Abstract
Somatostatin analogs ameliorate intestinal injury after localized irradiation. This study investigated whether SOM230, a novel, metabolically stable analog with broad receptor affinity, reduces intestinal injury and lethality in mice exposed to total-body irradiation (TBI). Male CD2F1 mice were exposed to 7–15 Gy TBI. Twice-daily administration of SOM230 (1, 4 or 10 mg/kg per day) or vehicle was started either 2 days before or 4 h after TBI and continued for either 14 or 21 days. Parameters of intestinal and hematopoietic radiation injury, bacterial translocation, and circulating cytokine levels were assessed. Animal survival was monitored for up to 30 days. SOM230 increased survival (P < 0.001) and prolonged survival time (P < 0.001) whether administration was initiated before or after TBI. There was no benefit from administration for 21 compared to 14 days. The survival benefit of SOM230 was completely reversed by co-administration of pancreatic enzymes (P = 0.009). Consistent with the presumed non-cytoprotective mechanism of action, SOM230 did not influence hematopoietic injury or intestinal crypt lethality. However, SOM230 preserved mucosal surface area (P < 0.001) and reduced bacterial translocation in a dose-dependent manner (P < 0.001). Circulating IL-12 levels were reduced in SOM230-treated mice (P = 0.007). No toxicity from SOM230 was observed. SOM230 enhances animal survival whether administration begins before or after TBI; i.e., it is effective both as a protector and as a mitigator. The mechanism likely involves reduction of intraluminal pancreatic enzymes. Because of its efficacy and favorable safety profile, SOM230 is a promising countermeasure against radiation and should undergo further development.
Introduction
The severity of hematopoietic/immune system injury and gastrointestinal (GI) injury is the main determinant of lethality after total-body irradiation (TBI). Significant progress has been made in the postexposure management of radiation-induced bone marrow injury with hematopoietic cytokines, blood transfusions, antimicrobial therapy and stem cell reconstitution (1, 2). In contrast, the management of GI radiation toxicity remains symptomatic and underdeveloped. Hence the relative importance of GI radiation toxicity is increasing, and the need to develop medical countermeasures against radiation injury of the GI tract has considerable significance.
The intestinal epithelium, although comprised of only a single layer of cells, has a surface area that is 200 times larger than that of the skin. Thus the epithelial lining of the gut constitutes the body's most extensive and important barrier to the exterior. Breakdown of the mucosal barrier during the acute phase of GI radiation injury exposes subepithelial tissues to the detrimental actions of the contents of the intestinal lumen. The significance of intestinal intraluminal contents in acute radiation-induced mucosal damage has been recognized for more than a century (3) and, not surprisingly, has been explored in the search for strategies that reduce GI radiation toxicity.
Among the various intraluminal factors, pancreatic enzymes exert a particularly prominent influence on development of intestinal radiation toxicity (4). Reducing pancreatic enzyme secretion by surgical or dietary methods attenuates acute mucosal injury and increases survival after abdominal irradiation in dogs (5–7), and pancreatic duct occlusion in rats protects against structural radiation injury of the intestine (8).
Clinically, the most relevant and feasible method of reducing intraluminal pancreatic secretions may be by administration of synthetic somatostatin receptor analogs. These drugs are used in the clinical treatment of acromegaly and in the treatment of patients with neuroendocrine tumors, notably carcinoid. In addition, somatostatin analogs are known as universal GI inhibitors and thus also strongly inhibit exocrine pancreatic secretion. We showed a number of years ago that short-term administration of the prototype somatostatin analog octreotide markedly ameliorates mucosal injury in small bowel after localized irradiation (9, 10). Subsequent work by others has confirmed our findings (11). However, the short half-life of octreotide represents a logistical obstacle to its use in mass casualty situations. The more recently developed somatostatin analog, SOM230 (pasireotide), on the other hand, has a more favorable pharmacokinetic profile and is thus a promising candidate as a medical countermeasure against gastrointestinal radiation injury.
In this study, the effects of SOM230 on animal survival and intestinal radiation injury in a mouse model of TBI were assessed. The results demonstrate that SOM230 confers a highly statistically significant survival benefit in this model, regardless of whether administration begins prior to or after TBI. Our data also suggest that the effectiveness of SOM230 as a radiation protective or radiation mitigating compound is related to inhibition of pancreatic enzyme secretion and that it involves preservation of the intestinal mucosal barrier.
Material and Methods
Reagents
SOM230 was kindly supplied by Novartis Pharma AG (Basel, Switzerland). Lyophilized SOM230 was stored at 4°C and was reconstituted in sterile deionized water just before use.
Pancrezyme, an enzyme preparation derived from porcine pancreas (25,500 USP units lipase, 139,000 USP units protease, and 164,000 USP units amylase per gram), was obtained from Virbac Animal Health (Forth Worth, TX). Pancrezyme was dissolved in sterile deionized water just before use.
Unless otherwise specified, all other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Animals
The experimental protocol was reviewed and approved by the Central Arkansas Veterans Healthcare System (CAVHS) Institutional Animal Care and Use Committee (IACUC) as well as by the IACUC at the University of Arkansas for Medical Sciences.
All experiments were carried out in randomly bred male CD2F1 mice (Harlan Sprague Dawley, Indianapolis, IN). The mice were 6–7 weeks of age at the initiation of the experiments (body weight 22–25 g). Animals were housed in conventional cages under standardized conditions with controlled temperature and humidity and a 12–12-h day-night light cycle. Animals had free access to water and chow (Harlan Teklad laboratory diet 7012, Purina Mills, St. Louis, MO).
A total of 608 mice were used for the experiments reported here. Mice were exposed to single-dose TBI, and groups of mice were subsequently killed humanely at set times after irradiation [0 h (no irradiation), 3.5 days, 7 days, 10 days, 21 days, 30 days] or were euthanized when moribund. For experiments that required longitudinal observation, a TBI dose of 8.0 Gy was given, because previous experiments in CD2F1 mice had shown that 8.0 Gy TBI induces substantial intestinal and hematopoietic injury, but with adequate 30-day survival rates.
To study the effect of the different treatments on postirradiation survival, mice were exposed to 7–15 Gy TBI. To reduce the number of animals required to a minimum, the design of these studies was optimized for efficiency and statistical power (12). While the experiments were ongoing, the mice were monitored twice daily by the investigators' support staff and twice daily by veterinary medical unit personnel for up to 30 days. The number of moribund/dead mice was recorded twice daily. Mice found to be moribund (defined as mice exhibiting excessive weight loss, lethargy, huddling, shivering, hunched posture or vocalization) were euthanized immediately by CO2 inhalation followed by cervical dislocation as recommended by the American Veterinary Medical Association (AVMA) in their 2007 Guidelines on Euthanasia.
Drug Treatment
To study the effect of SOM230 on radiation-induced injury, mice were randomly assigned to receive vehicle or 0.5 mg/kg SOM230 in 100 μl twice daily (b.i.d.) by subcutaneous (s.c.) injection. For subsequent dose optimization experiments, mice were randomly assigned to one of the following four treatment groups: vehicle control; 0.5 mg/kg SOM230 b.i.d.; 2.0 mg/kg SOM230 b.i.d.; or 5.0 mg/kg SOM230 b.i.d. SOM230 treatment was initiated either 2 days before TBI or 4 h after TBI; in both cases treatment was continued for 14 days. A side-by-side comparison of treatment for 14 days or 21 days was also performed as a separate experiment.
To determine whether the protective properties of SOM230 depended on inhibition of pancreatic enzyme secretion, experiments were performed with vehicle or SOM230 administration with and without co-administration of a commercial pancreatic enzyme preparation. For these experiments, mice were treated with SOM230 (5 mg/kg b.i.d.) by s.c. injection and pancreatic enzymes (200 mg/kg b.i.d.) by oral gavage. Mice were randomly assigned to one of the following treatment groups: vehicle s.c. combined with vehicle by gavage, vehicle s.c. combined with pancreatic enzymes by gavage, SOM230 s.c combined with vehicle by gavage, and SOM230 s.c. combined with pancreatic enzymes by gavage.
Irradiation and Dosimetry
Irradiation and dosimetry were performed as described elsewhere.4 Briefly, after confirmation of dose uniformity by thermoluminescence dosimetry, irradiation was performed with a Shepherd Mark I model 25 137Cs irradiator (J. L. Shepherd & Associates, San Fernando, CA). During irradiation, the animals were held in well-ventilated custom-made Plexiglas restrainers on a turntable rotating at five revolutions per minute. The average dose rate was 1.35 Gy per minute and was corrected for decay each day.
Intestinal Mucosal Surface Area (MSA)
Intestinal mucosal surface area is a well-validated, sensitive parameter of intestinal radiation injury. Mucosal surface area was measured in vertical sections of the jejunum stained with hematoxylin and eosin (H&E) using a projection/cycloid method as described by Baddeley et al. (13). We have validated the method previously specifically for surface area determination of the intestinal mucosa after irradiation (14).
Intestinal Crypt Colony Assay
Microcolony crypt cell survival was performed as described by Withers and Elkind (15). At 3.5 days after TBI (0, 9, 11, 13 and 15 Gy), mice were killed, and segments of proximal jejunum were obtained, fixed and stained with H&E. Surviving crypts, defined as crypts containing 10 or more adjacent chromophilic non-Paneth cells, were counted in transverse cross sections. Four circumferences were scored per mouse, and microcolony survival was expressed as the average number of crypts per circumference, with the average from each mouse considered as a single value for statistical purposes.
Plasma Citrulline Levels
Plasma citrulline is a well-validated, minimally invasive biomarker for functional enterocyte mass that can be performed in as little as 5 μl plasma (16).
At 0, 3.5 and 7 days after 8 Gy TBI, whole blood was collected in EDTA-coated tubes (Fisher Scientific, Pittsburgh, PA). Plasma was generated by centrifugation (12000 rpm, 5 min, 4°C) and stored at −80°C until analysis. Citrulline concentrations were determined using a reverse-phase HPLC-fluorimetry method with precolumn OPA/ME derivatization, as described previously by Pérez-Neri et al. (17).
Bacterial Translocation Assay
Radiation-induced bacterial translocation starts around day 7 after TBI and peaks about 2 weeks after TBI (18, 19). In the present study, analysis of bacterial translocation was performed on day 10 after TBI. Livers were removed aseptically and homogenized immediately. Bacterial translocation was quantified by real-time PCR as described by van Minnen et al. (20). Briefly, DNA was isolated from sterile livers using a DNA purification kit (Promega, Madison, WI), and real-time PCR was performed using Power SYBR green PCR master mix (Applied Biosystems, Foster City, CA) and 16S rRNA gene-targeted primers, forward (5′-AAC GCG AAG AAC CTT AC-3′) and reverse (5′-CGG TGT GTA CAA GAC CC-3′). Serially diluted bacterial genomic DNA was used to generate a standard curve. PCR-derived bacterial counts were expressed as nanograms of bacterial DNA per gram of mouse liver tissue.
Assessment of Hematopoietic Injury
Whole blood was collected in EDTA-coated tubes (Fisher Scientific). Peripheral blood cell counts were performed using a HEMAVET 950 system (Drew Scientific, Oxford, CA) according to the manufacturer's instructions.
Circulating Cytokine Levels
To explore the effect of TBI and SOM230 on the production of pro- and anti- inflammatory mediators, a multiplex based cytokine screen was used. Whole blood was collected in EDTA-coated tubes (Fisher Scientific). Plasma was generated by centrifugation (12,000 rpm, 5 min, 4°C) and stored at −80°C until analyzed. Plasma levels of 20 cytokines were measured by multiplexing using a Bioplex system (Bio-Rad Laboratories, Hercules, CA) and the BioSource mouse cytokine/chemokine 20-plex panel (Invitrogen, Carlsbad, CA).
Plasma Insulin-Like Growth Factor 1
SOM230 prominently inhibits the release of insulin-like growth factor 1 (IGF-1) (21). Hence plasma levels of IGF-1 were measured to verify biological activity of SOM230.
Three days after administration of SOM230 (5 mg/kg b.i.d.) was started, whole blood was collected in EDTA-coated tubes (Fisher Scientific). Plasma was generated by centrifugation (12,000 rpm, 5 min, 4°C) and stored at −80°C until tested. Plasma IGF-1 concentrations were measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Immunodiagnostic Systems, UK) as described previously (22).
Statistical Analysis
All statistical analyses were performed using NCSS 2004 for Windows (NCSS, Kaysville, UT). Data were presented as means ± SEM except for duration of survival, which was presented as median survival with interquartile range as measure of variability. Two-sided tests were used throughout, and differences were considered statistically significant when the P value was less than 0.05. Survival curves were constructed using the Kaplan-Meier method, and survival curves were compared with the log-rank test. Crypt colony assay data were compared using regression analysis with radiation dose and treatment group as independent variables. Pairwise (univariate) comparisons were performed with the Student's t test or Mann-Whitney U test as appropriate. Comparisons among several treatment groups and/or times were performed with analysis of variance (ANOVA) with post-hoc testing of group differences with Bonferroni's or Dunnet's test as indicated. LD50 values for estimation of dose reduction factors (DRFs) were calculated with logistic regression analysis; standard errors were calculated by use of the delta method.
Results
No signs of toxicity were observed, even at the highest SOM230 dose used (5 mg/kg b.i.d.) in this study.
Plasma concentrations of IGF-1 in vehicle-treated mice were 600 ng/ml (SEM: 26 ng/ml). In contrast, there was a highly significant decrease in plasma IGF-1 to 328 ng/ml (SEM: 16 ng/ml) after treatment with SOM230 (5 mg/kg b.i.d.) for 3 days (P < 10−6), thus confirming biological activity.
Overall Survival and Duration of Survival
Exposure to TBI induced signs of radiation sickness (i.e., radiation dose-dependent weight loss, lethargy) and mortality. Treatment with SOM230, starting 2 days before irradiation, significantly increased survival and prolonged survival time after TBI (Fig. 1). Importantly, starting administration of SOM230 4 h after TBI also resulted in substantially improved survival rates (Mantel Haenzel P < 0.00001) across the three different doses tested (8.5 Gy, 9 Gy and 9.5 Gy). Kaplan-Meier survival curves from the 9-Gy group are shown in Fig. 2. There was no added benefit from continuing SOM230 administration for 3 weeks instead of 2 weeks (data not shown).
FIG. 1.
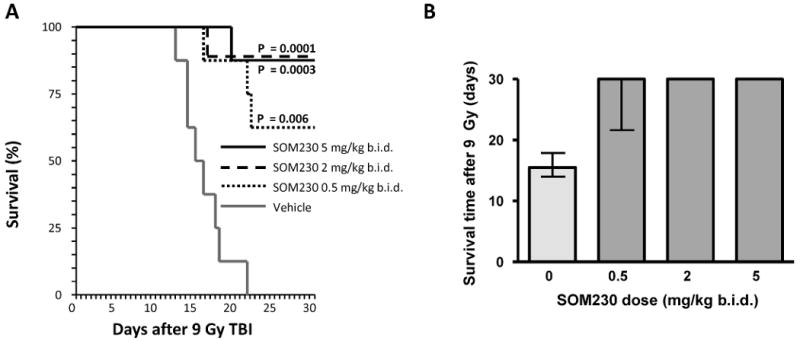
The effect of SOM230 on overall survival and median survival time with administration starting 2 h before and continuing for 2 weeks after TBI. Panel A : Kaplan-Meier survival curve from mice exposed to 9 Gy TBI. SOM230 significantly reduced lethality and prolonged survival (0.5 mg/kg b.i.d. P = 0.006; 2 mg/kg b.i.d. P = 0.0003; 5 mg/kg b.i.d. P = 0.0001). Panel B: Survival time (median and interquartile range) in mice exposed to 9 Gy TBI. SOM230 prolonged the median survival time after TBI (P < 0.05 at all doses). The animals were observed up to 30 days after TBI, n = 8.
FIG. 2.
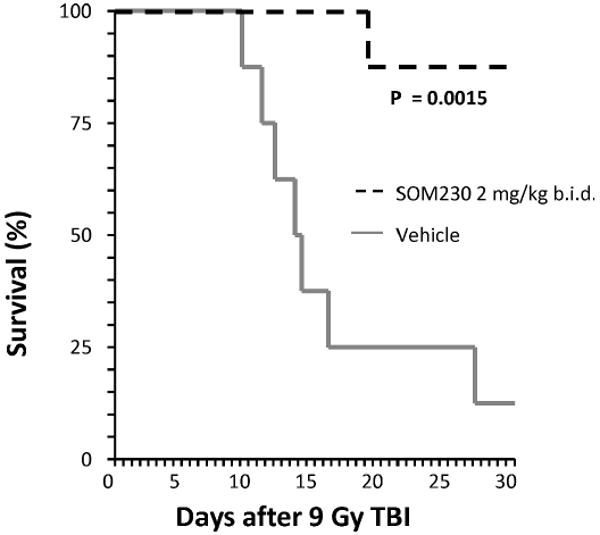
The effect of SOM230 on overall survival with drug administration beginning 4 h after TBI. Kaplan-Meier survival curve from mice exposed to 9 Gy TBI. SOM230, beginning 4 h after TBI, significantly reduced lethality (P = 0.0015). SOM230: 2 mg/kg b.i.d.; n = 8.
Overall survival rates at 10 days are generally considered appropriate for evaluating the contribution of the intestine to lethality after TBI (23) (in contrast to 7-day survival rates, which mainly reflect cell killing in the progenitor cell compartment in the intestinal crypts). The 10-day survival data are shown in Table 1. The confidence interval for the 5 mg/kg b.i.d. group was rather wide, but a conservative estimate of the DRF that can be achieved with SOM230 is at least of the order of 1.2.
TABLE 1. Estimates of Radiation Lethality with Standard Errors and Dose Reduction Factors for Two Different SOM230 Doses.
| LD50/10a (Gy) | SE (Gy) | DRF | |
|---|---|---|---|
| Vehicle | 10.4 | 0.3 | 1.0 |
| SOM230 (2 mg/kg b.i.d.) | 12.3 | 0.7 | 1.2 |
| SOM230 (5 mg/kg b.i.d.) | 13.3 | 1.4 | 1.3 |
Radiation dose associated with 50% lethality at 10 days.
Administration of pancreatic enzymes to mice did not affect survival after TBI compared to mice gavaged only with vehicle. In contrast, pancreatic enzyme administration in SOM230-treated animals strikingly reversed the beneficial effect of SOM230 (P = 0.009) (Fig. 3).
FIG. 3.

The effect of co-administration of SOM230 and pancreatic enzymes on overall survival after TBI. Kaplan-Meier survival curve from mice exposed to 9 Gy TBI. The beneficial effect of SOM230 on survival after TBI was reversed by co-administration of pancreatic enzymes (P = 0.009). V: vehicle; PE: pancreatic enzymes. SOM230: 5 mg/kg b.i.d.; pancreatic enzymes: 200 mg/kg b.i.d; n = 8.
Intestinal Radiation Injury
The effect of SOM230 (0.5 mg/kg b.i.d.) on intestinal mucosal injury was assessed at different times after 8 Gy TBI by measuring mucosal surface area and plasma citrulline levels. Radiation induced a highly significant decrease in mucosal surface area. As expected, the nadir of mucosal surface area was observed at 3.5 days after TBI with partial recovery at 21 days. Treatment with SOM230 significantly attenuated the effect of radiation on intestinal mucosal surface area (P < 10−6) (Fig. 4).
FIG. 4.

Effect of SOM230 administration on mucosal surface area. Panel A: TBI (8 Gy) induced a reduction in mucosal surface area. SOM230 administration significantly diminished the radiation-induced decrease in mucosal surface area (P < 10−6) (n = 6). Panel B: Representative image of intestine from a vehicle-treated animal on day 3.5 after 8 Gy TBI. Panel C: Representative image of intestine from an SOM230-treated animal on day 3.5 after 8 Gy TBI. SOM230: 0.5 mg/kg b.i.d.
Plasma citrulline levels were measured in unirradiated mice and at 3.5 and 7 days after 8 Gy TBI. TBI induced a significant decrease in plasma citrulline levels on day 3.5 (P = 0.01), while on day 7 levels had returned to baseline values. The difference between vehicle- and SOM230-treated animals did not reach statistical significance (Fig. 5).
FIG. 5.

Effect of SOM230 on plasma citrulline. TBI (8 Gy) induced a modest reduction in plasma citrulline at 3.5 days postirradiation (P = 0.01). SOM230 did not prevent the effect of TBI on plasma citrulline. SOM230: 0.5 mg/kg b.i.d.; n = 6.
Intestinal crypt survival was determined at 3.5 days after TBI (8–15 Gy). In contrast to the effect on mucosal surface area, SOM230 (0.5 mg/kg b.i.d.) did not affect radiation-induced crypt cell survival, consistent with the notion that SOM230 does not act as a cytoprotector (Fig. 6).
FIG. 6.
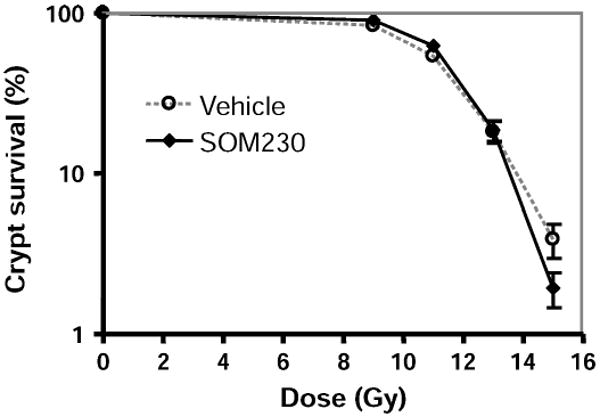
Effect of SOM230 on postirradiation crypt survival. SOM230 did not influence intestinal crypt survival after TBI. SOM230: 0.5 mg/kg b.i.d.; n = 4–6.
Bacterial Translocation
Bacterial translocation data, as quantified by real-time PCR of bacterial DNA in liver tissue, were obtained at 10 days after 9 Gy TBI. SOM230 administration was associated with a highly statistically significant dose-dependent reduction in bacterial translocation (P < 0.001) (Fig. 7). No bacterial DNA was detected in the livers of unirradiated mice.
FIG. 7.

Effect of SOM230 on postirradiation bacterial translocation. Ten days after 9 Gy TBI, significant amounts of bacterial DNA were observed in the livers of vehicle treated mice. There was a highly statistically significant dose-dependent reduction in bacterial translocation in SOM230-treated mice. n = 6.
Hematopoietic Toxicity and Recovery
Radiation induced a decrease in the numbers of circulating neutrophils, lymphocytes, erythrocytes and platelets (Fig. 8). Treatment with SOM230 (0.5 mg/kg b.i.d.) starting 2 days before irradiation did not significantly influence radiation-induced hematopoietic toxicity or recovery.
FIG. 8.

Effect of SOM230 on hematopoietic injury. SOM230 did not significantly alter the effects of TBI on numbers of circulating neutrophils, lymphocytes, erythrocytes or platelets. SOM230: 0.5 mg/kg b.i.d.; n = 6.
Circulating Cytokines and Chemokines
Among the cytokines and chemokines included in the multiplex assay, only plasma levels of IL-12 and CXCL9 were consistently above the detection limit (Fig. 9). SOM230 (0.5 mg/kg b.i.d.) significantly reduced the levels of IL-12 (P = 0.007), a cytokine that is known to sensitize the intestinal tract to ionizing radiation (24). CXCL9 levels gradually decreased after radiation (P = 0.003), but the levels were not influenced by SOM230 administration.
FIG. 9.

Effect of TBI and SOM230 on circulating levels of IL-12 and CXCL9. Panel A: SOM230-treated animals showed significantly lower circulating IL-12 levels than vehicle-treated animals (P = 0.007). Panel B: CXCL9 plasma levels gradually decreased after 8 Gy TBI (P = 0.003). No difference between vehicle- and SOM230-treated animals was observed. SOM230: 0.5 mg/kg b.i.d.; n = 6.
Discussion
Development of effective countermeasures against radiation-induced intestinal injury and lethality remains a significant capability shortfall and an unmet need. The results of the present study strongly suggest that the somatostatin analog SOM230 is useful as a non-toxic, highly effective pre-exposure protector as well as a postexposure mitigator of TBI-induced gastrointestinal injury and lethality. Moreover, our results suggest that there is no added benefit of prolonging SOM230 administration beyond 2 weeks after TBI and that the protective effect of SOM230 is mediated largely by inhibition of exocrine pancreatic secretion.
Endogenous somatostatin is a neuropeptide with receptors that are widely distributed throughout the gastrointestinal tract (25, 26). Somatostatin has a plethora of inhibitory effects on gastrointestinal physiology, including gastrointestinal hormone secretion, blood flow, motility and, notably, pancreatic secretion. Somatostatin analogs are widely used clinically (27, 28) and have an extremely favorable safety profile. Taken together, these findings make somatostatin analogs promising candidates for both prophylaxis and mitigation of intestinal radiation injury after TBI.
To our knowledge, all research to date investigating the efficacy of somatostatin analogs as radioprotective agents has been conducted with octreotide, the “prototype” somatostatin analog. Preclinical and clinical studies have shown that octreotide reduces acute side effects and the development of early as well as delayed structural radiation injury in small bowel after localized radiation exposure (9–11, 29, 30). Nevertheless, the potential usefulness of octreotide in the field or in a mass casualty situation is limited because of its short half-life (21, 31), thus requiring continuous infusion to maintain therapeutic drug levels. In contrast, SOM230 is a novel synthetic cyclohexapeptide that comprises modified unnatural amino acids and that may circumvent this obstacle (21). SOM230 has a half-life in excess of 11 h and can be administered by twice-daily subcutaneous injection. An additional potential advantage of SOM230 over octreotide is related to receptor selectivity. While octreotide binds only to the type 2 somatostatin receptor (sst2), SOM230 has affinity for somatostatin receptors sst1, sst2, sst3 and sst5 (32).
In addition to prolonging survival and reducing lethality after exposure to TBI, SOM230 preserved the intestinal mucosa surface area after irradiation and attenuated radiation-induced bacterial translocation. Plasma citrulline levels, on the other hand, did not increase significantly after SOM230 administration. Both mucosal surface area and plasma citrulline are indicators of enterocyte mass, but whereas the former is a morphological parameter, citrulline is a functional parameter that reflects total enterocyte metabolism (33, 34). It is conceivable that the discrepancy between mucosal surface area and plasma citrulline levels is due to the different nature of these two parameters. The fact that plasma citrulline levels are a somewhat less sensitive parameter of injury than mucosal surface area (i.e., more mucosal injury is required to achieve sufficient “resolution” of the assay) could also explain the discrepancy.
Consistent with the notion that SOM230 regulates the downstream pathophysiological manifestations of intestinal radiation injury rather than interfering directly with the initial radiochemical event, the present study did not show differences in crypt survival or in hematopoietic injury. This is also supported by the observation that SOM230 was effective when administration was started 4 h after irradiation, a time when the initial radiation-induced DNA strand breaks have already occurred.
Our study clearly indicates that the beneficial effects of SOM230 on intestinal injury and mortality are mediated at least in part by decreased pancreatic enzyme secretion. The specific importance of pancreatic enzymes in the pathogenesis of radiation-induced intestinal injury and subsequent lethality was proposed theoretically by Henry Quastler in the 1950s (35). Subsequently, Morgenstern's group, in a series of elegant articles, showed that reducing intraluminal pancreatic proteases in dogs lowers lethality after abdominal irradiation (5–7), and our and Delaney's groups demonstrated that pancreatic duct occlusion or inhibition of pancreatic enzymes ameliorates structural injury in the irradiated intestine (8, 36). More recently, Schoenbein et al. presented further evidence for the so-called auto-digestion hypothesis (37, 38). Hence, after events that reduce mucosal barrier function, pancreatic enzymes gain access to the intestinal wall where they initiate a process of auto-digestion. The auto-digestion products are highly inflammatory and cytotoxic and exacerbate tissue injury. Moreover, the pancreatic enzyme trypsin plays an important role in the activation of membrane receptors, notably proteinase-activated receptor 2 (PAR2), which plays a critical role in bowel inflammation and visceral nociception and is an important mediator of radiation-induced gut injury (39).
It is also conceivable that SOM230 confers some of its benefits by modulating immune cell function. Somatostatin receptors are abundant on both intestinal immune cells and nerve endings (25, 26). SOM230 may exert an immunomodulatory effect both by acting on immune cells directly, as in the somatostatin-INF-γ immunoregulatory circuit (40), and by neuroimmune interactions that have been shown to regulate the intestinal radiation response (41, 42). Another possibility is that SOM230, as shown for somatostatin/octreotide, regulates the vascular response to injury by stimulating protein-tyrosine phosphatase and/or serine/threonine phosphatases (43, 44), thus indirectly influencing the intestinal radiation response.
The present study showed that SOM230 reduced circulating levels of IL-12, a pro-inflammatory cytokine. These results are consistent with in vitro studies showing that octreotide reduces IL-12 production by macrophages and dendritic cells (45, 46). While IL-12 protects the hematopoietic system by enhancing endogenous hematopoiesis and stem cell engraftment after TBI, it exacerbates intestinal radiation injury (47, 48). Therefore, it is not unlikely that inhibition of IL-12 production by SOM230 contributes to its beneficial effects on intestinal radiation injury. The extent to which IL-12 plays a role in the net overall survival benefit after SOM230 administration remains to be elucidated.
Another interesting issue is the relationship between somatostatin analogs, IGF-1 and growth hormone (GH). SOM230 reduces the secretion of IGF-1 and GH (21), both of which have been suggested to protect against radiation-induced intestinal and hematopoietic injury (49–54). In the present study, we did find a substantial effect of SOM230 on IGF-1 levels. It is interesting to speculate that strategies to separate the beneficial effects of SOM230 from the effects on IGF-1 and GH will be able to further increase the efficacy of SOM230 as a radiation protector and radiation mitigator. Research is clearly needed to establish the significance of alterations in IGF-1 and GH during SOM230 therapy.
Considerable debate evolves around the role of endothelial dysfunction and endothelial apoptosis in the intestinal microvasculature during development of acute radiation-induced bowel injury. Acid sphingomyelinase-deficient mice are protected from radiation-induced endothelial cell apoptosis, exhibit decreased levels of crypt cell apoptosis and increased survival rates after TBI (55, 56). On the other hand, there is controversy related to the extent and significance of radiation-induced endothelial apoptosis in intestinal microvasculature and regarding whether a direct relationship exists between endothelial apoptosis and apoptosis in the crypt epithelium (57). Moreover, Qiu et al. (58) recently showed that mice deficient in p53 up-regulated modulator of apoptosis (PUMA) or mice in which PUMA was suppressed by antisense oligonucleotides exhibited decreased crypt cell apoptosis and prolonged postirradiation survival without an appreciable effect on endothelial apoptosis. Clearly, more work is needed to clarify the significance of interactions among the various cellular compartments in the gut during development of the acute gastrointestinal radiation syndrome.
Realistically, future strategies to ameliorate injury and lethality from TBI are likely going to consist of a combination of therapies rather than treatment with single compounds. SOM230 confers protection against TBI-induced injury by a mechanism that differs form many other strategies. Combining SOM230 with enteroprotective interventions that act through other mechanisms, for example, CBLB502 (a polypeptide that activates nuclear factor-κB (59), the vitamin E analog γ-tocotrienol (60), or orally administered IL-11 (61), should be explored. Further studies to define the postirradiation time window and the optimal SOM230 dose and duration of administration in this setting are also needed.
In conclusion, the present study demonstrates that SOM230 ameliorates TBI-induced intestinal injury and lethality when administered both before and after radiation exposure or only after irradiation. The beneficial effects of SOM230 do not appear to be mediated by free radical scavenging or traditional cytoprotective mechanisms but do depend to a large extent on reduction of exocrine pancreatic secretion. Because of the exceptionally favorable safety profile of somatostatin analogs, SOM230 is promising as a medical countermeasure against radiation in the mass casualty setting. More research is needed to further assess the potential of SOM230 as a mitigating agent and to determine the most appropriate dosing schedule, including the optimal drug concentration and postirradiation time window for administration.
Acknowledgments
The authors gratefully acknowledge Ivan Spasojević of the Clinical Research PK/PD Laboratory at Duke University Medical Center for performance of citrulline assays and Jeffrey C. Hale and Gregory D. Sempowski of the Immune Monitoring Core at Duke University Medical Center for performance of the Luminex assays. Dr. Berbee was enrolled in the Ph.D. program at the Department of Radiation Oncology, University of Maastricht. Support for this work was provided by grant CA71382 from the NCI and by the NIAID Centers for Medical Countermeasures against Radiation (CMCR) program to the RadCCORE Consortium at Duke University (grant U19 AI67798).
Footnotes
Q. Fu, M. Berbee, M. Boerma, J. Wang, K. S. Kumar and M. Hauer-Jensen, The vitamin E analog, gamma-tocotrienol, protects against tissue injury and lethality after total body irradiation partly via inhibition of HMG-CoA reductase. Presented at the Fifty-fourth Annual Meeting of the Radiation Research Society, Boston, MA, 2008.
References
- 1.Dainiak N, Waselenko JK, Armitage JO, MacVittie TJ, Farese AM. The hematologist and radiation casualties. Hematology Am Soc Hematol Educ Program. 2003:473–496. doi: 10.1182/asheducation-2003.1.473. [DOI] [PubMed] [Google Scholar]
- 2.Weisdorf D, Chao N, Waselenko JK, Dainiak N, Armitage JO, McNiece I, Confer D. Acute radiation injury: contingency planning for triage, supportive care, and transplantation. Biol Blood Marrow Transplant. 2006;12:672–682. doi: 10.1016/j.bbmt.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Krause P, Ziegler K. Experimentelle Untersuchungen ueber die Einwirkung der Roentgenstrahlen auf tierische Gewebe. A. Uebersicht ueber die in der Litteratur niedergelegten Angaben ueber die Wirkung der Roentgenstrahlen auf innere Organe. Fortschr Geb Roentgenstr. 1906;10:126–182. [Google Scholar]
- 4.Morgenstern L, Hiatt N. Injurious effect of pancreatic secretions on postradiation enteropathy. Gastroenterology. 1967;53:923–929. [PubMed] [Google Scholar]
- 5.Sokol AB, Lipson LW, Morgenstern L, Hiatt N. Protection against lethal irradiation injury by pancreatic enzyme exclusion. Surg Forum. 1967;18:387–389. [Google Scholar]
- 6.Morgenstern L, Patin CS, Krohn HL, Hiatt N. Prolongation of survival in lethally irradiated dogs. Arch Surg. 1970;101:586–589. doi: 10.1001/archsurg.1970.01340290042009. [DOI] [PubMed] [Google Scholar]
- 7.Rachootin S, Shapiro S, Yamakawa T, Goldman L, Patin S, Morgenstern L. Potent anti-protease from Ascaris lumbricoides: Efficacy in amelioration of post-radiation enteropathy. Gastroenterology. 1972;62:796. abstract. [Google Scholar]
- 8.Hauer-Jensen M, Sauer T, Berstad T, Nygaard K. Influence of pancreatic secretion on late radiation enteropathy in the rat. Acta Radiol Oncol. 1985;24:555–560. doi: 10.3109/02841868509134431. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Zheng H, Sung CC, Hauer-Jensen M. The synthetic somatostatin analogue, octreotide, ameliorates acute and delayed intestinal radiation injury. Int J Radiat Oncol Biol Phys. 1999;45:1289–1296. doi: 10.1016/s0360-3016(99)00293-x. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Zheng H, Hauer-Jensen M. Influence of short-term octreotide administration on chronic tissue injury, transforming growth factor β (TGF-β) overexpression, and collagen accumulation in irradiated rat intestine. J Pharmacol Exp Ther. 2001;297:35–42. [PubMed] [Google Scholar]
- 11.Abbasoglu SD, Erbil Y, Eren T, Giris M, Barbaros U, Yucel R, Olgac V, Uysal M, Toker G. The effect of heme oxygenase-1 induction by octreotide on radiation enteritis. Peptides. 2006;27:1570–1576. doi: 10.1016/j.peptides.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Kodell RL, Lensing SY, Landes RD, Kumar KS, Hauer-Jensen M. Determination of sample sizes for demonstrating efficacy of radiation countermeasures. Biometrics. doi: 10.1111/j.1541-0420.2009.01236.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baddeley AJ, Gundersen HJG, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc. 1986;142:259–276. doi: 10.1111/j.1365-2818.1986.tb04282.x. [DOI] [PubMed] [Google Scholar]
- 14.Langberg CW, Sauer T, Reitan JB, Hauer-Jensen M. Relationship between intestinal fibrosis and histopathologic and morphometric changes in consequential and late radiation enteropathy. Acta Oncol. 1996;35:81–87. doi: 10.3109/02841869609098484. [DOI] [PubMed] [Google Scholar]
- 15.Withers HR, Elkind MM. Microcolony survival assay for cells of mouse intestinal mucosa exposed to radiation. Int J Radiat Biol. 1970;17:261–267. doi: 10.1080/09553007014550291. [DOI] [PubMed] [Google Scholar]
- 16.Crenn P, Messing B, Cynober L. Citrulline as a biomarker of intestinal failure due to enterocyte mass reduction. Clin Nutr. 2008;27:328–339. doi: 10.1016/j.clnu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Perez-Neri I, Montes S, Boll MC, Ramirez-Bermudez J, Rios C. Liquid chromatographic-fluorimetric method for the estimation of nitric oxide biosynthesis in the central nervous system. J Chromatogr B Anal Technol Biomed Life Sci. 2004;806:133–139. doi: 10.1016/j.jchromb.2004.03.043. [DOI] [PubMed] [Google Scholar]
- 18.Brook I, MacVittie TJ, Walker RI. Recovery of aerobic and anaerobic bacteria from irradiated mice. Infect Immun. 1984;46:270–271. doi: 10.1128/iai.46.1.270-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobayashi T, Ohmori T, Yanai M, Kawanishi G, Mitsuyama M, Nomoto K. The analysis of the defense mechanism against indigenous bacterial translocation in X-irradiated mice. Microbiol Immunol. 1991;35:315–324. doi: 10.1111/j.1348-0421.1991.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 20.van Minnen LP, Timmerman HM, Lutgendorff F, Verheem A, Harmsen W, Konstantinov SR, Smidt H, Visser MR, Rijkers GT, Akkermans LM. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery. 2007;141:470–480. doi: 10.1016/j.surg.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146:707–716. doi: 10.1530/eje.0.1460707. [DOI] [PubMed] [Google Scholar]
- 22.Schmid HA, Silva AP. Short- and long-term effects of octreotide and SOM230 on GH, IGF-I, ACTH, corticosterone and ghrelin in rats. J Endocrinol Invest. 2005;28(Suppl):28–35. [PubMed] [Google Scholar]
- 23.Terry NHA, Travis EL. The influence of bone marrow depletion on intestinal radiation damage. Int J Radiat Oncol Biol Phys. 1989;17:569–573. doi: 10.1016/0360-3016(89)90108-9. [DOI] [PubMed] [Google Scholar]
- 24.Neta R, Stiefel SM, Finkelman F, Herrman S, Ali N. IL-12 protects bone marrow from and sensitizes intestinal tract to ionizing radiation. J Immunol. 1994;153:4230–4237. [PubMed] [Google Scholar]
- 25.Schafer J, Meyerhof W. sst1 mRNA is the prominent somatostatin receptor mRNA in the rat gastrointestinal tract: reverse transcription polymerase chain reaction and in situ-hybridization study. Neuropeptides. 1999;33:457–463. doi: 10.1054/npep.1999.0762. [DOI] [PubMed] [Google Scholar]
- 26.Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. Regulation and function of somatostatin receptors. J Neurochem. 2004;89:1057–1091. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- 27.Melen-Mucha G, Lawnicka H, Kierszniewska-Stepien D, Komorowski J, Stepien H. The place of somatostatin analogs in the diagnosis and treatment of the neuoroendocrine gland tumors. Recent Patents Anticancer Drug Discov. 2006;1:237–254. doi: 10.2174/157489206777442197. [DOI] [PubMed] [Google Scholar]
- 28.Murray RD, Melmed S. A critical analysis of clinically available somatostatin analog formulations for therapy of acromegaly. J Clin Endocrinol Metab. 2008;93:2957–2968. doi: 10.1210/jc.2008-0027. [DOI] [PubMed] [Google Scholar]
- 29.Yavuz MN, Yavuz AA, Aydin F, Can G, Kavgaci H. The efficacy of octreotide in the therapy of acute radiation-induced diarrhea: a randomized controlled study. Int J Radiat Oncol Biol Phys. 2002;54:195–202. doi: 10.1016/s0360-3016(02)02870-5. [DOI] [PubMed] [Google Scholar]
- 30.Olgac V, Erbil Y, Barbaros U, Oztezcan S, Giris M, Kaya H, Bilge H, Guler S, Toker G. The efficacy of octreotide in pancreatic and intestinal changes: radiation-induced enteritis in animals. Dig Dis Sci. 2006;51:227–232. doi: 10.1007/s10620-006-3113-3. [DOI] [PubMed] [Google Scholar]
- 31.Ma P, Wang Y, van der Hoek HJ, Nedelman J, Schran H, Tran LL, Lamberts SW. Pharmacokinetic-pharmacodynamic comparison of a novel multiligand somatostatin analog, SOM230, with octreotide in patients with acromegaly. Clin Pharmacol Ther. 2005;78:69–80. doi: 10.1016/j.clpt.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Lewis I, Bauer WC, Albert R, Chandramouli N, Pless J, Weckbecker G, Bruns C. A novel somatostatin peptidomimetic with broad somatotropin release inhibitory factor receptor binding and superior therapeutic potential. J Med Chem. 2003;46:2334–2344. doi: 10.1021/jm021093t. [DOI] [PubMed] [Google Scholar]
- 33.Lutgens L, Lambin P. Biomarkers for radiation-induced small bowel epithelial damage: an emerging role for plasma citrulline. World J Gastroenterol. 2007;13:3033–3042. doi: 10.3748/wjg.v13.i22.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutgens LC, Deutz NE, Gueulette J, Cleutjens JP, Berger MP, Wouters BG, von Meyenfeldt MF, Lambin P. Citrulline: a physiological marker enabling quantitation and monitoring of epithelial radiation-induced small bowel damage. Int J Radiat Oncol Biol Phys. 2003;57:1067–1074. doi: 10.1016/s0360-3016(03)00781-8. [DOI] [PubMed] [Google Scholar]
- 35.Quastler H. The nature of intestinal radiation death. Radiat Res. 1956;4:303–320. [PubMed] [Google Scholar]
- 36.Delaney JP, Bonsack M. Acute radiation enteritis in rats: bile salts and trypsin. Surgery. 1992;112:587–592. [PubMed] [Google Scholar]
- 37.Penn AH, Hugli TE, Schmid-Schonbein GW. Pancreatic enzymes generate cytotoxic mediators in the intestine. Shock. 2007;27:296–304. doi: 10.1097/01.shk.0000235139.20775.7f. [DOI] [PubMed] [Google Scholar]
- 38.Schmid-Schonbein GW, Hugli TE. A new hypothesis for microvascular inflammation in shock and multiorgan failure: self-digestion by pancreatic enzymes. Microcirculation. 2005;12:71–82. doi: 10.1080/10739680590896009. [DOI] [PubMed] [Google Scholar]
- 39.Wang J, Zheng H, Hollenberg MD, Vijesuriya SJ, Ou X, Hauer-Jensen M. Up-regulation and activation of proteinase-activated receptor-2 (PAR-2) in early and delayed radiation injury in the rat intestine: influence of biological PAR-2 activators. Radiat Res. 2003;160:524–535. doi: 10.1667/rr3080. [DOI] [PubMed] [Google Scholar]
- 40.Elliott DE, Li J, Blum AM, Metwali A, Patel YC, Weinstock JV. SSTR2A is the dominant somatostatin receptor subtype expressed by inflammatory cells, is widely expressed and directly regulates T cell IFN-gamma release. Eur J Immunol. 1999;29:2454–2463. doi: 10.1002/(SICI)1521-4141(199908)29:08<2454::AID-IMMU2454>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 41.Wang J, Zheng H, Kulkarni A, Ou X, Hauer-Jensen M. Regulation of early and delayed radiation responses in rat small intestine by capsaicin-sensitive nerves. Int J Radiat Oncol Biol Phys. 2006;64:1528–1536. doi: 10.1016/j.ijrobp.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Hauer-Jensen M. Neuroimmune interactions in the gut: potential target for mitigating or treating intestinal radiation injury. Br J Radiol. 2007;80:S41–S48. doi: 10.1259/bjr/33057885. [DOI] [PubMed] [Google Scholar]
- 43.Todisco A, Takeuchi Y, Yamada J, Sadoshima JI, Yamada T. Molecular mechanisms for somatostatin inhibition of c-fos gene expression. Am J Physiol. 1997;272:G721–G726. doi: 10.1152/ajpgi.1997.272.4.G721. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita M, Dimayuga P, Kaul S, Shah PK, Regnstrom J, Nilsson J, Cercek B. Phosphatase activity in the arterial wall after balloon injury: effect of somatostatin analog octreotide. Lab Invest. 1999;79:935–944. [PubMed] [Google Scholar]
- 45.Valatas V, Kolios G, Manousou P, Xidakis C, Notas G, Ljumovic D, Kouroumalis EA. Secretion of inflammatory mediators by isolated rat Kupffer cells: the effect of octreotide. Regul Pept. 2004;120:215–225. doi: 10.1016/j.regpep.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 46.Kao JY, Pierzchala A, Rathinavelu S, Zavros Y, Tessier A, Merchant JL. Somatostatin inhibits dendritic cell responsiveness to Helicobacter pylori. Regul Pept. 2006;134:23–29. doi: 10.1016/j.regpep.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 47.Neta R, Stiefel SM, Finkelman F, Herrmann S, Ali N. IL-12 protects bone marrow from and sensitizes intestinal tract to ionizing radiation. J Immunol. 1994;153:4230–4237. [PubMed] [Google Scholar]
- 48.Chen T, Burke KA, Zhan Y, Wang X, Shibata D, Zhao Y. IL-12 facilitates both the recovery of endogenous hematopoiesis and the engraftment of stem cells after ionizing radiation. Exp Hematol. 2007;35:203–213. doi: 10.1016/j.exphem.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-de-Segura IA, Prieto I, Grande AG, Garcia P, Guerra A, Mendez J, De Miguel E. Growth hormone reduces mortality and bacterial translocation in irradiated rats. Acta Oncol. 1998;37:179–185. doi: 10.1080/028418698429748. [DOI] [PubMed] [Google Scholar]
- 50.Alexandrides T, Spiliotis J, Mylonas P, Melachrinou M, Kardamakis D, Spiliopoulou I, Panagopoulos C, Kalfarentzos F. Effects of growth hormone and insulin-like growth factor-I on radiation enteritis. a comparative study. Eur Surg Res. 1998;30:305–311. doi: 10.1159/000008592. [DOI] [PubMed] [Google Scholar]
- 51.Vazquez I, Gomez-de-Segura IA, Grande AG, Escribano A, Gonzalez-Gancedo P, Gomez A, Diez R, De Miguel E. Protective effect of enriched diet plus growth hormone administration on radiation-induced intestinal injury and on its evolutionary pattern in the rat. Dig Dis Sci. 1999;44:2350–2358. doi: 10.1023/a:1026637611298. [DOI] [PubMed] [Google Scholar]
- 52.Mylonas PG, Matsouka PT, Papandoniou EV, Vagianos C, Kalfarentzos F, Alexandrides TK. Growth hormone and insulin-like growth factor I protect intestinal cells from radiation induced apoptosis. Mol Cell Endocrinol. 2000;160:115–122. doi: 10.1016/s0303-7207(99)00215-4. [DOI] [PubMed] [Google Scholar]
- 53.Wilkins HR, Ohneda K, Keku TO, D'Ercole AJ, Fuller CR, Williams KL, Lund PK. Reduction of spontaneous and irradiation-induced apoptosis in small intestine of IGF-I transgenic mice. Am J Physiol. 2002;283:G457–G464. doi: 10.1152/ajpgi.00019.2002. [DOI] [PubMed] [Google Scholar]
- 54.Raguso CA, Leverve X, Pichard C. Protective effects of recombinant growth hormone on intestinal mucosa in rats receiving abdominal radiotherapy. Clin Nutr. 2002;21:487–490. doi: 10.1054/clnu.2002.0579. [DOI] [PubMed] [Google Scholar]
- 55.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 56.Rotolo JA, Maj JG, Feldman R, Ren D, Haimovitz-Friedman A, Cordon-Cardo C, Cheng EH, Kolesnick R, Fuks Z. Bax and bak do not exhibit functional redundancy in mediating radiation-induced endothelial apoptosis in the intestinal mucosa. Int J Radiat Oncol Biol Phys. 2008;70:804–815. doi: 10.1016/j.ijrobp.2007.11.043. [DOI] [PubMed] [Google Scholar]
- 57.Schuller BW, Binns PJ, Riley KJ, Ma L, Hawthorne MF, Coderre JA. Selective irradiation of the vascular endothelium has no effect on the survival of murine intestinal crypt stem cells. Proc Natl Acad Sci USA. 2006;103:3787–3792. doi: 10.1073/pnas.0600133103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qiu W, Carson-Walter EB, Liu H, Epperly M, Greenberger JS, Zambetti GP, Zhang L, Yu J. PUMA regulates intestinal progenitor cell radiosensitivity and gastrointestinal syndrome. Cell Stem Cell. 2008;2:576–583. doi: 10.1016/j.stem.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Burdelya LG, Krivokrysenko VI, Tallant TC, Strom E, Gleiberman AS, Gupta D, Kurnasov OV, Fort FL, Osterman AL, Gudkov AV. An agonist of Toll-like receptor 5 has radioprotective activity in mouse and primate models. Science. 2008;320:226–230. doi: 10.1126/science.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berbee M, Fu Q, Boerma M, Wang J, Kumar KS, Hauer-Jensen M. Gamma-tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boerma M, Wang J, Burnett AF, Santin AD, Roman JJ, Hauer-Jensen M. Local administration of interleukin-11 ameliorates intestinal radiation injury. Cancer Res. 2007;67:9501–9506. doi: 10.1158/0008-5472.CAN-07-0810. [DOI] [PubMed] [Google Scholar]


