Abstract
Insulin-like growth factors (IGF-I, IGF-II) and their binding proteins (IGFBP-1-6) play a key role in cell proliferation, differentiation and apoptosis, suggesting possible involvement in carcinogenesis. Several epidemiological studies show associations of IGFs with prostate cancer. We searched the published literature for all studies relating levels of IGFs or IGFBPs with prostate cancer. We performed random effects meta-analysis to calculate summary odds ratios. The number of studies (prostate cancer cases) included in each meta-analysis were 42 (7,481) IGF-I; 10 (923) IGF-II; 3 (485) IGFBP-1; 5 (577) IGFBP-2; 29 (6,541) IGFBP-3; and 11 (3,545) IGF-1:IGFBP-3 ratio. The pooled odds ratios (95% confidence intervals) per standard deviation increase in peptide, were: IGF-I, OR = 1.21 (1.07, 1.36); IGF-II, OR = 1.17 (0.93, 1.47); IGFBP-1, OR = 1.21 (0.62, 2.33); IGFBP-2, OR = 1.18 (0.90, 1.54); IGFBP-3, OR = 0.88 (0.79, 0.98); IGFI:IGFBP-3 ratio, OR = 1.10 (0.97, 1.24). For all exposures, there was substantial heterogeneity (all I2 > 75%), partly explained by study design: the magnitude of associations was smaller in prospective versus retrospective studies, and for IGFBP-3 the inverse association with prostate cancer risk was seen in retrospective but not prospective studies. There was weak evidence that associations of IGF-I and IGFBP-3 with prostate cancer were stronger for advanced disease. Our meta-analysis confirms that raised circulating lGF-I is positively associated with prostate cancer risk. Associations between IGFBP-3 and prostate cancer were inconsistent, and there was little evidence for a role of IGF-II, IGFBP-1 or IGFBP-2 in prostate cancer risk.
Keywords: prostate cancer, meta-analysis, insulin-like growth factor, insulin-like growth factor binding protein
Introduction
Insulin-like growth factors (IGF-I and IGF-II) and their binding proteins (IGFBP-1-6) play a key role in cell proliferation, differentiation and apoptosis, in many tissues including the prostate. These processes are all involved in malignant transformation, and components of the IGF system may therefore be involved in the etiology and/or progression of prostate cancer [1].
Several epidemiological studies show positive associations of IGF levels with prostate cancer risk, but the results are inconsistent. A recently published analysis based on individual patient data from twelve prospective studies (n= 3700 prostate cancer cases) found an increased risk in the highest compared to the lowest quintiles of both IGF-I and IGFBP-3 (IGF-I odds ratio = 1.38, 96% confidence interval: 1.19,1.60; IGFBP-3 OR 1.23, 95% CI: 1.06,1.43), although the risk associated with IGFBP-3 was abolished in models controlling for IGF-I [2]. The authors found no association with IGF-II or IGFBP-2. Another meta-analysis of 9 prospective studies [3], which included 1512 men with prostate cancer, found an increased risk associated with the highest compared to the lowest quartile of IGF-I (OR = 1.31, 95% CI: 1.03,1.71), but no association of IGF-II or IGFBP-3. This association with IGF-I was weaker than that reported in an earlier meta-analysis published in 2004 [4], and based on 904 cases (OR = 1.83, 95% CI: 1.03,3.26).
We performed a systematic review of studies reporting on associations of IGFs and IGFBPs with the risk of prostate cancer. Unlike previous meta-analyses, we included retrospective and prospective studies. We have investigated several potential sources of heterogeneity, including separate analyses of prostate cancer by stage, grade and aggressiveness, and studied additional exposures not previously examined in meta-analyses of retrospective and prospective studies, including IGFBP-1. This review is the largest to date to assemble the published literature on the role of the IGF system in the aetiology of prostate cancer.
Material and Methods
Data Sources
We conducted a systematic search of all published papers, letters, abstracts and review articles on the association of insulin-like growth factors and measures of insulin resistance with prostate cancer. We searched the Medline (1966-2007), Embase (1980-2007), and Web of Science (1900-2007) bibliographic databases up to April 2007, using a combined text word and MESH heading search strategy (see supplementary material). The search was repeated on a weekly basis to identify any newly published studies up to December 2007. We also searched the reference lists from relevant articles and review articles, and previously published meta-analyses on the subject [3-5].
Titles and abstracts were assessed using pre-specified inclusion criteria (see below). Where abstracts were not available, the full papers were obtained and assessed. Full papers of all studies that were not clearly ineligible were obtained and two assessors (M-AR, RMM) independently reviewed all of these papers for inclusion. We identified duplicate publications by reviewing study name, authors, location, study population, dates, and study design. Where studies appeared to be published more than once, we took the most recent publication, or the publication that contained the most cases. If results were updated but did not include all previous exposures, we took the most recent publication that included that exposure.
Inclusion and exclusion criteria
In the present review, we included all studies reporting blood levels of at least one of the following peptides: IGF-I, IGF-II, IGFBP-I, IGFBP-2, IGFBP-3, IGF-I:IGFBP-3 ratio (an indicator of biologically available IGF-I [6]) - and reporting prostate cancer incidence or prevalence. To be included, studies had to present categories of peptide concentrations, or the mean or median and standard deviation of the peptide in prostate cancer case and control groups. We did not apply any language restrictions. We included population-based studies, whether retrospective or prospective. We excluded case reports, animal or in vitro studies, and research published as a conference abstract only. We excluded one paper where the age ranges of cases and controls did not overlap, because of the strong association of age with both IGF levels and prostate cancer [7].
Data extraction
Two investigators (M-AR, RMM or DG) extracted data from each paper independently using a standardised data extraction form. Data were extracted on study design, laboratory procedures, potential sources of heterogeneity (age of participants, date study conducted, inclusion of screen-detected cases, control group used (men with benign prostatic hyperplasia, healthy men, or a mixture of the two, and whether controls were selected from the general population or a hospital)), confounding factors controlled for and results from unadjusted, adjusted but excluding adjustment for one of the other peptides, and fully adjusted (including adjustment for one of the other peptides) models. Where studies included separate healthy and benign prostatic hyperplasia (BPH) control groups, we extracted data on the healthy control group only. Authors were contacted where data were missing or not clear, or where it was stated that subgroup analyses had been carried out but no results were presented in the publication. The two sets of extracted data were entered into an electronic database and checked for consistency using an automated procedure. Any disagreements were resolved by discussion between M-AR, RMM and DG.
Statistical analysis
To compare across studies, we calculated the log odds ratio (OR) or risk ratio (RR) per standard deviation (SD) increase in growth factor, with and without controlling for potentially confounding factors. For studies presenting results as a difference in means in healthy and diseased groups, we calculated the log odds ratio or risk ratio per unit increase in exposure using the method of Chêne and Thompson [8]. For studies presenting their results within categories of exposure (e.g. quantiles), we used the mean or median exposure in each category when they were reported, and calculated the log OR or RR per unit increase in exposure using the method of Greenland and Longnecker [9]. When the mean or median in each group was not reported, and a range of exposure in each group was given, we estimated the mean exposure in each group using the method of Chêne and Thompson, which assumes a normal distribution of the exposure in the population [8]. Where no information was presented on the exposure levels in each group, a normal distribution was assumed based on the number of subjects in each group, and the log OR or RR per SD increase calculated based on this assumption. Log OR or RR per unit increase and their standard errors were converted to a per SD increase by multiplying by the SD of the exposure. When this was not reported, the estimated SD from the Chêne and Thompson method was used [8]. Where studies only presented subgroup analyses and not an overall cancer group, we combined the subgroups statistically where possible, by calculating pooled means.
It is important to note that when comparing forest plots with previously published meta-analysis, some of the results for individual studies may appear to be on opposite sides of the OR = 1 (null) line. This is because our method of dose-response meta-analysis assumes a linear relationship between cancer risk and IGF/IGFBP, which can result in a different overall OR than a highest vs. lowest group analysis, which does not take into account the middle quantiles.
For papers presenting data in several ways, the order of preference for choosing the data to be pooled was: i) reported coefficients (log odds ratio or risk ratio (per unit increase) - in order of fully adjusted then unadjusted; ii) fully adjusted categorical data; iii) unadjusted categorical data; and iv) continuous data (presented as mean or median). To assess the effect of controlling for potential confounding factors on the results, we compared adjusted and unadjusted models from those prospective studies that presented both models.
Two studies presented their results in a way that was not possible to combine with other studies: one stated that there was “no difference” between cases and controls but did not provide raw data for the IGF-II and IGFBP-1 associations[10] and another presented IGFBP-2 as a percentage of total serum IGFBPs [11].
We performed random and fixed effects meta-analysis to calculate summary OR estimates, using the metan command [12]. The DerSimonian and Laird model relaxes the assumption of a common treatment effect. Effect sizes are assumed to have a Normal distribution with variance Τ2, which is based on Cochran's Q statistic for heterogeneity. This additional source of variation has the effect of making study weightings in the meta-analysis more similar in the presence of heterogeneity; the greater the heterogeneity, the more weight is given to smaller studies [13]. We calculated the I2 statistic as a quantitative measure of the degree of inconsistency across studies (heterogeneity) [14]. Small study effects were assessed by inspection of funnel plots and computation of Egger [15] and Begg [16] tests. Because there was evidence of substantial heterogeneity, we report random effects models throughout this paper, but for the main analyses we also present fixed effect models for comparison.
All statistical analyses were carried out using Stata statistical software (version 10.0. College Station, TX: Stata, 2007).
Subgroup analyses
Stage and grade
To investigate whether the IGFs or IGFBPs were associated more strongly with more advanced or clinically significant prostate cancers, we conducted separate meta-analyses of the association of these peptides with localised and advanced cancers, and with low and high Gleason grade cancers. Because definitions of “advanced”, “localised”, “low grade” and “high grade” differed between studies, and some studies grouped cancers as “aggressive” (a combination of high grade / advanced stage) or “non-aggressive” (a combination of low grade / localised stage), we analysed them in these groups and then created combined groups. Our combined groups were defined as: (i) “non-aggressive”, consisting of low Gleason grade and/or localised stage and/or “non-aggressive” cancer; and (ii) “aggressive”, defined as high Gleason grade and/or advanced stage and/or “aggressive” cancers. Where studies presented both stage and grade analyses, we included the stage analysis only in the pooled group, because it is an indicator of cancer that has or has not progressed, though we also repeated the grouping using the grade analysis and found similar results. Where authors stated that they carried out stratified analyses but did not present their results, we contacted authors for the original data, and received one response [17]. We used metaregression to investigate whether associations of prostate cancer with IGFs / IGFBPs differed between non-aggressive compared with aggressive cancers (heterogeneity) after excluding papers where both subgroups of prostate cancer were compared to one single control group. We repeated the analysis including such studies to check that the results were similar, although we recognise that including studies with non-independent control groups will give standard errors that are too small. One study [18] (n= 120 cases) provided results for localised and advanced cancers separately, but not an overall prostate cancer group. We could not easily combine these results to get an overall cancer group, because medians were presented. In this case we included the results for localised prostate cancer in our overall meta-analysis, because the other included studies contained a majority of localised cancers, and then included them in the localised subgroup analysis in addition. Another study only included advanced cancers so we excluded it from the overall meta-analyses but used the results in the advanced cancer subgroup analysis [19].
Study design characteristics and quality
We did not formally assess the quality of the included studies as there is no validated set of quality criteria for observational studies [20]. Instead, we first compared results from retrospective and prospective study designs using metaregression. Prospective studies are less likely than retrospective studies to be influenced by reverse causality (changes in IGF or IGFBP levels in response to cancer) and selection bias. We therefore conducted subgroup metaregression analyses to investigate the influence of the following, a priori defined study factors on effect estimates in prospective studies only: (i) mid-year the study was conducted, as this was considered an indicator of whether the study was conducted in the pre-PSA or post-PSA screening era (our cut-point for the pre-PSA era was 1993, as before this time period PSA screening was not widespread) [21], (ii) whether the study was population- or hospital-based, an indicator of potential selection bias; (iii) number of cases (split into two groups by the median of 128) to investigate small study effects [15]; (iv) sample type (serum or plasma), because other studies have found that this influences the overall estimate [4]; (v) assay type (ELISA or other), because the assay method has been reported as a source of variability [22] (vi) study location (North America, UK and Europe, or Other); (vii) whether cases were screen detected or clinically detected, or a combination of the two; (viii) whether the presence of prostate cancer was histologically confirmed or not; and (ix) whether models were mutually adjusted for IGF-I and IGFBP-3.
We also examined some study design features in retrospective studies separately, to see if those using BPH controls or hospital based controls (which might lead to selection bias: e.g. if IGFs are associated with BPH or the presence of BPH or other diseases alters levels of IGFs or IGFBPs), or having a smaller number of subjects (less than the median), gave different estimates than those that used healthy controls, population based controls, or more subjects (indicators of high-quality study design). We performed a sensitivity analysis to exclude one extreme outlying retrospective study [23].
Results
Systematic Review
The searches yielded 2135 references. After title and abstract review, 79 papers were selected as potentially relevant and were retrieved for more detailed assessment. Forty-seven of these studies provided an estimate of the association of at least one IGF peptide (IGF-I, IGF-II, IGFBP-I, IGFBP-2, or IGFBP-3) with prostate cancer occurrence, and were included in the meta-analysis (Figure 1). Sixteen were prospective and 31 were retrospective studies (Table 1).
Figure 1.
Flow diagram showing the number of studies included in and excluded from the meta-analysis
Table 1.
Characteristics of studies of IGFs, IGFBPs and prostate cancer included in the meta analysis of IGFs, grouped into prospective and retrospective study designs
| Author | Year | Study Name and location | N cases |
N controls |
Control Type |
Control Source |
IGF-I assay & sample type |
IGF-II assay & sample type |
IGFBP1 assay & sample type |
IGFBP2 assay & sample type |
IGFBP3 assay & sample type |
Time of sample collection (range in years) |
Prostate cancer confirmed by histology |
Mean or median (range) age at diagnosis of cases |
Mid-year of recruitment of cohort |
Ehtnicity |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective studies | ||||||||||||||||
| Schaefer | 1998 | Kaiser Permanente Health Plan USA |
45 | 720 | H | PBC | RIA, S | BD (1 to 21) |
Not stated | 1980 | Not stated |
|||||
| Chan | 1998 | Physicians Health Study, USA | 152 | 152 | H | PBC | ELISA, PE |
ELISA, PE |
ELISA, PE |
BD (.5 to 9.5) |
Not stated | 1987 | Not stated |
|||
| Stattin | 2000 | Northern Sweden Health & Disease Cohort, Sweden |
149 | 298 | H | PBC | IRMA, PE | RIA, PE | BD (.08 to 10) |
Some | 63 | 1992 | Not stated |
|||
| Harman | 2000 | Baltimore Longitudinal Study of Aging, USA |
72 | 127 | H | PBC | RIA, S | RIA, S | RIA, S | BD (3.1 to 16.2) |
Some | 74 | 2000 | Multi ethnic |
||
| Lacey | 2001 | Clue 1 population (Washington County serum bank), USA |
30 | 60 | H | PBC | ELISA, S | ELISA, S | BD (2 to 15) |
Yes | 70.6 (58 to 86) |
2001 | White | |||
| Chan | 2002 | Physician's Health Study, USA | 530 | 534 | H | PBC | ELISA, PE |
ELISA, PE | BD (.5 to 13) |
Some | 1988 | Not stated |
||||
| Woodson | 2003 | ATBC Trial, Finland | 100 | 400 | H | TC | ELISA, S | ELISA, S | BD (5 to 12) |
Yes | 68.6 | 1989 | Not stated |
|||
| Stattin | 2004 | Northern Sweden Health & Disease Cohort, Sweden |
281 | 560 | H | PBC | IRMA, PE | IRMA, PE | BD | Not stated | 63.6 | 2004 | Not stated |
|||
| Janssen | 2004 | ERSPC, Netherlands | 201 | 201 | H | TC | IRMA, S | IRMA, S | BD | Yes | 66.4 (58.9 to 74) |
1997 | Not stated |
|||
| Chen | 2005 | Cardiovascular Health Study, USA |
174 | 174 | H | PBC | IRMA, PE | IRMA, PE | BD (min 1) |
Not stated | 1994 | Multi ethnic |
||||
| Platz | 2005 | Health Professionals Follow Up Study, USA |
462 | 462 | H | PBC | ELISA, PE |
ELISA, PE | BD | Not stated | 68.6 (47.7 to 84.3) |
1995 | Not stated |
|||
| Meyer | 2005 | SUVIMAX Trial, Fance | 100 | 400 | H | TC | CLA, PH | IRMA, PH | RIA, PH | Other, PH | BD | Yes | 55.2 | 2005 | Not stated |
|
| Morris | 2006 | BUPA Study, UK | 141 | 423 | H | H | ELISA, S | ELISA, S | ELISA, S | BD (max 15) |
Not stated | 2006 | Not stated |
|||
| Severi | 2006 | Melbourne Collaborative Cohort Study, Australia |
524 | 2167 | H | PBC | ELISA, PU |
ELISA, PU | BD | Not stated | 67 (47 to 80) |
1996 | Not stated |
|||
| Allen | 2007 | EPIC, Europe | 630 | 630 | H | PBC | ELISA, S | ELISA, S | BD (.1 to 8.5) |
Not stated | 65 (47 to 82) |
1996 | Not stated |
|||
| Weiss | 2007 | PLCO screening trial, USA | 727 | 887 | H | PBC | ELISA, S | ELISA, S | BD | Not stated | 1997 | White | ||||
|
Retrospective studies | ||||||||||||||||
| Cohen | 1993 | USA | 32 | 16 | H | P | RIA, S | RIA, S | RIA, S | WB, S | AD | Yes | 1993 | Not stated |
||
| Kanety | 1993 | Israel | 14 | 6 | H | NS | RIA, S | RIA, S | AD | Yes | 66.5 | 1993 | Not stated |
|||
| Ho | 1997 | Australia | 16 | 7 | H | H | RIA, S | RIA, S | RIA, S | RIA, S | AD (.2 to 10) |
Not stated | 70.2 | 1997 | Not stated |
|
| Mantzoros | 1997 | Athens, Greece | 52 | 52 | H | P | RIA, S | D | Yes | 2007 | Not stated |
|||||
| Wolk | 1998 | Orebro County, Sweden | 210 | 224 | H | P | IRMA, S | IRMA, S | AD (max .25) |
Yes | 1998 | Not stated |
||||
| Cutting | 1999 | UK | 37 | 57 | H | H | IRMA, S | D | Yes | 73.2 | 1999 | Not stated |
||||
| Signorello | 1999 | Orebro, Sweden | 208 | 70 | H | P | RIA, S | AD (max .25) |
Yes | 1999 | Not stated |
|||||
| Djavan | 1999 | Austria | 71 | 174 | H | NS | IRMA, S | NS | Yes | 65.7 | 1999 | White | ||||
| Koliakos | 2000 | Greece | 34 | 113 | B | H | IRMA, S | BD | Yes | 67 | 2000 | Not stated |
||||
| Finne | 2000 | Finnish Prostate Cancer Screening Trial, Finland |
179 | 486 | HB | PBC | ELISA, S | IFMA, S | D | Yes | 62 | 2000 | Not stated |
|||
| Hill | 2000 | Czech Republic | 15 | 56 | B | H | IRMA, S | NS | Yes | 77.1 (64 to 86) |
2000 | Not stated |
||||
| Baffa | 2000 | USA | 57 | 39 | H | NS | ELISA, S | AD | Yes | 2000 | Not stated |
|||||
| Kurek | 2000 | Germany | 171 | 67 | H | NS | CLA, S | AD | Not stated | 66.2 | 2000 | Not stated |
||||
| Khosravi | 2001 | Canada | 84 | 75 | B | NS | ELISA, S | ELISA, S | NS | Yes | 2001 | Not stated |
||||
| Chokkalingam | 2001 | China | 128 | 306 | H | P | ELISA, PU |
ELISA, PU |
ELISA, PU | ELISA, PU | AD | Not stated | 71.9 | 2001 | Not stated |
|
| Shariat | 2002 | Baylor Prostate Cancer Center, USA |
120 | 44 | H | P | ELISA, PU |
RIA, PU | ELISA, PU | AD (min .8) |
Yes | 2002 | Not stated |
|||
| Hazem | 2002 | Uromed Prostate Cancer Detection Clinic, Canada |
244 | 408 | HB | H | ELISA, S | ELISA, S | D | Yes | 65.2 | 2002 | Not stated |
|||
| Peng | 2002 | Sichuan University, China | 81 | 55 | B | IRMA, S | Yes | 2002 | Not stated |
|||||||
| Li | 2003 | Cleveland and Detroit, USA | 408 | 437 | H | P | ELISA, S | ELISA, S | AD (.25 to 8) |
Yes | 61.3 | 2003 | Multi ethnic |
|||
| Miyata | 2003 | Nagasaki University, Japan | 112 | 32 | B | H | IRMA, S | RIA, S | D | Yes | 70.9 | 2003 | Japanese | |||
| Scorilas | 2003 | Padua, Italy | 171 | 174 | B | H | ELISA, S | Other | Yes | 68 (51 to 91) |
2003 | Not stated |
||||
| Lopez | 2004 | Kuala Lumpur, Malaysia | 16 | 46 | H | NS | ELISA, S | ELISA, S | NS | Yes | 69.7 | 2004 | Not stated |
|||
| Aksoy | 2004 | Turkey | 43 | 45 | B | NS | IRMA, S | IRMA, S | NS | Yes | (53 to 85) | 2004 | Not stated |
|||
| Oliver | 2004 | ProtecT, UK | 176 | 324 | H | P | ELISA, S | ELISA, S | RIA, S | AC, S | D | Yes | 62.2 (50 to 70) |
2004 | White | |
| Trapeznikova | 2004 | Russia | 36 | 80 | B | NS | ELISA | ELISA | NS | Not stated | 2004 | Not stated |
||||
| Kehinde | 2005 | Kuwait/Oman | 30 | 40* | H | P | IFMA, S | IRMA, S | D | Yes | 69.7 | 2005 | Arab | |||
| Marszalek | 2005 | Austria | 156 | 271 | HB | NS | IRMA, S | D | Yes | 66.7 | 2005 | Not stated |
||||
| Nam | 2005 | University Health Network, Canada | 483 | 205 | H | H | ELISA, S | ELISA, S | D | Yes | 66.6 (42.7 to 90.8) |
2005 | Multi ethnic |
|||
| Trojan | 2006 | Mannheim, Germany | 72 | 40 | B | H | ELISA, S | D | Yes | 62.8 (47 to 75) |
2006 | Not stated |
||||
| Hernandez | 2007 | Harvard University Hospital, USA | 401 | 366 | H | H | Other, S | Other, S | D | Not stated | 65.8 (40 to 85) |
2007 | Black | |||
| Zhigang | 2007 | Shantou University Medical College, China |
281 | 305 | HB | H | ELISA, S | ELISA, S | D | Yes | 65.5 (57 to 75) |
2007 | Chinese | |||
Control type: H = healthy B=BPH HB=mixed healthy BPH
Control source: H=hospital, PBC=population based cohort, TC=trial cohort, P=population, NS=not stated
Assay types: ELISA=enzyme-linked immunosorbent assay, IRMA=immunoradiometric assay, IFMA=immunofluorometric assay, RIA=radioimmunoassay, CLA=chemiluminescent assay, AC=acid chromatography, WB=Western blot
Sample types: S=serum, PH=heparin plasma, PE= EDTA plasma, PU=plasma unknown Time of sample collection: BD=before diagnosis, AD=after diagnosis, D=at diagnosis
This study presented several age categories of controls, and we chose the 60-69 group because this contained the mean age of the cases
The total number of prostate cancer cases per study ranged from 14 to 727 and the number of controls ranged from 6 to 2,167. All the identified studies were published from 1993 onwards, but covered recruitment periods beginning in 1960. Ethnicity was only reported in 34% of studies.
All prospective studies and 18 of the retrospective studies used healthy controls; of the remaining retrospective studies, nine used men with benign prostatic hyperplasia (BPH) as controls, and four used a mixture of both healthy and BPH controls. Prostate cancers were histologically confirmed in 33 studies.
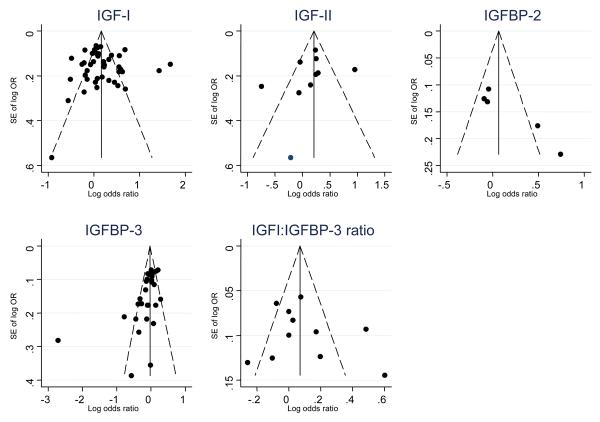
There was an indication of funnel plot asymmetry for IGFBP-2 and IGFBP-3 (Figure 2), and this was supported by results from the Egger (IGFBP-2 p=0.02, IGFBP-3 p=0.002) and Begg (IGFBP-2 p=0.09, IGFBP-3 p=0.004) tests. For IGFBP-3, the smaller studies were suggesting larger inverse associations with prostate cancer, while for IGFBP-2 the smaller studies provided larger positive associations. There was no indication of funnel plot asymmetry for IGF-I (Egger p=0.66, Begg p=0.66), IGF-II (Egger p=0.48, Begg p=0.11), or IGF-I:IGFBP-3 ratio (Egger p=0.67, Begg p=1.0). There were too few studies to assess funnel plot asymmetry for IGFBP-1.
Figure 2.
Funnel plots of standard errors (SE) of the log odds ratio (OR) of prostate cancer (Y axis) versus the log OR of prostate cancer (X axis) for IGF-I, IGF-II, IGFBP-2 and IGFBP-3. The vertical line is drawn at the pooled log OR. Diagonal lines indicate the pseudo 95% confidence interval.
Meta-analysis
The number of prostate cancer cases included in each meta-analysis were 7,481 for IGF-I [3;10;11;17;18;23-59]; 923 for IGF-II [3;11;27;29;30;34;46;49;55;60]; 485 for IGFBP-1 [29;61;62]; 577 for IGFBP-2 [18;30;46;49;62]; 6,541 for IGFBP-3 [3;10;17;18;23-25;27-30;33-36;38;39;42;43;46-49;54;56-59;63]; and 3,545 for the IGF-1:IGFBP-3 ratio [28;29;35;36;38;42;49;51;54;56;58].
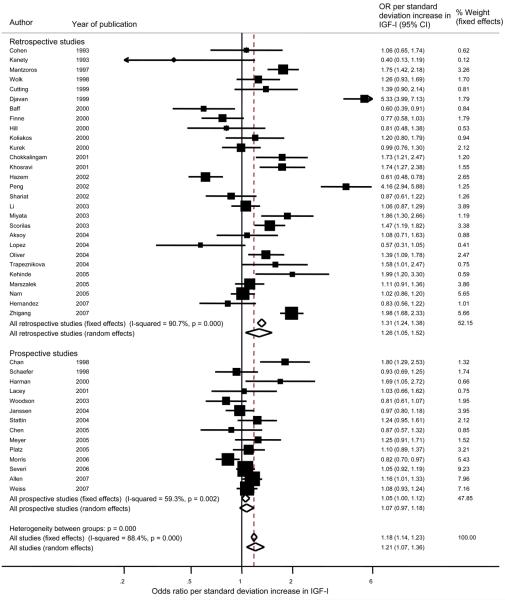
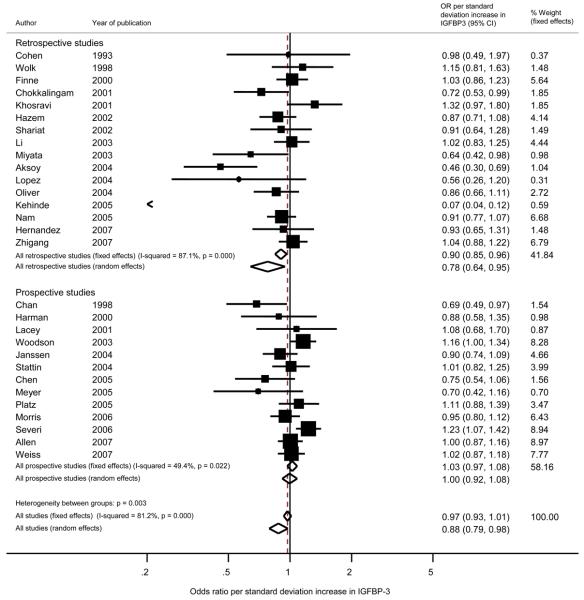
Overall (prospective and retrospective studies combined), the pooled random effects OR estimates (95% confidence intervals) per standard deviation increase in exposure, were: IGF-I, OR = 1.21 (1.07, 1.36), p < 0.003; IGF-II, OR = 1.17 (0.93, 1.47), p= 0.18; IGFBP-1, OR = 1.21 (0.62, 2.33), p = 0.58; IGFBP-2, OR = 1.18 (0.90, 1.54), p = 0.24; IGFBP-3, OR = 0.88 (0.79, 0.98), p < 0.02; IGFI:IGFBP-3 ratio, OR = 1.10 (0.97, 1.24), p = 0.12 (Figures 3, 4, 5). Forest plots for IGFBP-I, IGFBP-2, and the ratio of IGF-I:IGFBP-3 are available as supplementary figures (Figures 6, 7, 8). In those prospective studies that presented both unadjusted and fully adjusted effect estimates (14;23;24;43;48;53;60), pooled analysis indicated that adjustment for potential confounding factors, including mutual adjustment for IGF-I and IGFBP-3, did not impact on the overall OR for IGF-I, with the unadjusted OR = 1.14 (1.05, 1.25) and the adjusted OR = 1.17 (1.04, 1.31). Adjustment for IGF-I did shift the overall summary for IGFBP-3 from the unadjusted model being associated with increased risk (OR = 1.04 (95% CI 0.95, 1.13)) to the adjusted model being associated with slight reduced risk (OR = 0.95 (95% CI 0.85, 1.06)), however confidence intervals for both of these estimates include an OR of 1. As shown on the forest plot for IGFBP-3 (Figure 5), there was one extreme outlier with a vastly different OR to the other studies (OR 0.07, 95% CI 0.04, 0.12). A sensitivity analysis excluding this study resulted in a pooled OR of 0.95 (95% CI 0.89, 1.02) p=0.57.
Figure 3.
Forest plot of the association of IGF-I with prostate cancer showing the effect of a one standard deviation increase in IGF-I. Squares indicate the study-specific effect estimate, with the size proportional to the inverse of the variance, with horizontal lines showing 95% confidence intervals. The dashed vertical line and diamonds (95% confidence interval) are the pooled estimates based on random or fixed effects meta-analysis models.
Figure 4.
Forest plot of the association of IGF-II with prostate cancer showing the effect of a one standard deviation increase in IGF-II. Squares indicate the study-specific effect estimate, with the size proportional to the inverse of the variance, with horizontal lines showing 95% confidence intervals. The dashed vertical line and diamonds (95% confidence interval) are the pooled estimates based on random or fixed effects meta-analysis models.
Figure 5.
Forest plot of the association of IGFBP-3 with prostate cancer showing the effect of a one standard deviation increase in IGFBP-3. Squares indicate the study-specific effect estimate, with the size proportional to the inverse of the variance, with horizontal lines showing 95% confidence intervals. The dashed vertical line and diamonds (95% confidence interval) are the pooled estimates based on random or fixed effects meta-analysis models.
We found that retrospective study designs provided consistently stronger associations than prospective studies for all the peptides investigated; for example for IGF-I, the pooled OR was 1.26 (1.05, 1.52) from retrospective studies compared to a pooled OR of 1.07 (0.97, 1.18) from prospective studies (Figure 3). Statistical evidence for these differences was generally weak - the p-values for the differences comparing retrospective with prospective study designs for each exposure were: IGF-I, p=0.30; IGF-II, p=0.12; IGFBP-1, p=0.77, IGFBP-2, p=0.16; IGFBP-3, p=0.33; ratio of IGFI:IGFBP-3, p=0.02.
Subgroup analyses
For all exposures, there was evidence of substantial heterogeneity (all I2 > 75%) and we investigated potential sources of between study variation in a number of subgroup analyses shown below.
Stage and grade
Eight prospective studies investigated IGF-I and IGFBP-3 separately in subgroups of cancers, classified according to stage and/or Gleason grade and/or study-defined “aggressiveness” [17;25;27;28;46;51;54;56;64]. Table 2 shows the pooled OR and 95% confidence intervals for these subgroups, and for our combined group of “overall aggressiveness”.
Table 2.
Associations of IGF-I and IGFBP-3 with prostate cancer in prospective studies, stratified by cancer aggressiveness, stage, and Gleason grade.
| Number of studies |
IGF-I OR (95% CI) |
IGFBP-3 OR (95% CI) |
|
|---|---|---|---|
| Overall aggressiveness 1 | |||
| Non-aggressive | 8 | 1.05 (0.99, 1.12) | 1.03 (0.96, 1.11) |
| Aggressive | 8 | 1.21 (0.97, 1.51) | 0.78 (0.56, 1.10) |
| p for difference (a) (b) | 0.2 | 0.02 | |
|
| |||
| Stage 2 | |||
| Localised | 4 | 1.10 (0.98, 1.22) | 1.03 (0.92, 1.15) |
| Advanced | 4 | 1.41 (1.07, 1.85) | 0.82 (0.57, 1.18) |
| p for difference | 0.06 | 0.14 | |
|
| |||
| Gleason grade 3 | |||
| Low | 4 | 1.09 (1.00, 1.20) | 0.93 (0.85, 1.03) |
| High | 4 | 1.21 (0.90, 1.62) | 0.85 (0.49, 1.48) |
| p for difference | 0.5 | 0.6 | |
|
| |||
|
Aggressiveness (defined by individual studies)4 |
|||
| Non-aggressive | 3 | 1.03 (0.93, 1.13) | 1.12 (1.01, 1.23) |
| Aggressive | 3 | 0.93 (0.66, 1.33) | 0.92 (0.62, 1.37) |
| p for difference (b) | 0.59 | 0.38 | |
aggressive = advanced stage, high Gleason grade, or study-defined “aggressive”; non-aggressive = localised stage, low Gleason grade, or study-defined “non-aggressive”
localised = stage A or B (Whitmore Jewett), T1-T2 (sometimes also T3a), N0 M0 (AJCC classification); advanced = stage C or D (Whitmore Jewett), T3-4 or N1 or M1 or PSA>50ng/ml
low grade = Gleason grade <7; high grade = Gleason grade ≥7
non-aggressive = stage A or B (Whitmore Jewett) or Gleason grade <7; aggressive = stage C or D (Whitmore Jewett), or poorly differentiated (ICD grade 3), or Gleason grade ≥7
when a single study separately analysed both stage and grade, we chose to include the stage analysis in the pooled effect-estimate
excluding studies which did not use non-independent controls
IGF-I showed generally stronger associations with more aggressive (OR 1.21, 95% CI 0.97, 1.51) and advanced (OR 1.41, 95% CI 1.07, 1.85) cancers, compared to non-aggressive (OR 1.05, 95% CI 0.99, 1.12) and localised (OR 1.10, 95% CI 0.98, 1.22) cancers, but the statistical evidence for any differences was weak (p=0.20 for aggressiveness, p=0.06 for stage). A similar but opposite pattern was seen for IGFBP-3, which showed a greater reduction in odds of “aggressive” (OR = 0.78, 95% CI 0.56, 1.10) compared to “non-aggressive” (OR = 1.03, 95% CI 0.96, 1.11) cancers (p for difference= 0.02).
Study design characteristics and quality - investigating heterogeneity
When considering prospective studies alone, we still observed considerable heterogeneity in three of the four exposures (IGF-I: I2 = 59%, IGF-II: I2 = 81%, IGFBP-2: I2 = 0%, IGFBP-3: I2 = 49%). There were insufficient numbers of studies to do subgroup analyses on IGF-II, IGFBP-1, IGFBP-2, and the ratio of IGF-I:IGFBP-3 so we restricted the subgroup analyses to the 14 prospective studies presenting IGF-I and the 13 prospective studies presenting IGFBP-3.
There was little evidence that any of the nine factors investigated (e.g. date study conducted; study setting) explained the observed heterogeneity (all p values for heterogeneity between strata of each variable >0.09, all I2 values within strata >0.34). The lowest p value of 0.09 was for control source for IGF-I (hospital versus population-based control).
In meta-regression analyses of retrospective studies, investigating control type, control source, or number of prostate cancer cases, we found no difference between groups. Retrospective studies may be more likely to contain advanced cases, which could lead to an overestimation of associations as a result of reverse causality; however the reporting of cancer stage did not allow us to adequately investigate this in a subgroup analysis.
Discussion
Summary of findings
Our meta-analysis revealed that the body of the world-wide published literature is consistent with an average 21% increased risk of prostate cancer per standard deviation increase in IGF-I, and an average 12% reduced risk of prostate cancer per standard deviation increase in IGFBP-3. For IGF-II, IGFBP-2 and the ratio of IGF-I:IGFBP-3 there were positive but weaker associations with prostate cancer risk. Only the associations of IGF-I and IGFBP-3 excluded an odds ratio of 1 (no difference), and this was only the case for the random effects model for IGFBP-3. Our sensitivity analysis excluding one extreme outlying retrospective study resulted in a shift of the pooled IGFBP-3 OR towards the null (and therefore more in line with the prospective pooled result), with the associated p value changing from <0.02 to 0.57. The overall heterogeneity changed from 81.2% to 52.9%. We observed that effect estimates were consistently stronger in retrospective than prospective studies, suggesting that the true effect of IGF-I may be weaker than indicated by the retrospective studies, and that there is no association in prospective studies (positive or negative) of levels of IGF-II, IGFBP-2, IGFBP-3 and the ratio of IGFI:IGFBP3 with prostate cancer risk. This suggests they may not be involved in the aetiology of prostate cancer, but may be useful tumour markers as shown by the positive association in retrospective studies. IGFBP-1 showed no association with prostate cancer in either retrospective or prospective studies.
We observed considerable heterogeneity within both study types, as indicated by the I2 values which were generally greater than 50%. It is widely accepted that prospective studies are less likely than retrospective studies to be influenced by reverse causality (changes in IGF or IGFBP levels in response to cancer) and selection bias. We therefore conducted subgroup analyses to investigate several potential sources of between-study variation in effect-estimates in prospective studies. There was no strong evidence that any single factor played an important role in explaining the heterogeneity observed.
Eight prospective studies (totalling 3,428 cases) presented results stratified by subgroups of prostate cancer, either by stage, Gleason grade or aggressiveness and there was weak evidence that associations of IGF-I (p for difference = 0.2) and IGFBP-3 (p for difference = 0.02) were stronger with more advanced or aggressive disease.
Mechanisms
IGFs and IGFBPs play key roles in cell proliferation, differentiation, and apoptosis. IGFs are mitotic and anti-apoptotic, and their bioactivity is determined by their levels as well as the expression of IGFBPs and proteases. IGFBPs transport IGFs, protect IGFs from degradation, and regulate interactions between IGFs and the IGF receptor (IGF-IR) [1]. IGFBPs also have direct effects on cell growth and proliferation, independent of their IGF-binding properties. Men with higher levels of IGF-I and/or lower levels of IGFBP-3 may therefore have higher IGF-I bioactivity. This could contribute to carcinogenesis and neoplastic progression, via mechanisms such as enhancing the survival of partially transformed cells which would otherwise be forced into apoptosis, increasing the pool of cells available for subsequent hits and full transformation, or increasing the probability of a neoplasm progressing to metastatic or androgen-independent cancer [65]. Our finding that the associations of IGF-I and IGFBP-3 were stronger with more advanced or aggressive disease could imply that the IGF system plays a more important role in cancer progression than in initiation of the disease. These peptides may therefore have potential roles as markers of cancers that are more likely to become clinically important. Mouse models of prostate cancer have shown that progression from androgen-dependence to androgen-independence is associated with a major increase in IGF-I gene expression and decreased expression of IGFBP-3 [66] which supports the potential of IGF-I as a biomarker of more aggressive cancer.
Strengths
This systematic review presents associations of six measures of the IGF system with prostate cancer risk (IGF-I, IGF-II, IGFBP-1, IGFBP-2, IGFBP-3 and the ratio of IGFI:IGFBP-3) and the analysis includes nearly 7,500 prostate cancer cases. Both prospective and retrospective studies are included, and compared to previous meta-analyses [2-5] , that included a maximum of 14 studies, we have included data from 47 studies. Unlike previous meta-analyses, we included studies that presented results in a variety of different ways, and used proven statistical methods to calculate a log OR or RR per standard deviation increase in exposure for each study. This method also enabled inclusion of results that were presented in different measurement units. Therefore, we did not exclude any of the studies that we identified based solely on the way the results were presented.
Limitations
Meta-analyses of observational studies are subject to biases inherent in the original studies. Our study therefore has limitations that are based largely on the quality of the included studies.
We observed consistent differences in effect size reported from retrospective and prospective studies. These differences could be due to biases related to study design, but could also be due to more advanced or aggressive cases being included in retrospective studies. We investigated study-design issues and could not find differences between subgroups of retrospective studies. We lacked sufficient data to investigate the effect of the presence of advanced or aggressive cases in retrospective studies. If more advanced cases were abundant then this might explain the larger effect estimates seen in retrospective studies, a suggestion in keeping with the greater effect seen in advanced or aggressive subgroup analyses in prospective studies.
We found evidence of small study effects for two of our exposures (IGFBP-2 and IGFBP-3), suggesting caution when interpreting results for these peptides, because publication bias may have led to exaggerated pooled effect estimates.
The method of detecting cases was not always reported. Therefore, we have been unable to accurately examine the association of IGFs and IGFBPs with screen detected compared to clinically detected cancers. Nevertheless, we attempted to distinguish between cases diagnosed in the pre- and post-PSA era as a proxy for detection method, and found no evidence to suggest that studies conducted in the pre-PSA era (which involved clinically diagnosed cancers) yielded results that substantially differed from those conducted during the PSA-era (screen-detected cancers) for either IGF-I or IGFBP-3 (p for difference in effect-estimates by era > 0.95).
Tissue IGF bioactivity is determined not only by circulating levels but also by local expression of IGFs and the IGF-I receptor, IGFBPs and proteases that cleave IGFBPs to liberate free IGFs. A limitation is that all studies have investigated circulating IGF / IGFBPs, and it is possible that effects at the tissue level are stronger than those observed using circulating factors. However, there is accumulating evidence that serum IGF-I levels either represent a surrogate for tissue IGF bioactivity (for example, expression of genes that influence tissue IGF biological activity is regulated in parallel with circulating IGF-I [64;67], or, serum IGF-I levels may directly impact tumor biology: animal models indicate that genetically induced alterations to circulating IGF-I levels, which do not affect somatic growth, do affect tumor growth and progression [67;68].
Although we did not find that assay type explained the heterogeneity observed, there are related issues that we were unable to investigate that could affect results. For example, quality control, sample storage history, and other methodological concerns could have a role in explaining heterogeneity or differences between prospective and retrospective studies [68;69].
IGFBP-3 exists as both intact and fragmented forms in the blood, each of which may be present with multiple additional post-translational modifications. Furthermore these different forms may have complex differential actions, both IGF-dependent and IGF-independent, with both positive and negative effects on cancer cells [70]. Which of these forms is measured in any assay is poorly defined, and it is possible that different IGFBP-3 assays are measuring different forms of the peptide, leading to heterogeneity in effect estimates [22]. IGF-I assays require a separation step to avoid interference of IGFBPs, and the efficiency of such steps varies considerably between assays and can be operator-dependent. Although IGF peptides can be stable over many years in serum samples stored under ideal conditions [71], long-term storage has however been observed to result in 3- to 4-fold variations in measured IGF-I levels in some assays [72]. This problem with historical blood collections may have more effect in some prospective studies using stored blood samples, and may be more of an issue with large cohorts collected in many peripheral collection sites where training and adherence to lab protocols may be variable [68;69]. We could not investigate these issues in this meta-analysis because data are not available to allow stratification by such quality assurance variables.
Comparison with previous reviews
Our results for IGF-I, but not IGFBP-3, are in some agreement with previous meta-analyses. Roddam et al observed a 38% increased odds of prostate cancer comparing highest versus lowest quintiles of IGF-I (95% CI 1.19, 1.60) in their collaborative analysis of prospective studies [2]. This relationship is similar to our findings for all studies, but much larger than the 7% increased odds we calculated when considering only prospective studies. An essential point to note is the method of calculating our pooled odds ratios. Our odds ratios represent the odds per standard deviation increase in growth factor, whereas Roddam et al compared the highest versus lowest quintiles. This equates to a comparison of nearly 2.8 standard deviation increases in growth factor level (the difference between the mean in the highest and lowest quintiles of a normal distribution is nearly 2.8 standard deviations), which may explain why they observed a stronger increase in odds than in this study. If this were the case, our result scaled to an increase of 2.8 standard deviations would be an OR of 1.072.8 = 1.21, which is closer to the Roddam et al result [2]. In addition, our study included three further prospective studies which were not included in the Roddam collaborative analysis (two of which were published after they closed their database) ([3;56;58], providing an extra 968 cancer cases. These three studies all had weak odds ratios (close to OR = 1) which may explain our overall association being closer to 1. Roddam et al included one study in their prospective collaboration that we classified as a retrospective study (because the men underwent a prevalence screen and IGFs were measured at the same time as diagnosis), and therefore did not include in our prospective analysis [49]. This study provided an odds ratio of 1.78 which will have contributed to their greater positive odds ratio. A final point is that we did not have access to individual patient data, so our statistical methods are subject to the assumptions and adjustments that each study used, whereas Roddam et al had access to individual patient data. Although there is no reason to expect bias in any particular direction, this limitation in our study includes an extra source of variation.
The finding, by Roddam et al, of a weakly increased odds of prostate cancer with increased IGFBP-3 (OR 1.23, 95% CI 1.06, 1.43) was not consistent with our findings [2]. Again, they presented a high versus low quintile comparison, whereas we present the odds ratio per one standard deviation increase in growth factor level, and our analysis includes three additional studies [3;56;58]. Roddam et al included one extra study which we felt should be classified as a “retrospective” study design, which had a positive odds ratio (OR = 1.11) [49]. These considerations resulted in an overall smaller positive odds ratio in our study compared to Roddam et al. The inverse association for IGFBP-3 was only seen in retrospective but not prospective studies in our meta-analysis and was sensitive to one extreme outlier, so the overall data including three more studies than Roddam et al suggest no evidence of an association of prostate cancer with IGFBP-3. Renehan et al observed a 49% increased odds of prostate cancer with increasing IGF-I from the 25th to 75th percentile in a dose-response analysis (95% CI, 14%, 95%), but there was no evidence of an association with IGFBP-3 (OR = 0·95; 95% CI 0·70,1·28) [2]. In their highest versus lowest category meta-analysis, they showed an 83% increased odds of prostate cancer with increased IGF-I (95% CI 3%, 226%). Our findings that retrospective studies showed stronger effects are in agreement with Renehan's review of retrospective studies (OR for highest versus lowest category = 2.43, 95% CI 1.11, 5.32) compared with prospective studies (OR = 1.42, 95% CI 0.56, 3.60) [4]. Shi et al, analysing 14 case-control studies, report an OR of 1.47 for IGF-I (95% CI 1.27, 1.71%) comparing highest versus lowest categories, but also found a positive association for IGFBP-3, with an OR of 1.25 (95% CI 1.06, 1.47) [5]. Morris et al showed an increased odds for IGF-I when comparing the highest versus lowest quartile (OR=1.31, 95% CI 1.00, 1.71) but no relation with IGF-II (OR=0.72, 95% CI 0.36, 1.44) or IGFBP-3 (OR=1.05, 95% CI 0.82, 1.35) [3]. Our overview is more comprehensive and includes many more studies than previous meta-analyses, but all are consistent with higher circulating levels of IGF-I conferring an increased risk of prostate cancer. In contrast to the previous meta-analyses, we also found some evidence for a reduced risk of prostate cancer with increasing IGFBP-3. However, there was some evidence amongst prospective studies that this association could be restricted to clinically significant cancers (see Table 2). IGF-I showed generally stronger associations with more aggressive (OR 1.21, 95% CI 0.97, 1.51) and advanced (OR 1.41, 95% CI 1.07, 1.85) cancers. In contrast, Roddam et al [2] noted that IGF-I showed a stronger association with low-grade prostate cancer (OR 1.57, 95% CI 1.32,1.87) than high-grade cancer (OR 1.12, 95% CI 0.87, 1.43). Our analysis only included four prospective studies that presented IGF-I levels stratified by prostate cancer grade, so has limited power to detect differences in associations by grade; indeed the confidence intervals for the stratified associations overlapped somewhat and our results are inconclusive. Roddam et al may have included more than four studies because the collaborative nature of their analysis meant they had access to original patient data [2]. In both meta-analyses, these differences in associations by grade may have arisen by chance. In a formal test of the differences in ORs for low-grade versus high-grade cancer in our study, the p value was 0.2, and in the Roddam et al study it was much smaller at p = 0.027 [2].
Two previous meta-analyses have investigated the association of IGF-II with prostate cancer risk; one was based on only 4 studies, and provided a pooled OR of 0.72 (highest versus lowest quartile), but with confidence intervals that were wide and included the null value of 1 (95% CI 0.36, 1.44) [3], and the other was the pooled collaborative analysis of twelve prospective studies, in which the odds ratio was 1.12, again with very wide confidence intervals (95% CI 0.72, 1.74) [2]. Our results based on 10 studies show a trend towards a 17% increased risk of prostate cancer per SD increased IGF-II, but with confidence intervals also including 1 (−3%, 47%).
Renehan et al also investigated sources of heterogeneity, and found that the associations of IGF-I and IGFBP-3 depended on the type of sample medium analysed, with a stronger association seen with plasma than with serum [4]. We did not find this to be the case. Roddam et al [2] did not find any significant heterogeneity by patient or study characteristics.
Very few studies investigated IGF and IGFBP levels in relation to progression of prostate cancer. One study showed that IGFBP-2 predicted post-radical prostatectomy biochemical recurrence-free survival, independent of stage or Gleason score. It also showed that increased IGFBP-2 is associated with better survival in patients with neoadjuvant hormone therapy but worse survival in those without [69;73]. This study showed that IGF-I, IGFBP-1 and IGFBP-3 were not predictors of biochemical recurrence-free survival [69;73]. Another study showed no differences in IGF-I levels between patients with prostate cancer who were in remission and those who developed recurrent disease after radical prostatectomy, whereas levels of IGFBP-2 and IGFBP-3 were higher in the patients who were in remission [73;74]. IGFBP-2 levels increased over time in men in remission but did not change in the patients who had recurrence [73;74]. Further research is needed in this area before any conclusions about the relationship between IGF and IGFBP levels with prostate cancer progression can be drawn.
Conclusion
Even though we observed a modest increase in risk of prostate cancer associated with higher levels of IGF-I, and a slight reduced risk with higher levels of IGFBP-3, neither of these peptides are likely to be useful as additional measurements in prostate cancer PSA screening. The strength of the associations are too weak to have any value as a screening test, because at these odds ratios, the detection rate (sensitivity) is less than 8% for a 95% specificity (5% false positive rate) [3;74]. This issue has been investigated by Oliver et al. who found no evidence that measures of IGFs or IGFBPs enhanced the specificity of prostate cancer detection beyond that achievable by the currently used free/total PSA index [75]. Future research should be aimed at clarifying the associations of IGF-II, IGFBP-2 and IGFBP3 with prostate cancer, in large prospective studies.
The magnitude of the increased risk of prostate cancer per SD increase in IGF-I (21%), although modest, is likely to be aetiologically important as demonstrated by the fact that it is of the same order of magnitude as that for well-known ischemic heart disease (IHD) risk factors in population-based cohorts. For example, a one SD increase in diastolic blood pressure is associated with a 26% increased risk of IHD, and a one SD increase in total cholesterol is associated with a 44% increased risk of IHD [76]. So although IGF-I measurement is unlikely to increase the discriminatory accuracy of current prostate cancer screening methods (serum prostate specific antigen or digital rectal examination), it does represent a potentially modifiable risk factor for prostate cancer, and this could be achieved through dietary or lifestyle interventions which may alter IGF-I levels [77]. In addition, the IGF system is likely to become a potential therapeutic target either alone or in combination with other chemotherapeutic agents, and anticancer therapies that aim to target the IGF system are currently under investigation [1].
Supplementary Material
Acknowledgments
Funding: Cancer Research UK Project grant (C18281/A7062)
Abbreviations used
- IGF
insulin-like growth factor
- IGFBP
insulin-like growth factor binding protein
Footnotes
This meta-analysis includes up to 47 studies (7,000 cancer cases) investigating the association of IGF-I, IGF-II, IGFBP-1, IGFBP-2, IGFBP-3 and the ratio of IGF-I:IGFBP-3 with prostate cancer risk. Our results show a positive association of IGF-I and a slight inverse association of IGFBP-3 with prostate cancer risk, which although modest, suggest these are likely to be important risk factors and potential targets for interventions to reduce prostate cancer risk.
Reference List
- 1.Gennigens C, Menetrier-Caux C, Droz JP. Insulin-Like Growth Factor (IGF) family and prostate cancer. Critical Reviews in Oncology-Hematology. 2006;58(2):124–145. doi: 10.1016/j.critrevonc.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, Metter EJ, Chen C, Weiss NS, Fitzpatrick A, Hsing AW, Lacey JV, Jr., Helzlsouer K, Rinaldi S, Riboli E, Kaaks R, Janssen JAMJ, Wildhagen MF, Schroder FH, Platz EA, Pollak M, Giovannucci E, Schaefer C, Quesenberry CP, Jr., Vogelman JH, Severi G, English DR, Giles GG, Stattin P, Hallmans G, Johansson M, Chan JM, Gann P, Oliver SE, Holly JM, Donovan J, Meyer F, Bairati I, Galan P. Insulin-like Growth Factors, Their Binding Proteins, and Prostate Cancer Risk: Analysis of Individual Patient Data from 12 Prospective Studies. Ann Intern Med. 2008;149(7):461–471. doi: 10.7326/0003-4819-149-7-200810070-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris JK, George LM, Wu T, Wald NJ. Insulin-like growth factors and cancer: no role in screening. Evidence from the BUPA study and meta-analysis of prospective epidemiological studies. Br J Cancer. 2006;95(1):112–117. doi: 10.1038/sj.bjc.6603200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Renehan AG, Zwahlen M, Minder C, O'Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363(9418):1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 5.Shi R, Berkel HJ, Yu H. Insulin-like growth factor-I and prostate cancer: a meta-analysis. Br J Cancer. 2001;85(7):991–996. doi: 10.1054/bjoc.2001.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Juul A, Dalgaard P, Blum WF. Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: the relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index and pubertal maturation. J Clin Endocrinol Metab. 1995;80:2534–2542. doi: 10.1210/jcem.80.8.7543116. [DOI] [PubMed] [Google Scholar]
- 7.Perk H, Serel TA, Delibas N, Sutcu R. Prostatic fluid-free insulin-like growth factor-1 in relation to prostate cancer. BJU Int. 2001;88(9):946–949. doi: 10.1046/j.1464-4096.2001.02438.x. [DOI] [PubMed] [Google Scholar]
- 8.Chene G, Thompson SG. Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. American Journal of Epidemiology. 1996;144:610–621. doi: 10.1093/oxfordjournals.aje.a008971. [DOI] [PubMed] [Google Scholar]
- 9.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. American Journal of Epidemiology. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 10.Lacey JV, Jr., Hsing AW, Fillmore CM, Hoffman S, Helzlsouer KJ, Comstock GW. Null association between insulin-like growth factors, insulin-like growth factor-binding proteins, and prostate cancer in a prospective study. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1101–1102. [PubMed] [Google Scholar]
- 11.Kanety H, Madjar Y, Dagan Y, Levi J, Papa MZ, Pariente C, Goldwasser B, Karasik A. Serum insulin-like growth factor-binding protein-2 (IGFBP-2) is increased and IGFBP-3 is decreased in patients with prostate cancer: correlation with serum prostate-specific antigen. J Clin Endocrinol Metab. 1993;77(1):229–233. doi: 10.1210/jcem.77.1.7686915. [DOI] [PubMed] [Google Scholar]
- 12.Harris RJ, Bradburn MJ, Deeks JJ, Harbord RM, Altman DG, Sterne JAC. metan: fixed– and random–effects meta–analysis. The Stata Journal. 2008;8(1):3–28. [Google Scholar]
- 13.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin Trials. 1986;7(177):188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Thompson SG, Deeks JJ, Altman D. Measuring inconsistency in meta-analyses. British Medical Journal. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. British Medical Journal. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1011. [PubMed] [Google Scholar]
- 17.Stattin P, Rinaldi S, Biessy C, Stenman UH, Hallmans G, Kaaks R. High levels of circulating insulin-like growth factor-I increase prostate cancer risk: a prospective study in a population-based nonscreened cohort. J Clin Oncol. 2004;22(15):3104–3112. doi: 10.1200/JCO.2004.10.105. [DOI] [PubMed] [Google Scholar]
- 18.Shariat SF, Lamb DJ, Kattan MW, Nguyen C, Kim J, Beck J, Wheeler TM, Slawin KM. Association of preoperative plasma levels of insulin-like growth factor I and insulin-like growth factor binding proteins-2 and -3 with prostate cancer invasion, progression, and metastasis. J Clin Oncol. 2002;20(3):833–841. doi: 10.1200/JCO.2002.20.3.833. [DOI] [PubMed] [Google Scholar]
- 19.Ho PJ, Baxter RC. Insulin-like growth factor-binding protein-2 in patients with prostate carcinoma and benign prostatic hyperplasia. Clin Endocrinol (Oxf) 1997;46(3):333–342. [PubMed] [Google Scholar]
- 20.Juni P, Altman DG, Egger M. Assessing the quality of controlled clinical trials. British Medical Journal. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregorio DI, Kulldorff M, Sheenan J, Samociuk H. Geographic Distribution of Prostate Cancer Incidence in the Era of PSA Testing, Connecticut, 1984 to 1998. Urology. 2004;63:78–82. doi: 10.1016/j.urology.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Rinaldi S, Kaaks R, Zeleniuch-Jacquotte A, Arslan AA, Shore RE, Koenig KL, Dossus L, Riboli E, Stattin P, Lukanova A, Toniolo P. Insulin-like growth factor-I, IGF binding protein-3, and breast cancer in young women: A comparison of risk estimates using different peptide assays. Cancer Epidemiology Biomarkers & Prevention. 2005;14(1):48–52. [PubMed] [Google Scholar]
- 23.Kehinde EO, Akanji AO, Mojiminiyi OA, Bashir AA, Daar AS, Varghese R. Putative role of serum insulin-like growth factor-1 (IGF-1) and IGF binding protein-3 (IGFBP-3) levels in the development of prostate cancer in Arab men. Prostate Cancer Prostatic Dis. 2005;8(1):84–90. doi: 10.1038/sj.pcan.4500783. [DOI] [PubMed] [Google Scholar]
- 24.Aksoy Y, Aksoy H, Bakan E, Atmaca AF, Akcay F. Serum insulin-like growth factor-I and insulin-like growth factor-binding protein-3 in localized, metastasized prostate cancer and benign prostatic hyperplasia. Urol Int. 2004;72(1):62–65. doi: 10.1159/000075275. [DOI] [PubMed] [Google Scholar]
- 25.Allen N, Key T, Appleby PN, Travis R, Roddam A, Rinaldi S, Egevad L, Rohrmann S, Linseisen J, Pischon T, Boeing H, Johnsen NF, Tjonneland A, Gronbaek H, Overvad K, Kiemeny L, Bueno-de-Mesquita HB, Bingham SA, Khaw KT, Tumino R, Berrino F, Mattiello A, Sacerdote C, Palli D, Quiros JR, Ardanaz E, Navarro C, Larranaga N, Gonzales C, Sanchez MJ, Trichopolou A, Travezea C, Trichopoulos D, Jenab M, Ferrari P, Riboli E, Kaaks R. Serum insulin-like growth factor (IGF)-I and IGF-binding protein-3 concentrations and prostate cancer risk: results from the Eruopean Prospective Investigation into cancer and nutrition. Cancer Epidemiology, Biomarkers & Prevention. 2007;16(6):1121–1127. doi: 10.1158/1055-9965.EPI-06-1062. [DOI] [PubMed] [Google Scholar]
- 26.Baffa R, Reiss K, El-Gabry EA, Sedor J, Moy ML, Shupp-Byrne D, Strup SE, Hauck WW, Baserga R, Gomella LG. Low serum insulin-like growth factor 1 (IGF-1): a significant association with prostate cancer. Tech Urol. 2000;6(3):236–239. [PubMed] [Google Scholar]
- 27.Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279(5350):563–566. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Lewis SK, Voigt L, Fitzpatrick A, Plymate SR, Weiss NS. Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer. 2005;103(1):76–84. doi: 10.1002/cncr.20727. [DOI] [PubMed] [Google Scholar]
- 29.Chokkalingam AP, Pollak M, Fillmore CM, Gao YT, Stanczyk FZ, Deng J, Sesterhenn IA, Mostofi FK, Fears TR, Madigan MP, Ziegler RG, Fraumeni JF, Jr., Hsing AW. Insulin-like growth factors and prostate cancer: a population-based case-control study in China. Cancer Epidemiol Biomarkers Prev. 2001;10(5):421–427. [PubMed] [Google Scholar]
- 30.Cohen P, Peehl DM, Stamey TA, Wilson K, Clemmons D, Rosenfeld R. Elevated levels of insulin-like growth factor-binding protein-2 in the serum of prostate cancer patients. J Clin Endocrinol Metab. 1993;76:1031–1035. doi: 10.1210/jcem.76.4.7682560. [DOI] [PubMed] [Google Scholar]
- 31.Cutting CW, Hunt C, Nisbet JA, Bland JM, Dalgleish AG, Kirby RS. Serum insulin-like growth factor-1 is not a useful marker of prostate cancer. BJU Int. 1999;83(9):996–999. doi: 10.1046/j.1464-410x.1999.00088.x. see comment. [DOI] [PubMed] [Google Scholar]
- 32.Djavan B, Bursa B, Seitz C, Soeregi G, Remzi M, Basharkhah A, Wolfram R, Marberger M. Insulin-like growth factor 1 (IGF-1), IGF-1 density, and IGF-1/PSA ratio for prostate cancer detection. Urology. 1999;54(4):603–606. doi: 10.1016/s0090-4295(99)00280-0. [DOI] [PubMed] [Google Scholar]
- 33.Finne P, Auvinen A, Koistinen H, Zhang WM, Maattanen L, Rannikko S, Tammela T, Seppala M, Hakama M, Stenman UH. Insulin-like growth factor I is not a useful marker of prostate cancer in men with elevated levels of prostate-specific antigen. J Clin Endocrinol Metab. 2000;85(8):2744–2747. doi: 10.1210/jcem.85.8.6725. [DOI] [PubMed] [Google Scholar]
- 34.Harman SM, Metter EJ, Blackman MR, Landis PK, Carter HB, Baltimore Longitudinal Study on Aging Serum levels of insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-3, and prostate-specific antigen as predictors of clinical prostate cancer. J Clin Endocrinol Metab. 2000;85(11):4258–4265. doi: 10.1210/jcem.85.11.6990. [DOI] [PubMed] [Google Scholar]
- 35.Hazem IA, Pollak M, Behlouli H, Tanguay S, Begin LR, Aprikian AG. Insulin-like growth factor-1 and insulin-like growth factor binding protein-3 for prostate cancer detection in patients undergoing prostate biopsy. J Urol. 2002;168(6):2426–2430. doi: 10.1016/S0022-5347(05)64160-2. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez W, Grenade C, Santos ER, Bonilla C, Ahaghotu C, Kittles RA. IGF-I and IGFBP-3 gene variantes influence on serum levels and prostate cancer risk in African-Americans. Carcinogenesis. 2007;28(10):2154–2159. doi: 10.1093/carcin/bgm190. [DOI] [PubMed] [Google Scholar]
- 37.Hill M, Bilek R, Safarik L, Starka L. Analysis of relations between serum levels of epitestosterone, estradiol, testosterone, IGF-1 and prostatic specific antigen in men with benign prostatic hyperplasia and carcinoma of the prostate. Physiol Res. 2000;49(Suppl 1):S113–S118. [PubMed] [Google Scholar]
- 38.Janssen JA, Wildhagen MF, Ito K, Blijenberg BG, Van Schaik RH, Roobol MJ, Pols HA, Lamberts SW, Schroder FH. Circulating free insulin-like growth factor (IGF)-I, total IGF-I, and IGF binding protein-3 levels do not predict the future risk to develop prostate cancer: results of a case-control study involving 201 patients within a population-based screening with a 4-year interval. J Clin Endocrinol Metab. 2004;89(9):4391–4396. doi: 10.1210/jc.2004-0232. [DOI] [PubMed] [Google Scholar]
- 39.Khosravi J, Diamandi A, Mistry J, Scorilas A. Insulin-like growth factor I (IGF-I) and IGF-binding protein-3 in benign prostatic hyperplasia and prostate cancer. J Clin Endocrinol Metab. 2001;86(2):694–699. doi: 10.1210/jcem.86.2.7211. [DOI] [PubMed] [Google Scholar]
- 40.Koliakos G, Chatzivasiliou D, Dimopoulos T, Trachana V, Paschalidou K, Galiamoutsas V, Triantos A, Chitas G, Dimopoulos A, Vlatsas G. The significance of PSA/IGF-1 ratio in differentiating benign prostate hyperplasia from prostate cancer. Dis Markers. 2000;16(3-4):143–146. doi: 10.1155/2000/764851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurek R, Tunn UW, Eckart O, Aumuller G, Wong J, Renneberg H. The significance of serum levels of insulin-like growth factor-1 in patients with prostate cancer. BJU Int. 2000;85(1):125–129. doi: 10.1046/j.1464-410x.2000.00350.x. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Yu H, Schumacher F, Casey G, Witte JS. Relation of serum insulin-like growth factor-I (IGF-I) and IGF binding protein-3 to risk of prostate cancer (United States) Cancer Causes Control. 2003;14(8):721–726. doi: 10.1023/a:1026383824791. [DOI] [PubMed] [Google Scholar]
- 43.Lopez JB, Sahabudin RM, Chin LP. Are plasma insulin-like growth factor I (IGF-I) and IGF-binding protein 3 (IGFBP-3) useful markers of prostate cancer? Int J Biol Markers. 2004;19(2):164–167. doi: 10.1177/172460080401900213. [DOI] [PubMed] [Google Scholar]
- 44.Mantzoros C, Tzonou A, Signorello LB, Stampfer M, Trichopoulos D, Adami H-O. Insulin-like growth factor in relation to prostate cancer and benign prostatic hyperplasia. Br J Cancer. 1997;76(9):1115–1118. doi: 10.1038/bjc.1997.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marszalek M, Wachter J, Ponholzer A, Leitha T, Rauchenwald M, Madersbacher S. Insulin-like growth factor 1, chromogranin A and prostate specific antigen serum levels in prostate cancer patients and controls. Eur Urol. 2005;48(1):34–39. doi: 10.1016/j.eururo.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 46.Meyer F, Galan P, Douville P, Bairati I, Kegle P, Bertrais S, Czernichow S, Hercberg S. A prospective study of the insulin-like growth factor axis in relation with prostate cancer in the SU.VI.MAX trial. Cancer Epidemiol Biomarkers Prev. 2005;14(9):2269–2272. doi: 10.1158/1055-9965.EPI-05-0303. [DOI] [PubMed] [Google Scholar]
- 47.Miyata Y, Sakai H, Hayashi T, Kanetake H. Serum insulin-like growth factor binding protein-3/prostate-specific antigen ratio is a useful predictive marker in patients with advanced prostate cancer. Prostate. 2003;54(2):125–132. doi: 10.1002/pros.10175. [DOI] [PubMed] [Google Scholar]
- 48.Nam RK, Trachtenberg J, Jewett MA, Toi A, Evans A, Emami M, Narod SA, Pollak M. Serum insulin-like growth factor-I levels and prostatic intraepithelial neoplasia: a clue to the relationship between IGF-I physiology and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2005;14(5):1270–1273. doi: 10.1158/1055-9965.EPI-04-0430. [DOI] [PubMed] [Google Scholar]
- 49.Oliver SE, Gunnell D, Donovan J, Peters TJ, Persad R, Gillatt D, Pearce A, Neal DE, Hamdy FC, Holly J. Screen-detected prostate cancer and the insulin-like growth factor axis: results of a population-based case-control study. Int J Cancer. 2004;108:887–892. doi: 10.1002/ijc.11631. [DOI] [PubMed] [Google Scholar]
- 50.Peng L, Tang S, Xie J, Luo T, Dai B. Quantitative analysis of IGF-1 and its application in the diagnosis of prostate cancer. Hua Hsi I Ko Ta Hsueh Hsueh Pao. 2002;33(1):137–139. Chinese. [PubMed] [Google Scholar]
- 51.Platz EA, Pollak MN, Leitzmann MF, Stampfer MJ, Willett WC, Giovannucci E. Plasma insulin-like growth factor-1 and binding protein-3 and subsequent risk of prostate cancer in the PSA era. Cancer Causes Control. 2005;16(3):255–262. doi: 10.1007/s10552-004-3484-8. [DOI] [PubMed] [Google Scholar]
- 52.Schaefer C, Friedman GD, Quesenberry CP, Orentreich N, Vogelman JH. IGF-I and prostate cancer. Science. 1998;282:199a. [Google Scholar]
- 53.Scorilas A, Plebani M, Mazza S, Basso D, Soosaipillai AR, Katsaros N, Pagano F, Diamandis EP. Serum human glandular kallikrein (hK2) and insulin-like growth factor 1 (IGF-1) improve the discrimination between prostate cancer and benign prostatic hyperplasia in combination with total and %free PSA. Prostate. 2003;54(3):220–229. doi: 10.1002/pros.10186. [DOI] [PubMed] [Google Scholar]
- 54.Severi G, Morris HA, MacInnis RJ, English DR, Tilley WD, Hopper JL, Boyle P, Giles GG. Circulating insulin-like growth factor-I and binding protein-3 and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15(6):1137–1141. doi: 10.1158/1055-9965.EPI-05-0823. [DOI] [PubMed] [Google Scholar]
- 55.Trapeznikova MF, Shibaeva AN, Ianshin AA, Urenkov SB, Mironova OS, Kazantseva IA, Kushlinski NE. Vascular endothelial growth factor and insulin-like-growth factors in prostate cancer. Urologiia. 2004;(1):17–21. Russian. [PubMed] [Google Scholar]
- 56.Weiss JM, Huang W-Y, Rinaldi S, Fears TR, Chatterjee N, Chia D, Crawford ED, Kaaks R, Hayes RB. IGF-I and IGFBP-3: Risk of prostate cancer among men in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. International Journal of Cancer. 2007;121:2267–2273. doi: 10.1002/ijc.22921. [DOI] [PubMed] [Google Scholar]
- 57.Wolk A, Mantzoros CS, Andersson SO, Bergstrom R, Signorello LB, Lagiou P, Adami HO, Trichopoulos D. Insulin-like growth factor 1 and prostate cancer risk: a population-based, case-control study. J Natl Cancer Inst. 1998;90(12):911–915. doi: 10.1093/jnci/90.12.911. see comment. [DOI] [PubMed] [Google Scholar]
- 58.Woodson K, Tangrea JA, Pollak M, Copeland TD, Taylor PR, Virtamo J, Albanes D. Serum insulin-like growth factor I: tumor marker or etiologic factor? A prospective study of prostate cancer among Finnish men. Cancer Res. 2003;63(14):3991–3994. [PubMed] [Google Scholar]
- 59.Zhigang Z, Jieming L, Wenlu S. Serum insulin-like growth factor I/free prostate specific antigen (IGF-I/fPSA) ratio enhances prostate cancer detection in men with total PSA 4.0-10.0 ng/ml. Journal of Surgical Oncology. 2007;96(1):54–61. doi: 10.1002/jso.20784. [DOI] [PubMed] [Google Scholar]
- 60.Trojan L, Bode C, Weiss C, Mayer D, Grobholz R, Alken P, Michel MS. IGF-II serum levels increase discrimination between benign prostatic hyperplasia and prostate cancer and improve the predictive value of PSA in clinical staging. Eur Urol. 2006;49(2):286–292. doi: 10.1016/j.eururo.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 61.Signorello LB, Brismar K, Bergstrom R, Andersson SO, Wolk A, Trichopoulos D, Adami HO. Insulin-like growth factor-binding protein-1 and prostate cancer. J Natl Cancer Inst. 1999;91(22):1965–1967. doi: 10.1093/jnci/91.22.1965. [DOI] [PubMed] [Google Scholar]
- 62.Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, Stenman UH, Egevad L, Riboli E, Hallmans G, Kaaks R. Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst. 2000;92(23):1910–1917. doi: 10.1093/jnci/92.23.1910. [DOI] [PubMed] [Google Scholar]
- 63.Freedland SJ, Sokoll LJ, Platz EA, Mangold LA, Bruzek DJ, Mohr P, Yiu SK, Partin AW. Association between serum adiponectin, and pathological stage and grade in men undergoing radical prostatectomy. J Urol. 2005;174(4 I):1266–1270. doi: 10.1097/01.ju.0000173093.89897.97. [DOI] [PubMed] [Google Scholar]
- 64.Pollak M, Beamer W, Zhang JC. Insulin-like growth factors and prostate cancer. Cancer and Metastasis Reviews. 1998;17(4):383–390. doi: 10.1023/a:1006154108619. [DOI] [PubMed] [Google Scholar]
- 65.Pollak M. Insulin-like growth factors and prostate cancer. Epidemiol Rev. 2001;23(1):59–66. doi: 10.1093/oxfordjournals.epirev.a000796. [DOI] [PubMed] [Google Scholar]
- 66.Nickerson T, Chang F, Lorimer D, Smeekens SP, Sawyers CL, Pollak M. In vivo progression of LAPC-9 and LNCaP prostate cancer models to androgen independence is associated with increased expression of insulin-like growth factor I (IGF-I) and IGF-I receptor (IGF-IR) Cancer Res. 2001;61(16):6276–6280. [PubMed] [Google Scholar]
- 67.Yakar S, LeRoith D, Brodt P. The role of the growth hormone/insulin-like growth factor axis in tumor growth and progression: lessons from animal models. Cytokine & Growth Factor Reviews. 2005;16:407–420. doi: 10.1016/j.cytogfr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 68.Berrigan D, Potischman N, Dodd KW, Nicar M, McQuillan G, Lavigne JA, Barrett JC, Ballard-Barbash R. Serum levels of insulin-like growth factor-I and insulin-like growth factor-I binding protein-3: quality control for studies of stored serum. Cancer Epidemiol Biomarkers Prev. 2007;16(5):1017–1022. doi: 10.1158/1055-9965.EPI-07-0044. [DOI] [PubMed] [Google Scholar]
- 69.Inman BA, Harel F, Audet JF, Meyer F, Douville P, Fradet Y, Lacombe L. Insulin-like growth factor binding protein 2: an androgen-dependent predictor of prostate cancer survival. Eur Urol. 2005;47(5):695–702. doi: 10.1016/j.eururo.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 70.Holly J, Perks C. The role of insulin-like growth factor binding proteins. Neuroendocrinology. 2006;83:154–160. doi: 10.1159/000095523. [DOI] [PubMed] [Google Scholar]
- 71.Ito Y, Nakachi K, Imai K, Hashimoto S, Watanabe Y, Inaba Y, Tamakoshi A, Yoshimura T. Stability of Frozen Serum Levels of Insulin-like Growth Factor-I, Insulin-like Growth Factor-II, Insulin-like Growth Factor Binding Protein-3, Transforming Growth Factor?, Soluble Fas, and Superoxide Dismutase Activity for the JACC Study. Journal of Epidemiology. 2005;15(SI):S67–S73. doi: 10.2188/jea.15.S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khosravi J, Diamandi A, Bodani U, Khaja N, Krishna RG. Pitfalls of immunoassay and sample for IGF-1: Comparison of different assay methodologies using various fresh and stored serum samples. Clinical Biochemistry. 2005;38(7):659–666. doi: 10.1016/j.clinbiochem.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 73.Yu H, Nicar MR, Shi R, Berkel HJ, Nam R, Trachtenberg J, Diamandis EP. Levels of insulin-like growth factor I (IGF-I) and IGF binding proteins 2 and 3 in serial postoperative serum samples and risk of prostate cancer recurrence. Urology. 2001;57(3):471–475. doi: 10.1016/s0090-4295(00)01003-7. [DOI] [PubMed] [Google Scholar]
- 74.Wald NJ, Hackshaw AK, Frost CD. When can a risk factor be used as a worthwhile screening test? British Medical Journal. 1999;319:1562–1565. doi: 10.1136/bmj.319.7224.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oliver SE, Holly J, Peters TJ, Donovan J, Persad R, Gillatt D, Pearce A, Hamdy FC, Neal DE, Gunnell D. Measurement of insulin-like growth factor axis does not enhance specificity of PSA-based prostate cancer screening. Urology. 2004;64(2):317–322. doi: 10.1016/j.urology.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 76.Juul A, Scheike T, Davidsen M, Gyllenborg J, Jorgensen T. Low serum insulin-like growth factor I is associated with increased risk of ischemic heart disease: a population-based case-control study. Circulation. 2002;106(8):939–944. doi: 10.1161/01.cir.0000027563.44593.cc. [DOI] [PubMed] [Google Scholar]
- 77.Thissen J-P, Ketelslegers J-M, Underwood LE. Nutritional regulation of the insulin-like growth factors. Endocr Rev. 1994;15(1):80–101. doi: 10.1210/edrv-15-1-80. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.