Abstract
Objectives
To determine if bright light can improve sleep in older individuals with insomnia.
Design
Single-blind, placebo-controlled, twelve-week, parallel-group randomized design comparing four treatment groups representing a factorial combination of two lighting conditions and two times of light administration.
Setting
At-home light treatment, eight office therapy sessions.
Participants
Thirty six females, fifteen males (63.6 ± 7.1 years) meeting primary insomnia criteria, recruited from the community.
Interventions
A 12-week program of sleep hygiene and exposure either to bright (∼4000 lux) or dim light (∼65 lux) scheduled daily in the morning or evening for 45 minutes.
Measurements and Results
Within group changes were observed for subjective sleep measures (sleep logs, questionnaires) after morning or evening bright light, but were not significantly different from those observed after exposure to scheduled dim light. Objective sleep changes (actigraphy, polysomnography) after treatment were not significantly different between bright and dim light groups. Scheduled light exposure was able to shift circadian phase predictably, but was unrelated to changes in objective or subjective sleep measures. A polymorphism in CLOCK predicted morningness, but did not moderate the effects of light on sleep. The phase angle between the circadian system (melatonin midpoint) and sleep (darkness) was able to predict the magnitude of phase delays, but not phase advances, engendered by bright light.
Conclusion
Except for one subjective measure, scheduled morning or evening bright light effects were not different from those of scheduled dim light. Thus, support was not found for bright light treatment of older individuals with primary insomnia.
Keywords: Insomnia, bright light, circadian rhythm, melatonin, CLOCK, morningness/eveningness
Introduction
Older adults commonly complain of difficulty maintaining sleep; they experience unwanted wake time during their nocturnal sleep episode and/or wake for the day earlier than they wish.1 Pharmacologic treatment of sleep disruption in older individuals is unsatisfactory because of the potential for sleeping medications to interact in a negative way with other medications and significant side-effects. Given the effectiveness of bright light in inducing changes in hypothalamic functions related to sleep, including altering circadian rhythms and mood,2-6 a number of studies have examined the efficacy of bright light for treating insomnia in both community-dwelling and institutionalized older individuals. There has been considerable variability in the methodology used in the study of community-dwelling individuals. Light treatments conducted in the laboratory typically have lasted only for a few days,7, 8 but for relatively long exposure times (∼4 hours) on each day. However, home-based treatment studies typically involved more days of treatment but for generally shorter exposure times each day (10 to ∼60 days; 20 minutes to ∼2 hours of exposure per day.9-11 Under some study protocols light was administered within a fixed time period across subjects (e.g., always at 8pm),12-14 whereas in others, light exposure was timed relative to each individual's pre-treatment sleep-wake schedule (e.g., beginning two hours before typical bedtime).11, 15 Almost all studies included subjective outcome measures (sleep logs, questionnaires) and some studies also used objective measures of sleep such as polysomnography (PSG),9, 13, 14 wrist actigraphy,7, 10, 12, 15, 16 or both.8 These studies had mixed results with some showing beneficial effects of bright light on subjective sleep measures, but not on objective sleep measures (e.g., see reference 16) and others showing benefit on both subjective and objective measures of sleep (e.g., see references 10, 12). Although several studies demonstrated the benefit of light treatment on either subjective or objective measures of sleep within treatment conditions (i.e., between baseline and the end of treatment), Lack et al. (2005), Campbell et al. (1993) and Pallesen et al. (2005) were among the few studies that demonstrated the benefit of bright light on sleep when compared with a control treatment.
There are a number of circadian-based theories to explain why bright light might improve the sleep of older individuals with primary insomnia. It has been hypothesized that: (1) with aging, the circadian clock may initiate sleep-promoting mechanisms at an earlier time of day (phase advance)17-19, (2) aging is associated with a shortening of the endogenous period of the circadian pacemaker20, although Czeisler and colleagues21 found this not to be true for healthy older adults, and (3) in older adults the amplitude of the circadian variation of sleep propensity is decreased22, 23. Each of these three circadian factors (timing, period, amplitude) can be influenced by bright light. The effects of light on the circadian system are both intensity2, 3 and time-of-day4, 5 dependent. Importantly, light exposure during the late evening can delay and in the early morning can advance the timing of the circadian clock. Theoretically, decreased daytime light intensity can also increase the length of the circadian period.24 In cases in which the amplitude of the clock is low, properly timed light exposure can elicit a recovery in amplitude.25 Only a few of the previous studies that examined the utility of bright light for treatment of insomnia examined changes in the underlying biology (e.g., changes in thermoregulation or endocrine function) that might have mediated such changes.9, 12, 16 Similarly, few of these studies examined variables that might have influenced the interindividual variability in response to the light (i.e., moderators).
Given the variation in methodologies and results and typically small sample sizes, we designed a study of bright light for the treatment of insomnia in older individuals that was broadly inclusive, in terms of measures, and directly compared active treatment results to those of a control condition. Our specific goal was to test the hypothesis that bright-light administered in the morning or evening would cause an improvement in insomnia symptoms of older adults as a result of light-induced changes in circadian physiology. Specifically, we compared the impact of bright light-induced changes with those of dim light on nocturnal sleep in older individuals with a diagnosis of primary insomnia. We administered experimental light just before bedtime or just after awakening. These two time points were selected as light administered during these two times yields opposite effects on the circadian clock (evening light causing delays in timing and morning light causing advances). The light treatment was also scheduled relative to the individual's habitual sleep/wake schedule, as opposed to a fixed clock time, to ensure a more predictable circadian response. In our protocol, we examined older individuals with primary insomnia who, by definition, did not have a substantial phase advance or delay of their underlying circadian clock (i.e., Advanced or Delayed Sleep Phase Disorder). Our bright light exposure, therefore, was not hypothesized to cause large changes in the timing of the circadian clock but, rather, to either nudge the timing of the clock or to evoke a small change in the amplitude of the circadian clock. To generate a credible and valid placebo condition and to keep subjects engaged during the 12-week long protocol, we combined a comprehensive package of sleep hygiene instructions with the dim light exposure. To insure treatment comparability, the same sleep hygiene package was also included in the bright light protocol. Both subjective and objective measures of sleep were collected as were both physiological and genetic measures of the circadian clock.
Methods
Participants
Participants 55 years or older with insomnia complaints were recruited from the community through flyers, community newspapers and bulletins, and from the Stanford Sleep Disorders Clinic. Screening included: (1) in-home EdenTrace (Mallinckrodt, Hazelwood MO) recording to screen-out obstructive sleep apnea; (2) two nights of polysomnography (PSG) to detect and screen-out periodic limb movement disorder; (3) 14 days of baseline, at-home sleep logs to determine if participants met one of the following subjective insomnia criteria: average sleep latency (SL) >30 minutes or average sleep efficiency (SE) ≤80% or average total sleep time (TST) <6 hours, or wake after sleep onset (WASO) >30 minutes (this last criterion needed to occur on at least 5 of the 14 nights); (4) a brief physical and diagnostic evaluation by a sleep medicine fellow to determine if participants met International Classification of Sleep Disorders-1 (ICSD-1) diagnostic criteria for psychophysiological (primary) insomnia [insomnia due to other medical, neurologic, or other sleep disorders were excluded; those taking medications that significantly impact sleep (e.g., hypnotics, stimulants) were excluded unless on a stable dose for more than one year]; (5) approval from a licensed optometrist or ophthalmologist who had examined the participant within the past year; (6) testing of cognitive function (Mini-Mental State Exam26, exclusion <25) and executive function (Executive Interview27, >15) to ensure ability to meet protocol demands; and (7) depression (Geriatric Depression Scale28, scores ≥7 triggered an in-depth evaluation to exclude major depression). While many older individuals have insomnia secondary to depression, we wanted to have a population of primary insomnia that was not due to depression. As such we chose a relatively conservative threshold for GDS scores to screen out any cases of major depression as well as dysthymia from our sample. Participants were studied during all seasons of the year.
The experimental protocol received approval from the Institutional Review Board of Stanford University. All participants gave informed, written consent and were compensated for their participation. The experiment was conducted under the principles outlined in the Declaration of Helsinki.
Design
We used a parallel group design with four conditions (dim morning, dim evening, bright morning and bright evening) distinguished by whether participants received dim or bright light and by the time of day at which they received the specific treatment. Participants were randomly assigned, using a modification of the Efron procedure,29 in a ratio of 2 to 1 into a bright (2) or dim (1) treatment condition. Morning and evening exposure were evenly divided within each of the conditions. All treatments consisted of 12 weeks of once-daily light exposure, each lasting 45 minutes and starting 15 minutes after awakening (morning treatment) or starting one hour before scheduled bed time (evening treatment). The scheduled bed or wake times were based on the participant's average bed or wake times, respectively, during baseline. All participants received sleep hygiene instructions at eight individual sessions with an experienced sleep therapist (LF). The therapist, of necessity, was not blinded to participants' treatment assignments but was blind to their treatment responses on outcome measures.30
Objective (actigraphy, polysomnography) and subjective (questionnaires, logs) measures of sleep were monitored. PSG recordings, wrist actigraphy, and self-reports were taken during baseline and after twelve weeks of experimental light treatment. Measurement of overnight plasma melatonin for assessment of circadian phase was also done at these time points. Actigraphy, sleep logs, and light exposure measures were also collected at in the middle of the twelve-week treatment period (i.e., after about 5-10 weeks of treatment). Genetic measures were also obtained at baseline.
Interventions
Light Exposure
In all conditions, desk lights of the same size (48.26 × 45.72 × 27.94 cm), style (extendable arm with an adjustable lamp head positioned individually to maximize light exposure without shining light into participants' eyes) and manufacturer (SADelite Lamp, Northern Light Technologies, Montreal, Canada) were used. Every light held two fluorescent lamps each 36W and 3000 °K, behind a UV-filtering plastic screen (43.5 × 13.2 cm). A research assistant demonstrated correct use of the light equipment at the clinic and arranged it in participants' homes according to protocol. In both bright groups, light fixtures were calibrated to produce 10,000 lux full spectrum white light at the point of emission. The research assistant placed the light fixture at eye level, with 45.72 cm between the fixture face and middle of the participant's forehead, on a table or desk covered with white, reflective, photographic paper. Participants were instructed to avoid looking directly at the light and to read or to conduct any other activity provided that it could be accomplished sitting at the desk. Similar experimental conditions were used for dim groups except that filters reduced lamp output to <50 lux. After the experimental light source was activated, in both dim and bright conditions, the research assistant collected ten readings of illuminance at the outer canthi (Spectra Professional IV-A photometer, Spectra Cine, Burbank CA) with the light sensor positioned in the direction of the participant's gaze. The research assistant returned to participants' homes midway through the treatment to ensure that fixtures remained correctly positioned and emitted the proper illuminance. Lamps were replaced if they had dimmed. If the set-up changed during the study, subjects were instructed to contact the research team.
Sleep Hygiene
All participants received the same set of sleep hygiene instructions15 and given an instruction pamphlet to refer to at home. These instructions noted that for the duration of the treatment there was to be: 1) no sleeping medication; 2) no napping at any time; 3) no more than two cups of normal coffee or its equivalent with all caffeine consumption before lunch; 4) no more than one alcoholic drink with dinner and no more than one glass of liquid of any sort within three hours of bedtime; 5) regular schedules for eating and other daily activities; 6) a 30-minute walk outdoors at approximately 12 noon for the morning treatments and at approximately 4 PM for the evening treatments (both dim and bright); 7) a light snack eaten before bedtime; 8) moderate temperature and low noise levels in the bedroom; 9) the bedroom area kept separate from non-sleep activities; 10) writing down problems to be solved and tasks to be completed the next day; 11) a pleasant, regular bedtime routine; and 12) a hot bath taken within two hours of bedtime. Further, participants were instructed not to remain in bed for longer than 20 minutes without being able to initiate sleep or re-enter sleep if they woke during the night. If participants did arise at night (including for bathroom visits), they were instructed to avoid exposure to bright light. At subsequent treatment sessions, participants discussed the sleep hygiene practices they had been trying to change, any problems that had interfered with change, and the solutions they used to manage these problems.
Measures
Sleep measures
Daily Sleep Logs
Participants completed a dated sleep log for each day of the 2-week baseline and for 2 weeks at the end of treatment; this information was reported daily to a telephone answering machine. Baseline sleep log data determined if participants met study insomnia inclusion criteria described above. Sleep log “into” and “out of” bed times were used as the sleep period start and end times, respectively, for actigraphic sleep/wake scoring on matching nights. Sleep log data from baseline and end of treatment were also used as subjective outcome measures.
Actigraphy
The Actiwatch-L (MiniMitter, Bend OR), an ambulatory device for measuring arm movement, collected twenty-four hour activity data in 30-s epochs that were used as a proxy of sleep and wake.31, 32 Participants wore the device on their non-dominant wrist continuously for one week at each measurement time; data were averaged over the entirety of each measurement period. The Actiware-Sleep v.3.1 software program (Mini-Mitter, Bend OR) was used to derive wake after sleep onset (WASO), time in bed (TIB), total sleep time (TST), and sleep efficiency (SE), using the high sensitivity scoring option (found to be most appropriate for the disturbed sleep of insomnia patients).33
Polysomnography
At baseline and end of treatment, attended PSG (SensorMedics, Yorba Linda CA) was conducted at Stanford Hospital on the first two of a three-night stay. Subjects were in bed during the hours that they typically slept at home. Central and occipital electroencephalogram (EEG), submental electromyogram (EMG), and electro-oculogram (EOG) data were collected. An experienced sleep technologist, blinded to condition, determined sleep stage variables by visual scoring of the polygraph records according to the conventions established in the Rechtschaffen and Kales manual.34 Twenty percent of all records were double-scored by a senior sleep technologist. Reliability across scorers was maintained above 90 percent for all records. Measures included standard sleep variables including measures of all sleep stages, SL, TST, WASO, SE, and REM latency.
Additional Questionnaires
The following self-rating questionnaires or tests were administered at baseline and end of treatment: 1) the Spielman Insomnia Symptom Questionnaire35 (range 12-60, higher scores indicate greater severity of insomnia symptoms); 2) the Epworth Sleepiness Scale36 (ESS, range 0-24, higher scores indicate greater sleepiness); 3) Sleep Hygiene Questionnaire (range 0-31, lower scores indicating better hygiene, adapted from Blake and Gomez37); and 4) a single-item sleep satisfaction scale (“not at all rested” to “very rested” on a 7-point scale, higher scores are better) completed as part of the daily sleep log. The 19-item Horne-Östberg Morningness/Eveningness Questionnaire was given at baseline to determine diurnal preference.38
Quality of Life
All participants completed the Medical Outcomes Study Short Form Health Survey (SF-36) at baseline and end of treatment. The Mental Composite Score (range 0-100, higher scores are better) and Physical Composite Score39 (range 0-100, higher scores are better) were calculated and used as indicators of health-related quality of life.
Urine Toxicology
Participants taking hypnotic medications were instructed to withdraw (under supervision of prescribing physicians) a minimum of three weeks prior to study entry and remain free of them for the entire study. To monitor adherence, urine samples were collected at unannounced times during each study phase and screened by Quest Diagnostics (Van Nuys CA) for benzodiazepines and barbiturates.
Light Treatment Adherence and Measurement
Adherence to each morning or evening exposure was graded binomially by qualitatively comparing the light readings on the Actiwatch-L during the time of the scheduled experimental exposure to the prescribed levels of light. Thus, exposure to dim light was rated as compliant if most of the light readings fell under 100 lux during the prescribed exposure period. Exposure to bright light was rated as compliant if most of the light readings exceeded 2000 lux. Data (percentage of days with adherence, for seven days at mid-treatment and at end of treatment) were averaged within subjects and then among subjects in a treatment group. To estimate daily patterns of light exposure, for one week during the middle of the 12-week treatment and again for one week at the end of the 12-week treatment, actiwatch-collected illuminance data were quantified by calculating: average daily illumination (lux), standard deviation of the daily illumination (lux), maximum illumination (lux), integrated daily light exposure (lux*minutes), and time exposed to ≥1000 lux (minutes).
Plasma Melatonin
The daily rhythm in plasma melatonin concentrations was determined during pre-treatment baseline and at the end treatment. Blood for serum melatonin measurement was collected at the Stanford Hospital General Clinical Research Center (GCRC) on the third night of each of the in-hospital stays. Samples were obtained using an indwelling, intravenous catheter according to the following schedule: at 17:00, at 18:00, every fifteen minutes from 19:00 to 23:00, every 30 minutes from 23:00 to 05:00 and every 15 minutes from 05:00 to 09:00 for determination of the melatonin “onset” and “offset”.40 Subjects were in a constant, recumbent position and exposed to <10 lux light from 19:00 until the end of blood collection; subjects were ambulatory outside of the laboratory until 19:00. They were required to sleep in darkness during the times of their regular sleep episode. Plasma melatonin concentrations were determined by a single investigator (IZ) using a radioimmunoassay kit (Bühlmann Laboratories, Allschwil Switzerland) that employs the Kennaway G280 antibody.40 The limit of sensitivity of the melatonin assay is 0.5 pg/ml. Due to problems in either blood collection or melatonin assay, we were able to assess melatonin rhythmicity in only 44 subjects at all time points (n=18, bright AM; n=14, bright PM; n=6, dim AM; n=6, dim PM).
The phase of nocturnal melatonin secretion was defined as the midpoint between the time of the upward and downward crossing of the 16-hour mean melatonin concentration in each individual.41 In this quantification, we used a variably-defined phase marker as opposed to an absolutely defined phase marker (e.g., time at which melatonin rose above 10 pg/mL) because some older individuals have melatonin concentrations that are rhythmic, but lower than 10 pg/mL.42 A change in the timing of the melatonin midpoint (phase change, Δφ) was calculated as the difference between the clock time (phase) of the midpoint of the nocturnal melatonin secretion occurring at baseline and at the end of treatment in-laboratory visits. For example, a delay in the timing (phase) of melatonin from 3 am to 4 am would be a negative one-hour circadian phase change. The circadian phase angle (ψ), representing here the relationship between the timing of sleep and the circadian system, was calculated as the time between the midpoint of the scheduled sleep episode and the phase (midpoint) of melatonin secretion. The midpoint of the scheduled sleep episode was calculated as the average sleep midpoint during the seven nights of sleep log data collected prior to entry into the GCRC. A positive ψ indicates the midpoint of sleep occurred after the midpoint of melatonin; thus, a negative ψ indicates that the midpoint of sleep occurred before the midpoint of melatonin. ψ was calculated at baseline and end of treatment. The duration of melatonin secretion was defined as the time between the upward and downward crossing of the 16-hour mean melatonin concentration in each individual. Changes in melatonin duration are correlated with changes in the amplitude of the circadian clock.43
Genetics
Blood was also drawn for genetic analysis on the first (baseline) visit to the GCRC. We followed the methods outlined in Katzenberg et al. to obtain the human CLOCK (circadian locomotor output cycles kaput) gene allele status for the T3111C single nucleotide polymorphism (SNP).44 Genetic polymorphisms in CLOCK and baseline objective and subjective sleep characteristics were examined as possible moderators of observed responses of sleep to the light stimuli. We were unable to obtain blood for analysis from one subject.
Data Analyses
Sleep data were averaged over the course of the week of collection and analyzed first within (paired Student's t-tests) and then between groups (Kruskal-Wallis tests). The former test addressed whether a statistically significant change occurred between baseline and end of treatment within a given treatment. The latter test addressed whether the changes between baseline and end of treatment were different between the four groups. If a Kruskal-Wallis test was significant, we performed the Mann-Whitney U-test to compare differences between: 1) the AM bright and AM dim groups and 2) the PM bright and PM dim groups. If the Mann-Whitney U-test was significant, we also calculated the Number Needed to Treat (NNT) as a measure of medical efficacy, since this measure shows the number of subjects one would need to treat to have one subject experience improvement. Cohen's d is also given as a measure of effect size, such that 0.2 is indicative of a small effect, 0.5 a medium, and 0.8 a large effect size. ROC software (v.4.21, http://mirecc.stanford.edu) was used for the signal detection analyses45 and Origin (v.6.1, OriginLab, Northampton MA) was used for linear regression analyses. Other statistics were also calculated, as indicated in the text. Data were analyzed using SAS (v.9.1.3, Cary NC) and presented as mean ± SD unless otherwise noted.
Results
Participants
Of 1309 responders to our advertisements, 814 passed the initial phone screening and were sent a study description. After an in-depth telephone interview, 201 of the 390 respondents were deemed ineligible. Of the 189 eligible respondents, 128 were ineligible during in-office evaluations, of whom 24 were excluded for suspected obstructive sleep apnea (respiratory distress index greater than 10) based on overnight respiratory monitoring and another 12 for periodic limb movement disorder with associated arousals (greater than 10 per hour during PSG or by clinical judgment). Ineligible participants were given referral information and, when appropriate, sleep hygiene suggestions.
The remaining 61 participants were randomized to treatment; ten discontinued during treatment. No participants tested positive for barbiturates or benzodiazepines at any time. Subjects were 63.6 ± 7.1 years of age (54-78 years), 36 of whom were female, and had 16.2 ± 2.3 years of education. Scores on the Mini-Mental State Exam were 28.6 ± 1.4, 2.9 ± 2.2 on the Executive Interview, 4.1 ± 2.9 on the Geriatric Depression Scale, and 52.0 ± 7.8 on the Physical and 51.9 ± 7.9 on the Mental Composite Scores of the SF-36. Subjects were quite healthy for their age, 3.3 ± 0.6 on Self-Rated Health, and were empanelled if they had a chronic medical condition that was considered stable by a study physician (e.g., hypothyroidism treated with levothyroxine). At baseline there were no significant differences between treatment groups on these measures (ANOVA and chi-square tests). During a structured screening interview, our participants reported that they had a problem with waking during the night an average of 24 ± 9 nights a month and that they woke too early in the morning without being able to fall back to sleep an average of 13 ± 11 nights a month. During this interview, of the 51 participants who completed treatment, 27 said it took them 30 or more minutes to fall asleep initially and 33 said that it took them 30 or more minutes to fall asleep once they awoke during the night.
Participants woke 2.7 ± 1.7 times a night and this problem was self-rated as a 3.2 ± 1.0 on a 5-point scale (0-4, higher numbers being more problematic). Subjects reported going to sleep at 23:05 ± 1:07 (range, 20:00-04:35) and arising at 06:20 ± 1:25 (range, 0:30-13:20). On average, participants had a body mass index of 25.6 ± 3.2, a respiratory distress index of 3.1 ± 2.5, and duration of insomnia symptoms of 14.9 ± 15.4 years. Dropouts were the same as completing participants on background characteristics, except that drop-outs reported slightly better self-rated health (3.8 ± 0.2 versus 3.3 ± 0.3; p<0.01). Drop-out rates were not statistically different across treatments.
Light Therapy
Illuminance
During the first at-home light treatment, the average illuminance measured at the eye was 4000 ± 926 lux (bright morning), 3862 ± 464 lux (bright evening), 58 ± 21 lux (dim morning), and 81 ± 41 lux (dim evening). At mid-treatment, the average illuminance was 3754 ± 559 lux (bright morning), 3894 ± 750 lux (bright evening), 69 ± 18 lux (dim morning), and 77 ± 58 lux (dim evening). There were no significant differences in any group from beginning to mid-treatment in light exposure received during phototherapy.
Adherence
Data from the ambulatory Actiwatch-L light monitor indicated consistent adherence to the light treatment protocol across time points and groups In the bright morning group, 86 ± 21% (mid-treatment) and 82 ± 22% (end of treatment) adherence was achieved. In the bright evening light group, compliance was 83 ± 14% (mid-treatment) and 77 ± 22% (end of treatment). The dim morning group demonstrated compliance rates of 74 ± 38% (mid-treatment) and 69 ± 38% (end of treatment), while in the dim evening group compliance was 62 ± 30% (mid-treatment) and 61 ± 38% (end of treatment).
Average Daily Light Exposure
The pattern of light exposure in all the subjects recorded by the Actiwatch-L and described by the overall average daily light exposure (1156 ± 882.4 lux), variability in light exposure (5373 ± 3396 lux), maximum light exposure (80015 ± 42759 lux), area under the curve of light exposure (1528967 ± 1190960 lux*minute) and time spent in illuminance ≥1000 lux (109.6 ± 63.87 minutes), also did not differ between the groups at baseline (single factor ANOVAs). In none of the four groups was there a change between baseline measurements of total daily light exposure and the light exposure during treatment (paired t-tests), indicating that although the amount of morning or evening light was changed for 45 minutes, the experimental procedures did not change the overall pattern of daily light exposure as measured by the Actiwatch-L.
Objective Sleep Measures
Actigraphy
We tested the hypothesis that bright light therapy would improve objective measures of sleep as recorded by wrist actigraphy from baseline to end of treatment. First, we examined changes in the actigraph-determined sleep parameters within each of the four groups. The bright morning light group showed a significant decrease in TIB (Table 1); the bright evening light group experienced a similar decrease in TIB and a decline in TST. The dim morning and dim evening light groups showed no significant changes in actigraph-determined sleep parameters. Next, we compared the changes between baseline and end of treatment between the four groups and found that there were no significant differences among the sleep parameter changes associated with the four conditions. Thus, the changes found with bright light exposure were not significantly different from those obtained in the control (dim) conditions.
Table 1. Actigraphy-Determined Sleep Variables.
All data represent means of actigraphy collected over 7 days. Total sleep time (TST), wake after sleep onset (WASO), sleep efficiency (SE), and time in bed (TIB) are presented as average ± SD at baseline and the end of treatment. Significant differences from paired baseline (paired t-test) are shown by *p<0.05, **p<0.01, ***p<0.001.
| All (n=50) |
Dim Morning (n=7) |
Bright Morning (n=19) |
Dim Evening (n=7) |
Bright Evening (n=17) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | End of treatment |
Baseline | End of treatment |
Baseline | End of treatment |
Baseline | End of treatment |
Baseline | End of treatment |
|
| TST (min) |
403.3 ± 57.4 |
384.5*** ± 54.7 |
369.8 ± 46.0 |
365.2 ± 63.3 |
408.0 ± 47.3 |
392.6 ± 55.9 |
402.4 ± 63.3 |
383.3 ± 57.6 |
412.2 ± 68.5 |
384.0* ± 51.7 |
| WASO (min) |
60.8 ± 30.6 |
56.9 ± 28.1 |
61.3 ± 27.3 |
63.0 ± 19.2 |
66.2 ± 37.7 |
55.7 ± 35.1 |
54.7 ± 30.8 |
65.6 ± 24.4 |
57.2 ± 23.6 |
52.1 ± 24.3 |
| SE (%) |
80.4 ± 7.6 |
80.1 ± 8.5 |
78.9 ± 6.7 |
76.7 ± 7.3 |
80.6 ± 7.0 |
82.4 ± 8.2 |
82.3 ± 11.0 |
77.8 ± 9.3 |
80.1 ± 7.6 |
79.8 ± 8.9 |
| TIB (min) |
502.3 ± 56.7 |
478.4*** ± 39.0 |
470.2 ± 60.2 |
463.6 ± 48.1 |
508.8 ± 55.5 |
476.1*** ± 43.1 |
488.7 ± 26.0 |
491.8 ± 27.9 |
513.7 ± 63.7 |
481.4* ± 34.9 |
Polysomnnography
We repeated the analyses described above on the PSG data (Table 2) and found no significant changes between baseline and end of treatment within any of the four conditions on any sleep variables (TST, WASO, TIB, SE) or percentages of sleep stages (Stages 1, 2, 3, 4, and REM). There were also no significant differences in the changes between the four groups between baseline and end of treatment for any of these PSG sleep parameters.
Table 2. PSG-Determined Sleep Variables.
Total sleep time (TST), wake after sleep onset (WASO), sleep efficiency (SE), time in bed (TIB), stage 1 sleep percentage, stage 2 sleep percentage, stage 3 sleep percentage, stage 4 sleep percentage, and REM sleep percentage are presented as average ± SD from the first two in-lab nights at baseline and at the end of treatment. Significant difference from paired baseline (paired t-test) is shown by *p<0.05.
| All (n=49) |
Dim Morning (n=7) |
Bright Morning (n=18) |
Dim Evening (n=7) |
Bright Evening (n=17) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | End of treatment |
Baseline | End of treatment |
Baseline | End of treatment |
Baseline | End of treatment |
Baseline | End of treatment |
|
| TST (min) |
346.7 ± 54.4 |
346.5 ± 50.2 |
320.7 ± 62.8 |
320.0 ± 69.8 |
336.3 ± 44.5 |
345.5 ± 49.1 |
364.3 ± 47.6 |
358.9 ± 37.9 |
361.3 ± 60.7 |
353.3 ± 47.1 |
| WASO (min) |
81.9 ± 40.8 |
73.1 ± 34.3 |
82.9 ± 40.4 |
68.2 ± 28.0 |
88.8 ± 48.7 |
86.4 ± 44.5 |
73.4 ± 22.2 |
64.7 ± 21.2 |
77.8 ± 39.7 |
64.5 ± 25.3 |
| SE (%) |
74.4 ± 8.9 |
75.9 ± 7.1 |
72.2 ± 11.4 |
74.1 ± 8.8 |
72.5 ± 10.1 |
74.3 ± 8.4 |
75.9 ± 6.7 |
76.5 ± 3.7 |
76.8 ± 7.2 |
78.2 ± 5.8 |
| TIB (min) |
469.2 ± 58.8 |
457.2 ± 56.5 |
443.8 ± 48.5 |
428.9 ± 66.2 |
470.4 ± 42.3 |
466.2 ± 53.0 |
479.4 ± 44.5 |
470.8 ± 50.3 |
474.2 ± 80.6 |
453.7 ± 58.8 |
| Stage 1 (%) |
25.6 ± 10.3 |
27.7 ± 10.8 |
28.8 ± 14.7 |
34.3 ± 12.3 |
24.6 ± 9.0 |
25.6 ± 10.8 |
23.2 ± 7.7 |
26.2 ± 5.1 |
26.2 ± 11.0 |
27.8 ± 11.6 |
| Stage 2 (%) |
59.4 ± 9.7 |
57.1* ± 10.0 |
55.4 ± 12.0 |
52.3 ± 10.1 |
61.1 ± 7.7 |
59.3 ± 11.5 |
58.8 ± 9.4 |
57.5 ± 7.9 |
59.6 ± 10.9 |
56.6 ± 9.0 |
| Stage 3 (%) |
0.4 ± 0.7 |
0.8 ± 2.2 |
0.2 ± 0.2 |
0.1 ± 0.1 |
0.2 ± 0.4 |
0.3 ± 0.7 |
0.6 ± 0.8 |
0.3 ± 0.6 |
0.6 ± 0.9 |
1.6 ± 3.5 |
| Stage 4 (%) |
0 ± 0 |
0.1 ± 0.7 |
0 ± 0 |
0 ± 0 |
0 ± 0 |
0 ± 0 |
0 ± 0 |
0 ± 0 |
0 ± 0 |
0.3 ± 1.2 |
| REM (%) |
14.6 ± 4.6 |
14.4 ± 4.6 |
15.7 ± 4.0 |
13.4 ± 5.9 |
14.1 ± 5.0 |
14.8 ± 4.0 |
17.4 ± 3.1 |
16.0 ± 4.7 |
13.6 ± 4.5 |
13.6 ± 4.7 |
Subjective Sleep Measures
Sleep logs
Sleep log data (Table 3) shows significant changes in all four sleep variables at the end of treatment with either bright morning or bright evening light, with TST and SE increasing, and WASO and TIB decreasing. Except for an increase in TST following dim evening light exposure, there were no significant changes in any sleep variable after exposure to dim morning or dim evening light. However, the changes observed in sleep log data of the four groups were not significantly different from one another. Thus, the changes associated with exposure to bright light in the evening or morning were not significantly different from those obtained in the control (dim) conditions.
Table 3. Subjective Measures: Sleep Logs and Questionnaires.
From sleep logs, total sleep time (TST), wake after sleep onset (WASO), sleep efficiency (SE), and time in bed (TIB) are presented. From questionnaires, Spielman Insomnia Questionnaire, Epworth Sleepiness Scale, Sleep Hygiene Questionnaire and Sleep Satisfaction Scale, SF-36 Mental Composite Score and SF-36 Physical Composite Score are presented. Data are shown as average ± SD at baseline and at the end of treatment. Significant differences from baseline (paired t-test) are shown by *p<0.05, **p<0.01, ***p<0.001.
| All (n=51) |
Dim Morning (n=7) |
Bright Morning (n=19) |
Dim Evening (n=7) |
Bright Evening (n=18) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | End of treatment |
Baseline | End of treatment |
Baseline | End of treatment |
Baseline | End of treatment |
Baseline | End of treatment |
|
| TST (min) | 338.2 ± 50.6 |
363.1* ± 77.9 |
320.6 ± 34.0 |
291.1 ± 118.6 |
339.2 ± 51.8 |
367.5* ± 72.5 |
345.8 ± 37.0 |
378.9* ± 53.4 |
340.9 ± 60.4 |
380.3** ± 61.3 |
| WASO (min) |
68.6 ± 41.2 |
45.4*** ± 37.0 |
69.5 ± 46.2 |
63.3 ± 41.4 |
74.0 ± 37.3 |
48.9* ± 46.0 |
65.2 ± 27.5 |
55.6 ± 19.8 |
63.8 ± 49.3 |
30.8*** ± 25.1 |
| SE (%) |
68.0 ± 10.4 |
77.3*** ± 11.7 |
69.4 ± 12.4 |
73.1 ± 7.7 |
66.8 ± 9.1 |
77.0*** ± 12.9 |
71.6 ± 3.9 |
76.9 ± 9.2 |
67.4 ± 12.7 |
79.5** ± 12.8 |
| TIB (min) |
502.3 ± 59.3 |
478.4*** ± 38.2 |
470.2 ± 60.2 |
463.6 ± 48.1 |
512.4 ± 57.1 |
477.9** ± 41.1 |
484.4 ± 31.8 |
491.8 ± 27.9 |
511.2 ± 67.1 |
479.5* ± 35.3 |
| Spielman | 32.9 ± 5.1 |
26.9*** ± 6.1 |
30.9 ± 4.6 |
34.3 ± 5.8 |
31.4 ± 5.9 |
24.6*** ± 5.5 |
35.7 ± 3.9 |
28.4*** ± 5.3 |
34.2 ± 4.0 |
25.8* ± 4.8 |
| ESS | 7.6 ± 4.4 |
5.7*** ± 4.0 |
9.7 ± 2.0 |
8.4 ± 3.9 |
7.5 ± 4.3 |
5.4* ± 4.4 |
8.1 ± 3.5 |
6.0* ± 4.3 |
6.8 ± 5.3 |
4.8* ± 3.1 |
| Sleep Hygiene |
9.1 ± 3.1 |
3.4*** ± 2.0 |
7.9 ± 3.1 |
3.9* ± 2.2 |
9.1 ± 3.2 |
3.1*** ± 1.7 |
8.0 ± 2.2 |
3.6** ± 1.6 |
10.3 ± 3.2 |
3.4*** ± 2.3 |
| Sleep Satisfaction |
3.7 ± 1.0 |
4.3*** ± 1.1 |
3.7 ± 0.5 |
3.9 ± 1.3 |
3.7 ± 1.2 |
4.4** ± 1.0 |
3.6 ± 0.8 |
3.8 ± 0.6 |
3.8 ± 1.0 |
4.7** ± 1.0 |
| SF-36 Mental |
51.7 ± 8.0 |
55.1** ± 7.2 |
49.7 ± 8.7 |
53.9 ± 4.3 |
51.5 ± 8.2 |
55.7** ± 5.5 |
51.8 ± 5.4 |
54.0 ± 4.2 |
52.7 ± 8.8 |
55.4 ± 10.3 |
| SF-36 Physical |
51.4 ± 7.8 |
51.5 ± 7.4 |
53.8 ± 9.1 |
51.6 ± 7.3 |
51.6 ± 8.4 |
50.5 ± 10.1 |
52.3 ± 4.7 |
53.2 ± 5.6 |
50.1 ± 7.9 |
51.7 ± 4.8 |
Scales
Given the inherently subjective nature of insomnia, we also examined several scales that tapped participants' experience of their sleep (Table 3). Those exposed to dim morning light reported a non-significant worsening of their insomnia symptoms on the Spielman, while those exposed to dim evening, bright morning, and bright evening light all experienced significant improvements in symptoms. Subjects receiving bright morning light had significantly different responses on the Spielman from those receiving dim morning light (U=17, p=0.009, Mann Whitney U-test; NNT=1.34, Cohen's d=1.61). There were, however, no significant differences between the dim evening and bright evening groups.
For the Epworth Sleepiness Scale, there were significant improvements in the bright morning, bright evening, and dim evening groups but not in the dim morning (Table 3). There were, however, no significant differences between the changes observed in the Epworth responses between the four groups.
For the sleep hygiene questionnaire, each of the four groups exhibited improvements from baseline to end of treatment in their responses, but these improvements did not significantly differ between the four groups (Table 3).
On the sleep satisfaction scale, there were significant improvements after either bright condition but not after the dim conditions (Table 3). There were, however, no significant differences between the changes observed in sleep satisfaction scores between the four groups. On the Mental Composite score of the SF-36, there was a significant improvement in the bright morning but not in any of the other three conditions (Table 3). There were no significant changes after any condition for the Physical Composite score of the SF-36. There also were no significant between group differences observed in either the Mental or Physical SF-36 measures.
Comparisons without Group Stratification
We also examined all subjects grouped together, irrespective of bright/dim and morning/evening conditions to examine the effects of the sleep hygiene program, our “background” treatment to which all subjects were exposed. Changes between baseline and end of treatment were explored with paired t-tests (see Tables 1 to 4, under “All” columns). By actigraphy, TIB and TST decreased while WASO and SE exhibited no significant changes. None of the PSG-derived sleep variables significantly changed, except for a small reduction in stage 2 NREM sleep. All sleep log measures indicated a subjective improvement in sleep with a decrease in TIB, an increase in TST, an increase in SE, and a decrease in WASO. A similar consistency was found in subjective measures, with a decrease in the Spielman, a decrease in the ESS, a decrease in the sleep hygiene questionnaire and an increase in the sleep satisfaction score. There was also an improvement in the Mental Composite Score of the SF-36 but not the Physical Composite Score. Thus, while all subjects, taken together, appeared to have few significant improvements in objective sleep measures, there were many subjective improvements associated with participation in this protocol, regardless of treatment group. This effect may be attributable to the sleep hygiene component (and/or a participation effect) that all groups shared.
Table 4. Circadian Parameters at Baseline and at the End of 12 Weeks of Experimental Intervention.
Represented are the clock time of the melatonin midpoint, the duration of time (hours) melatonin is above the 16-hour average, and the phase angle (hours) between the midpoint of melatonin and the midpoint of habitual sleep.
| Dim Morning (n=6) | Bright Morning (n=18) | Dim Evening (n=6) | Bright Evening (n=14) | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | End of treatment | Baseline | End of treatment | Baseline | End of treatment | Baseline | End of treatment | |
| Melatonin Midpoint | 2:54 ± 1:11 | 3:10 ± 1:24 | 2:12 ± 0:42 | 1:42 ± 0:54 | 1:51 ± 0.22 | 1:36 ± 0:41 | 2:10 ± 0:51 | 2:42 ± 0:38 |
| Melatonin Duration | 8.19 ± 1.21 | 7.94 ± 1.00 | 8.31 ± 0.684 | 8.15 ± 0.737 | 8.77 ± 0.875 | 8.26 ± 0.665 | 8.35 ± 0.552 | 8.31 ± 0.527 |
| Circadian Phase Angle | 0.16 ± 1.0 | -0.25 ± 1.5 | 0.63 ± 0.71 | 0.58 ± 0.88 | 0.86 ± 0.98 | 0.92 ± 0.73 | 0.56 ± 0.69 | 0.10 ± 0.54 |
Individual Differences in Response (Moderators)
Moderator analyses were conducted in an effort to determine if certain factors were responsible for an individual subject responding to bright light treatment for insomnia.
Initial Sleep Efficiency Scores
Using a receiver operating characteristic (ROC) analysis, we determined that individuals who had an actigraphy-determined sleep efficiency of 84.14% or higher at baseline (n=10) were less likely than those who had a lower than 84.14% sleep efficiency at baseline (n=33) to experience an improvement in sleep efficiency after exposure to bright light scheduled either in the morning or evening. In this analysis, timing of bright light exposure was not differentiated into separate treatments (evening vs. morning) as at least 20 subjects are needed in each group for ROC analysis.
Baseline Light Exposure
We examined whether light exposure at baseline might predict sleep at baseline. There were no significant correlations (-0.10<r<0.07, Pearson correlations) when baseline sleep efficiency was compared, whether based on self-report or actigraphy, with any of the five measures of light exposure at baseline. Changes in sleep efficiency between baseline and the end of the 12-week treatment were also not correlated with any of the five measures of light exposure at baseline (-0.01<r<0.20, Pearson correlations).
Circadian Phase Angle
There was no significant difference in ψ (mid-melatonin to mid-sleep) at baseline among the four groups (F=0.84, df=3, p=0.48, ANOVA) (Table 4). There was also no significant correlation between ψ and “morningness” (r=0.11, p=0.47, Pearson correlation) at baseline. As ψ determines the relative position of the circadian pacemaker to darkness (sleep), we also explored whether ψ at baseline could predict the magnitude of the light-induced phase shifts (see Mediators below). The magnitude of the phase delay associated with scheduled evening bright light was indeed predicted by baseline ψ (p<0.02, r=-0.66, linear regression). Thus, the earlier the midpoint of melatonin occurred relative to the midpoint of sleep (i.e., the larger the ψ), the greater the phase delay in response to scheduled evening bright light, as a greater amount of the phase delay portion of the light sensitive phase response curve would be exposed to light. There was, however, no significant predictive value of baseline ψ for phase advances associated with scheduled morning bright light exposure (p=0.96, r=-0.01, linear regression) (see Figure 1 for a diagrammatic representation).
Figure 1. A Diagrammatic Representation of the Relationship between Circadian Light Sensitivity and Sleep.
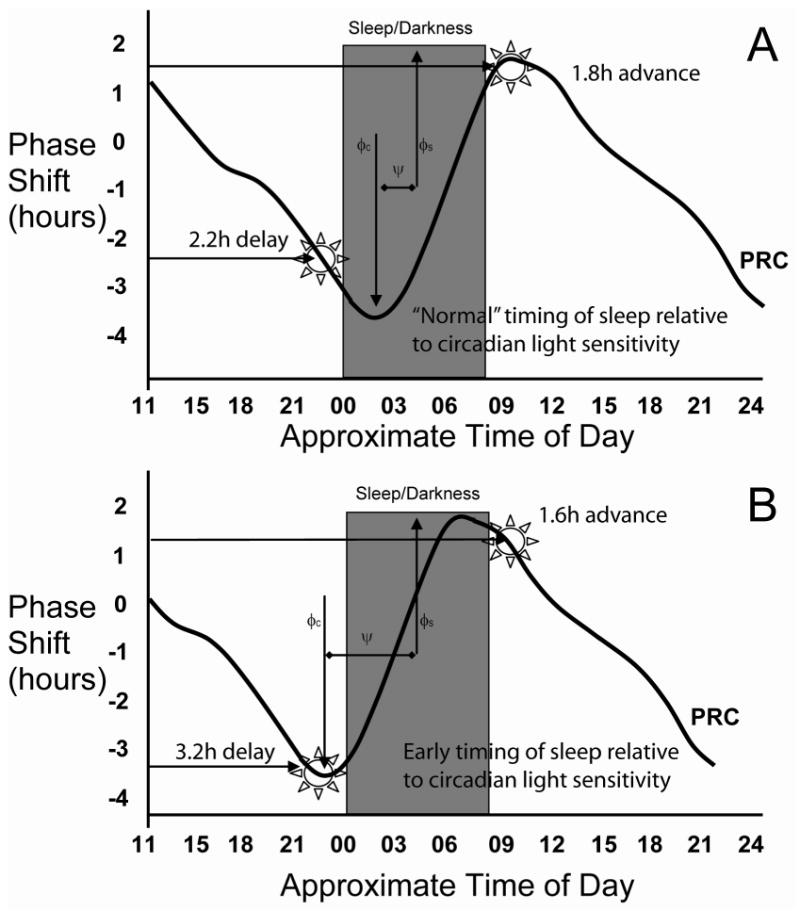
The effect of light exposure at different times of day (phase response curve, PRC, based on Khalsa et al., 2003) in “normal” individuals (top panel) and those with an altered relationship between their sleep and underlying circadian timing system (bottom panel) are diagrammatically represented. Note, this does not depict a phase advance, such as occurs in advanced sleep phase syndrome, rather it shows a change in the phase angle (ψ) between the sleep (φs, midpoint of the sleep episode shown as a grey box) and circadian (φc, timing of peak delay on PRC) systems. In both panels, bright light in the early night causes phase delays while light in the morning causes phase advances. The magnitude of the response of the circadian clock to light scheduled to start one hour before usual bedtime (lower horizontal arrows in both panels) is larger in the bottom panel than in the top panel while the magnitude of the response to morning light scheduled to start within 15 minutes of usual time of rising in the morning (upper arrows in both panels) is similar to that of the top panel.
Baseline ψ predicted the change in sleep efficiency after exposure to bright morning light, as measured by sleep logs (r=0.53, p<0.05, linear regression). In other words, for those individuals exposed to morning bright light, the more positive the ψ (i.e., the earlier the melatonin midpoint during the sleep episode), the greater the effectiveness of the bright light treatment was on log-determined sleep efficiency improvements. No other changes in actigraphic, sleep log or PSG sleep measures of either the morning or evening bright light groups were significantly predicted by baseline. This occurred even when the analysis group was limited to individuals with baseline sleep efficiency by actigraphy less than 85%.
CLOCK Polymorphism
As ψ is in part a fundamental property of the central circadian clock, we also wanted to determine if a known genetic polymorphism of the circadian clock would be of predictive value. The “C” allele of the CLOCK T3111C SNP was associated with less morningness: CC (n=6) genotypes had a Horne-Östberg score of 58.5 ± 8.2, CT (n=25) genotypes had a Horne-Östberg score of 61.6 ± 9.7, and TT (n=28) genotypes had a Horne-Östberg score of 66.3 ± 9.7 (n.b., higher Horne-Östberg scores are associated with increased morningness) (p<0.05, linear regression for number of T alleles). We extended the analysis of CLOCK polymorphisms to their relation to circadian variables such as ψ and the circadian phase change induced by bright light. Individuals homozygous for the C allele (n=5) had a larger ψ (i.e., melatonin midpoint occurring earlier in the sleep episode) (0.84 ± 0.61 h), but it was not significantly different from the ψ found in those with CT (n=17, 0.53 ± 0.87 h) or TT (n=22, ψ of 0.55 ± 0.76 h) genotypes (p=0.43, two-tailed T-test). CLOCK allele status did not significantly contribute to the amount of phase change that was observed under morning or evening bright light. In those exposed to evening bright light, there was no significant difference between CC (n=3, Δφ=-1.0 ± 1.1 h), CT (n=3, Δφ=-0.74 ± 0.66 h), or TT (n=8, Δφ=-0.27 ± 1.0 h) when the number of T alleles was regressed against phase change (p=0.23). In those exposed to morning bright light, we could not determine a three group difference, as only one person thus exposed had a genotype of CC (Δφ=1.7 h). The CT (n=8, Δφ=0.17 ± 0.42 h) and TT (n=7, Δφ=0.63 ± 0.74 h) genotypes were not significantly different by regression (p=0.16). Finally, we could find no differences between the three genetic subgroups in gains in sleep efficiency (baseline to end of treatment, in both bright light treatment groups), as measured by sleep logs (F= 0.78, df=2, p=0.47, ANOVA), wrist actigraphy (F=0.54, df=2, p=0.59; ANOVA), or PSG (F=1.45, df=2, p=0.25, ANOVA).
Mechanisms of Action (Mediators)
We found that both evening and morning bright light were able to evoke a significant change in the timing of the circadian pacemaker between baseline and the end of treatment (Table 4). Bright light in the morning was associated with an average phase advance (i.e., timing of the circadian clock moved earlier) of 0.49 ± 0.66 hrs and evening bright light was associated with an average phase delay of 0.53 ± 0.97 hrs. The phase change associated with bright light in the morning was significantly different from that associated with evening exposure to bright light (W=139, p<0.001, Wilcoxon) and morning dim light (W=43, p<0.05, Wilcoxon). There was also a significant difference between the phase shifting effects of evening bright light and evening dim light (W=88, p<0.05, Wilcoxon). Despite the significant effect of bright light on the circadian pacemaker, there was no relationship between the phase change induced by either morning or evening bright light exposure and any of the objective sleep measures (time in bed, total sleep time, sleep efficiency, wake after sleep onset), as determined by either actigraphy or PSG or subjective measures of sleep, as determined by sleep log, ESS, Spielman, or sleep satisfaction scale.
We also determined whether the experimental protocol had a significant impact on melatonin duration because changes in the duration of elevated plasma melatonin concentrations have been hypothesized to be a useful marker of changes in the amplitude of the circadian clock43 (Table 4). We, however, did not detect a significant change in melatonin duration (F=0.420, df=43, p=0.74, ANOVA) among the experimental groups from baseline to end of treatment.
Discussion
Following 12 weeks of daily exposure to 45-minutes of bright light in either the morning or the evening, we observed changes in several subjective measures of sleep. With the exception of the Spielman, none of these changes were significantly different from those observed after exposure to either dim morning or evening light. When the groups were collapsed across conditions, significant improvements in most measurements of subjective sleep quality were observed. This suggests that our sleep hygiene instructions, which were uniformly presented to all subjects, were able to significantly improve subjective sleep and that the addition of bright light did not result in further large, significant improvements to objective sleep and most measures of subjective sleep, over that engendered by sleep hygiene instructions. We did not include a third no-treatment group because we believed it was unethical to withhold a known effective treatment, particularly in the case of a 3-month long treatment such as ours. Thus, we are unable to determine if the within subject improvements were due to a “participation” effect rather than the sleep hygiene instructions. This also cautions interpretation of previous publications that did not include adequate control group comparisons.
Unlike the subjective effects of our treatments on sleep, we were unable to detect any consistent effects on objective measures of sleep, as determined through either wrist actigraphy or polysomnography. This is not surprising since these metrics measure sleep/wake differently and thus produce different information. The discrepancies in sleep evaluation produced by objective and subjective methods were noted early in the study of sleep.46, 47 Self report information is necessary for the evaluation of treatment interventions given that a subjective complaint is an essential component of an insomnia diagnosis48 and the experience of poor sleep can exist even when not supported by objective measures.49
The experimental bright light was able to change the timing of the circadian clock as predicted, with late evening light exposure causing phase delays and early morning light exposure causing phase advances. There were, however, no significant relationships between the changes in the timing of the circadian clock and objective or subjective changes in sleep, indicating that these circadian changes likely did not mediate changes in sleep. This result is similar to that reported by Suhner and colleagues who found no significant relationship between light-induced changes in circadian phase, measured by core body temperature, and PSG-based sleep efficiency.14 It is possible that the light stimulus in our study caused too small (0.5 hours) a circadian phase shift to evoke clinically relevant changes in sleep. However, our subjects were recruited on the basis of primary insomnia and not advanced or delayed sleep phase syndrome. Therefore, large phase changes would not seem necessary to align the circadian and sleep systems of these individuals. Rather, we hypothesized that a small “nudge” of the circadian system might have been enough to better align the two systems. The small circadian shifts that we generated, however, were unable to significantly improve sleep.
We observed a correlation between ψ and phase delays elicited by bright light, but not between ψ and phase advances. The shift of the PRC relative to the sleep episode (darkness) exposes a more sensitive portion of the PRC to the phase delaying effects of evening light exposure (Figure 1). This same shift, however, does not change the magnitude of the effects of light given in the morning as this is a relatively flat portion of the PRC. We saw a trend of those being homozygous for the C allele of CLOCK T3111C SNP also having a larger phase angle, thereby predisposing towards larger phase delays upon evening exposure to light. Given the relatively smaller number of individuals homozygous for the C allele and the observed variance, more subjects would be needed to examine this genetic correlation. Also, given the changes in phase angle that often occur during aging50, this relationship may vary depending on age. We did find that the C allele of the CLOCK T3111C SNP was associated with less morningness, which supports the findings of Katzenberg and colleagues44 and extends the correlation to older individuals with primary insomnia.
Baseline light exposure did not correlate with subjective or objective sleep measures at baseline nor did it correlate with changes in sleep measures between baseline and the end of treatment. At baseline, however, individuals who had a larger ψ, and were therefore more morning-type, had a greater increase in self-reported sleep efficiency after exposure to 12 weeks of bright morning light. This may have been due to the direct alerting affects of bright light6 (i.e., effects of light on the brain independent of its circadian effects) such that morning types receiving morning light felt more alert and, therefore, had a better impression of the quality of their previous night of sleep. Our light stimulus, while adding to the intensity of light in the morning or evening, did not change the overall, daily pattern of light exposure. This lack of change in overall light exposure may partially explain the lack of change in circadian amplitude as this would be hypothesized to increase with a greater differential between daytime and nighttime light exposure during treatment. We did not observe any correlation between changes in circadian amplitude, as measured by the duration of the nocturnal melatonin peak, and changes in objective or subjective measures of sleep. We were unable to engender a statistically consistent change in circadian amplitude, and any changes that were achieved in circadian amplitude were not correlated with changes in sleep. Thus, it remains possible that a treatment using different light parameters (e.g., timing or length of light exposure) that succeeded in achieving changes in circadian amplitude might be effective in relieving primary insomnia associated with aging.
It is possible that certain subtypes of insomnia (sleep onset, sleep maintenance, early morning awakening, dissatisfactory sleep, or a combination) might differentially benefit from either morning or evening bright light. Given the number of potential combinations, we were unable to examine such subtype-responsiveness in this study, but this would likely be an interesting path to pursue in future research. Another possible factor contributing to our failure to find greater benefit in the bright light groups is that we actively excluded subjects with signs of serious depression. Thus, if the improvement in sleep due to bright light exposure is secondary to an improvement in depressive symptoms, we would not expect to detect it in our subjects. Exploration of light-induced changes in mood associated with improvement in insomnia symptoms in future studies is warranted.
Acknowledgments
The authors would like to thank Beatriz Hernandez for data collection and management, Edward Wakabayashi and Thu Tu for collection and analysis of the polysomnography data, Dr. Ling Lin for assessment of the CLOCK polymorphism, Dr. Helena Kraemer for statistical consultation, Drs. John Brooks and Joy Taylor for helpful comments, the study subjects, and the staff of the Stanford GCRC, without whom this study would not have been possible.
This research is supported by grant R01 AG 12914 from the National Institutes of Health, by the Medical Research Service of the Veterans Affairs Palo Alto Health Care System, by the Department of Veterans Affairs Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), and in part by grant M01 RR-00070 from the National Center for Research Resources, National Institutes of Health
Footnotes
Conflict of Interest: The editor in chief has reviewed the conflict of interest checklist provided by the authors and has determined that the authors have no financial or any other kind of personal conflicts with this paper.
Authors Contributions: Leah Friedman had substantial contributions to all aspects of study design, conduct, analysis, and writing. Jamie Zeitzer had substantial contributions to study analysis and interpretation, as well as writing of the manuscript. Irina Zhdanova had substantial contributions to study design, conduct, and analysis. Clete Kushida had substantial contributions to study design and conduct, data acquisition, as well as editing of the manuscript. Tina Lee contributed to editing of the manuscript. Bret Schneider contributed to editing of the manuscript. Art Noda had substantial contributions to study analysis as well as editing of the manuscript. Christian Guilleminault had substantial contributions to study design as well as editing of the manuscript. Javaid Sheikh had substantial contributions to study design as well as editing of the manuscript. Jerome Yesavage had substantial contributions to study design and interpretation, as well as editing of the manuscript. All authors approve of this final version.
Sponsor's Role: None of the sponsors of this project were involved in the design, methods, subject recruitment, data collections, analysis, or preparation of this manuscript.
References
- 1.Bliwise D. Normal aging. 2nd. Philadelphia: Saunders Co; 1994. [Google Scholar]
- 2.Zeitzer JM, Khalsa SB, Duffy JF, et al. Dose-dependent response of the human circadian system to photic stimulation during the late biological night. Am J Physiol. 2005;289:R839–R844. doi: 10.1152/ajpregu.00232.2005. [DOI] [PubMed] [Google Scholar]
- 3.Zeitzer JM, Dijk DJ, Kronauer R, et al. Sensitivity of the human circadian pacemaker to nocturnal light: Melatonin phase resetting and suppression. J Physiol. 2000;526(Pt 3):695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czeisler CA, Kronauer RE, Allan JS, et al. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 5.Khalsa SB, Jewett ME, Cajochen C, et al. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cajochen C, Zeitzer JM, Czeisler CA, et al. Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115:75–83. doi: 10.1016/s0166-4328(00)00236-9. [DOI] [PubMed] [Google Scholar]
- 7.Lack L, Wright H. The effect of evening bright light in delaying the circadian rhythms and lengthening the sleep of early morning awakening insomniacs. Sleep. 1993;16:436–443. doi: 10.1093/sleep/16.5.436. [DOI] [PubMed] [Google Scholar]
- 8.Youngstedt SD, Kripke DF, Elliott JA, et al. Circadian phase-shifting effects of a laboratory environment: A clinical trial with bright and dim light. J Circadian Rhythms. 2005;3:11. doi: 10.1186/1740-3391-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41:829–836. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 10.Kirisoglu C, Guilleminault C. Twenty minutes versus forty-five minutes morning bright light treatment on sleep onset insomnia in elderly subjects. J Psychosom Res. 2004;56:537–542. doi: 10.1016/j.jpsychores.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Pallesen S, Nordhus IH, Skelton SH, et al. Bright light treatment has limited effect in subjects over 55 years with mild early morning awakening. Percept Mot Skills. 2005;101:759–770. doi: 10.2466/pms.101.3.759-770. [DOI] [PubMed] [Google Scholar]
- 12.Lack L, Wright H, Kemp K, et al. The treatment of early-morning awakening insomnia with 2 evenings of bright light. Sleep. 2005;28:616–623. doi: 10.1093/sleep/28.5.616. [DOI] [PubMed] [Google Scholar]
- 13.Murphy PJ, Campbell SS. Enhanced performance in elderly subjects following bright light treatment of sleep maintenance insomnia. J Sleep Res. 1996;5:165–172. doi: 10.1046/j.1365-2869.1996.t01-1-00001.x. [DOI] [PubMed] [Google Scholar]
- 14.Suhner AG, Murphy PJ, Campbell SS. Failure of timed bright light exposure to alleviate age-related sleep maintenance insomnia. J Am Geriatr Soc. 2002;50:617–623. doi: 10.1046/j.1532-5415.2002.50154.x. [DOI] [PubMed] [Google Scholar]
- 15.Guilleminault C, Clerk A, Black J, et al. Nondrug treatment trials in psychophysiologic insomnia. Arch Intern Med. 1995;155:838–844. [PubMed] [Google Scholar]
- 16.Palmer CR, Kripke DF, Savage HC, Jr, et al. Efficacy of enhanced evening light for advanced sleep phase syndrome. Behav Sleep Med. 2003;1:213–226. doi: 10.1207/S15402010BSM0104_4. [DOI] [PubMed] [Google Scholar]
- 17.Czeisler CA, Dumont M, Duffy JF, et al. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- 18.Duffy JF, Dijk DJ, Klerman EB, et al. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 19.Monk TH, Buysse DJ, Reynolds CF, 3rd, et al. Circadian temperature rhythms of older people. Exp Gerontol. 1995;30:455–474. doi: 10.1016/0531-5565(95)00007-4. [DOI] [PubMed] [Google Scholar]
- 20.Stepanski E, Zorick F, Roehrs T, et al. Daytime alertness in patients with chronic insomnia compared with asymptomatic control subjects. Sleep. 1988;11:54–60. doi: 10.1093/sleep/11.1.54. [DOI] [PubMed] [Google Scholar]
- 21.Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 22.Dijk DJ, Duffy JF, Riel E, et al. Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(Pt 2):611–627. doi: 10.1111/j.1469-7793.1999.0611v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Someren EJ, Mirmiran M, Swaab DF. Non-pharmacological treatment of sleep and wake disturbances in aging and Alzheimer's disease: Chronobiological perspectives. Behav Brain Res. 1993;57:235–253. doi: 10.1016/0166-4328(93)90140-l. [DOI] [PubMed] [Google Scholar]
- 24.Klerman EB, Dijk DJ, Kronauer RE, et al. Simulations of light effects on the human circadian pacemaker: Implications for assessment of intrinsic period. Am J Physiol. 1996;270:R271–282. doi: 10.1152/ajpregu.1996.270.1.R271. [DOI] [PubMed] [Google Scholar]
- 25.Jewett ME, Kronauer RE, Czeisler CA. Phase-amplitude resetting of the human circadian pacemaker via bright light: A further analysis. J Biol Rhythms. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- 26.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Royall DR, Mahurin RK, Gray KF. Bedside assessment of executive cognitive impairment: The executive interview. J Am Geriatr Soc. 1992;40:1221–1226. doi: 10.1111/j.1532-5415.1992.tb03646.x. [DOI] [PubMed] [Google Scholar]
- 28.Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1982;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 29.Kraemer HC. Coping strategies in psychiatric clinical research. J Consult Clin Psychol. 1981;49:309–319. doi: 10.1037//0022-006x.49.3.309. [DOI] [PubMed] [Google Scholar]
- 30.Morin CM, Bootzin RR, Buysse DJ, et al. Psychological and behavioral treatment of insomnia: Update of the recent evidence (1998-2004) Sleep. 2006;29:1398–1414. doi: 10.1093/sleep/29.11.1398. [DOI] [PubMed] [Google Scholar]
- 31.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 32.Morgenthaler T, Alessi C, Friedman L, et al. Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: An update for 2007. Sleep. 2007;30:519–529. doi: 10.1093/sleep/30.4.519. [DOI] [PubMed] [Google Scholar]
- 33.Kushida CA, Chang A, Gadkary C, et al. Comparison of actigraphic, polysomnographic, and subjective assessment of sleep parameters in sleep-disordered patients. Sleep Med. 2001;2:389–396. doi: 10.1016/s1389-9457(00)00098-8. [DOI] [PubMed] [Google Scholar]
- 34.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects (National Institute of Health Publ No 204) Washington DC: Government Printing Office; 1968. [Google Scholar]
- 35.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- 36.Johns MW. A new method for measuring daytime sleepiness: The Epworth sleepiness scale. Sleep. 1991;14:540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 37.Blake DD, Gomez MH. A scale for assessing sleep hygiene: Preliminary data. Psychol Rep. 1998;83:1175–1178. doi: 10.2466/pr0.1998.83.3f.1175. [DOI] [PubMed] [Google Scholar]
- 38.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 39.Ware JE, Jr, Kosinski M, Bayliss MS, et al. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: Summary of results from the Medical Outcomes Study. Med Care. 1995;33:AS264–279. [PubMed] [Google Scholar]
- 40.Zhdanova IV, Wurtman RJ, Balcioglu A, et al. Endogenous melatonin levels and the fate of exogenous melatonin: Age effects. J Gerontol A Biol Sci Med Sci. 1998;53:B293–298. doi: 10.1093/gerona/53a.4.b293. [DOI] [PubMed] [Google Scholar]
- 41.Zeitzer JM, Daniels JE, Duffy JF, et al. Do plasma melatonin concentrations decline with age? Am J Med. 1999;107:432–436. doi: 10.1016/s0002-9343(99)00266-1. [DOI] [PubMed] [Google Scholar]
- 42.Hughes RJ, Sack DA, Singer CM, et al. Preservation of normal endogenous melatonin profiles in older “low producers”. Sleep Research. 1998;26:718. [Google Scholar]
- 43.Shanahan TL, Kronauer RE, Duffy JF, et al. Melatonin rhythm observed throughout a three-cycle bright-light stimulus designed to reset the human circadian pacemaker. J Biol Rhythms. 1999;14:237–253. doi: 10.1177/074873099129000560. [DOI] [PubMed] [Google Scholar]
- 44.Katzenberg D, Young T, Finn L, et al. A CLOCK polymorphism associated with human diurnal preference. Sleep. 1998;21:569–576. doi: 10.1093/sleep/21.6.569. [DOI] [PubMed] [Google Scholar]
- 45.Kraemer HC. Evaluating medical tests: Objective and quantitative guidelines. Newbury Park, CA: Sage Publications; 1992. [Google Scholar]
- 46.Carskadon MA, Dement WC, Mitler MM, et al. Self-reports versus sleep laboratory findings in 122 drug-free subjects with complaints of chronic insomnia. Am J Psychiatry. 1976;133:1382–1388. doi: 10.1176/ajp.133.12.1382. [DOI] [PubMed] [Google Scholar]
- 47.Frankel BL, Coursey RD, Buchbinder R, et al. Recorded and reported sleep in chronic primary insomnia. Arch Gen Psychiatry. 1976;33:615–623. doi: 10.1001/archpsyc.1976.01770050067011. [DOI] [PubMed] [Google Scholar]
- 48.American Academy of Sleep Medicine. International Classification of Sleep Disorders. 2nd ed: Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 49.Sateia MJ, Doghramji K, Hauri PJ, et al. Evaluation of chronic insomnia. An American Academy of Sleep Medicine review. Sleep. 2000;23:243–308. [PubMed] [Google Scholar]
- 50.Duffy JF, Zeitzer JM, Rimmer DW, et al. Peak of circadian melatonin rhythm occurs later within the sleep of older subjects. Am J Physiol Endocrinol Metab. 2002;282:E297–303. doi: 10.1152/ajpendo.00268.2001. [DOI] [PubMed] [Google Scholar]


