Abstract
Objective
We have recently shown that ghrelin, a novel orexigenic hormone, is reduced in sepsis. Ghrelin treatment, mediated through ghrelin receptors in the brain, attenuates sepsis-induced inflammation and mortality. Gut barrier dysfunction is common in sepsis. High mobility group B1 (HMGB1) increases gut permeability both in vitro and in vivo. However, it remains unknown whether ghrelin has any effects on HMGB1 and gut barrier function in sepsis. We hypothesized that ghrelin decreases HMGB1 release and attenuates sepsis-induced gut barrier dysfunction through central ghrelin receptors.
Design
Prospective, controlled, and randomized animal study.
Setting
A research institute laboratory.
Subjects
Male Sprague-Dawley rats (275-325g).
Measurements and Main Results
Male adult rats were subjected to sepsis by cecal ligation and puncture (CLP). Five hours after CLP, a bolus intravenous injection of 2 nmol ghrelin was followed by a continuous infusion of 12 nmol ghrelin via an osmotic mini-pump for 15h. Twenty hours after CLP, brain ghrelin levels, serum HMGB1 levels, ileal mucosal permeability to fluorescein-isothiocyanate dextran (FD4), bacterial counts in the mesenteric lymph nodes complex, and gut water content were determined. In additional groups of animals, bilateral trunk vagotomy was performed at 5h after CLP before ghrelin injection. Moreover, to confirm the role of central ghrelin receptors in ghrelin's effect, ghrelin (1 nmol) was administered through intracerebroventricular injection at 5h after CLP. Our results showed that brain levels of ghrelin decreased by 34% at 20 h after CLP. Intravenous administration of ghrelin completely restored brain levels of ghrelin, significantly reduced the elevated HMGB1 levels, and attenuated gut barrier dysfunction. Vagotomy eliminated ghrelin's inhibition on HMGB1 and attenuation on gut barrier dysfunction. Intracerebroventricular injection of ghrelin decreased serum HMGB1 levels and ameliorated gut barrier dysfunction.
Conclusion
Ghrelin reduces serum HMGB1 levels and ameliorates gut barrier dysfunction in sepsis by vagus nerve activation via central ghrelin receptors. Ghrelin can be further developed as a novel agent to protect gut barrier function in sepsis.
Keywords: Sepsis, ghrelin, gut barrier function
Introduction
Sepsis remains a critical problem with significant morbidity and mortality even in the modern era of critical care management (1,2). Gut barrier dysfunction, manifested by increased mucosal permeability to hydrophilic macromolecules and increased bacterial translocation to mesenteric lymph nodes (MLN), occurs in sepsis (3-5). The decrease in gut barrier function has been associated with augmentation of systemic inflammation and distant organ dysfunction under such conditions (5,6). Thus, the therapeutic potential for an agent to prevent gut barrier compromise and attenuate gut-derived inflammatory response is significant.
Ghrelin, an orexigenic hormone, was first identified in the rat stomach in 1999 as an endogenous ligand for the growth hormone secretagogue receptor type 1a (GHSR-1a, i.e., ghrelin receptor) (7). Recent studies have indicated that ghrelin plays an important role in the regulation of pituitary hormone secretion, feeding, energy homeostasis, gastrointestinal function, and cardiovascular and immune system (8-10). Our recent studies have shown that circulating levels of ghrelin decreased significantly in a rat model of polymicrobial sepsis induced by cecal ligation and puncture (CLP), and ghrelin administration decreases TNF-α and IL-6, increases cardiac output, improves organ blood flow, reduces mortality under such conditions (10-13). The beneficial effect of ghrelin in sepsis is mediated through ghrelin receptors in the central nervous system (10,14).
High mobility group box 1 (HMGB1) is a late mediator of lethal sepsis (15). Once outside the cell, it acts as a potent mediator of inflammation (16). HMGB1 levels are significantly elevated in the blood of septic patients (15,17), suggesting that extracellular HMGB1 is involved in the pathogenesis of sepsis (18-20). A study has shown that HMGB1 increases gut permeability both in vitro and in vivo (21). Blockade of HMGB1 improves gut barrier function and decreases mortality after shock (22). However, it remains unknown whether ghrelin has any effects on HMGB1 levels and gut barrier function in polymicrobial sepsis. This study was conducted to test the hypothesis that ghrelin decreases HMGB1 levels and attenuates sepsis-induced gut barrier dysfunction through central ghrelin receptors.
Materials and Methods
Experimental animals
Male Sprague-Dawley rats (275-325g), purchased from Charles River Laboratories (Wilmington, MA), were used in this study. The rats were housed in a temperature-controlled room on a 12-h light/dark cycle and fed on a standard Purina rat chow diet. The rats were fasted for 12 h prior to the procedure. Animal experimentation was carried out in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources). This project was approved by the Institutional Animal Care and Use Committee of The Feinstein Institute for Medical Research.
Animal model of sepsis
Sepsis was induced by cecal ligation and puncture (CLP) as previously described (13). Briefly, the rats were anesthetized and a 2-cm ventral midline abdominal incision was performed. The cecum was then exposed, ligated just distal to the ileocecal valve to avoid intestinal obstruction, punctured twice with an 18-gauge needle, and returned to the abdominal cavity. The incision was then closed in layers. Sham-operated animals (i.e., control animals) underwent the same procedure with the exception that the cecum was neither ligated nor punctured. The animals were resuscitated with 3 ml/100g body weight (BW) normal saline subcutaneously immediately after surgery. The animals were then returned to their cages with free access to food and water.
Intravenous (IV) injection of ghrelin
Rat ghrelin (Phoenix Pharmaceuticals, Belmont, CA) was dissolved in normal saline to a final concentration of 100 μM. 200-μl mini-pumps (Alzet, infusion rate 8 μl/h) were primed with ghrelin solution or vehicle (normal saline) for 3 h prior to implantation. After a slow intravenous bolus injection of 2 nmol ghrelin (or 200 μl vehicle) at 5 h after CLP (i.e., post-treatment), the mini-pump was then connected to a jugular venous catheter and implanted subcutaneously. Twenty hours after CLP (i.e., 15 h after implantation of the mini-pump), the rats were sacrificed and blood and tissue samples were collected. The total dose of ghrelin each rat received was 14 nmol (i.e., ∼45 nmol/kg BW). The dose of ghrelin was determined based on our previous experience (10,12).
Vagotomy
In additional groups of animals, the trunks of the subdiaphragmatic vagus were transected as previously described (10). Briefly, the rats were re-anesthetized with isoflurane inhalation at 5 h after CLP or sham operation. The midline abdominal incision was reopened prior to the administration of ghrelin, and the dorsal and ventral branches of the vagus nerve were dissected from the esophagus. Ghrelin or vehicle (normal saline) was administrated intravenously immediately following vagotomy as described above in the vagus nerve intact animals. Twenty hours after CLP (i.e., 15 h after implantation of the mini-pump), the rats were sacrificed and blood and tissue samples were collected.
Intracerebroventricular (ICV) injection of ghrelin
ICV injection of ghrelin was performed as described recently (14). Briefly, rats were anesthetized with pentobarbital (intraperitoneal injection, 40 mg/kg BW) and placed in a stereotactic head frame (Stoelting, Wood Dale, IL) at 5 h after CLP. The incisor bar was adjusted until the plane defined by the lambda and bregma were parallel to the base plate. The musculature on the skull was removed, and the skull exposed. A hole (1-2 mm diameter) was drilled through the skull with a hand-operated drill (Dremel Robert Bosch Tool, Mount Prospect, IL). The needle of a Hamilton syringe (25 μl) was stereotactically guided into lateral ventricle (0.8 mm posterior to bregma, 1.5 mm lateral to midline, 3.0 mm below the dura). Ghrelin (1 nmol) was dissolved in sterile endotoxin-free normal saline (10 μl) and administered in 1 min. The hole was sealed by the use of cyanoacrylic glue. The rats were then removed from the stereotactic head frame and placed in a supine position on an acrylic board. Twenty hours after CLP, the rats were sacrificed and blood and tissue samples were collected. The location of ICV injections was confirmed by histological examination of the brain after the experiment.
Determination of brain ghrelin levels
At 20 h after CLP or sham operation, brain samples were rapidly harvested. The tissues were excised, rinsed of blood, homogenized with polytron in a homogenization buffer (phosphate-buffered saline solution, containing 0.05% Triton X-100 and a protease inhibitor cocktail; pH, 7.2; 4°C), and sonicated for 10 seconds. Homogenates were centrifuged at 12,000 g for 10 minutes, and ghrelin levels were quantified using an enzyme-linked immunosorbent assay (ELISA) kit specifically for human and rat active ghrelin (100% crossreactivity with rat active ghrelin, Linco Research, Inc, St. Charles, MO). The assay was carried out according to the instructions provided by the manufacturer. Brain levels of ghrelin were normalized to the protein concentration in the sample.
Determination of high mobility group box 1 (HMGB1)
The levels of HMGB1 in the serum were assayed by Western blot analysis using rabbit polyclonal antibodies. Briefly, serum (5 μl) was fractionated on 4-12% Bis-Tris gel and transferred to a 0.2 μm-nitrocellulose membrane. Nitrocellulose blots were blocked by incubation in TBST (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween 20) containing 5% milk for 1 h. Blots were incubated with rabbit anti-rat antibodies overnight at 4 °C. The blots were then washed in TBST for 5×10 min. Blots were incubated with HRP-labeled goat anti-rabbit IgG for 1 h at room temperature. The blots were then washed in TBST for 5×10 min. A chemiluminescent peroxidase substrate (ECL, Amersham Biosciences) was applied according to the manufacturer's instruction, and the membranes were exposed to X-ray film. The western blot relative band intensity was quantified by ChemiImager 5500 software. The levels of HMGB-1 were calculated with reference to standard curves generated from purified rHMGB-1.
Determination of intestinal mucosal permeability
Intestinal barrier function was assessed by measuring translocation of the fluorescent tracer, fluorescein isothiocyanate dextran with a molecular weight of 4000 Da (FD4, Sigma) by the everted gut sac method as described by Fink and associates (3,22-24) and recently used by us (25). Briefly, a ∼15 cm long ileal segment was harvested from each animal at 20 h after CLP or sham operation. Gut sacs were prepared in ice-cold modified Krebs-Henseleit bicarbonate buffer (KHBB, pH 7.4). One end of the gut segment was ligated with suture, and then the segment was everted onto a thin plastic rod. The resulting sac was secured with another suture to the grooved tip of a 3-ml plastic syringe containing KHBB. The sac was gently distended by injecting 1.5 ml KHBB and suspended for 30 min in a 50-ml beaker containing 40 ml KHBB plus FD4 (40 mg/ml). The solution in the beaker was temperature jacketed at 37 °C and was continuously bubbled with a gas mixture containing 95% O2, 5% CO2. The FD4 concentration of the fluid in the beaker and the inside of the sac was determined spectrofluorometrically, and permeability was expressed as the mucosal-to-serosal clearance of FD4.
Determination of bacterial translocation
The mesenteric lymph nodes (MLN) complex was harvested and rinsed carefully in a large volume of sterile normal saline to minimize the possibility of bacteria contamination from the peritoneal cavity. Equal amount of wet tissues was homogenized and briefly centrifuged to remove gross particulate matters. Serial log dilutions of tissue homogenates were applied. Five hundred μl of each dilution was then plated on chocolate agar plates (Fisher Scientific) and incubated at 37 °C for 24 h under aerobic conditions. The colony-forming units (CFU) were counted and results were expressed as CFU per gram of tissue.
Water content determination
Gut edema was estimated by comparing tissue water content. Briefly, a ∼5 cm long ileal segment was harvested from each animal at 20 h after CLP or sham operation. Gut tissues were dried in a 70 °C oven for 48 h. Gut water content was calculated as % H2O = (1 - dry wt/wet wt) × 100%.
Statistical analysis
All data are expressed as mean ± standard error (SE) and compared by one-way analysis of variance (ANOVA) and Student-Newman-Keuls test for multiple group analysis. Differences in values were considered significant if P<0.05.
Results
Brain levels of ghrelin are reduced in sepsis
Our previous study has shown that circulating ghrelin levels decreased significantly after CLP (11). However, the alteration in brain ghrelin levels remains unknown. In this regard, we have measured brain levels of ghrelin after CLP with or without intravenous ghrelin administration. As shown in Figure 1, brain levels of ghrelin decreased by 34% at 20 h after CLP. Intravenous administration of ghrelin restored the reduced brain levels of ghrelin completely. This result also confirms that ghrelin can cross the blood-brain barrier to produce its beneficial effects centrally.
Figure 1.
Alterations in brain levels of ghrelin in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Ghrelin or vehicle was administered intravenously at 5 h after CLP. Data are presented as means ± SE (n=6) and compared by one-way ANOVA and Student-Newman-Keuls method: * P<0.05 versus Sham group; # P<0.05 versus Vehicle group.
Ghrelin reduces serum HMGB1 levels and ameliorates gut barrier dysfunction in sepsis
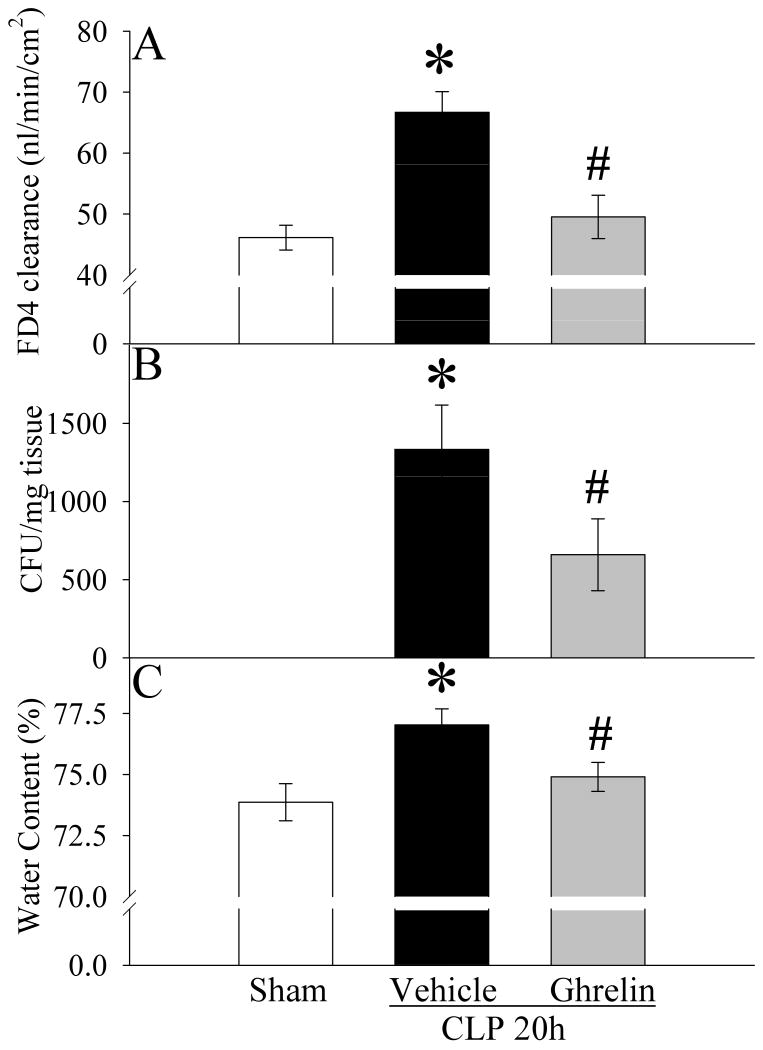
HMGB1 is a critical mediator of lethal sepsis (15). To examine the effect of ghrelin on HMGB1 release, HMGB1 levels were measured at 20 h after CLP with or without ghrelin treatment. As indicated in Figure 2, serum levels of HMGB1 increased by 1.5 fold at 20 h after CLP compared with sham-operated controls (P<0.05). Treatment with ghrelin reduced serum HMGB1 levels by 32% in septic animals (P<0.05, Fig. 2). As indicated in Figure 3A, ileal mucosal permeability to the fluorescent macromolecule, FD4, was significantly increased at 20 h after CLP in vehicle treated animals as compared with sham controls (P<0.05). Similarly, bacterial translocation to MLN was minimal in the sham group, but was extensive in the CLP vehicle treated group (P<0.05, Fig. 3B). Treatment with ghrelin, however, significantly ameliorated the development of both ileal mucosal hyperpermeability and bacterial translocation. As shown in Figure 3C, rats subjected to sepsis had a significant increase in gut water content (i.e., edema) as compared with sham-operated animals (P<0.05). When septic animals were treated with ghrelin, the water content of the gut (P<0.05, Fig. 3C) was reduced significantly and there was no statistical difference in gut water content between sham-operated and CLP ghrelin treated animals.
Figure 2.
Alterations in serum levels of HMGB1 in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Ghrelin or vehicle was administered intravenously at 5 h after CLP. Data are presented as means ± SE (n=6) and compared by one-way ANOVA and Student-Newman-Keuls method: * P<0.05 versus Sham group; # P<0.05 versus Vehicle group.
Figure 3.
Alterations in intestinal mucosal permeability (A) to fluorescein isothiocyanate dextran with a molecular weight of 4000 Da (FD4), bacterial translocation to mesenteric lymph nodes (B), and gut water content (C) in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Ghrelin or vehicle was administered intravenously at 5 h after CLP. Data are presented as means ± SE (n=6-8) and compared by one-way ANOVA and Student-Newman-Keuls method: * P<0.05 versus Sham group; # P<0.05 versus Vehicle group.
Ghrelin's beneficial effects in sepsis require the intact vagus nerve
To determine whether ghrelin's beneficial effects in sepsis require the intact vagus nerve, vagotomy was performed in sham and septic animals immediately prior to the administration of ghrelin. As indicated in Figure 4, vagotomy completely eliminated the beneficial effect of this agent on serum levels of HMGB1. Similarly, ghrelin's effects on ileal mucosal hyperpermeability (Fig. 5A), bacterial translocation (Fig. 5B) and gut water content (Fig. 5C) in sepsis were also completely abolished by vagotomy. Thus, ghrelin's beneficial effects sepsis require the intact vagus nerve. In addition, we also found that vagotomized rats had much higher serum HMGB1 levels (Fig. 4) than rats with intact vagus nerve (Fig. 2). This confirms Tracey and associates' early observation that vagotomy not only prevents the protective effect of the vagus nerve stimulation, but also sensitizes the animal to the lethal effect of endotoxin (26,27).
Figure 4.
Effects of vagotomy on alterations in serum levels of HMGB1 in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Ghrelin or vehicle was administered intravenously at 5 h after CLP. Vagotomy was performed in sham and septic animals immediately prior to the administration of ghrelin. Data are presented as means ± SE (n=6-8) and compared by one-way ANOVA and Student-Newman-Keuls method: * P<0.05 versus Sham group; # P<0.05 versus Vehicle group.
Figure 5.
Effects of vagotomy on alterations in intestinal mucosal permeability (A) to fluorescein isothiocyanate dextran with a molecular weight of 4000 Da (FD4), bacterial translocation to mesenteric lymph nodes (B), and gut water content (C) in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Ghrelin or vehicle was administered intravenously at 5 h after CLP. Vagotomy was performed in sham and septic animals immediately prior to the administration of ghrelin. Data are presented as means ± SE (n=6-8) and compared by one-way ANOVA and Student-Newman-Keuls method: * P<0.05 versus Sham group; # P<0.05 versus Vehicle group.
ICV administration of ghrelin is also protective in sepsis
To further confirm that ghrelin's beneficial effects in sepsis are mediated through the central nervous system, ICV administration of ghrelin was performed at 5 h after CLP. As shown in Figure 6, ICV injection of ghrelin significantly inhibited HMGB1 release (P<0.05). Similarly, ICV injection of ghrelin also ameliorated ileal mucosal hyperpermeability (P<0.05, Fig. 7A) and reduced bacterial translocation (P<0.05, Fig. 7B).
Figure 6.
Alterations in serum levels of HMGB1 in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Ghrelin or vehicle was administered intracerebroventricularly at 5 h after CLP. Data are presented as means ± SE (n=5-6) and compared by one-way ANOVA and Student-Newman-Keuls method: * P<0.05 versus Sham group; # P<0.05 versus Vehicle group.
Figure 7.
Alterations in intestinal mucosal permeability (A) to fluorescein isothiocyanate dextran with a molecular weight of 4000 Da (FD4) and bacterial translocation to mesenteric lymph nodes (B) in sham-operated animals (Sham) and septic animals treated with normal saline (Vehicle) or ghrelin (Ghrelin) at 20 h after cecal ligation and puncture (CLP). Ghrelin or vehicle was administered intracerebroventricularly at 5 h after CLP. Data are presented as means ± SE (n=6) and compared by one-way ANOVA and Student-Newman-Keuls method: * P<0.05 versus Sham group; # P<0.05 versus Vehicle group.
Discussion
Under normal conditions, the intestinal barrier acts as a selective route of entry, allowing movement of necessary molecules through the epithelium, but at the same time preventing the entry of potentially pathogenic organisms or their products. Gut barrier dysfunction has been identified in a number of local or systemic inflammatory disorders, including inflammatory bowel disease (28), thermal injury (5), multisystem trauma (22), endotoxemia (29), and sepsis (30). In this study, we found that although histologic sections of the small intestine showed little evidence of massive injury at 20 h after CLP (data not shown), gut barrier dysfunction, manifested by increased mucosal permeability to hydrophilic macromolecules (e.g., FD4) and increased bacterial translocation to MLN, presents in these animals. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill patients (4). It is suspected to be involved in the augmentation of systemic inflammation and distant organ dysfunction under such conditions (5,6). The principal findings of the current study are as follows: 1) ghrelin ameliorates sepsis-induced gut barrier dysfunction, which is paralleled by reduced serum levels of HMGB1; and 2) the beneficial effect of ghrelin in sepsis requires the intact vagus nerve involving the central nervous system.
Ghrelin, a novel gastrointestinal hormone, was originally reported to induce growth hormone release through pituitary GHSR-1a stimulation (7). However, a large body of evidence has indicated other physiological functions of ghrelin mediated by the central and peripheral ghrelin receptors (31). GHSR-1a is found in the brain stem, pituitary, hypothalamus, heart, blood vessels, lungs, stomach, pancreas, intestines, kidneys, and adipose tissue (32-34). The wide distribution of GHSR-1a suggests multiple paracrine, autocrine and endocrine roles of ghrelin (32-35). Besides the established effects on food intake and growth hormone production, ghrelin has also been shown to mediate a number of functions on a variety of other organ systems, including promoting adipogenesis, cardiomyocytes survival, enhancement of memory, neurogenesis, and promote thymic function (8,9,36). Recently, ghrelin has emerged as a potent immuno-regulatory and anti-inflammatory agent (36,37). In this study, we showed for the first time that ghrelin ameliorates gut barrier dysfunction in a clinically relevant model of sepsis.
Rodents challenged with LPS develop manifestations of impaired intestinal barrier function, including increased mucosal permeability to hydrophilic macromolecules and increased translocation of viable bacteria to mesenteric lymph nodes (MLN) (38,39). However, intestinal barrier dysfunction often is not apparent until many hours after the injection of LPS (40), suggesting that a late-acting mediator may be pathophysiologically responsible for this phenomenon (21). HMGB1, a 30-kDa nuclear and cytosolic ubiquitous protein, is a DNA-binding protein known as a transcription and growth factor (41,42). Structurally, HMGB1 is organized into two DNA-binding domains (named A-box and B-box), and a negatively charged C-terminus. Recently, extracellular HMGB1 was identified as a late-acting cytokine-like mediator of lipopolysaccharide (LPS)-induced (15) or sepsis-induced (19) lethality in mice. HMGB1 is actively secreted by immunostimulated macrophages (15,43) and enterocytes (44). It is also passively released by injured and necrotic cells and has been shown to trigger necrosis-induced inflammation (45). The proinflammatory actions of HMGB1 have been localized to the B box region of the molecule (46). High concentrations of HMGB1 can be detected in the serum of mice under endotoxemia, and delayed passive immunization of mice with antibodies against HMGB1 confers significant protection against LPS-induced mortality (15). Administration of highly purified recombinant HMGB1 to mice is lethal (15). Recently, Sappington et al have shown that recombinant human HMGB1 increases the permeability of Caco-2 human enterocytic monolayers to FD4 in a time- and dose-dependent fashion (21). The increase in permeability was reversible following removal of the recombinant protein. Administration of B box of HMGB1 to wild-type mice increases both ileal mucosal permeability to FD4 and bacterial translocation to MLN (21). On the other hand, treatment with a neutralizing anti-HMGB1 antibody ameliorates gut barrier dysfunction in a mouse model of hemorrhagic shock (22). In this regard, inhibition of HMGB1 release may contribute to ghrelin's attenuation of gut barrier dysfunction in sepsis. However, a cause-and-effect relationship can not be established in the current study.
Stimulation of the vagus nerve can rapidly attenuate systemic inflammatory responses through inhibiting the activation of macrophages and endothelial cells. This physiological mechanism, termed ‘the cholinergic anti-inflammatory pathway’, can reflexively monitor and adjust the inflammatory response to prevent excessive inflammation. Tracey and associates found that vagus nerve stimulation, via the activation of nicotinic acetylcholine receptors (α7 receptors), inhibits HMGB1 release and improves survival in animal models of polymicrobial sepsis and endotoxemia (47-49). Vagotomy, on the other hand, not only prevents the protective effect of the vagus nerve stimulation, but also sensitizes the animals to the lethal effects of endotoxin (26,49). It has been reported that ghrelin activates the vagus nerve and vagal blockade abolishes ghrelin-induced feeding and growth hormone secretion (50). To determine whether ghrelin's beneficial effects in sepsis involves vagus nerve stimulation, vagotomy was performed in sham and septic animals immediately prior to the administration of ghrelin. As anticipated, the protection of ghrelin on gut barrier function after CLP requires the intact vagus nerve, as vagotomy prevents its beneficial effects. Ghrelin can cross the blood-brain barrier (51-53); and ghrelin receptors are expressed at a high density in the brain (34,54). Our current study also showed that brain levels of ghrelin decreased significantly at 20 h after CLP, and intravenous administration of ghrelin restored brain levels of ghrelin completely. Moreover, ICV injection of ghrelin inhibited HMGB1 release and attenuated gut damage after CLP. Therefore, it appears that the stimulatory effect of ghrelin on the vagus nerve is mainly mediated via the central nervous system.
The method we used to determine bacterial translocation is a way to measure the number of bacteria translocated from the intestinal lumen to the MLB. Since no exogenous bacteria were provided, it is not intended to determine the percentage of translocation. Please note that other factors such as host immunity can also influence bacterial growth and survival of peritoneal bacteria as well as translocating bacteria. Our recently study has shown that peritoneal bacterial levels in ghrelin treated animals are lower than those in vehicle treated animals at 20 h after CLP (13). This is not surprising since ghrelin can improve host immunity and kill bacteria (55). Therefore, the bacterial translocation data can only be used as supporting evidence to reflect the degree of loss of gut barrier function. Our results showed that the bacteria translocation data are consistent with the gut permeability data, therefore, indirectly support our conclusion.
Our previous study has shown that sham vagotomy (i.e., the animals underwent the same surgical procedure as vagotomized animals with the exception that their vagus nerves were neither tied nor severed) had no impacts on ghrelin's effects on inflammation and organ injury after CLP (10). Moreover, as indicated in this paper, the percentage-wise increases in gut permeability, bacterial count in the MLN, and gut water content after CLP are similar between vagus nerve intact and vagotomized animals. Therefore, control groups of sham vagotomy were not included in this study.
Increased expression and activity of inducible nitric oxide synthase (iNOS) has been shown to play a role in sepsis-induced gut barrier dysfunction (56-58). Recent studies have shown that both central and peripheral administration of ghrelin can reduce iNOS expression in the mucosa and protect against gastrointestinal injury induced by either ethanol or ischemia-reperfusion (59,60). Therefore, attenuation of iNOS expression by this peptide may also contribute to ghrelin's intestinal protection.
The dose of ghrelin used for ICV injection was only about 7% of the dose for IV injection (i.e., 1 nmol vs. 14 nmol). Therefore, the impact of possible ghrelin leakage from the CNS to the general circulation after ICV injection is most likely neglectable. Moreover, the location of ICV injections was confirmed by histological examination of the brain after the experiment.
In summary, either IV or ICV injection of ghrelin significantly reduced the elevated HMGB1 levels, and attenuated gut barrier dysfunction at 20 h after CLP. The protection of ghrelin in sepsis requires the intact vagus nerve, as vagotomy eliminated ghrelin's inhibition on HMGB1 and attenuation on gut barrier dysfunction. Thus, ghrelin reduces serum HMGB1 levels and ameliorates gut barrier dysfunction in sepsis by vagus nerve activation via central ghrelin receptors. Ghrelin appears to be a novel agent to protect gut barrier function in sepsis.
Acknowledgments
This study was supported by National Institutes of Health grants R01 GM053008, R01 AG028352, and R21 AI080536 (PW).
Footnotes
The authors have not disclosed any potential conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Carlet J, Cohen J, Calandra T, et al. Sepsis: time to reconsider the concept. Crit Care Med. 2008;36:964–966. doi: 10.1097/CCM.0B013E318165B886. [DOI] [PubMed] [Google Scholar]
- 3.Fink MP. Effect of critical illness on microbial translocation and gastrointestinal mucosa permeability. Semin Respir Infect. 1994;9:256–260. [PubMed] [Google Scholar]
- 4.Doig CJ, Sutherland LR, Sandham JD, et al. Increased intestinal permeability is associated with the development of multiple organ dysfunction syndrome in critically ill ICU patients. Am J Respir Crit Care Med. 1998;158:444–451. doi: 10.1164/ajrccm.158.2.9710092. [DOI] [PubMed] [Google Scholar]
- 5.Magnotti LJ, Deitch EA. Burns, bacterial translocation, gut barrier function, and failure. J Burn Care Rehabil. 2005;26:383–391. doi: 10.1097/01.bcr.0000176878.79267.e8. [DOI] [PubMed] [Google Scholar]
- 6.Swank GM, Deitch EA. Role of the gut in multiple organ failure: bacterial translocation and permeability changes. World J Surg. 1996;20:411–417. doi: 10.1007/s002689900065. [DOI] [PubMed] [Google Scholar]
- 7.Kojima M, Hosoda H, Date Y, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 8.Kojima M, Kangawa K. Ghrelin: structure and function. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Lee HM, Englander E, et al. Ghrelin--not just another stomach hormone. Regul Pept. 2002;105:75–81. doi: 10.1016/s0167-0115(02)00012-5. [DOI] [PubMed] [Google Scholar]
- 10.Wu R, Dong W, Cui X, et al. Ghrelin down-regulates proinflammatory cytokines in sepsis through activation of the vagus nerve. Ann Surg. 2007;245:480–486. doi: 10.1097/01.sla.0000251614.42290.ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu R, Zhou M, Cui X, et al. Upregulation of cardiovascular ghrelin receptor occurs in the hyperdynamic phase of sepsis. Am J Physiol Heart Circ Physiol. 2004;287:H1296–H1302. doi: 10.1152/ajpheart.00852.2003. [DOI] [PubMed] [Google Scholar]
- 12.Wu R, Dong W, Zhou M, et al. Ghrelin improves tissue perfusion in severe sepsis via downregulation of endothelin-1. Cardiovasc Res. 2005;68:318–326. doi: 10.1016/j.cardiores.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Wu R, Dong W, Zhou M, et al. Ghrelin attenuates sepsis-induced acute lung injury and mortality in rats. Am J Respir Crit Care Med. 2007;176:805–813. doi: 10.1164/rccm.200604-511OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu R, Zhou M, Das P, et al. Ghrelin inhibits sympathetic nervous activity in sepsis. Am J Physiol Endocrinol Metab. 2007;293:E1697–E1702. doi: 10.1152/ajpendo.00098.2007. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Bloom O, Zhang M, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science. 1999;285:248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 16.Andersson U, Wang H, Palmblad K, et al. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. J Exp Med. 2000;192:565–570. doi: 10.1084/jem.192.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sunden-Cullberg J, Norrby-Teglund A, Rouhiainen A, et al. Persistent elevation of high mobility group box-1 protein (HMGB1) in patients with severe sepsis and septic shock. Crit Care Med. 2005;33:564–573. doi: 10.1097/01.ccm.0000155991.88802.4d. [DOI] [PubMed] [Google Scholar]
- 18.Fiuza C, Bustin M, Talwar S, et al. Inflammation-promoting activity of HMGB1 on human microvascular endothelial cells. Blood. 2003;101:2652–2660. doi: 10.1182/blood-2002-05-1300. [DOI] [PubMed] [Google Scholar]
- 19.Yang H, Ochani M, Li J, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci U S A. 2004;101:296–301. doi: 10.1073/pnas.2434651100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Liao H, Ochani M, et al. Cholinergic agonists inhibit HMGB1 release and improve survival in experimental sepsis. Nat Med. 2004;10:1216–1221. doi: 10.1038/nm1124. [DOI] [PubMed] [Google Scholar]
- 21.Sappington PL, Yang R, Yang H, et al. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology. 2002;123:790–802. doi: 10.1053/gast.2002.35391. [DOI] [PubMed] [Google Scholar]
- 22.Yang R, Harada T, Mollen KP, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chavez AM, Menconi MJ, Hodin RA, et al. Cytokine-induced intestinal epithelial hyperpermeability: role of nitric oxide. Crit Care Med. 1999;27:2246–2251. doi: 10.1097/00003246-199910000-00030. [DOI] [PubMed] [Google Scholar]
- 24.Yang R, Han X, Uchiyama T, et al. IL-6 is essential for development of gut barrier dysfunction after hemorrhagic shock and resuscitation in mice. Am J Physiol Gastrointest Liver Physiol. 2003;285:G621–G629. doi: 10.1152/ajpgi.00177.2003. [DOI] [PubMed] [Google Scholar]
- 25.Wu R, Dong W, Ji Y, et al. Orexigenic hormone ghrelin attenuates local and remote organ injury after intestinal ischemia-reperfusion. PLoS ONE. 2008;3:e2026. doi: 10.1371/journal.pone.0002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernik TR, Friedman SG, Ochani M, et al. Pharmacological stimulation of the cholinergic antiinflammatory pathway. J Exp Med. 2002;195:781–788. doi: 10.1084/jem.20011714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borovikova LV, Ivanova S, Zhang M, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–462. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- 28.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zayat M, Lichtenberger LM, Dial EJ. Pathophysiology of LPS-induced gastrointestinal injury in the rat: role of secretory phospholipase A2. Shock. 2008;30:206–211. doi: 10.1097/shk.0b013e318160f47f. [DOI] [PubMed] [Google Scholar]
- 30.Gosain A, Gamelli RL. Role of the gastrointestinal tract in burn sepsis. J Burn Care Rehabil. 2005;26:85–91. doi: 10.1097/01.bcr.0000150212.21651.79. [DOI] [PubMed] [Google Scholar]
- 31.Cowley MA, Grove KL. Ghrelin--Satisfying a Hunger for the Mechanism. Endocrinol. 2004;145:2604–2606. doi: 10.1210/en.2004-0346. [DOI] [PubMed] [Google Scholar]
- 32.Hattori N, Saito T, Yagyu T, et al. GH, GH receptor, GH secretagogue receptor, and ghrelin expression in human T cells, B cells, and neutrophils. J Clin Endocrinol Metab. 2001;86:4284–4291. doi: 10.1210/jcem.86.9.7866. [DOI] [PubMed] [Google Scholar]
- 33.Papotti M, Ghe C, Cassoni P, et al. Growth hormone secretagogue binding sites in peripheral human tissues. J Clin Endocrinol Metab. 2000;85:3803–3807. doi: 10.1210/jcem.85.10.6846. [DOI] [PubMed] [Google Scholar]
- 34.Shuto Y, Shibasaki T, Wada K, et al. Generation of polyclonal antiserum against the growth hormone secretagogue receptor (GHS-R): evidence that the GHS-R exists in the hypothalamus, pituitary and stomach of rats. Life Sci. 2001;68:991–996. doi: 10.1016/s0024-3205(00)01001-8. [DOI] [PubMed] [Google Scholar]
- 35.Sakata I, Yamazaki M, Inoue K, et al. Growth hormone secretagogue receptor expression in the cells of the stomach-projected afferent nerve in the rat nodose ganglion. Neurosci Lett. 2003;342:183–186. doi: 10.1016/s0304-3940(03)00294-5. [DOI] [PubMed] [Google Scholar]
- 36.Taub DD. Novel connections between the neuroendocrine and immune systems: the ghrelin immunoregulatory network. Vitam Horm. 2008;77:325–346. doi: 10.1016/S0083-6729(06)77014-5. [DOI] [PubMed] [Google Scholar]
- 37.Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114:57–66. doi: 10.1172/JCI21134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Unno N, Wang H, Menconi MJ, et al. Inhibition of inducible nitric oxide synthase ameliorates endotoxin-induced gut mucosal barrier dysfunction in rats. Gastroenterology. 1997;113:1246–1257. doi: 10.1053/gast.1997.v113.pm9322519. [DOI] [PubMed] [Google Scholar]
- 39.Deitch EA, Ma L, Ma WJ, et al. Inhibition of endotoxin-induced bacterial translocation in mice. J Clin Invest. 1989;84:36–42. doi: 10.1172/JCI114164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De B I, Deutz NE, van der Hulst RR, et al. Glutamine depletion and increased gut permeability in nonanorectic, non-weight-losing tumor-bearing rats. Gastroenterology. 1997;112:118–126. doi: 10.1016/s0016-5085(97)70226-9. [DOI] [PubMed] [Google Scholar]
- 41.Bianchi ME, Beltrame M, Paonessa G. Specific recognition of cruciform DNA by nuclear protein HMG1. Science. 1989;243:1056–1059. doi: 10.1126/science.2922595. [DOI] [PubMed] [Google Scholar]
- 42.Bianchi ME, Beltrame M. Upwardly mobile proteins. Workshop: the role of HMG proteins in chromatin structure, gene expression and neoplasia. EMBO Rep. 2000;1:109–114. doi: 10.1093/embo-reports/kvd030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rendon-Mitchell B, Ochani M, Li J, et al. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J Immunol. 2003;170:3890–3897. doi: 10.4049/jimmunol.170.7.3890. [DOI] [PubMed] [Google Scholar]
- 44.Liu S, Stolz DB, Sappington PL, et al. HMGB1 is secreted by immunostimulated enterocytes and contributes to cytomix-induced hyperpermeability of Caco-2 monolayers. Am J Physiol Cell Physiol. 2006;290:C990–C999. doi: 10.1152/ajpcell.00308.2005. [DOI] [PubMed] [Google Scholar]
- 45.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 46.Li J, Kokkola R, Tabibzadeh S, et al. Structural basis for the proinflammatory cytokine activity of high mobility group box 1. Mol Med. 2003;9:37–45. [PMC free article] [PubMed] [Google Scholar]
- 47.Wang H, Yu M, Ochani M, et al. Nicotinic acetylcholine receptor alpha7 subunit is an essential regulator of inflammation. Nature. 2003;421:384–388. doi: 10.1038/nature01339. [DOI] [PubMed] [Google Scholar]
- 48.Tracey KJ. The inflammatory reflex. Nature. 2002;420:853–859. doi: 10.1038/nature01321. [DOI] [PubMed] [Google Scholar]
- 49.Borovikova LV, Ivanova S, Nardi D, et al. Role of vagus nerve signaling in CNI-1493-mediated suppression of acute inflammation. Auton Neurosci. 2000;85:141–147. doi: 10.1016/S1566-0702(00)00233-2. [DOI] [PubMed] [Google Scholar]
- 50.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–1128. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 51.Banks WA, Tschop M, Robinson SM, et al. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302:822–827. doi: 10.1124/jpet.102.034827. [DOI] [PubMed] [Google Scholar]
- 52.Wu R, Zhou M, Cui X, et al. Ghrelin clearance is reduced at the late stage of polymicrobial sepsis. Int J Mol Med. 2003;12:777–781. [PubMed] [Google Scholar]
- 53.Diano S, Farr SA, Benoit SC, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–388. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 54.Cowley MA, Smith RG, Diano S, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 55.Chorny A, Anderson P, Gonzalez-Rey E, et al. Ghrelin protects against experimental sepsis by inhibiting high-mobility group box 1 release and by killing bacteria. J Immunol. 2008;180:8369–8377. doi: 10.4049/jimmunol.180.12.8369. [DOI] [PubMed] [Google Scholar]
- 56.Xu DZ, Lu Q, Deitch EA. Nitric oxide directly impairs intestinal barrier function. Shock. 2002;17:139–145. doi: 10.1097/00024382-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 57.Deitch EA, Shorshtein A, Houghton J, et al. Inducible nitric oxide synthase knockout mice are resistant to diet-induced loss of gut barrier function and intestinal injury. J Gastrointest Surg. 2002;6:599–605. doi: 10.1016/s1091-255x(01)00016-6. [DOI] [PubMed] [Google Scholar]
- 58.Han X, Fink MP, Yang R, et al. Increased iNOS activity is essential for intestinal epithelial tight junction dysfunction in endotoxemic mice. Shock. 2004;21:261–270. doi: 10.1097/01.shk.0000112346.38599.10. [DOI] [PubMed] [Google Scholar]
- 59.El Eter E, Al Tuwaijiri A, Hagar H, et al. In vivo and in vitro antioxidant activity of ghrelin: Attenuation of gastric ischemic injury in the rat. J Gastroenterol Hepatol. 2007;22:1791–1799. doi: 10.1111/j.1440-1746.2006.04696.x. [DOI] [PubMed] [Google Scholar]
- 60.Sibilia V, Pagani F, Rindi G, et al. Central ghrelin gastroprotection involves nitric oxide/prostaglandin cross-talk. Br J Pharmacol. 2008;154:688–697. doi: 10.1038/bjp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]