Abstract
In two conditioned lick suppression experiments using water-deprived rats, we examined the effects of following Pavlovian conditioned inhibition training (i.e., A–US/AX-NoUS) with pairings of the training excitor (A) and the unconditioned stimulus (US). Experiments 1 and 2 assessed the effects of this posttraining inflation treatment on Pavlovian conditioned inhibition using a summation test and a retardation test, respectively. Both experiments revealed that subjects exposed to inflation treatment demonstrated behavior indicative of enhanced conditioned inhibition compared to subjects that did not receive inflation treatment. The results in conjunction with other recent findings suggest that posttraining associative deflation (i.e., extinction of A) and inflation effects are more symmetrical than has previously been realized.
Stimulus interaction in the broad sense refers to phenomena in which stimuli (discrete or contextual) that were present during training of a target stimulus influence responding to the target stimulus at the time of test (e.g., overshadowing, blocking, and conditioned inhibition). There has been continuing debate between advocates of learning- and of performance-focused accounts of stimulus interaction, which continues today because of their very different views of acquisition, retrieval, and expression of relationships between environmental events (e.g., Miller & Matzel, 1988; Rescorla & Wagner, 1972). The empirical findings and theoretical implications generated by this debate have increased our understanding of the factors that influence learning and acquired performance.
One important finding was that behavioral control by a target conditioned stimulus (CS) can be influenced by posttraining changes in the associative status of a companion stimulus (one that was present when the target stimuli were paired). Kaufman and Bolles (1981) first demonstrated such posttraining effects in rats using an overshadowing procedure. Overshadowing treatment involves presentations of a compound stimulus AX, composed of the target conditioned stimulus (CS) X and a companion stimulus A, followed by an unconditioned stimulus (US), which reduces responding to the target CS at the time of test relative to subjects trained in the absence of A (i.e., X–US pairings). Kaufman and Bolles extinguished (i.e., associatively deflated) the companion stimulus (A) after overshadowing treatment. This posttraining manipulation produced increased responding to the target stimulus (X) at test (i.e., recovery from overshadowing was observed). Such observations posed a serious challenge to the then prevailing associative theories, which had assumed that responding to the target CS (i.e., performance) was a direct function of the associative strength accrued during training (i.e., learning). Miller and his colleagues replicated this phenomenon (Matzel, Schachtman, & Miller, 1985; Matzel, Shuster, & Miller, 1987) and provided the earliest theoretical account of it. Specifically, Miller and Matzel (1988) proposed the comparator hypothesis which posited that responding to X was the result of a comparison between expectancies of the US evoked by X and by its comparator (A) at the time of testing.
The comparator hypothesis distinguished itself from other associative models of acquired behavior because it focused on the interaction of associations at the time of performance to account for stimulus interaction and denied the existence of inhibitory associations (i.e., all associations are excitatory). In this framework, cues do not compete for associative strength at the time of training; rather, they compete for control of responding at the time of testing based on the excitatory status of each of the cues relative to one another. According to Miller and Matzel (1988), conditioned responding to the target CS at the time of testing depends on the strength of the target CS–US association relative to the strength of the associations between other stimuli that were present during target CS–US training (comparator stimuli; e.g., the context or other discrete stimuli) and the US. The relevant associations are depicted in Fig. 1; they include associations between the target CS and the US (Link 1), the target CS and the comparator stimulus (Link 2), and the comparator stimulus and the US (Link 3). During testing the presentation of the target stimulus directly activates a representation of the US through Link 1, which is then compared to a representation of the US that is indirectly evoked through activation of the representation of the comparator stimulus (Link 2) and the comparator stimulus' association with the US (Link 3). The strength of the expression of the target excitatory association is directly related to the strength of the target CS–US association (Link 1) and inversely related to the product of the CS–comparator association (Link 2) and the comparator–US association (Link 3). For example, in an overshadowing procedure the comparator hypothesis assumes that the overshadowed CS X forms a direct association with the US (Link 1) and that CS X also forms an association with the overshadowing CS A (Link 2) which in turn has its own association with the US (Link 3). At test, Link 1 augments responding to CS X, whereas the product of Links 2 and 3 attenuate responding to CS X. Therefore, at test, the presentation of the target CS elicits weaker responding compared to a situation in which the CS A was not present during training.
Fig. 1.
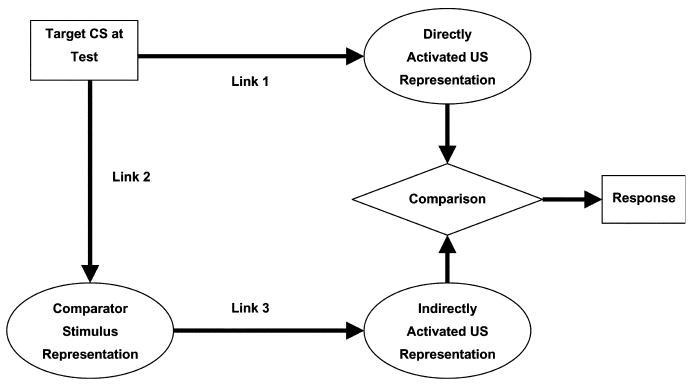
The original comparator hypothesis. Links represent: (1) the target CS–US association, (2) the target CS–comparator stimulus within-compound association, and (3) comparator stimulus–US association. At test, presentation of the target CS evokes two representations of the US, one directly (through Link 1) and another indirectly mediated through a representation of the comparator stimulus (Links 2 and 3). The strengths of the directly and indirectly activated US representations are compared to determine responding to the target CS.
As demonstrated by Kaufman and Bolles (1981) and others (e.g., Best, Dunn, Batson, Meachum, & Nash, 1985; Dickinson & Charnock, 1985; Kasprow, Schachtman, & Miller, 1987; Lysle & Fowler, 1985; Matzel et al., 1985; Miller, Hallam, Hong, & Dufore, 1991; Schachtman, Brown, Gordon, Catterson, & Miller, 1987; Wasserman & Berglan, 1998) and as initially uniquely predicted by the comparator hypothesis, many different stimulus interaction effects can be altered by posttraining manipulations of the associative status of the target CSs companion stimulus, which in turn influence responding to the target CS. The comparator hypothesis predicts that posttraining associative deflation of the comparator stimulus (nonreinforced presentations of the companion stimulus alone after training with the target CS; i.e., extinction) should enhance excitatory responding to the target, and conversely that posttraining inflation (reinforced presentations of the companion stimulus alone after training with the target CS) should reduce excitatory responding to the target. Moreover, the comparator hypothesis predicts that posttraining associative inflation of a conditioned inhibitor's companion stimulus (the inhibition training excitor) should enhance behavioral control by the target CS that is indicative of inhibition and conversely that posttraining deflation should attenuate behavioral control by the target CS that is indicative of inhibition. Based on these predictions, a 2 (excitation vs. inhibition training) × 2 (deflation vs. inflation of the companion stimulus) matrix can be created (see Fig. 2).
Fig. 2.
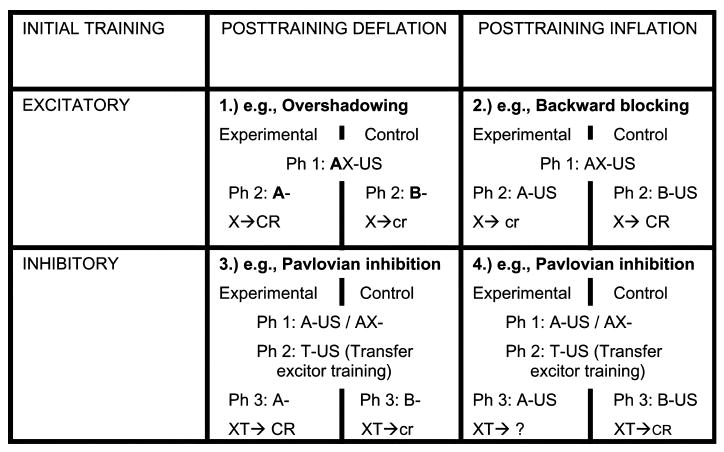
A 2 × 2 matrix of deflation and inflation effects upon both excitatory and inhibitory conditioning. ‘Ph’ followed by a number indicates a specific phase of training. ‘X’ represents the target stimulus, ‘A’ represents the companion stimulus, ‘US’ represents an unconditioned stimulus, and ‘-’ indicates that the stimulus was not followed by an unconditioned stimulus. ‘B’ represents the alternative stimulus that was presented to the control group. X or XT represents the test phase. ‘→’ indicates followed by. ‘CR’ and ‘cr’ indicate the expectation of robust and weak conditioned responding, respectively. Cell 1 (upper left cell) depicts an example of deflating the companion stimulus after conditioned excitation training. The boldface A indicates that A is of higher salience than X to encourage overshadowing. Cell 2 (upper right cell) depicts an example of inflating the companion stimulus after excitation training. To minimize overshadowing of X by A so there is the opportunity to see a decrease in responding to X, A is of relatively low salience. Cell 3 (lower left cell) shows an example of deflating the companion stimulus after conditioned inhibition training. Cell 4 (lower right cell) depicts an example of inflation of the companion stimulus after conditioned inhibition training. The effect anticipated in Cell 4 has yet to be demonstrated as denoted by the question mark.
Three of the cells of the matrix in Fig. 2 have been extensively examined, confirming the expectations of the comparator hypothesis in at least select circumstances. Cell 1 depicts posttraining associative deflation after excitatory conditioning with overshadowing as an example. In Cell 1, Phase 2 posttraining deflation (i.e., extinction) of companion stimulus A is expected to increase behavioral control by target stimulus X (relative to subjects given nonreinforced exposure to an alternative stimulus B during Phase 2). The previously described posttraining deflation effect in overshadowing observed by Kaufman and Bolles (1981) and replicated by others (e.g., Matzel et al., 1985; Matzel et al., 1987) affirms the expectation of Cell 1. Cell 2 depicts posttraining associative inflation after excitatory conditioning with backward blocking as an example. In Cell 2, the additional Phase 2 reinforced presentations of companion stimulus A alone are expected to decrease behavioral control of target stimulus X at the time of test relative to subjects that receive Phase 2 reinforced presentations of alternative stimulus B alone. Cell 2 expectations have been confirmed by the previous demonstrations of backward blocking (e.g., Chapman, 1991; Escobar, Pineño, & Matute, 2002; Miller & Matute, 1996; Shanks, 1985; Wasserman & Berglan, 1998).
Cell 3 of the matrix depicts posttraining associative deflation with Pavlovian conditioned inhibition training as an example. In Cell 3, Phase 3 posttraining deflation of companion stimulus A is expected to decrease behavior indicative of conditioned inhibition (here as assessed by a summation test) relative to subjects that receive nonreinforced exposure to alternative stimulus B during Phase 3. The demonstrations of such effects by Kasprow et al. (1987) and Hallam, Matzel, Sloat, and Miller (1990) fulfill this prediction of Cell 3. Cell 4 depicts posttraining associative inflation with Pavlovian conditioned inhibition training as an example. In Cell 4, Phase 3 posttraining reinforcement of the companion stimulus alone is expected to result in responding indicative of enhanced inhibition relative to subjects that do not experience the posttraining manipulation of the companion stimulus. As denoted by the question mark in the test location of this experimental condition, the prediction of this cell has not yet been extensively examined. However, some recent findings provide qualified evidence for the occurrence of this phenomenon.
Chapman (1991; also see Larkin, Aitken, & Dickinson, 1998; Williams & Docking, 1995), using a contingency learning task with humans, observed inflation effects on behavior indicative of backward conditioned inhibition (i.e., evidence supportive of the prediction of Cell 4). Specifically, Chapman required participants to rate the likelihood of a fictitious symptom (i.e., analogous to a CS) diagnosing the occurrence of a disease (i.e., analogous to a US) using a procedure akin to backward Pavlovian conditioned inhibition training. That is, she first gave participants trials of a compound stimulus composed of the target symptom and a companion symptom followed by no disease (i.e., analogous to Pavlovian nonreinforced AX trials). Next, she gave participants trials with the companion symptom alone followed by the disease (i.e., analogous to Pavlovian reinforced A trials). Chapman's results showed that participants gave a more negative rating to the target symptom, relative to participants who did not receive the additional reinforced presentations of the companion symptom, thereby indicating that the target symptom was less likely to diagnose the disease after additional reinforced presentations of the companion symptom alone. An important factor in Chapman's study is that she gave the participants' instructions that included explicit mention of the outcome, which conceivably created an expectation that was critical for observation of apparent backward inhibition. If so, this study does not demonstrate pure backward inhibition, but would still be an instance of inhibition being increased by posttraining associative inflation of the companion stimulus in humans. The same point applies to Williams and Docking (1995) and Larkin, Aitken, and Dickinson (1998). Because these instances of inflation effects were only observed in humans, the generalizabilty of the findings to nonhuman subjects, presumably limited to lower-order information processing, is questionable.
More recently, Baker, Murphy, and Mehta (2003), in an ancillary finding relative to the focus of their report, demonstrated backward conditioned inhibition in nonhuman subjects. Specifically, Baker et al. used a conditioned fear preparation to test interference effects arising from a learned irrelevance treatment. After the treatment sessions, Baker et al. used both a summation test and a retardation test to assess the inhibitory status of the CS for both groups and found greater inhibition in the experimental group than in the control group. However, these findings are not conclusive because trace conditioning of the presumably unpaired CSs in the control condition could have resulted in the CS acquiring a significant amount of excitatory value and consequently at test the control subjects responded more, giving the appearance of greater inhibition in the experimental group. Thus, Baker et al.'s seeming demonstration of backward conditioned inhibition is open to uncertainty as to whether it confirms the expectation of Cell 4 of the 2×2 matrix in Fig. 2.
These predictions concerning the effects of posttraining deflation and inflation of the companion stimulus on the behavioral control by a target stimulus are no longer unique to the comparator hypothesis. Modifications of learning-focused models such as the Rescorla and Wagner (1972) model and Wagner's (1981) SOP model now allow variants of these models to make similar predictions regarding posttraining manipulations (i.e., retrospective revaluation; e.g., Dickinson & Burke, 1996; Van Hamme & Wasserman, 1994). Nonetheless, the effects of posttraining deflation and inflation on stimulus interaction continue to be vigorously examined. These efforts have suggested an asymmetry in the effects of posttraining inflation and deflation. Specifically, the effects of posttraining deflation (Cell 1 and Cell 3 of Fig. 2) have been largely confirmed with a variety of procedures, tasks, and species (e.g., Arcediano, Escobar, & Matute, 2001; Blaisdell, Gunther, & Miller, 1999; Blaisdell, Denniston, & Miller, 2001; Cole, Barnet, & Miller, 1995; Hallam et al., 1990; Wasserman & Berglan, 1998). It should be noted there have been some failures to obtain this effect, presumably because the effect is parametrically delimited (e.g., Holland, 1999; Rauhut, McPhee, DiPietro, & Ayres, 2000; Schweitzer & Green, 1982). In contrast, effects of posttraining inflation of the companion stimulus have been more difficult to observe (Cell 2 and Cell 4 of Fig. 2). Several attempts to induce a decrement in the conditioned response to the target CS by inflating its companion stimulus have failed (e.g., Grahame, Barnet, & Miller, 1992; Larkin et al., 1998; Miller, Hallam, & Grahame, 1990).
Savastano and Miller (2003) investigated the asymmetry between these effects. In an assessment of the influence of biological significance on the asymmetry of inflation and deflation effects, Savastano and Miller concluded that the observed asymmetry is a result of stimuli in inflation and deflation procedures differentially acquiring biologically significant status during initial training, which protects stimuli against reductions in their response potential. They used the term biologically significant to refer to any stimulus with the potential to elicit vigorous responding because of its inherently excitatory status (i.e., primary reinforcers) or an excitatory status that has developed through an association with another biologically significant stimulus (i.e., a conditioned stimulus; e.g., Denniston, Miller, & Matute, 1996). Once a stimulus has the potential to elicit vigorous responding, it has proven difficult to attenuate this potential through indirect means such as posttraining inflation of its comparator stimulus (Denniston et al., 1996; Miller & Matute, 1996; Oberling, Bristol, Matute, & Miller, 2000; Savastano & Miller, 2003). The influence of biological significance in a deflation paradigm is minimal because one is not attempting to indirectly decrease the response potential of a target stimulus that is already capable of producing vigorous responding. However, in an inflation paradigm, this is exactly what one is attempting to do. Available data indicate that once a stimulus is capable of producing vigorous responding, one cannot readily attenuate such responding by presenting additional reinforced presentations of a companion stimulus that was present during initial training (i.e., associative inflation). Thus, the effects of inflation treatments on excitatory responding treatments are most reliably observed with non-human subjects using sensory preconditioning procedures (e.g., Miller & Matute, 1996) and with humans (Chapman, 1991; Shanks, 1985; Van Hamme & Wasserman, 1994; Wasserman & Berglan, 1998; Williams, Sagness, & McPhee, 1994), presumably because of the absence of pairings of the target stimulus with a biologically significant stimulus prior to the inflation treatment. Consistent with this view, Savastano and Miller (2003) observed symmetrical inflation and deflation effects on excitatory responding in a sensory preconditioning preparation.
A few demonstrations of inflation effects when the target stimulus has acquired some, but weak, control of behavior (e.g., Chang, Stout, & Miller, 2004) suggest that it is possible to observe at least small effects of posttraining associative inflation of companion stimuli on stimulus competition in first-order Pavlovian conditioning. That is, in at least a few select conditions, inflation effects can be observed in Pavlovian conditioning despite the target stimulus acquiring biological significance prior to inflation. One Pavlovian procedure for assessing the effects of posttraining associative inflation of a companion stimulus that avoids the potential influences of biological significance is Pavlovian conditioned inhibition. In a conventional Pavlovian conditioned inhibition procedure, the subjects receive A–US pairings interspersed with nonreinforced presentations of the compound AX (i.e., A–US/AX-), which establishes stimulus X as an inhibitor of the US (Pavlov, 1927). In this situation the target stimulus X is never directly presented with a primary reinforcer, but only with a second-order reinforcer which should result in X accruing little if any biological significance (although second-order conditioning is a possibility with appropriate parameters). Therefore, in this procedure one might expect to observe effects of posttraining inflation. That is, after the initial A–US/ X- training, if additional reinforced trials of the companion stimulus A are presented (i.e., A–US), then one might expect stimulus X to become a stronger inhibitor.
The purpose of this experimental series was to investigate in nonhuman subjects the effects of posttraining inflation of the companion stimulus on a stimulus that has already undergone limited inhibition training. The observation of increased inhibition with a posttraining inflation manipulation after Pavlovian condition inhibition treatment would confirm the expectation depicted in Cell 4 of the matrix in Fig. 2. This would serve as the last step in demonstrating that posttraining deflation or inflation of a companion stimulus can have an inverse effect on either excitatory or inhibitory stimulus control by a target stimulus at least under some conditions.
Experiment 1: Summation test
In assessments of conditioned inhibition, one common means of assessing of the inhibitory status of the target stimulus is the summation test. The summation test involves the presentation of the target stimulus in compound with a separately trained excitor stimulus (called a transfer excitor) at test. For example, after initial Pavlovian conditioned inhibition training (i.e., interspersed trials of A–US/AX-, which should establish stimulus X as an inhibitor), subjects receive separate training with a transfer excitor (i.e., T–US pairings). Then at test, the target stimulus X and the transfer excitor T are presented in compound (XT) to one group of subjects and the level of responding to this compound is compared to responding by a group of subjects that is tested on stimulus T alone or a group tested on XT but which did not receive the putative conditioned inhibition treatment. If weaker responding is observed in the compound test situation with the experimental group relative to these two control groups, this is said to be behavior indicative of inhibition. Importantly, in the above example, the comparator hypothesis anticipates that at test the target stimulus X will activate a strong indirect representation of the US (i.e., Links 2 and 3) which should negate the strong direct representation of the US activated by T, thereby resulting in behavior indicative of inhibition. The purpose of Experiment 1 was to assess, with a summation test, the effects of posttraining inflation of the comparator stimulus (i.e., the training excitor) following Pavlovian conditioned inhibition training. That is, we were interested in determining if additional reinforced presentations of the inhibition training excitor (A–US) after interspersed A–US/AX-training would result in weaker responding to the compound of the target stimulus X and T (i.e., enhanced conditioned inhibition) compared to a situation in which additional reinforced presentations of A alone did not occur.
The design of Experiment 1 is summarized in Table 1. In developing the current design for Experiment 1 and the subsequent design for Experiment 2, we assumed that inflation effects would be parameter dependent because our procedure was similar to those known to result in sensory preconditioning. Hence, our parameters were selected with the anticipation of maximizing the likelihood of our obtaining a stimulus-specific posttraining inflation effect. In order to achieve greater stimulus-specificity, we included an additional stimulus during both conditioned inhibition training and subsequent inflation training to enhance discrimination between stimuli and therefore, stimulus specificity. Specifically, in Phase 1 nonreinforced presentations of stimulus B were administered to enhance stimulus specificity of the excitatory learning with respect to the training excitor (A), and similarly in Phase 2 nonreinforced presentations of stimulus D were included to enhance stimulus specificity of the excitatory learning with respect to the stimulus inflated in that phase. The control groups received inflation treatment with stimulus C (an irrelevant stimulus), rather than A. Reinforced stimulus C presentations in the control groups served to equate subjects in terms of experience with signaled reinforcement without inflating CS A. Moreover, to maximize sensitivity to any possible inflation effects in Pavlovian conditioned inhibition, in Phase 1 we used parameters that would produce only weak conditioned inhibition so that we would not lose sensitivity owing to a ceiling effect for conditioned inhibition. Typically strong conditioned inhibition is observed with a large number of A–US trials interspersed among the AX nonreinforced trials. Therefore, we administered relatively fewer A–US trials in an effort to produce, at most, only weak conditioned inhibition in the noninflation groups.
Table 1.
Design of Experiment 1
| Group | Phase 1 | Phase 2 | Phase 3 | Test |
|---|---|---|---|---|
| Inflate-XT | 9 B-/9 A–US/84 AX- | 30 A–US/30 D- | 4 T–US | XT |
| Inflate-T | 9 B-/9 A–US/84 AX- | 30 A–US/30 D- | 4 T–US | T |
| Inflate-GD | 9 B-/9 A–US/84 BX- | 30 A–US/30 D- | 4 T–US | XT |
| NoInfl-XT | 9 B-/9 A–US/84 AX- | 30 C–US/30 D- | 4 T–US | XT |
| NoInfl-T | 9 B-/9 A–US/84 AX- | 30 C–US/30 D- | 4 T–US | T |
| NoInfl-GD | 9 B-/9 A–US/84 BX- | 30 C–US/30 D- | 4 T–US | XT |
Note. The numbers preceding the letters in Phases 1–3 indicate the total number of presentations of the stimuli (A, B, C, D, T, and X). Slashes represent interspersed trials. The US was footshock. ‘-’ represents nonreinforced presentations of a CS. ‘XT’ and ‘T’ indicate the type of stimulus that was presented at test, compound or element, respectively.
Method
Subjects
The subjects were 36 male (225–340g) and 36 female (175–225 g) Sprague–Dawley rats, bred in our own colony. Subjects were individually housed in wire-mesh cages in a vivarium maintained on a 16 h light/8 h dark cycle. The experiment was conducted approximately midway through the light portion of the cycle. A progressive water deprivation schedule was imposed over the week prior to the beginning of the experiment until water availability was limited to 30 min per day, provided approximately an hour after any treatment scheduled for that day. All subjects were handled for 30 s three times per week from weaning until the initiation of the study. Subjects were randomly assigned to one of six groups (ns =12), counterbalanced for sex.
Apparatus
Two different types of experimental chambers were used, six copies of each. Chamber rectangular (R) was a clear, Plexiglas, rectilinear chamber, measuring 23.0×8.5×12.5 cm (length×width×height). The floor was constructed of 0.48-cm diameter stainless-steel rods, spaced 1.5 cm apart, center-to-center. The rods were connected by NE-2H neon bulbs that allowed a constant-current footshock to be delivered by means of a high voltage AC circuit in series with a 1.0-MΩ resistor. Each copy of Chamber R was housed in a separate light- and sound-attenuating environmental isolation chest, which was dimly illuminated by a 2-W (nominal at 120 VAC) incandescent bulb driven at 60 VAC. The houselight was mounted on the ceiling of the environmental chest approximately 26 cm from the center of the experimental chamber.
Chamber V-shaped (V) was a 22.1cm long box in the shape of a vertical truncated-V (25.3 cm height, 21.3 cm wide at the top, 5.1 cm wide at the bottom). The floor and long sides were constructed of stainless-steel sheets, the short sides were constructed of black Plexiglas, and the ceiling was constructed of clear Plexiglas. The floor consisted of two parallel metal plates, each 2.0 cm wide, with a 1.1-cm gap between them, which permitted delivery of constant-current footshock. Each V-shaped chamber was housed in its own environmental isolation chest, which was dimly illuminated by a 7.5 (nominal at 120 VAC) incandescent houselight driven at 60 VAC mounted on an inside wall of the environmental chest approximately 30 cm from the center of the experimental chamber. The light entering the animal chamber was primarily that reflected from the roof of the environmental chest, which was white insulating material. The light intensities in the two types of chambers (R and V) were approximately equal, due to the differences in opaqueness of the walls.
Each chamber (R and V) was also equipped with three 45-Ω speakers widely separated on the inside walls of the environmental chest. Each speaker could deliver a different auditory stimulus. One speaker mounted on the right sidewall was used to deliver a complex tone stimulus (800 and 1000 Hz), 6dB above background, which served as stimuli A, B, or C, counterbalanced within groups. A second speaker mounted on the back sidewall of the experimental chamber was used to deliver a click stimulus (6/s) 8 dB above background, which served as the transfer excitor (T). A third speaker mounted on the left sidewall of the chamber was used to deliver a white noise stimulus 6dB above background, which served as stimuli A, B, or C, counterbalanced within groups. A SonAlert and a buzzer mounted on the top of the experimental chamber were used to deliver an elemental tone (1900 Hz) or buzzing sound 6 dB above the background. These two stimuli served as X and D, counterbalanced within groups. A flashing visual stimulus (0.5-s on/0.5-s off) consisted of a 25-W (nominal at 120 VAC but driven at 100 VAC) light in Chamber R and a 100-W light in Chamber V mounted on the interior back side of each environmental chest approximately 30 cm from the center of the experimental chamber, these served as stimuli A, B, or C, counterbalanced within groups. Ventilation fans in each enclosure provided a constant 74-dB background noise. All CS durations except during testing were 5 s.
Each chamber (R and V) could be equipped with a water-filled lick tube that extended 1 cm from the rear of a cylindrical niche, 4.5 cm in diameter, left–right centered in one short wall, with its axis perpendicular to the wall, and positioned with its center 4.25 cm above the floor of the chamber. Each niche had a horizontal infrared photobeam traversing it parallel to the wall on which the niche was mounted, 1 cm in front of the lick tube. To drink from the tube, subjects had to insert their heads into the niche, thereby breaking the photobeam. Thus, we could record when subjects had their heads in the niche to access the water tube. Ordinarily, they did this only when they were drinking. Disruption of ongoing drinking by a test stimulus served as our dependent variable. Chambers R and V were counterbalanced within groups, and each subject was trained and tested exclusively in one chamber. The use of the two different types of chambers merely reflects the available equipment.
Procedure
Subjects were randomly assigned to one of three test conditions: T, which was tested on the transfer excitor alone; XT, which was tested on a compound of T and the putative inhibitor (X); and generalization decrement (GD) which was also tested on XT but for which X was not trained as a conditioned inhibitor. Additionally, all subjects were assigned to one of two inflation conditions, inflation (Inflate) and no inflation (NoInfl), see Table 1. Groups in the XT condition were tested with the compound comprised of the target stimulus X and the transfer excitor T, and behavior indicative of conditioned inhibition was expected relative to the control groups that were tested with the excitatory transfer excitor (T) alone. The GD groups were included to assess the alternative explanation that any differences observed between the XT and the T conditions were due to the potential influence of generalization decrement from training with T and testing with the XT compound. Groups in the inflate condition received additional reinforced presentations of the companion stimulus A alone. Groups in the ‘NoInfl’ condition did not receive additional reinforced presentations of the companion stimulus A alone but instead received reinforced presentations of an alternative stimulus C alone (i.e., no inflation). The central question was whether inhibition would be enhanced in the Inflate-XT group compared to the NoInfl-XTgroup.
Acclimation
On Day 1, all subjects were exposed to the experimental context for 60min with lick tubes present. No nominal stimuli were presented. At the end of this session the lick tubes were removed.
Conditioned Inhibition Training
On Days 2–7, two different schedules (1 and 2) were used on alternate days. Two schedules of training were used in order to reduce potential timing of the US that would be more apt to occur by having the CS–US presentations always occurring at the same time over the course of training. That is, time could serve as a predictor of the US presentations, thereby reducing responding to the CS. Therefore, two schedules were used to prevent this potential influence of time. In schedule 1, all subjects received two daily A–US presentations and two daily nonreinforced B presentations. In schedule 2, all subjects received one daily A–US presentation and one daily nonreinforced stimulus B presentation. During both schedules, for groups Inflate-XT, Inflate-T, NoInfl-XT, and NoInfl-T these trials were interspersed with 14 daily presentations of the AX compound without reinforcement, whereas for groups Inflate-GD and NoInfl-GD these trials were interspersed with 14 daily presentations of the BX compound without reinforcement. Schedule 1 was used on Days 2, 4, and 6, and schedule 2 was used on Days 3, 5, and 7. In schedule 2, stimulus A onset occurred at 5 min and 35 min into the 60-min session, with the US being presented immediately after termination of A, and stimulus B onset occurred at 9 and 30 min into the session. In schedule 2, stimulus A onset occurred at 30 min into the session, with the US being presented immediately after termination of A, and stimulus B onset occurred at 35 min into the session. In schedule 1, the AX or BX compound presentations occurred at 7, 11, 14, 17, 23, 27, 33, 38, 43, 48, 50, 54, 56, and 59 min into the session. In schedule 2, the AX or BX compound presentations occurred at 5, 8, 12, 14, 17, 23, 27, 33, 38, 43, 47, 53, 55, and 57 min into the session.
Inflation training
On Days 8–13, subjects in the inflate condition received five daily presentations of A–US interspersed with five daily nonreinforced stimulus D presentations. Subjects in the NoInfl condition received five daily presentations of C–US interspersed with five daily nonreinforced stimulus D presentations during the 60-min session. Two different schedules (1 and 2) were used on alternate days. Schedule 1 was used on Days 8, 10, and 12 and, schedule 2 was used on Days 9, 11, and 13. In schedule 1, stimulus A or stimulus C presentations occurred at 11, 18, 24, 34, and 46 min into the 60-min session, with the US being presented immediately after termination of A or C. In schedule 2, stimulus A or stimulus C presentations occurred at 5, 15, 21, 38, and 45 min into the 60-min session, with the US being presented immediately after termination of A or C. The stimulus D presentations occurred in schedule 1 at 8, 15, 28, 39, and 51 min into the 60-min session and in schedule 2 at 10, 18, 26, 31, and 41 min into the session.
Transfer stimulus training
On Day 14, all subjects were exposed to the experimental context for 60min and were given four T–US pairings. Stimulus T presentations occurred 10, 25, 35, and 48 min into the 60-min session. US onset coincided with CS T termination.
Reacclimation
On Days 15, 16, and 17, all subjects were exposed to the experimental context for 60 min with lick tubes again available. No discrete stimuli were presented. Each subject's latency to complete the first 5 cumulative seconds of licking (i.e., the sum of the time spent completing its first 5-s of cumulative licking) was recorded. Subjects that did not reach the criterion of completing these five cumulative seconds within the first 60s on Day 15 received an extra 30-min session on that day.
Summation test
On Day 18, subjects in groups Inflate-XT, NoInfl-XT, Inflate-GD, and NoInfl-GD were tested for conditioned lick suppression to XT. Groups Inflate-T and Group NoInfl-T were tested on conditioned lick suppression to T. The test stimulus was presented after completion of the first five cumulative seconds of licking behavior and latency to complete five more cumulative seconds of licking, this time in the presence of the test stimulus, was recorded. Thus, all subjects were licking at the time of CS onset. All subjects were exposed to the test stimulus for 10min, thereby creating a ceiling score of 600 s. Following the established practice in our laboratory, the data from all animals that took longer than 60-s to complete their first five cumulative seconds of drinking in the test session (i.e., before CS onset) were eliminated from the analyses because such long latencies presumably reflect unusual fear of the experimental context. Based on this criterion, no animals were eliminated from the statistical analyses. However, the data from one animal in group NoInfl-XT and from one animal in group NoInfl-T were lost due to an equipment failure. Our critical measure was the time to complete five cumulative seconds of licking in the presence of the test stimulus. To better approximate the normal within-group distributions of scores assumed by parametric statistics, a log (base 10) transformation was performed on each measured latency in Experiments 1 and 2.
Results and discussion
A 2 (Posttraining Treatment: Inflation vs. No Inflation)×3 (Test Stimulus: XT vs. T vs. GD) analysis of variance (ANOVA) on the baseline scores (i.e., time to complete five cumulative seconds of drinking prior to CS onset) recorded during testing revealed a main effect of posttraining treatment, F(2, 64)=7.05, MSE=0.04, p< .01. Means were 1.23, 1.24, and 1.28 for groups Inflate-XT, Inflate-T, Inflate-GD, and 1.12, 1.16, 1.11 for groups NoInfl-XT, NoInfl-T, and NoInfl-GD, respectively. Therefore, the baseline scores were used as a covariate in all subsequent analyses. A 2 (Posttraining Treatment: Inflation vs. No Inflation)×3 (Test Stimulus: XT vs. T vs. GD) analysis of covariance (ANCOVA) was used to assess conditioned fear during the test presentation of the test stimuli (i.e., XT and T). This analysis revealed a main effect of test stimulus, F(2, 63)=10.02, MSE=0.16, p<.001, and an interaction between the posttraining condition and test stimulus, F(2, 63)=4.65, MSE=0.16, p=.01, (see Fig. 3). Planned comparisons were conducted to better understand the basis of the interaction. These comparisons revealed that group Inflate-XT suppressed less to the XT compound than did group NoInfl-XT, F(1, 63)=12.25, p<.001, thereby suggesting that the inflation treatment increased the inhibitory status of X. An additional planned comparison of the difference between group Inflate-XT and group Inflate-T, F(1, 63)=14.13, p<.001, revealed that after inflation treatment X passed a summation test for conditioned inhibition. In contrast, the difference between group NoInfl-XT and group NoInfl-T, F(1, 63)=0.18, p>.05, was not significant. Although, the comparison between group NoInfl-XT and group NoInfl-T did not reveal a significant difference, p>.05, there was a tendency for group NoInfl-XT to respond less than group NoInfl-T. The lack of a significant difference between these groups is likely due to our intentionally using parameters intended to produce weak conditioned inhibition, so that the study would be maximally sensitive to potential effects of inflation. An ANCOVA omitting the GD groups also detected an interaction, F(1,41)=5.14, MSE=0.17, p<.03. These comparisons revealed that posttraining associative inflation of A (relative to posttraining inflation of control stimulus C) enhanced behavior indicative of conditioned inhibition. Moreover, inspection of the GD groups in Fig. 3 indicates that passage of the summation test by group Inflate-XT was not due to generalization decrement and that inflating A did not increase generalization decrement.
Fig. 3.
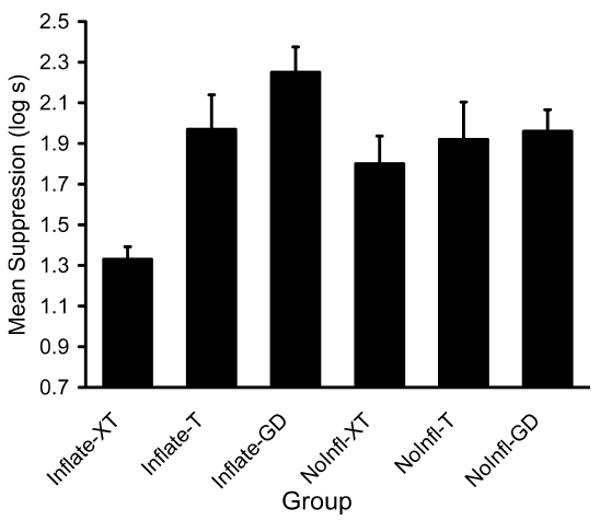
Means of Experiment 1 (summation test). The XT groups were tested with Stimulus X in compound with the transfer excitor T. The T groups were tested with the transfer excitor T alone. The GD groups served as controls for generalization decrement. Enhanced conditioned inhibition is evident in the lower responding to the compound XT in group Inflate-XT than in group NoInfl-XT relative to each of their controls, group Inflate-T and group NoInfl-T, respectively.
Overall, Experiment 1 demonstrated conditioned inhibition was enhanced when posttraining associative inflation of X's companion stimulus (A) occurred. That is, group Inflate-XT, which received inflation of companion stimulus A, demonstrated weaker excitatory responding to T in the presence of the inhibitory stimulus X than the group that did not receive inflation treatment of the companion stimulus A, group NoInfl-XT.
Experiment 2: Retardation test
Experiment 1 demonstrated, using a summation test, that posttraining inflation of the companion stimulus can enhance Pavlovian conditioned inhibition. However, Hearst (1972) and Rescorla (1969) have reasonably argued that a single test for inhibition, such as the summation test, is not sufficient to assess the inhibitory status of a stimulus because the use of a summation test alone allows for an alternative attentional account. That is, in Experiment 1 the decreased responding to the XT compound relative to T alone could arise from the putative inhibition treatment instead causing subjects to pay increased attention to X at the cost of attention to T. Therefore, it is common practice to conduct an additional test of inhibition, specifically a retardation test, which assesses resistance by the putative inhibitor in coming to control responding in the face of reinforcement. Thus, Experiment 2 used a retardation test to assess changes in conditioned inhibition as a result of posttraining associative inflation of A (see Table 2 for a summary of the design). Importantly, the comparator hypothesis anticipates that at test the high excitatory status of the X–A association (i.e., a strong Link 2) as a consequence of the inhibitory training and the A–US association (i.e., a strong Link 3) as consequence of the inflation treatment will result in retarded responding to X. Unlike the summation test, the retardation test does not use a compound stimulus during testing and consequently does not require a generalization decrement control. Therefore, the generalization decrement control groups of Experiment 1were excluded from the design of Experiment 2.
Table 2.
Design of Experiment 2
| Group | Phase 1 | Phase 2 | Phase 3 | Test |
|---|---|---|---|---|
| Inflate-CI | 9 B-/9 A–US/84 AX- | 30 A–US/30 D- | 6 X–US | X |
| Inflate-Cntl | 9 B-/9 A–US/84 BX- | 30 A–US/30 D- | 6 X–US | X |
| NoInfl-CI | 9 B-/9 A–US/84 AX- | 30 C–US/30 D- | 6 X–US | X |
| NoInfl-Cntl | 9 B-/9 A–US/84 BX- | 30 C–US/30 D- | 6 X–US | X |
Note. The numbers preceding the letters in Phases 1–3 indicate the total number of presentations of the stimuli (A, B, C, D, T, and X). Slashes represent interspersed trials. The US was a footshock. ‘-’ represents nonreinforced presentations of a conditioned stimulus (CS). ‘X’ indicates the stimulus that was presented at test.
Subjects and apparatus
The subjects were 48 male (253–401g) and 48 female (182–296 g), experimentally naïve, Sprague–Dawley descended rats, bred in our colony, which were housed and maintained as described in Experiment 1. The apparatus and parameters were the same as those used in Experiment 1 except for the absence of the transfer excitor.
Procedure
Subjects were randomly assigned to one of four groups: Inflate Conditioned Inhibition (Inflate-CI), Inflate Control (Inflate-Cntl), NoInflate Conditioned Inhibition (NoInfl-CI), NoInflate Control (NoInfl-Cntl), counterbalanced for sex, (ns=24). Groups in the CI condition were expected to exhibit behavior indicative of conditioned inhibition relative to the Cntl condition. Groups in the Inflate condition received posttraining reinforced presentations of the companion stimulus A alone. Groups in the NoInfl condition did not receive additional reinforced presentations of the companion stimulus A (i.e., no inflation), but instead received additional reinforced presentations of an alternative stimulus C alone. Enhanced inhibition in the form of retardation of X in acquiring control of responding was expected in the Inflate-CI group compared to the NoInfl-CI group.
The procedure used in Experiment 2 was the same as that in Experiment 1 except for Phase 3 and testing. In Phase 3 of Experiment 2 (Days 14 and 15), the training of the transfer excitor of Experiment 1 was replaced by excitatory training in which all subjects were exposed to the experimental context for 60min per day and were given three X–US pairings per session. Onset of CS X occurred at 10, 30, and 40 min into the 60-min session, with the US being presented immediately after termination of stimulus X. On Days 16 and 17, all subjects were reacclimated to the experimental context with lick tubes restored. Finally, on Day 18, the inhibitory status of stimulus X was tested using a lick suppression test to assess retarded emergence of conditioned excitation. The test stimulus was presented immediately upon completion of the first five cumulative seconds of licking behavior. A 600-s ceiling was imposed on suppression scores. Subjects that failed to complete their first five cumulative seconds of licking (i.e., prior to CS onset) within 60 s were scheduled to be eliminated from the study. Based on this baseline criterion which was explained in Experiment 1, again no data from any animals had to be eliminated from the analyses. However, one animal in group Inflate-Cntl was eliminated because of an equipment failure prior to testing.
Results and discussion
A 2 (Posttraining Treatment: Inflation vs. No Inflation) × 2 (Group: Conditioned Inhibition vs. No Conditioned Inhibition) ANOVA on the baseline scores (i.e., time to complete five cumulative seconds of drinking prior to CS onset) recorded during testing revealed no differences in initiation of drinking on test day, Fs<1. Nevertheless, to adjust for any (nonsignificant) differences in baseline drinking and to maintain consistency with the analysis in Experiment 1, we used the baseline scores as a covariate. Therefore, a 2 (Posttraining Treatment: Inflation vs. No Inflation) × 2 (Group: Conditioned Inhibition vs. No Conditioned Inhibition) ANCOVA was conducted on suppression scores during CS X presentation. This revealed a main effect of group, F(1, 90)=33.73, MSE=0.09, p<.001 and an interaction between the posttraining condition and group, F(1,90)=4.75, MSE=0.09, p=.03 (see Fig. 4). As in Experiment 1, planned comparisons were conducted to better understand the basis of the interaction. These comparisons revealed that group Inflate-CI suppressed less to stimulus X than did group NoInfl- CI, F(1,90)=6.31, p<.01, thereby suggesting that the inflation treatment increased the inhibitory status of X. An additional planned comparison of the difference between group Inflate-CI and group Inflate-Cntl, F(1,90)=31.60, p<.001, revealed that after inflation treatment X passed a retardation test for conditioned inhibition. Moreover, the difference between group NoInfl-CI and its control, group NoInfl-Cntl, F(1,90)=6.64, p<.01, also indicates that X passed a retardation test. Thus, the interaction and the planned comparisons found that posttraining associative inflation of A increased retardation, which is indicative of enhanced conditioned inhibition. In other words, group Inflate-CI, which received inflation of the companion stimulus A, demonstrated stronger retardation of stimulus control by the X–US association when inflation training was conducted after Pavlovian conditioned inhibition training than did group NoInfl-CI, which did not receive inflation treatment.
Fig. 4.
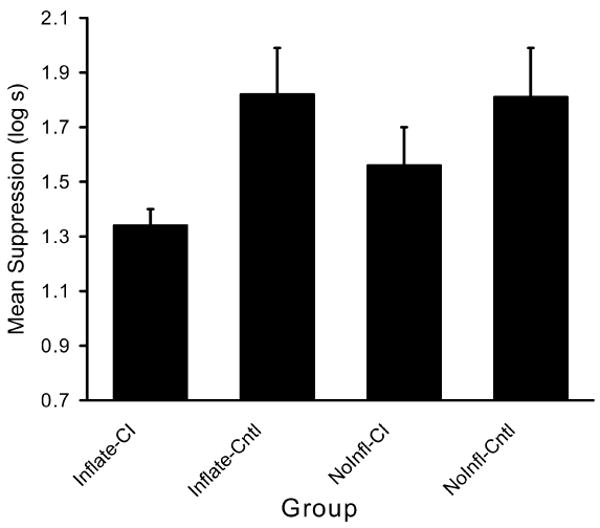
Means of Experiment 2 (retardation test). The CI groups were those in which retardation of conditioned inhibition was expected due to inhibition training and posttraining inflation of companion stimulus A. The Cntl groups served as controls for the retardation test. Enhanced conditioned inhibition is evident in the lower responding to the target stimulus X in groups Inflate-CI than in group NoInfl-CI relative to each of their controls, group Inflate-CI and group NoInfl-Cntl, respectively.
General discussion
Experiment 1 successfully demonstrated, using a summation test, inflation effects in Pavlovian conditioned inhibition as a result of posttraining associative inflation of the excitatory CS that was used in inhibition training. Specifically, a group that received additional reinforced posttraining presentations of the companion stimulus A after initial Pavlovian conditioned inhibition training (i.e., A–US/AX- followed by A–US pairings) showed behavior indicative of enhanced inhibition compared to a group that did not receive additional A–US pairings. To further corroborate the findings of Experiment 1, a retardation test for conditioned inhibition was conducted in Experiment 2. The findings of Experiment 2 also indicated that conditioned inhibition as assessed by a retardation test was enhanced by posttraining associative inflation of the training excitor. That is, subjects that were exposed to inflation treatment after initial Pavlovian conditioned inhibition treatment displayed greater retardation of the acquisition of the X–US association than subjects that did not receive the inflation treatment.
Prior research has demonstrated an asymmetry between the posttraining effects of deflation and inflation of the companion stimuli in first-order Pavlovian conditioning (e.g., Savastano & Miller, 2003). Increases in responding to a target stimulus as a result of posttraining decreases in the associative strength (i.e., associative deflation) of a companion stimulus have proven relatively easy to obtain (Blaisdell et al., 1999; Hallam et al., 1990; Matzel et al., 1985), whereas decreases in responding to a target stimulus as a result of posttraining increases in the associative strength (i.e., associative inflation) of a companion stimulus have been more difficult to obtain (Grahame et al., 1992; Larkin et al., 1998; Miller et al., 1990). This asymmetry has posed a challenge to both learning-focused models that were designed to account for the effects of posttraining inflation and deflation (e.g., Van Hamme & Wasserman, 1994) and performance-focused models (e.g., Miller & Matzel, 1988). However, recent reports (Baker et al., 2003; Melchers, Lachnit, & Shanks, 2004) in conjunction with the current findings alleviate this challenge by confirming the expectations of Cell 4 of the matrix in Fig. 2, thereby demonstrating that both deflation and inflation effects do occur, although seemingly with different degrees of ease. Importantly, it should be noted that some contemporary models predict a distinction between inflation and deflation effects when considering a more detailed assessment of the processes that are potentially involved. For example, Dickinson and Burke's (1996) revised SOP model and the comparator hypothesis both predict, albeit for different reasons, a greater potential to observe deflation than inflation effects at least with select parameters.
The comparator hypothesis anticipates that inflation effects will be weaker than deflation effects if the inflation treatment that strengthens Link 3 (see Fig. 1) also weakens Link 2. That is, the comparator hypothesis predicts that a weakening of Link 2 will attenuate inflation effects but may also facilitate deflation effects. For example, in the current experiments, following Pavlovian conditioned inhibition training the inflation of the companion stimulus (A) might have served not only to strengthen the A–US association (Link 3) but also to weaken the association between the target stimulus (X) and companion stimulus (i.e., Link 2). Because Link 2 is necessary for the companion stimulus to play a modulatory role, the weakening of Link 2, if it occurred, would work against observation of inflation effects. In contrast, the weakening of Link 2 during deflation treatment might work synergically with the weakening of Link 3. However, despite the potential influence of a weakened Link 2, in the present research we were able to establish parameters that allowed us to observe inflation effects on conditioned inhibition. One might be concerned that such specific parameters render the model predictively weak. On the contrary, because the compartor hypothesis incorporates Link 2 it allows one to better anticipate more specific parameters needed so that Link 2 is not weakened to the point of not observing inflation effects.
As mentioned above, Dickinson and Burke's (1996) revised SOP model offers an alternative account of the observed effects. Their model suggests that the increased inhibitory status of X is the result of the within-compound association between stimuli A and X mediating the acquisition of an inhibitory association between X and the outcome. The model incorporates Wagner's (1981) original concept of an event representation being distributed across an inactive state (I), a highly active state (A1), and a low activity state (A2). When both elements are in A1 or in A2, an increment in the excitatory association occurs, but, if one element is in A1 and the other is in A2, an increment in the inhibitory relationship occurs. During the inflation treatment of the present experiments, the reinforced presentations of the competing cue A presumably activates A and the US into state A1 and the representation of X into state A2. The occurrence of X in state A2 while the US is in state A1 results in X accruing increased inhibitory value.
Centrally, the present findings speak to Cell 4 of Fig. 2, which concerns the effects on a test for conditioned inhibition of posttraining inflation of a stimulus that was present during target training. As previously mentioned, Chapman (1991) did observe such inflation effects in a human contingency judgment task, and recently Melchers et al. (2004), in a test of the importance of within-compound associations in retrospective revaluation, also observed inflation effects on conditioned inhibition using Chapman's (1991) design. Although these findings are compelling, a difficulty in determining the generalizability of the findings by both Chapman (1991) and Melchers et al. (2004) is that only humans were used in these studies. Hence, these observations may depend on higher-level processing available only to humans. This lack of generalizability makes it difficult to conclude that the prediction in Cell 4 of the matrix in Fig. 2 is fulfilled in general. However, the observation of inflation effects by Baker et al. (2003) with nonhuman subjects in a negative contingency procedure does speak to the generalizability of inflation effects. Importantly, the conclusions based on the current findings are congruent with those of Baker et al., but the lack of a true baseline control in their study creates difficulty in interpreting their results. That is, one can not definitively conclude that inhibition was enhanced in the group that received independent exposure of the CS and US compared to the group that received interspersed presentations of the CS and US because there was not a proper control group. As suggested in the introduction, without this control the observed difference is potentially attributable to an increase in the excitatory value of target CS in the group that received interspersed CS and US exposures because the CS and US presentations were close enough to possibly support excitatory trace conditioning. The current experiments allow for a comparison between the experimental groups, each relative to a proper control group, and thus, preclude alternative explanations of the results. Therefore, the importance of the current findings is that, in conjunction with the findings of Chapman (1991), Baker et al. (2003), and Melchers et al. (2004), there is now stronger evidence that the anticipated increase in inhibitory control as a result of posttraining associative inflation actually occurs (i.e., the prediction in Cell 4 of the matrix in Fig. 2 is fulfilled).
It is worth noting that the apparent demonstrations of backward conditioned inhibition (i.e., with all of the nonreinforced AX trials followed by all of the reinforced A trials) mentioned above do not provide unambiguous evidence for the enhancement of inhibition during A-outcome trials. That is, the initial training in backward conditioned inhibition (i.e., nonreinforced AX trials) presumably results in no association between either A or X and the outcome. However, the initial status of the X-outcome association may have been nonzero prior to the A-outcome trials in the apparent demonstrations of backward conditioned inhibition in humans by Chapman (1991) and Melchers et al. (2004) or in nonhuman subjects by Baker et al. (2003). In Chapman and Melchers et al., the status of X was potentially influenced by mention of the outcome (i.e., disease) in the instructions. Participants were instructed about the occurrence of cues and an outcome and consequently may have started training with a nonzero association. In Chapman and Melchers et al., if mentioning the outcome in the instructions gave X an excitatory status (i.e., it predicts the outcome), then it is possible that the observed apparent inhibition was due to a decrease in the excitatory status of X rather than enhanced inhibition. It should be noted that the present research did not use a procedure that could have possibly demonstrated backward conditioned inhibition because we were interested in enhanced conditioned inhibition rather than in creating backward inhibition from a point at which the associative status of A and X was zero.
Additionally, as suggested previously, in Baker et al. (2003) the observed difference in inhibition between the two groups they used (i.e., one that received separate days of exposure to the CS and US and one that received interspersed exposures to the CS and the US in the same session) may have resulted from the CS being more excitatory in the interspersed exposure group. That is, interspersing the CS and US presentations within the same session may have increased the excitatory status of the CS in that group, whereas the CS could have remained more neutral for the group that received presentations of the CS and US on separate days. Thus, the apparent inhibition was potentially due to the excitatory status of the CS increasing in the interspersed group rather than the inhibitory status of X increasing in the group that received presentations of the CS and US on separate days.
One might ask why prior attempts to observe inflation effects on conditioned inhibition were unsuccessful (e.g. Grahame et al., 1992). Likely differences in parameters are a major factor. The introduction to Experiment 1 provided a rationale for the present parameters. Specifically, the parameters used were intended to establish a low but finite level of inhibition to the target stimulus because a previous attempt by Grahame et al. (1992) failed to observe inflation effects in the form of increased conditioned inhibition when the pre-inflation target training involved no established Pavlovian inhibition (i.e., no A–US trials). The parameters used here were intended to establish a nonzero level of inhibition so that posttraining associative inflation of the companion stimulus A could enhance the inhibitory status of the already inhibitory target stimulus X. The fact that the NoInfl-XT group in Experiment 1 showed a (nonsignificant) tendency for conditioned inhibition suggests that some minimal inhibition was established.
In summary, the current findings in conjunction with previous findings (Baker et al., 2003; Chapman, 1991; Larkin et al., 1998; Melchers et al., 2004; Williams & Docking, 1995) effectively complete the 2 (excitation vs. inhibition training) × 2 (deflation vs. inflation of the comparator) matrix shown in Fig. 2. Thus, there is now good evidence that the phenomena in all four cells of this matrix do occur.
Footnotes
Support for this research was provided by NIMH Grant 33881. We thank Gonzalo Urcelay, Kouji Urushihara, Miguel Vadillo, and Daniel Wheeler for their comments on a preliminary version of the manuscript. Special thanks are due to James Esposito and Daniel Gutter for their assistance with the collection of the data.
References
- Arcediano F, Escobar M, Matute M. Reversal from blocking in humans as a result of posttraining extinction of the blocking stimulus. Animal Learning & Behavior. 2001;29:354–366. [Google Scholar]
- Baker AG, Murphy RA, Mehta R. Learned irrelevance and retrospective correlation learning. Quarterly Journal of Experimental Psychology B. 2003;56:90–101. doi: 10.1080/02724990244000197. [DOI] [PubMed] [Google Scholar]
- Best MR, Dunn DP, Batson JD, Meachum CL, Nash SM. Extinguishing conditioned inhibition in flavour-aversion learning: Effects of repeated testing and extinction of the excitatory element. Quarterly Journal of Experimental Psychology B. 1985;37:359–378. doi: 10.1080/14640748508401175. [DOI] [PubMed] [Google Scholar]
- Blaisdell AP, Gunther LM, Miller RR. Recovery from blocking achieved by extinguishing the blocking CS. Animal Learning & Behavior. 1999;27:63–76. [Google Scholar]
- Blaisdell AP, Denniston JC, Miller RR. Recovery from the overexpectation effect: Contrasting performance-focused and acquisition-focused models of retrospective revaluation. Animal Learning & Behavior. 2001;29:367–380. [Google Scholar]
- Chang RC, Stout S, Miller RR. Comparing excitatory backward and forward conditioning. Quarterly Journal of Experimental Psychology B. 2004;57:1–23. doi: 10.1080/02724990344000015. [DOI] [PubMed] [Google Scholar]
- Chapman GB. Trial order affects stimulus interaction in contingency judgment. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1991;17:837–854. doi: 10.1037//0278-7393.17.5.837. [DOI] [PubMed] [Google Scholar]
- Cole RP, Barnet RC, Miller RR. Effect of relative stimulus validity: Learning or performance deficit? Journal of Experimental Psychology: Animal Behavior Processes. 1995;21:293–303. doi: 10.1037//0097-7403.21.4.293. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Miller RR, Matute H. Biological significance as determinant of stimulus competition. Psychological Science. 1996;7:325–331. [Google Scholar]
- Dickinson A, Charnock DJ. Contingency effects with maintained instrumental reinforcement. Quarterly Journal of Experimental Psychology. 1985;37B:397–416. [Google Scholar]
- Dickinson A, Burke J. Within-compound associations mediate the retrospective revaluation of causality judgments. Quarterly Journal of Experimental Psychology B. 1996;49:60–80. doi: 10.1080/713932614. [DOI] [PubMed] [Google Scholar]
- Escobar M, Pineño O, Matute H. A comparison between elemental and compound training of cues in retrospective revaluation. Animal Learning & Behavior. 2002;30:228–238. doi: 10.3758/bf03192832. [DOI] [PubMed] [Google Scholar]
- Grahame NJ, Barnet RC, Miller RR. Pavlovian inhibition cannot be obtained by posttraining A–US pairings: Further evidence for the empirical asymmetry of the comparator hypothesis. Bulletin of the Psychonomic Society. 1992;30:399–402. [Google Scholar]
- Hallam SC, Matzel LD, Sloat JS, Miller RR. Excitation and inhibition as a function of posttraining extinction of the excitatory stimulus used in Pavlovian inhibition training. Learning and Motivation. 1990;21:59–84. [Google Scholar]
- Hearst E. Some persistent problems in the analysis of conditioned inhibition. In: Boakes RA, Halliday MS, editors. Inhibition and learning. London: Academic Press; 1972. [Google Scholar]
- Holland PC. Overshadowing and blocking as acquisition deficits: No recovery after extinction of overshadowing or blocking stimulus. Quarterly Journal of Experimental Psychology. 1999;52:307–334. doi: 10.1080/027249999393022. [DOI] [PubMed] [Google Scholar]
- Kasprow WJ, Schachtman TR, Miller RR. The comparator hypothesis of conditioned response generation: Manifest conditioned excitation and inhibition as a function of relative excitatory strengths of CS and conditioning context at the time of testing. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:395–406. [PubMed] [Google Scholar]
- Kaufman MA, Bolles RC. A nonassociative aspect of overshadowing. Bulletin of the Psychonomic Society. 1981;18:318–320. [Google Scholar]
- Larkin MJW, Aitken MRF, Dickinson A. Retrospective revaluation of causal judgements under positive and negative contingencies. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1331–1352. [Google Scholar]
- Lysle DT, Fowler H. Inhibition as a “slave” process: Deactivation of conditioned inhibition through extinction of conditioned excitation. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:71–94. doi: 10.1037//0097-7403.11.1.71. [DOI] [PubMed] [Google Scholar]
- Matzel LD, Schachtman TR, Miller RR. Recovery of an overshadowed association achieved by extinction of the overshadowing stimulus. Learning and Motivation. 1985;16:398–412. [Google Scholar]
- Matzel LD, Shuster K, Miller RR. Covariation in conditioned response strength between stimuli trained in compound. Animal Learning & Behavior. 1987;15:437–439. [Google Scholar]
- Melchers KG, Lachnit H, Shanks DR. Within-compound associations in retrospective revaluation and in direct learning: A challenge for comparator theory. Quarterly Journal of Experimental Psychology: Comparative & Physiological Psychology B. 2004;57:25–53. doi: 10.1080/02724990344000042. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matzel LD. The comparator hypothesis: A response rule for the expression of associations. In: Bower GH, editor. The psychology of learning and motivation. Vol. 22. San Diego, CA: Academic Press; 1988. pp. 51–92. [Google Scholar]
- Miller RR, Hallam SC, Grahame NJ. Inflation of comparator stimuli following CS training. Animal Learning & Behavior. 1990;18:434–443. [Google Scholar]
- Miller RR, Hallam SC, Hong JY, Dufore DS. Associative structure of differential inhibition: Implications for models of conditioned inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 1991;17:141–150. doi: 10.1037//0097-7403.17.2.141. [DOI] [PubMed] [Google Scholar]
- Miller RR, Matute H. Biological significance in forward and backward blocking: Resolution of a discrepancy between animal conditioning and human causal judgment. Journal of Experimental Psychology: General. 1996;125:370–386. doi: 10.1037//0096-3445.125.4.370. [DOI] [PubMed] [Google Scholar]
- Oberling P, Bristol AS, Matute H, Miller RR. Biological significance attenuates overshadowing, relative validity, and degraded contingency effects. Animal Learning & Behavior. 2000;28:172–186. [Google Scholar]
- Pavlov IP. Conditioned reXexes. London: Oxford University Press; 1927. [Google Scholar]
- Rauhut AS, McPhee JE, DiPietro NT, Ayres JJB. Conditioned inhibition training of the competing stimulus after compound conditioning does not reduce stimulus competition. Animal Learning & Behavior. 2000;28:92–108. [Google Scholar]
- Rescorla RA. Pavlovian condition inhibition. Psychological Bulletin. 1969;72:77–94. [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current theory and research. New York: Appleton-Century Crofts; 1972. pp. 64–99. [Google Scholar]
- Savastano HI, Miller RR. Biological significance and posttraining changes in conditioned responding. Learning and Motivation. 2003;34:303–324. [Google Scholar]
- Schachtman TR, Brown AM, Gordon EL, Catterson DA, Miller RR. Mechanisms underlying retarded emergence of conditioned responding following inhibitory training: Evidence for the comparator hypothesis. Journal of Experimental Psychology: Animal Behavior Processes. 1987;13:310–322. [PubMed] [Google Scholar]
- Schweitzer L, Green L. Reevaluation of things past: A test of the “retrospection hypothesis” using a CER procedure with rats. Pavlovian Journal of Biological Science. 1982;17:62–68. [PubMed] [Google Scholar]
- Shanks DR. Forward and backward blocking in human contingency judgment. Quarterly Journal of Experimental Psychology B. 1985;37:1–21. [Google Scholar]
- Van Hamme LJ, Wasserman EA. Stimulus competition in causality judgments: The role of nonpresentation of compound stimulus elements. Learning and Motivation. 1994;25:127–151. [Google Scholar]
- Wasserman EA, Berglan LR. Backward blocking and recovery from overshadowing in human causality judgment: The role of within-compound associations. Quarterly Journal of Experimental Psychology B. 1998;51:121–138. doi: 10.1080/713932675. [DOI] [PubMed] [Google Scholar]
- Wagner AR. SOP: A model of automatic memory processing in animal behavior. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 5–47. [Google Scholar]
- Williams DA, Docking GL. Associative and normative accounts of negative transfer. Quarterly Journal of Experimental Psychology: Human Experimental Psychology A. 1995;48:976–998. [Google Scholar]
- Williams DA, Sagness KE, McPhee JE. Configural and elemental strategies in predictive learning. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20:694–709. [Google Scholar]


