Abstract
Gastrulation is a critical morphogenetic event during vertebrate embryogenesis, and it is comprised of directional cell movement resulting from the polarization and reorganization of the actin cytoskeleton. The non-canonical Wnt signaling pathway has emerged as a key regulator of gastrulation. However, the molecular mechanisms by which the Wnt pathway mediates changes to the cellular actin cytoskeleton remains poorly defined. We had previously identified the Formin protein Daam1 and an effector molecule XProfilin1 as links for Wnt-mediated cytoskeletal changes during gastrulation. We report here the identification of XProfilin2 as a non-redundant and distinct effector of Daam1 for gastrulation. XProfilin2 interacts with FH1 domain of Daam1 and temporally interacts with Daam1 during gastrulation. In the Xenopus embryo, XProfilin2 is temporally expressed throughout embryogenesis and it is spatially expressed in cells undergoing morphogenetic movement during gastrulation. While we have previously shown XProfilin1 regulates blastopore closure, overexpression or depletion of XProfilin2 specifically affects convergent extension movement independent of mesodermal specification. Specifically, we show that XProfilin2 modulates cell polarization and axial alignment of mesodermal cells undergoing gastrulation independent of XProfilin1. Together, our studies demonstrate XProfilin2 and XProfilin1 are non-redundant effectors for Daam1 for non-canonical Wnt signaling and that they regulate distinct functions during vertebrate gastrulation.
Keywords: Wnt, non-canonical signaling, Profilin1, Profilin2, Daam1, gastrulation, morphogenesis, Xenopus
Introduction
The establishment of the vertebrate body plan during embryogenesis results from a series of exquisite and tightly-controlled cell polarization and cell migration events during gastrulation (Keller, 2002). A full understanding of the molecular mechanisms and signaling pathways that regulate these important processes of cell polarization and motility during gastrulation remains a daunting challenge to developmental biologists.
While a number of signaling pathways including BMPs and FGFs have been shown to play crucial roles in cell fate determination and motility during gastrulation (Heisenberg and Solnica-Krezel, 2008), The Wnt pathway has emerged as a key regulator of gastrulation. Wnt proteins comprise a large family of secreted glycoproteins with conserved functions from invertebrates to vertebrates (Komiya and Habas, 2008). Notably, Wnt signaling regulates a variety of developmental processes including cell fate determination, cell proliferation, cell motility, and the establishment of the primary axis during vertebrate embryogenesis (Logan and Nusse, 2004).
An important aspect of Wnt signaling involves the regulation of cell polarity and cell motility via the non-canonical signaling cascade (Komiya and Habas, 2008). For non-canonical Wnt signaling, the Wnt signal is transduced via the Wnt-receptor Frizzled (Fz) to the cytoplasmic protein Dishevelled (Dvl), and this pathway regulates cell movement during gastrulation via modification of the actin cytoskeleton (Veeman et al., 2003; Wallingford and Habas, 2005). A number of molecular components for non-canonical Wnt signaling have been identified (Habas and He, 2007; Komiya and Habas, 2008; Veeman et al., 2003; Wallingford and Habas, 2005). Downstream of Dvl, the small GTPases Rho and Rac are activated by two independent pathways (Habas et al., 2003; Habas et al., 2001). Signaling to Rho occurs via the molecule Daam1 (Dishevelled associated activator of morphogenesis) that binds to the PDZ domain of Dvl (Habas et al., 2001). The Daam1 protein exists in an auto-inhibited state and upon Dvl-binding, this auto-inhibition is relieved and Daam1 is activated (Liu et al., 2008). Activated Daam1 subsequently activates Rho, and Daam1 can also interact with actin-binding proteins (Sato et al., 2006). The Daam1/Rho pathway leads to the activation of the Rho associated kinase ROCK, which mediates cytoskeletal re-organization (Semenov et al., 2007). In the second pathway, the DEP domain of Dvl leads to activation of a second GTPase, Rac, which in turn leads to activation of Jun Kinase (Habas et al., 2003; Semenov et al., 2007). Together, the Rho and Rac branches of signaling are integrated to regulate modification of the actin cytoskeleton for modification of cell adhesion and directional migration during gastrulation (Komiya and Habas, 2008; Wallingford and Habas, 2005).
Although several molecular components of the non-canonical Wnt pathway have been identified, many questions remain on the mechanism by which signaling proceeds from the plasma membrane to the actin cytoskeleton for cell polarization and motility. We had previously identified the Formin homology protein, Daam1, as a crucial link between Dishevelled (Dvl) and the small GTPase Rho (Habas et al., 2001; Mlodzik, 2002). Furthermore, we established that Daam1 functions to mediate Wnt-induced cytoskeletal changes required for gastrulation cell movement; we also identified XProfilin1 as a Daam1-binding partner required for blastopore closure independent of convergent extension movement during gastrulation (Sato et al., 2006).
Profilins are evolutionarily conserved proteins that play important roles in the polymerization of actin filaments (Watanabe and Higashida, 2004; Witke, 2004). In mammals, there are four Profilin genes (1-4) and of these, Profilin 1 is ubiquitously expressed, Profilin 2 is expressed only in the nervous system, and Profilin 3 and Profilin 4 appear to be testis-specific (Birbach, 2008; Witke, 2004). Profilin1 mutant mice die at the 8-cell stage, showing that Profilin1 is essential for cytokinesis (Witke et al., 2001). Mutant studies in Drosophila have revealed a role for Profilin1 in oogenesis, spermatogenesis, and in bristle and eye formation (Cooley et al., 1992; Verheyen and Cooley, 1994). In Xenopus, Profilin1 was shown to regulate blastopore closure during gastrulation (Sato et al., 2006) and a recent study in zebrafish points to a role for Profilin1 in gastrulation (Lai et al., 2008). Profilin2 mouse mutants show deficiencies in synaptic physiology with no embryonic defects noted (Pilo Boyl et al., 2007).
The Profilin proteins bind to monomeric actin and this Profilin-actin complex serves as a major source of actin monomers for actin polymerization and for growth of unbranched actin filaments (Birbach, 2008). The Profilin proteins contain two major surfaces, one that binds to actin and the other that binds to polyproline-rich sequences such as those found in the FH1 domain of Formin proteins (Goode and Eck, 2007). Upon actin binding, Profilin activates actin monomers by stimulating ADP to ATP exchange; Profilin then binds to the FH1 domain of Formin proteins and provides these activated actin monomers for the growth of the actin filament (Goode and Eck, 2007; Paul and Pollard, 2008). The FH2 domain of Formin proteins, including Daam1, has been shown to nucleate actin and to function in processive capping; however the ability of an FH1/FH2 fragment of Daam1 to promote actin assembly in vitro was approximately 100-fold less than that of other Formins such as mDia1 (Goode and Eck, 2007; Higashi et al., 2008; Li and Higgs, 2003; Lu et al., 2007; Moseley et al., 2006; Pollard, 2007; Yamashita et al., 2007). To date, it remains unclear whether precise changes to the actin cytoskeleton require specific or regulated interactions between different Profilin isoforms and distinct Formin proteins.
We identified Profilin1 in a screen for Daam1 interacting proteins, and showed that it regulated blastopore closure during gastrulation (Sato et al., 2006). However, as gastrulation is comprised of a complex series of dynamic events including cell migrations, and cell rearrangements that drive convergence and extension during axial elongation, neural tube closure, and blastopore closure (Keller, 2002; Keller et al., 2003; Wallingford et al., 2002), we reasoned that additional binding-partners for Daam1 must exist for its function. From the screen that netted Profilin1 (Sato et al., 2006), the second most abundant isolated clone was Profilin2. We therefore chose to characterize the potential role of Profilin2 in non-canonical Wnt-regulated signaling during gastrulation. Here we show XProfilin2 binds to Daam1 via its FH1 domain. In the Xenopus embryo, we show that XProfilin2 is temporally expressed throughout embryogenesis and is spatially enriched in cells undergoing morphogenetic movement during gastrulation.
Interestingly, we find that overexpression or depletion of XProfilin2 specifically affects convergent extension movement and this effect is independent of mesodermal specification. To further delineate how XProfilin2 regulates convergent-extension movement, we show that XProfilin2 regulates cell polarization and axial alignment of mesodermal cells undergoing gastrulation and this function is independent of XProfilin1. Additionally, overexpression of XProfilin2 promotes actin fiber formation and conversely a reduction of XProfilin2 reduces actin fibers in Xenopus dorsal marginal explants. Our studies show that Profilin2 and Profilin1 are non-redundant proteins for Daam1-mediated for non-canonical Wnt signaling, and that they have distinct roles in regulating cell behavior during vertebrate gastrulation.
Materials and Methods
Antibodies
Monoclonal antibodies (mAbs) against HA (F-7), Myc (9E10), RhoA (26C4), Dvl2 (10B5), and polyclonal Abs (pAbs) against Myc (N-262) were obtained from Santa Cruz Biotechnology. The hDaam1 polyclonal antibody was previously reported (Sato et al., 2006). Alexa Fluor anti-mouse and anti-rabbit Abs and Texas Red X-Phalloidin were obtained from Molecular Probes (Eugene, OR).
Plasmids and oligonucleotides
The human Daam1 and fragments of Daam1 were generated by restriction digest or a PCR approach, and subcloned in pCS2+MT (for the Myc tag at the N terminus) or pcDNA-HA (for the HA tag at the amino terminus), or pCS2+GFP vector (kindly provided by Dr Jeffrey Miller, University of Minnesota). Full length, 5′UTR-XProfilin2-Myc and 5′end deleted (ΔN-Profilin) versions of Xenopus Profilin2 (isolated by a PCR approach from a Xenopus Stage 10.5 cDNA library) were cloned into pCS2+MT or pCS2+GFP. Details of plasmids are available upon request.
An XProfilin1 Morpholino oligonucleotide (MO) complementary to the translational initiation site, 5′-TGTAGCCGTTCCAAGACATTGTTGT-3′ (Sato et al., 2006) and an XProfilin2 Morpholino oligonucleotide (MO) complementary to the translational initiation site, 5′-CGCACTGAAGATGTCCGGCTGGCAG-3′ were synthesized by Gene Tools. A MO of similar length but with a random sequence was used as the negative control.
Yeast two-hybrid screen
A rat brain cDNA library (Clonetech) was screened using the C-Daam1 fragment of Daam1 as the bait. 3.9 million independent clones were screened, and 11 overlapping Profilin2 cDNAs, in addition to other positives, were obtained.
Transfections
HEK293T cells were employed for all transfections. Briefly, cells in a six well plate were transfected using either the calcium-phosphate method or Polyfect reagent (Qiagen) with 1-2 μg of each indicated plasmid. Transfected DNA amounts were equalized using plasmid vectors without any inserts.
Embryo Manipulations and Explant Assays
Embryo manipulations and explant assays were performed as described (Sato et al., 2006). Embryo injections were performed using in vitro transcribed RNAs, cDNAs or Morpholino oligonucleotides. Convergent extension assays in explants were performed as described (Sato et al., 2006).
Embryo Dissection and in Vivo Imaging
Microinjection and micro-dissection of Xenopus embryos were performed as previously described (Sato et al., 2006). Briefly, RNAs (0.5–2 ng) encoding GFP-CAAX (kindly provided by Dr. Karen Symes, Boston University) or membrane-tethered Cherry (Dr. Chenbei Chang, University of Alabama) were microinjected separately into dorsal blastomeres of four-cell stage embryos, alone or with XProfilin2 MO and RNAs encoding ΔN-XProfilin1 and ΔN-XProfilin2. Dorsal explants of the neurula stage embryos were prepared and kept flat with mesoderm layer up using a coverslip.
Phalloidin Staining of Xenopus explants
Dorsal mesodermal explants of Xenopus embryos cultured until neurula stage were fixed in 3.7% paraformaldehyde for two hours followed by washing in 1XPBS/0.1% Triton X-100 and incubated with 4 U/ml of Oregon Green 488 Phalloidin (Invitrogen) for 3 hours. The samples were then washed with 1XPBS/0.1% Triton X-100 and mounted on a glass slide with FluoroGel mounting media (Electron Microscopy Sciences).
Confocal microscopy and data scoring
An Olympus IX-50 confocal microscope with a 63X objective was used for image acquisition. Images were scored for abundance of actin fibers as normal (average of the uninjected control sample), increased and decreased (compared to the normal).
Results
Identification of Profilin2 as a binding partner for Daam1
We had previously reported the identification and characterization of XProfilin1 as an effector for Daam1 that is required for blastopore closure during gastrulation (Sato et al., 2006). As Daam1-depletion results in defects in convergent extension movement along with a failure of neural tube closure and blastopore closure (Habas et al., 2001), we reasoned that additional effectors must exist downstream of Daam1 for its function. From the yeast two-hybrid screen that netted Profilin1 as a binding partner for Daam1, the second most abundant interactor isolated was Profilin2. We therefore sought to characterize the possible role for Profilin2 in Daam1-mediated non-canonical Wnt signaling.
We first cloned the Xenopus homologue of Profilin2. The XProfilin2 protein comprises 140 amino acids and shares a 52% identity and 65% similarity with XProfilin1 protein and an 80% identity and 87% similarity the mouse homologue (Figure 1A). Interestingly, a phylogenetic analysis of mouse and Xenopus Profilin1 and Profilin2 proteins reveal that XProfilin2 is more closely related to mouse Profilin2 and Profilin1 than to XProfilin1 (Figure 1B).
Figure 1. Profilin2 is a Daam1-interacting protein.
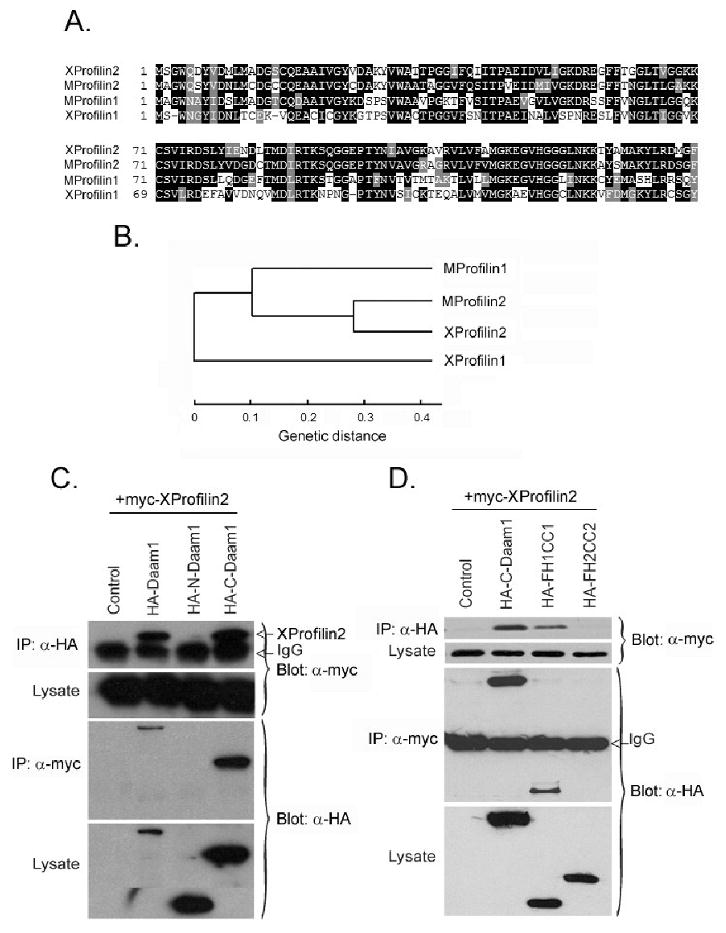
(A) Amino acid sequence alignment of mouse Profilin1 (MProfilin1) and Profilin2 (MProfilin2) and Xenopus Profilin1 (XProfilin1) and Profilin2 (XProfilin2) shows stronger homology among XProfilin2, MProfilin1 and MProfilin2. (B) Phylogenetic analysis of mouse Profilin1 and Profilin2 and Xenopus Profilin1 and Profilin2 shows XProfilin2 to be more closely related to MProfilin1, MProfilin2 than to XProfilin1. ClustalW 1.83 program was used for obtaining the phylogram. (C) Co-immunoprecipitation assays reveal XProfilin2 binds to Daam1, to C-Daam1 (which harbors the FH1, FH2 and DAD domains, (Liu et al., 2008)) but not to N-Daam1 (which contains the GBD domain, (Liu et al., 2008)). (D) XProfilin2 binds to the FH1 but not the FH2 domain of Daam1. Plasmids encoding epitope tagged-Daam1 and XProfilin2 cDNAs were co-transfected into HEK293T cells, and cell lysates were immunoprecipitated (IP) with and immunoblotted with indicated the Abs.
In order to demonstrate interactions between XProfilin2 and Daam1 outside of yeast, we examined the binding between XProfilin2 and Daam1 via co-immunoprecipitation assays using epitope-tagged wild type or truncated proteins expressed in mammalian HEK293T culture cells (Figure 1C–D). We found that XProfilin2 binds to full length Daam1 and C-Daam1, which contains the FH1 and FH2 domains, but not to N-Daam1, which contains the amino-terminal GBD domain (Figure 1C–D). Using smaller fragments of C-Daam1 harboring the FH1 or FH2 domains separately, we were able to determine that XProfilin2 binds to the FH1-containing fragment of Daam1 but not to the FH2-containing fragment (Figure 1D). Thus, like XProfilin1, XProfilin2 also interacts with the FH1 domain of Daam1 and is a bona-fide binding partner.
Expression pattern of XProfilin2 during Xenopus embryogenesis
To help elucidate the in vivo role of XProfilin2, we examined its temporal and spatial expression pattern during Xenopus embryogenesis. RT-PCR analysis showed that XProfilin2 was strongly expressed maternally, and this level of expression persisted throughout development (Figure 2A). The spatial pattern of XProfilin2 gene expression was next visualized by in situ hybridization. These studies revealed a strong and ubiquitous expression of XProfilin2 throughout all stages in the developing embryo (Figure 2B). XProfilin2 was observed in the animal pole of the fertilized egg and around the blastopore lip during the blastula stage. At the neurula stage, XProfilin2 was highly expressed within the neural plate and neural folds, and as development progressed its expression became restricted to the brain, eyes and spinal cord regions (Figure 2B). This expression pattern overlaps with that for XProfilin1 and XDaam1 (Nakaya et al., 2004; Sato et al., 2006).
Figure 2. Temporal and spatial expression pattern of XProfilin2.
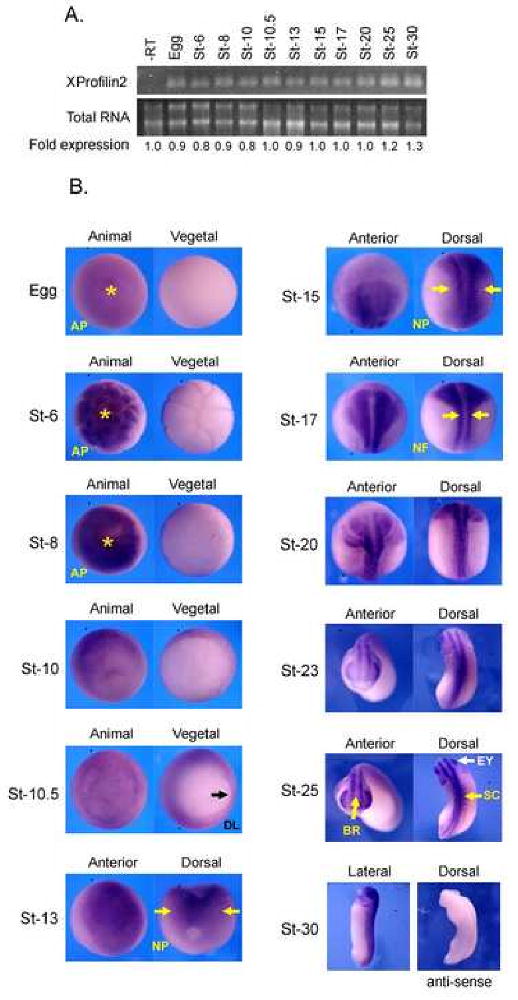
(A). XProfilin2 is expressed throughout Xenopus development as monitored by RT-PCR analysis; total extracted mRNA is shown as a loading control, -RT without reverse transcriptase. Quantitation of the relative RNA expression levels is shown below (B). The spatial expression pattern of XProfilin2 is dynamic with the highest level of expression refined to the neural fold and nervous system during neurula-stage embryo. Stages and views of the embryos are shown in each panel; AP = animal pole, DL = dorsal lip, NP= neural plate, NF = neural fold, BR = brain, EY = eye, and SC = spinal cord. No signal is detected using an XProfilin2 sense probe.
Profilin2 is required for gastrulation
To elucidate the function of XProfilin2 in vivo, we examined the effects of overexpression and loss of function of XProfilin2 in the Xenopus embryo. Injection of XProfilin2 RNA into the two ventral marginal zones of the four-cell embryo at the highest dose of 2ng or injection of 2 ng LacZ RNA had no effect on Xenopus development (Figure 3A–B). In contrast, injection of 1 ng and 2 ng of XProfilin2 RNA but not 2 ng LacZ RNA into the two dorsal marginal zone of the four-cell embryo resulted in profound gastrulation defects. The injected embryos had reduced anterior structures, open blastopores, shortened anterior-posterior axis and a failure of neural fold closure. This phenotype suggests that XProfilin2 may regulate gastrulation, as interference with this process results in such phenotypes (Keller, 2002; Veeman et al., 2003; Wallingford et al., 2002).
Figure 3. XProfilin2 is required for gastrulation.
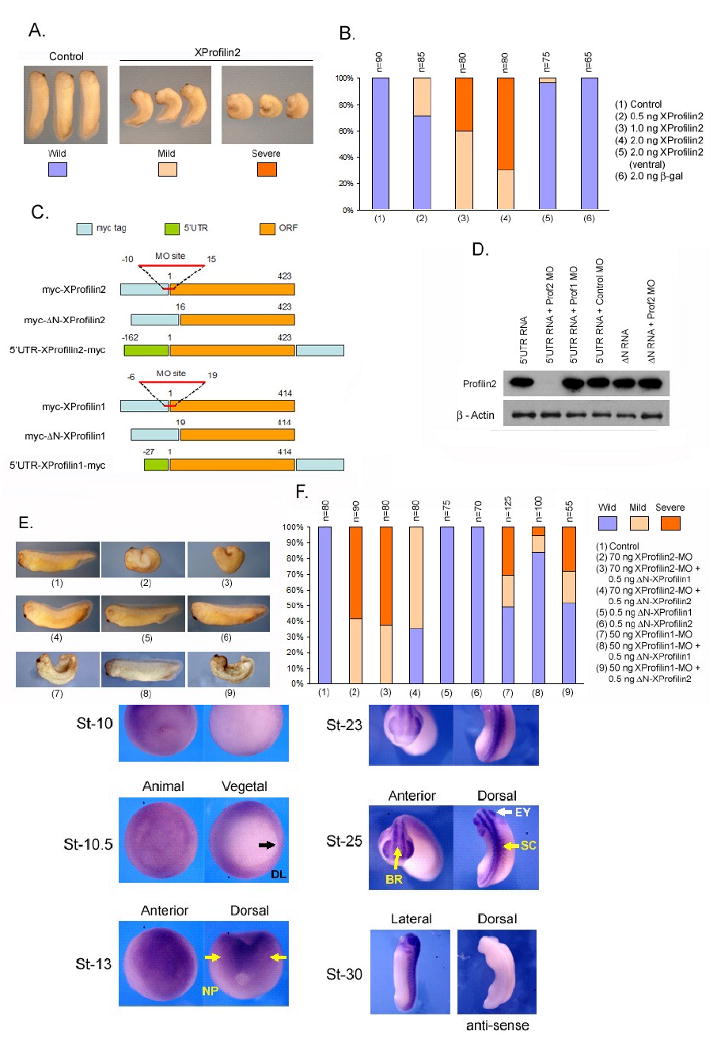
(A) Injection of XProfilin2 RNA dorsally but not ventrally inhibits gastrulation with the resulting embryos having open neural folds and reduced anterior structures (Severe) or delayed blastopore closure and a curved/bent axis (Mild). (B). Quantitation of the phenotypic results from overexpression studies of XProfilin2. Number of embryos scored (n) is shown at the top of each bar. (C) Schematic representation of the XProfilin2 constructs and targeted-Morpholino site. (D). Injection of the XProfilin2 MO but not XProfilin1 MO or a control MO inhibits translation of Myc-tagged 5′UTR-XProfilin2. The ΔN-XProfilin2 cDNA lacking the XProfilin2 MO recognition sequence is insensitive to the effects of the XProfilin2 MO. (E). Injection of XProfilin2 MO inhibits gastrulation and results in a similar gastrulation-defect phenotype as overexpression of XProfilin2 RNA (see A). This phenotype is reversed by injection of ΔN-XProfilin2 but not ΔN-XProfilin1 (F). Quantitation of phenotypic results of XProfilin2-depletion studies. Number of embryos scored (n) is shown at the top of each bar.
To delineate the role of XProfilin2 using a loss-of-function approach, we designed an anti-sense Morpholino oligonucleotide (MO) that recognizes and overlaps with the translational initiation codon to deplete the endogenous XProfilin2 protein (Figure 3C). As commercial Profilin2 antisera did not recognize endogenous XProfilin2 by Western Blot analysis (not shown), we tested the efficiency of the XProfilin2 MO (70 ng) to inhibit translation of a 5′UTR-XProfilin2-Myc (1 ng) or 5′UTR-XProfilin1-Myc (1 ng) construct injected into Xenopus embryos. Protein translation via injection of 5′UTR-XProfilin2-Myc, but not that of 5′UTR-XProfilin1-Myc RNA was abrogated by co-injection with the XProfilin2 MO but not by the co-injected control MO (Figure 3D). Deletion of the XProfilin2 MO binding site within the ΔN-XProfilin2 (1 ng) construct was further insensitive to the effects of the XProfilin2 MO, and XProfilin1 MO (70 ng) did not affect the translation of 5′UTR-XProfilin2-Myc (Figure 3D). As a control, we observed no effects with the injected Morpholinos on the levels of endogenous β-actin protein (Figure 3D). These studies demonstrate that the XProfilin2 MO was specific, and it effectively inhibits the translation of XProfilin2.
We next utilized the XProfilin2 MO to delineate the role of Profilin2 during development. Xenopus embryos injected with XProfilin2 MO (70 ng) into both dorsal blastomeres at the 4-cell stage showed a failure of neural tube closure, short and curved anterior-posterior axis, and open blastopores similar to the XProfilin2 RNA injection (Figure 3E–F). This XProfilin2 MO result was observed to be dose-dependent using doses ranging from 50 to 100 ng (not shown). Importantly, the XProfilin2 MO-induced phenotype could be rescued by co-injecting XProfilin2 MO (70 ng) along with XProfilin2 RNA (0.5 ng) lacking MO-binding site (ΔN-XProfilin2), but not by co-injection with LacZ RNA (0.5 ng) (Figure 3E–F). Additionally, co-injection of ΔN-XProfilin1 RNA (0.5 ng) (Sato et al., 2006) failed to rescue the phenotype induced by the XProfilin2 MO-injection, indicating that this phenotype is specific to the endogenous function of the XProfilin2 protein. These results together suggest that XProfilin2 is required for gastrulation and that XProfilin1 and XProfilin2 have non-redundant functions during gastrulation.
We further examined the effect of overexpression of XProfilin2 by injecting Myc-XProfilin2 RNA in a similar fashion as above. RNAs (2 ng) from either full length, 5′UTR-XProfilin2-Myc and MO site deleted (ΔN-XProfilin2) constructs of XProfilin2 showed phenotype similar to the loss of function phenotype. In order to confirm the specificity of the effect, we employed a “reverse-rescue” method by co-injecting 5′UTR-XProfilin2-Myc (2 ng) and a low dose of XProfilin2 MO (25 ng) or XProfilin1 MO (25 ng) together. Co-injection of XProfilin2 MO but not XProfilin1 MO rescued the overexpression of 5′UTR-XProfilin2-Myc phenotype (data not shown). Altogether, these results confirmed that proper levels of XProfilin2 protein are required for gastrulation and that the function of XProfilin2 is non-redundant with that of XProfilin1.
Profilin2 is not required for mesodermal specification
As a failure of mesodermal specification can result in defective gastrulation, we sought to determine whether XProfilin2 might regulate mesodermal cell fate determination. We first examined whether gain-of-function or loss-of-function of XProfilin2 impacted mesodermal gene expression by RT-PCR analysis. Injection of XProfilin2 RNA (2 ng), XProfilin2 MO (70 ng), control MO (70 ng) or XProfilin2 MO (70 ng) along with ΔN-XProfilin2 RNA (0.5 ng) into the dorsal blastomeres at the 4-cell stage had no effect on the expression of the pan-mesodermal marker brachyury (Xbra), the dorsal mesodermal marker goosecoid (Gsc), or the ventrolateral mesodermal marker Xwnt8 (Figure 4A).
Figure 4. XProfilin2 does not interfere with mesoderm induction.
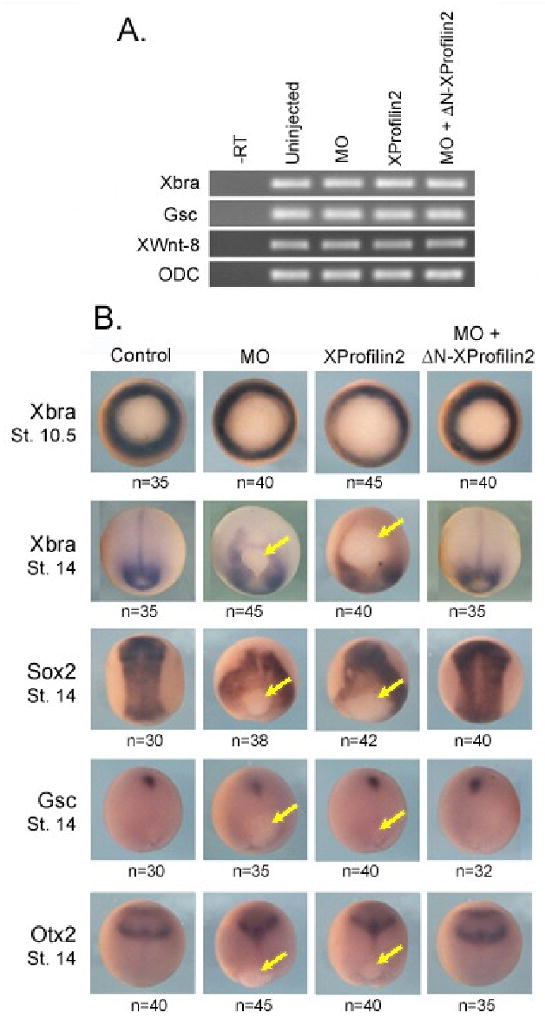
Embryos injected dorsally with Profilin2 (2 ng), XProfilin2 MO (70 ng), XProfilin2 MO + ΔN-XProfilin2 (70 ng+ 100 pg) have no defects in expression of mesodermal marker genes Xbra, Gsc and XWnt8 as monitored by RT-PCR analysis; Ornithine decarboxylase (ODC) is used as a loading control, -RT without reverse transcriptase. (B) Embryos injected dorsally with XProfilin2 (2 ng), XProfilin2 MO (70 ng), XProfilin2 MO + ΔN-XProfilin2 (70 ng+ 100 pg) show abnormal tissue localization due to gastrulation defects. Injected embryos show normal expression of Xbra at st 10.5 but Xbra is observed trapped around the blastopore that does not close at st 14. Sox-2 is expressed in the neural plate at st 14 in un-injected embryos, but in the injected embryos, Sox-2 expression surrounds the open blastopore. Gsc expression is observed in anterior mesendoderm away from the closed blastopore in control embryos at st 14, but in the injected embryos remains trapped near the open blastopore. Otx-2 is expressed anteriorly in both mesodermal and overlying neural tissues in control embryos at st 14, but in the injected embryos, the Otx-2 expression domain remains closer to the blastopore. Number of embryos scored is shown on each panel and yellow arrow indicates position of the blastopore.
We further examined the spatial expression pattern of mesodermal (Xbra and Gsc) and neural maker (Sox2 and Otx2) genes in response to over-expression or loss of XProfilin2 using whole embryo in situ hybridization analysis. Using doses of RNAs and MOs similar to the RT-PCR analysis above showed that the expression of mesodermal markers was unaffected. However, we observed a positional shifting of the gene expression territories owing to the defective gastrulation in both embryos overexpressing XProfilin2 or embryos depleted of XProfilin2 (Figure 4B). Together, these results show that XProfilin2 does not regulate mesodermal cell fate specification, but affects the proper localization of mesodermal and neural genes due to abnormal cell movement during gastrulation.
Profilin2 is required for convergent extension cell movement
The phenotypes observed with our gain-of-function and loss-of-function studies with XProfilin2 may result from defects in convergent-extension movement during gastrulation. In order to test the function of XProfilin2 for convergent extension movement in developing embryos, we employed the Keller explant assay and in vivo imaging of migrating mesodermal cells (Figure 5A–E). In Keller explant assays, the elongation of the dorsal mesodermal explants was significantly inhibited when embryos were injected with XProfilin2 MO (70 ng), but not with the control MO (70 ng) (Figure 5A–B). A dominant negative mutant of Dishevelled (Xdd1, 2 ng) known to result in the defective convergent extension movement was employed as a positive control (Figure 5A–B and (Sokol, 1996; Wallingford and Harland, 2001)). Additionally, the inhibition of explant elongation by the XProfilin2 MO (70 ng) was effectively rescued by co-injecting ΔN-XProfilin2 RNA (0.5 ng) (Figure 5A–B).
Figure 5. XProfilin2 regulates cell behavior responsible for convergent extension movement.
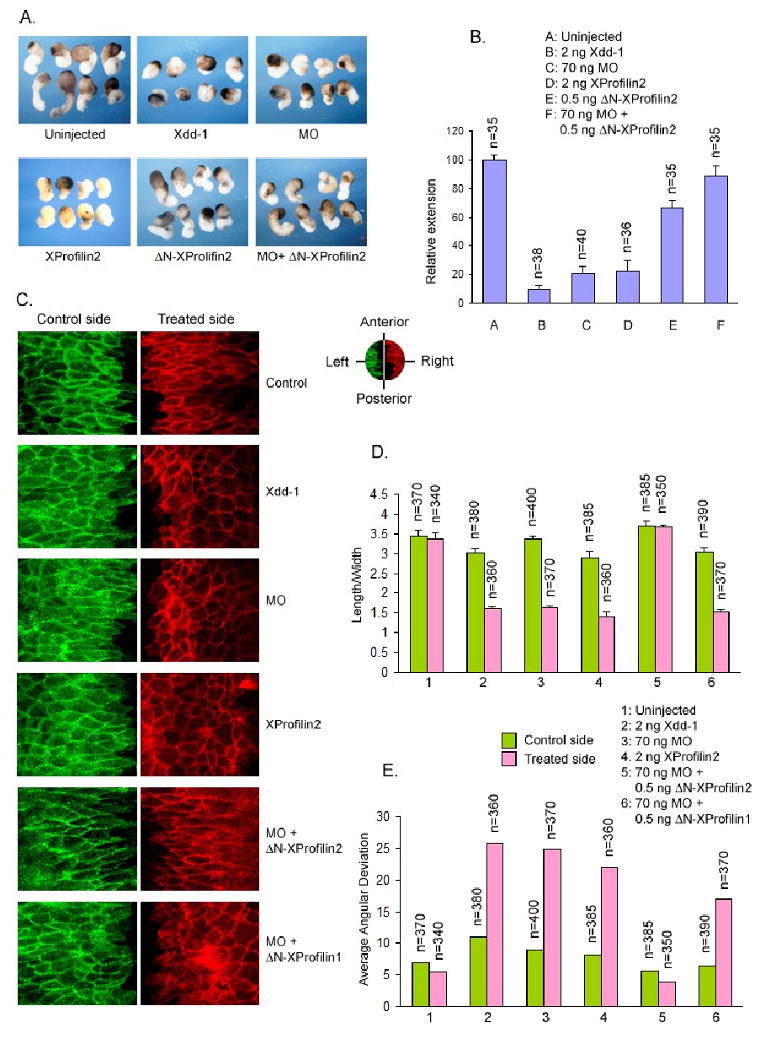
(A) Overexpression (2 ng RNA) or depletion of XProfilin2 (70 ng MO) inhibit convergent extension in Keller explants and dominant negative Dishevelled (Xdd1, 2 ng) is used as a positive control. The effects of the XProfilin2 MO are rescued by co-expression of ΔN-XProfilin2. (B) Quantitation of the Keller explants. The elongation of explants was measured using ImageJ and the values were expressed relative to that of the uninjected control sample (C). Overexpression (2 ng RNA) or depletion of XProfilin2 (70 ng MO) impairs polarization, elongation and mediolateral alignment of dorsal mesodermal cells undergoing convergent extension movement similar to expression of Xdd1 (2 ng). The induced defects in cell behaviors by XProfilin2 MO are rescued by co-expression of ΔN-XProfilin2 but not ΔN-XProfilin1 and the control MO has no effects. The orientation of the explants is shown in the upper right; anterior, posterior and left and right lateral. (D and E). Quantification of the effects of XProfilin2 on cell polarization, elongation and mediolateral alignment. Numbers of cells examined are shown at the top of each bar. Average angular deviation is the average deviation of the angular orientation of the length of cells along the midline.
We next studied the behaviors of mesodermal cells of cultured dorsal explant of embryos injected into the two dorsal blastomeres at the 4-cell embryo with XProfilin2 RNA (2 ng) or XProfilin2 MO (70 ng) co-injected along with a membrane-targeted fluorescent construct (cherry and GFP, 0.5 ng) as tracer. Similar to the results obtained with the Keller explant assays, the explants from XProfilin2 MO-injected embryos displayed irregular shaped cells similar as those from Xdd1 (2 ng) injected embryos indicating that convergent extension movement was affected (Figure 5C–E). The defects in cell morphology due to XProfilin2 MO-injection were rescued by co-expression of ΔN-XProfilin2 RNA (0.5 ng). The length/width ratios of the cells from the control, Xdd1-injected, XProfilin2 MO-injected, and rescued explants were 3.3, 1.6, 1.6 and 3.6 respectively (Figure 5D). We measured the degree of disorientation of the cells by calculating the average deviation of the lengthwise angular orientation of the cells, which were 5.5, 25.6, 24.8 and 3.9 for the control, Xdd1, XProfilin2 MO and rescued cells respectively (Figure 5E). Together, these results showed that XProfilin2 is required for convergent extension movement during gastrulation.
Profilin2 can modulate the actin cytoskeleton
As Daam1 regulates cytoskeletal changes during non-canonical Wnt signaling (Habas et al., 2001), this prompted us to test whether the gastrulation defects observed with overexpression or depletion of XProfilin2 were due to effects on the actin cytoskeleton. We thus examined the effects of overexpression or depletion of XProfilin2 on the actin cytoskeleton in explanted dorsal mesodermal cells. We found that injection of XProfilin2 RNA (2ng) led to a pronounced increase of actin fibers while injection of the XProfilin2 MO (70 ng) led to a marked decrease in the number of actin fibers in these cells (Figure 6A–B). Importantly the loss of actin fibers resultant from the XProfilin2 MO (70 ng) was efficiently rescued by co-injection of the XProfilin2 MO-insensitive ΔN-XProfilin2 RNA (0.5 ng) (Figure 6A–B).
Figure 6. Profilin2 modulates actin fibers.
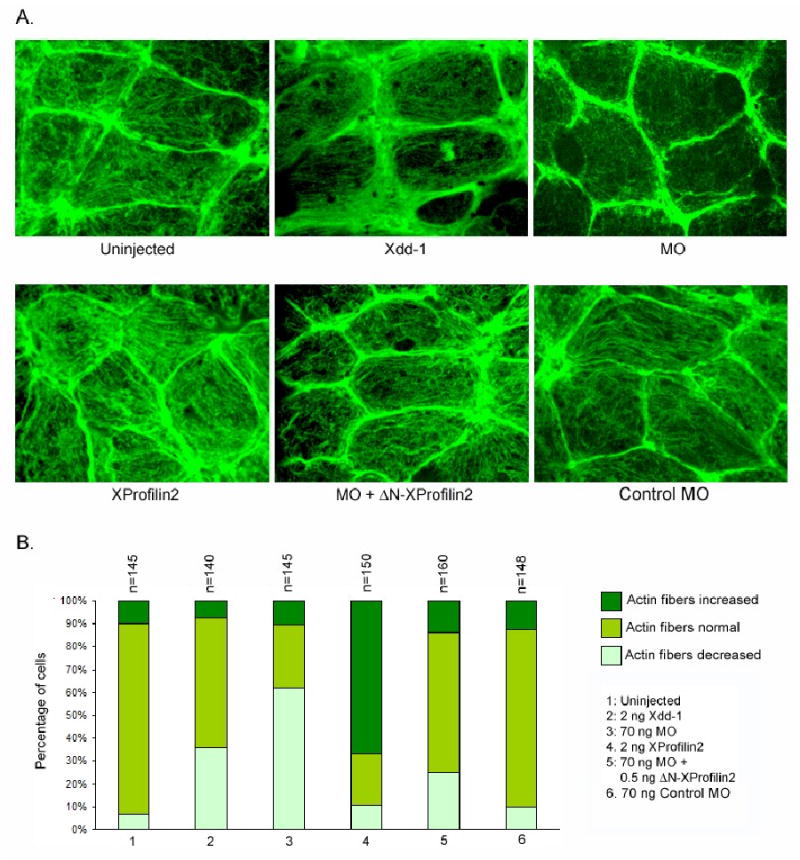
(A) Overexpression of XProfilin2 (2 ng RNA) increases the actin fibers in dorsal mesodermal cells of Xenopus embryos at neurula stage, while the depletion of XProfilin2 (70 ng MO) depletes the actin fibers. The MO mediated depletion of the actin fibers is rescued by co-expression of ΔN-XProfilin2. B. Quantitation of the effects of Profilin2 on the abundance of actin fibers. Numbers of the cells counted are shown at the top of each bar.
Distinct temporal interaction of Profilin2 with Daam1 during gastrulation
Because of the distinct functional roles observed for XProfilin2 for convergent extension movement and Profilin1 for blastopore closure during gastrulation, we asked whether there was a preferential association between XProfilin1 and XProfilin2 with XDaam1 during gastrulation. We injected Myc-tagged XProfilin1 (1ng) and Myc-tagged XProfilin2 (1ng) and performed co-immunoprecipitation with endogenous Daam1 from Xenopus lysates at distinct temporal stages during gastrulation. We found no difference between the ability of XProfilin1 to interact with Daam1 during stages 10.5 to 17 (Figure 7A) and a preferential increase in the association between XProfilin2 and Daam1 commencing around stage 12 (Figure 7B). This difference in temporal complex formation between XProfilin1, XProfilin2, and Daam1 suggests Daam1 differentially binds to and likely employs XProfilin1 and XProfilin2 for discrete roles during gastrulation consistent with the phenotypes observed with gain-of-function or loss-of-function analysis with XProfilin1 and XProfilin2.
Figure 7. Distinct temporal association of XProfilin2 with Daam1 during gastrulation.
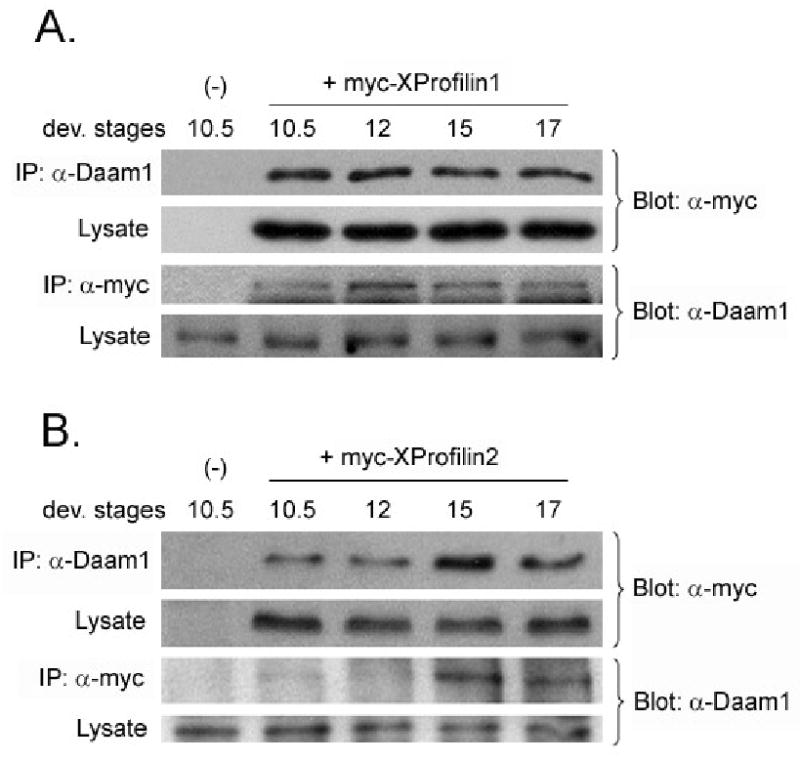
(A) Co-immunoprecipitation assays reveal XProfilin1 binds to endogenous Daam1 throughout gastrulation while (B) XProfilin2 binds to endogenous Daam1 preferentially during stages 12-15 during gastrulation. mRNAs encoding tagged-XProfilin1 and XProfilin2 were injected into Xenopus embryos and embryo lysates were immunoprecipitated (IP) with and immunoblotted with indicated Abs.
Discussion
In this study, we characterized the role of XProfilin2 in non-canonical Wnt signaling and Xenopus gastrulation. We identified XProfilin2 as a Daam1-interacting molecule and show XProfilin2 specifically binds to the FH1 domain of Daam1. In the Xenopus embryo, we find XProfilin2 is expressed in a temporal and spatial manner consistent with a role in gastrulation and show via gain-of-function and loss-of-function analysis that XProfilin2 is required for convergent extension movement during gastrulation. Importantly, XProfilin2 regulates cell polarization and axial alignment of mesodermal cells undergoing gastrulation and XProfilin1 cannot compensate for this function. These findings together demonstrate XProfilin2 is a non-redundant regulator of cell motility downstream of Daam1 during vertebrate gastrulation.
Profilin2 is an effector for Daam1
The non-canonical Wnt signaling pathway plays central roles during vertebrate gastrulation (Wallingford et al., 2002), and we have shown that the Formin protein Daam1 is a crucial regulator of non-canonical Wnt signaling for gastrulation (Habas et al., 2001). Daam1 binds to and is activated by the Dishevelled protein for its function modulating cytoskeletal changes and one effector identified as a downstream component for Daam1's effect on the actin cytoskeleton was the actin-binding protein XProfilin1 (Liu et al., 2008). However, as XProfilin1 specifically regulated blastopore closure, we reasoned additional downstream effectors for Daam1-mediated regulation of gastrulation must exist.
We now show that XProfilin2, similar to XProfilin1, binds to the FH1 domain of Daam1 (Figure 1D and (Sato et al., 2006)). Profilin2 can serve a similar biochemical function as Profilin1 in providing actin monomers for Formin-mediated actin filament elongation (Goode and Eck, 2007). While the expression pattern of Profilin2 in the mouse shows predominant expression in the nervous system (Gareus et al., 2006), we find that XProfilin2 RNA is maternally present, and its temporal expression pattern remains largely unchanged during embryogenesis. Spatially, XProfilin2 is enriched in dorsal mesodermal cells undergoing gastrulation, and it becomes refined to the neural plate and later in the eye, brain and spinal cord during embryogenesis. The maternal and zygotic expression of XProfilin2 suggested an early role in development and we note its expression pattern overlaps with that of XProfilin1 during early development (Sato et al., 2006).
Profilin2 regulates vertebrate gastrulation
The non-canonical Wnt pathway and Daam1 play important roles in cell polarization and cytoskeletal reorganization during gastrulation (Habas et al., 2001; Mlodzik, 2002). The effectors for Daam1 required for gastrulation remain poorly deciphered, and we find that RNA-based overexpression or MO-based depletion of XProfilin2 leads to severe gastrulation defects hallmarked by a shortened antero-posterior axis, curved body axis, and open neural tubes (Figure 3A–B). These results are in stark contrast to functional studies of XProfilin1 that only affects blastopore closure (Sato et al., 2006). Additionally, the effects of XProfilin2 on gastrulation were independent of mesodermal cell fate specification suggesting a direct effect on cell behavior (Figure 4A). The gastrulation defects observed by the depletion of XProflin2 were not rescued by expression of XProfilin1 RNA, which is resistant to the effects of the XProfilin2 MO. These results therefore strongly suggest distinct and non-redundant roles for XProfilin1 and XProfilin2 proteins during embryogenesis.
Profilin2 regulates dynamic cell behavior during convergent extension movement
Morphogenesis during gastrulation relies on a series of dynamic cell polarization and migratory events termed convergent extension movement that mediate axial extension, neural fold closure, and blastopore closure and convergent extension movement are regulated by non-canonical Wnt signaling (Wallingford et al., 2002; Wallingford and Habas, 2005). As the phenotypes resultant from overexpression or depletion of XProfilin2 resembled those of convergent extension-defective embryos, we examined whether XProfilin2 regulated convergent-extension movement. Using Keller explants, we find overexpression or depletion of XProfilin2 strongly suppressed elongation of mesodermal explants suggesting that XProfilin2 can regulate cell movement during gastrulation (Figure 5A–B).
Cell shape changes and polarization of dorsal mesodermal cells undergoing gastrulation are required for convergent extension movement, such that these cells adopt an elongated and polarized shape with a long axis oriented towards the midline. We find using overexpression and depletion of XProfilin2 that both length-to-width ratios and angular deviation from the midline are observed in these cells (Figure 5C–E). Thus XProfilin2 functions to regulate cell behavior including polarization and mediolateral orientation responsible for normal convergent extension movement.
Profilin modulates the actin cytoskeleton
Cell motility during gastrulation requires modulation of the actin cytoskeleton for directed cell polarity, and polarization of the migrating cells associated with mediolateral intercalation and convergent extension movement. Profilin2 is therefore an excellent candidate for linking Daam1's function to the actin cytoskeleton (Keller, 2002; Keller et al., 2003; Wallingford et al., 2002). The Profilin family members have been shown to regulate actin polymerization together with Formin proteins (Goode and Eck, 2007; Witke, 2004). Indeed we find that expression of XProfilin2 strongly induced actin fiber formation in dorsal mesodermal explants while depletion of XProfilin2 strongly depleted actin fibers in these cells (Figure 6A–B). As gain-of-function and loss-of-function of XProfilin2 in the Xenopus embryo inhibits convergent extension movement, we propose XProfilin2 functions downstream of Daam1 to regulate actin polymerization for the polarization and directional cell movement required for convergent extension movement. Whether additional factors downstream of Daam1 are required for distinct aspects of gastrulation remains to be investigated.
Distinct effectors for Daam1 regulate discrete aspects of gastrulation
Our studies together show that XProfilin2 and XProfilin1 function with Daam1 to regulate distinct aspects of gastrulation; XProfilin2 regulates convergent extension movement while XProfilin1 regulates blastopore closure (Sato et al., 2006). This however raises a deeper question: how does Daam1 engage these factors for these distinct functions? We note that both XProfilin2 and XProfilin1 RNAs are expressed throughout embryogenesis and even in overlapping regions, but we do not know how this RNA expression relates to their protein expression. Currently, the available antibodies against Profilin2 and Profilin1 either do not recognize or cannot distinguish between these two isoforms in Xenopus.
Additionally, we note that in the mouse, a knockout of Profilin1 reveals a critical function as the embryos die during the first cleavage stages (Witke et al., 2001), while in Xenopus XProfilin1 regulates blastopore closure (Sato et al., 2006). In the mouse, a knockout of Profilin2 shows defects in neuronal excitability (Pilo Boyl et al., 2007), whereas our current study in Xenopus revealed that XProfilin2 is critical for cell motility during gastrulation. The apparent discrepancy between the function of Profilin in mouse and Xenopus could possibly be due to the fact that our Morpholino approach may only partially deplete these proteins, thereby producing a hypomorphic phenotype. Alternatively, the Profilin isoforms may simply function differently in the two species. It should be noted, however, that in our XProfilin2 knockdown studies in Xenopus, the expression of XProfilin1 could not compensate for the loss of XProfilin2. Moreover, phylogenetic analysis of the Xenopus and mouse Profilin2 and Profilin1 isoforms reveal that Xenopus Profilin2 is more closely related at the amino acid sequence level to mouse Profilin1. Therefore, based on phenotypic analysis and sequence homology, it remains possible that Xenopus XProfilin2 functions more like mouse Profilin1 than mouse Profilin 2.
In summary, we provide evidence that Daam1 utilizes distinct effectors for discrete aspects of gastrulation. Daam1 binds to XProfilin1 and this Daam1/XProfilin1 complex function is required for blastopore closure (Sato et al., 2006). Daam1 can also bind to XProfilin2 and this Daam1/XProfilin2 complex function is required for cell polarization and motility during convergent extension movement. How Daam1 temporally and biochemically utilizes these different actin binding proteins for these effects remains to be investigated. We are further pursuing whether additional effectors are required downstream of Daam1 during gastrulation.
Acknowledgments
We thank Loren Runnels and the Habas laboratory for critical comments. We thank Dr. Jeffrey Miller, Karen Symes and Chenbei Chang for reagents and we are grateful to Dr. William Wadsworth and Gabriella D'Arcangelo for use of their microscopes. This work is supported by grants from the March of Dimes, an NSF (#0544061) and an NIH (GM078172) grant to R.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Birbach A. Profilin, a multi-modal regulator of neuronal plasticity. Bioessays. 2008;30:994–1002. doi: 10.1002/bies.20822. [DOI] [PubMed] [Google Scholar]
- Cooley L, Verheyen E, Ayers K. chickadee encodes a profilin required for intercellular cytoplasm transport during Drosophila oogenesis. Cell. 1992;69:173–84. doi: 10.1016/0092-8674(92)90128-y. [DOI] [PubMed] [Google Scholar]
- Gareus R, Di Nardo A, Rybin V, Witke W. Mouse profilin 2 regulates endocytosis and competes with SH3 ligand binding to dynamin 1. J Biol Chem. 2006;281:2803–11. doi: 10.1074/jbc.M503528200. [DOI] [PubMed] [Google Scholar]
- Goode BL, Eck MJ. Mechanism and function of formins in the control of actin assembly. Annu Rev Biochem. 2007;76:593–627. doi: 10.1146/annurev.biochem.75.103004.142647. [DOI] [PubMed] [Google Scholar]
- Habas R, Dawid IB, He X. Coactivation of Rac and Rho by Wnt/Frizzled signaling is required for vertebrate gastrulation. Genes Dev. 2003;17:295–309. doi: 10.1101/gad.1022203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas R, He X. Cell signaling: moving to a Wnt-Rap. Curr Biol. 2007;17:R474–7. doi: 10.1016/j.cub.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Habas R, Kato Y, He X. Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell. 2001;107:843–54. doi: 10.1016/s0092-8674(01)00614-6. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Solnica-Krezel L. Back and forth between cell fate specification and movement during vertebrate gastrulation. Curr Opin Genet Dev. 2008 doi: 10.1016/j.gde.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi T, Ikeda T, Shirakawa R, Kondo H, Kawato M, Horiguchi M, Okuda T, Okawa K, Fukai S, Nureki O, Kita T, Horiuchi H. Biochemical characterization of the Rho GTPase-regulated actin assembly by diaphanous-related formins, mDia1 and Daam1, in platelets. J Biol Chem. 2008;283:8746–55. doi: 10.1074/jbc.M707839200. [DOI] [PubMed] [Google Scholar]
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. doi: 10.1126/science.1079478. [DOI] [PubMed] [Google Scholar]
- Keller R, Davidson LA, Shook DR. How we are shaped: the biomechanics of gastrulation. Differentiation. 2003;71:171–205. doi: 10.1046/j.1432-0436.2003.710301.x. [DOI] [PubMed] [Google Scholar]
- Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4:68–75. doi: 10.4161/org.4.2.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Chan TH, Lin MJ, Huang WP, Lou SW, Lee SJ. Diaphanous-related formin 2 and profilin I are required for gastrulation cell movements. PLoS ONE. 2008;3:e3439. doi: 10.1371/journal.pone.0003439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Higgs HN. The mouse Formin mDia1 is a potent actin nucleation factor regulated by autoinhibition. Curr Biol. 2003;13:1335–40. doi: 10.1016/s0960-9822(03)00540-2. [DOI] [PubMed] [Google Scholar]
- Liu W, Sato A, Khadka D, Bharti R, Diaz H, Runnels LW, Habas R. Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A. 2008;105:210–5. doi: 10.1073/pnas.0707277105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt Signaling Pathway in Development and Disease. Annu Rev Cell Dev Biol. 2004 doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Lu J, Meng W, Poy F, Maiti S, Goode BL, Eck MJ. Structure of the FH2 domain of Daam1: implications for formin regulation of actin assembly. J Mol Biol. 2007;369:1258–69. doi: 10.1016/j.jmb.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlodzik M. Planar cell polarization: do the same mechanisms regulate Drosophila tissue polarity and vertebrate gastrulation? Trends Genet. 2002;18:564–71. doi: 10.1016/s0168-9525(02)02770-1. [DOI] [PubMed] [Google Scholar]
- Moseley JB, Maiti S, Goode BL. Formin proteins: purification and measurement of effects on actin assembly. Methods Enzymol. 2006;406:215–34. doi: 10.1016/S0076-6879(06)06016-2. [DOI] [PubMed] [Google Scholar]
- Nakaya MA, Habas R, Biris K, Dunty WC, Jr, Kato Y, He X, Yamaguchi TP. Identification and comparative expression analyses of Daam genes in mouse and Xenopus. Gene Expr Patterns. 2004;5:97–105. doi: 10.1016/j.modgep.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Paul AS, Pollard TD. The role of the FH1 domain and profilin in formin-mediated actin-filament elongation and nucleation. Curr Biol. 2008;18:9–19. doi: 10.1016/j.cub.2007.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilo Boyl P, Di Nardo A, Mulle C, Sassoe-Pognetto M, Panzanelli P, Mele A, Kneussel M, Costantini V, Perlas E, Massimi M, Vara H, Giustetto M, Witke W. Profilin2 contributes to synaptic vesicle exocytosis, neuronal excitability, and novelty-seeking behavior. Embo J. 2007;26:2991–3002. doi: 10.1038/sj.emboj.7601737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct. 2007;36:451–77. doi: 10.1146/annurev.biophys.35.040405.101936. [DOI] [PubMed] [Google Scholar]
- Sato A, Khadka DK, Liu W, Bharti R, Runnels LW, Dawid IB, Habas R. Profilin is an effector for Daam1 in non-canonical Wnt signaling and is required for vertebrate gastrulation. Development. 2006;133:4219–31. doi: 10.1242/dev.02590. [DOI] [PubMed] [Google Scholar]
- Semenov MV, Habas R, Macdonald BT, He X. SnapShot: Noncanonical Wnt Signaling Pathways. Cell. 2007;131:1378. doi: 10.1016/j.cell.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Sokol SY. Analysis of Dishevelled signalling pathways during Xenopus development. Curr Biol. 1996;6:1456–67. doi: 10.1016/s0960-9822(96)00750-6. [DOI] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–77. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Verheyen EM, Cooley L. Profilin mutations disrupt multiple actin-dependent processes during Drosophila development. Development. 1994;120:717–28. doi: 10.1242/dev.120.4.717. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Fraser SE, Harland RM. Convergent extension: the molecular control of polarized cell movement during embryonic development. Dev Cell. 2002;2:695–706. doi: 10.1016/s1534-5807(02)00197-1. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–36. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- Wallingford JB, Harland RM. Xenopus Dishevelled signaling regulates both neural and mesodermal convergent extension: parallel forces elongating the body axis. Development. 2001;128:2581–92. doi: 10.1242/dev.128.13.2581. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Higashida C. Formins: processive cappers of growing actin filaments. Exp Cell Res. 2004;301:16–22. doi: 10.1016/j.yexcr.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Witke W. The role of profilin complexes in cell motility and other cellular processes. Trends Cell Biol. 2004;14:461–9. doi: 10.1016/j.tcb.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Witke W, Sutherland JD, Sharpe A, Arai M, Kwiatkowski DJ. Profilin I is essential for cell survival and cell division in early mouse development. Proc Natl Acad Sci U S A. 2001;98:3832–6. doi: 10.1073/pnas.051515498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M, Higashi T, Suetsugu S, Sato Y, Ikeda T, Shirakawa R, Kita T, Takenawa T, Horiuchi H, Fukai S, Nureki O. Crystal structure of human DAAM1 formin homology 2 domain. Genes Cells. 2007;12:1255–65. doi: 10.1111/j.1365-2443.2007.01132.x. [DOI] [PubMed] [Google Scholar]


