Recent studies suggest that persistent intravascular haemolysis, a defining feature of sickle cell disease (SCD), is associated with severe long-term consequences, including pulmonary hypertension, which carries a 2-year mortality rate of up to 50% (Castro et al, 2003). Thus, the development of therapies that correct intravascular haemolysis and its complications are increasingly recognized as important for the long-term prognosis of the SCD patient. Haematopoietic stem cell transplantation (HSCT) is a potentially curative therapy for SCD, and recent improvements in supportive care and the development of reduced-intensity conditioning regimens have substantially lessened the severity of the immediate toxicities of the transplant procedure (Locatelli, 2006). When lower intensity conditioning regimens are utilized, host haematopoiesis is incompletely eliminated, and mixed haematopoietic chimerism frequently results. The effects of persistent recipient erythro-poiesis on intravascular haemolysis are unknown, but would be anticipated to perpetuate intravascular haemolysis. Several serum biomarkers have been recently identified to strongly correlate with endothelial damage, pulmonary hypertension and prospective early mortality in SCD patients. These include increased soluble vascular cellular adhesion molecule 1 (sVCAM-1) levels, increased nitric oxide (NO) consumption, increased plasma free haemoglobin (Hb), and inverted argi-nine/ornithine ratio (Solovey et al, 1997; Reiter et al, 2002; Morris et al, 2005). Using these parameters, we examined the potential of partial donor engraftment to correct intravascular haemolysis in nine SCD patients, and hence to potentially avert the development of long-term SCD-associated complications.
Of the 19 patients who underwent matched related donor HSCT for SCD across four transpalnt centres, each with a different conditioning regimen, nine developed mixed haematopoietic chimerism and were studied in detail. Heparinized blood was obtained from these nine patients upon enrolment onto Institutional Review Board-approved protocols. Overall mononuclear cell engraftment for all nine patients was measured by standard analysis of short tandem repeats from genomic DNA extracted from peripheral blood mononuclear cells. Indications for HSCT ranged from acute chest syndrome and strokes to intractable recurrent pain crises. As shown in Table I, patients 1–7 and 9 underwent nonmyeloablative transplant, while patient 8 underwent a fully myeloablative transplant. For patients 1–3, this period of partial donor engraftment, with levels ranging from 20% to 50%, lasted 5–11 months following HSCT. For patients 4–9, stable mixed chimerism was achieved with levels ranging from 42% to 90%. For patients 4–8, mixed chimerism was maintained even off immunosuppression. Patients 7 and 8 continue to stably demonstrate 55% and 42% peripheral blood donor engraftment, respectively, >4 years following transplant. Patient 9 has been continuously maintained on immunosuppression, and remains a mixed chimera. None of the subjects required red blood cell (RBC) transfusion beyond 1 month post-transplant unless they relapsed and none, while engrafted, experienced any SCD-related events.
Table I.
Patient clinical characteristics.
| Patient | Age (years) /sex |
Donor genotype |
Conditioning regimen |
Stem cell source |
GVHD prophylaxis |
Time to stopping of immunosuppression/ to graft loss (months) |
Time of post-transplant sampling (months) |
% donor engraftment at time of post-transplant sampling |
|
|---|---|---|---|---|---|---|---|---|---|
| Overall PBMC* | RBC specific | ||||||||
| 1 | 34/F | AS | Flu/Bu† | PBSC | FK506/pred | 7/7 | 6 | 25 | 66 |
| 2 | 52/M | AS | Flu/Bu† | PBSC | FK506/pred | 11/11 | 8 | 50 | 100 |
| 3 | 27/M | AS | Campath/TBI¶ | PBSC | Sirolimus | 6/5 | 3 | 20 | Not available |
| 4 | 5/M | AS | Flu/Bus/ATG/TLI‡ | BM | CsA/MMF | 6/NA | 18 | 50 | 100 |
| 5 | 8/M | A/βthal | Flu/Bus/ATG/TLI‡ | BM | CsA/MMF | 11/NA | 6 | 85 | 100 |
| 6 | 18/F | AS | Flu/Bus/ATG/TLI‡ | BM | CsA/MMF | 7.5/NA | 6 | 90 | 100 |
| 7 | 8/M | AA | Flu/Bus/ATG/TLI‡ | BM | CsA/MMF | 10/NA | 48 | 55 | 100 |
| 8 | 8/M | AS | Bu/Cy/ATG§ | BM | CsA/MTX | 7/NA | 42 | 42 | 86 |
| 9 | 24/F | AA | Campath/TBI¶ | PBSC | Sirolimus | Not discontinued/NA | 12 | 52 | 100 |
Overall mononuclear cell chimerism was measured by analysis of short tandem repeat regions from genomic DNA obtained from peripheral blood mononuclear cells (PBMC).
PBSC, peripheral blood derived stem cells; BM, bone marrow; RBC, red blood cells; MMF, mycophenolate mofetil; MTX, methotrexate; CsA, cyclosporine A; NA, not applicable.
Fludarabine 30 mg/m2 × 4 d; Busulfex 3.2 mg/kg.
Fludarabine 30 mg/m2 × 4 d; Busulfan 6.4 mg/kg; antithymocyte globulin 30 mg/kg/dose IV daily × 5 d and total lymphoid irradiation 500 cGy with shielding of the liver, lungs, heart and gonads.
Busulfan 11.2 mg/kg; Cyclophosphamide 200 mg/kg; Equine antithymocyte globulin 90 mg/kg.
CAMPATH 1 mg/kg total dose; total body irradiation 200–300 cGy.
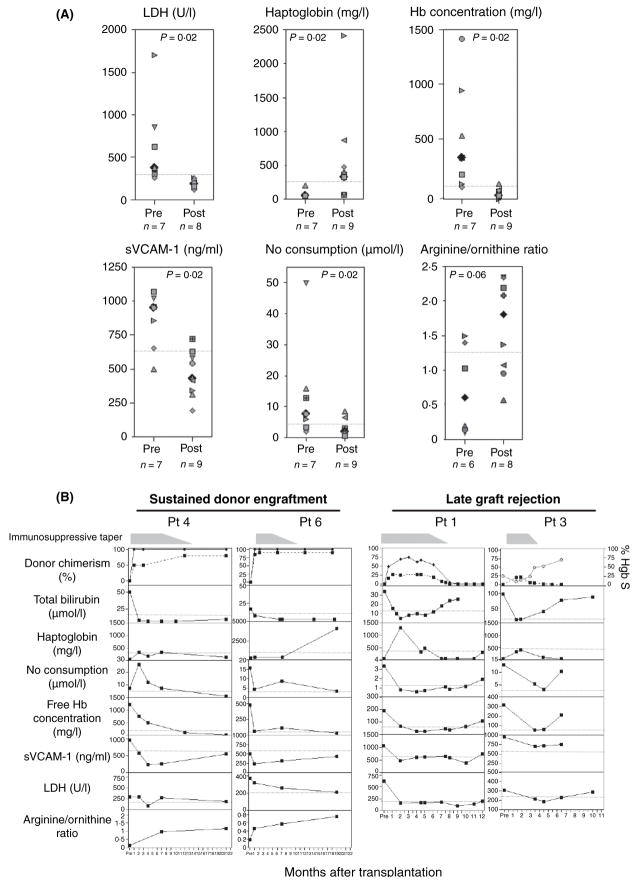
Despite differences in the conditioning regimens, all patients similarly demonstrated dramatic improvements in parameters of intravascular haemolysis while partially donor-engrafted. The median pretransplant values for indices of intravascular haemolysis of our cohort were elevated, similar to the general SCD population (Reiter et al, 2002). Following transplantation, significant normalization of these parameters was observed for each patient, to levels similar to normal donors. As shown in Fig 1A, lactate dehydrogenase (LDH) and free Hb concentration decreased from a median of 375 U/l (range: 255–1699) to 184 U/l (range: 122–260; P = 0.02), and from 19.7 μmol/l (range 5.5–75.9) to 1.6 μmol/l (range: 0.2–7.3; P = 0.02) respectively. Haptoglobin increased from a median value of 50 mg/l (range: 50–200) to 330 mg/l (range: 70–2420; P = 0.02).
Fig 1.
(A) Pre- and post-transplant plasma values for haptoglobin; LDH; free Hb concentration; nitric oxide (NO) consumption; sVCAM-1; and arginine/ornithine ratio. Median levels for each parameter are represented by the black filled diamonds. The dotted line represents the upper limit of the range of normal for each parameter. For each parameter, at least two post-transplant samples, obtained at the time of mixed chimerism, were measured per patient, and each symbol represents the mean of two or more measurements per patient. Plasma haptoglobin was measured immunonephelometrically (Behring Nephelometer II, Newark, DE, USA). Total bilirubin (panel B) and LDH were measured using a Hitachi Autoanalyser (Roche Diagnostics, Indianapolis, IN, USA). Plasma arginine and ornithine were measured by ion exchange chromatography (Hitachi Amino Acid Analyser L-8800; San Jose, CA, USA). Plasma sVCAM-1 levels were measured by ELISA (R&D Systems, Minneapolis, MN, USA). Plasma free haemoglobin and NO consumption were measured as previously described (Reiter et al, 2002). For all parameters, upper range of normal was defined as two standard deviations above the average of values obtained from 30 normal donors. On the seven of nine patients for whom both pre- and postsamples were available, paired analysis using the two-sided Wilcoxon signed-rank test was performed. (B) Serial plasma measurements of parameters of intravascular haemolysis and endothelial function following HSCT in the setting of sustained donor engraftment following immunosuppressive taper (patients 4 and 6), and late graft rejection (patients 1 and 3). Shown are measurements of donor chimerism (overall donor chimerism denoted by the dashed line; erythroid lineage specific chimerism denoted by the solid line); total bilirubin; haptoglobin; NO consumption; free Hb concentration; sVCAM-1; LDH and arginine/ornithine ratio (only collected from patients 4 and 6) following HSCT. The fine dotted line represents the upper limit of the range of normal for each parameter. For patient 3, erythroid lineage-specific chimerism measurements were unavailable; the % HbS levels are shown, represented by grey circles and lines.
Parameters of vascular function post-transplant similarly improved. Plasma NO consumption and sVCAM-1 levels significantly decreased from a median of 7.6 μmol/l (range: 1.9–49.7) to 1.3 μmol/l (range: 0.6–8.6; P = 0.02), and 952.1 ng/ml (range: 498.2–1067) to 543.1 ng/ml (range: 193.9–724; P = 0.02) respectively. The median arginine/ornithine ratio increased from 0.6 (range: 0.1–1.5) to 1.8 (range: 0.6–2.3; P = 0.06) following HSCT.
The observed improvements in parameters of intravascular haemolysis and endothelial function were not related to presence of immunosuppression, which is routinely administered in the early post-transplant period as graft-vs.-host disease prophylaxis. Patients 4 and 6 maintained unchanged or improved levels of donor chimerism and extent of intravascular haemolysis and vascular function, both on and off immunosuppression (Fig 1B).
We had sufficient samples from two of the three patients who experienced graft rejection (patients 1 and 3) to analyse in detail the impact of absence or presence of donor erythropoiesis on intravascular haemolysis. Temporary normalization of these parameters occurred with the presence of donor haematopoiesis, with reversion to abnormal baseline values following loss of the donor graft (Fig 1B). Direct indices of intravascular haemolysis (haptoglobin and total bilirubin) were the first to revert to abnormal, coincident with loss of donor engraftment. Free Hb concentration and NO consumption subsequently rose, consistent with the loss of the haemoglobin binding and clearance capacity of haptoglobin. Finally, a few months after graft rejection, a slight rise in LDH was observed. These findings directly demonstrate the dependence upon donor cells to achieve improvement in intravascular haemolysis and potentially on vascular function.
To reconcile these results in the face of persistent recipient haematopoiesis, erythroid lineage-specific chimerism was analysed by RNA β-globin pyrosequencing. This method evaluates chimerism by quantitative sequencing of donor vs. host-derived erythroid lineage transcripts, based on detection of the sickle mutation (Wu et al, 2005). Peripheral blood RBCs were fully replaced by donor-derived erythrocytes for patients with at least 50% donor nucleated cell engraftment (Table I). For patients with <50% engraftment, 70–85% donor RBCs expression was observed. Post-transplant donor RBC enrichment relative to the degree of nucleated cell engraftment is probably related to factors such as decreased survival of SS erythrocytes and intrinsic SCD-associated ineffective erythropoiesis (Kean et al, 2003; Wu et al, 2005). Fig 1B shows that normalization of the parameters of haemolysis and endothelial dysfunction was coincidental with expression of donor RBCs, thus directly demonstrating the beneficial effect of donor erythrocytes. Although patients 1, 3 and 8 continued to produce recipient RBCs, which would be expected to perpetuate intravascular haemolysis, this degree of haemolysis is apparently within the buffering capacity of the system. Supporting these findings, RBC exchange and hyper-transfusion that reduce peripheral blood %HbS to 20–30% are clinically protective (Swerdlow, 2006). Taken together, our data mechanistically support mixed chimerism as a suitable endpoint of stem cell-based therapies for SCD.
Acknowledgments
We thank Betty Leef, RN, Sandie Edwards and Phil Zorich, PhD for assistance in sample procurement. We thank Paul Armi-stead, MD, Mehrdad Mohseni, MD and Wandi Zhang for their help in sample processing. We thank Gregory Kato, MD and Carlo Brugnara, MD for their insightful discussions. Supported by NIH grants HL070149-01, and AI29530; and the Ted and Eileen Pasquarello Research Fund. Dr Wu is a recipient of a Doris Duke Clinical Scientist Development Award.
References
- Castro O, Hoque M, Brown BD. Pulmonary hypertension in sickle cell disease: cardiac catheterization results and survival. Blood. 2003;101:1257–1261. doi: 10.1182/blood-2002-03-0948. [DOI] [PubMed] [Google Scholar]
- Kean LS, Manci EA, Perry J, Balkan C, Coley S, Holtzclaw D, Adams AB, Larsen CP, Hsu LL, Archer DR. Chimerism and cure: hematologic and pathologic correction of murine sickle cell disease. Blood. 2003;102:4582–4593. doi: 10.1182/blood-2003-03-0712. [DOI] [PubMed] [Google Scholar]
- Locatelli F. Reduced-intensity regimens in allogeneic hemato-poietic stem cell transplantation for hemoglobinopathies. American Society of Hematology Education Program. 2006:398–401. doi: 10.1182/asheducation-2006.1.398. [DOI] [PubMed] [Google Scholar]
- Morris CR, Kato GJ, Poljakovic M, Wang X, Blackwelder WC, Sachdev V, Hazen SL, Vichinsky EP, Morris SM, Jr, Gladwin MT. Dysregulated arginine metabolism, hemolysis-associated pulmonary hypertension, and mortality in sickle cell disease. JAMA. 2005;294:81–90. doi: 10.1001/jama.294.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter CD, Wang X, Tanus-Santos JE, Hogg N, Cannon RO, III, Schechter AN, Gladwin MT. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nature Medicine. 2002;8:1383–1389. doi: 10.1038/nm1202-799. [DOI] [PubMed] [Google Scholar]
- Solovey A, Lin Y, Browne P, Choong S, Wayner E, Hebbel RP. Circulating activated endothelial cells in sickle cell anemia. New England Journal of Medicine. 1997;337:1584–1590. doi: 10.1056/NEJM199711273372203. [DOI] [PubMed] [Google Scholar]
- Swerdlow PS. Red cell exchange in sickle cell disease. American Society of Hematology Education Program. 2006:48–53. doi: 10.1182/asheducation-2006.1.48. [DOI] [PubMed] [Google Scholar]
- Wu CJ, Krishnamurti L, Kutok JL, Biernacki M, Rogers SA, Zhang W, Antin JH, Ritz J. Evidence for ineffective erythropoiesis in severe sickle cell disease. Blood. 2005;106:1–7. doi: 10.1182/blood-2005-04-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]