Abstract
The agmatine deiminase system was identified in seven strains of mutans streptococci. Genes encoding the AgDS of Streptococcus rattus FA-1 were sequenced and found to share homology with the agu genes of Streptococcus mutans UA159. Agmatine inhibited bacterial growth, suggesting that the AgDS degrades a deleterious substance into useful compounds.
Keywords: acid tolerance, biofilm, caries
To persist in the oral cavity, bacteria must possess sophisticated strategies for coping with frequent changes in oxygen tension, pH, and nutrient source and availability (8). During the development of dental caries, repeated and sustained acidification of oral biofilms results in the increases in the proportions of organisms with a potent capacity to lower the pH through glycolysis and to grow and continue to metabolize carbohydrates at extreme pH values (9). As a counterbalance to the forces of acidification in oral biofilms, a variety of species have retained the capacity to produce alkali from salivary substrates, which appears to have a major impact on pH homeostasis and microbial ecology (2). The two primary sources of plaque ammonia production are through the hydrolysis of urea by urease enzymes or of arginine via the arginine deiminase system (ADS); and both of these systems are thought to play an integral role in caries prevention (2, 5, 10, 12, 13).
We recently described an ammonia-generating system in the cariogenic organism, Streptococcus mutans (7). The agmatine deiminase system (AgDS) is highly similar to the ADS and catabolizes agmatine to produce putrescine, ammonia and carbon dioxide, with the concomitant production of ATP (Fig. 1). As with the ADS of other plaque streptococci, the AgDS of S. mutans augments ΔpH and provides ATP, thereby contributing to acid tolerance. However, activity in the AgDS is generally lower than that in the ADS and ammonia production from agmatine in the oral cavity probably does not result in significant alkalinization of the environment, as does arginine catabolism via the ADS. Thus, while possession of the AgDS would benefit S. mutans, it is unlikely to have a positive effect on the persistence of acid sensitive species in the oral cavity, as does ammonia production from arginine or urea (2). Interestingly, agmatine is inhibitory to the growth of S. mutans and a functional AgDS is required for the optimal growth in the presence of agmatine, which is readily measurable in dental plaque samples (7). Thus, in addition to providing bioenergetic benefits, the AgDS of S. mutans detoxifies a growth-inhibitory substance that can be produced by some plaque bacteria (7). The AgDS is a potentially attractive target for anti-caries strategies, so additional information about the distribution and physiologic role of the system in cariogenic bacteria is needed.
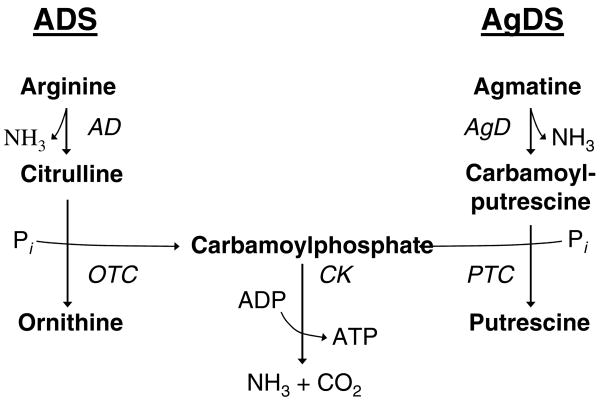
Fig. 1.
The AD and AgD pathways are highly analogous.
Identification of streptococcal aguA homologs
To assess the sequence similarity of the agmatine deiminase-encoding gene, aguA, among the streptococci, S. mutans aguA was used in Blast searches of the available streptococcal genomic sequences in the NCBI, TIGR and Sanger databases. Homologs were identified in the genomes of S. sobrinus (72% ID), Streptococcus uberis (46% ID), Streptococcus pneumoniae (45% ID), and Streptococcus mitis (44% ID), Lactobacillus salivarius (38%) and Lactobacillus brevis (54%). An aguA homolog was not found in the partial genome of S. gordonii, nor in the genomes of Streptococcus suis, Streptococcus thermophilis, Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus sanguinis, Streptococcus equi, or Streptococcus zooepidemicus. The genomic sequences of S. rattus, S. oralis, and S. salivarius are not currently available.
Measurement of AgD enzyme activity in oral streptococci
To determine if the AgDS was widely distributed among the oral streptococci, production of N-carbamoylputrescine from agmatine was assayed in S. mutans UA159, six related strains of mutans streptococci, and five strains of non-mutans streptococci, including Streptococcus salivarius, Streptococcus oralis, Streptococcus sanguinis genospecies 1 and 2, and Streptococcus gordonii. Bacterial strains were grown in TV medium (4) containing 25 mM glucose, galactose or sorbitol, with or without 10 mM agmatine, at 37°C in 5% CO2 and 95% air. AgD activity was measured by colorimetric determination of N-carbamoylputrescine production from agmatine using the method of Archibald (1). Chemical reagents were obtained from Sigma. Interestingly, AgD enzyme activity was detected in the non-mutans strains S. salivarius, S. oralis, and S. sanguinis genospecies 2 but not in S. gordonii or in S. sanguinis genospecies 1, even in the presence of non-repressing sugars (data not shown). The remainder of the study focused on AgD activity in mutans streptococci, many of which are associated directly associated with human dental caries.
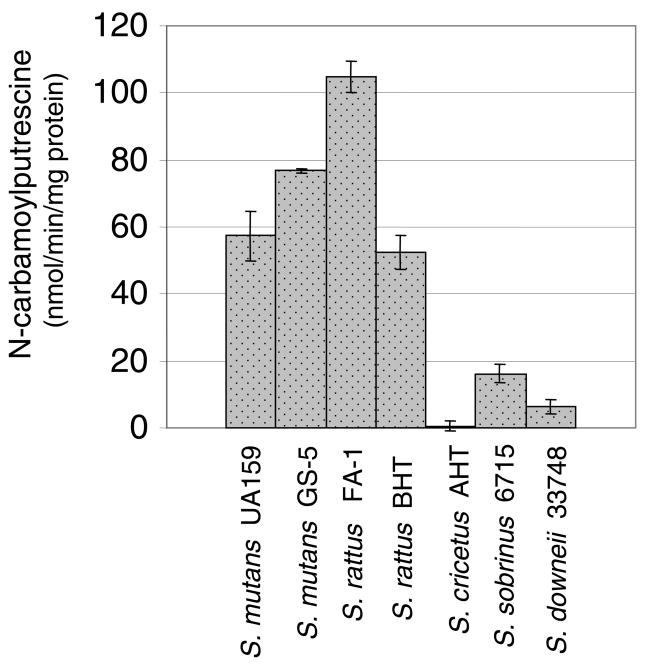
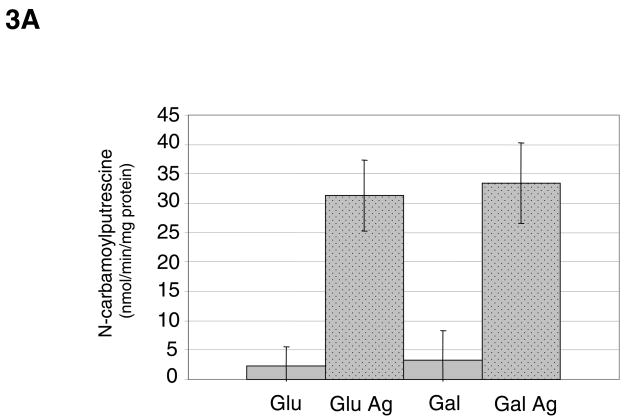
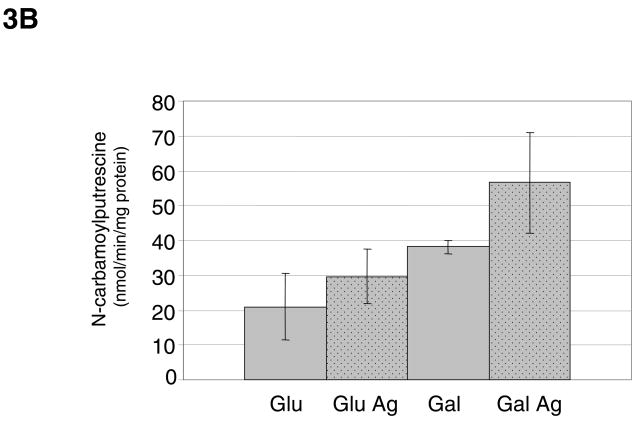
AgD activity was detected in each of the mutans streptococci, with the highest levels observed in S. mutans strains UA159 and GS-5, and S. rattus strains FA-1 and BHT (Fig. 2). As previously demonstrated with S. mutans, the AgDS in S. rattus was induced by agmatine (Fig. 3A). Unlike in S. mutans, carbohydrate catabolite repression (CCR) did not appear to regulate the S. rattus AgDS, as observed by comparing activity in cells grown in the repressing sugar glucose versus the poorly-repressing sugar galactose. However, when S. rattus was grown in 2% glucose, the effects of catabolite repression were apparent and began to mask the effects of agmatine induction (Fig. 3B), consistent with previous studies on the ADS of S. rattus (3, 6).
Fig. 2.
AgD enzyme activity in mutans streptococci grown in TV media containing 25 mM glucose and 10 mM agmatine. Results shown are the average and standard deviations (error bars) of a minimum of 9 separate cultures for each strain and condition.
Fig. 3.
(A) AgD enzyme activity of S. rattus FA-1 grown in TV medium containing 0.2 % glucose (Glu) or galactose (Gal), with or without the addition of 10 mM agmatine (Ag). Results shown are the average and standard deviations (error bars) of a minimum of 9 separate cultures for each strain and condition. (B) AgD enzyme activity of S. rattus FA-1 grown in TV medium containing 2% glucose or galactose, with or without 10 mM agmatine. Results shown are the average and standard deviations (error bars) of a minimum of 9 separate cultures for each strain and condition.
The AgDS of S. cricetus appeared to be tightly regulated by carbon catabolite repression, as enzyme activity was only measurable when the cells were grown in a non-repressing sugar, such as sorbitol (Table 2). Streptococcus sobrinus displayed low, albeit consistently detectable, AgD activity regardless of whether repressing or non-repressing carbohydrates were included in the growth medium (Fig. 2 and Table 2). Thus, the AgDS has been conserved among the mutans streptococci and, with the exception of S. sobrinus, appears to be sensitive to carbon catabolite repression.
Table 2.
AgD enzyme activity in selected oral streptococci grown in TV media containing 25 mM galactose or 25 mM sorbitol and 10 mM agmatine (Ag). Results shown are the average and standard deviations of a minimum of 9 separate cultures for each strain and condition. ND = Not determined.
| Strain: | TV Sorbitol + Ag | TV Galactose + Ag |
|---|---|---|
| S. mutans UA159 | 476.2 ± 120.3 | 170.0 ± 11.4 |
| S. sobrinus 6715 | 9.7 ± 2.0 | ND |
| S. cricetus AHT | 10.9 ± 3.7 | ND |
Sequencing of the S. rattus agu operon
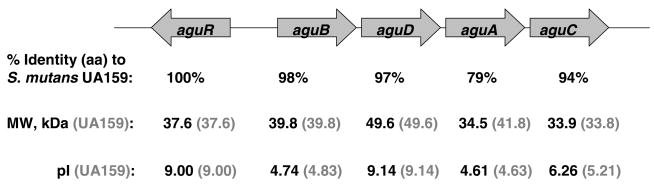
S. rattus is the closest genetic relative of S. mutans, yet this organism is poorly cariogenic in experimental animals. To examine the genetic relationships of the agu genes of mutans streptococci and to dissect the basis for differential sensitivity of the operons to CCR, the agu operon of S. rattus FA-1 was sequenced using the primers in Table 1. The S. rattus agu genes are organized in an aguRBDAC cluster and the predicted amino acid sequences are nearly identical to their counterparts in S. mutans (Fig. 5).
Table 1.
Primers used to sequence the S. rattus agu operon.
| Primer a | Sequence | Locus |
|---|---|---|
| 5-S | 5′-TATTTCCAATTTACGGGTGTTCT-3′ | aguR |
| 100-S | 5′-GTGAATGTGAGTTTTTACTGTGC-3′ | aguR |
| 560-S | 5′-TTGATTTGGTAGGTAATAGAGGT-3′ | aguR |
| 1050-S | 5′-GGCTTTGTAAAAAAGGCATAAAC-3′ | aguR-aguB |
| 1760-S | 5′-GAATGGAATTTGTTCACTTTGGA-3′ | aguB |
| 2300-S | 5′-CGCAATCATGTCTGTCCTAAAC-3′ | aguB-aguD |
| 3080-S | 5′-GCTTTTGGTATTGGCGTCTCA-3′ | aguD |
| 3540-S | 5′-CGTCCTTTTAAGGTTAGTGGC-3′ | aguD-aguA |
| 4260-S | 5′-GTGTCTGTTACATCTTAGTCGG-3′ | aguA |
| 4840-S | 5′-CAGTGAAAATGGAGAAAATGTATG-3′ | aguA-aguC |
| 5340-S | 5′-GAGGTTGGCGAAAAGTAGTTG-3′ | aguC |
| 560-AS | 5′-TACCTCTATTACCTACCAAATCAA-3′ | aguR |
| 1050-AS | 5′-GTTTATGCCTTTTTTACAAAGCC-33 | aguR |
| 1760-AS | 5′-GTCCAAAGTGAACAAATTCCA-3′ | aguR-aguB |
| 2300-AS | 5′-GTTTAGGACAGACATGATTGCG-3′ | aguB |
| 3080-AS | 5′-GAGACGCCAATACCAAAAGCA-3′ | aguB-aguD |
| 3540-AS | 5′-GCCACTAACCTTAAAAGGACG-3′ | aguD |
| 4260-AS | 5′-CCGACTAAGATGTAACAGACAC-3′ | aguD-aguA |
| 4840-AS | 5′-CATACATTTTCTCCATTTTCACTG-3′ | aguA |
| 5340-AS | 5′-CAACTACTTTTCGCCACCTC-3′ | aguA-aguC |
| 5780-AS | 5′-CCGTAATTTGTGTGCCACTTC-3′ | aguC |
Number denotes the position of the primer relative to the 5′ end of the agu operon.
Fig. 5.
The agu operon of S. rattus FA-1 and homology of each ORF to the corresponding gene in S. mutans UA159. The molecular weight and pI of each predicted AgD protein is shown for S. rattus (black) and S. mutans (parentheses, grey). AguR is a transcriptional activator of the agmatine deiminase operon in the presence of agmatine. The nucleotide sequences bear the GenBank accession number EF104920.
Similar to ADS enzymes, AguA shares the least similarity (79 %) between the two bacteria of enzymes in the AgDS, which may contribute in part to measured differences in the AgD activities in these organisms. The functional domains of AgD have not yet been identified, but the conserved [GGGNIHCITQQ] sequence (11) present in all known AgD enzymes was identified at the C-terminus of S. rattus AguA, as well as in the AguA sequences of S. sobrinus, S. uberis, S. pneumoniae, and S. mitis.
Growth inhibition by agmatine
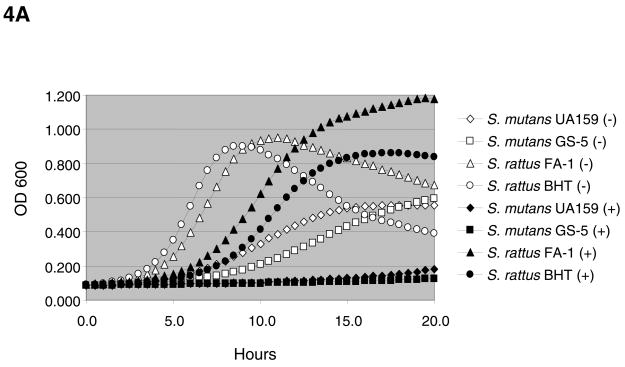
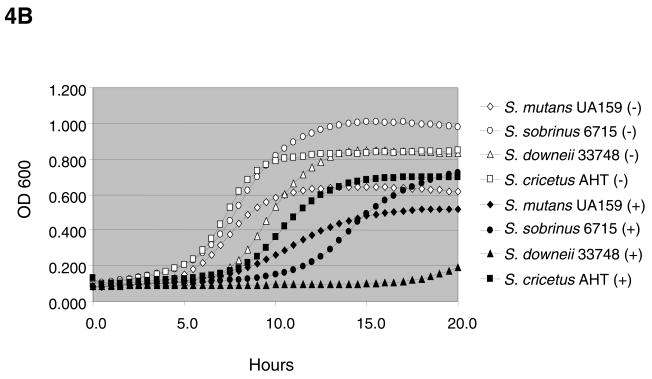
Consistent with data previously obtained with S. mutans UA159, the presence of 20 mM agmatine slowed the doubling times of all strains tested (Figs. 4A – 4B) with the exception of the non-mutans streptococci (data not shown). Enhanced yields, as measured by final OD, in the presence of agmatine and the non-preferred sugar, galactose, likely resulted from neutralization of the cytoplasm and environment by ammonia, coupled with increased ATP generation through the AgDS. Interestingly, S. rattus is the only mutans streptococci capable of generating ammonia via the ADS, in addition to the AgDS (2). We previously demonstrated that agmatine is present in biologically relevant amounts in dental plaque and saliva (0.75 μmol and 0.2 μmol of agmatine per mg of protein, respectively) (7). Thus, in addition to enhancing ammonia and energy production, the AgDS may function primarily to break down a growth-inhibitory component present in saliva and dental plaque.
Fig. 4.
(A) Growth of S. mutans and S. rattus strains in TV medium containing 25 mM galactose and 0 mM (open symbols) or 20 mM (closed symbols) agmatine. Standard deviations for data points shown were <0.02 for cultures grown in 0 mM agmatine and <0.06 for cultures grown in 20 mM agmatine. (B) Growth of selected mutans streptococci in TV medium containing 25 mM glucose and 0 mM (open symbols) or 20 mM (closed symbols) agmatine. Standard deviations for data points shown were <0.02 for cultures grown in 0 mM agmatine and <0.06 for cultures grown in 20 mM agmatine.
Summary
In the dental plaque biofilm, where the source and amount of nutrients is highly variable, organisms benefit from the ability to metabolize a wide variety of substrates. Here, we show that a system for agmatine utilization has been conserved among a genetically and physiologically diverse group of oral streptococci with distinct niches in oral biofilms. Thus, the ability to hydrolyze agmatine into ATP and ammonia may confer a significant selective advantage to bacterial species residing in the oral cavity.
References
- 1.Archibald RM. Determination of citrulline and allantoin and demonstration of citrulline in blood plasma. J Biol Chem. 1944;156:121–142. [Google Scholar]
- 2.Burne RA, Marquis RE. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 2000;193:1–6. doi: 10.1111/j.1574-6968.2000.tb09393.x. [DOI] [PubMed] [Google Scholar]
- 3.Burne RA, Parsons DT, Marquis RE. Environmental variables affecting arginine deiminase expression in oral streptococci. In: Dunny GM, McKay LL, editors. Genetics and molecular biology of streptococci, lactococci, and enterococci. Washington, DC: Am Soc Microbiol; 1991. pp. 276–280. [Google Scholar]
- 4.Burne RA, Wen ZT, Chen YY, Penders JE. Regulation of expression of the fructan hydrolase gene of Streptococcus mutans GS-5 by induction and carbon catabolite repression. J Bacteriol. 1999;181:2863–2871. doi: 10.1128/jb.181.9.2863-2871.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy KA, Pearson S, Bowen WH, Burne RA. Characterization of recombinant, ureolytic Streptococcus mutans demonstrates an inverse relationship between dental plaque ureolytic capacity and cariogenicity. Infect Immun. 2000;68:2621–2629. doi: 10.1128/iai.68.5.2621-2629.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griswold A, Chen YY, Snyder JA, Burne RA. Characterization of the arginine deiminase operon of Streptococcus rattus FA-1. Appl Environ Microbiol. 2004;70:1321–1327. doi: 10.1128/AEM.70.3.1321-1327.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Griswold AR, Jameson-Lee M, Burne RA. Regulation and physiologic significance of the agmatine deiminase system of Streptococcus mutans UA159. J Bacteriol. 2006;188:834–841. doi: 10.1128/JB.188.3.834-841.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemos JA, Abranches J, Burne RA. Responses of cariogenic streptococci to environmental stresses. Current Issues in Molecular Biology. 2005;7:95–107. [PubMed] [Google Scholar]
- 9.Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Margolis HC, Duckworth JH, Moreno EC. Composition and buffer capacity of pooled starved plaque fluid from caries-free and caries-susceptible individuals. J Dent Res. 1988;67:1476–1482. doi: 10.1177/00220345880670120701. [DOI] [PubMed] [Google Scholar]
- 11.Nakada Y, Jiang Y, Nishijyo T, Itoh Y, Lu CD. Molecular characterization and regulation of the aguBA operon, responsible for agmatine utilization in Pseudomonas aeruginosa PAO1. J Bacteriol. 2001;183:6517–6524. doi: 10.1128/JB.183.22.6517-6524.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Wuyckhuyse BC, Perinpanayagam HE, Bevacqua D, Raubertas RF, Billings RJ, Bowen WH, Tabak LA. Association of free arginine and lysine concentrations in human parotid saliva with caries experience. J Dent Res. 1995;74:686–690. doi: 10.1177/00220345950740021001. [DOI] [PubMed] [Google Scholar]
- 13.Wijeyeweera RL, Kleinberg I. Arginolytic and ureolytic activities of pure cultures of human oral bacteria and their effects on the pH response of salivary sediment and dental plaque in vitro. Arch Oral Biol. 1989;34:43–53. doi: 10.1016/0003-9969(89)90045-9. [DOI] [PubMed] [Google Scholar]