Abstract
BACKGROUND
While electroconvulsive therapy (ECT) in major depression is effective, cognitive effects limit its use. Reducing the width of the electrical pulse and using the right unilateral electrode placement may decrease adverse cognitive effects, while preserving efficacy.
METHODS
In a double-masked study, we randomly assigned 90 depressed patients to right unilateral ECT at 6 times seizure threshold or bilateral ECT at 2.5 times seizure threshold, using either a traditional brief pulse (1.5 ms) or an ultrabrief pulse (0.3 ms). Depressive symptoms and cognition were assessed before, during, and immediately, two, and six months after therapy. Patients who responded were followed for a one-year period.
RESULTS
The final remission rate for ultrabrief bilateral ECT was 35 percent, compared with 73 percent for ultrabrief unilateral ECT, 65 percent for standard pulse width bilateral ECT, and 59 percent for standard pulse width unilateral ECT (all P’s<0.05 after covariate adjustment). The ultrabrief right unilateral group had less severe cognitive side effects than the other 3 groups in virtually all primary outcome measures assessed in the acute postictal period, and during and immediately following therapy. Both the ultrabrief stimulus and right unilateral electrode placement produced less short- and long-term retrograde amnesia. Patients rated their memory deficits as less severe following ultrabrief right unilateral ECT compared to each of the other three conditions (P<0.001).
CONCLUSIONS
The use of an ultrabrief stimulus markedly reduces adverse cognitive effects, and when coupled with markedly suprathreshold right unilateral ECT, also preserves efficacy. (ClinicalTrials.gov number, NCT00487500.)
Keywords: Depression, Electroconvulsive Therapy, Efficacy, Electrical Stimulation, Side Effects
Electroconvulsive therapy (ECT) is a highly effective antidepressant treatment, but its use is limited by adverse cognitive effects.1 Patients experience a variable period of disorientation following each treatment and may have difficulty remembering new information for several weeks following the treatment course (anterograde amnesia).2, 3 There may also be persistent memory gaps for events that occurred prior to the treatment course (retrograde amnesia).4–7
Until recently, the width of the rectangular pulse delivered by many ECT devices ranged from 0.5 to 2 ms. In contrast, the optimal width for neuronal depolarization is estimated to be at most 0.1 to 0.2 ms.8, 9 Excessively wide pulses should be inefficient, with most of the energy administered during the refractory period following depolarization. The movement away from sine wave stimulation, with a phase period of 8.33 ms (60 Hz), to rectangular brief pulses (0.5 to 2.0 ms duration), resulted in a dramatic decrease in adverse cognitive effects.7, 10 It is not known whether similar improvement would obtain with a shift from brief (0.5–2 ms) to ultrabrief (<0.5 ms) stimulation.
Short-term cognitive deficits are greater with the bilateral (BL) than right unilateral (RUL) electrode placement.5, 11 This difference may extend to long-term cognitive effects.6 There has been longstanding controversy whether RUL ECT achieves the efficacy of BL ECT. The extent to which electrical dosage exceeds seizure threshold strongly influences the efficacy of RUL ECT.5, 11–14 Thus, another key issue is whether RUL ECT retains advantages with respect to cognitive side effects when its dosage is optimized to exert maximal efficacy. We report a prospective, random assignment, double-masked trial of the effects of pulse width and electrode placement on the efficacy and safety of ECT in 90 patients.
METHODS
General Description
We conducted a prospective, randomized, double-masked trial contrasting 4 forms of ECT in 90 patients with major depression. All were inpatients at the New York State Psychiatric Institute, whose Institutional Review Board approved the study. An independent data and safety monitoring board monitored the safety of the study.
The first author (HAS), who is also the senior scientist, designed the study in conjunction with the co-authors. Patient enrollment and data collection were supervised by the authors and the authors analyzed, interpreted, and had full access to the data; were responsible for the manuscript; and verify the completeness and accuracy of the data. Analyses were conducted on locked data sets.
Patients
The 90 study patients met Research Diagnostic Criteria15 and Diagnostic and Statistical Manual IV (DSM-IV)16 criteria for major depressive episode, had a pretreatment score of 18 or greater on the Hamilton Rating Scale for Depression (HRSD, 24-item),17 clinical indication for ECT,1 and provided written informed consent. Patients were excluded with a history of schizophrenia, schizoaffective disorder, other functional psychosis, rapid-cycling bipolar disorder, neurological illness or insult, alcohol or other drug abuse within the past year, ECT within the past 6 months, or severe medical illness.
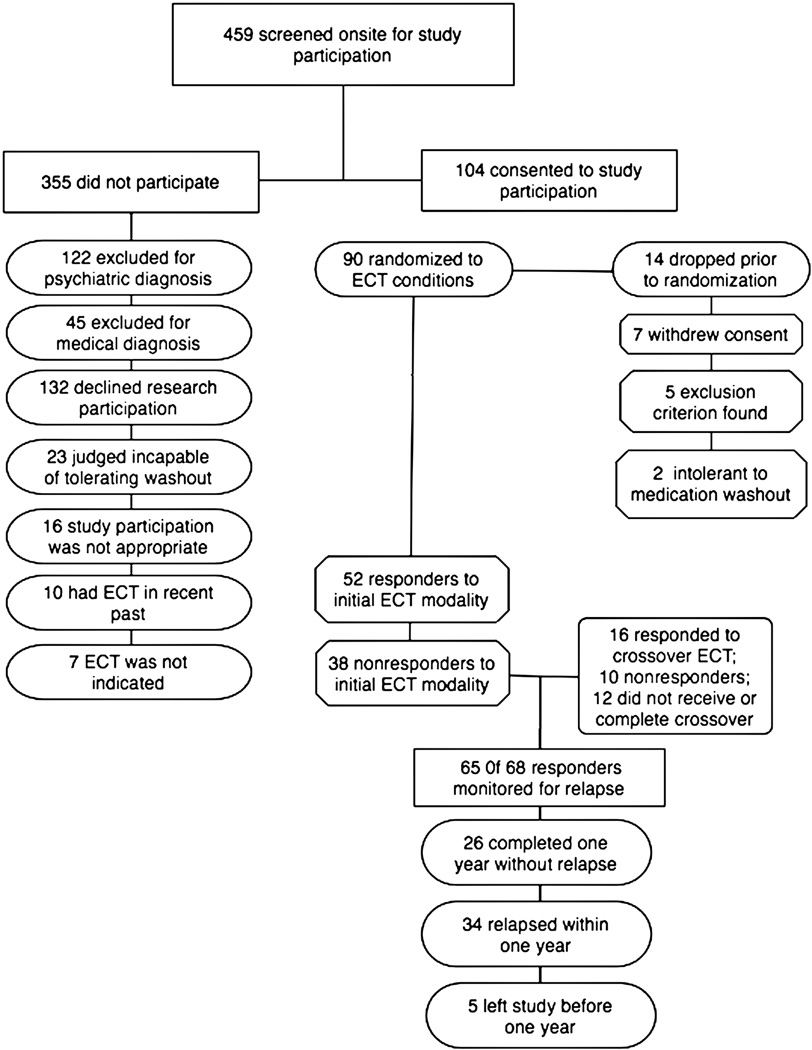
From 1999–2005, on-site screening interviews were conducted for 459 consecutive potential participants who were referred for treatment with ECT by a mental health professional (Figure 1). Recruitment strategies were limited to increasing awareness of protocol availability among health professionals. Of those screened, 104 (23%) patients were offered and consented to protocol participation. Fourteen patients left the study prior to randomization and start of therapy, resulting in the intent-to-treat sample of 90.
Figure 1.
Flow of Participants in the Study.
Except for lorazepam (up to 3 mg/d), psychotropic medications were discontinued at least 5 days before preECT research evaluations [(mean±SD) 14±7 days prior to starting ECT] until one week after completing ECT. The average dose of lorazepam during ECT was 1.1±1.0 mg/d, and was similar in the four treatment groups.
Electroconvulsive Therapy
Using a two-by-two factorial design, patients were randomly assigned in permuted blocks of 12 to treatment conditions differing in electrode placement (BL vs. RUL) and pulse width (0.3 vs. 1.5 ms). Based on the Antidepressant Treatment History Form,18 the randomization was stratified by whether or not the patient had received one or more adequate medication trials in the current episode. Atropine (0.4 mg), methohexital (1.0 mg/kg), and succinylcholine (0.75 mg/kg) were the anesthetic medications. ECT was administered three times per week with a customized, bidirectional, rectangular pulse, constant current device (MECTA Corporation, Tualatin, Oregon). The standard bifrontotemporal and d’Elia electrode placements were used for BL and RUL ECT, respectively.1 Seizure threshold was quantified at the first and last treatments using the empirical titration procedure.19 At all other treatments electrical dosage was 2.5 or 6.0 times the initial seizure threshold for BL and RUL ECT, respectively. Except for the pulse width manipulation, stimulation parameters were identical in the ultrabrief and brief pulse groups. Duration of motor convulsive movements and two channels of prefrontal electroencephalogram were recorded. The criterion for an adequate seizure was at least 20 s of tonic-clonic movement or 25 s of electroencephalographic seizure activity.
Clinical Evaluations
The patients and the clinical evaluation team (research psychiatrist and social worker) were masked to treatment assignment. The team completed HRSD ratings before the first treatment, twice weekly during the treatment course, and within two days and one week after the end of treatment. Inter-rater reliability coefficients exceeded 0.98 at all intervals.
Patients were considered initial responders if their HRSD scores decreased at least 60 percent from pretreatment to immediately after the final treatment, and had a maximum post-treatment score of 16. Final responders maintained this level of improvement for at least one week after ECT. Remission was defined at these two time points as a HRSD score of 10 or less.
Response was also defined as a score immediately following the treatment course of 1 or 2 on the Clinical Global Impression — Improvement (CGI-I) Scale,20 corresponding to a judgment that residual symptoms were at most minimal. The Global Assessment Scale,21 a clinician-based assessment of overall functioning, and the Beck Depression Inventory-II,22 a self-report measure of depressive symptom severity, were completed at baseline and during the week after ECT termination.
Patients who showed benefit continued to receive ECT until they were asymptomatic or had no further improvement over two treatments. Patients received at least 10 treatments before classification as a nonresponder. This minimum was lowered to 8 treatments for those who had HRSD reductions of 20% or less. Nonresponders received an open, crossover course of brief pulse (1.5 ms) BL ECT, with dosage 2.5 times the re-determined seizure threshold. Patients who met final response criteria to randomized or crossover ECT were followed until relapse or for one year. HRSD interviews were conducted every two weeks for the first two months after ECT and monthly thereafter. During follow-up, all patients received maintenance pharmacotherapy with antidepressant medications. As defined previously,23 relapse was declared when HRSD scores increased over two consecutive interviews at least one week apart by 10 points relative to the score at ECT termination, with a minimum score of at least 16, or if the patient was hospitalized for symptomatic worsening, or presented with psychotic features or suicidal intent.
Cognitive Evaluations
Acute neuropsychological effects were assessed at each treatment. Before anesthesia induction, patients memorized sets of words, geometric and ‘nonsense’ shapes, and emotionally-neutral faces.11, 24, 25 After seizure termination, recovery of orientation was assessed continuously for 90 minutes,26 followed by testing retrograde memory for the material learned earlier. This postictal battery also included tasks assessing recognition memory for newly learned affective facial expressions,11 and memory for newly learned sentences and their order of presentation.27 Attention was assessed using cancellation tasks with syllables, geometric shapes, and nonsense shapes as targets.28 Verbal fluency tasks involved generating words based on initial letter and semantic category.29 Language was assessed with brief tasks requiring naming based on verbal description and visual presentation.30 Most tasks had 12 equivalent stimuli sets that were randomized to testing occasion.
Assessments of global cognitive status and anterograde and retrograde memory were made at pretreatment baseline, the day after specific treatments, during the week after the end of both the randomized and crossover phases, as well as two months and six months later. The tests included a modified version of the Mini-Mental State Examination,31 reproduction of a complex figure 20 minutes after copying,32 learning and memory for a word list using the Buschke Selective Reminding Test with assessment 2 hr after initial list learning,5, 33 recall of the gist of a story read 24 hr earlier,34 recall of diverse personal events on the Columbia University Autobiographical Memory Interview (AMI),4, 11, 26 and recall of famous people and events, using the Goldberg Remote Memory Test.35, 36
Statistical Analyses
The results are expressed as means±SD. Statistical tests were two-tailed. Comparability of the treatment groups in baseline measures was tested with analyses of variance (ANOVAs) on continuous variables and log-linear analyses on dichotomous variables, with pulse width (ultrabrief vs. brief) and electrode placement (BL vs. RUL) and their interaction as between-subject terms.
The primary efficacy outcome measures were HRSD scores and the remission rate one week following ECT. As an omnibus test of short-term efficacy differences, a repeated measures analysis of covariance (ANCOVA) was conducted on HRSD scores with the pulse width and electrode placement conditions as between-subject factors, time point (after six treatments, immediate post-treatment, and one-week post-treatment) as the repeated measures factor, and preECT HRSD score, age, and number of adequate antidepressant medication trials in the depressive episode serving as covariates. This was followed by ANCOVAs on HRSD scores at the individual time points. Post-hoc pair-wise group differences were identified with t-tests on covariate-adjusted means. Log-linear analyses compared the groups in rates of response and remission, controlling for age and number of adequate medication trials in the current episode. The efficacy analyses were conducted both in the intent-to-treat (N=90) and study completer (N=83) samples. Survival analyses, using both regression models and life table methods, provided tests of treatment group differences in likelihood and speed of relapse. The covariates in the regression models were age, number of adequate medication trials and HRSD scores one week following ECT.
The acute neuropsychological battery, other than orientation recovery assessment, was administered twice during the pretreatment baseline period. Baseline scores were standardized so that the sample mean was 0, with a standard deviation of 1. Scores on each test assessed in the postictal period were averaged for each patient and expressed in SD units relative to the baseline distribution. Scores on each of the short- and long-term neuropsychological tests were similarly standardized relative to their baseline distributions. For measures obtained during and immediately following the randomized phase, ANCOVAs were conducted with pulse width and electrode placement conditions as between-subject factors, and number of treatments, age, and the baseline score as covariates. For the long-term follow-up assessments, the analyses were conducted treating patients who participated in the crossover phase as a separate group. As indicated in the Tables, a subset of measures at each assessment interval had been declared a priori as primary outcomes. In the short-term efficacy and cognitive analyses, outcomes were compared after a fixed number of treatments, as well as following the end of the treatment course. There were no missing data in the efficacy analyses following the termination of ECT. Missing cognitive data were not imputed but resulted in reduced sample size.
RESULTS
Patient Characteristics and Treatment Parameters
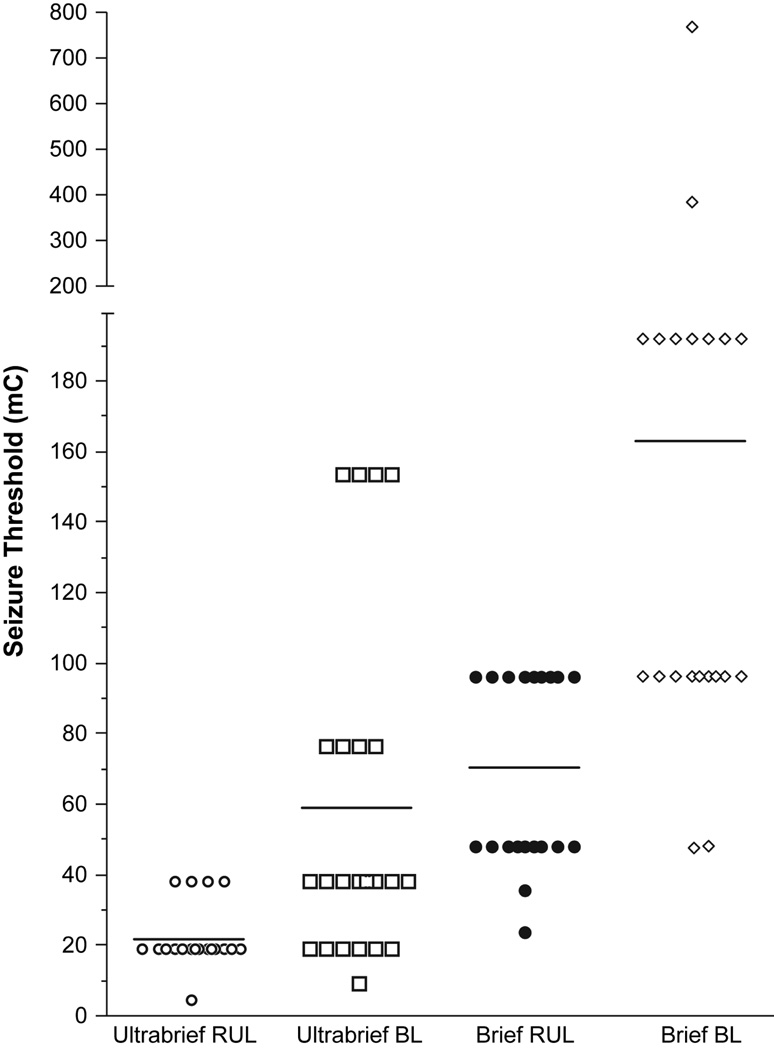
Demographic and clinical characteristics of the treatment groups were comparable (P > 0.05 for each comparison), except for a higher representation of psychotic depression in the ultrabrief pulse groups. (Table 1). The doses of methohexital and succinylcholine and the measures of seizure duration were also comparable (Table 2). Replicating previous findings,5, 11, 19 initial seizure threshold was higher in the patients treated with BL compared to RUL ECT. However, pulse width exerted a larger effect; seizure threshold was approximately three times greater in brief compared to ultrabrief pulse groups (Figure 2). The groups did not differ in motor or EEG seizure duration.
Table 1.
Demographic and Clinical Characteristics at Study Entry, According to Treatment Group.*
| VARIABLE | Ultrabrief Pulse Right Unilateral ECT (N = 22) | Ultrabrief Pulse Bilateral ECT (N = 23) | Brief Pulse Right Unilateral ECT (N = 22) | Brief Pulse Bilateral ECT (N = 23) |
|---|---|---|---|---|
| Age (yr) | 54±16 | 51±17 | 45±14 | 53±18 |
| Sex (% female) | 55 | 57 | 59 | 56 |
| Education (yr) | 15±3 | 15±3 | 15±3 | 15±3 |
| Verbal IQ | 112±19 | 107±16 | 108±19 | 109±16 |
| Familial socioeconomic status (range 1–5)† | 2.1±1.0 | 1.5±0.8 | 1.9±0.9 | 1.8±0.9 |
| Psychotic depression (%)‡ | 23 | 26 | 9 | 4 |
| Unipolar/bipolar (% bipolar depression)‡ | 23 | 26 | 36 | 35 |
| Pretreatment HRSD | 30±7 | 32±8 | 31±7 | 29±7 |
| History of past ECT (%) | 18 | 30 | 27 | 35 |
| Duration of current episode (mo)§ | 36±38 | 28±32 | 24±30 | 27±33 |
| No. of previous affective episodes¶ | 3±3 | 3±3 | 3±4 | 3±3 |
| No. of previous psychiatric hospitalizations¶ | 1±2 | 2±2 | 1±2 | 2±3 |
| Age at onset of affective disorder (yr) | 27±21 | 26±20 | 23±12 | 32±21 |
| No. of medication trials during episode | 5±4 | 6±4 | 6±3 | 5±4 |
| No. of adequate antidepressant trials‖ | 2±2 | 2±1 | 3±2 | 2±2 |
| Medication resistance rating (0–5 range)‖ | 3.2±1.3 | 3.1±1.4 | 3.5±1.1 | 3.6±1.1 |
Plus–minus values are means ±SD. There were no significant differences among the groups on any variable other than psychotic depression. The ultrabrief pulse groups had a higher rate of psychotic depression than the brief pulse groups (P=0.03).
Hollingshead Four-Factor Index (1, highest socioeconomic status; 5 lowest socioeconomic status).
Research Diagnostic Criteria23 probable or definite.
Upper limit of 120 months used.
Upper limit of 10 used.
Each antidepressant medication trial during the index episode before ECT was rated for potency (1 = definitely inadequate due to insufficient dose and/or duration; 3 = trial meets threshold criteria for adequacy of dose and duration using established antidepressant medication; 5 = definitely adequate trial of sufficient dose and duration with established antidepressant medication and an established augmentation strategy).26 Total number of adequate trials was determined, while the medication resistance rating corresponded to the rating of the most potent trial.
Table 2.
ECT Parameters, According to Treatment Group.*
| PARAMETER | Ultrabrief Pulse Right Unilateral ECT (N = 22) | Ultrabrief Pulse Bilateral ECT (N = 23) | Brief Pulse Right Unilateral ECT (N = 22) | Brief Pulse Bilateral ECT (N = 23) |
|---|---|---|---|---|
| Atropine (mg)† | 0.4±0.0 | 0.4±0.0 | 0.4±0.0 | 0.4±0.0 |
| Methohexital (mg)† | 80±9 | 88±9 | 74±9 | 81±9 |
| Succinylcholine (mg)† | 58±3 | 59±3 | 54±3 | 58±3 |
| Initial seizure threshold (mC) | 22±8a | 59±49b | 70±27b | 162±151c |
| Charge to induce seizure (mC)† | 103±38a | 165±144a | 318±123b | 285±157b |
| Motor seizure duration (sec)† | 40±10 | 43±9 | 40±9 | 46±17 |
| EEG seizure duration (sec)† | 50±14 | 55±15 | 53±18 | 60±26 |
Plus-minus values are means±SD. mC denotes millicoulombs or charge. ANCOVA indicated that there significant differences among the treatment groups for parameters superscript letters attached to each value. Values with different letter superscripts differed significantly from each other in post hoc t-tests on least-squares adjusted means (P<0.05).
Average per treatment during the entire ECT course.
Figure 2.
Seizure Threshold at the Start of ECT. An analysis of covariance was conducted on log-transformed (base10) seizure threshold values, with pulse width and electrode placement conditions and their interaction as between-subject terms and age as a covariate. Seizure threshold was approximately three times higher in patients treated with a brief pulse compared to an ultrabrief pulse stimulus (P<0.001, F = 85.8, df = 1, 85). Seizure threshold was approximately two times higher in patients treated with bilateral compared to right unilateral ECT (P<0.001, F = 32.5, df = 1, 85). The relationship between age and seizure threshold was marginal (P=0.073, F = 3.3, df = 1, 85).
Efficacy
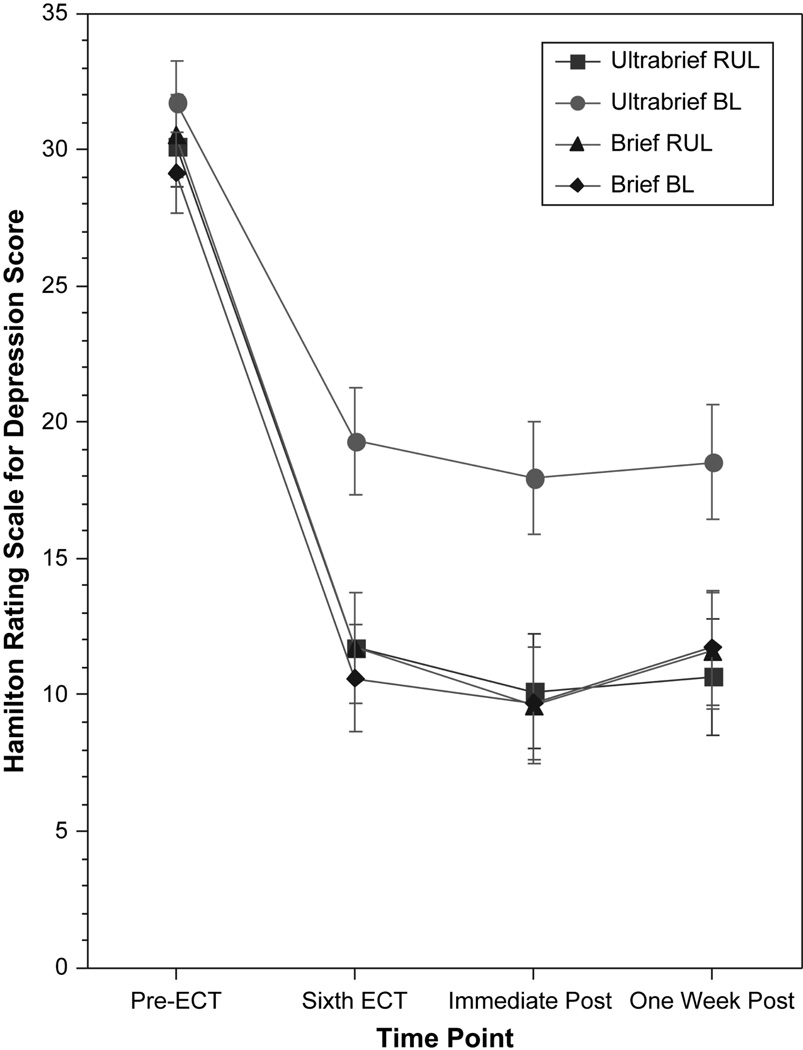
The omnibus repeated measures ANCOVA on the HRSD scores indicated that the effects of pulse width (P=0.03), electrode placement (P=0.04), and their interaction (P=0.03) were significant (Figure 3). After the sixth treatment, immediately after the end of treatment, and one week after ECT, the antidepressant response in the ultrabrief BL group was significantly poorer than in the other three groups (all P’s<0.001). The ultrabrief RUL group and the two brief pulse groups did not differ in HRSD scores at any time point.
Figure 3.
Scores on the Hamilton Rating Scale for Depression Before, During and Following the Treatment Course. A repeated measures analysis of covariance on serial depression severity ratings used pulse width and electrode placement conditions and their interaction as between-subject terms, and age, preECT depression score, and number of adequate medication trials as covariates. In this omnibus analysis depression severity over time varied with the pulse width and electrode placement conditions, as well as their interaction (P’s<0.05). Post hoc analyses indicated that each time point, the ultrabrief bilateral ECT group had inferior outcome relative to the other three groups (P’s <0.05), none of which differed from each other.
The log-linear analysis indicated that the final remission rate was contingent on both pulse width and electrode placement (P=0.04). Only 35 percent of the ultrabrief BL group were final remitters (Table 3). After adjustment for covariates, the final remission rate was lower in the ultrabrief BL than the ultrabrief RUL group (P=0.009), and brief pulse BL (P=0.02) and RUL (P=0.048) groups. Indeed, a significant interaction between pulse width and electrode placement conditions was obtained for all the efficacy measures in Table 3 (P’s<0.05), other than initial response rate (P=0.12). In each case, the ultrabrief BL group had inferior outcome than each of the other groups (P’s<0.05), and the remaining groups did not differ.
Table 3.
Initial and Final Response and Remission Rates, Ratings of Global Functioning and Depression Severity, and Number of Treatments According to Treatment Group.
| Intent-to-Treat Sample | Ultrabrief Pulse Right Unilateral ECT (N = 22) | Ultrabrief Pulse Bilateral ECT (N = 23) | Brief Pulse Right Unilateral ECT (N = 22) | Brief Pulse Bilateral ECT (N = 23) |
|---|---|---|---|---|
| Response Rates | ||||
| Immediate post-ECT (%) | 77 | 48 | 73 | 70 |
| One-week post-ECT (%) | 73a | 35b | 59a | 65a |
| Remission Rates | ||||
| Immediate post-ECT (%) | 77a | 43b | 73a | 70a |
| One-week post-ECT (%)‖ | 73a | 35b | 59a | 65a |
| CGI-I Response Rate (%) | 82a | 35b | 64a | 65a |
| Post-ECT GAS | 64±12a | 51±15b | 63±11a | 62±12a |
| Post-ECT BDI | 10±11a | 19±19b | 11±8a | 10±12a |
| Number of Treatments | 8.7±2.4a | 8.9±2.5a | 8.5±2.5a | 6.2±2.4b |
Plus–minus values are means±SD. The treatment groups differed significantly in log-linear analyses or ANCOVAs, except in immediate response rate. Values with different letter superscripts for the same variable indicate that the corresponding treatment groups differed significantly from each other in post hoc pair-wise comparisons (P<0.05). Groups did not differ if they shared a superscript letter.
Declared a priori as a primary efficacy outcome measure.
Seven patients withdrew consent for treatment during the randomized phase: 4 received brief pulse BL ECT, 2 received ultrabrief BL ECT, and 1 received brief pulse RUL ECT. When the efficacy analyses were restricted to completers (N=83), the pattern of significant effects in the intent-to-treat sample was accentuated (data not shown). The poor efficacy in the ultrabrief BL group was also not attributable to a difference in the number of treatments administered (Table 3). ANOVAs on treatment number yielded an interaction between the electrode placement and pulse width conditions both in the intent-to-treat (P=0.02) and completer (P=0.01) samples. In both samples, the brief pulse BL group received fewer treatments than each of the other groups (P’s<0.05) and the remaining three groups did not differ.
Crossover Phase
Of the 31 nonresponders who completed the randomized phase, 26 (84 percent) completed the crossover phase. They received 8.7±3.0 brief pulse BL treatments. The HRSD scores of this group were 33±8 at baseline, 19±11 after the randomized phase, and 13±7 one week after the crossover phase; 62 percent and 54 percent were responders and remitters, respectively, one week after the crossover phase. Symptomatic improvement was equivalent regardless of randomized assignment.
Relapse
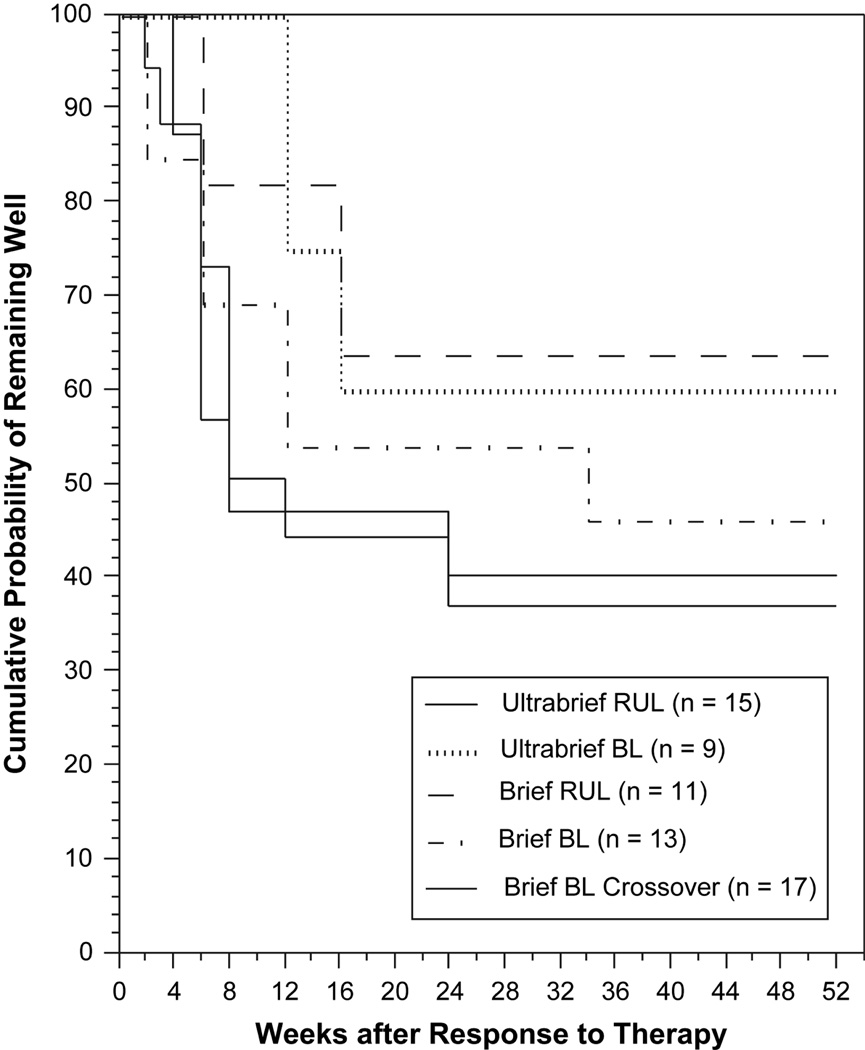
Of the 68 patients who responded to randomized (N=52) or crossover (N=16) treatment, 65 were followed for relapse and 3 refused monitoring. ECT treatment conditions were unrelated to relapse (P>0.3, Figure 4), while younger patients (P=0.04) and those with a higher degree of medication resistance (P=0.01) relapsed at a higher rate. Twenty-six of the 65 patients (40 percent) completed the one-year monitoring without relapse, 34 patients (52 percent) relapsed, and 5 patients (8 percent) left the study before the one-year observation period was completed. Of the 65 patients monitored for relapse, 61 received continuation pharmacotherapy. The most common strategies were use of a tricylcic antidepressant combined with lithium carbonate (N=28), another class of antidepressant medication combined with lithium (N=10), monotherapy with an antidepressant medication (10), combination of an antidepressant and antipsychotic medication (N=5), other regimens (N=2) and no medication (N=4). In addition, 9 patients received continuation treatment with ECT. Form of continuation pharmacotherapy or use of continuation ECT appeared unrelated to relapse. These findings replicate previous observations from our group that the form of ECT that produces remission is largely irrelevant to likelihood or timing of relapse, while relapse rates are elevated in patients with established medication resistance.5, 11, 23 However, we did not replicate the observation that continuation pharmacotherapy with a TCA and lithium exerted protective effects.11, 23 This failure to replicate may have been due in part to the fact that patients who had relapsed on robust continuation therapies following successful treatment with ECT were especially likely to be represented in this study.
Figure 4.
Kaplan–Meier Estimates of the Proportion of Patients Who Remained Well One Year after Electroconvulsive Therapy, According to Treatment Group. Survival analyses applying regression models and life-table methods showed no significant differences between the treatment groups (P>0.3).
Acute Cognitive Side Effects
Compared to ultrabrief stimulation, brief pulse stimulation produced greater cognitive impairment in the postictal period, and these effects were consistently more marked than those of electrode placement (Table 4). All the primary acute cognitive measures were influenced by pulse width, while only time to orientation recovery was also affected by electrode placement. Compared with ultrabrief RUL ECT, recovery of orientation took 36 percent longer with ultrabrief BL ECT (P<0.01), 108 percent longer with brief pulse RUL ECT (P<0.001), and 212 percent longer with brief pulse BL ECT (P<0.001). Relative to the ultrabrief groups, the brief pulse groups had significantly greater impairment in all measures of retrograde amnesia, anterograde memory for affective faces and sentences, omission and commission errors on the cancellation tasks, verbal fluency for categories, and verbal naming based on visual confrontation. Scores on several of the acute measures were associated with the number of treatments administered and/or patient age (Table 4). Cognitive performance was poorer with higher numbers of treatments or advancing age.
Table 4.
Acute Effects of ECT on Recovery of Orientation and Cognitive Functions, According to Treatment Group.*
| Ultrabrief Pulse Right Unilateral ECT | Ultrabrief Pulse Bilateral ECT | Brief Pulse Right Unilateral ECT | Brief Pulse Bilateral ECT | PW | EP | EPxPW | No. of Treatments | Age | |
|---|---|---|---|---|---|---|---|---|---|
| Post-ictal Orientation Recovery | |||||||||
| Time to recovery of orientation (min)‖ | 10±6a | 14±7b | 22±9c | 33±21d | 45.6¶ | 9.6‡ | 0.2 | 0.0 | 0.7 |
| Treatment sessions with prolonged disorientation (%) | 0.0±0.0a | 0.4±1.7a | 1.1±3.5a | 10.0±24.6b | 5.1† | 2.5 | 1.3 | 0.0 | 7.6‡ |
| Retrograde Memory | |||||||||
| Word recall | −0.5±0.8a | −0.7±0.5a,b | −1.0±0.4b | −1.1±0.4b | 16.3¶ | 1.4 | 0.3 | 4.2† | 0.3 |
| Word recall and recognition‖ | −0.4±0.9a | −0.7±0.8a | −1.3±1.1b | −1.4±0.9b | 19.1¶ | 0.2 | 0.9 | 7.3‡ | 0.8 |
| Geometric shape recognition‖ | −0.1±0.4a | −0.2±0.6a | −0.8±0.9b | −0.8±0.8b | 25.7¶ | 0.0 | 0.8 | 1.3 | 7.9‡ |
| Nonsense shape recognition‖ | 0.0±0.6a | 0.0±0.5a | −0.3±0.8a,b | −0.4±0.7b | 8.8‡ | 0.1 | 0.1 | 2.7 | 6.4† |
| Neutral face recognition | −0.9±0.9a | −1.3±1.0a,b | −1.5±1.1b | −1.7±0.9b | 9.1‡ | 1.6 | 0.4 | 0.6 | 4.0† |
| Anterograde Memory | |||||||||
| Affective face recognition‖ | −0.8±1.1 | −0.8±0.8 | −1.1±1.0 | −1.2±0.8 | 4.0† | 0.1 | 0.1 | 8.4‡ | 13.7§ |
| Sentence recognition‖ | −0.6±0.8a | −1.1±0.6b | −2.0±0.8c | −1.7±0.9c | 32.2§ | 0.3 | 0.3 | 1.6 | 0.2 |
| Sentence temporal order | −0.7±0.5 | −0.7±0.5 | −0.6±0.9 | −0.8±0.7 | 0.0 | 0.5 | 0.5 | 0.5 | 6.9‡ |
| Cancellation Performance | |||||||||
| Omission Errors | −0.8±0.9a | −0.9±1.0a,b | −1.4±1.0b | −1.1±1.1a,b | 4.6† | 0.1 | 0.9 | 0.4 | 24.9§ |
| Commission Errors | −0.3±0.8a | −0.4±0.9a | −1.0±1.2b | −0.8±1.0a,b | 5.5† | 0.0 | 0.6 | 1.2 | 1.5 |
| Verbal Fluency | |||||||||
| Letter | −0.7±0.7 | −0.6±0.8 | −0.6±0.7 | −0.9±0.7 | 0.2 | 0.2 | 1.1 | 4.9† | 2.2 |
| Category | −0.3±1.1a | −0.6±0.7a,b | −0.8±0.7b | −0.9±0.6b | 5.6† | 1.4 | 0.3 | 11.7§ | 4.9† |
| Language | |||||||||
| Visual confrontation naming | 0.2±0.8a | −0.2±1.0a,b | −0.6±1.3b | −0.4±1.2a,b | 3.9† | 0.5 | 1.5 | 0.1 | 12.8§ |
| Naming from verbal description | −0.0±0.9 | −0.1±0.6 | −0.3±1.3 | −0.5±0.9 | 3.2 | 0.0 | 0.4 | 2.3 | 16.5¶ |
Plus–minus values are means±SD. PW is the F-value for the main effect of pulse width from the ANCOVA. EP is F-value for the main effect of electrode placement. EPxPW is the F-value for the interaction between pulse width and electrode placement. No. of Treatments is the F-value for the covariate, number of ECT treatments. Age is the F-value for the covariate, patient age. Higher values indicate grater impairment for the 3 orientation measures. For all other measures, lower values indicate greater impairment. Values with different letter superscripts differed significantly from each other in post hoc t-tests on least-squares adjusted means (P<0.05).
F-value was significant in the ANCOVA (P<0.05)
F-value was significant in the ANCOVA (P<0.01)
F-value was significant in the ANCOVA (P<0.001)
F-value was significant in the ANCOVA (P<0.0001).
Declared a priori as a primary outcome measure.
Short-Term Cognitive Side Effects
The treatment groups were equivalent in baseline neuropsychological measures. The brief had greater impairment than the ultrabrief pulse groups for nearly all assessments during and immediately after treatment, including each of the primary cognitive outcomes (Table 5). Compared to the ultrabrief groups, the brief pulse groups had greater impairment in modified Mini-Mental State scores after the sixth ECT (P<0.001) and end of treatment (P<0.001), delayed recall on the Buschke Selective Reminding Task after the seventh treatment (P=0.006) and end of treatment (P<0.001), delayed reproduction on the Complex Figure Test after the seventh treatment (P=0.007) and end of treatment (P<0.001), and retrograde memory at end of treatment for autobiographical events (P<0.001) and public events (P=0.002). In contrast, the effects involving electrode placement indicated that post-treatment retrograde amnesia on both the Autobiographical Memory Interview (P<0.001) and the Goldberg Public Events Test (P=0.002) was greater with BL than RUL ECT. The ultrabrief RUL ECT group did not show a statistically significant impairment on any of the measures obtained during or immediately following the ECT course relative to their own pre-ECT baseline. When rating the global impact of ECT on memory, 71% of patients reported a negative effect, 26% reported no effect, and only 2% reported a positive effect. Patients who received ultrabrief RUL ECT differed from each of the other three groups in having the least negative global assessment (Table 5).
Table 5.
Short-term Effects of ECT on Cognitive Measures, According to Treatment Group.*
| Ultrabrief Right Unilateral ECT |
Ultrabrief Bilateral ECT |
Brief Pulse Right Unilateral ECT |
Brief Pulse Bilateral ECT |
PW | EP | EPxPW | No. of Treatments |
Age | Baseline Score |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Global Cognitive Status: Modified Mini-Mental State Exam | ||||||||||
| Post-Six ECT | +0.0±1.1a | −0.1±0.9a | −1.3±1.9b | −1.0±1.7b | 24.4¶ | 0.2 | 0.8 | — | 2.1 | 55.9¶ |
| Post-Treatment Course‖ | +0.2±1.0a | −0.2±1.0b | −0.6±1.2b | −0.6±1.1b | 13.2§ | 1.8 | 2.2 | 0.0 | 5.3† | 47.1¶ |
| Anterograde Memory: Delayed Reproduction Complex Figure Test | ||||||||||
| Post-Six ECT | −0.1±0.9a | −0.1±0.9a | −0.3±0.8a,b | −0.5±0.6b | 4.3† | 0.1 | 0.4 | — | 0.5 | 30.2¶ |
| Post-Treatment Course‖ | +0.2±1.0a | +0.1±0.8a | −0.3±0.9b | −0.4±0.7b | 11.4§ | 0.4 | 0.1 | 3.4 | 0.1 | 40.5¶ |
| Anterograde Memory: Delayed Recall Buschke Selective Reminding Test | ||||||||||
| Post-Six ECT | +0.4±1.1a | +0.3±0.9a | −0.4±1.0b | −0.3±0.7b | 18.3¶ | 0.2 | 0.4 | — | 1.9 | 37.2¶ |
| Post-Treatment Course‖ | +0.3±1.0a | +0.2±0.9a,b | −0.2±1.2b | −0.1±0.7b | 5.6† | 0.3 | 0.1 | 0.4 | 0.3 | 33.3¶ |
| Anterograde Memory: Randt Story Recall at 24 Hr Delay | ||||||||||
| Post-Six ECT | −0.2±0.9 | −0.3±1.2 | −0.9±0.7 | −0.7±1.1 | 4.5† | 0.3 | 0.3 | — | 0.7 | 11.4§ |
| Post-Treatment Course | +0.1±1.1a | −0.2±1.2a,b | −0.6±0.8a,b | −0.7±1.2b | 4.7† | 0.4 | 0.1 | 1.7 | 0.0 | 12.4§ |
| Retrograde Memory: Autobiographical Memory Interview | ||||||||||
| Post-Treatment Course‖ | 0.0±1.0a | −0.4±1.1b | −1.0±1.0c | −1.4±1.0d | 44.7¶ | 10.0‡ | 0.0 | 31.6¶ | 1.7 | 81.9¶ |
| Retrograde Memory: Goldberg Public Events | ||||||||||
| Post-Treatment Course‖ | 0.0±1.2a | −0.2±0.9a | −0.2±1.2a | −0.6±1.0b | 9.8‡ | 9.4‡ | 1.5 | 2.7 | 0.1 | 264.4¶ |
| Subjective Cognitive Evaluation | ||||||||||
| Global Memory Assessment‖ | −0.3±1.0a | −1.1±0.9b | −1.4±1.0b | −1.4±1.0b | 15.8§ | 7.5‡ | 0.7 | 8.6‡ | 10.0‡ | — |
Plus–minus values are means±SD. PW is the F-value for the main effect of pulse width from the ANCOVA. EP is F-value for the main effect of electrode placement. EPxPW is the F-value for the interaction between pulse width and electrode placement. No. of Treatments is the F-value for the covariate, number of ECT treatments. Age is the F-value for the covariate, patient age. For all measures lower values indicate greater impairment. Values with different letter superscripts differed significantly from each other in post hoc t-tests on least-squares adjusted means (P<0.05).
F-value was significant in the ANCOVA (P<0.05)
F-value was significant in the ANCOVA (P<0.01)
F-value was significant in the ANCOVA (P<0.001)
F-value was significant in the ANCOVA (P<0.0001).
Declared a priori as a primary outcome measure.
Long-term Cognitive Side Effects
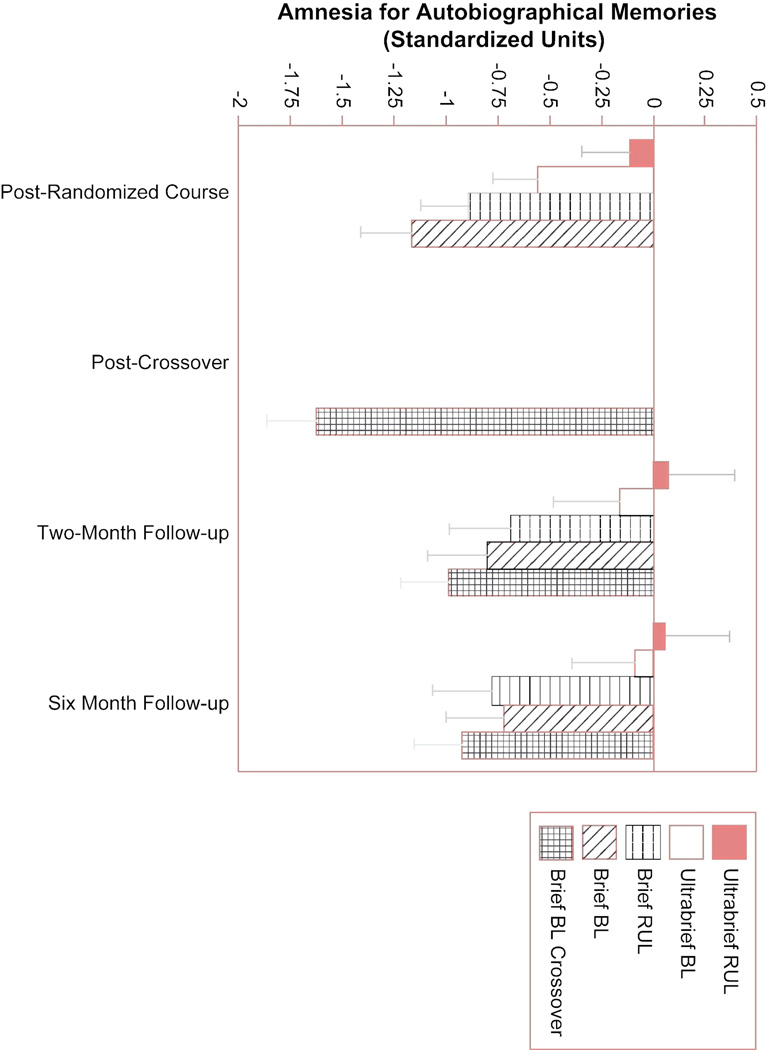
The measures of retrograde amnesia were tested for long-term differences among the treatment groups. The AMI performance for each treatment group at each assessment interval is depicted in Figure 5. The ANCOVA on two-month follow-up AMI scores produced effects of treatment group (P<0.001), number of treatments in the randomized phase (P<0.001), and baseline AMI score (P<0.001). Post hoc comparisons indicated the ultrabrief RUL group differed from all but the ultrabrief BL group. The ultrabrief BL group had less amnesia than the groups that received brief BL ECT in the randomized or crossover phases. The ANCOVA on these scores at the six-month time point produced effects of treatment group (P=0.003), number of randomized phase treatments (P<0.001), and baseline score (P<0.001). Post hoc comparisons indicated that each of the ultrabrief groups differed from each of the brief pulse groups without any other differences. At both time points, more treatments and advancing age were associated with greater retrograde amnesia. The treatment groups also differed at the two-month time point in memory for public events (P=0.04). The two brief pulse BL ECT groups (randomized and crossover) had poorer scores than each of the remaining groups. There were no effects of treatment number or age. At the six-month follow-up, the treatment groups continued to differ (P=0.02) in the same fashion (data not shown).
Figure 5.
Scores on the Columbia University Autobiographical Memory Interview. Retrograde amnesia for autographical events was assessed immediately following the end of the randomized and crossover phases and at two- and six-month followup, after completing all ECT. At each time point, Analyses of covariance indicated that each of the ultrabrief ECT conditions resulted in less retrograde amnesia than any of the brief pulse conditions (P’s<0.05). Thus, effects of pulse width on extent of retrograde amnesia persisted at least six month following completion of ECT.
There was 1 serious adverse event among the 104 patients who enrolled in this study. One patient while on a pass from the inpatient unit committed suicide during the period of research evaluation prior to the start of ECT.
DISCUSSION
This study demonstrated that the use of an ultrabrief pulse in ECT results in more efficient electrical stimulation than a traditional brief pulse. Further, ultrabrief pulse stimulation markedly reduces the acute, short-term, and long-term adverse cognitive effects of the treatment. This study confirmed that at high dosage relative to seizure threshold, RUL ECT administered with a traditional brief pulse does not differ in efficacy compared to a form of high dosage bilateral ECT, but retains advantages with respect to the acute recovery of orientation and short- and long-term retrograde amnesia. However, ultrabrief RUL ECT, administered at a high dosage relative to threshold, showed robust therapeutic effects, not differing from the traditional brief pulse conditions in any efficacy measure, including potential for relapse. Patients receiving this form of treatment did not show deterioration in any cognitive measure relative to baseline when assessed during and immediately following the treatment course or at long-term follow-up. Patients receiving this treatment also reported that the subjective impact of ECT on memory was less adverse than patients in each of the other conditions. Thus, the use of an ultrabrief stimulus coupled with high dosage RUL stimulation is a strategy that appears to retain the therapeutic properties of ECT, while substantially reducing its potential for adverse cognitive side effects. The option of ultrabrief stimulation is now standard with the ECT devices produced by the two US manufacturers, and, in some cases, can be implemented as an upgrade to older devices.
Retrograde amnesia for information about one’s life is the best-documented long-term adverse effect of ECT, and is typically reported by patients as the most distressing aspect of the treatment. This study used an instrument especially sensitive to this type of amnesia, with 118 questions about autobiographical events.4, 11, 26 The differences among the treatment groups in retrograde amnesia at the 6 month follow-up were statistically robust and of clinical significance (Figure 5). The patients treated with BL brief pulse ECT, whether as a first and only course or as crossover treatment, could not recall any information or gave inconsistent responses to more than 22% of the questions they answered at baseline. These error rates were 10%, 6%, and 6% in the groups treated with RUL brief pulse, BL ultrabrief, and RUL ultrabrief ECT, respectively. Thus, the magnitude of long-term retrograde amnesia is a function of treatment technique.
For decades it had been assumed that the efficacy of ECT depended on the elicitation of the generalized seizure, while the cognitive side effects were also sensitive to electrical parameters.37, 38 The demonstrations that the efficacy of RUL ECT is contingent on electrical dosage contradicted this view and showed that generalized seizures could be reliably produced that lacked efficacy.5, 11–13 In this study, BL ECT administered with an ultrabrief stimulus at 2.5 times the initial seizure threshold had inferior efficacy. It is unlikely that this effect was due to imbalances among the groups in baseline characteristics. Neither psychotic depression nor history of prior ECT was associated with clinical outcome, whether or not statistically controlling for treatment condition (data not shown). The imbalances likely reflected the large number of comparisons of the treatment conditions in baseline characteristics. Indeed, this loss of efficacy for BL ECT had been observed in earlier attempts to use ultrabrief stimulation to minimize electrical dosage.39, 40 It is likely that with ultrabrief stimulation, electrical dosage relative to seizure threshold also impacts on the efficacy of BL ECT, and in this study the dosage 2.5 times the initial seizure threshold was insufficient to maintain therapeutic effects.
Of course, the reason why the efficacy of BL ECT would be undermined at these parameter settings is matter of considerable interest. It should be recognized that the marked reduction in pulse width changed the size and geometry of the population of neurons engaged in the ictal process. The use of a 0.3 ms pulse did not result in a 5-fold gain in efficiency in seizure induction with either BL or RUL ECT, but rather an impressive gain of 3–4 fold. Since the amplitude of the pulse falls off with distance from the surface stimulation, the traditional wide pulse width was intrinsically more likely to trigger depolarization from a larger population of neurons. The size and location of the population of neurons engaged in the ictal process and the local intensity of the ictal discharge may be critical efficacy. We have long suggested that the seizure triggers an anticonvulsant response that, in terminating the seizure, also is responsible for the antidepressant properties of the treatment.41 RUL ECT has a considerably lower seizure threshold than BL ECT, suggesting less shunting of current. This fact, and the use of a markedly suprathreshold stimulus with RUL ECT, may have protected its efficacy when using an ultrabrief stimulus as a sufficiently large population of neurons may have been engaged in the ictal process, thus resulting in the anticonvulsant response needed to exert maximal therapeutic benefit. Had the dosage of the ultrabrief BL ECT condition been substantially increased, it is likely that efficacy would have been preserved.
Regardless, enhancing the efficacy of ultrabrief BL ECT through increased electrical dosage has doubtful clinical utility. Such an increase should only intensify an already inferior side effect profile compared to ultrabrief RUL ECT. Even though ECT results in a generalized seizure, manipulations of electrical parameters profoundly impact on safety and efficacy. This should also be the case with forms of brain stimulation that do not result in seizures, e.g., deep brain stimulation (DBS), transcranial magnetic stimulation (TMS) and vagus nerve stimulation (VNS). It is noteworthy that, due to safety issues, the widest pulse width used with these modalities is 0.5 ms, while wider pulses have been routine with ECT. It may be a general principle that restricting the pulse width of stimulation to the range close to the chronaxie for depolarization is critical in minimizing the side effects of any form of brain stimulation. Indeed, this study contrasted the use of a “traditional” 1.5 ms pulse width with a 0.3 ms ultrabrief pulse. The 1.5 ms pulse was especially wide and had the comparison with ultrabrief stimulation been made to a 1.0 ms pulse, as widely used in clinical practice, it is likely that the effects on cognitive measures would not have been as dramatic. This possibility only underscores the fact that basic principles of the physiological and behavioral effects of brain stimulation need to be proposed and verified, and parameter optimization carefully determined. It is of considerable practical and theoretical interests why reducing pulse width to a duration closer to that optimal in producing neuronal depolarization had such a dramatic effect on cognitive measures. We might speculate that with the repetitive pulses given in ECT, there is largely synchronous depolarization of the large population of neurons needed to produce a self-sustaining seizure. Consequently, increasing pulse width is an especially inefficient strategy for seizure production. Further, stimulation during the refractory period following depolarization may have biological effects distinct from stimulation that has yet to result in depolarization.
Undoubtedly, the findings of this study will raise considerable debate about the optimal algorithm in the use of ECT in mood disorders. The findings strongly support initial use of high dosage ultrabrief RUL ECT. However, some patients will show poor or slow response to this intervention. There is insufficient information to decide at this point whether subsequent treatment should involve an increase of dosage of the ultrabrief RUL stimulus, a switch to a traditional pulse width with high dosage RUL ECT, or a switch to some form of BL ECT. Previous reports from our group have shown long-term differences between BL and RUL ECT in the extent of retrograde amnesia.5, 6 This was also the case in this study with the measures obtained immediately and two months following the ECT course. Nonetheless, the effects of the pulse width manipulation were more profound than the effects of electrode placement at all time points and for most cognitive measures.
The findings of this study also demonstrate that the therapeutic and cognitive effects of ECT are dissociable. In general, correlational studies have not found associations between the extent of amnesia and the therapeutic effects of ECT.2, 4 This is the first study to show that a form of ECT can result in both superior efficacy and less cognitive disturbance than other types of ECT.
Acknowledgements
Supported in part by grants R01 MH35636 and R01 MH47739 from the National Institute of Mental Health. We are indebted to Dr. D. Hardesty, Ms. H. Chow, Ms. K. Schreiber, Ms. E. Dillingham and the staffs of the General Clinical Research Service without whose help this study could not have been carried out.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
Dr. Sackeim serves as an unpaid consultant to the MECTA Corporation, which donated the electroconvulsive therapy devices used in this study.
References
- 1.American Psychiatric Association. The Practice of ECT: Recommendations for Treatment, Training and Privileging. Second Edition. Washington, D.C.: American Psychiatric Press; 2001. [Google Scholar]
- 2.Sackeim HA. The cognitive effects of electroconvulsive therapy. In: Moos WH, Gamzu ER, Thal LJ, editors. Cognitive Disorders: Pathophysiology and Treatment. New York: Marcel Dekker; 1992. pp. 183–228. [Google Scholar]
- 3.Squire L. Memory functions as affected by electroconvulsive therapy. Ann NY Acad Sci. 1986;462:307–314. doi: 10.1111/j.1749-6632.1986.tb51265.x. [DOI] [PubMed] [Google Scholar]
- 4.McElhiney MC, Moody BJ, Steif BL, et al. Autobiographical memory and mood: Effects of electroconvulsive therapy. Neuropsychology. 1995;9:501–517. [Google Scholar]
- 5.Sackeim HA, Prudic J, Devanand DP, et al. A prospective, randomized, double-blind comparison of bilateral and right unilateral electroconvulsive therapy at different stimulus intensities. Arch Gen Psychiatry. 2000;57:425–434. doi: 10.1001/archpsyc.57.5.425. [DOI] [PubMed] [Google Scholar]
- 6.Sackeim HA, Prudic J, Fuller RB, Keilp J, Lavori PW, Olfson M. The cognitive effects of electroconvulsive therapy in community settings. Neuropsychopharmacology. 2007;32:244–254. doi: 10.1038/sj.npp.1301180. [DOI] [PubMed] [Google Scholar]
- 7.Weiner RD, Rogers HJ, Davidson JR, Squire LR. Effects of stimulus parameters on cognitive side effects. Ann NY Acad Sci. 1986;462:315–325. doi: 10.1111/j.1749-6632.1986.tb51266.x. [DOI] [PubMed] [Google Scholar]
- 8.Geddes L. Optimal stimulus duration for extracranial cortical stimulation. Neurosurgery. 1987;20:94–99. doi: 10.1097/00006123-198701000-00023. [DOI] [PubMed] [Google Scholar]
- 9.Ranck JB., Jr Which elements are excited in electrical stimulation of mammalian central nervous system: A review. Brain Res. 1975;98:417–440. doi: 10.1016/0006-8993(75)90364-9. [DOI] [PubMed] [Google Scholar]
- 10.Squire L, Zouzounis J. ECT and memory: brief pulse versus sine wave. Am J Psychiatry. 1986;143:596–601. doi: 10.1176/ajp.143.5.596. [DOI] [PubMed] [Google Scholar]
- 11.Sackeim HA, Prudic J, Devanand DP, et al. Effects of stimulus intensity and electrode placement on the efficacy and cognitive effects of electroconvulsive therapy. N Engl J Med. 1993;328:839–846. doi: 10.1056/NEJM199303253281204. [DOI] [PubMed] [Google Scholar]
- 12.Sackeim HA, Decina P, Kanzler M, Kerr B, Malitz S. Effects of electrode placement on the efficacy of titrated, low-dose ECT. Am J Psychiatry. 1987;144:1449–1455. doi: 10.1176/ajp.144.11.1449. [DOI] [PubMed] [Google Scholar]
- 13.McCall WV, Reboussin DM, Weiner RD, Sackeim HA. Titrated moderately suprathreshold vs fixed high-dose right unilateral electroconvulsive therapy: acute antidepressant and cognitive effects. Arch Gen Psychiatry. 2000;57:438–444. doi: 10.1001/archpsyc.57.5.438. [DOI] [PubMed] [Google Scholar]
- 14.Stoppe A, Louza M, Rosa M, Gil G, Rigonatti S. Fixed high-dose electroconvulsive therapy in the elderly with depression: a double-blind, randomized comparison of efficacy and tolerability between unilateral and bilateral electrode placement. J ECT. 2006;22:92–99. doi: 10.1097/00124509-200606000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: Rationale and reliability. Arch Gen Psychiatry. 1978;35:773–782. doi: 10.1001/archpsyc.1978.01770300115013. [DOI] [PubMed] [Google Scholar]
- 16.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders — Patient Edition (with Psychotic Screen) (SCID-I/P); New York: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 17.Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. doi: 10.1111/j.2044-8260.1967.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 18.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62 Suppl 16:10–17. [PubMed] [Google Scholar]
- 19.Sackeim HA, Decina P, Prohovnik I, Malitz S. Seizure threshold in electroconvulsive therapy. Effects of sex, age, electrode placement, and number of treatments. Arch Gen Psychiatry. 1987;44:355–360. doi: 10.1001/archpsyc.1987.01800160067009. [DOI] [PubMed] [Google Scholar]
- 20.Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, D.C.: Superintendent of Documents, U.S. Government Printing Office, U.S. Department of Health, Education, and Welfare Publication, No. 76-338; 1976. [Google Scholar]
- 21.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 22.Beck AT, Steer RA, Brown GK. Beck Depression Inventory Manual. 2nd ed. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 23.Sackeim HA, Haskett RF, Mulsant BH, et al. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. JAMA. 2001;285:1299–1307. doi: 10.1001/jama.285.10.1299. [DOI] [PubMed] [Google Scholar]
- 24.Sackeim HA, Portnoy S, Neeley P, Steif BL, Decina P, Malitz S. Cognitive consequences of low-dosage electroconvulsive therapy. Ann N Y Acad Sci. 1986;462:326–340. doi: 10.1111/j.1749-6632.1986.tb51267.x. [DOI] [PubMed] [Google Scholar]
- 25.Prudic J, Sackeim HA, Devanand DP, Krueger RB, Settembrino JM. Acute cognitive effects of subconvulsive electrical stimulation. Convuls Ther. 1994;10:4–24. [PubMed] [Google Scholar]
- 26.Sobin C, Sackeim HA, Prudic J, Devanand DP, Moody BJ, McElhiney MC. Predictors of retrograde amnesia following ECT. Am J Psychiatry. 1995;152:995–1001. doi: 10.1176/ajp.152.7.995. [DOI] [PubMed] [Google Scholar]
- 27.Squire L, Nadel L, Slater P. Anterograde amnesia and memory for temporal order. Neuropsychologia. 1981;19:141–145. doi: 10.1016/0028-3932(81)90054-3. [DOI] [PubMed] [Google Scholar]
- 28.Sackeim HA, Nobler MS, Prudic J, et al. Acute effects of electroconvulsive therapy on hemispatial neglect. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1992;5:151–160. [Google Scholar]
- 29.Benton AL, Hamsher K, Sivan AB. Multilingual Aphasia Examination. AJA Associates: Iowa City, IA; 1983. [Google Scholar]
- 30.Goodglass H, Kaplan E. The Assessment of Aphasia and Related Disorders. 2nd Edition. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 31.Stern Y, Sano M, Pauson J, Mayeux R. Modified mini-mental status examination: validity and reliability. Neurology. 1987;37 suppl 1:179. [Google Scholar]
- 32.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. New York: Oxford University Press; 1998. [Google Scholar]
- 33.Buschke H. Selective reminding for analysis of memory and learning. Journal of Verbal Learning and Verbal Behavior. 1973;12:543–550. [Google Scholar]
- 34.Randt CT, Brown RE. Randt Memory Test. Bayport, New York: Life Science; 1983. [Google Scholar]
- 35.Goldberg E, Barnett J. The Goldberg-Barnett Remote Memory Questionnaire. New York: Albert Einstein Medical College; 1985. [Google Scholar]
- 36.Barr WB, Goldberg E, Wasserstein J, Novelly RA. Retrograde amnesia following unilateral temporal lobectomy. Neuropsychologia. 1990;28:243–255. doi: 10.1016/0028-3932(90)90018-j. [DOI] [PubMed] [Google Scholar]
- 37.Fink M. Convulsive Therapy: Theory and Practice. New York: Raven Press; 1979. [Google Scholar]
- 38.d'Elia G, Ottosson JO, Strömgren LS. Present practice of electroconvulsive therapy in Scandinavia. Arch Gen Psychiatry. 1983;40:577–581. doi: 10.1001/archpsyc.1983.01790050103013. [DOI] [PubMed] [Google Scholar]
- 39.Cronholm B, Ottoson J-O. Ultrabrief stimulus technique in electroconvulsive therapy. II. Comparative studies of therapeutic effects and memory disturbances in treatment of endogenous depression with the Elther ES electroshock apparatus and Siemens Konvulsator III. J Nerv Ment Dis. 1963;137:268–276. doi: 10.1097/00005053-196309000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Robin A, De Tissera S. A double-blind controlled comparison of the therapeutic effects of low and high energy electroconvulsive therapies. Br J Psychiatry. 1982;141:357–366. doi: 10.1192/bjp.141.4.357. [DOI] [PubMed] [Google Scholar]
- 41.Sackeim HA, Decina P, Prohovnik I, Malitz S, Resor S. Anticonvulsant and antidepressant properties of ECT: A proposed mechanism of action. Biological Psychiatry. 1983;18:1301–1310. [PubMed] [Google Scholar]