Abstract
Telomeres consist of nucleotide repeats and a protein complex at chromosome ends that are essential to maintaining chromosomal integrity. Several studies have suggested that subjects with shorter telomeres are at increased risk of bladder and lung cancer. In comparison to normal tissues, telomeres are shorter in high-grade intraepithelial neoplasia and prostate cancer. We examined prostate cancer risk associated with relative telomere length as determined by quantitative PCR on pre-diagnostic buffy coat DNA isolated from 612 advanced prostate cancer cases and 1049 age-matched, cancer-free controls from the PLCO Cancer Screening Trial. Telomere length was analyzed as both a continuous and a categorical variable with adjustment for potential confounders. Statistically significant inverse correlations between telomere length, age and smoking status were observed in cases and controls. Telomere length was not associated with prostate cancer risk (at the median, OR = 0.85, 95% CI 0.67, 1.08); associations were similar when telomere length was evaluated as a continuous variable or by quartiles. The relationships between telomere length and inflammation-related factors, diet, exercise, body mass index, and other lifestyle variables were explored since many of these have previously been associated with shorter telomeres. Healthy lifestyle factors (i.e., lower BMI, more exercise, tobacco abstinence, diets high in fruit and vegetables) tended to be associated with greater telomere length. This study found no statistically significant association between leukocyte telomere length and advanced prostate cancer risk. However, correlations of telomere length with healthy lifestyles were noted, suggesting the role of these factors in telomere biology maintenance and potentially impacting overall health status.
Keywords: Telomere, prostate cancer, risk, lifestyle, epidemiology
Introduction
Telomeres are specialized structures located at eukaryotic chromosome ends composed of several thousand (TTAGGG)n nucleotide repeats and an ordered protein complex (deLange, 2005; Moon & Jarstfer, 2007). They protect chromosomes from degradation, end-to-end fusion, and atypical recombination (Moon & Jarstfer, 2007). In most normal cells, telomeric repeats shorten by 50 to 200 base pairs with each cell division, because of ineffective replication of the 3′ end by DNA polymerases (Collins & Mitchell, 2002). Progressive telomeric attrition eventually results in critically short telomeres, prompting cellular senescence or cellular crisis (Maser & DePinho, 2002; Gilley et al., 2005). In contrast, cancer cells, lacking a normal DNA damage response, continue to divide by up-regulating telomerase or through the alternative lengthening of telomeres (ALT) pathway. This can lead to continued cellular proliferation despite chromosomal instability. Most normal tissues express no or very low telomerase; however, telomerase activity has been found in approximately 90% of human cancers (Shay & Bacchetti, 1997; Shay & Roninson, 2004). Mouse models show an increased frequency of tumor formation in mice with shorter telomeres (Blasco et al., 1997; Rudolph et al., 1999; Artandi et al., 2000), suggesting that short telomeres increase the risk of cancer.
Several studies have shown an association between shorter telomeres in peripheral blood leukocytes and/or buccal cells and risk of bladder, head and neck, lung, and renal cell cancers (Wu et al., 2003; Broberg et al., 2005; McGrath et al., 2007; Shao et al., 2007; Jang et al., 2008). Leukocyte and buccal cell telomere shortening has also been associated with aging and age-related diseases, inflammatory processes, regeneration, and many other non-neoplastic diseases (including diabetes mellitus, coronary artery disease, and ulcerative colitis) (reviewed in Wong & Collins, 2003; Aubert & Lansdorp, 2008). Decreased telomere length has also been associated with lower vitamin D intake (Richards et al., 2007), increased oxidative stress (von Zglinicki, 2002; Tchirkov & Lansdorp, 2003), increased body mass index (Gardner et al., 2005; Valdes et al., 2005; O’Donnell et al., 2008; Zannolli et al., 2008; Aviv et al., 2009), smoking (Nawrot et al., 2004; Valdes et al., 2005; Morlà et al., 2006; McGrath et al., 2007; Aviv et al., 2009), low socio-economic status (Cherkas et al., 2006), and decreased physical activity (Cherkas et al., 2008), although the strength of the data varies between studies.
Prostate cancer is the most common non-cutaneous malignancy among men in developed countries. It is estimated that there will be approximately 186,000 new cases in the U.S. reported in 2008 (American Cancer Society, 2008). The molecular mechanisms underlying prostate cancer pathogenesis remain largely unknown. The data suggest that prostate cancer has a complex genetic and environmental basis (Schaid, 2004). Several large studies of prostate cancer suggest that genetic variation in the 8q24 chromosome locus and several other loci are associated with increased risk (Eeles et al., 2008; Thomas et al., 2008). However, the direct biological relevance of these associations has yet to be determined.
Telomeres, telomere shortening and telomerase activity have emerged as potentially important factors in prostate carcinogenesis. Telomere length in epithelial cells from high-grade prostatic intra-epithelial neoplasia (HGPIN) lesions were strikingly shorter than those of adjacent normal appearing epithelial cells (Meeker et al., 2002; Joshua et al., 2007). Increased telomere attrition in prostate cancer tumor tissue has also been associated with poor clinical outcome and increased rates of disease progression (Donaldson et al., 1999; Fordyce et al., 2005).
This study evaluated the role of pre-diagnostic leukocyte telomere length as a marker of prostate cancer risk among participants in the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial (Prorok et al., 2000). Associations between smoking, diet, obesity, physical activity, inflammation, and other selected lifestyle variables were explored, since many of these have previously been associated with telomere shortening.
Results
Subject characteristics
The characteristics of the 612 cases of advanced prostate cancer and 1049 matched controls are shown in Table 1. Age and smoking habits were similar in cases and controls. A family history of prostate cancer was more common in prostate cancer cases (12.0%) than controls (5.9%) (OR = 2.18, 95% CI 1.45, 3.27). As expected, statistically significant inverse correlations were found in controls between relative telomere length and age (r = −0.47, P < 0.0001). These age correlations were consistent when cases and controls were combined (Table 2) or when analyzed separately (Supplemental Table 1). Statistically significant inverse correlations were also found with pack-years smoked (in controls: r = −0.095, P = 0.003).
Table 1.
Distribution of select study subjects’ characteristics.
| Controls |
Patients |
|||
|---|---|---|---|---|
| Variables | No. (%) | Relative LTL† | No. (%) | Relative LTL† |
| Overall | 1049 (63.2) | 15.45 | 612 (36.8) | 15.49 |
| Age | ||||
| ≤64 | 539 (51.4) | 19.76 | 313 (51.1) | 19.33 |
| >64 | 510 (48.6) | 10.76 | 299 (48.9) | 10.98 |
| Smoking pack-year tertiles | ||||
| 0 | 300 (31.9) | 16.26 | 202 (37.3) | 15.58 |
| 0.1–30 | 355 (37.8) | 15.69 | 210 (38.8) | 15.18 |
| >30 | 285 (30.3) | 14.92 | 129 (23.8) | 15.33 |
| Family history of cancer | ||||
| none | 478 (45.6) | 15.22 | 275 (44.9) | 15.64 |
| any cancer | 508 (48.5) | 15.63 | 263 (43.0) | 15.19 |
| Father PC | 38 (3.6) | 18.27 | 46 (7.5) | 14.08 |
| Brother PC | 23 (2.2) | 16.12 | 26 (4.2) | 16.17 |
| Father and brother PC | 1 (0.1) | 14.99 | 2 (0.3) | 12.87 |
PC = prostate cancer;
leukocyte telomere length adjusted for age (all variables except age).
Table 2.
Correlations with relative telomere length in all subjects combined (n = 1661).
| Variables | r‡ (P value) |
|---|---|
| Age | −0.477 (<0.0001) |
| Pack-years of smoking | −0.072 (0.004) |
| BMI (kg/m2) | 0.016 (0.517) |
| Alcohol intake (g/day) | 0.006 (0.799) |
| Saturated fat (g/day) | −0.008 (0.770) |
| Lycopene (μg/day) | 0.010 (0.696) |
| Vitamin E (i.u./day) | 0.002 (0.427) |
| B-carotene (μg/day) | 0.007 (0.784) |
| Selenium (μg/day) | 0.047 (0.202) |
| Vitamin D (nmol/L) | −0.015 (0.773) |
| Vegetable (servings/day) | 0.028 (0.278) |
| Fruit (servings/day) | 0.019 (0.443) |
| Physical activity (hrs/wk) | 0.029 (0.262) |
| Education | 0.034 (0.171) |
| Marital status | −0.022 (0.384) |
| Aspirin usage (pill/month) | −0.001 (0.955) |
| Ibuprofen usage (pills/month) | −0.014 (0.572) |
| Aspirin and Ibuprofen usage | −0.018 (0.474) |
r = Spearman rank correlation coefficient;
adjusted for age and/or smoking pack-years (age was only adjusted for smoking pack-years, and pack-years of smoking only adjusted for age).
Prostate cancer risk and telomere length
Prostate cancer cases and controls did not differ with respect to mean relative telomere length (mean for cases = 15.49 [95% CI 14.8, 16.2] and mean for controls =15.45 [95% CI 14.9, 16.0] (Pwilcoxon = 0.452). When the subjects were categorized into quartiles of telomere length based on the telomere length distribution of the controls, the ORs for prostate cancer, compared to the 4th (highest) quartile, decreased from 1.28 (95% CI 1.00, 1.58), 0.97 (95% CI 0.81, 1.15), and 0.81 (95% CI 0.64, 1.02) with successively shorter telomere lengths (3rd to the 1st quartile; Ptrend 0.341). In logistic regression models conditioning on the matching factors and adjusting for smoking status (Table 3), shorter telomere length (less than the median) was associated with a statistically non-significant decreased risk of prostate cancer (OR = 0.85, 95% CI 0.67, 1.08). Comparison of telomere length as a continuous variable did not yield a statistically significant association (OR per unit length = 0.99, 95% CI 0.98, 1.01).
Table 3.
Relative risk of prostate cancer associated with relative telomere length.
| Telomere status | Controls n (%) | Patients n (%) | OR†(95% CI) | P value |
|---|---|---|---|---|
| Categorized by quartile | ||||
| 4th quartile | 253 (24.1) | 144 (23.5) | 1.00 (ref) | |
| 3rd quartile | 253 (24.1) | 177 (28.9) | 1.28 (1.00, 1.58) | |
| 2nd quartile | 251 (23.9) | 149 (24.4) | 0.97 (0.81, 1.15) | |
| 1st quartile | 292 (27.8) | 142 (23.2) | 0.81 (0.64, 1.02) | 0.341‡ |
| Categorized by median | ||||
| Long | 506 (48.2) | 321 (52.4) | 1.0 (ref) | |
| Short | 543 (51.8) | 291 (47.6) | 0.85 (0.67, 1.08) | 0.179 |
ref = referent group;
from logistic regression models conditioning on age, fiscal year of cohort entry, and time since initial screen, adjusting for pack-years of smoking;
Ptrend.
Associations of shorter relative telomere length (categorized by the median) with lower prostate cancer risk tended to be stronger in men who were older (>64 years), smoked more (>30 pack-years), and reported no family history of prostate cancer (Table 4), but results were also not statistically significant for any sub-group (Table 4). Only in men with a family history of prostate cancer did shorter telomeres tend to be associated with increased risk of prostate cancer, but these findings were also not statistically significant.
Table 4.
Risk estimates for relative telomere length dichotomized at the median within each subset.
| Telomere length strata | Control n (%) | Patient n (%) | OR (95% CI) | P ChiSq | |
|---|---|---|---|---|---|
| Age§ | |||||
| ≤64 | Short† | 154 (28.6) | 81 (25.9) | 0.83 (0.59, 1.18) | 0.298 |
| Long | 385 (71.4) | 232 (74.1) | 1.0 (ref) | ||
| >64 | Short | 389 (76.3) | 210 (70.2) | 0.74 (0.52, 1.05) | 0.089 |
| Long | 121 (23.7) | 89 (29.8) | 1.0 (ref) | ||
| Pack years in tertiles¶ | |||||
| 0 | Short | 151 (50.3) | 90 (44.6) | 1.01 (0.64, 1.57) | 0.976 |
| Long | 149 (49.7) | 112 (55.4) | 1.0 (ref) | ||
| 0.1–30 | Short | 177 (49.9) | 101 (48.1) | 0.95 (0.62, 1.45) | 0.807 |
| Long | 178 (50.1) | 109 (51.9) | 1.0 (ref) | ||
| >30 | Short | 154 (54.0) | 63 (48.8) | 0.66 (0.39, 1.12) | 0.122 |
| Long | 131 (45.9) | 66 (51.2) | 1.0 (ref) | ||
| No family history of prostate cancer‡ | |||||
| Short | 247 (51.7) | 122 (44.4) | 0.69 (0.45, 1.08) | 0.103 | |
| Long | 231 (48.3) | 153 (55.6) | 1.0 (ref) | ||
| Family history of prostate cancer‡ | |||||
| Short | 28 (45.2) | 38 (51.4) | 2.02 (0.76, 5.31) | 0.156 | |
| Long | 34 (54.8) | 36 (48.7) | 1.0 (ref) | ||
OR = odds ratio (95% confidence intervals); ref = referent group;
telomere length categorized by the median value;
conditioning on fiscal year of cohort entry and time since initial screen, adjusting for pack-years of smoking;
conditioning on age, fiscal year of cohort entry, and time since initial screen;
conditioning on age, fiscal year of cohort entry, and time since initial screen, adjusting for pack-years of smoking.
Lifestyle-related factors and telomere length in all subjects
Since statistically significant differences in telomere length between prostate cancer cases and controls were not observed, we evaluated the relationship of lifestyle factors to telomere length, combining data from cases and controls (Table 2 and Supplemental Tables 1 and 2). There was no significant correlation between relative telomere length and dietary variables, alcohol intake, BMI, physical activity, or medical history (including Crohn’s disease, ulcerative colitis, diabetes, coronary heart disease, high blood pressure, stroke, gonorrhea, syphilis, HPV, HSV, CMV, HHV, or Chlamydia; data not shown), when these factors were evaluated individually (Table 2). Telomere length was longer with heavier anti-inflammatory drug usage (aspirin and ibuprofen) in cases only (P=0.001) (Supplemental Table 2).
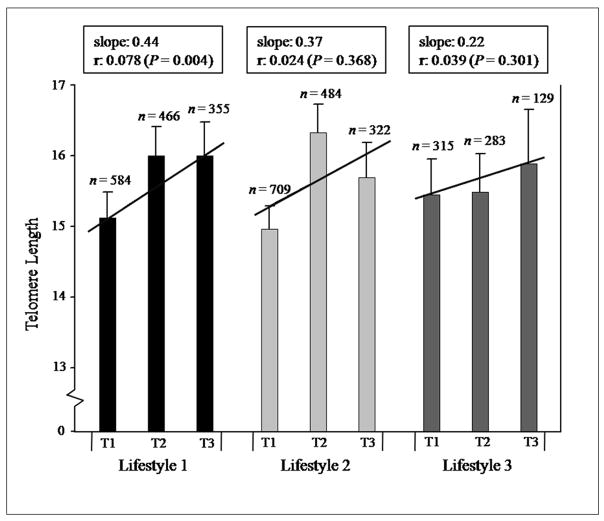
Although individual lifestyle factors were unrelated to relative telomere length, a sum score of healthier lifestyle and diet factors, defined as lifestyle 1 (low or no cigarette use, higher fruit and vegetable intake, lower BMI, and more physical activity) was correlated with longer telomere length (r = 0.078, P = 0.004, Figure 1). When smoking was removed from this lifestyle score, the correlation remained statistically significant (r = 0.056, P = 0.038). Similar but statistically non-significant trends were found with lifestyle 2, groupings of fruit, vegetable, and fat intake (r = 0.024, P = 0.368) as well as lifestyle 3, β-carotene, vitamin E, lycopene, and selenium intake (r = 0.039, P = 0.301).
Figure 1.
Mean relative telomere length for lifestyle scores in tertiles for all subjects combined (n = 1661). Total sample sizes vary for each lifestyle due to missing information for one or more of the selected variables (only persons with information for all of the combined variables were included in the analysis). T1 = tertile 1; T2 = tertile 2; T3 = tertile 3. Lifestyle 1 includes the following variables: pack-years smoked, fruit and vegetable intake, BMI, and physical activity; lifestyle 2: fruit, vegetable, and fat intake; and, lifestyle 3: β-carotene, vitamin E, lycopene, and selenium intake. See the Methods section for an explanation of the lifestyle score determination. Error bars show the standard error of each mean estimate. A regression line, slope, sample size (n), and Spearman rank correlation coefficients (r) are shown for each lifestyle after adjustment for age and/or pack-years smoked (lifestyle 1 was only adjusted for age).
Discussion
This large nested case-control study showed no association between relative telomere length and risk of aggressive (Gleason ≥ 7) prostate cancer; with 612 cases and 1049 controls, this study had adequate statistical power (>0.8) to detect an odds ratio of 1.31, with telomere length considered as a dichotomous variable. Shorter telomeres were correlated in our study with greater age, consistent with prior studies (Nawrot et al., 2004; Valdes et al., 2005; Mayer et al., 2006; McGrath et al., 2007; Richards et al., 2007; Aubert & Lansdorp, 2008; Cherkas et al., 2008; Jang et al., 2008), and greater pack-years smoked, as found in some (Valdes et al., 2005; Cherkas et al., 2006; Cherkas et al., 2008; O’Donnell et al., 2008; Vasan et al., 2008; Aviv et al., 2009) but not all studies, several of which may have been too small to detect that association (Wu et al., 2003; Bischoff et al., 2006; Harris et al., 2006; McGrath et al., 2007; Risques et al., 2007; Jang et al., 2008; Wang et al., 2008). The small significant correlation observed between telomere length and smoking is likely only detectable with larger sample sizes.
Strengths of this study include the large sample size, the collection of blood samples prior to diagnosis, and the systematic approach to prostate cancer detection in this screening trial for prostate and other cancers. The prospective collection avoids potential reverse causation bias whereby the presence of cancer might have impacted leukocyte telomere length. It should be noted that the use of different methods of telomere length measurement (Q-PCR, Southern blots, and Q-FISH), different tissue sources (blood or buccal cells), and differences in sample collection (before or after cancer diagnosis) make direct comparisons between our results in prostate cancer and studies of other cancers challenging. Studies of intra-individual peripheral blood leukocyte and buccal cell telomere length years before and after cancer diagnosis are required to evaluate potential systemic effects that the presence of cancer could have on telomere stability.
A recent study found a decreasing risk of breast cancer associated with shorter telomeres (Svenson et al., 2008), similar to our prostate cancer findings. Svenson et al. (2008) also suggested that shorter blood telomere lengths were associated with a better prognosis in breast cancer patients. Our study was part of a prospective cohort in a cancer screening trial and we were unable to evaluate telomere length in relation to prognosis. Future studies of telomere length as a prognostic factor in prostate and other cancers will be informative. Comparisons of leukocyte telomere lengths in low and high grade prostate cancer cases, as well as in pre- and post-diagnostic specimens would be helpful in order to more definitively show that leukocyte telomere length is not associated with prostate cancer. There do appear to be tissue-specific differences in telomere length between HGPIN lesions compared to normal surrounding tissue (Meeker et al., 2002; Joshua et al., 2007). This suggests that telomere shortening may occur early in the pathogenesis of prostate cancer. It could be reflective of the intra-prostatic micro-environment since our study did not identify significant differences in leukocyte telomere length between prostate cancer cases and controls.
Several case-control studies have found that peripheral blood leukocyte or buccal cell telomere length, determined after cancer diagnosis, is shorter in bladder, lung, head and neck, and renal cancer patients compared with controls (Wu et al., 2003; Broberg et al., 2005; McGrath et al., 2007; Shao et al., 2007; Jang et al., 2008). In general, these cancers have diverse underlying carcinogenic pathways. Smoking is a strong risk factor in bladder, lung, and head and neck cancer whereas it does not appear to be a significant prostate cancer risk factor, either in the present study or in many others (US Department of Health and Human Services, 2004; Cox et al., 2006; Kristal et al., 2007; Darlington et al., 2007). Inflammation may contribute to telomere shortening and play an important role in the pathogenesis of several cancers, including bladder and lung cancer (reviewed in Coussens & Werb, 2002; Kundu & Surh, 2008). It may also play a role in prostate cancer (Sutcliffe & Platz, 2008; Vasto et al., 2008) but our results do not support leukocyte telomere shortening as a causal pathway.
A family history of prostate cancer is associated with increased prostate cancer risk. Our data suggested a modest, but not statistically significant, association of shorter telomeres in individuals with a family history of prostate cancer. Others have found similar, not statistically significant, associations between short telomeres and family history of breast cancer in breast cancer cases (Shen et al., 2007). This finding may be partly explained by the fact that telomere length is an inherited trait. One could also hypothesize that some of the increased risk conferred by a family history of cancer is related to the presence of shorter telomeres in those individuals.
It has been suggested by others that leukocyte or buccal cell telomere lengths may be associated with chronic inflammation (Aikata et al., 2000; Bekaert et al., 2007), diabetes (Jeanclos et al., 1998; Sampson et al., 2006), hypertension and cardiovascular disease (Serrano & Andres, 2004; Balasubramanyam et al., 2007; Lung et al., 2008), vitamin D intake (Richards et al., 2007), socio-economic status (Cherkas et al., 2006), physical activity (Cherkas et al., 2008), and BMI (Gardner et al., 2005; Valdes et al., 2005; O’Donnell et al., 2008; Zannolli et al., 2008; Aviv et al., 2009). We evaluated these factors in an effort to understand their role in telomere shortening. Our results did not support these associations, showing no correlation between relative telomere length and these factors. However, we did find significant differences in telomere length with aspirin and ibuprofen drug usage in cases, which perhaps could reflect a protective role of anti-inflammatory drugs on telomere length in those with prostate cancer. On the other hand, our data for many of these factors was limited in scope and focused studies would be needed to definitively evaluate these relationships.
We found that lifestyle 1, the summary lifestyle score of pack-years smoked, fruit and vegetable intake, BMI, and physical activity, was related to relative telomere length, a finding that is consistent with recent reports that healthy lifestyle changes (e.g., improved diet and increased exercise) were associated with a significant increase in telomerase activity (Ornish et al., 2008) and longer telomeres (Bekaert et al., 2007). Obesity, smoking, and fruit and vegetable intake have been related to systemic oxidative stress (Alberg, 2002; Block et al., 2002; Keaney et al., 2003) which may contribute to telomere shortening. Telomeres are very sensitive to damage by oxidative stress and telomeric DNA is deficient in the repair of single-strand breaks induced by oxidative DNA damage (von Zglinicki, 2002; Houben et al., 2008). Based on this, it is possible that the rate of telomere shortening may vary between individuals with different degrees of exposure to oxidative stress. Individuals with high levels of oxidative stress caused by smoking which generates reactive oxygen species, and diets low in fruits and vegetables which are natural antioxidant sources may have shorter telomeres, as supported by our data and others (Bekaert et al., 2007).
In summary, this nested case-control study in a prospective cohort showed no association between relative telomere length in peripheral blood cells and risk of aggressive prostate cancer. However, healthier lifestyle parameters were related to longer telomere lengths which may have significance for risk of other diseases.
Experimental procedures
Study population
The PLCO Cancer Screening Trial is an ongoing randomized, community-based controlled trial in which 154,942 persons ages 55 to 74 were enrolled at 10 screening centers nationwide between September1993 and July 2001 (Prorok et al., 2000). Participants were randomly assigned to two study arms: half to undergo cancer screening (intervention group) and half to continue their normal health care routine (control group). Data on age, race, education level, height, weight, adult occupation, physical activity level, smoking history, dietary information (including food items, multivitamins and single-nutrient supplements), medical history (including use of selected medications), family history of cancer, and screening history were collected by questionnaire from all subjects at baseline. Participants provide biologic samples (blood and tissue) for etiologic studies of cancer and answer annual questionnaires about their cancer status; detailed information about the PLCO screening tests and eligibility criteria are described elsewhere (Gohagan et al., 2000). Institutional review boards at the U.S. National Cancer Institute and the 10 screening centers approved the PLCO protocol, and all participants provided written informed consent.
Case and control subjects for this study were men in the PLCO Trial who were selected for NCI Cancer Genetic Markers of Susceptibility (CGEMS) study of prostate cancer risk. The details of the CGEMS study are described elsewhere (Yeager et al., 2007; Ahn et al., 2008). In brief, men were selected from the screening arm of the trial who (a) were of non-Hispanic white race/ethnicity; (b) had a prostate cancer screen (PLCO PSA test) prior to October 1, 2003; (c) completed a baseline questionnaire about cancer risk factors; and, (d) provided a blood sample. CGEMS included 1,200 aggressive (clinical stage III and IV tumors and/or tumors with Gleason sum ≥ 7) and non-aggressive (clinical stage I and II tumors with Gleason sum < 7) prostate cancer cases, and controls were matched by age at cohort entry (5-year intervals), year since initial screen (1-year time window), and fiscal year of cohort entry, with a case-control ratio of 1:1. Further eligibility criteria for inclusion in our analysis were (1) age (55–74 years); (2) consented blood sample obtained between one month and three years prior to diagnosis of prostate cancer; (3) no history of cancer (other than non-melanoma skin cancer) prior to study entry; and, (4) only aggressive cases with confirmed prostate cancer and a Gleason score of ≥ 7. The current study consists of 616 CGEMS aggressive prostate cancer cases with a Gleason score of ≥ 7 (mean age = 64, standard deviation [SD] 5.09) and 1061 matched male controls (mean age = 64, SD 5.1).
Telomere length measurement
The blood and DNA samples were coded and analyzed by laboratory personnel blinded to case-control status. Genomic DNA was extracted from buffy coat samples using the QIAmp (Qiagen, Chatsworth, CA) 96-spin blood protocol. The DNA concentration was quantified using a Nanodrop SD-1000 spectrophotometer, and subsequently dried down and re-suspended to ensure accurate and uniform DNA concentrations. Telomere length was measured by a modified version of the quantitative real time polymerase chain reaction (PCR)-based assay previously described (Cawthon, 2002; McGrath et al., 2007). In brief, the ratio of telomere repeat copy number to single-gene (β-globin, 36B4) copy number (T/S) was determined using an Applied Biosystems 7900HT thermocycler in a 384-well format. Five nanograms of genomic DNA was dried down and then re-suspended in 10 μL of either the telomere or 36B4 PCR reaction mixture for 2 hours at 4°C. The telomere reaction mixture contained 1Χ Qiagen Quantitect Sybr Green Master Mix, 2.5 mM of DTT, 270 nM of Tel-1b primer (GGTTTTTGAGGGTGAGGGTGAGGGTGAGGGTGAGGGT), and 900 nM of Tel-2b primer (TCCCGACTATCCCTATCCCTATCCCTATCCCTATCCCTA). The PCR reaction ran for 1 cycle at 95°C for 5 minutes, followed by 40 cycles at 95°C for 15 seconds, and 54°C for 2 minutes. The 36B4 reaction mixture consisted of 1Χ Qiagen Quantitect Sybr Green Master Mix, 300 nM of 36B4U primer (CAGCAAGTGGGAAGGTGTAATCC), and 500 nM of 36B4D primer (CCCATTCTATCATCAACGGGTACAA). The 36B4 PCR reaction ran for 1 cycle at 95°C for 5 minutes, followed by 40 cycles at 95°C for 15 seconds, and 58°C for 1 minute 10 seconds.
All samples for both the telomere and single-copy gene reactions were performed in triplicate, and the threshold value for both reactions was set to 0.5. In addition, each 384-well plate included an 8-point standard curve from 1.25 to 50 ng using pooled buffy-coat derived genomic DNA. The standard curve is used to assess and compensate for interpolated variations in PCR efficiency. The slope of the standard curve was −3.2 for both the telomere and 36B4 reactions, and the linear correlation coefficient value for both reactions were 0.98 and 0.99, respectively. Forty-six blind replicate samples were interspersed with the samples to assess inter-plate and intra-plate variability of threshold cycle (Ct) values. The relative average telomere length was calculated as the ratio of telomere repeat copy number to single-gene copy number (T/S) in the study subjects compared with that of a reference DNA sample. It was derived from exponentiating the T/S ratio (−dCt) between the average telomere Ct and average 36B4 Ct values. The coefficients of variation (CV) within triplicates of the telomere and single-gene assay were 1.11% and 0.77%, respectively, and the inter-assay CVs were 5.6% and 2.6%, respectively.
Statistical analyses
The final sample size was 612 cases and 1049 controls, after removal of failed Q-PCR reactions or samples with T/S values greater than two standard deviations from the mean. Telomere length was analyzed as a continuous and as a categorical variable. The Wilcoxon rank-sum test was used to compare telomere length among case and controls as a continuous variable. Conditional logistic regression was used to obtain the odds ratio (OR) and 95% confidence intervals (CI) for the strength of the association between telomere length and risk of prostate cancer, conditioning on the matching factors (age, fiscal year of cohort entry, and time since initial screen) and adjusting for smoking status. Telomere length was considered as a categorical variable, in quartiles according to its distribution in control subjects, with the highest quartile as the referent. Explorations were also carried out for telomere length dichotomized at the median value, based on the distribution in control subjects. Tests for trend were conducted by including telomere length as a continuous variable in the conditional logistic regression model.
Spearman rank correlations and general linear models (GLM) were used to investigate associations between telomere length and age, pack-years of smoking, anti-inflammatory drug usage (aspirin and ibuprofen), alcohol intake (g/day), body mass index (BMI in kg/m2), medical history [if have/had Crohn’s disease, ulcerative colitis, diabetes, coronary heart disease, high blood pressure, stroke, sexually transmitted diseases (gonorrhea, syphilis, HPV, HSV, CMV, HHV, or Chlamydia)], physical activity (hrs/week), education (years of high school and/or college), marital status (married, unmarried, or widowed/divorced/separated), dietary fat (total, monounsaturated, polyunsaturated, and saturated fatty acids in g/day), dietary fruit (pyramid servings/day), dietary vegetables (pyramid servings/day), dietary and supplemented β-carotene (μg/day), dietary and supplemented vitamin E (i.u./day), dietary lycopene (μg/day), circulating vitamin D (serum 25-hydroxyvitamin D [25(OH)D] levels, nmol/L), and dietary selenium (μg/day). GLMs contained telomere length as the dependent variable, and were adjusted for age and pack-years smoked. A family history of prostate cancer is defined as a first-degree relative (father, brother, son) with prostate cancer. All variables that were not categorical were categorized; specifically, dietary variables and BMI values were grouped into quartiles, smoking pack-years into tertiles (0, 0.1–30, >30), and age into quartiles (≤59, 60–64, 65–69, 70–74) and also dichotomized at the 50% level (≤64, >64). Statistical significance refers to a P ≤ 0.05 or a 95% CI for the OR that excludes 1.0.
Lifestyle and diet sum scores were created to evaluate these effects on telomere length. Individual variables (pack-years smoked, BMI, physical activity, fruit, vegetable, fat, β-carotene, vitamin E, lycopene, and selenium intake) were first categorized into four groups or quartiles. All variables were categorized such that the highest score pertains to the healthiest group, e.g., nonsmokers, the 1st quartile (lowest) BMI, the 4th quartile (highest) physical activity. The sum of these scores was then considered the lifestyle and diet score, with a higher score indicating a healthier lifestyle or diet, using an approach modified from Bekaert et al. (2007). These scores were categorized into quartiles and tertiles, and Spearman rank correlations were used to investigate their associations with telomere length. Lifestyle 1 included the following variables: pack-years smoked, fruit and vegetable intake, BMI, and physical activity; lifestyle 2 included fruit, vegetable, and fat intake; and, lifestyle 3: β-carotene, vitamin E, lycopene, and selenium intake. All tests were two-sided. Statistical significance refers to a P ≤ 0.05 or a 95% CI for the OR that excludes 1.0. All analyses were carried out using SAS software version 9.1 (SAS Institute, Cary, NC).
Supplementary Material
Acknowledgments
The authors thank Drs. Christine Berg and Philip Prorok, Division of Cancer Prevention, National Cancer Institute, the Screening Center investigators and staff of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, Mr. Tom Riley and staff, Information Management Services, Inc., Ms. Barbara O’Brien and staff, Westat, Inc., and the staff of the telomere testing laboratory. We thank Dr. Mark Greene, National Cancer Institute, for helpful discussions. Most importantly, we acknowledge the study participants for their contributions to making this study possible. This work was supported [in part] by the Intramural Research Program of the Division of Cancer Epidemiology and Genetics and contracts from the Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Department of Health and Human Services; National Institutes of Health (grant CA82838); and American Cancer Society (grant RSG-00-061-04-CCE).
Footnotes
Author contributions
L. Mirabello did the statistical analysis and wrote the manuscript. W.Y. Huang participated in the sample selection and statistical analysis. J.Y.Y. Wong and I. De Vivo performed the telomere length measurements. N. Chatterjee contributed to interpretation of the data. D. Reding and E.D. Crawford participated in patient recruitment and management of the PLCO study. S.A. Savage and R.B. Hayes designed the study, analyzed data, and wrote the manuscript. All authors approved the final version.
References
- Ahn J, Peters U, Albanes D, Purdue MP, Abnet CC, Chatterjee N, Horst RL, Hollis BW, Huang WY, Shikany JM, Hayes RB Prostate Lung Colorectal and Ovarian Cancer Screening Trial Project Team . Serum vitamin D concentration and prostate cancer risk: a nested case-control study. J Natl Cancer Inst. 2008;100:796–804. doi: 10.1093/jnci/djn152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikata H, Takaishi H, Kawakami Y, Takahashi S, Kitamoto M, Nakanishi T, Nakamura Y, Shimamoto F, Kajiyama G, Ide T. Telomere reduction in human liver tissues with age and chronic inflammation. Exp Cell Res. 2000;256:578–582. doi: 10.1006/excr.2000.4862. [DOI] [PubMed] [Google Scholar]
- Alberg A. The influence of cigarette smoking on circulating concentrations of antioxidant micronutrients. Toxicology. 2002;180:121–137. doi: 10.1016/s0300-483x(02)00386-4. [DOI] [PubMed] [Google Scholar]
- American Cancer Society Inc. Cancer facts and figures 2008. Atlanta: 2008. [Google Scholar]
- Artandi SE, Chang S, Lee SL, Alson S, Gottlieb GJ, Chin L, DePinho RA. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature. 2000;406:641–645. doi: 10.1038/35020592. [DOI] [PubMed] [Google Scholar]
- Aubert G, Lansdorp PM. Telomeres and aging. Physiol Rev. 2008;88:557–579. doi: 10.1152/physrev.00026.2007. [DOI] [PubMed] [Google Scholar]
- Aviv A, Chen W, Gardner JP, Kimura M, Brimacombe M, Cao X, Srinivasan SR, Berenson GS. Leukocyte telomere dynamics: longitudinal findings among young adults in the Bogalusa Heart Study. Am J Epidemiol. 2009;169:323–329. doi: 10.1093/aje/kwn338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanyam M, Adaikalakoteswari A, Monickaraj SF, Mohan V. Telomere shortening & metabolic/vascular diseases. Indian J Med Res. 2007;125:441–450. [PubMed] [Google Scholar]
- Bekaert S, De Meyer T, Rietzschel ER, De Buyzere ML, De Bacquer D, Langlois M, Segers P, Cooman L, Van Damme P, Cassiman P, Van Criekinge W, Verdonck P, De Backer GG, Gillebert TC, Van Oostveldt P, Asklepios investigators Telomere length and cardiovascular risk factors in a middle-aged population free of overt cardiovascular disease. Aging Cell. 2007;6:639–647. doi: 10.1111/j.1474-9726.2007.00321.x. [DOI] [PubMed] [Google Scholar]
- Bischoff C, Petersen HC, Graakjaer J, Andersen-Ranberg K, Vaupel JW, Bohr VA, Kolvraa S, Christensen K. No association between telomere length and survival among the elderly and oldest old. Epidemiology. 2006;17:190–194. doi: 10.1097/01.ede.0000199436.55248.10. [DOI] [PubMed] [Google Scholar]
- Blasco MA, Lee HW, Hande MP, Samper E, Lansdorp PM, DePinho RA, Greider CW. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- Block G, Dietrich M, Norkus EP, Morrow JD, Hudes M, Caan B, Packer L. Factors Associated with Oxidative Stress in Human Populations. Am J Epidemiol. 2002;156:274–285. doi: 10.1093/aje/kwf029. [DOI] [PubMed] [Google Scholar]
- Broberg K, Bjork J, Paulsson K, Hoglund M, Albin M. Constitutional short telomeres are strong genetic susceptibility markers for bladder cancer. Carcinogenesis. 2005;26:1263–1271. doi: 10.1093/carcin/bgi063. [DOI] [PubMed] [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkas LF, Aviv A, Valdes AM, Hunkin JL, Gardner JP, Surdulescu GL, Kimura M, Spector TD. The effects of social status on biological aging as measured by white-blood-cell telomere length. Aging Cell. 2006;5:361–365. doi: 10.1111/j.1474-9726.2006.00222.x. [DOI] [PubMed] [Google Scholar]
- Cherkas LF, Hunkin JL, Kato BS, Richards JB, Gardner JP, Surdulescu GL, Kimura M, Lu X, Spector TD, Aviv A. The association between physical activity in leisure time and leukocyte telomere length. Arch Intern Med. 2008;168:154–158. doi: 10.1001/archinternmed.2007.39. [DOI] [PubMed] [Google Scholar]
- Collins K, Mitchell JR. Telomerase in the human organism. Oncogene. 2002;21:564–579. doi: 10.1038/sj.onc.1205083. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B, Sneyd MJ, Paul C, Skegg DC. Risk factors for prostate cancer: A national case-control study. Int J Cancer. 2006;119:1690–1694. doi: 10.1002/ijc.22022. [DOI] [PubMed] [Google Scholar]
- Darlington GA, Kreiger N, Lightfoot N, Purdham J, Sass-Kortsak A. Prostate cancer risk and diet, recreational physical activity and cigarette smoking. Chronic Dis Can. 2007;27:145–153. [PubMed] [Google Scholar]
- deLange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- Donaldson L, Fordyce CA, Gilliland F, Smith A, Feddersen R, Joste N, Moyzis R, Griffith J. Association between outcome and telomere DNA content in prostate cancer. J Urol. 1999;162:1788–1792. [PubMed] [Google Scholar]
- Eeles RA, Kote-Jarai Z, Giles GG, Olama AA, Guy M, et al. Multiple newly identified loci associated with prostate cancer susceptibility. Nat Genet. 2008;40:316–321. doi: 10.1038/ng.90. [DOI] [PubMed] [Google Scholar]
- Fordyce CA, Heaphy CM, Joste NE, Smith AY, Hunt WC, Griffith JK. Association between cancer-free survival and telomere DNA content in prostate tumors. J Urol. 2005;173:610–614. doi: 10.1097/01.ju.0000143195.49685.ce. [DOI] [PubMed] [Google Scholar]
- Gardner JP, Li S, Srinivasan SR, Chen W, Kimura M, Lu X, Berenson GS, Aviv A. Rise in insulin resistance is associated with escalated telomere attrition. Circulation. 2005;111:2171–2177. doi: 10.1161/01.CIR.0000163550.70487.0B. [DOI] [PubMed] [Google Scholar]
- Gilley D, Tanaka H, Herbert BS. Telomere dysfunction in aging and cancer. Int J Biochem Cell Biol. 2005:37. doi: 10.1016/j.biocel.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Gohagan JK, Prorok PC, Hayes RB, Kramer BS. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials. 2000;21:251–272S. doi: 10.1016/s0197-2456(00)00097-0. [DOI] [PubMed] [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Whalley LJ, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett. 2006;406:260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Houben JM, Moonen HJ, van Schooten FJ, Hageman GJ. Telomere length assessment: biomarker of chronic oxidative stress? . Free Radic Biol Med. 2008;44:235–246. doi: 10.1016/j.freeradbiomed.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Jang JS, Choi YY, Lee WK, Choi JE, Cha SI, Kim YJ, Kim CH, Kam S, Jung TH, Park JY. Telomere length and the risk of lung cancer. Cancer Sci. 2008 doi: 10.1111/j.1349-7006.2008.00831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanclos E, Krolewski A, Skurnick J, Kimura M, Aviv H, Warram JH, Aviv A. Shortened telomere length in white blood cells of patients with IDDM. Diabetes. 1998;47:482–486. doi: 10.2337/diabetes.47.3.482. [DOI] [PubMed] [Google Scholar]
- Joshua AM, Vukovic B, Braudey I, Husseinb S, Zielenska M, Srigleyb J, Evans A, Squire JA. Telomere attrition in isolated high-grade prostaticintraepithelial neoplasia and surrounding stroma is predictive of prostate cancer. Neoplasia. 2007;9:81–89. doi: 10.1593/neo.06745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keaney JF, Jr, Larson MG, Vasan RS, Wilson PWF, Lipinska I, Corey D, Massaro JM, Sutherland P, Vita JA, Benjamin EJ. Obesity and systemic oxidative stress: clinical correlates of oxidative stress in the Framingham Study. Arterioscler Thromb Vasc Biol. 2003;23:434–439. doi: 10.1161/01.ATV.0000058402.34138.11. [DOI] [PubMed] [Google Scholar]
- Kristal AR, Arnold KB, Schenk JM, Neuhouser ML, Weiss N, Goodman P, Antvelink CM, Penson DF, Thompson IM. Race/ethnicity, obesity, health related behaviors and the risk of symptomatic benign prostatic hyperplasia: results from the prostate cancer prevention trial. J Urol. 2007;177:1395–1400. doi: 10.1016/j.juro.2006.11.065. [DOI] [PubMed] [Google Scholar]
- Kundu JK, Surh YJ. Inflammation: Gearing the journey to cancer. Mutat Res. 2008;659:15–30. doi: 10.1016/j.mrrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Lung FW, Ku CS, Kao WT. Telomere length may be associated with hypertension. J Hum Hypertens. 2008;22:230–232. doi: 10.1038/sj.jhh.1002314. [DOI] [PubMed] [Google Scholar]
- Maser RS, DePinho RA. Connecting Chromosomes, Crisis, and Cancer. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- Mayer S, Bruderlein S, Perner S, Waibel I, Holdenried A, Ciloglu N, et al. Sex-specific telomere length profiles and age-dependent erosion dynamics of individual chromosome arms in humans. Cytogenet Genome Res. 2006;112:194–201. doi: 10.1159/000089870. [DOI] [PubMed] [Google Scholar]
- McGrath M, Wong JYY, Michaud D, Hunter DJ, De Vivo I. Telomere length, cigarette smoking, and bladder cancer risk in men and women. Cancer Epidemiol Biomarkers Prev. 2007;16:815–819. doi: 10.1158/1055-9965.EPI-06-0961. [DOI] [PubMed] [Google Scholar]
- Meeker AK, Hicks JL, Platz EA, et al. Telomere shortening is an early somatic DNA alteration in human prostate tumorigenesis. Cancer Res. 2002;62:6405–6409. [PubMed] [Google Scholar]
- Moon IK, Jarstfer MB. The human telomere and its relationship to human disease, therapy, and tissue engineering. Frontiers in Bioscience. 2007;12:4595–4620. doi: 10.2741/2412. [DOI] [PubMed] [Google Scholar]
- Morlà M, Busquets X, Pons J, Sauleda J, MacNee W, Agusti AG. Telomere shortening in smokers with and without COPD. Eur Respir J. 2006;27:525–528. doi: 10.1183/09031936.06.00087005. [DOI] [PubMed] [Google Scholar]
- Nawrot TS, Staessen JA, Gardner JP, Aviv A. Telomere length and possible link to X chromosome. Lancet. 2004;363:507–510. doi: 10.1016/S0140-6736(04)15535-9. [DOI] [PubMed] [Google Scholar]
- O’Donnell CJ, Demissie S, Kimura M, Levy D, Gardner JP, White C, D’Agostino RB, Wolf PA, Polak J, Cupples LA, Aviv A. Leukocyte Telomere Length and Carotid Artery Intimal Medial Thickness. The Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.107.154849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, Kemp C, Magbanua MJ, Marlin R, Yglecias L, Carroll PR, Blackburn EH. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 2008;9:1048–1057. doi: 10.1016/S1470-2045(08)70234-1. [DOI] [PubMed] [Google Scholar]
- Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, Fogel R, Gelmann EP, Gilbert F, Hasson MA, Hayes RB, Johnson CC, Mandel JS, Oberman A, O’Brien B, Oken MM, Rafla S, Reding D, Rutt W, Weissfeld JL, Yokochi L, Gohagan JK Prostate Lung Colorectal and Ovarian Cancer Screening Trial Project Team. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000;21:273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- Richards JB, Valdes AM, Gardner JP, Paximadas D, Kimura M, Nessa A, Lu X, Surdulescu GL, Swaminathan R, Spector TD, Aviv A. Higher serum vitamin D concentrations are associated with longer leukocyte telomere length in woman. Am J Clin Nutr. 2007;86:1420–1425. doi: 10.1093/ajcn/86.5.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risques RA, Vaughan TL, Li X, Odze RD, Blount PL, Ayub K, Gallaher JL, Reid BJ, Rabinovitch PS. Leukocyte telomere length predicts cancer risk in Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2007;16:2649–2655. doi: 10.1158/1055-9965.EPI-07-0624. [DOI] [PubMed] [Google Scholar]
- Rudolph KL, Chang S, Lee HW, Blasco M, Gottlieb GJ, Greider C, DePinho RA. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA. Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care. 2006;29:283–289. doi: 10.2337/diacare.29.02.06.dc05-1715. [DOI] [PubMed] [Google Scholar]
- Schaid DJ. The complex genetic epidemiology of prostate cancer. Hum Mol Genet. 2004;13:R103–121. doi: 10.1093/hmg/ddh072. [DOI] [PubMed] [Google Scholar]
- Serrano AL, Andres V. Telomeres and cardiovascular disease: does size matter? . Circ Res. 2004;94:575–584. doi: 10.1161/01.RES.0000122141.18795.9C. [DOI] [PubMed] [Google Scholar]
- Shao L, Wood CG, Zhang D, Tannir NM, Matin S, Dinney CP, Wu X. Telomere dysfunction in peripheral lymphocytes as a potential predisposition factor for renal cancer. J Urol. 2007;178:1492–1496. doi: 10.1016/j.juro.2007.05.112. [DOI] [PubMed] [Google Scholar]
- Shay JW, Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Shay JW, Roninson IB. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004;23:2919–2933. doi: 10.1038/sj.onc.1207518. [DOI] [PubMed] [Google Scholar]
- Shen J, Terry MB, Gurvich I, Liao Y, Senie RT, Santella RM. Short telomere length and breast cancer risk: a study in sister sets. Cancer Res. 2007;67:5538–5544. doi: 10.1158/0008-5472.CAN-06-3490. [DOI] [PubMed] [Google Scholar]
- Sutcliffe S, Platz EA. Inflammation and prostate cancer: a focus on infections. Curr Urol Rep. 2008;9:243–249. doi: 10.1007/s11934-008-0042-z. [DOI] [PubMed] [Google Scholar]
- Svenson U, Nordfjall K, Stegmayr B, Manjer J, Nilsson P, Tavelin B, Henriksson R, Lenner P, Roos G. Breast cancer survival is associated with telomere length in peripheral blood cells. Cancer Res. 2008;68:3618–3623. doi: 10.1158/0008-5472.CAN-07-6497. [DOI] [PubMed] [Google Scholar]
- Tchirkov A, Lansdorp PM. Role of oxidative stress in telomere shortening in cultured fibroblasts from normal individuals and patients with ataxia-telangiectasia. Hum Mol Genet. 2003;12:227–232. doi: 10.1093/hmg/ddg023. [DOI] [PubMed] [Google Scholar]
- Thomas G, Jacobs KB, Yeager M, Kraft P, Wacholder S, Orr N, Yu K, Chatterjee N, Welch R, Hutchinson A, Crenshaw A, Cancel-Tassin G, Staats BJ, Wang Z, Gonzalez-Bosquet J, Fang J, Deng X, Berndt SI, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cussenot O, Valeri A, Andriole GL, Crawford ED, Tucker M, Gerhard DS, Fraumeni JF, Hoover R, Hayes RB, Hunter DJ, Chanock SJ. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40:310–315. doi: 10.1038/ng.91. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon Generaled. Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2004. [Google Scholar]
- Valdes AM, Andrew T, Gardner JP, Kimura M, Oelsner E, Cherkas LF, Aviv A, Spector TD. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366:662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Demissie S, Kimura M, Cupples LA, Rifai N, White C, Wang TJ, Gardner JP, Cao X, Benjamin EJ, Levy D, Aviv A. Association of leukocyte telomere length with circulating biomarkers of the renin-angiotensin-aldosterone system. The Framingham Heart Study. Circulation. 2008;117:1138–1144. doi: 10.1161/CIRCULATIONAHA.107.731794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasto S, Carruba G, Candore G, Italiano E, Di Bona D, Caruso C. Inflammation and prostate cancer. Future Oncol. 2008;4:637–645. doi: 10.2217/14796694.4.5.637. [DOI] [PubMed] [Google Scholar]
- von Zglinicki T. Oxidative stress shortens telomeres. Trends Biochem Sci. 2002;27:339–344. doi: 10.1016/s0968-0004(02)02110-2. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen H, Gao X, McGrath M, Deer D, Immaculata DV, Schwarzschild MA, Ascherio A. Telomere length and risk of Parkinson’s disease. Movement Disorders. 2008;23:302–305. doi: 10.1002/mds.21867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JM, Collins K. Telomere maintenance and disease. Lancet. 2003;362:983–988. doi: 10.1016/S0140-6736(03)14369-3. [DOI] [PubMed] [Google Scholar]
- Wu X, Amos CI, Zhu Y, Zhao H, Grossman BH, Shay JW, Luo S, Hong WK, Spitz MR. Telomere dysfunction: a potential cancer predisposition factor. J Natl Cancer Inst. 2003;95:1211–1218. doi: 10.1093/jnci/djg011. [DOI] [PubMed] [Google Scholar]
- Yeager M, Orr N, Hayes RB, Jacobs KB, Kraft P, Wacholder S, Minichiello MJ, Fearnhead P, Yu K, Chatterjee N, Wang Z, Welch R, Staats BJ, Calle EE, Feigelson HS, Thun MJ, Rodriguez C, Albanes D, Virtamo J, Weinstein S, Schumacher FR, Giovannucci E, Willett WC, Cancel-Tassin G, Cussenot O, Valeri A, Andriole GL, Gelmann EP, Tucker M, Gerhard DS, Fraumeni JF, Hoover R, Hunter DJ, Chanock SJ, Thomas G. Genome-wide association study of prostate cancer identifies a second risk locus at 8q24. Nat Genet. 2007;39:645–649. doi: 10.1038/ng2022. [DOI] [PubMed] [Google Scholar]
- Zannolli R, Mohn A, Buoni S, Pietrobelli A, Messina M, Chiarelli F, Miracco C. Telomere length and obesity. Acta Paediatrica. 2008;97:952–954. doi: 10.1111/j.1651-2227.2008.00783.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.