Abstract
The peripheral nervous system detects different somatosensory stimuli including pain, temperature and touch. Merkel receptors are touch receptors composed of sensory afferents and Merkel cells. The role that Merkel cells play in light touch responses has been the center of controversy for over 100 years. We used Cre-loxP technology to conditionally delete the transcription factor Atoh1 from the body skin and foot pads of mice. Merkel cells are absent from these areas in Atoh1CKO animals. Ex vivo skin/nerve preparations from Atoh1CKO animals demonstrate complete loss of the characteristic neurophysiologic responses normally mediated by Merkel receptors. Merkel cells are therefore required for the proper encoding of Merkel receptor responses, suggesting that these cells form an indispensible part of the somatosensory system.
Different qualities of touch are encoded by discrete touch receptors, each with distinctive coding properties (1–3). One form of light touch important for tactile discrimination of shapes and textures is mediated by Merkel receptors, which exhibit a characteristic response to light skin indentation (4, 5). Merkel receptors are composed of nerve fibers associated with Merkel cells, an enigmatic skin cell population first described in 1875 (6). In mammalian skin, Merkel cells are normally found in whisker follicles of the face, specialized epithelial structures of the hairy skin called touch domes, and epidermal invaginations of the plantar foot surface called rete ridges (7). Merkel cells have been proposed to be the sensory receptor cells of the complexes because they form synaptic contacts with somatosensory afferents (8, 9); however, studies that indirectly tested this model have yielded conflicting results (10–16).
Atoh1 is a basic helix-loop-helix transcription factor expressed by Merkel cells in all areas of the skin (17). Atoh1 null mice die within minutes of birth, which prevents a detailed assessment of non-lethal phenotypes resulting from deletion of the gene. We used the Hoxb1Cre allele (18), which is expressed throughout the dermis and epidermis of body but not head skin (Fig. 1A, A’ and A”), to delete a floxed allele of Atoh1 (Atoh1flox) (19) in transgenic mice (Materials and method are available as supporting material on Science online20). Conditional knockout (Atoh1CKO) animals were born in the expected Mendelian ratio, but roughly 50% of these animals died within 24–36 hours of birth.
Fig. 1. Hoxb1Cre abolishes Atoh1flox expression in the body but not the face.
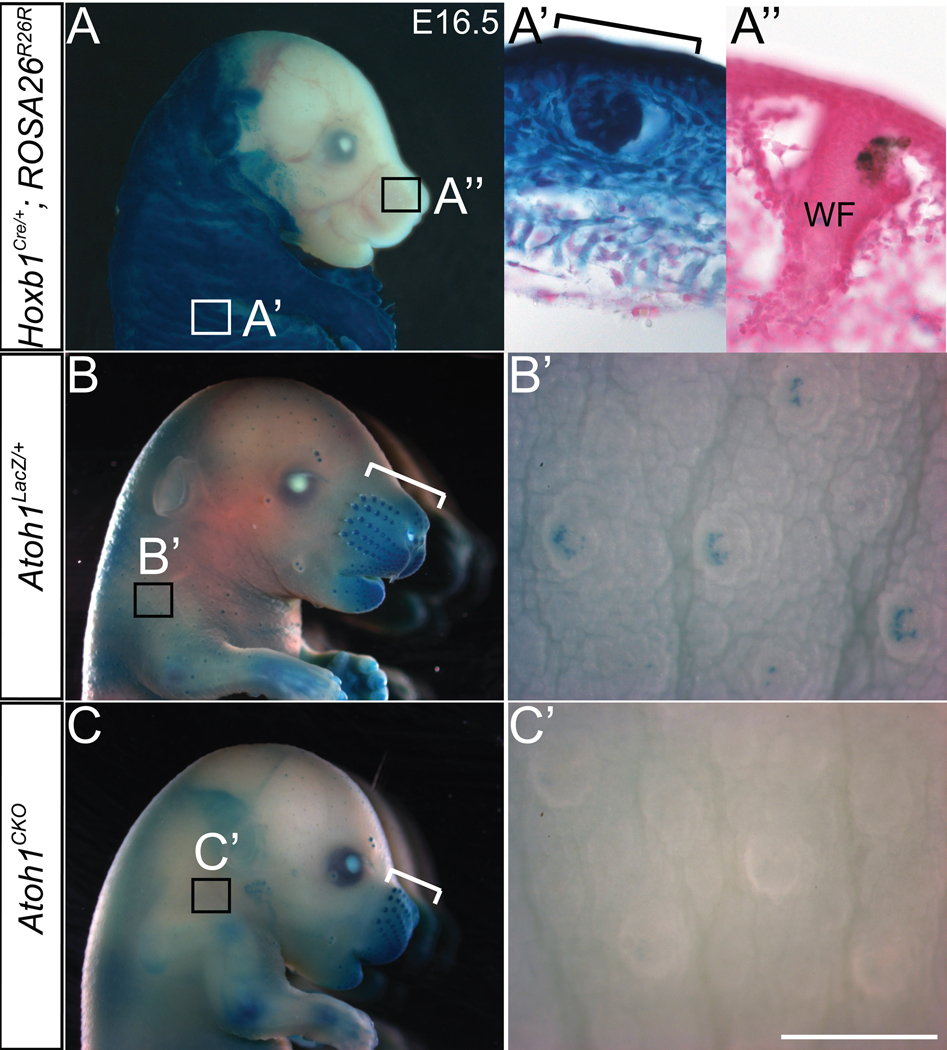
Wholemount X-gal staining of E16.5 embryos revealed β-galactosidase expression driven from the ROSA26R26R (A-A”) or Atoh1LacZ (B–C’) loci. A) Hoxb1Cre/+; ROSA26R26R embryo. Hoxb1Cre expression is absent from the majority of the head. Boxes denote regions shown in (A’) and (A”). A’) and A”) Body skin and whisker pad, respectively, from a Hoxb1Cre/+; ROSA26R26R embryo counterstained with nuclear fast red. Staining is present throughout the epidermis and dermis of the body skin, while virtually no staining is seen in the whisker pad. Bracket in (A’) denotes a developing touchdome. WF – whisker follicle. B) Atoh1LacZ/+ embryo showing β-galactosidase expression in the whisker pad (bracket) and touch domes of the skin. Box denotes region shown in (B’). B’) Hoxb1+/+; Atoh1LacZ/flox embryo body skin showing staining in individual touch domes. C) Atoh1CKO embryo showing β-galactosidase expression driven from the Atoh1 locus in the whisker pad (bracket), but absent from touch domes of the skin (box). C’) Atoh1CKO embryo body skin. Touch domes are present but lack the wildtype staining pattern. Scale bar: 5 mm (A, B, C); 400 µm (B’, C’); 100µm (A’, A”).
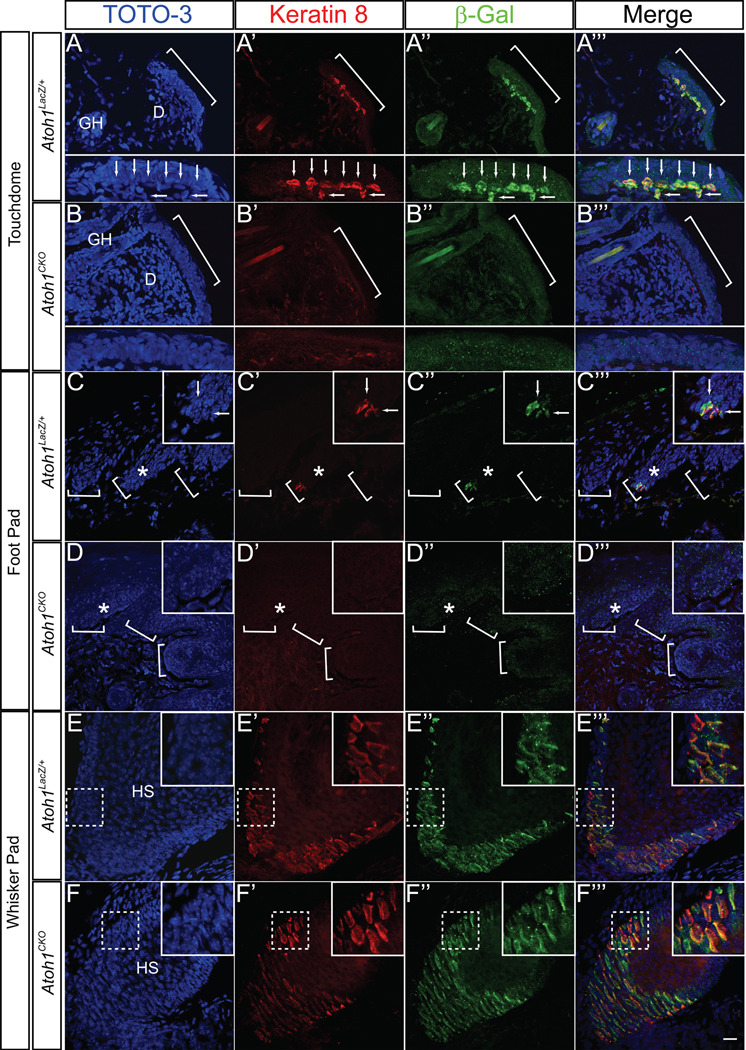
The overall structure of the touch dome, including the palisading epithelium and location of the guard hair, was preserved in Atoh1CKO animals (Fig. 1B, C and Fig. 2A, B). Atoh1 is a positive autoregulator of its own expression (21), so we analyzed β-galactosidase expression driven by the Atoh1LacZ knock-in allele (22) to demonstrate that Atoh1 was deleted from the skin of E16.5 Atoh1CKO mice (Fig. 1B and C). Xgal staining was found in touch domes and foot pads of heterozygous Atoh1LacZ/+ mice but not Atoh1CKO animals (Fig. 1B’ and C’). To determine whether Merkel cells were present, we used immunocytochemistry to compare the expression pattern of Keratin 8, an intermediate filament protein specifically expressed by Merkel cells in adult mammalian skin (23, 24), with that of β-galactosidase protein expression driven from the Atoh1LacZ locus (Fig. 2). Both proteins were expressed by Merkel cells in all regions of Atoh1LacZ/+ animals, but were absent throughout the body of Atoh1CKO animals except for the whisker pads where Hoxb1Cre is not expressed. We also found that VGLUT2, a synaptic vesicle protein that robustly labels Merkel cells and terminal afferent branches that innervate them (8, 25, 26), was absent from the body skin and foot pads but was present in whisker pads of Atoh1CKO animals (Fig. 3A, B). We confirmed these findings by examining over 100 touch domes and 16 foot pads from four adult Atoh1CKO mice. These results differ from a previous report from our group suggesting that Merkel cells are present in Atoh1-null embryos (17). That study was limited by the necessity of examining prenatal mice because Atoh1-null animals die within minutes of birth secondary to respiratory failure (22). Here, our use of conditional knockout animals enabled us to examine specific Merkel-cell markers in fully developed skin and to show Merkel cell loss in Atoh1CKO mice. To our knowledge Atoh1 is the first gene shown to be necessary for the specification of Merkel cells. Our data also demonstrate that Merkel cells are not necessary to specify or maintain touch dome ultrastructure, as guard hairs and the overlying keratinocytes appear completely normal in the hairy skin of Atoh1CKO mice.
Fig. 2. Merkel cells are absent from the body skin and foot pads of Atoh1CKO animals.
All tissue is from P22 animals, and all images are z-stack projections of confocal images. A–B”’) Skin from Atoh1LacZ/+ (A-A”’) and Atoh1CKO (B-B”’) animals. Brackets denote a single touch dome. Subpanels in A-A”’ show Merkel cells (arrows) from the wildtype touch dome denoted by the bracket, while none are seen in the Atoh1CKO animal (subpanels B-B”’). D – dermis, GH – guard hair. C–D”’) Hind foot pad from Atoh1LacZ/+ (C-C”’) and Atoh1CKO (D-D”’) animals. Brackets denote epidermal rete ridges, asterisks denote areas shown in insets, and arrows in (C-C”’) mark individual Merkel cells. Cells positive for Keratin 8 and β-galactosidase are absent from Atoh1CKO body skin and foot pad. E–F”’) Whisker pad from Atoh1LacZ/+ (E-E”’) and Atoh1CKO (F-F”’) animals. There is no difference in the immunostaining patterns between the two genotypes. Dotted boxes outline areas shown in insets. HS – whisker hair shaft. All Keratin 8-positive cells were also β-galactosidase-positive and vice versa in all tissues of both genotypes. Scale bar: 25µm in all panels, 12.5 µm in subpanels and insets.
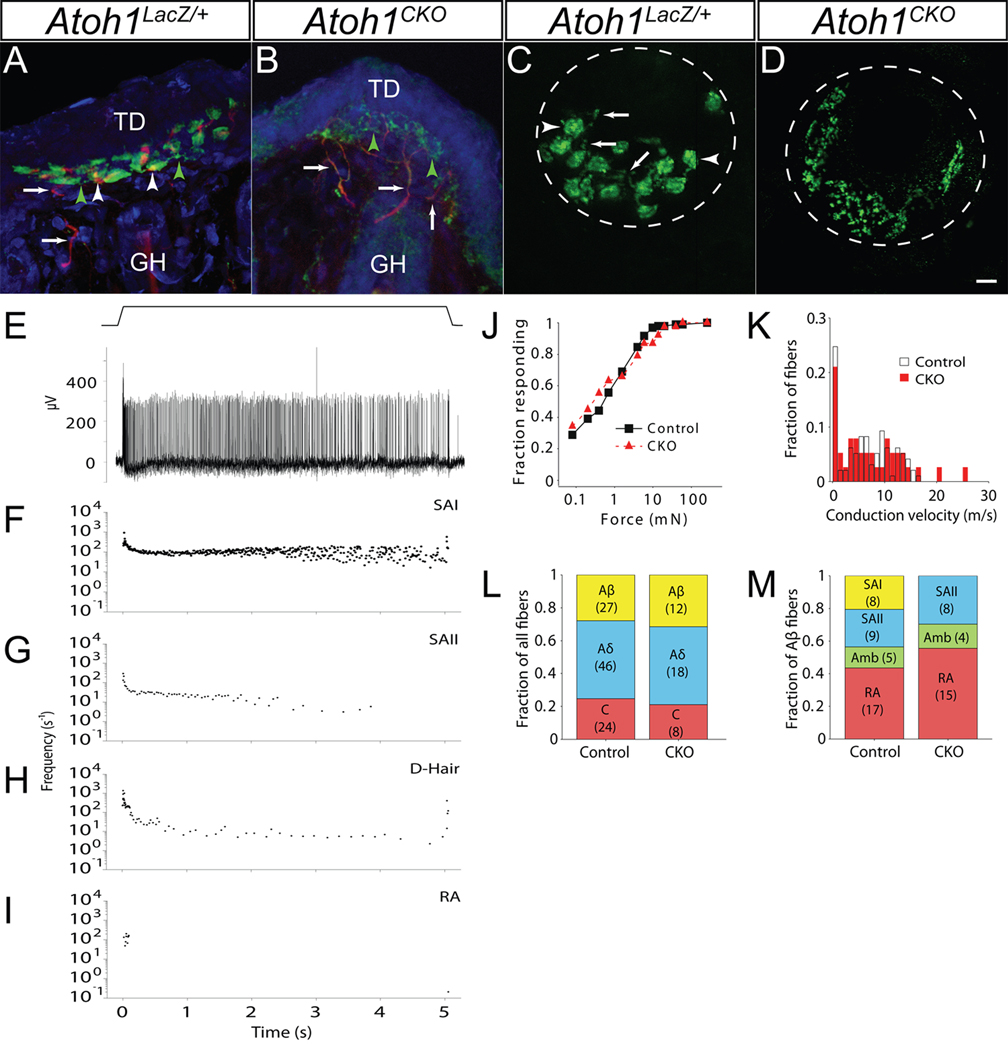
Fig. 3. Touch dome afferents are present but SAI responses are absent in Atoh1CKO mice.
A, B) Touch dome sections from P22 Atoh1LacZ/+ (A) and Atoh1CKO (B) animals immunostained for NF200 (red) and VGLUT2 (green). Arrows mark nerve branches innervating the touch dome, white arrowheads mark nerve terminal branches contacting individual Merkel cells, and green arrowheads mark VGLUT2-positive, NF200-negative nerve terminal branches. Counterstain (blue) is TOTO3. Note the lack of cellular staining but increased nerve branching pattern in (B). GH – guard hair, TD – touch dome. C, D) In vivo FM 1–43 dye labeling of adult Atoh1LacZ/+ (C) and Atoh1CKO (D) touch domes. Dotted lines delineate touch dome boundaries. Arrows (C) show sensory nerve branches; arrowheads mark Merkel cells. Note the excessive sensory nerve branching but absence of Merkel cells in (D). Scale bar: 12.5µm. E) Semi-intact recordings from touch-sensitive afferents innervating hairy skin of wildtype mice. Top: Displacement trace showing a 5-s touch to the skin surface. Bottom: slowly-adapting type I (SAI) response to the 5-s mechanical stimulus shown above. F–I) Representative plots of instantaneous firing frequency vs. time for 5-s mechanical stimuli of wildtype afferent fibers. F) Aβ SAI fiber. G) Aβ slowly-adapting type II (SAII) fiber. H) Aδ down hair fiber (D-Hair). I) Aβ rapidly-adapting (RA) fiber. J, K) Atoh1CKO fiber populations did not differ from wildtype littermates in mechanical sensitivity (by von Frey threshold, J) or conduction velocity (K) (Atoh1CKO n=38, wildtype n=97; p>0.10, Mann-Whitney U-test). L) A survey of all mechanosensitive afferents revealed that fiber type proportions (Aβ, Aδ and C) were not significantly different in Atoh1CKO mice compared to wildtype littermate control animals (p>0.10, Fisher exact test). M) Directed survey of Aβ-subtypes in Atoh1CKO and control mice. No SAI responses were found among Aβ fibers in Atoh1CKO mice (p<0.02, Fisher exact test). Number of fibers in (L) and (M) are shown in parentheses.
Crescent-shaped clusters of Merkel cells are normally found within each touch dome, where they are innervated by a single sensory afferent that expresses neurofilament 200 (NF200) in large and medium-sized branches and VGLUT2 in terminal branches (26) (Fig. 3A). Innervation of touch domes was present in wildtype and Atoh1CKO animals as revealed by NF200 immunocytochemistry (Fig. 3A and B). However, there was exuberant branching of the VGLUT2-positive, NF200-negative terminal ends of touch dome afferent fibers of Atoh1CKO animals (Fig. 3B). We confirmed this observation using in vivo subcutaneous injections of the styryl dye FM 1–43. FM 1–43 incorporates into the outer portion of the lipid bilayer, and it strongly labels Merkel cells and their afferent fibers in vivo (27) in wildtype mice, permitting wholemount analysis of Merkel receptor structure (Fig. 3C). Afferent terminal branches were labeled in the touch domes of Atoh1CKO animals despite the absence of Merkel cells (Fig. 3D). Both of these methods demonstrated that touch domes in Atoh1CKO animals display excessive terminal branching compared to wildtype animals. These data suggest that, although Merkel cells are not necessary for the development or maintenance of touch dome innervation, they play a role in the acquisition of the typical terminal arborization pattern of touch dome afferents. Several neurotrophins have been implicated in the development and maintenance of Merkel cell innervation (28, 29). Our data also demonstrate that Merkel cells cannot be the primary source of these trophic factors.
Many different afferent somatosensory fiber types innervate the skin (30). These fibers can be grouped by conduction velocity into three broad categories: Aβ-, Aδ- and C-fibers (Table S1). Nociceptors and temperature receptors are primarily of the Aδ- and C-subtypes, whereas light touch and joint position sense are mediated by Aβ-fibers. The Aβ-fiber subclass can be further subdivided by the adaptation characteristics of the fibers: slowly adapting type I (SAI) fibers innervate Merkel receptors (5), SAII fibers are thought to innervate Ruffini corpuscles, and rapidly adapting (RA) fibers innervate Meissner and Pacinian corpuscles (1). Each of these subclasses is important for detecting a specific form of touch (1). The absence of Merkel cells in Atoh1CKO animals together with the presence of touch dome innervation provided the perfect opportunity to test whether Merkel cells are required for mechanotransduction by their innervating nerve fibers.
The overall population of cutaneous afferent receptors is normal in Atoh1CKO animals (Fig. 3J–L). We applied electrical and mechanical stimuli to the epidermal surface of ex vivo skin/saphenous nerve preparations from wildtype and Atoh1CKO animals and simultaneously recorded extracellular responses from teased afferent fibers (Fig. 3E–M). There were no differences between wildtype and Atoh1CKO mice in the mechanical thresholds (Fig. 3J), conduction velocities (Fig. 3K), or proportions (Fig. 3L) of touch-sensitive fibers (p>0.1 by Mann-Whitney U-test for all tests).
We next focused specifically on the Aβ-fiber population. The distribution of Aβ subtypes revealed a conspicuous loss of SAI responses among slowly adapting Aβ-afferents in Atoh1CKO animals (N=0/27 afferents) compared with wildtype animals (N=8/39 afferents; Fig. 3M). We observed a proportional expansion of other Aβ-afferent subtypes (20). These data are consistent with a complete loss of mechanosensitive SAI fibers. Thus, canonical SAI responses elicited by touch require Merkel cells. It is possible that touch dome afferents lacking Merkel cells are capable of detecting somatosensory stimuli and are represented in the “ambiguous” class (Fig. S1); however, they are unlikely to constitute the whole population because we observed similar responses in wildtype mice. We did not observe an overall loss of Aβ fibers by electrophysiological testing. This finding further supports our immunocytochemical data indicating that Merkel cells are not necessary for touch dome innervation, but that they are likely required for proper pruning and maturation of these somatosensory neurons.
For more than a century, neurobiologists have postulated that Merkel cells are responsible for the specialized coding properties that allow their afferent nerves to resolve fine spatial details. Our genetic knockout approach has allowed us to directly test this hypothesis and to demonstrate that Merkel cells are indeed essential for these responses. Since Merkel cells fail to develop in Atoh1CKO mice, a key question that remains is whether Merkel cells, somatosensory neurons or both are sites of mechanotransduction at the skin surface. Selective and acute control of Merkel-cell signaling will be necessary to determine whether Merkel cells act as sensory receptor cells or serve another role in touch.
Supplementary Material
References and Notes
- 1.Johnson KO. Curr Opin Neurobiol. 2001 Aug;11:455. doi: 10.1016/s0959-4388(00)00234-8. [DOI] [PubMed] [Google Scholar]
- 2.Johnson KO, Lamb GD. J Physiol. 1981 Jan;310:117. doi: 10.1113/jphysiol.1981.sp013540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson KO, Yoshioka T, Vega-Bermudez F. J Clin Neurophysiol. 2000 Nov;17:539. doi: 10.1097/00004691-200011000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Iggo A, Muir AR. J Physiol. 1969 Feb;200:763. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woodbury CJ, Koerber HR. J Comp Neurol. 2007 Dec 10;505:547. doi: 10.1002/cne.21517. [DOI] [PubMed] [Google Scholar]
- 6.Merkel FS. Arch Mikrosc Anat. 1875;11:636. [Google Scholar]
- 7.Halata Z, Grim M, Bauman KI. Anat Rec A Discov Mol Cell Evol Biol. 2003 Mar;271:225. doi: 10.1002/ar.a.10029. [DOI] [PubMed] [Google Scholar]
- 8.Haeberle H, et al. Proc Natl Acad Sci U S A. 2004 Oct 5;101:14503. doi: 10.1073/pnas.0406308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hartschuh W, Weihe E. J Invest Dermatol. 1980 Aug;75:159. doi: 10.1111/1523-1747.ep12522555. [DOI] [PubMed] [Google Scholar]
- 10.Cahusac PM, Senok SS. Synapse. 2006 Mar 15;59:235. doi: 10.1002/syn.20236. [DOI] [PubMed] [Google Scholar]
- 11.Diamond J, Mills LR, Mearow KM. Prog Brain Res. 1988;74:51. doi: 10.1016/s0079-6123(08)62997-0. [DOI] [PubMed] [Google Scholar]
- 12.Fagan BM, Cahusac PM. Neuroreport. 2001 Feb 12;12:341. doi: 10.1097/00001756-200102120-00032. [DOI] [PubMed] [Google Scholar]
- 13.Gottschaldt KM, Vahle-Hinz C. Science. 1981 Oct 9;214:183. doi: 10.1126/science.7280690. [DOI] [PubMed] [Google Scholar]
- 14.Kinkelin I, Stucky CL, Koltzenburg M. Eur J Neurosci. 1999 Nov;11:3963. doi: 10.1046/j.1460-9568.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 15.Pacitti EG, Findlater GS. Prog Brain Res. 1988;74:37. doi: 10.1016/s0079-6123(08)62995-7. [DOI] [PubMed] [Google Scholar]
- 16.Senok SS, Baumann KI, Halata Z. Exp Brain Res. 1996 Aug;110:325. doi: 10.1007/BF00229133. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Arie N, et al. Development. 2000 Mar;127:1039. doi: 10.1242/dev.127.5.1039. [DOI] [PubMed] [Google Scholar]
- 18.Arenkiel BR, Gaufo GO, Capecchi MR. Dev Dyn. 2003 Jul;227:379. doi: 10.1002/dvdy.10323. [DOI] [PubMed] [Google Scholar]
- 19.Shroyer NF, et al. Gastroenterology. 2007 Jun;132:2478. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 20.Materials and methods are available as supporting material on Science online.
- 21.Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Development. 2000 Mar;127:1185. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Arie N, et al. Nature. 1997 Nov 13;390:169. doi: 10.1038/36579. [DOI] [PubMed] [Google Scholar]
- 23.Moll R, Moll I, Franke WW. Differentiation. 1984;28:136. doi: 10.1111/j.1432-0436.1984.tb00277.x. [DOI] [PubMed] [Google Scholar]
- 24.Vielkind U, Sebzda MK, Gibson IR, Hardy MH. Acta Anat (Basel) 1995;152:93. doi: 10.1159/000147688. [DOI] [PubMed] [Google Scholar]
- 25.Hitchcock IS, Genever PG, Cahusac PM. Neurosci Lett. 2004 May 27;362:196. doi: 10.1016/j.neulet.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 26.Nunzi MG, Pisarek A, Mugnaini E. J Neurocytol. 2004 May;33:359. doi: 10.1023/B:NEUR.0000044196.45602.92. [DOI] [PubMed] [Google Scholar]
- 27.Meyers JR, et al. J Neurosci. 2003 May 15;23:4054. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cronk KM, et al. Development. 2002 Aug;129:3739. doi: 10.1242/dev.129.15.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fundin BT, et al. Dev Biol. 1997 Oct 1;190:94. doi: 10.1006/dbio.1997.8658. [DOI] [PubMed] [Google Scholar]
- 30.Lumpkin EA, Caterina MJ. Nature. 2007 Feb 22;445:858. doi: 10.1038/nature05662. [DOI] [PubMed] [Google Scholar]
- 31.We would like to thank Dr. M. Capecchi for generously providing us with Hoxb1Cre animals; N. Ao, K. Firozi, E. Mathes, K. Morrison and S. Vaishav for technical assistance; and Drs. G. Landreth, L. Landmesser and R. Miller for critical reading of the manuscript. Funding sources: SMM (NIH 5K08NS53419), AMN (McNair Scholars Program), DRL (NLM T15LM009462), GJG (Defense Advanced Research Projects Agency HR0011-08-1-0072), EAL (NIH AR051219), and HYZ (investigator, HHMI).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.