Abstract
We examined the association between complexity of the main lifetime occupation and changes in cognitive ability in later life. Data on complexity of work with data, people, and things and on four cognitive factors (verbal, spatial, memory, and speed) were available from 462 individuals in the longitudinal Swedish Adoption/Twin Study of Aging. Mean age at the first measurement wave was 64.3 (s.d. = 7.2) and 65% of the sample had at least 3 waves of data. Occupational complexity with people and data were both correlated with cognitive performance. Individuals with more complex work demonstrated higher mean performance on the verbal, spatial, and speed factors. Latent growth curve analyses indicated that, after correcting for education, only complexity with people was associated with differences in cognitive performance and rate of cognitive change. Continued engagement as a result of occupational complexity with people helped to facilitate verbal function before retirement, while a previous high level of complexity of work with people was associated with faster decline after retirement on the spatial factor.
Keywords: Occupational complexity, cognitive change, retirement
As the prevalence of cognitive impairment continues to rise in parallel with increasing life expectancy, preserving cognitive health has become a growing concern among older adults. Associated with this concern has been an effort to identify factors that may help maintain cognitive function into older adulthood.
Despite the fact that most people spend a substantial portion of their lives at work, our understanding of the relationship between occupational activity and cognition is limited. Schooler and colleagues’ concept of “environmental complexity” provides some clues (Schooler, 1984; Schooler, Mulatu, & Oates, 2004). They posit that exposure to complex environments at work or during leisure enables continued practice of cognitive skills and hence facilitates cognitive functioning. Several studies have supported the environmental complexity hypothesis with respect to work environment and cognitive function. Using data from the Maastricht Aging Study based in the Netherlands, Bosma et al. (2003) found that higher mental work demands were associated with lower risk of cognitive impairment. Potter, Helms, and Plassman (2008) found that greater general intellectual demands and greater human interaction and communication were associated with better cognitive performance in over 1,000 members of the Duke Twins Study of Memory in Aging, which consists of male World War II veterans. This result was particularly pronounced in individuals with relatively low intelligence scores in early adulthood. Using almost 4,000 male twins from the same study, Potter, Plassman, Helms, Foster, and Edwards (2006) reported that greater general intellectual demands at work were associated with more stable cognitive performance in older adulthood when assessed over approximately 7 years of follow-up. Andel, Kåreholt, Parker, Thorslund, and Gatz (2007) found that complexity of work with data and people was associated with cognitive function above and beyond age, sex, and childhood socioeconomic status. The results were sustained when either education or adult socioeconomic status was added into the regression models. Finally, using a U.S.-based nationally representative sample of older men, Wight, Aneshensel, and Seeman (2002) found a positive association between post educational training on the job or elsewhere and cognitive function in older adulthood, again underscoring that complexity of environment at work may play a role in maintaining cognitive function in older adulthood.
Studies with clinically defined dementia support the notion that environmental complexity during working life may also relate to cognitive status. Stern et al. (1995) found that high interpersonal demands of primary lifetime occupation delayed the onset of Alzheimer’s disease independent of age and education. Smyth et al. (2004) found that participants with jobs characterized by lower mental and higher physical occupational demands were more likely to be diagnosed with Alzheimer’s disease after controlling for race, sex, year of birth, and education. Andel et al. (2005) found that more complex work with people was associated with reduced risk of Alzheimer’s disease controlling for age, gender, and education in a twin sample from the population-based Swedish Twin Registry. Co-twin control analysis showed that more complex work with people and with data was protective. Similarly, Kröger et al. (2008) recently reported that higher complexity of work with people and also with things may reduce the risk of incident dementia or Alzheimer’s disease.
The presumed basis of the relationship between intellectually demanding activity in adulthood and cognition seems to be expressed well in the adage “use it or lose it” (Katzman, 1995; Orrell & Sahakian, 1995), such that intellectual stimulation by means of daily activities facilitates maintenance of cognitive skills into old age. Additional support for this line of thought comes from animal and human research suggesting that high levels of neuronal activation brought about by intellectually stimulating activity can buffer against neurodegeneration and cognitive impairment in old age (e.g., Churchill et al., 2002).
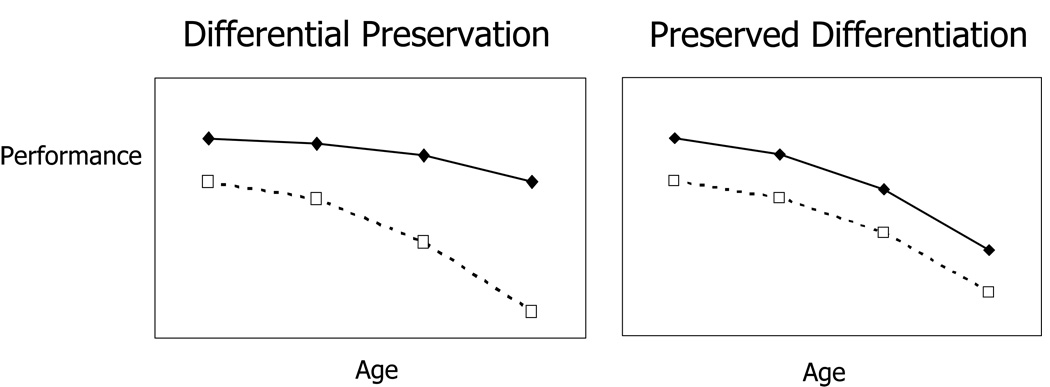
However, some (e.g., Salthouse, 2006; Salthouse, Berish, & Miles, 2002) have cautioned that the possible effect of intellectual activity on cognitive functioning has not been sufficiently substantiated. This critique can be summarized by separating “differential preservation” from “preserved differentiation” (Salthouse, 2006). The differential preservation hypothesis suggests that individuals who exercise their cognitive skills show superior preservation of their baseline cognitive functioning, with mental activity affecting not only initial level of performance but also slowing the rate of decline (see Figure 1). On the other hand, the preserved differentiation hypothesis suggests parallel aging trajectories (i.e., differences in average level but the same rate of decline) for individuals who do and individuals who do not exercise their cognitive skills. The preserved differentiation hypothesis would also be consistent with reverse causation such that better cognitive functioning leads to greater engagement in intellectual exercise rather than vice versa. Salthouse (2006) suggested that there is little evidence in the literature to support differential preservation while others have supported the opposite view (Schooler, 2007). For example, in one study applicable to the test of differential preservation, Schooler and Mulatu (2001) evaluated reciprocal effects of leisure activity and intellectual functioning and found that engagement in leisure activities in old age continued to have a positive effect on intellectual flexibility.
1.
Differential preservation reflects group differences in both intercepts and slopes. Preserved differentiation results when initial group differences are maintained over age.
The role of retirement in the association between occupational complexity and cognitive change has also not been resolved. The initial support for the role of occupation complexity in intellectual functioning came from analyses of a large sample of older workers (Schooler, 1984; Schooler, Mulatu, & Oates, 2004). Although empirical support for the positive association between occupational complexity and cognitive health has more recently been extended to retired workers, it is still unclear how retirement may affect the relation of mental exercise associated with occupational complexity to cognitive functioning pre- and post-retirement. Schaie (2005) examined cognitive function over a 7-year period in over 1,000 participants in the Seattle Longitudinal Study. The results indicated that complex work and low routine in the work-place support stable cognitive function. Retirement appeared to have an adverse effect on cognitive function in individuals who had held more complex jobs but not those previously in more routine jobs. Such a result could be viewed as illustrating “use it or lose it” if those who retired from more complex occupations suffered a greater drop in level of mental activity with retirement compared to those with less complex occupations. A similar interpretation was offered by Salthouse in a review of training data from the ACTIVE project, in which those who received cognitive training retained a higher mean performance than those who were not trained, but had a steeper trajectory of loss.
Using data from the Swedish Adoption/Twin Study of Aging, we examined (a) whether complexity of the main lifetime occupation predicts level and trajectory of change in cognitive functioning and (b) the impact of retirement on the association between occupational complexity and cognitive aging. We measured occupational complexity as complexity of work with data, people, and things. Although occupational complexity is partly determined by occupational status, the measure offers advantages with respect to capturing intellectual exercise provided by work. Specifically, occupational complexity can distinguish between work activity related to complex data manipulations (e.g., data analyst), complex interactions with people (e.g., counselor or social worker), or precision work with things (e.g., watch repairman). We used principal components analysis to generate latent components for verbal, spatial, memory, and speed domains. To our knowledge, this is the first study to test whether complexity of work with data, people, and things may predict change in specific cognitive domains, and one of the first to specifically test for the effect of retirement. Based on preserved differentiation, we hypothesized that individuals with more complex lifetime occupations will show greater preservation of cognitive functioning over the study period. We also tested for differential preservation through examining change in cognitive functioning for those with more and less complex lifetime occupations. Finally, we hypothesized that retirement will be associated with greater cognitive decline in individuals who had held more complex occupations.
Method
Participants
Ascertainment procedures for SATSA have been described previously (Finkel & Pedersen, 2004; Pedersen et al, 1991). In brief, the sample is a subset of twins from the population-based Swedish Twin Registry (Lichtenstein et al., 2002). The base population comprises all pairs of twins who indicated that they had been separated before the age of 11 and reared apart, and a sample of twins reared together matched on the basis of gender and date and county of birth. Twins were mailed questionnaires and a sample of those pairs age 50 years or older in which both twins responded was invited to participate in an additional in-person examination of health and cognitive abilities. In-person testing (IPT1) took place in a location convenient to the twins. Testing was completed during a single 4-hour visit. The second (IPT2) and third (IPT3) waves of in-person testing occurred after three-year intervals. In-person testing did not occur during wave 4; therefore, the next wave of in-person testing is labeled IPT5 and occurred after a 7-year interval (see Finkel & Pedersen, 2004). The fifth wave of in-person testing (IPT6) took place 3 years after IPT5.
Dementia status was determined by clinical diagnosis based on current diagnostic criteria (Gatz, et al., 1997). To avoid possible confounds resulting from including data from individuals experiencing pre-clinical cognitive declines, none of the cognitive data from participants who developed dementia at any point during their participation is SATSA was included in the current analyses. The number of participants at each in-person testing occasion who remained free of dementia as of IPT6 is reported in Table 1. In total, 774 non-demented individuals had cognitive data available from at least one testing occasion. Occupational information was available for 1025 individuals who participated in a questionnaire sent by SATSA in 1984. Combining the data from the IPTs and the 1984 questionnaire resulted in a sample of 462 individuals with both cognitive and occupational data (see Table 1). Mean age at each wave of measurement did not change monotonically from IPT1 to IPT3 because during the first three measurement waves SATSA continued to add twin pairs who reached the age of 50 years. Over the course of the 5 measurement waves testing ages range from 50 to 91 years. 55% of the individuals included in the current analyses are female. Twelve percent of the current sample participated in only 1 measurement wave, 15% participated in 2 waves, 20% participated in 3 waves, 19% participated in 4 waves, and 34% participated in all 5 measurement waves. Older adults are somewhat more likely to have more waves of participation because some younger adults were added to the sample at later waves.
Table 1.
Description of the SATSA sample.
| Wave | Years | Total N1 | Sample N2 | Mean Age (s.d.)3 |
|---|---|---|---|---|
| IPT1 | 1986–1988 | 565 | 303 | 64.3 (7.2) |
| IPT2 | 1989–1991 | 503 | 300 | 63.9 (8.2) |
| IPT3 | 1992–1994 | 483 | 291 | 65.9 (8.6) |
| IPT5 | 1999–2001 | 375 | 302 | 67.4 (8.1) |
| IPT6 | 2002–2004 | 423 | 280 | 71.1 (8.5) |
| At least one IPT | 1986–2004 | 774 | 462 | 66.1 (7.5) |
Number of participants who remained free of dementia.
Subset of the total participants for whom occupational and cognitive data were available.
Mean age of participants included in the current analysis.
Measures
Cognitive Components
Four cognitive domains are represented in the SATSA cognitive test battery (see Nesselroade et al., 1988; Pedersen et al., 1992): verbal, spatial, memory and processing speed abilities. Verbal abilities are tapped by Information, Synonyms, and Analogies. Figure Logic, Block Design, and Card Rotations assess spatial abilities. Memory tests include Digit Span, Picture Memory, and Names & Faces. Finally, Symbol Digit and Figure Identification measure processing speed. Reliabilities for these tests range from .82 to .96 (Pedersen, et al., 1992). Principal components analysis was used within each domain to construct latent components from the individual tests: verbal, spatial, memory, and speed. For the verbal, spatial, and speed components, loadings ranged from .78 to .92 and the components explained 74%, 67%, and 85% of the variance among the individual measures. The memory component was more diverse, including measures of short-term, long-term, and picture memory. Loadings ranged from .64 to .78 and the component explained 53% of the variance. Previous comparisons of component structure between cohorts and across testing occasions indicate that the structure does not vary systematically across age or time (Finkel et al., 2005). Therefore, to ensure that the cognitive components were defined in exactly the same manner at each wave of testing (cf. Wicherts et al., 2004), an invariant definition of components at each testing occasion was created by standardizing the cognitive measures relative to the respective means and variances at IPT1 and the loadings from the principal components analyses conducted at IPT1 were used to construct the verbal, spatial, and memory components. The speed measures were combined into a speed component using unit weighting. Finally, for ease of interpretation all component scores were translated to T-scores, using means and variances from IPT1.
Subject Variables
Occupational Complexity
The independent variable in this study was complexity of the primary lifetime occupation collected during the 1984 SATSA mailed questionnaire. The respondents were asked “What kind of occupation did you have during the major part of your working life?” The measure of complexity of work included three specific dimensions—complexity of work with data, people, and things. Occupation was originally coded according to categories from the 1980 Swedish Population and Housing Census. To assess complexity of work, we first matched each occupational category from the 1980 Swedish Census to the best-fitting category in the 1970 U.S. Census (Roos & Treiman, 1980; U.S. Bureau of the Census, 1973) using category descriptions. Then we used the score matrix for complexity of work with data, people, and things available in the 1970 U.S. Census (see Roos & Treiman, 1980). A detailed description of the conversion method and general characteristics of complexity measures can be found in Andel et al. (2005). In the original US Census files lower scores reflected higher complexity. We used reversed scores where higher scores reflect higher complexity. Descriptive data for the occupational complexity measures are presented in Table 2. Mean occupational complexity was significantly higher for men than women for complexity with data (t(460) = 4.00, p < .01) and complexity with things (t(460) = 2.42, p < .05), but not for complexity with people (t(460) = −0.98, ns).
Table 2.
Description of occupational complexity levels and descriptive statistics
| Dimension | Complexity with Data | Complexity with People | Complexity with Things |
|---|---|---|---|
| Function | 6 Synthesizing | 8 Mentoring | 7 Setting up |
| 5 Coordinating | 7 Negotiating | 6 Precision working | |
| 4 Analyzing | 6 Instructing | 5 Operating | |
| 3 Compiling | 5 Supervising | 4 Driving/Operating | |
| 2 Computing | 4 Diverting | 3 Manipulating | |
| 1 Copying | 3 Persuading | 2 Tending | |
| 0 Comparing | 2 Speaking/Signaling | 1 Feeding/Offbearing | |
| 1 Serving | 0 Handling | ||
| 0 Taking instructions | |||
| Median | 2.75 | 1.75 | 2.80 |
| Overall | |||
| Mean (SD)a | 2.88 (1.5) | 1.79 (1.5) | 2.59 (2.2) |
| Men | |||
| Mean (SD) | 3.18 (1.6) | 1.71 (1.6) | 2.37 (2.3) |
| Women | |||
| Mean (SD) | 2.62 (1.4) | 1.85 (1.5) | 0.48 (2.0) |
Total sample size = 462; 213 men and 249 women.
Retirement Age
Questions about retirement were included in SATSA questionnaires in 1987, 1990, 1993, and 2003. In addition, the same set of questions was included as part of questionnaires administered at IPT2 (1989–1991) and IPT3 (1992–1994). Included in the set of questions were items that asked respondents whether they were retired and if so, the year in which they retired. Combining this information with birth year, we were able to calculate retirement age for 368 individuals from the current sample of 462. Swedish retirement policy includes partial retirement after age 60 and full retirement benefits at age 65 without any earnings test. As a result, the majority of Swedish citizens retires by age 65, such that the unemployment rate after age 65 is functionally zero (Ginsburg, 1985). The median retirement age in this sample was 64, the mode retirement age was 65 (31% of the sample), and 89% of the sample had retired by the age of 65. Of the 94 individuals in the sample who had not reported a retirement year, 81 had not participated in an IPT measurement occasion after the typical Swedish retirement age of 65. For these individuals, retirement was estimated at the typical retirement age of 65 and their growth models were based on how many years prior to estimated retirement they had been tested (e.g., 5 years before retirement, 2 years before retirement). The remaining 13 individuals either were not retired or had failed to complete that item on the questionnaire (note that all individuals included in the current analyses worked outside the home at some point). Therefore, we estimated a retirement age of 65 for these 13 individuals as well. As a result, 236 individuals in the current sample (51%) had a retirement age of 65, 26 (6%) retired after age 65, and 200 (43%) retired before age 65. Mean retirement age was 62.3 (s.d. = 4.8) with a range of 23 to 75. Sex differences in mean retirement age were not significant (t(460) = 0.98, ns); sex differences in variability in retirement age were also not significant (F(212,248) = 1.00, ns). Note that using the median retirement age (64) instead of the typical retirement age (65) or simply excluding individuals without retirement age data had no significant impact on the conclusions drawn from the model-fitting analyses reported below.
Education
Education was included as a covariate in the growth curve models. In SATSA, education is rated on a 4-point scale from 1 (elementary school) to 4 (university or higher). Mean education was 1.77 (s.d. = 0.9), with significantly higher mean education for males (mean = 1.89, s.d. = 1.1) than for females (mean = 1.66, s.d. = 0.8); t(460) = 2.70, p < .01.
Statistical Method
A growth curve model was used to examine the impact of occupational complexity on cognitive aging. The structural model can be considered as a multi-level random coefficients model (Bryk & Raudenbush, 1992; Laird & Ware, 1982; McArdle & Anderson, 1990). The model provides estimation of fixed effects, i.e. fixed population parameters as estimated by the average growth model of the entire sample, and random effects, i.e., interindividual variability in intraindividual change in growth model parameters. Growth curve models take into account missing data by giving more weight to individuals with the most time points.
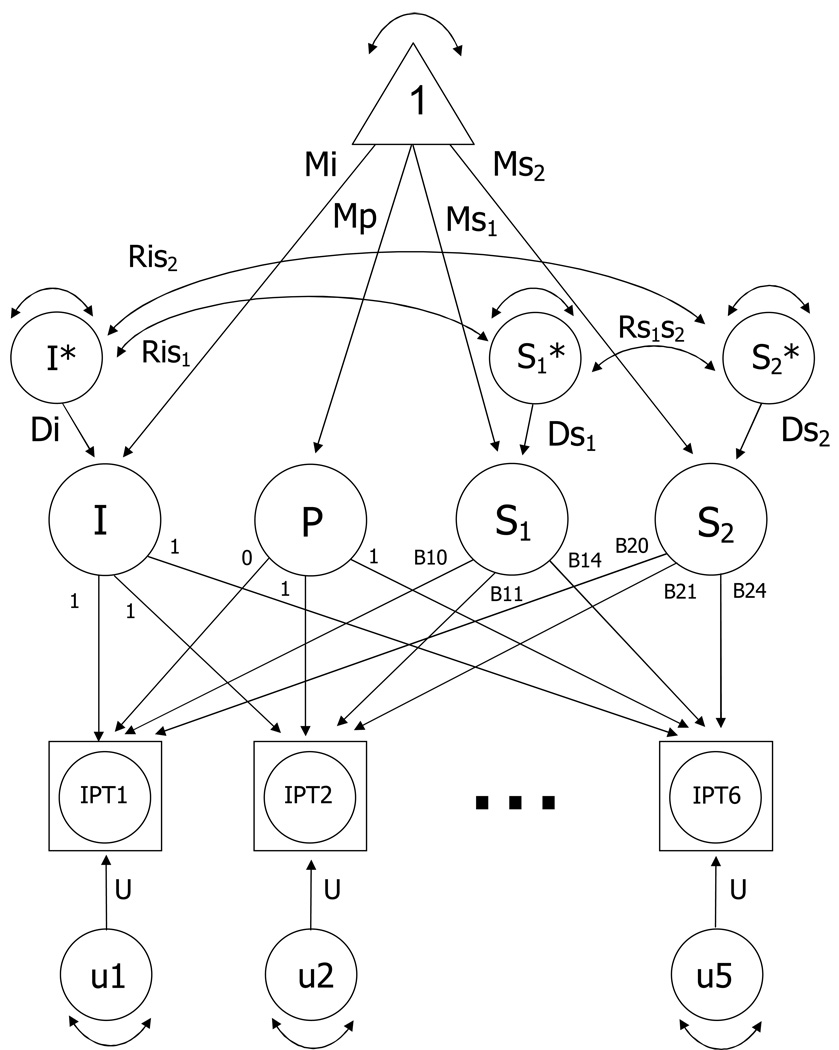
Because the focus of the current investigation was to examine the impact of occupation as indicated both by group differences in occupational complexity and by qualitative changes in aging trajectories after retirement, a two-slope latent growth curve model was applied to the data (Bryk & Raudenbush, 1992; Finkel et al., 2003): centering age was set at each individual’s retirement age with one linear slope before retirement age and a separate linear slope after retirement age. As a result, retirement age serves as the intercept, or pivot point, between the two estimated slopes.1 The two-slope growth curve model is presented in Figure 2. Following the standards of structural equation models, observed data are represented by circles within squares (to indicate that data may be missing), latent variables are indicated by circles, and estimation of phenotypic means is indicated via a triangle. Double-headed areas between variables indicate correlations, whereas double-headed arrows within variables indicate variance. Individual scores at any one time are a function of a latent intercept (I), practice or retest effects (P), the first slope (S1), the second slope (S2), and random error (U1–U5). The paths from the latent slope factors to the observed scores are the age basis coefficients, B1t and B2t. Age basis coefficients are calculated separately for each individual, based on age at testing and age of retirement. Values of B1 were set to zero for any age greater than retirement age, thereby defining S1 as the rate of change up to retirement. Similarly, values of B2 were set to zero for any age less than retirement age, defining S2 as the rate of change after retirement. I*, S1*, and S2* refer to the standardized scores of I, S1, and S2. Standardized practice effects can also be included. The effect of practice on cognitive performance is not a simple matter and practice effects can be modeled in a variety of ways (e.g., Ferrer et al., 2005). Previous LGC analyses in the SATSA dataset have indicated small but significant mean practice effects, but no significant interindividual variance in practice effects (e.g., Finkel at al., 2005). As a result, we have selected one of the simpler methods for modeling practice and we include a practice term in the fixed effects for the current analysis but not in the random effects.
2.
Two-slope Latent Growth Curve Model. Observed data are denoted by IPT1 through IPT6. Mi = mean intercept; Ms1 = mean slope 1; Ms2 = mean slope 2; U1 through U5 indicate random error. I*, S1*, and S2* refer to the standardized scores of I, S1, and S2. Di denotes deviations from the group intercept and Ds1 and Ds2 denote deviations from the group slopes. The correlations among the growth curve parameters are indicated by Ris1, Ris2, and Rs1s2. The paths from the latent slopes to the observed scores are the age basis coefficients, B1t and B2t, which define the intervals of change over age.
The model fitting procedure entails fitting individual growth models to all available data; repeated measurements are indicated by the y0 through y4 variables. Paths from practice to the observed scores indicate that the entire practice effect was assumed to occur at the first retest. The random errors or uniquenesses (u0–u4) represent unaccounted variation from fitting the growth model to the cognitive measures; these time-specific residual variances were constrained to be equal over time. The means (Mi = mean intercept; Mp = mean practice; Ms1 = mean slope 1; Ms2 = mean slope 2) are the estimates of the average performance and average amount of change. Standard deviations of the interindividual differences in the intercept and slope parameters are indicated by Di, Ds1, and Ds2. Finally, the relationships among the intercept and rates of change are represented by the correlations Ris1, Ris2, and Rs1s2.
Because of the skew apparent in the occupational complexity variables, individuals were divided into groups of high and low occupational complexity using a median split (c.f. Andel et al., 2006).2 The current analyses focus on individual performance, making it necessary to eliminate any bias resulting from the inclusion of twins. All models were fit to a sample that included a randomly selected member of each twin pair (sample A). Analyses were then replicated in a sample consisting of the other member of each twin pair (sample B). Individuals from incomplete pairs were randomly assigned to either sample A or sample B.3 The random and fixed effects parameter estimates were obtained using PROC Mixed in SAS 8.0 (SAS, 1999).
Results
Correlations
Before estimating growth curve models for the effect of occupational complexity on cognitive aging, we explored the relationships among the occupational and cognitive variables. Correlations among the measures of occupational complexity and between these variables and the cognitive components at IPT1 are presented in Table 3. The occupational complexity measures are all significantly correlated with each other, although the correlation between complexity with people and complexity with things is significantly negative (r = −.33). Not surprisingly, education level is also significantly correlated with the occupational measures, although again the correlation with complexity with things is significantly negative (r = −.13). However, the magnitude of the correlation indicates that only 1.7% of the variance in occupational complexity with things is explained by education level. Modest significant positive correlations were found between the cognitive components and occupational complexity with data and people, but no significant correlations were indicated between the cognitive components and occupational complexity with things. As a result, growth curve analyses focused on occupational complexity with data and people.
Table 3.
Correlations between the measures of occupational complexity and the cognitive components.
| Variable | Complexity with Data |
Complexity with People |
Complexity with Things |
|---|---|---|---|
| People | .53* | ||
| Things | .14* | −.33* | |
| Education | .36* | .47* | −.13* |
| Verbal | .28* | .32* | −.04 |
| Spatial | .20* | .15* | .03 |
| Memory | .19* | .25* | −.06 |
| Speed | .19* | .19* | −.01 |
Correlation is significantly different from zero at p < .01.
Growth Curve Models
The purpose of growth curve model fitting was to determine the impact of occupational complexity on the parameters of the two-slope growth curve model; therefore, six growth curve models were fit to the data in a sequential fashion. Nested models were compared using the difference chi-square test obtained by taking the difference between the obtained model fits [i.e., -2ln(Likelihood)] and testing its significance with the degrees of freedom equal to the difference in the number of parameters of the two models. First, a basic two-slope model was fit to the data with education included as a covariate to account for the relationship between educational level and occupational complexity. In the second model, the dichotomous occupational complexity variable was added as a covariate for the intercept term, only. Comparing the fit of models 1 and 2 provided a direct test of group differences in the intercept (i.e., mean performance at retirement age). Model 3 added the complexity covariate for the practice (or retest) effect and comparing it to model 2 provided a direct test of group differences in practice. Group differences in the two slopes were tested individually: model 4 included the complexity covariate for intercept, practice, and slope 1, whereas model 5 included the complexity covariate for intercept, practice, and slope 2. Comparing models 4 and 5 to model 3 meets the requirement of nested model comparisons and provides a direct test of group differences in slope 1 and slope 2, respectively. Finally, model 6 represents the full model, with the complexity covariate incorporated for all parameters of the two-slope growth curve model (intercept, practice, slope1, and slope2).
Results of fitting these six models to the growth curves for the four cognitive components are presented in Table 4; the top half of the table presents the results for using complexity with data as a covariate and the bottom half of the table presents the results for complexity with people. The results indicate that after including education as a covariate, occupational complexity with data does not significantly impact any of the parameters of the two-slope latent growth curve model for any of the cognitive components. All parameter estimates were functionally equivalent for groups with high and low occupational complexity with data. In contrast, occupational complexity with people did have an impact on the aging trajectories for the three of the four cognitive components, even after education was included in the growth curve model. No impact of complexity of work with people was found for any part of the aging trajectory for the memory component.
Table 4.
Results of comparing growth curve models: -2LL (df).
| Variable and modela | Verbal | Spatial | Memory | Speed |
|---|---|---|---|---|
| Complexity with Data | ||||
| 1: No group differences | 4025 (669) | 4344 (678) | 4623 (665) | 4463 (683) |
| 2: Group differences in I | 4021 (667) | 4343 (676) | 4622 (663) | 4460 (681) |
| 3: Group differences in I & P | 4017 (665) | 4343 (674) | 4618 (661) | 4460 (679) |
| 4: Group differences in I & P & S1 | 4015 (663) | 4341 (672) | 4614 (659) | 4460 (677) |
| 5: Group differences in I & P & S2 | 4014 (663) | 4343 (672) | 4614 (659) | 4460 (677) |
| 6: Group differences in I & P & S1 & S2 | 4013 (661) | 4341 (670) | 4614 (657) | 4459 (675) |
| Complexity with People | ||||
| 1: No group differences | 4025 (669) | 4344 (678) | 4623 (665) | 4463 (683) |
| 2: Group differences in I | 4025 (667) | 4337 (676)b | 4619 (663) | 4456 (681)b |
| 3: Group differences in I & P | 4020 (665) | 4331 (674)c | 4618 (661) | 4455 (679) |
| 4: Group differences in I & P & S1 | 4013 (663)d | 4331 (672) | 4615 (659) | 4452 (677) |
| 5: Group differences in I & P & S2 | 4018 (663) | 4324 (672)d | 4615 (659) | 4454 (677) |
| 6: Group differences in I & P & S1 & S2 | 4010 (661) | 4324 (670) | 4612 (657) | 4452 (675) |
Model fitting is described in the text: I = Intercept, P = Practice, S1 = Slope 1, S2 = Slope 2
Model fit is significantly different from model 1 at p < .05.
Model fit is significantly different from model 2 at p < .05.
Model fit is significantly different from model 3 at p < .05.
Note: -2LL is the log likelihood indicator of model fit. Education was included as a covariate in all models.
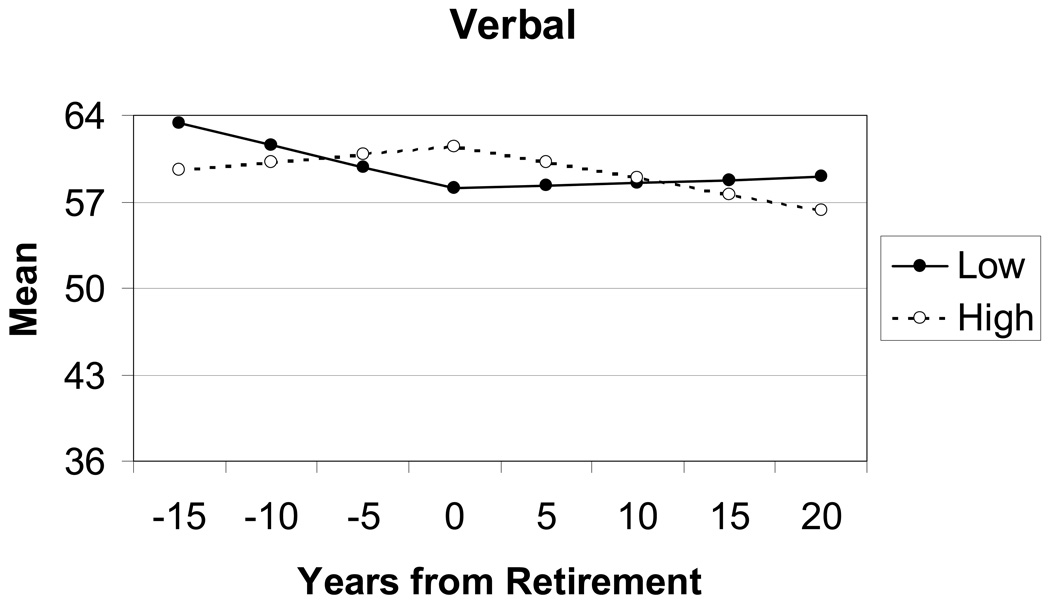
Model fitting for the verbal component indicated a significant difference between groups with occupations high and low in complexity with people for the first slope parameter, i.e., the rate of change up to retirement age. Parameter estimates resulting from fitting the full model (model 6) to the verbal component were used to calculate the change trajectories presented in Figure 3. Parameter estimates and standard errors are provided in the appendix. The growth curve model was centered on retirement age; therefore, the horizontal axis indicates how many years before (−15, −10, −5) and after (5, 10, 15) retirement age verbal performance was estimated. Although the intercept (at retirement age) and both slopes appear to differ between the two groups, only the group difference in slope 1 (before retirement) achieved significance. Individuals with occupations high in complexity with people demonstrated increases in verbal performance up to the age of retirement, whereas individuals with occupations low in complexity with people showed decreases in verbal performance from age 50 up to retirement.
3.
Verbal Ability. Trajectories estimated by the two-slope latent growth curve model for individuals with occupations high and low in complexity with people.
Appendix.
Parameter estimates and standard errors resulting from fitting full group differences in complexity of work with people (model 6) to the cognitive components. Model parameters are anchored for the low complexity of work with people group (lo) and then the change (Δ) in parameter for the high complexity of work with people group (hi) is indicated. For example, the mean intercept on the verbal component for the low complexity group is 53.71 and the mean intercept for the high complexity group is 55.30 (53.71 + 1.59).
| Parameter | Verbal | Spatial | Memory | Speed |
|---|---|---|---|---|
| Intercept (lo) | 53.71 (1.2) | 51.36 (1.1) | 54.51 (1.2) | 52.91 (1.1) |
| Δ Intercept (hi) | 1.59 (1.8) | 3.48 (1.6) | 1.25 (1.7) | 2.56 (1.3) |
| Education (lo) | 2.96 (1.7) | −1.62 (1.5) | 2.11 (1.7) | 0.80 (1.5) |
| Δ Education (hi) | 3.09 (2.1) | −1.83 (1.8) | 0.72 (2.0) | 2.42 (1.8) |
| Practice (lo) | 1.72 (0.9) | 2.14 (0.8) | 0.22 (0.9) | 0.92 (0.8) |
| Δ Practice (hi) | −0.94 (1.3) | −0.86 (0.9) | 1.95 (1.3) | 1.02 (1.0) |
| Slope 1 (lo) | −0.13 (0.2) | −0.35 (0.1) | 0.04 (0.2) | −0.27 (0.2) |
| Δ Slope 1 (hi) | 0.20 (0.2) | 0.00 (0.2) | −0.25 (0.2) | −0.25 (0.2) |
| Slope 2 (lo) | −0.10 (0.1) | −0.29 (0.1) | −0.24 (0.1) | −0.60 (0.1) |
| Δ Slope 2 (hi) | −0.10 (0.1) | −0.22 (0.1) | −0.13 (0.1) | −0.09 (0.1) |
| Practice × Education (lo) | 1.66 (1.2) | 1.65 (1.1) | −0.51 (1.2) | 0.05 (1.1) |
| Δ Practice × Education (hi) | −2.11 (1.5) | −1.83 (1.3) | 0.91 (1.5) | −0.75 (1.3) |
| Slope 1 × Education (lo) | −0.32 (0.2) | 0.00 (0.2) | −0.15 (0.2) | 0.10 (0.2) |
| Δ Slope 1 × Education (lo) | 0.35 (0.2) | 0.14 (0.2) | 0.19 (0.2) | 0.07 (0.2) |
| Slope 2 × Education (hi) | 0.05 (0.1) | 0.13 (0.1) | 0.15 (0.1) | −0.03 (0.1) |
| Δ Slope 2 × Education (lo) | −0.11 (0.1) | −0.22 (0.1) | −0.08 (0.1) | −0.06 (0.1) |
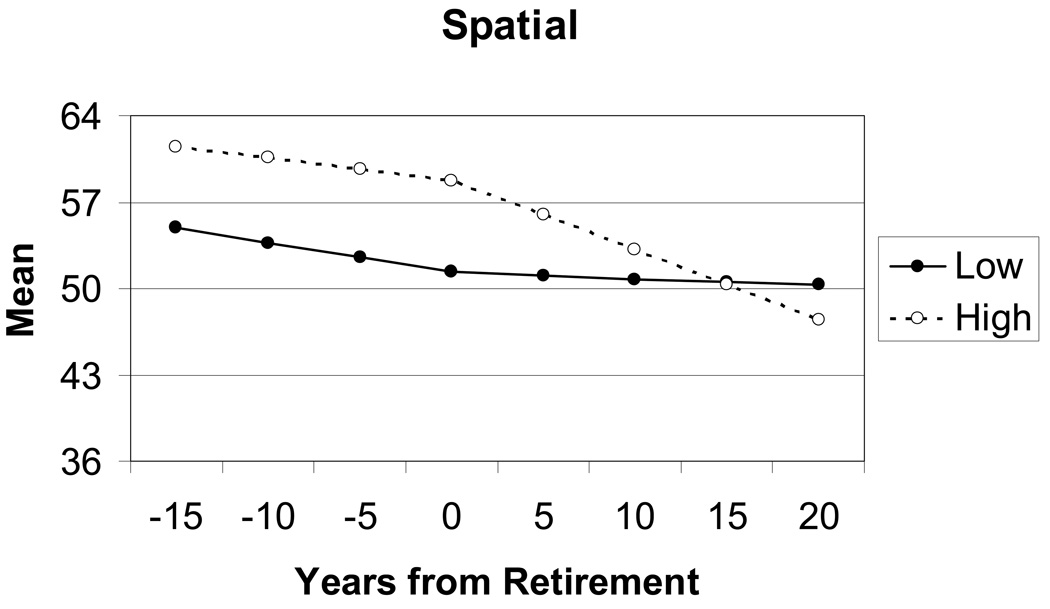
For the spatial component, retirement had a larger impact on rate of change in performance for the group high on complexity with people than for the low complexity group. The high complexity group is performing at a significantly higher overall level at retirement age, as indicated by the significant difference between models 1 and 2. Although the rate of decline in spatial ability was equivalent for both the high and low complexity groups prior to retirement (e.g., no significant difference between models 3 and 4), after retirement the situation changes, as presented in Figure 4. Individuals in the high complexity group demonstrated a significantly steeper rate of decline after retirement than individuals in the low complexity group. Mean group differences in the practice (or retest) effect were also found for the spatial component: the low complexity group demonstrated a larger mean improvement from first to second measurement occasion than the high complexity group, but the effect size was quite small (.16).
4.
Spatial Ability. Trajectories estimated by the two-slope latent growth curve model for individuals with occupations high and low in complexity with people.
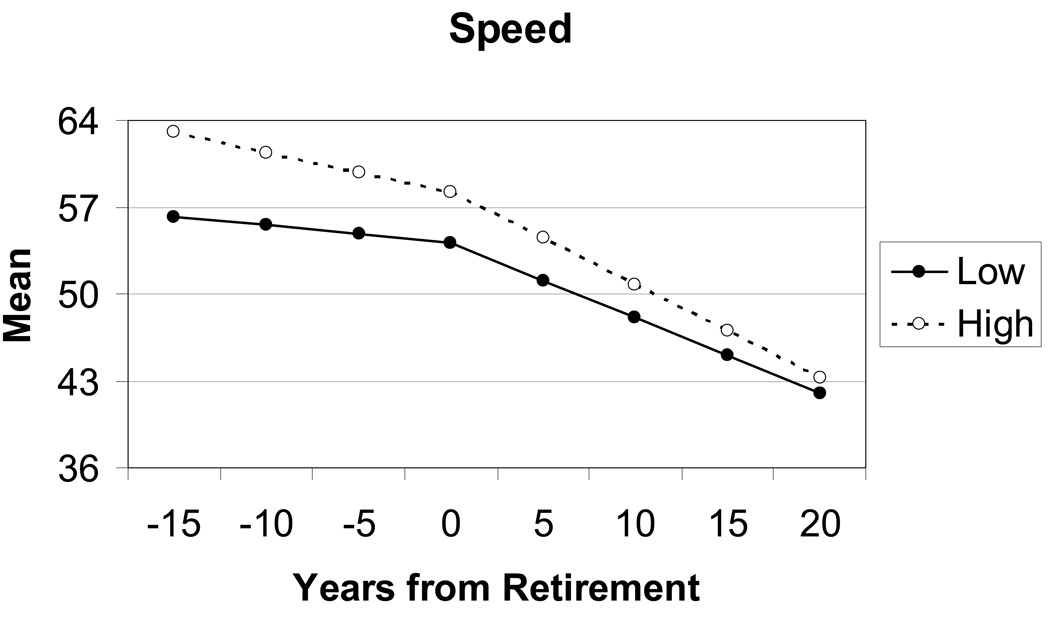
Finally, although loss of speed with age is apparent, the effect of occupational complexity for the processing speed component was limited to a significant difference in the intercept, or level of performance at retirement age. As we can see in Figure 5, performance on the processing speed tasks is declining before retirement, and the rate of decline accelerates after retirement for both high and low complexity groups.
5.
Processing Speed. Trajectories estimated by the two-slope latent growth curve model for individuals with occupations high and low in complexity with people.
Discussion
The purpose of this study was to examine the association between complexity of the main lifetime occupation, measured as complexity of work with data, people, and things, and trajectories of cognitive aging. We also considered the potential impact of retirement on this association. We measured cognitive aging across four latent components: verbal, spatial, memory, and speed. We first tested the hypothesis of preserved differentiation (Salthouse, 2006), i.e., that there would be higher levels of cognitive performance for those with more complex occupations. We then tested for differential preservation (Salthouse, 2006), i.e., whether mental practice offered by complex occupation would lead to differential preservation of cognitive skills, or slower cognitive aging. Finally, we tested the hypothesis that retirement has a more negative impact on cognitive skills in individuals retiring from complex occupations, as previously suggested by Schaie (2005).
We found that only one measure of complexity, complexity of work with people, was associated with cognitive aging. Within this complexity measure, for three of the four cognitive components we found support for the hypothesis of greater preservation of cognitive function for those with more complex lifetime occupations. Differences in trajectories were found in the verbal and spatial components. Specifically, we found that individuals holding occupations with high complexity of work with people experienced greater improvement in verbal skills up until retirement, suggesting continued facilitation of cognitive skills potentially attributable to mental practice at work. As hypothesized, following retirement, individuals previously holding jobs with high complexity of work exhibited faster rate of decline, although only on spatial ability.
Change with age in processing speed followed the pattern predicted by the preserved differentiation hypothesis. That is, individuals with occupations high in complexity with people demonstrated significantly faster processing speed, on average, than individuals whose occupations were low in complexity with people, but the mean difference was maintained over the age range studied. Parallel patterns of decline were identified for the two groups. Previous investigations of aging trajectories for the processing speed factor have reported strong genetic influences on rates of decline, with little contribution of environmental factors to variance in the slope parameters of the latent growth curve model (e.g., Finkel et al., 2005; 2007). It is not surprising, then, that the present analyses found that the cognitive stimulation provided by an environment high in complexity with people failed to slow the rate of change with aging for processing speed.
In general, these findings provide further support for the notion that complexity of work plays a role in cognitive aging (Andel et al., 2007; Bosma et al., 2003; Schooler et al., 2004; Potter et al., 2006) and provide new information about how individual cognitive components may be affected by work complexity, as well as about the potentially detrimental effect of retirement from a complex job on several cognitive domains. Our findings regarding the role of complexity of work with people in verbal and spatial skills can be interpreted as providing some support for the differential preservation hypothesis (Salthouse, 2006), as well as for the “use it or lose it” hypothesis, from two points of view. First, the favorable trajectory of cognitive change in verbal skills before retirement among individuals with complex occupations suggests that mental practice through complex work may facilitate verbal ability, leading to differential preservation of this skill. Second, the relatively precipitous decline in spatial skills following retirement from complex work with people implies a potentially detrimental effect that taking away this source of mental exercise may have on cognitive aging.
In our results, although occupational complexity was related to a higher level of cognitive performance, there was no evidence that occupational complexity protected against cognitive decline after retirement in any cognitive domain. These findings seem to be a logical extension of differential preservation. In this statement of the theory, intellectual stimulation at work plays a role in differential preservation of cognitive skills, while disuse of cognitive skills after work is discontinued may contribute to accelerated loss among those whose prior use had been greatest. It is important to note that these effects were limited to complexity of work with people; the results for complexity for work with data and things suggest no differentiation of any kind in cognitive aging trajectories.
Complexity of work with people, which has a relatively strong social component, was associated with cognitive aging, whereas complexity of work with data was not. Only a few studies examined complexity of work with data, people, and things in relation to cognitive aging. In one such study (Andel et al., 2007) both complexity of work with data and people yielded positive cross-sectional associations with cognitive functioning in old age. Previous findings with clinical populations support the importance of complex work with people. For example, Stern et al. (1995) found that interpersonal demands of main lifetime occupation delayed the onset of Alzheimer’s disease. Andel et al. (2005) found that complexity of work with people was associated with lower risk of dementia and Alzheimer’s disease. Kröger et al.(2008) recently replicated these findings and found a particularly strong effect of complexity in individuals who held their main occupation for at least 23 years.
Our finding that only complexity of work with people, not data or things, impacts cognitive aging parallels recent investigations of the relationship between social activities and cognitive aging. Applying dual change score models that allow investigation of the leading indicators of change, researchers found evidence that social participation influences subsequent changes in perceptual speed (Lövdén, Ghisletta, and Lindenberger, 2005). A similar analysis included measures of both perceptual speed and verbal fluency and various measures of activity engagement (Ghisletta, Bickel, & Lövdén, 2006). Results indicated that media and leisure activity (but not social activity) contribute to maintaining performance on perceptual speed measures, whereas verbal fluency was unaffected. In the current study, we found effects for verbal ability, spatial ability, and speed. Clearly, evidence is accumulating that the interactive component of engaging with people contributes to maintaining cognitive functioning.
We cannot exclude psychological factors as a plausible alternative explanation of our finding that retirement from a job with high complexity of work with people may lead to an accelerated cognitive decline compared to retiring from a job with low complexity. Individuals in complex (and likely relatively prestigious) jobs may be more socially and psychologically attached to these jobs than individuals in less complex types of jobs. Consequently, retirement from a complex job may carry a certain psychological burden projected as loss of social support and increased psychological distress, which by itself may adversely affect cognitive aging and decline (Wilson et al., 2006). Future research should aim to examine this possibility.
Several limitations should be noted. First, the hypothetical nature of occupational complexity precludes the possibility of a direct measurement, and it is difficult to assess the level of intellectual effort exerted by different individuals in the same occupation. As a result, the true effects of occupational complexity may be underestimated. Another potential concern is that a subjective measure of complexity may yield different results than an objective measure like the one used in this study. We also do not know whether additional control for occupational status would affect the results, although we did control for education, a proxy for occupational status. Second, because about 40% of the original SATSA sample did not report gainful occupations, the sample size was restricted. Although considerable variability in occupational complexity remained, it is possible that the uncoded occupations do not represent a random subset of the sample. Third, it is important to note that both education and gender differences exist in occupational achievement in the cohorts represented in the SATSA dataset. Gender differences in educational level explain most but not all of the gender differences in occupation. However, gender differences in occupational complexity with people were not significant. As a result, it was not necessary to include gender as a covariate in the latent growth curve models. Interestingly, although men demonstrated higher levels of complexity with data and things compared to women, women and men in the work force demonstrated equivalent levels of occupational complexity with people.
The relationships among occupation, education, and cognitive aging are complex (e.g., Powell & Whitla, 1994) and an argument could be made for alternate methods of modeling the impact of education. Comparing the results with and without education included as a covariate indicates one significant difference: complexity of work with data does impact cognitive aging when education is not included (Finkel, Andel, & Pedersen, 2007). Interestingly, even 40 years after education has been completed, including education as a covariate in the growth curve model eliminates the impact of complexity of work with data on trajectories of cognitive aging. Education may have a stronger role as pre-requisite for attaining a job defined as high in complexity of work with data than it has in occupations that involve complex work with people. It is difficult to estimate the continuing impact of initial educational on occupational success; therefore, it is possible that controlling for education may have resulted in underestimating job condition effects. It is important to note, however, that the ordinal measure of education used in the current analysis limits our ability to draw inferences.
In conclusion, this study supports the notion that high complexity of work with people may facilitate cognitive function, as evidenced by improved performance in verbal skills until retirement and indirectly by a faster rate of decline in spatial skills after retirement, when intellectual stimulation through complex work with people is removed. The possibility that complex work may lead to differential preservation of cognitive skills deserves further investigation. Gender and socioeconomic differences in access to occupations clearly exist: two-thirds of those who did not report gainful occupations were women. As a result, other measures of mental activity may provide additional insight in to the possible protective advantage of exercising cognitive skills. For example, participation in mentally challenging leisure activities is limited by neither gender nor retirement. We may find that evidence for relationships between mental activity and cognitive decline reported here is supported by additional analyses of the impact of leisure activities (e.g., Crowe, Andel, Pedersen, Johansson, & Gatz, 2003).
Acknowledgments
The Swedish Adoption/Twin Study of Aging (SATSA) is supported by NIA (AG04563, AG10175), The MacArthur Foundation Research Network on Successful Aging, the Swedish Council for Social Research (97:0147:1B), and the Swedish Research Council.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/pag.
All analyses were repeated using a quadratic growth curve model. The quadratic and two-slope growth curve models resulted in similar fit to the data and similar residual variance. Furthermore, the results of model comparisons between groups high and low in occupational complexity resulted in the same conclusions when either the quadratic or two-slope models were used.
Log transformation of the occupational complexity variables was also completed and the transformed variables were included as continuous covariates in the growth curve models. These analyses result in the same conclusions about group differences in trajectories of cognitive aging.
It is also possible to include all individuals in the model and include a correction for twin pairs in the modeling. The analyses were repeated using this method and resulted in the same conclusions about group differences.
References
- Andel R, Crowe M, Pedersen NL, Mortimer JA, Crimmins E, Johansson B, Gatz M. Complexity of work and risk of Alzheimer’s disease: a population-based study of Swedish twins. Journal of Gerontology: Psychological Sciences. 2005;60B:P251–P258. doi: 10.1093/geronb/60.5.p251. [DOI] [PubMed] [Google Scholar]
- Andel R, Kåreholt I, Parker M, Thorslund M, Gatz M. Complexity of main lifetime occupation and cognition in advanced old age. Journal of Aging and Health. 2007;19:397–415. doi: 10.1177/0898264307300171. [DOI] [PubMed] [Google Scholar]
- Bosma H, van Boxtel MPJ, Ponds RWHM, Houx PJ. Mental work demands protect against cognitive impairment: MAAS prospective cohort study. Experimental Aging Research. 2003;29:33–45. doi: 10.1080/03610730303710. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models. London: Sage Publications; 1992. [Google Scholar]
- Churchill JD, Galvez R, Colcombe S, Swain RA, Kramer AF, Greenough WT. Exercise, experience and the aging brain. Neurobiology of Aging. 2002;23:941–955. doi: 10.1016/s0197-4580(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer's Disease? A prospective study of Swedish Twins. Journals of Gerontology: Psychological Sciences. 2003;58B:P249–P255. doi: 10.1093/geronb/58.5.p249. [DOI] [PubMed] [Google Scholar]
- Ferrer E, Salthouse TA, McArdle JJ, Stewart WF, Schwartz BS. Multivariate modeling of age and retest in longitudinal studies of cognitive abilities. Psychology and Aging. 2005;20:412–422. doi: 10.1037/0882-7974.20.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Andel R, Pedersen NL. The role of occupational complexity in trajectories of cognitive aging. In: Reed S, Rantanen T, editors. Psychological Aging in Twins. San Francisco, CA: Symposium presented at the annual meeting of the Gerontological Society of America; 2007. Nov, (Chairs) [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging, Neuropsychology, and Cognition. 2004;11:325–345. [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. The longitudinal relationship between processing speed and cognitive ability: Genetic and environmental influences. Behavior Genetics. 2005;35:535–549. doi: 10.1007/s10519-005-3281-5. [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Gatz M, Pedersen NL. Latent growth curve analyses of accelerating decline in cognitive abilities in late adulthood. Developmental Psychology. 2003;39:535–550. doi: 10.1037/0012-1649.39.3.535. [DOI] [PubMed] [Google Scholar]
- Finkel D, Reynolds CA, McArdle JJ, Pedersen NL. Cohort differences in trajectories of cognitive aging. Journals of Gerontology: Psychological Sciences. 2007;62B:P286–P294. doi: 10.1093/geronb/62.5.p286. [DOI] [PubMed] [Google Scholar]
- Gatz M, Fiske A, Reynolds CA, Wetherell JL, Johansson B, Pedersen NL. Sex differences in genetic risk for dementia. Behavior Genetics. 2003;33:95–105. doi: 10.1023/a:1022597616872. [DOI] [PubMed] [Google Scholar]
- Gatz M, Pedersen NL, Berg S, Johansson B, Johansson K, Mortimer JA, Posner SF, Viitanen M, Winblad B, Ahlbom A. Heritability of Alzheimer’s Disease: The Study of Dementia in Swedish Twins. Journal of Gerontology: Medical Sciences. 1997;52:M117–M125. doi: 10.1093/gerona/52a.2.m117. [DOI] [PubMed] [Google Scholar]
- Ghisletta P, Bickel JF, Lövdén M. Does activity engagement protect against cognitive decline in old age? Methodological considerations. Journal of Gerontology: Psychological Sciences. 2006;61B:P253–P261. doi: 10.1093/geronb/61.5.p253. [DOI] [PubMed] [Google Scholar]
- Ginsburg H. Flexible and partial retirement for Norwegian and Swedish workers. Monthly Labor Review. 1985;108:33–43. [Google Scholar]
- Katzman R. Can late-life social and leisure activities delay the onset of dementia? Journal of the American Geriatrics Society. 1995;43:583–584. doi: 10.1111/j.1532-5415.1995.tb06112.x. [DOI] [PubMed] [Google Scholar]
- Kröger E, Andel R, Lindsay J, Benounissa Z, Verreault R, Laurin D The Canadian Study of Health and Aging. Is complexity of work associated with risk of dementia or Alzheimer’s disease? The American Journal of Epidemiology. doi: 10.1093/aje/kwm382. (in press) [DOI] [PubMed] [Google Scholar]
- Laird NM, Ware H. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- Lövdén M, Ghisletta P, Lindenberger U. Social participation attenuates decline in perceptual speed in old and very old age. Psychology and Aging. 2005;20:423–434. doi: 10.1037/0882-7974.20.3.423. [DOI] [PubMed] [Google Scholar]
- McArdle JJ, Anderson E. Latent variable growth models for research on aging. In: Birren JE, Schaie KW, editors. Handbook of the Psychology of Aging. 3rd Ed. New York: Academic Press; 1990. pp. 21–44. [Google Scholar]
- Miller AR, Treiman DJ, Cain PS, Roos PA. Work, jobs, and occupations: A critical review of occupational titles. Washington, DC: National Academy Press; 1980. [Google Scholar]
- Nesselroade JR, Pedersen NL, McClearn GE, Plomin R, Bergeman CS. Factorial and criterion validities of telephone-assessed cognitive ability measures: Age and gender comparisons in adult twins. Research on Aging. 1988;10:220–234. doi: 10.1177/0164027588102004. [DOI] [PubMed] [Google Scholar]
- Orrell M, Sahakian B. Education and dementia. British Journal of Medicine. 1995;310:951–952. doi: 10.1136/bmj.310.6985.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, de Faire U. The Swedish Adoption/Twin Study of Aging: An update. Acta Geneticae Medicae et Gemellologiae. 1991;40:7–20. doi: 10.1017/s0001566000006681. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. Quantitative genetic analysis of cognitive abilities during the second half of the lifespan. Psychological Science. 1992;3:346–353. [Google Scholar]
- Potter GG, Helms MJ, Plassman BL. Associations of job demands and intelligence with cognitive performance among men in late life. Neurology. 2008;70:1803–1808. doi: 10.1212/01.wnl.0000295506.58497.7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter GG, Plassman BL, Helms MJ, Foster SM, Edwards NW. Occupational characteristics and cognitive performance among elderly male twins. Neurology. 2006;67:1377–1382. doi: 10.1212/01.wnl.0000240061.51215.ed. [DOI] [PubMed] [Google Scholar]
- Powel DH, Whitla DK. Profiles in cognitive aging. Cambridge, MA: Harvard University Press; 1994. [Google Scholar]
- Roos PA, Treiman DJ. DOT scales for the 1970 Census classification. In: Miller AR, Treiman DJ, Cain PS, Roos PA, editors. Work, jobs, and occupations: A critical review of occupational titles. Washington, DC: National Academy Press; 1980. [Google Scholar]
- Salthouse TA. Mental exercise and mental aging: Evaluating the validity of the “use it or lose it” hypothesis. Perspectives on Psychological Science. 2006;1:68–87. doi: 10.1111/j.1745-6916.2006.00005.x. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Berish BE, Miles JD. The role of cognitive stimulation on the relations between age and cognitive functioning. Psychology and Aging. 2002;17:548–557. doi: 10.1037//0882-7974.17.4.548. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS system for Microsoft Windows Version 9 [Computer Software] Gary, NC: SAS Institute Inc; 2003. [Google Scholar]
- Schaie KW. Developmental influences on adult intelligence: The Seattle Longitudinal Study. New York: Oxford University Press; 2005. [Google Scholar]
- Schooler C. Psychological effects of complex environment during the life span: A review and theory. Intelligence. 1984;8:259–281. [Google Scholar]
- Schooler C. Use it-and keep it, longer, probably. A reply to Salthouse 2006. Perspectives on Psychological Science. 2007;2:24–29. doi: 10.1111/j.1745-6916.2007.00026.x. [DOI] [PubMed] [Google Scholar]
- Schooler C, Mulatu SM. The reciprocal effects of leisure time activities and intellectual functioning in older people: A longitudinal analysis. Psychology and Aging. 2001;16:466–482. doi: 10.1037//0882-7974.16.3.466. [DOI] [PubMed] [Google Scholar]
- Schooler C, Mulatu MS, Oates G. Occupational self-direction, intellectual functioning, and self-directed orientation in older workers: Findings and implications for individuals and societies. American Journal of Sociology. 2004;110:161–197. [Google Scholar]
- Smyth KA, Fritsch T, Cook TB, McLendon MJ, Santillan CE, Friedland RP. Worker functions and traits associated with occupations and the development of AD. Neurology. 2004;63:498–503. doi: 10.1212/01.wnl.0000133007.87028.09. [DOI] [PubMed] [Google Scholar]
- Stern Y, Alexander GE, Prohovnik I, Stricks L, Link B, Lennon MC, Mayeux R. Relationship between lifetime occupation and parietal flow: Implications for a reserve against Alzheimer’s disease pathology. Neurology. 1995;45 doi: 10.1212/wnl.45.1.55. [DOI] [PubMed] [Google Scholar]
- U.S. Bureau of the Census. Washington, D.C: U.S. government Printing Office; Census of population 1970. 1973 Subject Reports, Final Report PC(2)-7A. Occupational Characteristics.
- Wicherts JM, Dolan CV, Hessen DJ, Oosterveld P, van Baal GCM, Boomsma DI, Span MM. Are intelligence tests measurement invariant over time? Investigating the nature of the Flynn effect. Intelligence. 2004;32:509–537. [Google Scholar]
- Wight RG, Aneshensel CS, Seeman TE. Educational attainment, continued learning experience, and cognitive function among older men. Journal of Aging and Health. 2002;14:211–236. doi: 10.1177/089826430201400203. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Arnold SE, Schneider JA, Kelly JF, Tang Y, Bennett DA. Chronic psychological distress and risk of Alzheimer's disease in old age. Neuroepidemiology. 2006;27:143–153. doi: 10.1159/000095761. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Hoganson G, Bienias JL, Evans DA, Bennett DA. Early life socioeconomic status and late life risk of Alzheimer’s disease. Neuroepidemiology. 2005;25:8–14. doi: 10.1159/000085307. [DOI] [PubMed] [Google Scholar]