google Scholar]
- Blondeau F, Ritter B, Allaire PD, Wasiak S, Girard M, Hussain NK, Angers A, Legendre-Guillemin V, Roy L, Boismenu D, Kearney RE, Bell AW, Bergeron JJ, McPherson PS. Tandem MS analysis of brain clathrin-coated vesicles reveals their critical involvement in synaptic vesicle recycling. Proc Natl Acad Sci U S A. 2004;101:3833–3838. doi: 10.1073/pnas.0308186101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boekhoff I, Braunewell K-H, Andreini I, Breer H, Gundelfinger ED. The calcium-binding protein VILIP in olfactory neurons: regulation of second messenger signaling. Eur J Cell Biol. 1997;72:151–158. [PubMed] [Google Scholar]
- Brackmann M, Schuchmann S, Anand R, Braunewell K-H. Neuronal Ca2+ sensor protein VILIP-1 affects cGMP signalling of guanylyl cyclase B by regulating clathrin-dependent receptor recycling in hippocampal neurons. J Cell Sci. 2005;118:2495–2505. doi: 10.1242/jcs.02376. [DOI] [PubMed] [Google Scholar]
- Braunewell K-H, Gundelfinger ED. Intracellular neuronal calcium sensor proteins: a family of EF-hand calcium-binding proteins in search of a function. Cell Tissue Res. 1999;299:1–12. doi: 10.1007/s004410051207. [DOI] [PubMed] [Google Scholar]
- Braunewell K-H, Brackmann M, Schaupp M, Spilker C, Anand R, Gundelfinger ED. Intracellular neuronal calcium sensor (NCS) protein VILIP-1 modulates cGMP signalling pathways in transfected neural cells and cerebellar granule neurones. J Neurochem. 2001;78:1277–1286. doi: 10.1046/j.1471-4159.2001.00506.x. [DOI] [PubMed] [Google Scholar]
- Braunewell K-H, Riederer P, Spilker C, Gundelfinger ED, Bogerts B, Bernstein HG. Abnormal localization of two neuronal calcium sensor proteins, visinin-like proteins (VILIPs)-1 and -3, in neocortical brain areas of Alzheimer disease patients. Dement Geriatr Cogn Disord. 2001;2:110–115. doi: 10.1159/000051244. [DOI] [PubMed] [Google Scholar]
- Braunewell K-H, Gundelfinger ED. Low level expression of calcium-sensor protein VILIP induces cAMP- dependent differentiation in rat C6 glioma cells. Neurosci Lett. 1997;234:139–142. doi: 10.1016/s0304-3940(97)00696-4. [DOI] [PubMed] [Google Scholar]
- Braunewell K-H, Spilker C, Behnisch T, Gundelfinger ED. The neuronal calcium-sensor protein VILIP modulates cyclic AMP accumulation in stably transfected C6 glioma cells: amino-terminal myristoylation determines functional activity. J Neurochem. 1997;68:2129–2139. doi: 10.1046/j.1471-4159.1997.68052129.x. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, O’Callaghan DW, Hasdemir B, Haynes LP, Tepikin AV. Neuronal Ca2+-sensor proteins: multitalented regulators of neuronal function. Trends Neurosci. 2004;27:203–209. doi: 10.1016/j.tins.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Weiss JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD. Neuronal calcium sensor proteins: generating diversity in neuronal Ca2+ signalling. Nat Rev Neurosci. 2007;8:182–193. doi: 10.1038/nrn2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion AM, Link WA, Ledo F, Mellstrom B, Naranjo JR. DREAM is a Ca2+-regulated transcriptional repressor. Nature. 1999;398:80–84. doi: 10.1038/18044. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Pitcher GM, Laviolette SR, Whishaw IQ, Tong KI, Kockeritz LK, Wada T, Joza NA, Crackower M, Goncalves J, Sarosi I, Woodgett JR, Oliveira-dos-Santos AJ, Ikura M, van der Kooy D, Salter MW, Penninger JM. DREAM is a critical transcriptional repressor for pain modulation. Cell. 2002;108:31–43. doi: 10.1016/s0092-8674(01)00629-8. [DOI] [PubMed] [Google Scholar]
- Coussen F, Mulle C. Kainate receptor-interacting proteins and membrane trafficking. Biochem Soc Trans. 2006;34:927–930. doi: 10.1042/BST0340927. [DOI] [PubMed] [Google Scholar]
- Coussen F, Perrais D, Jaskolski F, Sachidhanandam S, Normand E, Bockaert J, Marin P, Mulle C. Co-assembly of two GluR6 kainate receptor splice variants within a functional protein complex. Neuron. 2006;47:555–566. doi: 10.1016/j.neuron.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Cox JA, Durussel I, Comte M, Nef S, Nef P, Lenz SE, Gundelfinger ED. Cation binding and conformational changes in VILIP and NCS-1, two neuron-specific calcium-binding proteins. J Biol Chem. 1994;269:32807–32813. [PubMed] [Google Scholar]
- Dai FF, Zhang Y, Kang Y, Wang Q, Gaisano HY, Braunewell KH, Chan CB, Wheeler MB. The neuronal Ca2+ sensor protein visinin-like protein-1 is expressed in pancreatic islets and regulates insulin secretion. J Biol Chem. 2006;281:21942–2153. doi: 10.1074/jbc.M512924200. [DOI] [PubMed] [Google Scholar]
- De Raad S, Comte M, Nef P, Lenz SE, Gundelfinger ED, Cox JA. Distribution pattern of three neural calcium-binding proteins (NCS-1, VILIP and recoverin) in chicken, bovine and rat retina. Histochem J. 1995;27:524–535. doi: 10.1007/BF02388752. [DOI] [PubMed] [Google Scholar]
- Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolley D, Walsh KA, Philipov PP, Hurley JB, Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991;251:915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- Duda T, Sharma RK. ONE-GC membrane guanylate cyclase, a trimodal odorant signal transducer. Biochem Biophys Res Commun. 2008;367:440–445. doi: 10.1016/j.bbrc.2007.12.153. [DOI] [PubMed] [Google Scholar]
- Duda T, Fik-Rymarkiewicz E, Venkataraman V, Krishnan A, Sharma RK. Calcium-modulated ciliary membrane guanylate cyclase transduction machinery: constitution and operational principles. Mol Cell Biochem. 2004;267:107–122. doi: 10.1023/b:mcbi.0000049372.33965.4f. [DOI] [PubMed] [Google Scholar]
- Duda T, Jankowska A, Venkataraman V, Nagele RG, Sharma RK. A novel calcium-regulated membrane guanylate cyclase transduction system in the olfactory neuroepithelium. Biochemistry. 2001;40:12067–12077. doi: 10.1021/bi0108406. [DOI] [PubMed] [Google Scholar]
- Few AP, Lautermilch NJ, Westenbroek RE, Scheuer T, Catterall WA. Differential regulation of CaV2.1 channels by calcium-binding protein 1 and visinin-like protein-2 requires N-terminal myristoylation. J Neurosci. 2005;25:7071–7080. doi: 10.1523/JNEUROSCI.0452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu J, Fong K, Bellacosa A, Ross E, Apostolou S, Bassi DE, Jin F, Zhang J, Cairns P, de Caceres II, Braunewell KH, Klein-Szanto AJ. VILIP-1 downregulation in non-small cell lung carcinomas: mechanisms and prediction of survival. PLoS ONE. 2008;3:e1698. doi: 10.1371/journal.pone.0001698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierke P, Zhao C, Bernstein H-G, Noack C, Anand R, Heinemann U, Braunewell K-H. Implication of neuronal Ca2+-Sensor Protein VILIP-1 in the glutamate hypothesis of Schizophrenia. NBD. 2007 doi: 10.1016/j.nbd.2008.07.008. in press. [DOI] [PubMed] [Google Scholar]
- Gierke P, Zhao C, Linke B, Brackmann M, Heinemann U, Braunewell K-H. Expression analysis of members of the neuronal calcium sensor protein family: combining bioinformatics and Western blot analysis. Biochem Biophys Res Commun. 2004;323:38–43. doi: 10.1016/j.bbrc.2004.08.055. [DOI] [PubMed] [Google Scholar]
- Gonzalez Guerrico AM, Jaffer ZM, Page RE, Braunewell K-H, Chernoff J, Klein-Szanto AJP. Visinin-like protein-1 is a potent inhibitor of cell adhesion and migration in squamous carcinoma cells. Oncogene. 2005;24:2307–2316. doi: 10.1038/sj.onc.1208476. [DOI] [PubMed] [Google Scholar]
- Grant AL, Jones A, Thomas KL, Wisden W. Characterization of the rat hippocalcin gene: the 5′ flanking region directs expression to the hippocampus. Neuroscience. 1996;75:1099–1115. doi: 10.1016/0306-4522(96)00344-2. [DOI] [PubMed] [Google Scholar]
- Hamashima H, Tamaru T, Noguchi H, Kobayashi M, Takamatsu K. Immunochemical assessment of neural visinin-like calcium-binding protein 3 expression in rat brain. Neurosci Res. 2001;39:133–143. doi: 10.1016/s0168-0102(00)00208-x. [DOI] [PubMed] [Google Scholar]
- Haynes LP, Fitzgerald DJ, Wareing B, O’Callaghan DW, Morgan A, Burgoyne RD. Analysis of the interacting partners of the neuronal calcium-binding proteins L-CaBP1, hippocalcin, NCS-1 and neurocalcin delta. Proteomics. 2006;6:1822–1832. doi: 10.1002/pmic.200500489. [DOI] [PubMed] [Google Scholar]
- Haynes LP, Thomas GM, Burgoyne RD. Interaction of neuronal calcium sensor-1 and ADP-ribosylation factor 1 allows bidirectional control of phosphatidylinositol 4-kinase {beta} and trans-Golgi network-plasma membrane traffic. J Biol Chem. 2005;280:6047–6054. doi: 10.1074/jbc.M413090200. [DOI] [PubMed] [Google Scholar]
- Hyun JK, Yon C, Kim YS, Noh DY, Lee KH, Han JS. Role of hippocalcin in Ca2+-induced activation of phospholipase D. Mol Cells. 2000;10:669–677. doi: 10.1007/s10059-000-0669-1. [DOI] [PubMed] [Google Scholar]
- Ivings L, Pennington SR, Jenkins R, Weiss JL, Burgoyne RD. Identification of Ca2+-dependent binding partners for the neuronal calcium sensor protein neurocalcin delta: interaction with actin, clathrin and tubulin. Biochem J. 2002;363:599–608. doi: 10.1042/0264-6021:3630599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivings L, Pennington SR, Jenkins R, Weiss JL, Burgoyne RD. Identification of Ca2+-dependent binding partners for the neuronal calcium sensor protein neurocalcin delta: interaction with actin, clathrin and tubulin. Biochem J. 2002;363:599–608. doi: 10.1042/0264-6021:3630599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jheng FF, Wang L, Lee L, Chang LS. Functional contribution of Ca2+ and Mg2+ to the intermolecular interaction of visinin-like proteins. Protein J. 2006;25:250–256. doi: 10.1007/s10930-006-9008-5. [DOI] [PubMed] [Google Scholar]
- Kajimoto Y, Shirai Y, Mukai H, Kuno T, Tanaka C. Molecular cloning of two additional members of the neural visinin-like Ca(2+)-binding protein gene family. J Neurochem. 1993;61:1091–1096. doi: 10.1111/j.1471-4159.1993.tb03624.x. [DOI] [PubMed] [Google Scholar]
- Kamide K, Kokubo Y, Yang J, Tanaka C, Hanada H, Takiuchi S, Inamoto N, Banno M, Kawano Y, Okayama A, Tomoike H, Miyata T. Hypertension susceptibility genes on chromosome 2p24-p25 in a general Japanese population. J Hypertens. 2005;23:955–960. doi: 10.1097/01.hjh.0000166835.70935.3c. [DOI] [PubMed] [Google Scholar]
- Kamiyama M, Kobayashi M, Araki S, Iida A, Tsunoda T, Kawai K, Imanishi M, Nomura M, Babazono T, Iwamoto Y, Kashiwagi A, Kaku K, Kawamori R, Ng DP, Hansen T, Gaede P, Pedersen O, Nakamura Y, Maeda S. Polymorphisms in the 3′ UTR in the neurocalcin delta gene affect mRNA stability, and confer susceptibility to diabetic nephropathy. Hum Genet. 2007;122:397–407. doi: 10.1007/s00439-007-0414-3. [DOI] [PubMed] [Google Scholar]
- Kato M, Watanabe Y, Iino S, Takaoka Y, Kobayashi S, Haga T, Hidaka H. Cloning and expression of a cDNA encoding a new neurocalcin isoform (neurocalcin alpha) from bovine brain. Biochem J. 1998;331:871–876. doi: 10.1042/bj3310871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Masaki T, Hori K, Masuo Y, Miyamoto M, Tsubokawa H, Noguchi H, Nomura M, Takamatsu K. Hippocalcin-deficient mice display a defect in cAMP response element-binding protein activation associated with impaired spatial and associative memory. Neuroscience. 2005;133:471–484. doi: 10.1016/j.neuroscience.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Takamatsu K, Saitoh S, Nogushi T. Myristoylation of hippocalcin is linked to its membrane association properties. J Biol Chem. 1993;268:18898–18904. [PubMed] [Google Scholar]
- Korhonen L, Hansson I, Kukkonen JP, Brannvall K, Kobayashi M, Takamatsu K, Lindholm D. Hippocalcin protects against caspase-12-induced and age-dependent neuronal degeneration. Mol Cell Neurosci. 2005;28:85–95. doi: 10.1016/j.mcn.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Kraut N, Frampton J, Graf T. Rem-1, a putative direct target gene of the Myb-Ets fusion oncoprotein in haematopoietic progenitors, is a member of the recoverin family. Oncogene. 1995;10:1027–1036. [PubMed] [Google Scholar]
- Krishnan A, Venkataraman V, Fik-Rymarkiewicz E, Duda T, Sharma RK. Structural, biochemical, and functional characterization of the calcium sensor neurocalcin delta in the inner retinal neurons and its linkage with the rod outer segment membrane guanylate cyclase transduction system. Biochemistry. 2004;43:2708–2723. doi: 10.1021/bi035631v. [DOI] [PubMed] [Google Scholar]
- Kumar VD, Vijay-Kumar S, Krishnan A, Duda T, Sharma RK. A second calcium regulator of rod outer segment membrane guanylate cyclase, ROS-GC1: neurocalcin. Biochemistry. 1999;38:12614–12620. doi: 10.1021/bi990851n. [DOI] [PubMed] [Google Scholar]
- Kuno T, Kajimoto Y, Hashimoto T, Mukai H, Shirai Y, Saheki S, Tanaka C. cDNA cloning of a neural visinin-like Ca(2+)-binding protein. Biochem Biophys Res Commun. 1992;184:1219–25. doi: 10.1016/s0006-291x(05)80012-9. [DOI] [PubMed] [Google Scholar]
- Ladant D. Calcium and membrane binding properties of bovine neurocalcin delta expressed in Escherichia coli. J Biol Chem. 1995;270:3179–3185. [PubMed] [Google Scholar]
- Lautermilch NJ, Few AP, Scheuer T, Catterall WA. Modulation of CaV2.1 channels by the neuronal calcium-binding protein visinin-like protein-2. J Neurosci. 2005;25:7062–7070. doi: 10.1523/JNEUROSCI.0447-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterza OF, Modur VR, Crimmins DL, Olander JV, Landt Y, Lee JM, Ladenson JH. Identification of novel brain biomarkers. Clin Chem. 2006;52:1713–1721. doi: 10.1373/clinchem.2006.070912. [DOI] [PubMed] [Google Scholar]
- Lederer CW, Torrisi A, Pantelidou M, Santama N, Cavallaro S. Pathways and genes differentially expressed in the motor cortex of patients with sporadic amyotrophic lateral sclerosis. BMC Genomics. 2007;8:26. doi: 10.1186/1471-2164-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledo F, Carrion AM, Link WA, Mellstrom B, Naranjo JR. DREAM-alphaCREM interaction via leucine-charged domains derepresses downstream regulatory element-dependent transcription. Mol Cell Biol. 2000;20:9120–9126. doi: 10.1128/mcb.20.24.9120-9126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledo F, Kremer L, Mellstrom B, Naranjo JR. Ca2+-dependent block of CREB-CBP transcription by repressor DREAM. EMBO J. 2002;21:4583–4592. doi: 10.1093/emboj/cdf440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz SE, Braunewell KH, Weise C, Nedlina-Chittka A, Gundelfinger ED. The neuronal EF-hand Ca(2+)-binding protein VILIP: interaction with cell membrane and actin-based cytoskeleton. Biochem Biophys Res Commun. 1996;225:1078–1083. doi: 10.1006/bbrc.1996.1298. [DOI] [PubMed] [Google Scholar]
- Lenz SE, Jiang S, Braun K, Gundelfinger ED. Localization of the neural calcium-binding protein VILIP (visinin-like protein) in neurons of the chick visual system and cerebellum. Cell Tissue Res. 1996;283:413–424. doi: 10.1007/s004410050552. [DOI] [PubMed] [Google Scholar]
- Lenz SE, Henschel Y, Zopf D, Voss B, Gundelfinger ED. VILIP, a cognate protein of the retinal calcium binding proteins visinin and recoverin, is expressed in the developing chicken brain. Brain Res Mol Brain Res. 1992;15:133–140. doi: 10.1016/0169-328x(92)90160-d. [DOI] [PubMed] [Google Scholar]
- Lenz SE, Zuschratter W, Gundelfinger ED. Distribution of visinin-like protein (VILIP) immunoreactivity in the hippocampus of the Mongolian gerbil (Meriones unguiculatus) Neurosci Lett. 1996;206:133–136. doi: 10.1016/s0304-3940(96)12444-7. [DOI] [PubMed] [Google Scholar]
- Lin L, Jeanclos EM, Treuil M, Braunewell KH, Gundelfinger ED, Anand R. The Calcium Sensor Protein Visinin-like Protein-1 Modulates the Surface Expression and Agonist-Sensitivity of the a4β2 Nicotinic Acetylcholine Receptor. J Biol Chem. 2002a;277:41872–41878. doi: 10.1074/jbc.M206857200. [DOI] [PubMed] [Google Scholar]
- Lin L, Braunewell KH, Gundelfinger ED, Anand R. Functional Analysis of Calcium-Binding EF-Hand Motifs of Visinin-like Protein-1. Biochem Biophys Res Commun. 2002b;296:827–832. doi: 10.1016/s0006-291x(02)00943-9. [DOI] [PubMed] [Google Scholar]
- Lindholm D, Mercer EA, Yu LY, Chen Y, Kukkonen J, Korhonen L, Arumae U. Neuronal apoptosis inhibitory protein: Structural requirements for hippocalcin binding and effects on survival of NGF-dependent sympathetic neurons. Biochim Biophys Acta. 2002;1600:138–147. doi: 10.1016/s1570-9639(02)00454-5. [DOI] [PubMed] [Google Scholar]
- Link WA, Ledo F, Torres B, Palczewska M, Madsen TM, Savignac M, Albar JP, Mellstrom B, Naranjo JR. Day-night changes in downstream regulatory element antagonist modulator/potassium channel interacting protein activity contribute to circadian gene expression in pineal gland. J Neurosci. 2004;24:5346–5355. doi: 10.1523/JNEUROSCI.1460-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahloogi H, Gonzalez-Guerrico AM, De Cicco RL, Bassi DE, Goodrow T, Braunewell KH, Klein-Szanto AJP. Graduate decrease of VILIP-1 Expression during mouse skin tumor progression and its role in regulating tumor cell invasive behavior. Cancer Res. 2003;63:4997–5004. [PubMed] [Google Scholar]
- Mammen A, Simpson PJ, Nighorn A, Imanishi Y, Palczewski K, Ronnett GV, Moon C. Hippocalcin in the olfactory epithelium: a mediator of second messenger signaling. Biochem Biophys Res Commun. 2004;322:1131–1139. doi: 10.1016/j.bbrc.2004.07.123. [DOI] [PubMed] [Google Scholar]
- Martinez-Guijarro FJ, Brinon JG, Blasco-Ibanez JM, Okazaki K, Hidaka H, Alonso JR. Neurocalcin-immunoreactive cells in the rat hippocampus are GABAergic interneurons. Hippocampus. 1998;8:2–23. doi: 10.1002/(SICI)1098-1063(1998)8:1<2::AID-HIPO2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Masuo Y, Ogura A, Kobayashi M, Masaki T, Furuta Y, Ono T, Takamatsu K. Hippocalcin protects hippocampal neurons against excitotoxin damage by enhancing calcium extrusion. Neuroscience. 2007;145:495–504. doi: 10.1016/j.neuroscience.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Mathisen PM, Johnson JM, Kawczak JA, Tuohy VK. Visinin-like protein (VILIP) is a neuron-specific calcium-dependent double-stranded RNA-binding protein. J Biol Chem. 1999;274:31571–3156. doi: 10.1074/jbc.274.44.31571. [DOI] [PubMed] [Google Scholar]
- Mellstrom B, Savignac M, Gomez-Villafuertes R, Naranjo JR. Ca2+-operated transcriptional networks: molecular mechanisms and in vivo models. Physiol Rev. 2008;88:421–449. doi: 10.1152/physrev.00041.2005. [DOI] [PubMed] [Google Scholar]
- Mercer EA, Korhonen L, Skoglosa Y, Olsson PA, Kukkonen JP, Lindholm D. NAIP interacts with hippocalcin and protects neurons against calcium-induced cell death through caspase-3-dependent and -independent pathways. EMBO J. 2000;19:3597–3607. doi: 10.1093/emboj/19.14.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfort P, Munoz MD, Kosenko E, Felipo V. Long-term potentiation in hippocampus involves sequential activation of soluble guanylate cyclase, cGMP-dependent protein kinase, and cGMP-degrading phosphodiesterase. J Neurosci. 2002;22:10116–10124. doi: 10.1523/JNEUROSCI.22-23-10116.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K, Puls A, Futter C, Aspenstrom P, Schaefer E, Nakata T, Hirokawa N, Hall A. The MAP kinase kinase kinase MLK2 co-localizes with activated JNK along microtubules and associates with kinesin superfamily motor KIF3. EMBO J. 1998;17:149–158. doi: 10.1093/emboj/17.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano A, Terasawa M, Watanabe M, Usuda N, Morita T, Hidaka H. Neurocalcin, a novel calcium binding protein with three EF-hand domains, expressed in retinal amacrine cells and ganglion cells. Biochem Biophys Res Commun. 1992;186:1207–1211. doi: 10.1016/s0006-291x(05)81534-7. [DOI] [PubMed] [Google Scholar]
- Nef P. Neuron-specific calcium sensors (the NCS subfamily) In: Celio MR, editor. Guidebook to the calcium-binding proteins. Oxford University Press; New York: 1996. pp. 94–98. [Google Scholar]
- Noguchi H, Kobayashi M, Miwa N, Takamatsu K. Lack of hippocalcin causes impairment in Ras/extracellular signal-regulated kinase cascade via a Raf-mediated activation process. J Neurosci Res. 2007;85:837–844. doi: 10.1002/jnr.21180. [DOI] [PubMed] [Google Scholar]
- O’Callaghan DW, Ivings L, Weiss JL, Ashby MC, Tepikin AV, Burgoyne RD. Differential use of myristoyl groups on neuronal calcium sensor proteins as a determinant of spatio-temporal aspects of Ca2+ signal transduction. J Biol Chem. 2002;277:14227–14237. doi: 10.1074/jbc.M111750200. [DOI] [PubMed] [Google Scholar]
- O’Callaghan DW, Tepikin AV, Burgoyne RD. Dynamics and calcium sensitivity of the Ca2+-myristoyl switch protein hippocalcin in living cells. J Cell Biol. 2003;163:715–721. doi: 10.1083/jcb.200306042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Callaghan DW, Hasdemir B, Leighton M, Burgoyne RD. Residues within the myristoylation motif determine intracellular targeting of the neuronal Ca2+ sensor protein KChIP1 to post-ER transport vesicles and traffic of Kv4 K+ channels. J Cell Sci. 2003b;116:4833–4845. doi: 10.1242/jcs.00803. [DOI] [PubMed] [Google Scholar]
- O’Callaghan DW, Haynes LP, Burgoyne RD. High-affinity interaction of the N-terminal myristoylation motif of the neuronal calcium sensor protein hippocalcin with phosphatidylinositol 4,5-bisphosphate. Biochem J. 2005;391:231–238. doi: 10.1042/BJ20051001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh DY, Yon C, Oh KJ, Lee KS, Han JS. Hippocalcin increases phospholipase D2 expression through extracellular signal-regulated kinase activation and lysophosphatidic acid potentiates the hippocalcin-induced phospholipase D2 expression. J Cell Biochem. 2006;97:1052–1065. doi: 10.1002/jcb.20665. [DOI] [PubMed] [Google Scholar]
- Oh DY, Cho JH, Park SY, Kim YS, Yoon YJ, Yoon SH, Chung KC, Lee KS, Han JS. A novel role of hippocalcin in bFGF-induced neurite outgrowth of H19-7 cells. J Neurosci Res. 2008;86:1557–1565. doi: 10.1002/jnr.21602. [DOI] [PubMed] [Google Scholar]
- Ohya S, Horowitz B. Differential transcriptional expression of Ca2+ BP superfamilies in murine gastrointestinal smooth muscles. Am J Physiol Gastrointest Liver Physiol. 2002;283:1290–1297. doi: 10.1152/ajpgi.00101.2002. [DOI] [PubMed] [Google Scholar]
- Oikawa K, Kimura S, Aoki N, Atsuta Y, Takiyama Y, Nagato T, Yanai M, Kobayashi H, Sato K, Sasajima T, Tateno M. Neuronal calcium sensor protein visinin-like protein-3 interacts with microsomal cytochrome b5 in a Ca2+-dependent manner. J Biol Chem. 2004;279:15142–1552. doi: 10.1074/jbc.M312766200. [DOI] [PubMed] [Google Scholar]
- Palmer CL, Lim W, Hastie PG, Toward M, Korolchuk VI, Burbidge SA, Banting G, Collingridge GL, Isaac JT, Henley JM. Hippocalcin functions as a calcium sensor in hippocampal LTD. Neuron. 2005;47:487–494. doi: 10.1016/j.neuron.2005.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterlini M, Revilla V, Grant AL, Wisden W. Expression of the neuronal calcium sensor protein family in the rat brain. Neuroscience. 2000;99:205–216. doi: 10.1016/s0306-4522(00)00201-3. [DOI] [PubMed] [Google Scholar]
- Pribanic S, Gisler S, Bacic D, Kocher O, Braunewell K-H, Nakadai T, Murer H, Biber J. Expression of visinin-like protein 3 in mouse kidney. Nephron -Physiology. 2003;95:76–82. doi: 10.1159/000074844. [DOI] [PubMed] [Google Scholar]
- Rivas M, Mellstrom B, Naranjo JR, Santisteban P. Transcriptional repressor DREAM interacts with thyroid transcription factor-1 and regulates thyroglobulin gene expression. J Biol Chem. 2004;279:33114–33122. doi: 10.1074/jbc.M403526200. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Kobayashi M, Kuroki T, Noguchi T, Takamatsu K. The development of neural visinin-like Ca(2+)-binding protein 2 immunoreactivity in the rat neocortex and hippocampus. Neurosci Res. 1995;23:383–388. doi: 10.1016/0168-0102(95)00968-Y. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Takamatsu K, Kobayashi M, Noguchi T. Expression of hippocalcin in the developing rat brain. Brain Res Dev Brain Res. 1994;80:199–208. doi: 10.1016/0165-3806(94)90105-8. [DOI] [PubMed] [Google Scholar]
- Saitoh S, Takamatsu K, Kobayashi M, Noguchi T. Distribution of hippocalcin mRNA and immunoreactivity in rat brain. Neurosci Lett. 1993;157:107–110. doi: 10.1016/0304-3940(93)90654-4. [DOI] [PubMed] [Google Scholar]
- Sanz C, Mellstrom B, Link WA, Naranjo JR, Fernandez-Luna JL. Interleukin 3-dependent activation of DREAM is involved in transcriptional silencing of the apoptotic Hrk gene in hematopoietic progenitor cells. EMBO J. 2001;20:2286–2292. doi: 10.1093/emboj/20.9.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- Spilker C, Richter K, Smalla K-H, Manahan-Vaughan D, Gundelfinger ED, Braunewell K-H. The neuronal EF-hand calcium-binding protein VILIP-3 is expressed in cerebellar Purkinje cells and shows a calcium-dependent membrane association. Neurosci. 2000;96:121–129. doi: 10.1016/s0306-4522(99)00536-9. [DOI] [PubMed] [Google Scholar]
- Spilker C, Braunewell K-H. The calcium-myristoyl switch of neuronal calcium sensor (NCS) proteins: same biochemical principle but different calcium-dependent localization of VILIP-3 and -1 in hippocampal neurons. Mol Cell Neurosci. 2003;24:766–778. doi: 10.1016/s1044-7431(03)00242-2. [DOI] [PubMed] [Google Scholar]
- Spilker C, Dresbach T, Braunewell K-H. Reversible translocation and activity-dependent localization of the calcium-myristoyl switch protein VILIP-1 to different membrane compartments in living hippocampal neurons. J Neurosci. 2002b;22:7331–7339. doi: 10.1523/JNEUROSCI.22-17-07331.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilker C, Gundelfinger ED, Braunewell K-H. Different properties of the intracellular neuronal Calcium sensor proteins VILIP-1 and VILIP-3: from subcellular localization to cellular function BBA-proteins and proteomics. 2002a;1600:118–127. doi: 10.1016/s1570-9639(02)00452-1. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ames JB, Harvey TS, Stryer L, Ikura M. Sequestration of the membrane-targeting myristoyl group of recoverin in the calcium-free state. Nature. 1995;376:444–447. doi: 10.1038/376444a0. [DOI] [PubMed] [Google Scholar]
- Telegdy G. The action of ANP, BNP and related peptides on motivated behavior in rats. Rev Neurosci. 1994;5:309–315. doi: 10.1515/revneuro.1994.5.4.309. [DOI] [PubMed] [Google Scholar]
- Teruel MN, Meyer T. Translocation and reversible localization of signaling proteins: a dynamic future for signal transduction. Cell. 2000;103:181–184. doi: 10.1016/s0092-8674(00)00109-4. [DOI] [PubMed] [Google Scholar]
- Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Kobayashi M, Takamatsu K, Nicoll RA. Hippocalcin gates the calcium activation of the slow afterhyperpolarization in hippocampal pyramidal cells. Neuron. 2007;53:487–493. doi: 10.1016/j.neuron.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman V, Duda T, Ravichandran S, Sharma RK. Neurocalcin delta Modulation of ROS-GC1, a New Model of Ca(2+) Signaling. Biochemistry. 2008 doi: 10.1021/bi800394s. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Fibuch EE, Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- Weiss JL, Archer DA, Burgoyne RD. NCS-1/Frequenin functions in an autocrine pathway regulating Ca2+ channels in bovine adrenal chromaffin cells. J Biol Chem. 2000;275:40082–40087. doi: 10.1074/jbc.M008603200. [DOI] [PubMed] [Google Scholar]
- Wickborn C, Klein-Szanto AJ, Schlag PM, Braunewell KH. Correlation of visinin-like-protein-1 expression with clinicopathological features in squamous cell carcinoma of the esophagus. Mol Carcinog. 2006;45:572–581. doi: 10.1002/mc.20201. [DOI] [PubMed] [Google Scholar]
- Xie Y, Chan H, Fan J, Chen Y, Young J, Li W, Miao X, Yuan Z, Wang H, Tam PK, Ren Y. Involvement of visinin-like protein-1 (VSNL-1) in regulating proliferative and invasive properties of neuroblastoma. Carcinogenesis. 2007;28:2122–2130. doi: 10.1093/carcin/bgm147. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Goto K, Kuo CH, Kondo H, Miki N. Visinin: a novel calcium binding protein expressed in retinal cone cells. Neuron. 1990;4:469–476. doi: 10.1016/0896-6273(90)90059-o. [DOI] [PubMed] [Google Scholar]
- Zaidi NF, Kuplast KG, Washicosky KJ, Kajiwara Y, Buxbaum JD, Wasco W. Calsenilin interacts with transcriptional co-repressor C-terminal binding protein(s) J Neurochem. 2006;98:1290–1301. doi: 10.1111/j.1471-4159.2006.03972.x. [DOI] [PubMed] [Google Scholar]
- Zhao C, Braunewell K-H. Expression of the neuronal calcium sensor VILIP-1 in the rat hippocampus. Neuroscience. 2008;153:1202–1212. doi: 10.1016/j.neuroscience.2007.10.067. [DOI] [PubMed] [Google Scholar]
- Zhao X, Varnai P, Tuymetova G, Balla A, Toth ZE, Oker-Blom C, Roder J, Jeromin A, Balla T. Interaction of neuronal calcium sensor-1 (NCS-1) with phosphatidylinositol 4-kinase beta stimulates lipid kinase activity and affects membrane trafficking in COS-7 cells. J Biol Chem. 2001;276:40183–40189. doi: 10.1074/jbc.M104048200. [DOI] [PubMed] [Google Scholar]
- Zozulya S, Stryer L. Calcium-myristoyl protein switch. Proc Natl Acad Sci U S A. 1992;89:11569–11573. doi: 10.1073/pnas.89.23.11569. [DOI] [PMC free article] [PubMed] [Google Scholar]