Abstract
Background
Heel stick is the most common painful procedure for preterm infants in neonatal intensive care units. Resultant pain causes adverse physiological effects in major organ systems. Kangaroo Care (KC), involving mother-infant skin-to-skin contact is a promising analgesic for infant pain; however, the effect of KC on the autonomic nervous system's response to pain is unknown.
Aim
To determine if KC results in improved balance in autonomic responses to heel stick pain than the standard method where infants remain in an incubator care (IC) for the heel stick.
Study Design
A randomized cross-over trial.
Subjects
Fourteen preterm infants, 30-32 weeks gestational age and less than 9 days postnatal age.
Outcome Measures
Infant behavioral state, heart rate, heart rate variability (HRV) indices including low frequency (LF) and high frequency (HF) power, and the LF/HF ratio measured over Baseline, Heel Warming, Heel Stick, and Recovery periods in KC and IC conditions.
Results
HRV differences between KC and IC were that LF was higher in KC at Baseline (p<.01) and at Heel Stick (p< .001), and HF was higher in KC at Baseline than in the IC condition (p< .05). The LF/HF ratio had less fluctuation across the periods in KC than in IC condition and was significantly lower during Recovery in KC than in IC (p< .001).
Conclusions
Infants experienced better balance in response in KC than IC condition as shown by more autonomic stability during heel stick. KC may be helpful in mediating physiologic response to painful procedures in preterm infants.
Keywords: Pain, Heel Stick, Kangaroo Care, Heart Rate Variability, Preterm Infants
1. Introduction
Preterm infants are subjected to numerous invasive procedures as part of their care during stays in neonatal intensive care units (NICUs). In several studies, preterm neonates had a mean of 10 to 16 painful procedures per day during their first several days of life; heel sticks were the most common source of pain and most frequently untreated for pain relief [1-7]. Preterm infants can detect, process, and respond to painful stimuli since autonomic ascending pathways for pain transmission develop as early as the 20th week of gestation [8, 9]. Simultaneously, infants may actually have a 30-50% lower pain threshold than that of adults and a lower pain tolerance than older children. Thus the preterm infant is at greater risk for pain than a full-term infant due to immaturity of the descending pathway to inhibit or dampen nociception at birth, leading to hypersensitivity to pain [10, 11]. Excessive and prolonged unrelieved pain in the infant causes adverse physiological effects in all major organ systems, can be life threatening and can have long-term cumulative outcomes [12, 13]. Effective, non-pharmacological interventions are valuable alternatives for pain relief during invasive procedures in neonates [14, 15].
Kangaroo Care (KC), also called mother-infant skin-to-skin contact, has been shown so consistently to be an analgesic for procedural pain [16-20] that the American Academy of Pediatrics [21] and others [22-27] recommend KC as an effective non-pharmacologic pain intervention. KC's proposed action as a pain treatment is supported by the Neuromatrix Theory of Pain [28], in which pain is postulated as a multidimensional output produced by a widely distributed neural network in the brain and determined by many factors, such as context, company, competitive stimuli, and meaning [29]. During KC, the mother's skin-to-skin contact with her preterm infant provides multi-sensory stimulation including emotional, tactile, proprioceptive, vestibular, olfactory, auditory, visual, and thermal stimulation in a unique interactive style. When the infant undergoes a heel stick, KC and its multi-sensory inputs may act on the pain matrix programs to modulate and inhibit pain perception, and to contribute to the outflowing neurosignature in such a way that pain responses are minimized. KC's action occurs through multi-sensory input to the brain, activation of the neuro-chemical system, and modulation of the stress-regulation system involved in pain experience [19].
KC has been shown to reduce both physiologic and behavioral responses to pain in preterm infants. Physiologic changes documented have included a decreased variation in heart rate (HR) [16, 19, 30], a diminished increase in HR [17, 18, 31], an increased level of oxygenation [30] and increased stability in oxygen saturation [18, 32], stability in respiratory rate [32], decreased central venous pressures [30], and a shortened recovery time as indicated by return to baseline physiological values [30, 33]. Behavioral changes due to KC have been a decrease in crying time [18, 19, 34], as well as a decrease in and a shortened duration of facial expressions of pain [16-18, 31].
Infants often show differences in behavioral and physiologic responses to pain [35-37]. Behavioral responses may diminish, but physiological responses may remain elevated or increase. Without a reduction of physiological responses, infants' organs remain exposed to adverse effects of pain [35]. Because the autonomic nervous system responds to the environment by increasing (in the stressful environment) or decreasing (in a calming environment) cardio-respiratory parameters, the most relevant measure of KC's pain reduction ability is assessment of KC's effects on the autonomic nervous system. Heart rate variability (HRV) has been hypothesized to be a sensitive indicator of autonomic function in relation to pain and has been used as a non-invasive measure of parasympathetic and sympathetic reactivity to pain in preterm infants [38-41]. The frequency domain analysis of HRV delineates parasympathetic from sympathetic components of autonomic control, i.e. by using power spectral analysis [42]. The spectral power of the high-frequency (HF) band (0.15 -1.0 Hz) is related to respiratory sinus arrhythmia and reflects parasympathetic activity. The spectral power of the low-frequency (LF) band (0.04 -0.15 Hz) is an index of primarily sympathetic activity with some parasympathetic input [42-45]. HRV is a recommended indicator to be examined in response to a painful event shortly after birth [46]. However, the effect of KC on the HRV response to pain is not known.
Thus, the purpose of this randomized cross-over experimental study was to determine if a heel stick performed in a KC intervention condition showed different autonomic responses to pain than a heel stick in the standard incubator care condition. Pain responses were measured by use of spectral analysis of heart rate variability.
2. Methods
2.1. Design
A prospective cross-over with random assignment by permuted block design was used. A statistician helped the investigator generate a list of randomization codes using the SAS® procedure PLAN. The list of random codes consisted of the subject's number and the treatment assignment. According to the random codes, infants were assigned to two groups, determined by the sequence of the KC and current standard of care – routine incubator care (IC) conditions. Group A received routine IC on the first day of the study and KC on the second day. Group B received KC on the first day and routine IC on the second day. Infants served as their own controls. A 24-hour routine IC washout period was incorporated into the design for both groups. Twenty-four-hours was sufficient to allow any lingering effects of KC to dissipate. Previous research has shown that KC effects on cardiorespiratory and behavioral state outcomes disappear within three hours [47-49], and KC's blunting effects on plasma and salivary cortisol were not sustained a day later [50], suggesting that all carry-over effects of the first day's treatment would be absent 24 hours later.
2.2. Subjects
Institutional Review Board approval was obtained and mothers gave written informed consent. Inclusion criteria were infants 30 through 32 weeks gestational age (GA), two through nine postnatal days old, cared-for in an incubator, and whose mothers were English speaking. Exclusion criteria were infants with known congenital anomalies, periventricular/intraventricular hemorrhage (≥ Grade III), history of surgery, having received sedation, vasopressor, or analgesics, exposure to drug abuse during pregnancy, multiples at birth, and showing signs of severe tissue breakdown of either heel as measured by the Neonatal Skin Condition Score [51].
No study was found that measured HRV as an outcome of KC in relation to preterm infant pain responses. Based on Lindh's finding [41] of an increase in low frequency power with M = .30, SD = .35 log mHz2 in the response of preterm infants to heel stick compared to baseline, as well as medium effect size of KC on pain scores in Johnston's [18] study, power analysis showed that 14 subjects were needed to detect the effect of KC (effect size = 0.40) on HRV, with α = .05 and power = .80. Eighteen infants and their mothers were approached in a Level II NICU in a non–profit community hospital located in Washington state and 14 healthy preterm infants (8 male and 6 female, Group A = 7, Group B = 7) and their mothers were included in the final sample (Figure 1).
Figure 1.
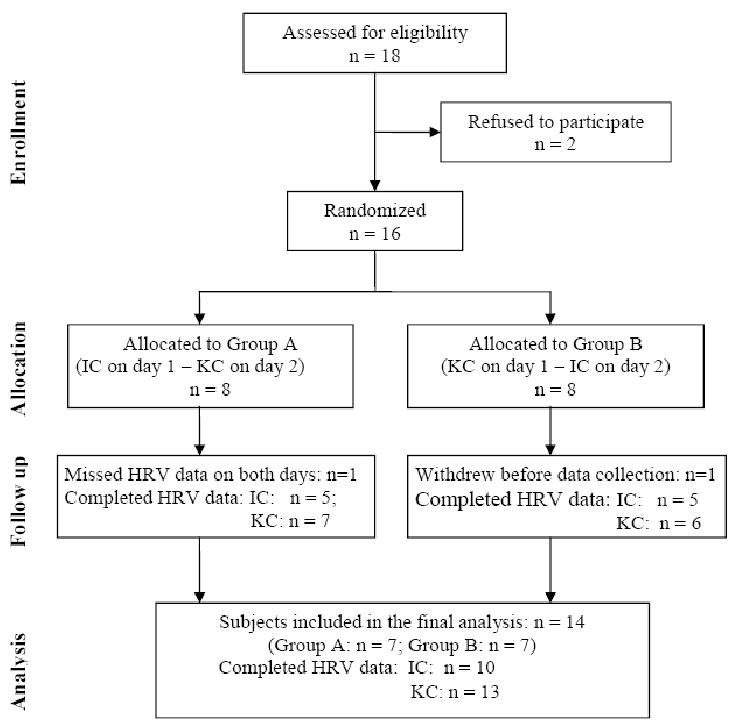
Consort diagram of enrollment, allocation, follow-up, and data analysis. KC = Kangaroo Care heel stick condition; IC = Incubator heel stick condition.
2.3. Measures
Infant's behavioral state
The Anderson Behavioral State Scoring System (ABSS) [52] was used to measure infant state. The ABSS has 12 categories with behavioral states of increasing agitation: 1 = regular, quiet sleep; 2 = irregular sleep; 3 = active sleep; 4 = very active sleep; 5 = drowsy; 6 = alert inactive; 7 = quiet awake; 8 = active awake; 9 = very awake; 10 = fussing; 11 = crying and 12 = hard crying. Content and convergent validity of the ABSS have been reported extensively [53, 54]. Interrater reliability has been estimated at .71 to .95 in two studies [53, 55]. For each assessment, an infant was observed for 30 seconds, and the number corresponding to the highest behavioral state observed was recorded [55].
Heart rate and heart rate variability
Continuous electrocardiogram (ECG) data were recorded from two surface chest electrodes throughout baseline, heel warm, heel stick, and recovery periods. R-R intervals and respiratory activity were captured and measured using the ANS-R1000 system (Ansar, Inc., Philadelphia, PA), a non-invasive signal monitor, which is an accessory connecting to the infant cardio-respiratory monitor that captures ECG and respiratory data on line. The ECG was converted by the computer software to digital values and a time series curve of the R-R intervals was generated. Heart rate and respiratory activity graphs displayed the 128 second (512 points) segment required to produce the HR and respiratory activity spectra. Following smoothing of the curve, the computer re-sampled at regular intervals over the same period using a Fast Fourier Transform (FFT) [56]. Spectral analysis of the transformed data generated two components of clinical interest: the HF (0.15 - 1.0 Hz) component, which is identified with vagal tone and is determined by the respiratory rhythm; and LF (0.04 to 0.15 Hz) component, which is mediated by both sympathetic and parasympathetic parts of the autonomic system. The ratio of the LF/HF spectra represents an index of parasympathetic-sympathetic balance. Movement and artifact were eliminated by comparing amplitude (height) of the R-wave to be included with the amplitude for the last acceptable R-wave. Waves of more or less than 38% deviance from the previous wave were automatically eliminated. The researcher or research assistant who extracted the HRV data was not blinded from the study conditions. Although the bias was likely minimal, still it is important. A proper blinded data extraction process would be necessary to guard against bias pertaining to knowledge of study conditions in the future study.
2.4. Procedures
Based on ethical considerations and pain management guidelines from the American and Canadian Pediatric Societies [21], only heel sticks that were clinically warranted and ordered by physicians or neonatal nurse practitioners were used as the painful procedure in the study. Such procedures are typically ordered on at least a daily basis, as was the case for the neonates in this study. No additional bloodwork was used in the research procedure.
The demographic data and the number of previous invasive procedures were obtained from the patients' medical records. Data collection on infants' responses to pain in KC and in IC was conducted on two consecutive mornings between 8:30 am and 10:20 am. The heel stick and subsequent blood draw were standardized and performed in accordance with the guidelines and step-by-step procedure developed by National Association of Neonatal Nurses [57]. A Tenderfoot™ device was used to lance the heel. One consistent person, the neonatal unit phlebotomist, did all the heel sticks and blood draws. In both KC and IC conditions, data were collected across four periods: (1) Baseline (BL), 20 minutes immediately prior to heel warming, (2) Heel Warming (HW) with a warm pack, 5 minutes, (3) Heel Stick (HS), 15 seconds, and blood collection with possible further squeezes, 0.5 to 10 minutes, and (4) Recovery (RC), 20 minutes after a band-aid was placed on heel.
Procedure for KC Condition
The infant was transferred from the incubator into KC after a naso-gastric tube or bottle feeding was completed. KC was carried out with the mother holding her preterm infant prone, clothed only in a diaper, skin-to-skin, between her breasts. All infants were held upright at a 30 – 40 degree angle. The infant's back was covered with a receiving blanket folded in fourths and placed beneath the mother's cover gown to insure infant temperatures were sustained within a neutral thermal zone. Mother and infant were seated in a recliner next to the infant's incubator. Mothers were encouraged to rest during KC, but could talk softly. Mothers were encouraged to leave the infant alone if he/she was sleeping and to leave their hands clasped behind the infant's back. Mothers and infants had 60 minutes of undisturbed KC before heel warming began. Heel Warming, then Heel Stick, and then the Recovery period were executed in the KC position.
Procedure for IC condition
At the end of the morning feeding, the diaper-clad infant was placed prone and nested in a servo-control incubator inclined at 30°-40°. Mothers were absent during the incubator periods. Infants remained undisturbed for 60 minutes, and then Heel Warming, Heel Stick, and Recovery periods were executed while the infant remained in the incubator in the same position.
During both the KC and IC conditions, one of three research nurses observed and coded behavioral states every minute during the four data collection periods. These nurses were trained in the behavioral state assessment procedures before the study. Assessed inter-rater reliability (Cohen's kappa) among the three research nurses was 95% at the beginning of the study and was maintained at 91%-95% agreement during the study by reestablished after every 5th participant. HRV data were collected during four periods in both the KC and IC conditions.
2.5. Data analysis
Infant behavioral states were described as the percentage of time in each state for each of the data collection periods (i.e. BL, HW, HS, and RC). The HRV indices were not significantly different among the behavioral states, and so the HRV data were combined for each data collection period; arithmetic means were calculated for HR and geometric means were calculated for HRV indices for each of the four data collection periods in the KC and IC conditions. The geometric mean [GM = (a1 · … · an)1/N] was used instead of the arithmetic mean [M = (a1 + … + an)/N] for calculating HRV indices, because the GM results in an average rate of change [58]. Repeated measures analysis of variance (RM-ANOVA) was used to compare HR and HRV indices across BL, HW, HS, and RC periods using SPSS 13.0 (Chicago, IL). To address possible carry-over effects and dependence of observations within subjects, the Generalized Estimating Equations (GEE) model analyzed treatment (KC vs. IC) and carry-over effects (assignment to KC day 1 vs. KC day 2) using SAS 8.0 (Cary, NC). The logarithm transformation was applied before the analyses, because the HRV data were not normally distributed.
3. Results
3.1. Characteristics of subjects
Eighteen infant-mother dyads were approached and 16 were enrolled (Figure 1). After randomized allocation, one subject was withdrawn prior to any treatment administration and data collection and one infant missed HRV data in both IC and KC days due to equipment issues. Fourteen subjects included in the final sample. Seven were randomly assigned to group A (IC Day 1, KC Day 2) and seven to group B (KC Day 1, IC Day 2). Because no statistical differences between the groups were found on demographic and medical characteristics, and because all babies served as their own controls, the data are presented for all participants as one group. Demographic and medical characteristics are in Table 1. The mean number of previous painful procedures was 29.15 ± 6.44 with a range of 20 - 40. Previous painful procedures were heel sticks, intubation, placement of an umbilical line or a percutaneous venous catheter or peripheral intravenous lines, arterial punctures, lumbar puncture, chest tubes, urinary catheterization for bladder tap, and vitamin K injections. Heel stick was the most frequently performed previous painful procedure, constituting a mean of 16 out of the 29 (54%) of the procedures, and every baby had at least one heel stick procedure during their NICU stay.
Table 1.
Demographic and Medical Characteristics of Infants (n = 14)
| Variables | Values | |
|---|---|---|
| Gestational age: | 30 weeks | 5 (36%) |
| 31 weeks | 3 (21%) | |
| 32 weeks | 6 (43%) | |
| Gender: | Male | 8 (57%) |
| Female | 6 (43%) | |
| IVH incidence: | None | 13 (93%) |
| Grade I | 1 (7%) | |
| Race/Ethnicity: | Caucasian NonHisp | 7 (50%) |
| NonCauc Hispanic | 7 (50%) | |
| Postnatal age (days) | 6 ± 1 | |
| Birth weight (g) | 1775 ± 292 | |
| Weight on the day of study (g) | 1706 ± 293 | |
| 1 minute APGAR score | 7 ± 2 | |
| 5 minutes APGAR score | 8 ± 1 | |
| Number of previous pain experiences | 29 ± 7 | |
Note: IVH= intraventricular hemorrhage
3.2. Behavioral state
During BL, neonates were in Quiet Sleep 65% of the time in KC and 60% in IC, and in Active Sleep 24% of the time in both KC and IC. During HW, neonates were in Quiet Sleep 65% of the time and in Active Sleep 22% of the time in both KC and IC. During HS, most neonates cried (65% in KC; 64% in IC). During RC, neonates were predominantly in Quiet Sleep (57% in KC, 48% in IC) or Active Sleep (21% in KC, 25% in IC). No significant differences in behavioral states were found between KC and IC conditions during the BL, HW, HS, and RC periods.
3.3. Heart rate and heart rate variability indices
Heart rate and HRV data were available for all days except one KC and four IC days due to equipment problems. The pairwise deletion was used for missing data; therefore, the final data were from 13 babies in KC and 10 babies in IC. To test infants' pain responses for the heel stick across the four study periods (BL, HW, HS, and RC), individual RM-ANOVA with study periods as the repeated factor was conducted for each study condition (KC and IC). HR increased significantly during HS from the BL and HW periods in both KC (p < .05) and IC conditions (p < .001), and returned to BL values during RC in both conditions (Table 2 and Figure 2.A). Comparing HR between KC and IC heel stick conditions using the GEE model, HR was significantly lower in the KC condition (146 ± 9 beats/min) than in IC (152 ± 13 beats/min) during BL period (p < .05) and HS period (KC 159 ± 14 beats/min vs. IC 165 ± 14 beats/min, p < .05).
Table 2.
Values for HR, LF, HF, and LF/HF during Four Periods in KC and IC Conditions1
| KC (n = 13) | IC (n = 10) | Comparison KC vs. IC 3 | |||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | Z | df | p-value | |
| HR (beats/min) | |||||||
| BL | 146.46 | 9.36 | 152.07 | 12.83 | 2.20 | 1 (19) | < .05 |
| HW | 152.10 | 11.65 | 153.71 | 11.91 | 0.11 | 1 (18) | > .05 |
| HS | 158.64 | 13.58 | 164.87 | 14.48 | 2.43 | 1 (16) | < .05 |
| RC | 146.42 | 14.17 | 153.86 | 14.12 | 1.44 | 1 (18) | > .05 |
| Comparison across 4 periods 2 | F (3, 12) = 4.43, p < .05 | F (3, 9) = 12.16, p < .001 | |||||
| LF (ms2) | |||||||
| BL | 6.30 | 10.38 | 2.83 | 2.65 | 2.59 | 1 (19) | < .01 |
| HW | 19.44 | 54.37 | 6.84 | 6.38 | 0.60 | 1 (18) | > .05 |
| HS | 30.05 | 41.26 | 17.62 | 24.55 | 3.43 | 1 (17) | < .001 |
| RC | 15.16 | 29.85 | 3.13 | 2.46 | 1.46 | 1 (18) | > .05 |
| Comparison across 4 periods 2 | F (3, 10) = 4.06, p < .05 | F (3, 9) = 4.75, p < .01 | |||||
| HF (ms2) | |||||||
| BL | 2.05 | 3.77 | 1.38 | 3.10 | 2.00 | 1 (19) | < .05 |
| HW | 22.91 | 70.57 | 5.96 | 8.62 | 1.12 | 1 (18) | > .05 |
| HS | 48.50 | 71.04 | 23.52 | 35.96 | 1.24 | 1 (17) | > .05 |
| RC | 19.89 | 54.42 | 0.50 | 0.52 | 1.71 | 1 (18) | > .05 |
| Comparison across 4 periods 2 | F (3, 10) = 6.18, p < .01 | F (3, 9) = 10 .88, p < .001 | |||||
| LF/HF ratio | |||||||
| BL | 6.32 | 4.56 | 11.18 | 9.00 | 0.77 | 1 (20) | > .05 |
| HW | 5.12 | 5.71 | 4.23 | 4.90 | 0.95 | 1 (18) | > .05 |
| HS | 3.89 | 6.69 | 1.75 | 1.84 | 0.07 | 1 (17) | > .05 |
| RC | 7.61 | 5.26 | 10.60 | 8.63 | 3.59 | 1 (18) | < .001 |
| Comparison across 4 periods 2 | F (3, 10) = 6.07, p < .01 | F (3, 9) = 11.63, p < .001 | |||||
HR = arithmetic mean, LF, HF, L/HF = geometric means;
Comparison across 4 periods: Repeated measures analysis of variance was used;
Comparison KC vs. IC: Generalized Estimating Equations model was used; KC = Kangaroo Care condition; IC = Incubator care condition; BL = Baseline; HW = Heel Warming; HS = Heel Stick; RC = Recovery; LF = low frequency power; HF = high frequency power; LF/HF = Low/High frequency power ratio.
This study was supported by AWHONN to the first author and National Institute of Nursing Research, NIH (RO3NR08587-01) to the third author.
Figure 2.
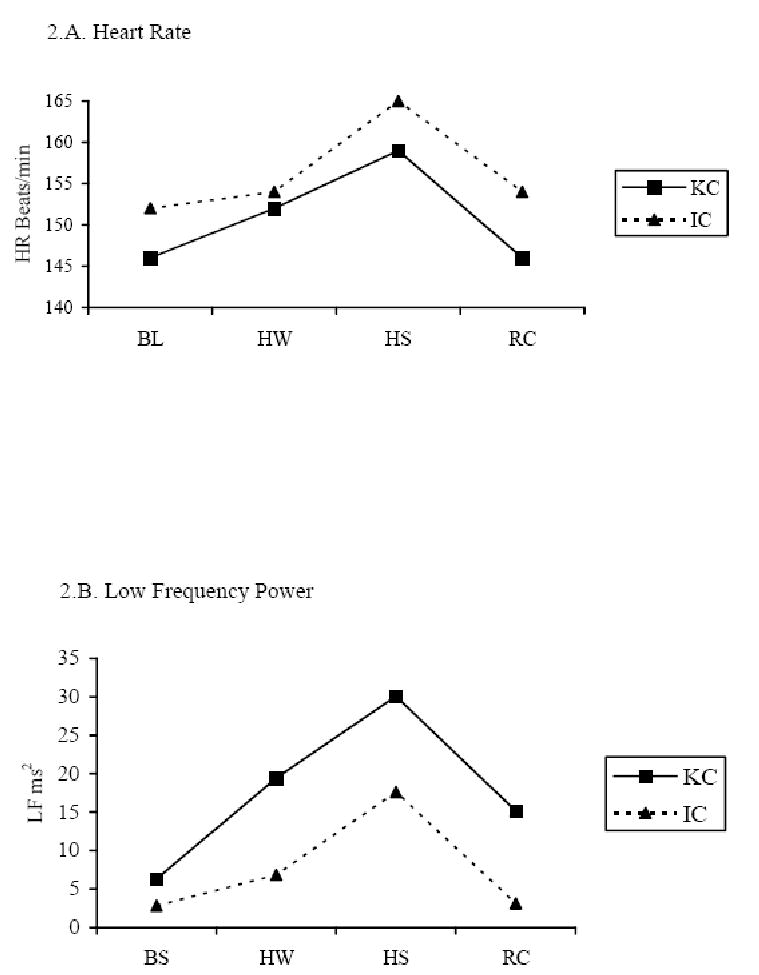
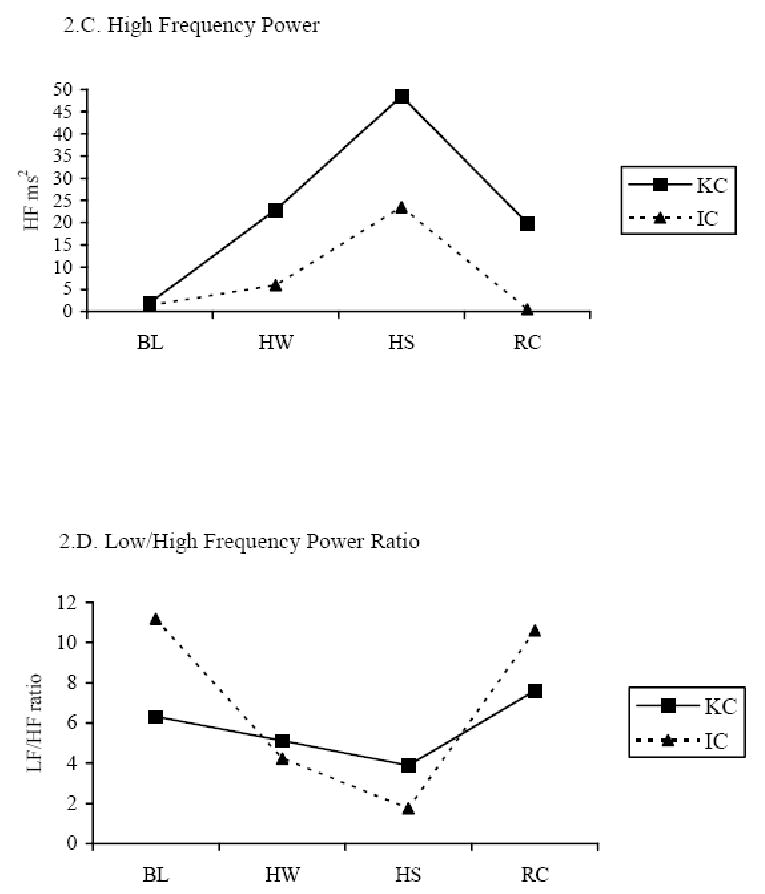
HRV indices across four periods in the KC and IC conditions. KC = Kangaroo Care condition; IC = incubator care condition; LF = low frequency power (predominately sympathetic activity); HF = high frequency power (parasympathetic activity); LF/HF = Low/High frequency power ratio (balance of sympathetic-parasympathetic activity).
There were significant differences in HRV indices across BL, HW, HS, and RC periods in both KC and IC conditions using RM-ANOVA with study periods as the repeated factor, and the differences followed similar patterns in both study conditions (Table 2; Figure 2.B, 2.C, and 2.D). Both LF and HF were increased during HS from the BL and HW, and dropped in the RC period in both KC (LF, p < .05 and HF, p < .01) and IC (LF, p < .01 and HF, p < .001) conditions. The LF/HF ratio was lower during HS than during BL, HW, and RC in both KC (p < .01) and IC (p < .001) conditions. When testing the KC effect on the heel stick compared to the IC condition using the GEE model, HRV differences between KC and IC indicated that LF was higher in KC at BL (p<.01) and at HS (p < .001) than in IC, and HF was higher in KC at BL than in IC (p < .05). The LF/HF ratio was significantly lower during RC in the KC than IC condition (p< .001). Results showed medium effect size (0.35 to 0.40) of KC on modifying HRV indices during heel stick procedure.
4. Discussion
In this study, the patterns shown over time in the HR and HRV indices provided a noninvasive measure of sympathovagal balance during heel stick among 14 preterm infants 30 – 32 weeks GA in KC and IC conditions. To our knowledge, the investigation reported here is the first study examining the effect of KC on heel stick pain responses measured by HRV indices in preterm infants. Consistent with previous studies [41, 59-61], HR increased from baseline to heel stick and decreased in the recovery period in both the KC and IC conditions, indicating a clear pain response caused by the heel stick procedure. The LF and HF responses were also found similar in both KC and IC with increases from BL to HS and decreases from HS to RC. In relation to painful events in preterm infants, previous studies showed that total HRV [41], the LF [41, 59] and HF bands of HRV [59] were reduced during heel lance, but other studies did not show the correlation of HRV to pain [38, 39]. Our sample differed in the LF and HF responses with increases at heel stick instead of decreases as reported in these previous studies. The reason for this difference is not clear as our infants' positions and behavioral responses were similar to those in other studies. It may be that the length of the data collection period during Heel Stick varied among the studies so that the intervals are not comparable. In our study, data were collected across the HS period which ranged from 3.5 – 4.5 minutes; the length of data collection was not reported in the two Oberlander studies. The LF/HF ratio response in our sample was the same as that reported by Oberlander and colleagues [59, 60] with a decrease in the LF/HF ratio at heel stick. In both KC and IC, the LF/HF ratio decreased from BL to HS, and increased in RC, reflecting an increase in parasympathetic influence in order to balance sympathetic response to the heel stick.
When comparing infants' pain responses between KC and IC conditions, HR was significantly lower in KC than IC for the BL and HS periods. LF and HF were higher in the BL period and LF was also increased in the HS period in KC compared to the IC condition with a medium effect size (Figure 2.B and 2.C). The results are consistent with Schrod and Walter's [62] findings, in which LF and HF increased when infants were tilted into the KC position. Another study by Begum and associates [63] found that LF was significantly increase while HF was decreased during KC in low birth weight infants. However, none of these previous studies tested the effect of KC on HRV in a procedural pain condition. Increases in LF (predominately sympathetic tone) and HF (parasympathetic tone) may be explained by several factors, i.e., maturation (gestational age and postnatal age), change in body position, sleep state, and maternal presence and body temperature [44, 62, 64, 65]. In the present cross-over design study, infants in both KC and IC condition have equivalent GA and postnatal age, similarly inclined 30 – 40 degree prone position, and no difference in behavioral states during the study periods. One explanation for higher LF and HF in KC than in IC may be maternal presence and touch. Changes in LF have been found in response to thermoregulation influences and stimulation in preterm infants. Infant sympathetic activity is increased in a mother-infant bed-sharing environment compared to solitary-sleeping, which might be partly explained by thermal stimulation and thermoregulation when mothers are present [66]. Increased environmental air temperature due to the mother's body temperature also causes LF to rise in KC. The most likely explanation is that increased environmental temperature in KC increased infants' central temperature, thus causing a concurrent increase in LF compared to IC. KC also activates pressure receptors that can increase parasympathetic activity. Studies of moderate-pressure massage therapy show that parasympathetic activity peaks during massage and remains significantly higher throughout the 15-minute post-massage period compared with infants who receive sham, light-pressure massage [67, 68]. Animal studies also indicate that tactile interactions between rat pups and their mother, a type of pressure receptor stimulation, activates pups' parasympathetic responses and prevents all the changes associated with maternal deprivation [69-71]. Increased HF in KC may be due to KC's stimulating infants' pressure receptors, because the pressure receptors located in an infant's chest, abdomen, and extremities are activated by the full body touch between the infant and mother in KC, and the intensity of pressure in KC may be similar to moderate pressure.
For LF/HF ratio, there was more stability in KC and greater fluctuations in IC across BL, HW, HS, and RC periods (Figure 2.D). The ratio was significantly lower in KC than in IC condition during the RC period with a medium effect size. A more mature, balanced response (lower LF/HF ratio) to the painful heel stick during recovery was present in KC compared to that in IC. Balance between parasympathetic and sympathetic tone is associated with maturation and the ability to react effectively to stress, such as heel stick pain. Sympathetic tone is dominant in preterm infants, whereas parasympathetic tone increases with maturation [64, 72]. A lower LF/HF ratio indicates more maturity and a greater balance between the two systems [73]. The findings suggest a more balanced, and stable autonomic response to a painful heel stick in KC compared to that in IC. The fluctuations in autonomic response in IC were due to greater swings in sympathetic activity, suggesting that KC has a stabilizing, or balancing, effect during a painful procedure such as heel stick.
The central underlying mechanisms of KC as an analgesic and comforting experience on reducing infant pain may be through multi-sensory stimulation inputs and modulation of the stress-regulation system involved in pain. Mother-infant physical contact has been found to trigger release of beta-endorphins that are critically involved in mediation of the pain signal by blocking the perception of pain in rat pups [74, 75] and in human newborns [76]. Dieter [77] and Harrison [78] suggested that systematic gentle human touch may stimulate peripheral nerves that activate the vagus nerve, thus promoting infant comfort and reducing stress, resulting in positive immediate and long term outcomes. In addition, KC compared to the incubator condition provides stimulation through the infant's prone positioning [79-83], maternal warmth [84], containment and swaddling [85-87], maternal heart sounds [88, 89], vestibular movement (mother's chest respiratory movement) [90], maternal body odor [91-93], and mother's voice [94]. Infants are apparently familiar with their mothers' odor, voice, respiratory and heart beat rhythm from the uterine environment [92] and these have soothing effects on infants. Following KC, preterm infants showed a more rapid maturation of vagal tone as compared to controls, underscoring the effect of KC on the autonomic and circadian systems in preterm infants [19, 58, 95]. These findings support the premise that maternal effects reduce infant pain by blunting sympathetic responses, accelerating parasympathetic recovery of autonomic activation, and activating the endogenous opioid system.
These HRV findings of better balance and autonomic stability in the Kangaroo Care condition during heel stick for preterm infants lend further support the findings about KC decreasing HR, crying, and grimacing during painful procedures [16-19, 31, 34, 96-98]. Our study's findings add to the continuing evidence for KC as a non-pharmacologic intervention to alleviate preterm infant pain responses related to the heel stick.
Acknowledgments
This study was supported by AWHONN to the first author and National Institute of Nursing Research, NIH (RO3NR08587-01) to the second author.
Footnotes
Conflict of interest statement: Authors have reported no relevant financial and personal relationships with other people or organizations that could inappropriately influence their work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Xiaomei Cong, Assistant Professor, University of Connecticut School of Nursing, 231 Glenbrook Road, U-2026, Storrs, CT 06269-2026, Office: 860-486-2694, Fax: 860-486-0001, Email: xiaomei.cong@uconn.edu.
Susan M. Ludington-Hoe, Professor and Walters Chair of Pediatric Nursing, Bolton School of Nursing, Case Western Reserve University, 10900 Euclid Ave., Room 322D, Cleveland, OH 44106-4904, Phone: 216-368-6490, Fax: 216-368-3542, Email: Susan.Ludington@case.edu.
Gail McCain, Professor, University of Miami School of Nursing and Health Studies, 5801 Red Road, Coral Gables, FL 33143-3850, Office: 305-284-2904, Fax: 305-284-4370, Email: gmccain@miami.edu.
Pingfu Fu, Statistician, Dept of Epidemiology and Biostatistics, Medical School, Case Western Reserve University, 11100 Euclid Ave., Cleveland, OH 44106-4915, 216-368-3911 pxf16@case.edu.
References
- 1.Stevens B, McGrath P, Gibbins S, Beyene J, Breau L, Camfield C, et al. Procedural pain in newborns at risk for neurologic impairment. Pain. 2003 Sep;105(12):27–35. doi: 10.1016/s0304-3959(03)00136-2. [DOI] [PubMed] [Google Scholar]
- 2.Barker DP, Rutter N. Exposure to invasive procedures in neonatal intensive care unit admissions. Arch Dis Child Fetal Neonatal Ed. 1995 Jan;72(1):F47–8. doi: 10.1136/fn.72.1.f47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simons SH, van Dijk M, Anand KS, Roofthooft D, van Lingen RA, Tibboel D. Do we still hurt newborn babies? A prospective study of procedural pain and analgesia in neonates. Arch Pediatr Adolesc Med. 2003 Nov;157(11):1058–64. doi: 10.1001/archpedi.157.11.1058. [DOI] [PubMed] [Google Scholar]
- 4.Porter FL, Anand KJS. Epidemiology of pain in neonates. Research & Clinical Forums. 1998;20:9–16. [Google Scholar]
- 5.Evans JC, McCartney EM, Lawhon G, Galloway J. Longitudinal comparison of preterm pain responses to repeated heelsticks. Pediatr Nurs. 2005 May-Jun;31(3):216–21. [PubMed] [Google Scholar]
- 6.Lago P, Guadagni A, Merazzi D, Ancora G, Bellieni CV, Cavazza A. Pain management in the neonatal intensive care unit: a national survey in Italy. Paediatr Anaesth. 2005 Nov;15(11):925–31. doi: 10.1111/j.1460-9592.2005.01688.x. [DOI] [PubMed] [Google Scholar]
- 7.Carbajal R, Rousset A, Danan C, Coquery S, Nolent P, Ducrocq S, et al. Epidemiology and treatment of painful procedures in neonates in intensive care units. Jama. 2008 Jul 2;300(1):60–70. doi: 10.1001/jama.300.1.60. [DOI] [PubMed] [Google Scholar]
- 8.Anand KJ, Carr DB. The neuroanatomy, neurophysiology, and neurochemistry of pain, stress, and analgesia in newborns and children. Pediatr Clin North Am. 1989 Aug;36(4):795–822. doi: 10.1016/s0031-3955(16)36722-0. [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald M. Development of pain mechanisms. Br Med Bull. 1991 Jul;47(3):667–75. doi: 10.1093/oxfordjournals.bmb.a072499. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald M, Beggs S. The neurobiology of pain: developmental aspects. Neuroscientist. 2001 Jun;7(3):246–57. doi: 10.1177/107385840100700309. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald M. The development of nociceptive circuits. Nature reviews. 2005 Jul;6(7):507–20. doi: 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- 12.Taddio A, Shah V, Gilbert-MacLeod C, Katz J. Conditioning and hyperalgesia in newborns exposed to repeated heel lances. JAMA. 2002 Aug 21;288(7):857–61. doi: 10.1001/jama.288.7.857. [DOI] [PubMed] [Google Scholar]
- 13.Anand KJ. Neonatal analgesia and anesthesia. Introduction. Semin Perinatol. 1998 Oct;22(5):347–9. doi: 10.1016/s0146-0005(98)80051-7. [DOI] [PubMed] [Google Scholar]
- 14.Franck LS, Lawhon G. Environmental and behavioral strategies to prevent and manage neonatal pain. Semin Perinatol. 1998 Oct;22(5):434–43. doi: 10.1016/s0146-0005(98)80059-1. [DOI] [PubMed] [Google Scholar]
- 15.Tsao JC, Evans S, Meldrum M, Altman T, Zeltzer LK. A Review of CAM for Procedural Pain in Infancy: Part II. Other Interventions. Evid Based Complement Alternat Med. 2008 Dec;5(4):399–407. doi: 10.1093/ecam/nem089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freire NB, Garcia JB, Lamy ZC. Evaluation of analgesic effect of skin-to-skin contact compared to oral glucose in preterm neonates. Pain. 2008 Sep 30;139(1):28–33. doi: 10.1016/j.pain.2008.02.031. [DOI] [PubMed] [Google Scholar]
- 17.Johnston CC, Filion F, Campbell-Yeo M, Goulet C, Bell L, McNaughton K, et al. Kangaroo mother care diminishes pain from heel lance in very preterm neonates: a crossover trial. BMC Pediatics. 2008;8:13. doi: 10.1186/1471-2431-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnston CC, Stevens B, Pinelli J, Gibbins S, Filion F, Jack A, et al. Kangaroo care is effective in diminishing pain response in preterm neonates. Arch Pediatr Adolesc Med. 2003 Nov;157(11):1084–8. doi: 10.1001/archpedi.157.11.1084. [DOI] [PubMed] [Google Scholar]
- 19.Ludington-Hoe S, Hosseini R, Torowicz DL. Skin-to-skin contact (Kangaroo Care) analgesia for preterm infant heel stick. AACN Clin Issues. 2005 Jul-Sep;16(3):373–87. doi: 10.1097/00044067-200507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000 Jan;105(1):e14. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- 21.American Academy of Pediatrics CoFaNaSoS, Canadian Paediatric Society and Fetus and Newborn Committee. Prevention and management of pain in the neonate: an update. Pediatrics. 2006 Nov;118(5):2231–41. doi: 10.1542/peds.2006-2277. [DOI] [PubMed] [Google Scholar]
- 22.Carbajal R, Gall O, Annequin D. Pain management in neonates. Expert Rev Neurother. 2004 May;4(3):491–505. doi: 10.1586/14737175.4.3.491. [DOI] [PubMed] [Google Scholar]
- 23.Cignacco E, Hamers JP, Stoffel L, van Lingen RA, Gessler P, McDougall J, et al. The efficacy of non-pharmacological interventions in the management of procedural pain in preterm and term neonates. A systematic literature review. European journal of pain (London, England) 2007 Feb;11(2):139–52. doi: 10.1016/j.ejpain.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Golianu B, Krane E, Seybold J, Almgren C, Anand KJ. Non-pharmacological techniques for pain management in neonates. Semin Perinatol. 2007 Oct;31(5):318–22. doi: 10.1053/j.semperi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Leslie A, Marlow N. Non-pharmacological pain relief. Seminars in fetal & neonatal medicine. 2006 Aug;11(4):246–50. doi: 10.1016/j.siny.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 26.Tsao JC, Evans S, Meldrum M, Altman T, Zeltzer LK. A Review of CAM for Procedural Pain in Infancy: Part II. Other Interventions. Evid Based Complement Alternat Med. 2007 Oct 25; doi: 10.1093/ecam/nem089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Anand KJ. Analgesia for skin-breaking procedures in newborns and children: what works best? CMAJ. 2008 Jul 1;179(1):11–2. doi: 10.1503/cmaj.080834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melzack R. Pain and the neuromatrix in the brain. J Dent Educ. 2001 Dec;65(12):1378–82. [PubMed] [Google Scholar]
- 29.Moseley GL. A pain neuromatrix approach to patients with chronic pain. Man Ther. 2003 Aug;8(3):130–40. doi: 10.1016/s1356-689x(03)00051-1. [DOI] [PubMed] [Google Scholar]
- 30.Gazzolo D, Masetti P, Meli M. Kangaroo care improves post-extubation cardiorespiratory parameters in infants after open heart surgery. Acta Paediatr. 2000 Jun;89(6):728–9. doi: 10.1080/080352500750044098. [DOI] [PubMed] [Google Scholar]
- 31.Castral TC, Warnock F, Leite AM, Haas VJ, Scochi CG. The effects of skin-to-skin contact during acute pain in preterm newborns. European journal of pain (London, England) 2008 May;12(4):464–71. doi: 10.1016/j.ejpain.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 32.Ludington-Hoe S. Kangaroo Care to Blunt Pain in Premature Infants. Ceveland, OH: Frances Payne Bolton School of Nursing, Case Western Reserve University; 2005. NINR-08587-01. [Google Scholar]
- 33.Johnston CC, Filion F, Campbell-Yeo M, Goulet C, Bell L, McNaughton K, et al. Enhanced kangaroo mother care for heel lance in preterm neonates: a crossover trial. J Perinatol. 2008 Sep 4; doi: 10.1038/jp.2008.113. [DOI] [PubMed] [Google Scholar]
- 34.Kostandy RR, Ludington-Hoe SM, Cong X, Abouelfettoh A, Bronson C, Stankus A, et al. Kangaroo Care (skin contact) reduces crying response to pain in preterm neonates: pilot results. Pain Manag Nurs. 2008 Jun;9(2):55–65. doi: 10.1016/j.pmn.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anand KJ, Aranda JV, Berde CB, Buckman S, Capparelli EV, Carlo W, et al. Summary proceedings from the neonatal pain-control group. Pediatrics. 2006 Mar;117(3 Pt 2):S9–S22. doi: 10.1542/peds.2005-0620C. [DOI] [PubMed] [Google Scholar]
- 36.Stevens B, Franck L, Gibbins S, McGrath PJ, Dupuis A, Yamada J, et al. Determining the structure of acute pain responses in vulnerable neonates. Can J Nurs Res. 2007 Jun;39(2):32–47. [PubMed] [Google Scholar]
- 37.Barr RG. Reflections on measuring pain in infants: dissociation in responsive systems and “honest signalling”. Arch Dis Child Fetal Neonatal Ed. 1998 Sep;79(2):F152–6. doi: 10.1136/fn.79.2.f152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grunau RE, Holsti L, Haley D, Oberlander T, Weinberg J, Solimano A, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005 Feb;113(3):293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens B, McGrath P, Gibbins S, Beyene J, Breau L, Camfield C, et al. Determining behavioural and physiological responses to pain in infants at risk for neurological impairment. Pain. 2007 Jan;127(12):94–102. doi: 10.1016/j.pain.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 40.Oberlander T, Saul JP. Methodological considerations for the use of heart rate variability as a measure of pain reactivity in vulnerable infants. Clin Perinatol. 2002 Sep;29(3):427–43. doi: 10.1016/s0095-5108(02)00013-1. [DOI] [PubMed] [Google Scholar]
- 41.Lindh V, Wiklund U, Sandman PO, Hakansson S. Assessment of acute pain in preterm infants by evaluation of facial expression and frequency domain analysis of heart rate variability. Early Hum Dev. 1997 Apr 25;48(12):131–42. doi: 10.1016/s0378-3782(96)01851-8. [DOI] [PubMed] [Google Scholar]
- 42.Cowan MJ. Measurement of heart rate variability. West J Nurs Res. 1995 Feb;17(1):32–48. doi: 10.1177/019394599501700104. discussion 101-11. [DOI] [PubMed] [Google Scholar]
- 43.de Beer N, Andriessen P, Berendsen R, Oei S, Wijn P, Oetomo S. Customized spectral band analysis compared with conventional Fourier analysis of heart rate variability in neonates. Physiol Meas. 2004 Dec;25(6):1385–95. doi: 10.1088/0967-3334/25/6/004. [DOI] [PubMed] [Google Scholar]
- 44.Longin E, Schaible T, Lenz T, Konig S. Short term heart rate variability in healthy neonates: Normative data and physiological observations. Early Hum Dev. 2005 Aug;81(8):663–71. doi: 10.1016/j.earlhumdev.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 45.Task Force of the European Society of Cardiology, North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation and clinical use. Circulation. 1996 Mar 1;93(5):1043–65. [PubMed] [Google Scholar]
- 46.Gibbins S, Stevens B, McGrath PJ, Yamada J, Beyene J, Breau L, et al. Comparison of pain responses in infants of different gestational ages. Neonatology. 2008;93(1):10–8. doi: 10.1159/000105520. [DOI] [PubMed] [Google Scholar]
- 47.Bohnhorst B, Heyne T, Peter CS, Poets CF. Skin-to-skin (kangaroo) care, respiratory control, and thermoregulation. J Pediatr. 2001 Feb;138(2):193–7. doi: 10.1067/mpd.2001.110978. [DOI] [PubMed] [Google Scholar]
- 48.Ludington-Hoe S, Ferreira CN, Goldstein MR. Kangaroo care with a ventilated preterm infant. Acta Paediatr. 1998 Jun;87(6):711–3. doi: 10.1080/080352598750014201. [DOI] [PubMed] [Google Scholar]
- 49.Ludington-Hoe S, Nguyen N, Swinth JY, Satyshur RD. Kangaroo care compared to incubators in maintaining body warmth in preterm infants. Biol Res Nurs. 2000 Jul;2(1):60–73. doi: 10.1177/109980040000200107. [DOI] [PubMed] [Google Scholar]
- 50.Modi N, Glover V. Non-pharmacological reduction of hypercortisolaemia in preterm infants. Infant Behavior and Development. 1998;21(Special ICIS Issue):86. [Google Scholar]
- 51.Lund CH, Osborne JW. Validity and reliability of the neonatal skin condition score. JOGNN. 2004;33(3):320–7. doi: 10.1177/0884217504265174. [DOI] [PubMed] [Google Scholar]
- 52.Andersen GC, Behnke M, Gill NE, Cohen M, Mearel C, McDonie TE. Self-regulatory gauge to bottle feeding for preterm infants: Effect on behavioral state, energy, expenditure, and weight gain. In: Funk FG, Turnquist MT, Champagne LA, Coop RA, Wiere T, editors. Key aspects of recovery: Nutrition, Rest, and Mobility. NY: Springer; 1990. pp. 83–97. [Google Scholar]
- 53.McCain GC. Facilitating inactive awake states in preterm infants: a study of three interventions. Nurs Res. 1992 May-Jun;41(3):157–60. [PubMed] [Google Scholar]
- 54.Ludington S. Energy conservation during skin-to-skin contact between premature infants and their mothers. Heart Lung. 1990 Sep;19(5 Pt 1):445–51. [PubMed] [Google Scholar]
- 55.Gill NE, Behnke M, Conlon M, McNeely JB, Anderson GC. Effect of nonnutritive sucking on behavioral state in preterm infants before feeding. Nurs Res. 1988 Nov-Dec;37(6):347–50. [PubMed] [Google Scholar]
- 56.Cowan MJ, Pike K, Burr RL, Cain KC, Narayanan SB. Description of time- and frequency- domain-based measures of heart rate variability in individuals taking antiarrhythmics, beta blockers, calcium channel blockers, and/or antihypertensive drugs after sudden cardiac arrest. J Electrocardiol. 1993;26(Suppl):1–13. [PubMed] [Google Scholar]
- 57.National Association of Neonatal Nurses. Neonatal Capillary Blood Draw: Performance of a Heelstick. NANN Technical Bulletin. 1995:1–9. [Google Scholar]
- 58.McCain GC, Ludington-Hoe SM, Swinth JY, Hadeed AJ. Heart rate variability responses of a preterm infant to kangaroo care. J Obstet Gynecol Neonatal Nurs. 2005 Nov-Dec;34(6):689–94. doi: 10.1177/0884217505281857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oberlander T, Grunau RE, Fitzgerald C, Whitfield MF. Does parenchymal brain injury affect biobehavioral pain responses in very low birth weight infants at 32 weeks' postconceptional age? Pediatrics. 2002 Sep;110(3):570–6. doi: 10.1542/peds.110.3.570. [DOI] [PubMed] [Google Scholar]
- 60.Oberlander T, Grunau RE, Whitfield MF, Fitzgerald C, Pitfield S, Saul JP. Biobehavioral pain responses in former extremely low birth weight infants at four months' corrected age. Pediatrics. 2000 Jan;105(1):e6. doi: 10.1542/peds.105.1.e6. [DOI] [PubMed] [Google Scholar]
- 61.Grunau RE, Oberlander TF, Whitfield MF, Fitzgerald C, Lee SK. Demographic and therapeutic determinants of pain reactivity in very low birth weight neonates at 32 weeks' postconceptional age. Pediatrics. 2001 Jan;107(1):105–12. doi: 10.1542/peds.107.1.105. [DOI] [PubMed] [Google Scholar]
- 62.Schrod L, Walter J. Effect of head-up body tilt position on autonomic function and cerebral oxygenation in preterm infants. Biol Neonate. 2002;81(4):255–9. doi: 10.1159/000056756. [DOI] [PubMed] [Google Scholar]
- 63.Begum EA, Bonno M, Ohtani N, Yamashita S, Tanaka S, Yamamoto H, et al. Cerebral oxygenation responses during kangaroo care in low birth weight infants. BMC pediatrics. 2008;8:51. doi: 10.1186/1471-2431-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chatow U, Davidson S, Reichman BL, Akselrod S. Development and maturation of the autonomic nervous system in premature and full-term infants using spectral analysis of heart rate fluctuations. Pediatr Res. 1995 Mar;37(3):294–302. doi: 10.1203/00006450-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 65.Rosenstock EG, Cassuto Y, Zmora E. Heart rate variability in the neonate and infant: analytical methods, physiological and clinical observations. Acta Paediatr. 1999 May;88(5):477–82. doi: 10.1080/08035259950169422. [DOI] [PubMed] [Google Scholar]
- 66.Richard C, Mosko S. Mother-infant bedsharing is associated with an increase in infant heart rate. Sleep. 2004 May 1;27(3):507–11. doi: 10.1093/sleep/27.3.507. [DOI] [PubMed] [Google Scholar]
- 67.Diego MA, Field T, Hernandez-Reif M. Vagal activity, gastric motility, and weight gain in massaged preterm neonates. J Pediatr. 2005 Jul;147(1):50–5. doi: 10.1016/j.jpeds.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 68.Ireland M, Olson M. Massage therapy and therapeutic touch in children: state of the science. Altern Ther Health Med. 2000 Sep;6(5):54–63. [PubMed] [Google Scholar]
- 69.Pauk J, Kuhn CM, Field TM, Schanberg SM. Positive effects of tactile versus kinesthetic or vestibular stimulation on neuroendocrine and ODC activity in maternally-deprived rat pups. Life Sci. 1986 Dec 1;39(22):2081–7. doi: 10.1016/0024-3205(86)90359-0. [DOI] [PubMed] [Google Scholar]
- 70.Kuhn CM, Schanberg SM, Field T, Symanski R, Zimmerman E, Scafidi F, et al. Tactile-kinesthetic stimulation effects on sympathetic and adrenocortical function in preterm infants. J Pediatr. 1991 Sep;119(3):434–40. doi: 10.1016/s0022-3476(05)82059-1. [DOI] [PubMed] [Google Scholar]
- 71.Schanberg SM, Ingledue VF, Lee JY, Hannun YA, Bartolome JV. PKC alpha mediates maternal touch regulation of growth-related gene expression in infant rats. Neuropsychopharmacology. 2003 Jun;28(6):1026–30. doi: 10.1038/sj.npp.1300125. [DOI] [PubMed] [Google Scholar]
- 72.Smith S. Heart period variability of intubated very-low-birth-weight infants during incubator care and maternal holding. Am J Crit Care. 2003 Jan;12(1):54–64. [PubMed] [Google Scholar]
- 73.Whitley JA, Rich BL. A double-blind randomized controlled pilot trial examining the safety and efficacy of therapeutic touch in premature infants. Adv Neonatal Care. 2008 Dec;8(6):315–33. doi: 10.1097/01.ANC.0000342764.71864.28. [DOI] [PubMed] [Google Scholar]
- 74.Machelska H. Functional evidence of pain control by the immune system. Adv Exp Med Biol. 2003;521:88–97. [PubMed] [Google Scholar]
- 75.Blass EM, Shide DJ, Zaw-Mon C, Sorrentino J. Mother as shield: differential effects of contact and nursing on pain responsivity in infant rats--evidence for nonopioid mediation. Behav Neurosci. 1995 Apr;109(2):342–53. doi: 10.1037//0735-7044.109.2.342. [DOI] [PubMed] [Google Scholar]
- 76.Mooncey S, Giannakoulopoulos X, Glover V, Acolet D, Modi N. The effect of mother-infant skin-to-skin contact on plasma cortisol and β-endorphin concentrations in preterm newborns. Infant Behavior and Development. 1997;20(4):553–7. [Google Scholar]
- 77.Dieter JN, Emory EK. Supplemental stimulation of premature infants: a treatment model. J Pediatr Psychol. 1997 Jun;22(3):281–95. doi: 10.1093/jpepsy/22.3.281. [DOI] [PubMed] [Google Scholar]
- 78.Harrison LL, Williams AK, Berbaum ML, Stem JT, Leeper J. Physiologic and behavioral effects of gentle human touch on preterm infants. Res Nurs Health. 2000 Dec;23(6):435–46. doi: 10.1002/1098-240X(200012)23:6<435::AID-NUR3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 79.Chang YJ, Anderson GC, Lin CH. Effects of prone and supine positions on sleep state and stress responses in mechanically ventilated preterm infants during the first postnatal week. J Adv Nurs. 2002 Oct;40(2):161–9. doi: 10.1046/j.1365-2648.2002.02358.x. [DOI] [PubMed] [Google Scholar]
- 80.Chang YJ, Anderson GC, Dowling D, Lin CH. Decreased activity and oxygen desaturation in prone ventilated preterm infants during the first postnatal week. Heart Lung. 2002 Jan-Feb;31(1):34–42. doi: 10.1067/mhl.2002.120241. [DOI] [PubMed] [Google Scholar]
- 81.Sahni R, Saluja D, Schulze KF, Kashyap S, Ohira-Kist K, Fifer WP, et al. Quality of diet, body position, and time after feeding influence behavioral states in low birth weight infants. Pediatr Res. 2002 Sep;52(3):399–404. doi: 10.1203/00006450-200209000-00016. [DOI] [PubMed] [Google Scholar]
- 82.Maynard V, Bignall S, Kitchen S. Effect of positioning on respiratory synchrony in non-ventilated pre-term infants. Physiother Res Int. 2000;5(2):96–110. doi: 10.1002/pri.189. [DOI] [PubMed] [Google Scholar]
- 83.Grunau RE, Linhares MB, Holsti L, Oberlander TF, Whitfield MF. Does prone or supine position influence pain responses in preterm infants at 32 weeks gestational age. Clin J Pain. 2004 Mar-Apr;20(2):76–82. doi: 10.1097/00002508-200403000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stringer M, Shaw VD, Savani RC. Comfort care of neonates at the end of life. Neonatal Netw. 2004 Sep-Oct;23(5):41–6. doi: 10.1891/0730-0832.23.5.41. [DOI] [PubMed] [Google Scholar]
- 85.Huang CM, Tung WS, Kuo LL, Ying-Ju C. Comparison of pain responses of premature infants to the heelstick between containment and swaddling. J Nurs Res. 2004 Mar;12(1):31–40. doi: 10.1097/01.jnr.0000387486.78685.c5. [DOI] [PubMed] [Google Scholar]
- 86.Fearon I, Kisilevsky BS, Hains SM, Muir DW, Tranmer J. Swaddling after heel lance: age-specific effects on behavioral recovery in preterm infants. J Dev Behav Pediatr. 1997 Aug;18(4):222–32. [PubMed] [Google Scholar]
- 87.Ward-Larson C, Horn RA, Gosnell F. The efficacy of facilitated tucking for relieving procedural pain of endotracheal suctioning in very low birthweight infants. MCN Am J Matern Child Nurs. 2004 May-Jun;29(3):151–6. doi: 10.1097/00005721-200405000-00004. quiz 7-8. [DOI] [PubMed] [Google Scholar]
- 88.Kurihara H, Chiba H, Shimizu Y, Yanaihara T, Takeda M, Kawakami K, et al. Behavioral and adrenocortical responses to stress in neonates and the stabilizing effects of maternal heartbeat on them. Early Hum Dev. 1996 Sep 20;46(12):117–27. doi: 10.1016/0378-3782(96)01749-5. [DOI] [PubMed] [Google Scholar]
- 89.Kawakami K, Takai-Kawakami K, Kurihara H, Shimizu Y, Yanaihara T. The effect of sounds on newborn infants under stress. Infant Behavior and Development. 1996;19:375–9. [Google Scholar]
- 90.Johnston CC, Stremler RL, Stevens BJ, Horton LJ. Effectiveness of oral sucrose and simulated rocking on pain response in preterm neonates. Pain. 1997 Aug;72(12):193–9. doi: 10.1016/s0304-3959(97)00033-x. [DOI] [PubMed] [Google Scholar]
- 91.Goubet N, Rattaz C, Pierrat V, Bullinger A, Lequien P. Olfactory experience mediates response to pain in preterm newborns. Dev Psychobiol. 2003 Mar;42(2):171–80. doi: 10.1002/dev.10085. [DOI] [PubMed] [Google Scholar]
- 92.Porter RH, Winberg J. Unique salience of maternal breast odors for newborn infants. Neurosci Biobehav Rev. 1999;23(3):439–49. doi: 10.1016/s0149-7634(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 93.Sullivan RM, Toubas P. Clinical usefulness of maternal odor in newborns: soothing and feeding preparatory responses. Biol Neonate. 1998 Dec;74(6):402–8. doi: 10.1159/000014061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Johnston CC, Filion F, Nuyt AM. Recorded maternal voice for preterm neonates undergoing heel lance. Adv Neonatal Care. 2007 Oct;7(5):258–66. doi: 10.1097/01.ANC.0000296634.26669.13. [DOI] [PubMed] [Google Scholar]
- 95.Feldman R, Eidelman AI. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev Med Child Neurol. 2003 Apr;45(4):274–81. doi: 10.1017/s0012162203000525. [DOI] [PubMed] [Google Scholar]
- 96.Ferber SG, Makhoul IR. Neurobehavioural assessment of skin-to-skin effects on reaction to pain in preterm infants: a randomized, controlled within-subject trial. Acta Paediatr. 2008 Feb;97(2):171–6. doi: 10.1111/j.1651-2227.2007.00607.x. [DOI] [PubMed] [Google Scholar]
- 97.Cong X. Kangaroo Care for Analgesia in Preterm Infants Undergoing Heel Stick Pain. Cleveland, OH: Case Western Reserve University; 2006. [Google Scholar]
- 98.Johnston CC, Filion F, Campbell-Yeo M, Goulet C, Bell L, McNaughton K, et al. Enhanced kangaroo mother care for heel lance in preterm neonates: a crossover trial. J Perinatol. 2009 Jan;29(1):51–6. doi: 10.1038/jp.2008.113. [DOI] [PubMed] [Google Scholar]


