Abstract
Although highly active retroviral therapy (HAART) has been extremely effective in lowering AIDS incidence among patients infected with HIV, certain drugs included in HAART can cause serious mitochondrial toxicities. One of the most frequent adverse events is lipoatrophy, which is the loss of subcutaneous fat in the face, arms, buttocks and/or legs as an adverse reaction to nucleoside reverse transcriptase inhibitors (NRTIs). The clinical symptoms of lipoatrophy resemble those of inherited mitochondrial diseases, which suggests that host mitochondrial genotype may play a role in susceptibility. We analyzed the association between mitochondrial haplogroup and severity of lipoatrophy in HIV-infected European American patients on HAART in the Multicenter AIDS cohort Study (MACS) and found that mitochondrial haplogroup H was strongly associated with increased atrophy (arms: p = 0.007, OR = 1.77, 95% CI = 1.17–2.69 legs: p = 0.037, OR = 1.54 95% CI = 1.03–2.31, and buttocks: p = 0.10, OR = 1.41 95% CI = 0.94–2.12). We also saw borderline significance for haplogroup T as protective against lipoatrophy (p = 0.05, OR = 0.52, 95% CI = 0.20–1.00). These data suggest that mitochondrial DNA haplogroup may influence the propensity for lipoatrophy in patients receiving NRTIs.
Keywords: lipoatrophy, mitochondrial haplogroup, NRTI, mitochondrial toxicity
Introduction
Highly active anti-retroviral therapy (HAART) markedly decreases AIDS progression among HIV-infected individuals 1. However mitochondrial toxicity resulting from the use of specific antiretrovirals used as part of HAART has been linked to several adverse effects including lipodystrophy, peripheral neuropathy, hepatic steatosis, myopathy, cardiomyopathy, pancreatitis, bone-marrow suppression, and lactic acidosis 2–6. Nearly all of these adverse effects resemble clinical symptoms seen in inherited mitochondrial diseases 7, 8, suggesting that host mitochondrial genotype may play a role in their development. This hypothesis is supported by previous studies of patients receiving antiretroviral therapy for whom mitochondrial DNA haplogroup T was overrepresented among patients with peripheral neuropathy in the ACTG [AIDS Clinical Trials Group] cohort 9, 10, and in five patients with haplogroup J who had higher median limb fat change post-therapy compared to other haplogroups 11.
One of the most common clinical pathologies associated with HAART is lipoatrophy, a physically disfiguring mitochondriotoxicity that occurs in 13–63% of patients on HAART 12–17. Associated primarily with the thymidine analogue nucleoside reverse transcriptase inhibitors (NRTIs) zidovudine (AZT) and stavudine (d4T), lipoatrophy is the loss of subcutaneous fat from the face, extremities, and buttocks 18. The distinctive sunken cheeks and wasted appearance can have profound social and psychological impacts for affected persons and can lead to decreased therapy adherence 19–22. Lipoatrophy has also been observed as a prelude to other health risks such as hypertension 23 and coronary heart disease24.
Lipoatrophy is caused by a combination of cellular mechanisms including inhibition of mitochondrial gamma-polymerase, depleted mtDNA, acquired mtDNA mutations, and oxidative stress 8, 25–27. Because mitochondria are critical for energy production and for control of cellular apoptosis, disruption of mitochondrial processes has serious metabolic consequences. Through oxidative phosphorylation (OXPHOS), mitochondria convert calories to ATP, release heat that maintains body temperature, and generate reactive oxygen species (ROS). Many mitochondrial diseases occur when energy production drops below the energetic threshold for a given process in the cell 28–31. NRTI- induced mtDNA depletion disrupts OXPHOS, likely causing energy deprivation 32. Compromised ATP production in turn may lower fat production since ATP is needed for triglyceride synthesis in adipocytes 33. Further, mitochondrial perturbation and oxidative stress can result in the release of apoptosis-inducing factors causing apoptosis of adipocytes and consequent peripheral fat loss 26, 34.
The mitochondrial genome encodes thirteen proteins that participate in OXPHOS. Variation in these polypeptides may influence energy production efficiency, ROS generation, and levels of apoptosis 35. Mitochondrial variation has been associated with climate adaptation 28, 36, susceptibility to neurodegenerative disease 37–40, energy deficiency disease 41, 42, longevity 43–45, sperm motility 46, 47, sprint performance 48, and microbial infection 49. In a previous study, we also showed that specific mtDNA genotypes are associated with AIDS progression in untreated HIV-infected patients50.
Hence, we sought to determine whether the host mtDNA genotype was associated with propensity for development of lipoatrophy in HIV-infected patients on HAART in the Multicenter AIDS Cohort Study (MACS). We determined the mitochondrial DNA haplogroup of 410 male European American patients who had been assessed for lipoatrophy by clinical examination of fat in the limbs and buttocks, and investigated whether severity of fat loss was associated with mtDNA genotype.
Methods
Cohort
The Multicenter AIDS Cohort Study (MACS) is a United States- based ongoing prospective study of HIV-1 infection in adult (ages 18–70) men who have sex with men (MSM) in Baltimore, Chicago, Pittsburgh, and Los Angeles 51. This study focused on men who self-reported as “white”. White-hispanic men were not included due to different genetic background.
Details of atrophy assessment
The severity of peripheral atrophy was quantified by a standardized physical exam assessment scale which used mild, moderate and severe gradations for each of the affected body areas (arms, legs, face and buttocks). Mild was recorded for atrophic changes that were evident to the MACS clinician upon close inspection; moderate was recorded for changes that were evident without close inspection and severe atrophy was recorded for atrophic changes that were evident to a non-medical person by casual observation. Specific anatomic features noting presence of moderate or greater atrophy were prominence of the nasolabial folds, hollowing of the cheeks (“sunken cheeks”), peripheral venous prominence and apparent bony landmarks. Physical exams were performed during the HAART era between 1999 and 2006.
Genotyping
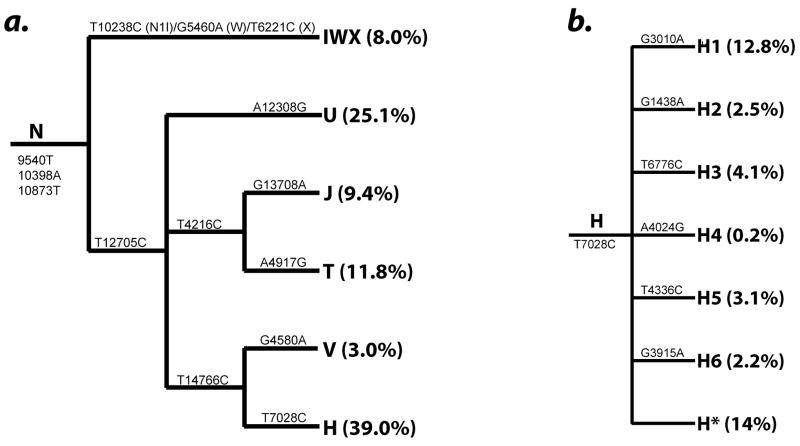
DNAs were extracted from immortal lymphoblastoid B cell lines for each patient. Six haplotype-tagging SNPs were used initially to classify individuals as mitochondrial macro-haplogroups N, M and L groups. Haplogroups within the Western European (N) subset were further parsed into haplogroups using SNPs in the Mitochondrial Haplogrouping using Candidate Functional Variants (MHCFV) approach as described in Hendrickson et al. (2008), and the subset used in this study to identify the major haplogroups and subgroups within H are shown in Figure 1. Based on the hierarchical nature of the human mtDNA tree we devised a strategy for identifying haplotypes by subdividing the samples using highly conserved polymorphic sites located at key haplogroup branch points. Genotyping was performed using TaqMan Assays-by-Design(SM). Thermocycling conditions were an initial 95°C hold for 3 minutes, followed by 30 cycles of 92°C for 15s, and 56°–62°C annealing for 1 minute depending on primer specificity. Haplogroups were compared against the remaining haplogroups in statistical analyses. Rare, loosely associated haplogroups R*, HV* and JT* were excluded from individual analyses but included in controls.
Figure 1.
Relationships between the European mtDNA haplogroups surveyed in this study and the SNPs used to identify them: (a.) Macro-haplogroup N, and (b.) a detail of the subhaplogroups within haplogroup H and the SNPs supporting each branch.
Statistical analysis
Associations between mitochondrial haplogroup and severity of lipoatrophy were assessed with proportional odds logistic regression (POLR) 52, 53. All analyses were performed with SAS version 9.1 (SAS Institute, Inc, Cary NC). Sensitivity appeared to vary between the measures of atrophy on arms, legs and buttocks; therefore we analyzed each lipoatrophy assessment separately. Further, we attempted an analysis of the average of these values; however the score test was significant suggesting it violated the model assumptions, likely due to small cell size in the uppermost levels of severity54. We used backward selection to test environmental variables to include in our POLR models. Age at HAART initiation (p = 0.03), BMI at the time of lipoatrophy assessment (p < 0.0001), and AZT and d4T use (both p < 0.0001) were all significant at the p ≤ 0.05. In a previous study, cumulative exposure to NRTIs was associated with decreases in BMI and body circumference over 5 years of follow-up among HIV-infected men in the MACS cohort 55, therefore we used a continuous variable to account to the number of visits (6 month intervals) prior to assessment at which a patient was taking either d4T or AZT.
Results
HIV-1 infected Caucasian men have an increased prevalence of lipoatrophy 56; therefore the European American men on HAART in the MACS represent a high-risk group. We successfully genotyped 536 patients who self identified as “white” and had a Western European, or “N”, mitochondrial macro-haplogroup. Individuals who had L or M macro-haplogroups (found in Africa and East Asia) were excluded from the study, and those within the N haplogroup were further parsed into N haplogroups H, T, IWX, J, T, V, and U. A complete clinical data set for all variables used in the final analyses of lipoatrophy and mitochondrial haplogroup association was available from 410 of these patients.
Clinical characteristics of study participants are shown in Table 1. Age at HAART initiation, BMI at the time of lipoatrophy assessment, and cumulative AZT and d4T exposure were all significantly associated with an increased incidence of lipoatrophy, consistent with previous studies33 (Table 2). Age was only strongly significant in for atrophy in the legs according to our models, but because of its known importance in previous studies, we included it in all models. We also evaluated whether tenofovir, which has been reported to cause lipodystrophy in a small percentage of patients 57, or nelfinavir were associated with lipoatrophy, but found no associations between their use and lipoatrophy in the MACS.
Table 1.
Basic characteristics of European American patients in the MACS cohort in this study. Clinical visits for each patient were approximately 6 months apart.
| Variable | Mean (sd) |
|---|---|
| Age at HAART | 43.8 (7.0) |
| Baseline BMI | 24.9 (3.5) |
| Baseline HIV-1 RNA (copies/mL) | 87507 (193956) |
| Baseline CD4 (cells/mL) | 365.5 (298.12) |
| AIDS prior to HAART | 20.0% |
| Weight1 (kg) | 78.8 (13.3) |
| Visits on AZT2 | 7.2 (6.3) |
| Visits on d4TD4T2 | 6.2 (5.6) |
| Total Visits2 | 32.0 (7.5) |
at time of atrophy assessment
prior to assessment visit
sd, standard deviation.
Table 2.
Odds ratios (OR), 95% confidence interval, p-values (p), and population frequency for covariates included in the mtDNA haplogroup models for severity of lipoatrophy in three anatomical sites.
| mtDNA haplogroup | Buttocks |
Legs |
Arms |
|||
|---|---|---|---|---|---|---|
| p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | |
| Age | 0.17 | 1.02 (0.99–1.05) | 0.03 | 1.03 (1.00–1.06) | 0.06 | 1.03 (1.00–1.06) |
| AZT visits | <0.0001 | 1.06 (1.03–1.09) | <0.0001 | 1.07 (1.04–1.10) | <0.0001 | 1.07 (1.04–1.10) |
| D4T visits | <0.0001 | 1.07 (1.04–1.10) | <0.0001 | 1.08 (1.04–1.11) | 0.001 | 1.05 (1.02–1.09) |
| BMI | <0.0001 | 0.44 (0.33–0.59) | <0.0001 | 0.52 (0.40–0.68) | <0.0001 | 0.41 (0.30–0.55) |
All tests were done using Proportional Odds Logistic Regression (POLR) models with lipoatrophy scored as 0=no atrophy, 1=mild, 2=moderate, and 3=severe. BMI at the time of the assessment was scored as underweight(<18.5), normal (≥18.5 to <24.9), overweight (24.9<BMI<29.9), and obese (BMI ≥29.9).
Mitochondrial haplogroup H was strongly associated with significant increases in extremity lipoatrophy (arms: p = 0.007, OR = 1.77, 95% CI = 1.17–2.69; legs: p = 0.03, OR = 1.54, 95% CI = 1.03–2.31) (Table 3). We also observed a trend for increased lipoatrophy in the closely related V haplogroup (p = 0.07 OR = 2.59, 95% CI = 0.93–7.26). The phylogenetic tree of the major haplogroups is shown in Figure 1. In contrast, weak significance suggesting a protective effect against lipoatrophy were observed with haplogroup T (p = 0.05). No significant associations were observed for haplogroup J, which is closely related to T, however, odds ratios were consistently protective.
Table 3.
Odds ratios (OR), 95% confidence interval, p-values (p), and population frequency for major European mtDNA haplogroups with severity of lipoatrophy in three anatomical sites.
| mtDNA haplogroup | Freq(%) | Buttocks |
Legs |
Arms |
|||
|---|---|---|---|---|---|---|---|
| p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | ||
| IWX | 8.0 | 0.10† | 1.75 (0.89–3.42) | 0.38 | 1.35 (0.69–2.65) | 0.51 | 1.27 (0.63–2.55) |
| U | 25.1 | 0.33 | 0.79 (0.49–1.27) | 0.40 | 0.82 (0.52–1.30) | 0.14 | 0.69 (0.42–1.12) |
| H | 39.0 | 0.10 | 1.41 (0.94–2.12) | 0.03 | 1.54 (1.03–2.31) | 0.007** | 1.77 (1.17–2.69) |
| V | 3.0 | 0.07 | 2.59 (0.93–7.26) | 0.13 | 2.2 (0.79–6.22) | 0.23 | 1.94 (0.66–5.66) |
| J | 9.4 | 0.52 | 0.80 (0.40–1.59) | 0.43† | 0.76 (0.39–1.50) | 0.30 | 0.68 (0.33–1.41) |
| T | 11.8 | 0.05 | 0.52 (0.2–1.00) | 0.12 | 0.61 (0.33–1.14) | 0.43 | 0.77 (0.41–1.46) |
All tests were done using Proportional Odds Logistic Regression (POLR) models with lipoatrophy scored as 0=no atrophy, 1=mild, 2=moderate, and 3=severe. The age at of the patients at the beginning of treatment, BMI at the time of the assessment [scored as underweight(<18.5), normal (≥18.5 to <24.9), overweight (24.9<BMI<29.9), and obese (BMI ≥29.9)], and number of visits with AZT and d4T use were included as covariates in the model. N=410. Rare, Loosely associated haplotypes R*, HV* and JT* were excluded from individual analysis and therefore the total of frequencies shown is 96.3%. Likewise, NIb, W, and X were not analyzed in individual analyses due to low sample size.
POLR model had a significant score test, which suggests the model assumptions were violated, likely due to small cell size.
Indicates p-value remained significant when BMI was removed from the POLR model.
Because BMI is a confounding variable during atrophy assessment but is also biologically related to atrophy, we repeated the analysis without BMI as a covariate in the model. Results were generally the same but with slightly weaker associations observed. The association between haplogroup H and increased arm atrophy remained significant (p = 0.021, OR = 1.60, 95% CI = 1.07–2.38), but associations with buttock and leg lipoatrophy became non-significant (p-values of 0.15 and 0.08 respectively). The association between haplogroup T and buttock lipoatrophy diminished to borderline significance (p = 0.07). All other results were non-significant.
Haplogroup H is composed of 6 distinct subhaplogroups (H1-H6) which are separated by SNPs 3010 G>A in 16S rRNA (non-coding), 1438 A > G in the 12S gene (consensus), 6776 C > T in the Cytochrome Oxidase I (synonymous), 4024G > A in ND1 (T240A), 4336 C > T in TQ (tRNA), and 3915 A > G in NDI (synonymous) as shown in Figure 1b. The haplogroups defined by 3010, 4336, and the H* (the remaining unclassified H mtDNA) haplogroup demonstrated significant associations with lipoatrophy in the same direction as the H haplogroup. The other haplogroups occurred infrequently (< 4%) in our sample; therefore any lack of association may simply be a consequence of their low prevalence and lack of power (for genotypes with frequency ~4%, power is only 13% for a OR=1.5 at α=0.05).
Lipoatrophy and lipo-accumulation may arise via different mechanisms because they represent extremes in metabolism and are often independent 22. In our patients, the presence lipo-accumulation in the back of the neck, known as a dorsocervical fat pad or “buffalo hump”, was correlated with lipoatrophy with a Pearson correlation coefficient of 0.2 (p < 0.0001). We saw a trend for T to be protective against the presence buffalo hump (p = 0.06, OR = 0.30, 95% CI = 0.09–1.04), but no other statistically significant associations were observed.
Discussion
We examined the genetic association between six major European mitochondrial DNA haplogroups and clinical severity of lipoatrophy in 410 patients receiving HAART in the MACS cohort. We found a significant association between haplogroup H and increased risk for lipoatrophy among men graded on lipoatrophy presence and severity in legs, arms and buttocks. We also observed a borderline significant association between the presence of haplogroup T and protection against lipoatrophy. In the context of previous studies of fat accumulation conducted in the ACTG cohort, we did not observe a significant association between the presence of haplogroup J and protection against fat loss as reported by Hulgan et al. 2008; however odds ratios in our study suggest J may be protective against lipoatrophy.
We recently investigated the relationship between mitochondrial haplogroups and rate of progression to AIDS in untreated patients infected with HIV where we observed an association between the J haplogroup and accelerated AIDS progression, as well as associations between certain U haplogroups and IWX and progression to disease 50. Although the effects of HIV-virus and drugs would likely influence mitochondrial function through different mechanisms, it was important to determine whether mtDNA haplogroup risk associated with disease progression in untreated patients were later affiliated with adverse events in patients on HAART. The data in the present study suggest that risk associated in untreated patients is not a factor in risk for lipodystrophy in patients on NRTIs. In patients on HAART in the present study, no significant associations were found between J and lipoatrophy despite the strong association between J and accelerated AIDS progression in untreated patients. Further, in the present study, we found a strong associations between haplogroup H and increased risk of lipoatrophy, however, we saw no over-all association between haplogroup H and progression in untreated patients, and one group within H (H3, which contains a mutation 6776 C>T) was found to be protective against AIDS progression and death in transfusion patients. These data suggest the risk of drug-toxicities associated with different mitochondrial haplogroups in patients on NRTIs is not effected by and independent of associations between mitochondrial haplogroups and disease progression in untreated patients.
Although the mechanism by which lipoatrophy develops during NRTI exposure has not been elucidated, one of the proposed mechanisms is that the release of apoptosis-inducing factors by damaged mitochondria cause apoptosis of adipocytes and lead to peripheral fat loss 26, 34. Variation in mtDNA among haplogroups may influence energy production efficiency, ROS generation, and levels of apoptosis. H in particular is thought to be tightly coupled to the production of ATP and consequently more ROS, while J and T are partially uncoupled, thus produce less ATP and therefore less ROS 35. In patients with the H haplogroup, oxidative stress and subsequent apoptosis could be exacerbated by increased baseline ROS production compared to other mitochondrial haplogroups. Further, oxidative stress has also been proposed as a principle mechanism operative in NRTI-related mitochondrial toxicity that leads to mtDNA depletion and subsequent mitochondrial energetic deficiencies 27. Again, the threshold effect of increased ROS production in the tightly coupled H haplogroup may worsen this effect. On the other hand, haplogroups J and T, which are uncoupled, had less atrophy, consistent with this hypothesis. However, the consistency in the protection of the T haplotype against both lipoatrophy and the presence of “buffalo hump” (p=0.07) appears to support the hypothesis that fat loss and accumulation are part of a single syndrome 58, 59, and a better understanding of the pathology of NRTI-related lipodystrophy, as well as additional genetic studies are needed to elucidate a mechanistic relationship between mitochondrial haplogroup and altered fat distribution.
One other potential explanation for the observed associations involves consideration of the strong phylo-geographic structure of mitochondrial haplogroups. Because of this phenomenon, it is possible that the associations observed in our study are correlated with background nuclear genetic effects that are distinctive between geographically separated populations. However, population stratification analysis using 304 autosomal markers in a previous study50, and ongoing work in our lab based on a genome scan did not reveal significant geographic structure in the mitochondrial haplogroups associated with lipoatrophy. Regardless, it will be important to repeat these associations in populations of different ethnic background.
Our results would no longer be significant if a conservative Bonferoni correction was performed, however we did see more significant associations than would be expected by chance, which implies that the mitochondrial genotype has at least a moderate effect on lipoatrophy. We also realize that lipoatrophy is difficult to assess, and therefore the results could potentially mask a stronger relationship. An indication that this may be the case is the very strong association between low BMI and severe atrophy. This inverse correlation may reflect the slowed diagnosis of moderate to severe atrophy in those with very high BMIs and hence very high peripheral fat depots.
Although many mechanisms are likely involved in the development mitochondrial dysfunction and subsequent lipoatrophy, this study demonstrates that mitochondrial haplogroup may be an important genetic factor in the development of lipoatrophy associated with NRTI treatment in HIV-infected persons.
Acknowledgments
The Multicenter AIDS Cohort Study (MACS) includes the following: Baltimore: The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (Principal Investigator), Haroutune Armenian, Barbara Crain, Adrian Dobs, Homayoon Farzadegan, Joel Gallant, John Hylton, Lisette Johnson, Shenghan Lai, Ned Sacktor, Ola Selnes, James Shepard, Chloe Thio. Chicago: Howard Brown Health Center, Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: John P. Phair (Principal Investigator), Joan S. Chmiel (Co-Principal Investigator), Sheila Badri, Bruce Cohen, Craig Conover, Maurice O’Gorman, David Ostrow, Frank Palella, Daina Variakojis, Steven M. Wolinsky. Los Angeles: University of California, UCLA Schools of Public Health and Medicine: Roger Detels (Principal Investigator), Barbara R. Visscher (Co-Principal Investigator), Aaron Aronow, Robert Bolan, Elizabeth Breen, Anthony Butch, Thomas Coates, Rita Effros, John Fahey, Beth Jamieson, Otoniel Martínez-Maza, Eric N. Miller, John Oishi, Paul Satz, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang.
Pittsburgh: University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (Principal Investigator), Lawrence Kingsley (Co-Principal Investigator), James T. Becker, Robert L. Cook, Robert W. Evans, John Mellors, Sharon Riddler, Anthony Silvestre. Data Coordinating Center: The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (Principal Investigator), Alvaro Muñoz (Co-Principal Investigator), Keri Althoff, Christopher Cox, Gypsyamber D’Souza, Stephen J. Gange, Elizabeth Golub, Janet Schollenberger, Eric C. Seaberg, Sol Su. NIH: National Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez; National Heart, Lung and Blood Institute: Cheryl McDonald. Website located at http://www.statepi.jhsph.edu/macs/macs.html. We also thank Mike Malasky and Mary McNally of the Laboratory of Genomic Diversity Genotyping Core and Holli Hutcheson who was involved in the initial genotyping of patients.
Sources of support: MACS work was supported by NIH grants UO1-AI-35042, 5-MO1-RR-00722 (GCRC), UO1-AI-35043, UO1-AI-37984, UO1-AI-35039, UO1-AI-35040, UO1-AI-37613, UO1-AI-35041 and M01 RR00425 (National Center for Research Resources grant awarded to the GCRC at Harbor-UCLA Research and Education Institute). Mitochondrial work was also supported by NIH Grants AG24373, DK73691, NS21328, AG16573 awarded to DCW and postdoctoral fellowship grant AG25638 awarded to JP and Spanish Fondo de Investigacion Sanitaria grants FIS-PI08-0264 and DGA-PM-083/2008 (ER-P).
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Kohler JJ, Lewis W. A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environ Mol Mutagen. 2007 Apr-May;48(3–4):166–172. doi: 10.1002/em.20223. [DOI] [PubMed] [Google Scholar]
- 3.Lewis W. Nucleoside reverse transcriptase inhibitors, mitochondrial DNA and AIDS therapy. Antivir Ther. 2005;10 Suppl 2:M13–27. [PubMed] [Google Scholar]
- 4.Lewis W, Kohler JJ, Hosseini SH, et al. Antiretroviral nucleosides, deoxynucleotide carrier and mitochondrial DNA: evidence supporting the DNA pol gamma hypothesis. AIDS. 2006 Mar 21;20(5):675–684. doi: 10.1097/01.aids.0000216367.23325.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinkman K, Smeitink JA, Romijn JA, Reiss P. Mitochondrial toxicity induced by nucleoside-analogue reverse-transcriptase inhibitors is a key factor in the pathogenesis of antiretroviral-therapy-related lipodystrophy. Lancet. 1999 Sep 25;354(9184):1112–1115. doi: 10.1016/S0140-6736(99)06102-4. [DOI] [PubMed] [Google Scholar]
- 6.Chapplain JM, Beillot J, Begue JM, et al. Mitochondrial abnormalities in HIV-infected lipoatrophic patients treated with antiretroviral agents. J Acquir Immune Defic Syndr. 2004 Dec 1;37(4):1477–1488. doi: 10.1097/01.qai.0000138982.68106.6c. [DOI] [PubMed] [Google Scholar]
- 7.Brinkman K, ter Hofstede HJ, Burger DM, Smeitink JA, Koopmans PP. Adverse effects of reverse transcriptase inhibitors: mitochondrial toxicity as common pathway. Aids. 1998 Oct 1;12(14):1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lewis W, Dalakas MC. Mitochondrial toxicity of antiviral drugs. Nat Med. 1995 May;1(5):417–422. doi: 10.1038/nm0595-417. [DOI] [PubMed] [Google Scholar]
- 9.Hulgan T, Haas DW, Haines JL, et al. Mitochondrial haplogroups and peripheral neuropathy during antiretroviral therapy: an adult AIDS clinical trials group study. Aids. 2005 Sep 2;19(13):1341–1349. doi: 10.1097/01.aids.0000180786.02930.a1. [DOI] [PubMed] [Google Scholar]
- 10.Canter JA, Haas DW, Kallianpur AR, et al. The mitochondrial pharmacogenomics of haplogroup T: MTND2*LHON4917G and antiretroviral therapy-associated peripheral neuropathy. Pharmacogenomics J. 2008 Feb;8(1):71–77. doi: 10.1038/sj.tpj.6500470. [DOI] [PubMed] [Google Scholar]
- 11.Hulgan T, Tebas P, Canter JA, et al. Hemochromatosis gene polymorphisms, mitochondrial haplogroups, and peripheral lipoatrophy during antiretroviral therapy. J Infect Dis. 2008 Mar 15;197(6):858–866. doi: 10.1086/528697. [DOI] [PubMed] [Google Scholar]
- 12.Saint-Marc T, Partisani M, Poizot-Martin I, et al. A syndrome of peripheral fat wasting (lipodystrophy) in patients receiving long-term nucleoside analogue therapy. AIDS. 1999 Sep 10;13(13):1659–1667. doi: 10.1097/00002030-199909100-00009. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson DL, Knox T, Spiegelman D, Skinner S, Gorbach S, Wanke C. Prevalence of, evolution of, and risk factors for fat atrophy and fat deposition in a cohort of HIV-infected men and women. Clin Infect Dis. 2005 Jun 15;40(12):1837–1845. doi: 10.1086/430379. [DOI] [PubMed] [Google Scholar]
- 14.Miller J, Carr A, Emery S, et al. HIV lipodystrophy: prevalence, severity and correlates of risk in Australia. HIV Med. 2003 Jul;4(3):293–301. doi: 10.1046/j.1468-1293.2003.00159.x. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein KA, Ward DJ, Moorman AC, et al. Clinical assessment of HIV-associated lipodystrophy in an ambulatory population. AIDS. 2001 Jul 27;15(11):1389–1398. doi: 10.1097/00002030-200107270-00008. [DOI] [PubMed] [Google Scholar]
- 16.Palella FJ, Jr, Cole SR, Chmiel JS, et al. Anthropometrics and examiner-reported body habitus abnormalities in the multicenter AIDS cohort study. Clin Infect Dis. 2004 Mar 15;38(6):903–907. doi: 10.1086/381684. [DOI] [PubMed] [Google Scholar]
- 17.Villarroya F, Domingo P, Giralt M. Lipodystrophy in HIV 1-infected patients: lessons for obesity research. Int J Obes (Lond) 2007 Dec;31(12):1763–1776. doi: 10.1038/sj.ijo.0803698. [DOI] [PubMed] [Google Scholar]
- 18.Calmy A, Hirschel B, Cooper DA, Carr A. Clinical update: adverse effects of antiretroviral therapy. Lancet. 2007 Jul 7;370(9581):12–14. doi: 10.1016/S0140-6736(07)61027-7. [DOI] [PubMed] [Google Scholar]
- 19.Golin C, Isasi F, Bontempi JB, Eng E. Secret pills: HIV-positive patients’ experiences taking antiretroviral therapy in North Carolina. AIDS Educ Prev. 2002 Aug;14(4):318–329. doi: 10.1521/aeap.14.5.318.23870. [DOI] [PubMed] [Google Scholar]
- 20.Rintamaki LS, Davis TC, Skripkauskas S, Bennett CL, Wolf MS. Social stigma concerns and HIV medication adherence. AIDS Patient Care STDS. 2006 May;20(5):359–368. doi: 10.1089/apc.2006.20.359. [DOI] [PubMed] [Google Scholar]
- 21.Duran S, Saves M, Spire B, et al. Failure to maintain long-term adherence to highly active antiretroviral therapy: the role of lipodystrophy. AIDS. 2001 Dec 7;15(18):2441–2444. doi: 10.1097/00002030-200112070-00012. [DOI] [PubMed] [Google Scholar]
- 22.Kingsley LA. Body habitus changes in HIV-associated lipodystrophy syndrome (HIV-LS). IAPAC sessions 2001, July 18–19, 2001 - Chicago. IAPAC Mon. 2001 Aug;7(8):246–250. [PubMed] [Google Scholar]
- 23.Sattler FR, Qian D, Louie S, et al. Elevated blood pressure in subjects with lipodystrophy. AIDS. 2001 Oct 19;15(15):2001–2010. doi: 10.1097/00002030-200110190-00013. [DOI] [PubMed] [Google Scholar]
- 24.Hadigan C, Meigs JB, Corcoran C, et al. Metabolic abnormalities and cardiovascular disease risk factors in adults with human immunodeficiency virus infection and lipodystrophy. Clin Infect Dis. 2001 Jan;32(1):130–139. doi: 10.1086/317541. [DOI] [PubMed] [Google Scholar]
- 25.Martin AM, Hammond E, Nolan D, et al. Accumulation of mitochondrial DNA mutations in human immunodeficiency virus-infected patients treated with nucleoside-analogue reverse-transcriptase inhibitors. Am J Hum Genet. 2003 Mar;72(3):549–560. doi: 10.1086/367849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kakuda TN, Brundage RC, Anderson PL, Fletcher CV. Nucleoside reverse transcriptase inhibitor-induced mitochondrial toxicity as an etiology for lipodystrophy. AIDS. 1999 Nov 12;13(16):2311–2312. doi: 10.1097/00002030-199911120-00019. [DOI] [PubMed] [Google Scholar]
- 27.Lewis W, Day BJ, Copeland WC. Mitochondrial toxicity of NRTI antiviral drugs: an integrated cellular perspective. Nat Rev Drug Discov. 2003 Oct;2(10):812–822. doi: 10.1038/nrd1201. [DOI] [PubMed] [Google Scholar]
- 28.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet. 2005;39:359–407. doi: 10.1146/annurev.genet.39.110304.095751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace DC. Diseases of the mitochondrial DNA. Annu Rev Biochem. 1992;61:1175–1212. doi: 10.1146/annurev.bi.61.070192.005523. [DOI] [PubMed] [Google Scholar]
- 30.Johns DR. The other human genome: mitochondrial DNA and disease. Nat Med. 1996 Oct;2(10):1065–1068. doi: 10.1038/nm1096-1065. [DOI] [PubMed] [Google Scholar]
- 31.Moraes CT, Shanske S, Tritschler HJ, et al. mtDNA depletion with variable tissue expression: a novel genetic abnormality in mitochondrial diseases. Am J Hum Genet. 1991 Mar;48(3):492–501. [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis W, Copeland WC, Day BJ. Mitochondrial dna depletion, oxidative stress, and mutation: mechanisms of dysfunction from nucleoside reverse transcriptase inhibitors. Lab Invest. 2001 Jun;81(6):777–790. doi: 10.1038/labinvest.3780288. [DOI] [PubMed] [Google Scholar]
- 33.McComsey GA, Walker UA. Role of mitochondria in HIV lipoatrophy: insight into pathogenesis and potential therapies. Mitochondrion. 2004 Jul;4(2–3):111–118. doi: 10.1016/j.mito.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 34.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998 Aug 28;281(5381):1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 35.Wallace DC, Lott MT, Procaccio V. Mitochondrial Genes in Degenerative Diseases, Cancer and Aging. In: Rimoin DL, Connor JM, Pyeritz RE, Korf BR, editors. Emery and Rimoin’s Principles and Practice of Medical Genetics. 5. Vol. 1. Philadelphia, PA: Churchill Livingstone Elsevier; 2007. pp. 194–298. Chapter 13. [Google Scholar]
- 36.Ruiz-Pesini E, Mishmar D, Brandon M, Procaccio V, Wallace DC. Effects of purifying and adaptive selection on regional variation in human mtDNA. Science. 2004 Jan 9;303(5655):223–226. doi: 10.1126/science.1088434. [DOI] [PubMed] [Google Scholar]
- 37.Ross OA, McCormack R, Maxwell LD, et al. mt4216C variant in linkage with the mtDNA TJ cluster may confer a susceptibility to mitochondrial dysfunction resulting in an increased risk of Parkinson’s disease in the Irish. Exp Gerontol. 2003 Apr;38(4):397–405. doi: 10.1016/s0531-5565(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 38.van der Walt JM, Nicodemus KK, Martin ER, et al. Mitochondrial polymorphisms significantly reduce the risk of Parkinson disease. Am J Hum Genet. 2003 Apr;72(4):804–811. doi: 10.1086/373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wallace DC. Mitochondrial DNA mutations in diseases of energy metabolism. J Bioenerg Biomembr. 1994 Jun;26(3):241–250. doi: 10.1007/BF00763096. [DOI] [PubMed] [Google Scholar]
- 40.Torroni A, Petrozzi M, D’Urbano L, et al. Haplotype and phylogenetic analyses suggest that one European-specific mtDNA background plays a role in the expression of Leber hereditary optic neuropathy by increasing the penetrance of the primary mutations 11778 and 14484. Am J Hum Genet. 1997 May;60(5):1107–1121. [PMC free article] [PubMed] [Google Scholar]
- 41.McMahon FJ, Chen YS, Patel S, et al. Mitochondrial DNA sequence diversity in bipolar affective disorder. American Journal of Psychiatry. 2000;157(7):1058–1064. doi: 10.1176/appi.ajp.157.7.1058. [DOI] [PubMed] [Google Scholar]
- 42.van der Walt JM, Dementieva YA, Martin ER, et al. Analysis of European mitochondrial haplogroups with Alzheimer disease risk. Neuroscience Letters. 2004 Jul 15;365(1):28–32. doi: 10.1016/j.neulet.2004.04.051. [DOI] [PubMed] [Google Scholar]
- 43.Dato S, Passarino G, Rose G, et al. Association of the mitochondrial DNA haplogroup J with longevity is population specific. Eur J Hum Genet. 2004 Dec;12(12):1080–1082. doi: 10.1038/sj.ejhg.5201278. [DOI] [PubMed] [Google Scholar]
- 44.Niemi AK, Hervonen A, Hurme M, Karhunen PJ, Jylha M, Majamaa K. Mitochondrial DNA polymorphisms associated with longevity in a Finnish population. Hum Genet. 2003 Jan;112(1):29–33. doi: 10.1007/s00439-002-0843-y. [DOI] [PubMed] [Google Scholar]
- 45.Ross OA, McCormack R, Curran MD, et al. Mitochondrial DNA polymorphism: its role in longevity of the Irish population. Exp Gerontol. 2001 Jul;36(7):1161–1178. doi: 10.1016/s0531-5565(01)00094-8. [DOI] [PubMed] [Google Scholar]
- 46.Ruiz-Pesini E, Lapena AC, Diez-Sanchez C, et al. Human mtDNA haplogroups associated with high or reduced spermatozoa motility. Am J Hum Genet. 2000 Sep;67(3):682–696. doi: 10.1086/303040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Montiel-Sosa F, Ruiz-Pesini E, Enriquez JA, et al. Differences of sperm motility in mitochondrial DNA haplogroup U sublineages. Gene. 2006 Mar 1;368:21–27. doi: 10.1016/j.gene.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 48.Niemi AK, Majamaa K. Mitochondrial DNA and ACTN3 genotypes in Finnish elite endurance and sprint athletes. Eur J Hum Genet. 2005 Aug;13(8):965–969. doi: 10.1038/sj.ejhg.5201438. [DOI] [PubMed] [Google Scholar]
- 49.Baudouin SV, Saunders D, Tiangyou W, et al. Mitochondrial DNA and survival after sepsis: a prospective study. Lancet. 2005 Dec 17;366(9503):2118–2121. doi: 10.1016/S0140-6736(05)67890-7. [DOI] [PubMed] [Google Scholar]
- 50.Hendrickson SL, Hutcheson HB, Ruiz-Pesini E, et al. Mitochondrial DNA haplogroups influence AIDS progression. Aids. 2008;22(18):2429–2439. doi: 10.1097/QAD.0b013e32831940bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phair J, Jacobson L, Detels R, et al. Acquired immune deficiency syndrome occurring within 5 years of infection with human immunodeficiency virus type-1: the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr. 1992;5(5):490–496. [PubMed] [Google Scholar]
- 52.Mccullagh P. Regression Models for Ordinal Data. Journal of the Royal Statistical Society, Series B. 1980;42(2):109–142. [Google Scholar]
- 53.Bender R, Grouven U. Using binary logistic regression models for ordinal data with non-proportional odds. J Clin Epidemiol. 1998 Oct;51(10):809–816. doi: 10.1016/s0895-4356(98)00066-3. [DOI] [PubMed] [Google Scholar]
- 54.Scott SC, Goldberg MS, Mayo NE. Statistical assessment of ordinal outcomes in comparative studies. J Clin Epidemiol. 1997 Jan;50(1):45–55. doi: 10.1016/s0895-4356(96)00312-5. [DOI] [PubMed] [Google Scholar]
- 55.Brown TT, Chu H, Wang Z, et al. Longitudinal increases in waist circumference are associated with HIV-serostatus, independent of antiretroviral therapy. AIDS. 2007 Aug 20;21(13):1731–1738. doi: 10.1097/QAD.0b013e328270356a. [DOI] [PubMed] [Google Scholar]
- 56.Lichtenstein KA, Delaney KM, Armon C, et al. Incidence of and risk factors for lipoatrophy (abnormal fat loss) in ambulatory HIV-1-infected patients. J Acquir Immune Defic Syndr. 2003 Jan 1;32(1):48–56. doi: 10.1097/00126334-200301010-00007. [DOI] [PubMed] [Google Scholar]
- 57.Law M, Puls R, Cheng AK, Cooper DA, Carr A. Evaluation of the HIV lipodystrophy case definition in a placebo-controlled, 144-week study in antiretroviral-naive adults. Antivir Ther. 2006;11(2):179–186. [PubMed] [Google Scholar]
- 58.Palacios R, Galindo MJ, Arranz JA, et al. Cervical lipomatosis in HIV-infected patients: a case-control study. HIV Med. 2007 Jan;8(1):17–21. doi: 10.1111/j.1468-1293.2007.00421.x. [DOI] [PubMed] [Google Scholar]
- 59.Mallon PW, Wand H, Law M, Miller J, Cooper DA, Carr A. Buffalo hump seen in HIV-associated lipodystrophy is associated with hyperinsulinemia but not dyslipidemia. J Acquir Immune Defic Syndr. 2005 Feb 1;38(2):156–162. doi: 10.1097/01.qai.0000147527.64863.1a. [DOI] [PubMed] [Google Scholar]