Abstract
The indirect immunoperoxidase antibody technique (IPA) has been applied to determine immunoglobulin (Ig)G to humans cytomegalovirus (CMV) antibodies in 114 blood donor sera, four cases of congenital cytomegalic inclusion disease, and four cases of acquired CMV infection. The results have been compared with those obtained with the CMV complement fixation (CF) test and indirect fluorescent antibody technique (IFA) for broad spectrum CMV antibody (sigmaAb) detection. IgG antibody has been detected by both CF and IPA. In healthy adult people IPA titers are usually higher than CF titers. In addition, IFA sigmaAb titers are generally higher than CF titers. Some sera negative by CF and IPA are positive at low dilutions by IFA sigmaAb antibody determination, due to the detection of small amounts of IgA or noncomplement-fixing IgG. Nonspecific results seem unlikely, since only nuclear inclusion fluorescence was interpreted as specific, as demonstrated by blocking tests. In acute CMV infection, the IFA sigmaAb and IPA IgG titers are essentially the same, except during the first weeks of infection, when IFA titers are higher and IgM is detectable. No cross-reactivity with other herpes group viruses, herpes simplex and varicella-zoster, was observed. Although some problems of nonspecific staining of cytoplasmic inclusions are shared by both methods, the IPA technique seems to possess the same degree of sensitivity and specificity as the IFA technique, but interpretation is easier and various procedural steps can be delayed without the technical problems associated with fluorescence microscopy.
Full text
PDF


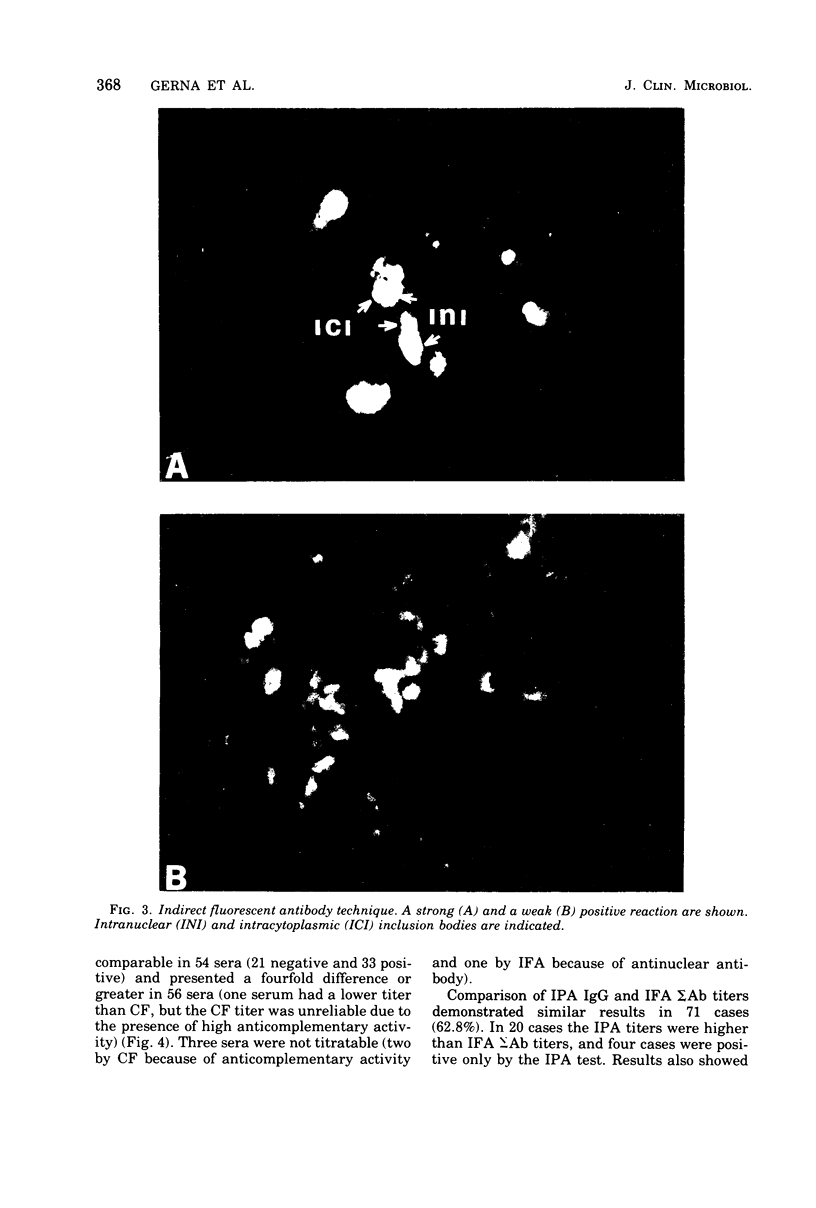
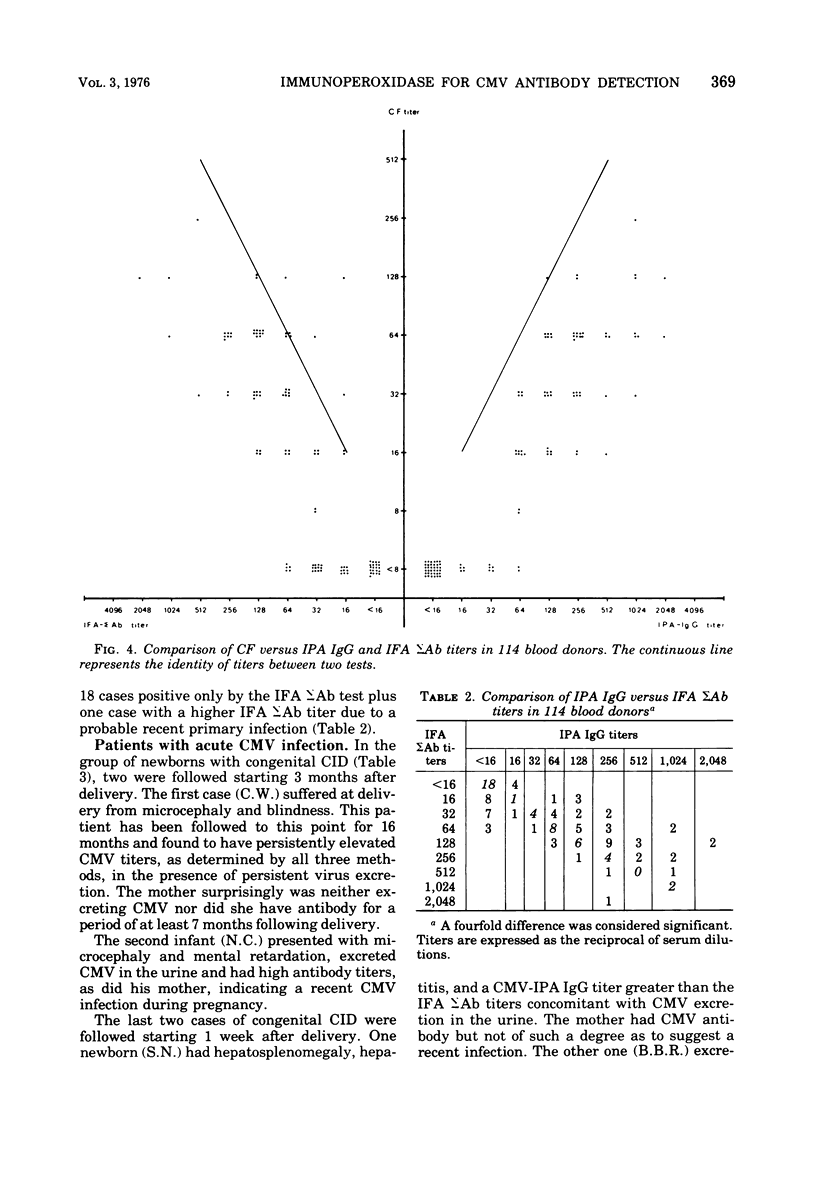
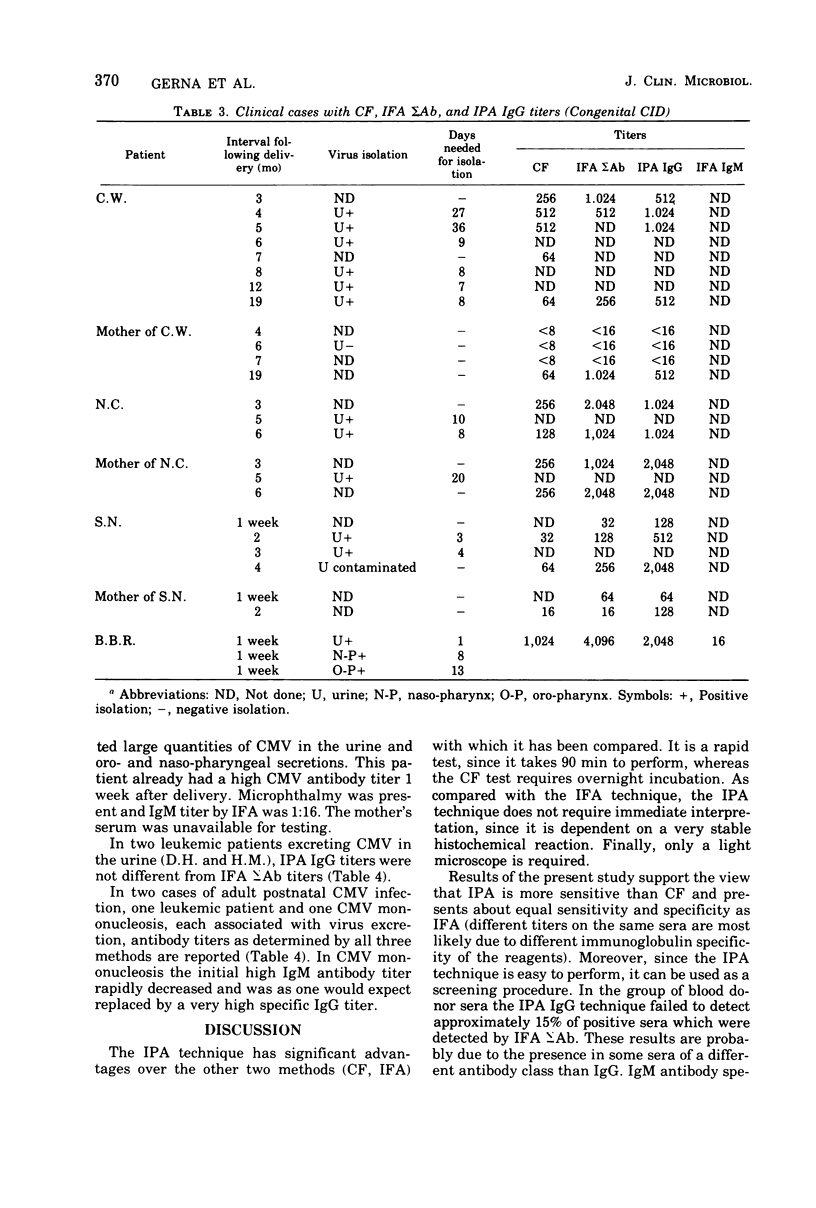
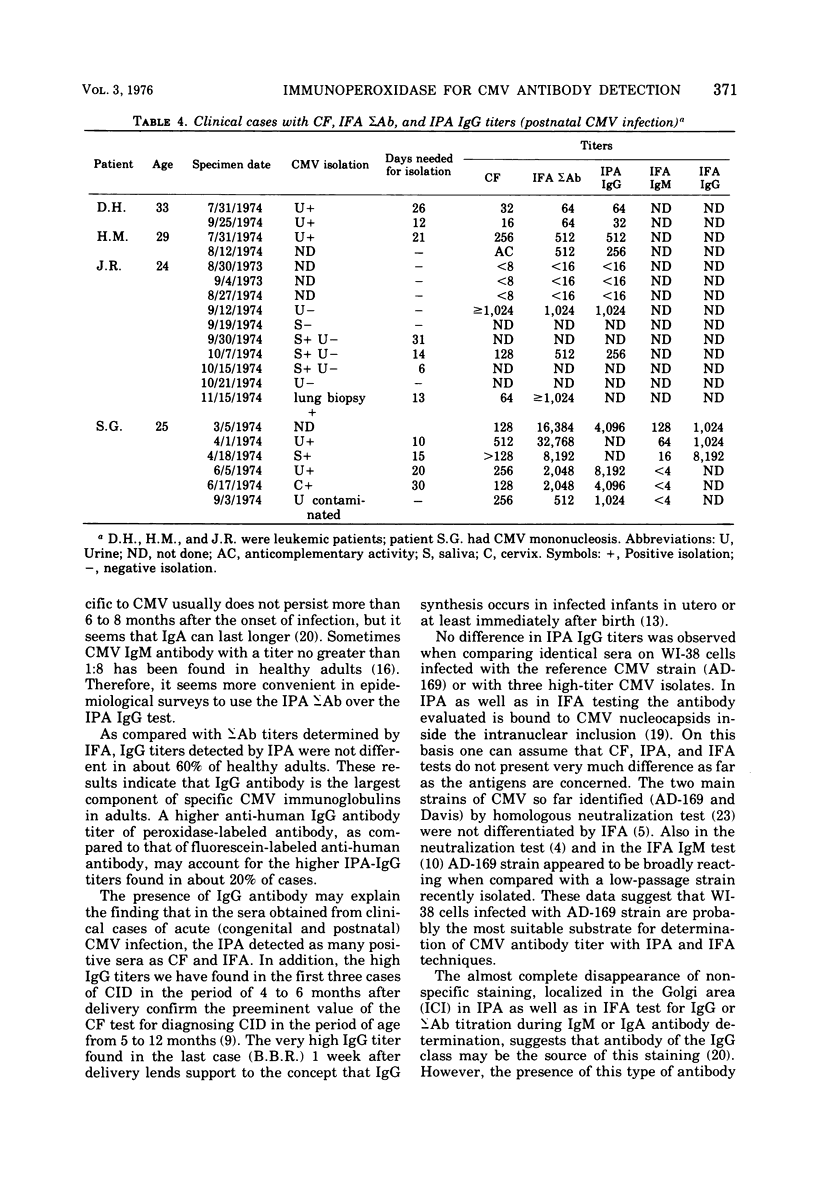

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avrameas S. Coupling of enzymes to proteins with glutaraldehyde. Use of the conjugates for the detection of antigens and antibodies. Immunochemistry. 1969 Jan;6(1):43–52. doi: 10.1016/0019-2791(69)90177-3. [DOI] [PubMed] [Google Scholar]
- Benyesh-Melnick M., Vonka V., Probstmeyer F., Wimberly I. Human cytomegalovirus: properties of the complement-fixing antigen. J Immunol. 1966 Feb;96(2):261–267. [PubMed] [Google Scholar]
- Bernstein M. T., Stewart J. A. Indirect hemagglutination test for detection of antibodies to Cytomegalovirus. Appl Microbiol. 1971 Jan;21(1):84–89. doi: 10.1128/am.21.1.84-89.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum G., Lynch J. I., Margileth A. M., Lonergan W. M., Sever J. L. Cytomegalovirus infections in newborn infants. J Pediatr. 1969 Nov;75(5):789–795. doi: 10.1016/s0022-3476(69)80301-x. [DOI] [PubMed] [Google Scholar]
- Chiang W. T., Wentworth B. B., Alexander E. R. The use of an immunofluorescence technique for the determination of antibodies to cytomegalovirus strains in human sera. J Immunol. 1970 Apr;104(4):992–999. [PubMed] [Google Scholar]
- Fuccillo D. A., Moder F., Traub R. G., Hensen S., Sever J. L. Micro indirect hemagglutination test for Cytomegalovirus. Appl Microbiol. 1971 Jan;21(1):104–107. doi: 10.1128/am.21.1.104-107.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Hanshaw J. B., Steinfeld H. J., White C. J. Fluorescent-antibody test for cytomegalovirus macroglobulin. N Engl J Med. 1968 Sep 12;279(11):566–570. doi: 10.1056/NEJM196809122791102. [DOI] [PubMed] [Google Scholar]
- Hebert G. A., Pelham P. L., Pittman B. Determination of the optimal ammonium sulfate concentration for the fractionation of rabbit, sheep, horse, and goat antisera. Appl Microbiol. 1973 Jan;25(1):26–36. doi: 10.1128/am.25.1.26-36.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Salmon S., Fudenberg H. Biologic activities of aggregated gamma-globulin. 8. Aggregated immunoglobulins of different classes. J Immunol. 1967 Jul;99(1):82–91. [PubMed] [Google Scholar]
- Kurstak E., Belloncik S., Onji P. A., Montplaisir S., Martineau B. Localisation par l'immunoperoxydase des antigènes du virus cytomégalique en culture cellulaire de fibroblastes humains. Microscopie photonique et électronique. Arch Gesamte Virusforsch. 1972;38(1):67–76. [PubMed] [Google Scholar]
- Lang D. J., Noren B. Cytomegaloviremia following congenital infection. J Pediatr. 1968 Dec;73(6):812–819. doi: 10.1016/s0022-3476(68)80233-1. [DOI] [PubMed] [Google Scholar]
- Langenhuysen M. M., The T. H., Nieweg H. O., Kapsenberg J. G. Demonstration of IgM cytomegalovirus-antibodies as an aid to early diagnosis in adults. Clin Exp Immunol. 1970 Mar;6(3):387–393. [PMC free article] [PubMed] [Google Scholar]
- Numazaki Y., Yano N., Morizuka T., Takai S., Ishida N. Primary infection with human cytomegalovirus: virus isolation from healthy infants and pregnant women. Am J Epidemiol. 1970 Apr;91(4):410–417. doi: 10.1093/oxfordjournals.aje.a121151. [DOI] [PubMed] [Google Scholar]
- PLUMMER G., BENYESH-MELNICK M. A PLAQUE REDUCTION NEUTRALIZATION TEST FOR HUMAN CYTOMEGALOVIRUS. Proc Soc Exp Biol Med. 1964 Oct;117:145–150. doi: 10.3181/00379727-117-29520. [DOI] [PubMed] [Google Scholar]
- Schmitz H., Haas R. Determination of different cytomegalovirus immunoglobulins (IgG, IgA, IgM) by immunofluorescence. Arch Gesamte Virusforsch. 1972;37(1):131–140. doi: 10.1007/BF01241158. [DOI] [PubMed] [Google Scholar]
- Stiehm E. R., Ammann A. J., Cherry J. D. Elevated cord macroglobulins in the diagnosis of intrauterine infections. N Engl J Med. 1966 Nov 3;275(18):971–977. doi: 10.1056/NEJM196611032751801. [DOI] [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B., SCOTT D. E. Serologic differentiation of viruses responsible for cytomegalic inclusion disease. Virology. 1960 Sep;12:130–132. doi: 10.1016/0042-6822(60)90156-2. [DOI] [PubMed] [Google Scholar]