Abstract
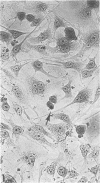
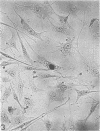
The time course of formation of inclusion bodies produced by Chlamydia in 5-iodo-2-deoxyuridine (IUdR)-treated McCoy's cells was studied with the use of a known isolate of Chlamydia trachomatis D/UW-184/Ur and 47 frozen clinical urethral specimens previously shown to be either positive or negative for chlamydial inclusions after 3 days of incubation. Subsequent examination of 369 clinical specimens from the genitourinary tract over a 6-month period revealed 47 (13%) Chlamydia-positive cultures, all of which demonstrated inclusion bodies by iodine staining at 40 and 64 h postinoculation. Another 146 similar detected by iodine staining from 22 (15%). This study indicates that, although Chlamydia subgroup A inclusions are larger at 64 h, they can be readily detected from clinical specimens in IUdR-treated McCoy's cells at 40 h postinfection.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Croy T. R., Kuo C. C., Wang S. P. Comparative susceptibility of eleven mammalian cell lines to infection with trachoma organisms. J Clin Microbiol. 1975 May;1(5):434–439. doi: 10.1128/jcm.1.5.434-439.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan V. S., Jenkin H. M. Glycogen metabolism in Chlamydia-infected HeLa-cells. J Bacteriol. 1970 Oct;104(1):608–609. doi: 10.1128/jb.104.1.608-609.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. ISOLATION OF THE TRACHOMA AGENT IN CELL CULTURE. Proc Soc Exp Biol Med. 1965 Feb;118:354–359. doi: 10.3181/00379727-118-29841. [DOI] [PubMed] [Google Scholar]
- Gordon F. B., Harper I. A., Quan A. L., Treharne J. D., Dwyer R. S., Garland J. A. Detection of Chlamydia (Bedsonia) in certain infections of man. I. Laboratory procedures: comparison of yolk sac and cell culture for detection and isolation. J Infect Dis. 1969 Oct;120(4):451–462. doi: 10.1093/infdis/120.4.451. [DOI] [PubMed] [Google Scholar]
- Holmes K. K., Handsfield H. H., Wang S. P., Wentworth B. B., Turck M., Anderson J. B., Alexander E. R. Etiology of nongonococcal urethritis. N Engl J Med. 1975 Jun 5;292(23):1199–1205. doi: 10.1056/NEJM197506052922301. [DOI] [PubMed] [Google Scholar]
- Kaufman R. E., Wiesner P. J. Nonspecific urethritis. N Engl J Med. 1974 Nov 28;291(22):1175–1177. doi: 10.1056/NEJM197411282912208. [DOI] [PubMed] [Google Scholar]
- Reeve R., Taverne J. Strain differences in the behavior of TRIC agnets in cell cultures. Am J Ophthalmol. 1967 May;63(5 Suppl):1167–1173. doi: 10.1016/0002-9394(67)94099-8. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Weed L. A., Segura J. W., Pettersen G. R., Washington JA I. I. Isolation of Chlamydia from patients with urethritis. Mayo Clin Proc. 1975 Mar;50(3):105–110. [PubMed] [Google Scholar]
- Thygeson P. The matrix of the epithelial cell inclusion body of trachoma. Am J Pathol. 1938 Jul;14(4):455–462.1. [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., Alexander E. R. Isolation of Chlamydia trachomatis by use of 5-iodo-2-deoxyuridine-treated cells. Appl Microbiol. 1974 May;27(5):912–916. doi: 10.1128/am.27.5.912-916.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]