Abstract
Background
The etiology, clinical course and outcome of fever and neutropenia (FN) in children with cancer using the current FN guidelines and diagnostic resources in the United States have not been well described.
Patients and Methods
Medical records of one randomly selected FN episode per patient during 2004–2005 at a pediatric oncology center were reviewed. Patients were managed as per institutional FN guidelines and blood cultures collected in continuously-read BACTEC™ bottles.
Results
Of 337 FN episodes, infection was proven in 86 (25%) and probable in 75 (22%). 177 episodes (53%) were judged fever of unknown origin (FUO). Bacteremia accounted for most (41) of the proven bacterial episodes, with viridans streptococci (13), Pseudomonas spp (6) and E. coli (6) the most frequently isolated organisms. The median time to positivity of blood cultures was 12 hrs (range 5.4 – 143.7) with 93% positive within 24 hours of incubation. Viral pathogens were identified in 29 (34%) episodes. Compared to other patients, those with FUO had shorter median duration of fever (0.5 vs. 2.0 days; p<0.0001) and hospitalization (3 vs. 6 days; p<0.0001), longer median duration since last chemotherapy (6.0 vs. 4.0 days; p=0.01) and were less likely to have a diagnosis of acute myelogenous leukemia (AML) (11% vs 22%; p=0.009) or develop a clinical complication (5.1% vs 24.4%; p<0.0001).
Conclusion
Despite currently available diagnostic resources, the majority of patients with FN have FUO marked by a low rate of clinical complications and no infection-related mortality. Emergence of viridans streptococci as the most common blood isolate has affected FN treatment recommendations. Study findings will help further development of strategies for risk stratified management of fever with neutropenia in pediatric patients.
Keywords: Fever, Neutropenia, Children, Microbiology, Etiology
INTRODUCTION
Despite a considerable reduction over the past decades in infection-related mortality in patients with cancers who present with fever and neutropenia (FN), infections remain a major cause of morbidity and mortality in this susceptible population 1. The strategy of using empiric antibiotics has greatly influenced the outcome of fever in a neutropenic host 2. Critical to this strategy is its ongoing update based on the spectrum and resistance patterns of pathogens isolated from patients and known etiologies as determined by the diagnostic modalities currently available to clinicians. While in neutropenic patients an overall shift from gram-negative to gram-positive bacteria 1, 3–6 has been well documented, there are considerable site- and region-specific differences in incidence of resistant organisms such as methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococcus (VRE); differences that would influence the initial choice of empiric antibiotic therapy. This study was conducted to describe the etiology and clinical course of fever in neutropenic children with cancer managed with currently available resources in the United States; information that was found lacking in literature and that has potential implications for optimizing care.
METHODS
Study population and Design
All children and adolescents (< 22 years old) with an underlying malignancy who were seen in the outpatient clinic for FN and admitted to St. Jude Children's Research Hospital (SJCRH) in Memphis, TN during the study period from 1/1/2004 to 12/31/2005 were identified. SJCRH is a 60 inpatient bed referral center with approximately 1,800 admissions per year for children with catastrophic diseases including malignancy and other primary or acquired immunodeficiency syndromes. Patients who had received a hematopoietic stem cell transplant (HSCT) or developed FN while hospitalized were excluded from this study. The medical records were reviewed retrospectively for demographic data, medical history including underlying cancer diagnosis, relapse, duration of neutropenia, granulocyte-colony stimulating factor (G-CSF) use, steroid use within the past 14 days, presence of an indwelling central venous catheter (CVC), interval since last chemotherapy, and other infection-related factors. These factors included antifungal therapy for a probable or proven fungal infection within the past 6 months; colonization within the past 4 weeks with MRSA, VRE or Pseudomonas aeruginosa; prophylactic antibiotics or antifungal agents; and clinical and laboratory information including vital signs, absolute neutrophil count (ANC), absolute monocyte count (AMC), and results of cultures from the blood and other sites as indicated by the clinical assessment. Blood culture sets were collected at admission in continuously-read BACTEC™ bottles, and routinely included both AEROBIC/F bottles for the detection of aerobic organisms and the MYCO/F Lytic bottles for the detection of fungi and mycobacteria. The volume of blood inoculated in the culture bottles was based on the patient's weight 7. Viral etiologies were identified using direct fluorescent antibody (DFA) assays for antigen detection and viral cultures for respiratory, stool or mucosal specimens. Information was collected about duration of hospitalization, development of clinical complications during the period until the patient had been afebrile for five consecutive days, and infection-related mortality. This study was approved by the Institutional Review Board at SJCRH. Patients with FN were managed as inpatients based on institutional FN guidelines consistent with the IDSA recommendations 2. Intravenous (IV) cefepime 1500 mg/m2/dose (maximum dose of 2000 mg) every 8 hours was empirically started upon presentation. Vancomycin was added for any of the following: obvious CVC or soft tissue infection, history of recent cytarabine treatment, quinolone prophylaxis or therapy at the time of fever, known colonization with MRSA, evidence of sepsis, suspected central nervous system shunt infection, and known or suspected infection with Bacillus cereus. Empiric antifungal therapy was added if the patient remained febrile on day 5 of antibiotics and neutropenia was expected to last longer than 5–7 days. Patients could be discharged on oral cefpodoxime or IV cefepime if blood cultures remained sterile after 24 – 48 hours and there was no evidence of pneumonia, focus of suspected bacterial infection, evidence of sepsis during the episode, and no vomiting, stomatitis or diarrhea at the time of discharge. Antibiotics were continued until resolution of fever for at least 24 hours and recovery of neutropenia, or, if neutropenia persisted, for a minimum of five afebrile days with no evidence of documented infection or signs and symptoms of sepsis. The chemotherapeutic protocol for AML patients required administration of voriconazole for antifungal prophylaxis.
Definitions
Fever was defined as a single oral temperature of ≥ 38.3 °C or an oral temperature of ≥ 38.0 °C that persists for over one hour. Neutropenia was defined as an ANC ≤ 500 cells/mm3. Hypotension was defined as systolic blood pressure <5th percentile for age and sex, or need for vasopressor support to maintain blood pressure. Respiratory failure was defined as arterial oxygen pressure < 60 mmHg on room air or need for mechanical ventilation or oxygen requirement (unless patient known to have oxygen requirement at baseline). Bacteremia was defined as a growth of an organism that was judged not to be a contaminant in a blood culture drawn during a febrile episode. Organisms were considered contaminants if they were part of skin flora (e.g., diphtheroids other than Corynebacterium jeikeium, Bacillus spp. not B. cereus, Propionibacterium spp., coagulase-negative staphylococcus (CNS) or Micrococcus spp.) and if they were isolated from only one culture receptacle. All other organisms were regarded as pathogens. Proven infection was defined as isolation of a pathogen from a sterile body site (blood, urine, CSF, or lung biopsy) in the clinical setting of suspected infection or isolation of a pathogen from the skin or gastrointestinal tract with a clinical setting consistent with infection at that site. Probable infection was defined as clinical or radiological findings of infections where the patient shows a prompt response to antimicrobials and there is no laboratory evidence of any infectious etiology. Fever of unknown origin (FUO) was defined as fever without any focus or etiology identified by clinical history, physical examination, radiological or microbiologic testing. Culture-negative sepsis was defined, in the absence of a positive culture, as a systemic response to a possible infection by hemodynamic instability, focal or multiple organ involvement such as poor skin perfusion, oliguria, hypoxemia, and/or altered mental status or lethargy. At the time of conducting the study, invasive mycoses were classified using the 2002 European Organization for Research and Treatment of Cancer/National Institute of Allergy and Infectious Diseases Mycoses Study Group criteria 8. Targeted clinical complications were altered mental status, arrhythmia or electrocardiographic (ECG) changes, bleeding requiring transfusion, congestive heart failure, disseminated intravascular coagulation (DIC), hypotension, intensive care unit (ICU) admission, respiratory failure, renal failure, and other complications judged serious and clinically significant by the investigator.
Statistical analysis
While all patients during the indicated study period were included in the study, only one randomly selected FN episode per patient was included for data analysis. This was done to avoid potential bias related to the inclusion of multiple, not necessarily independent, FN episodes per patient in the analysis. Frequency tables and descriptive statistics were calculated. Continuous variables of 2 groups were compared using Mann Whitney U test while proportions were compared using Chi-square or Fisher's Exact test as appropriate. All tests were two-tailed and a p-value of < 0.05 was considered to be statistically significant. SAS version 9.1.3 (SAS institute, Cary, NC) was used for the analysis.
RESULTS
Baseline characteristics
337 patients with febrile neutropenia (one randomly selected FN episode per patient) met the study inclusion criteria. Baseline characteristics of patients with FN are shown in Table 1. The ethnicity distribution of the patients was consistent with the composition of the patient population at SJCRH. Over 60% of the episodes were noted in patients with an underlying hematological malignancy. Over half of the patients (56%) had profound neutropenia (ANC < 100 cells/mm3) and 44% thrombocytopenia (platelets < 50,000 / mm3) at initial assessment.
Table 1.
Baseline characteristics of patients with fever and neutropenia (N = 337)
| Characteristics | Value (%) |
|---|---|
| Age (median, range) | 5.9 yrs (2.4 months–21.6 yrs) |
| Sex, males | 182 (54) |
| Race | |
| Caucasian | 252 (75) |
| African American | 50 (15) |
| Others | 34 (10) |
| Diagnosis | |
| Leukemia/Lymphoma/MDS* | 221 (66) |
| Solid Tumor | 116 (34) |
| Relapse (malignancy) | 60 (18) |
| Central Venous Line | 321 (95) |
| Steroids in the past 14 days | 118 (35) |
| On G-CSF* | 96 (28) |
| Antifungal therapy in the past 6 months | 26 (8) |
| Antibacterial prophylaxis other than TMP/SMX* | 30 (9) |
| Antifungal prophylaxis with Voriconazole | 59 (17) |
| Interval since last chemotherapy (median, range) | 5.0 days (0 days–1.4 yrs) |
| Duration of neutropenia preceding presentation (median, range) | 11.0 days (1 day – 4.9 months) |
VRE, vancomycin-resistant enterococcus; MRSA, methicillin-resistant Staphylococcus aureus;
URI, upper respiratory infection; ANC, absolute neutrophil count; Plts, platelets; TMP/SMX, trimethoprim/sulfamethoxazole; G-CSF, granulocyte-colony stimulating factor; MDS, myelodysplastic syndrome.
Etiology of fever with neutropenia
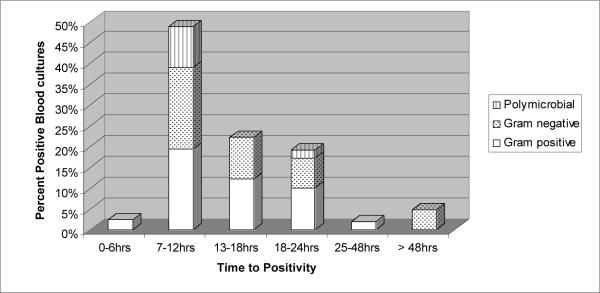
Even with the currently available diagnostic modalities, 177 (53%) episodes were categorized as FUO (Table 2). 74 (22%) episodes were attributed to an infectious etiology based on clinical findings alone (probable infections) and in 86 (25%) episodes a pathogen was identified based on laboratory tests (Table 3; proven infections). The majority (54; 63%) of the episodes with an identified pathogen was categorized as bacterial infections, and most of these (41 episodes) were bacteremias. Viral pathogens were identified in 29 (34%) episodes, fungal in 2 (2.4%) and one episode was of mixed bacterial and fungal etiology. Bacteria most frequently isolated from blood cultures were viridans streptococci (13 episodes), Pseudomonas spp (6) and E. coli (6). The median time to positivity (TTP) of the blood cultures collected at the time of admission was 12 hours (range, 5.4–143.7 hours) and 93% of the bacteremias were detected in the first 24 hours of incubation (Figure 1). In three episodes (7%) the admission blood cultures turned positive after 24 hours of incubation - Capnocytophaga sputigena was isolated in two and coagulase-negative staphylococcus (CNS) in one of these patients. The latter patient had herpes simplex virus (HSV) mucositis and was clinically septic one hour after presentation. Of interest, there were no episodes of candidemia identified. 12 patients were diagnosed with fungal infections, 4 were proven and 8 were probable based on radiological findings of pulmonary and/or splenic nodules detected after resolution of neutropenia. The proven invasive fungal infections were caused by Pseudallescheria boydii (1), Cunninghamella spp. (1), and Aspergillus fumigatus (1) (all pulmonary and culture proven). One additional case of disseminated aspergillosis involving the lungs, liver and central nervous system was diagnosed based on CT scan findings in a patient with rising serum galactomannan antigen (1). The 2 patients with Pseudallescheria boydii and Cunninghamella spp infections were receiving voriconazole prophylaxis at the time of diagnosis, whereas those diagnosed with aspergillosis were not. Three other patients receiving voriconazole prophylaxis were diagnosed with probable breakthrough invasive pulmonary fungal infection, one of whom died.
Table 2.
Etiology and Outcome of fever with neutropenia (N = 337)
| Outcome | Value |
|---|---|
| Etiology of fever, n (%) | |
| FUO | 177 (53) |
| Proven Infections | 86 (25) |
| Bacteremia | 41 |
| Viral URI | 10 |
| GI infection | 10 |
| HSV mucositis | 8 |
| Pneumonia | 7 |
| Fungal infection | 4 |
| Skin/soft tissue infection | 3 |
| Other† | 3 |
| Probable Infections* | 74 (22) |
| URI | 37 |
| GI infection | 16 |
| Culture-negative sepsis | 14 |
| S/ST infection | 13 |
| Fungal infection | 8 |
| Pneumonia | 7 |
| Duration of fever in days (median, range) | 1.1 (0.4–73.4) |
| Duration of hospitalization in days (median, range) | 4.0 (0.25–146) |
| Clinical complications (as per study definitions), n (%) | 48 (14) |
| Mortality within 2 weeks of FN episode, n (%) | 5 (1.5) |
A patient may have more than one diagnosis
The “Other” category includes 2 UTIs and 1 infectious mononucleosis.
FUO, fever of unknown origin; URI, upper respiratory infection; GI, gastrointestinal; S/ST, skin and soft tissue; HSV, herpes simplex virus; UTI, urinary tract infection
Table 3.
Isolated pathogens in the episodes with demonstrated infectious etiology
| Pathogens isolated by sites§ | Number of isolates (%) |
|---|---|
| BLOOD | |
|
| |
| Viridans streptococci | 12 (29) |
| Escherichia coli | 5 (12) |
| Pseudomonas aeruginosa | 4 (10) |
| Coagulase-negative staphylococci | 3 (8) |
| Klebsiella pneumoniae | 3 (8) |
| Capnocytophaga sputigena | 2 (5) |
| Staphylococcus aureus | 1 (2) |
| Bacillus cereus | 1 (2) |
| Bacillus licheniformis | 1 (2) |
| Enterococcus gallinarum | 1 (2) |
| Enterobacter cloacae | 1 (2) |
| Moraxella nonliquifaciens | 1 (2) |
| Eikenella corrodens | 1 (2) |
| EBV* | 1 (2) |
| Polymicrobial | 5 (12) |
|
| |
| URINE | |
|
| |
| Pseudomonas fluorescens/putida | 1 (50) |
| Escherichia coli | 1 (50) |
|
| |
| STOOL† | |
|
| |
| Clostridium difficile | 7 (70) |
| Adenovirus | 1 (10) |
| Rota virus | 2 (20) |
|
| |
| WOUND† | |
|
| |
| Bacillus cereus | 1 (8) |
| Pseudomonas aeruginosa | 2 (17) |
| HSV* | 9 (75) |
|
| |
| NASOPHARYNGEAL† | |
|
| |
| RSV* | 3 (20) |
| Parainfluenza virus | 9 (60) |
| Influenza | 2 (13) |
| Rhinovirus | 1 (7) |
|
| |
| TRACHEAL OR LUNG TISSUE | |
|
| |
| Staphylococcus aureus | 1 (25) |
| Pseudallescheria boydii | 1 (25) |
| Cunninghamella spp. | 1 (25) |
| GP bacteria and fungus | 1 (25) |
HSV, herpes simplex virus; RSV, respiratory syncytial virus; EBV, Epstein Barr virus.
C. difficile in stool was detected by a toxin assay, EBV in blood by PCR assay and viral etiologies in stool, nasopharyngeal or wound specimens by direct fluorescent antibody (DFA) assays for antigen detection and viral cultures.
30–53% of the invasive Staphylococcus aureus isolates were MRSA; 66–89% of the invasive Staphylococcus epidermidis isolates were MRSE; 14–41% of the enterococcus isolates were VRE (including both invasive and surveillance isolates); < 5% of the E.coli and Enterobacter spp. isolates were ESBL producers.
Figure 1.
Distribution of positive blood cultures collected on admission according to time to detection and microbiologic etiology.
Clinical course and outcome
Patients remained febrile for a median of 1.1 days (range, 0.4–73.4) and hospitalized for a median of 4.0 days (range, 0.25–146). Clinical complications were noted in 48 (14%) of the episodes and 5 patients died within 2 weeks of admission for FN. Two of these deaths were related to infection (B. cereus sepsis and presumed disseminated fungal infection) and the rest to underlying disease or co-morbidities.
There were no significant differences in baseline characteristics, TTP of blood cultures, clinical course or outcome of patients with gram-negative bacteremia (17; 5.0%) versus those with gram-positive bacteremia (19; 5.6%) except for duration of fever which was longer in the latter group (data not shown).
Of the 177 patients with FUO, 116 (66%) had fever of < 24 hours; often a single fever spike not repeated after admission. Patients with FUO had a longer duration since last chemotherapy (6.0 vs. 4.0 days, p=0.01), and were less likely to have an underlying diagnosis of AML (11% vs. 22%, p=0.009) as compared to those for whom an infectious etiology was clinically or microbiologically identified. They also had significantly shorter duration of fever (median 0.5 vs. 2.0 days, p<0.0001), hospitalization (median 3.0 vs. 6.0 days, p<0.0001) and were less likely to develop clinical complications (5% vs. 24%, p<0.0001).
DISCUSSION
Infectious complications are an important cause of morbidity and mortality in pediatric cancer patients presenting with FN. The choice of empiric antimicrobial therapy and site of care (inpatient versus outpatient) is in part determined by the etiology of FN, time to establish the diagnosis and risk of related complications and death. The objective of this study was to describe the etiology and clinical outcome of FN in children with cancer in the US. Similar to previous studies in pediatric oncology patients in Malaysia, Italy and Germany 9–12 that have reported 56 to 79% of FN episodes as FUO, we find that in just over half of all FN episodes, the etiology remains unknown despite use of currently available diagnostic tools including continuously-read blood cultures, optimizing blood culture practices 7, direct fluorescent antibody (DFA) assays for antigen detection and viral cultures for respiratory viruses. When comparing FUO rates across studies, one has to be cognizant of the differences in how FUO is defined, the standard of care diagnostic work-up that was used to exclude etiologies prior to determining an episode is FUO, and the timing of FUO assessment (at admission or after how many days after admission). In one study, 28% of FN patients who ultimately were found to have an underlying infectious etiology were considered to have FUO based on initial assessment 13. As defined in our study, we find patients with an FUO have a less severe clinical course and are at low risk for related morbidity and mortality. Previous studies have reported similar outcomes 10, 14–16. This information combined with our finding that 93% of bacteremias are diagnosed within the first 24 hours of admission could potentially influence discharge recommendations after an initial period of observation. While we can speculate that FUOs might represent undetected infections (presumably viral) or be the result of an inflammatory process triggered by non-infectious factors such as the patient's underlying oncological process, chemotherapeutic agents or other medications, or mucosal irritation, studies to address this question need to be designed.
A viral etiology based on laboratory-based confirmation was established in one third of the microbiologically documented infections. This is much higher than what has been reported previously and emphasizes for clinicians the importance of optimizing use of viral diagnostics as part of FN work-up. Besides the obvious infection control implications, confirmation of a viral infection in a patient with FN potentially could reduce the otherwise empiric antibiotic or antifungal changes that are made in patients with an FUO. In their prospective study, Castagnola and colleagues documented that 4 (5%) of the 78 microbiologically documented infections (other than invasive mycosis) occurring during primary FN episodes were due to viral etiology 10. Similarly, in another study involving pediatric AML patients, viral pathogens were isolated in 22 (8%) of 275 microbiologically documented infections 11. The most likely explanation for the striking difference in episodes attributed to a viral etiology between our study and those previously reported may be the improvement and better availability of diagnostic tests (antigen detection and cultures) and/or more frequent use of laboratory methods to confirm a clinically suspected viral infection. We anticipate an even further increase in the proportion of infections deemed to be viral with routine use of molecular diagnostic tools (PCR) when processing respiratory samples; a process recently initiated at our institute.
We found bacteremia as the most common microbiologically documented type of infection, with gram-positive bacteria being the predominant pathogen. This shift from gram-negative to gram-positive bacteria has been well documented over the past decade and is similar to what has been described in adult patients 1, 4, 5, 10, 11, 17, 18. Klastersky and colleagues reviewed 2142 adult patients with febrile neutropenia, of whom 499 (23%) had bacteremia 15. In that study, the relative frequencies of gram-positive, gram-negative and polymicrobial bacteremias were 57%, 34% and 10%, respectively. Similarly, the previously described frequency of gram-positive bacteremias in pediatric FN episodes has ranged from 57% to 80% 10, 11. These findings were in contrast with other studies that showed that gram-negative bacteria remain the predominant pathogens in multiple centers 9,14, 19–23. Hospital/community based differences in predominant antimicrobial flora, in part influenced by differences in antimicrobial pressure, might be an explanation for this difference and highlight the importance of remaining cognizant of the local trend. Our finding of Pseudomonas spp and E. coli as the most common gram-negative bacteria isolated in bloodstream infections is consistent with previous reports 15, 19, 22. However, instead of coagulase-negative staphylococci, we observed viridans streptococci as the most common cause of gram-positive bacteremia 1, 19, 22. Viridans streptococcal bacteremia has been associated with high-dose cytarabine 11, 24–28 and in our study 8 of the 13 patients with this diagnosis had received high-dose cytarabine as part of their AML chemotherapy protocol. The latter observation explains in part the likely risk factor behind the high incidence of viridans bacteremia in our study and supports our institutional FN guidelines that recommend empiric vancomycin use in patients at high risk for viridans streptococci bacteremia such as those with AML. Viridans streptococci as the major pathogen for bacteremias in patients receiving therapy for AML and the role of prophylactic vancomycin use in this patient population has recently been described 29 in our center.
Our study showed that most bacteremias diagnosed by blood cultures (BACTEC™ culture media) taken upon admission were detected within 24 hours of incubation emphasizing the value of an initial observation period as a step down approach to stratified, risk based approach to FN management. A previous review of 360 febrile patients at our institution with blood cultures drawn on two or more consecutive days highlighted the value of media type and blood volume to optimize yield of blood cultures 7 and revealed that the majority (81 of 94; 87%) of bloodstream infections were diagnosed by day one cultures (personal communication with Aditya Gaur, MD). The high yield of day one cultures combined with detection in those positive within 24 hours of culture incubation further supports the early hospital discharge policy suggested by other studies 12,30–34.
The absence of fungemia in our study population may be the result of antifungal prophylaxis in high risk patients or a chance finding despite random selection of the FN episodes. While the overall risk of invasive mycosis (including fungemia) has been low in other pediatric FN studies 10, 35, the emergence of breakthrough fungal infections in those receiving prophylaxis with broad spectrum antifungal agents such as voriconazole is of concern 36–38. Three such confirmed mould breakthrough infections were seen in our study.
Finally, the 0.6 % infection-related mortality seen in this study is on the lower end of the previously reported rates ranging from 0.5 to 6.6% in pediatric 9, 11, 39, 40 FN studies and 1% to 25% in adult episodes 16, 19. The rate of clinical complications (14%) is also low compared to that (32%) reported in the only previous study that evaluated clinical complications in children with FN 41. This may be related to increased vigilance for fever and aggressive parenteral antibiotic therapy when fever is documented in the presence of neutropenia in our study population.
In conclusion, this study finds that half of FN episodes in children with cancer seen at an oncology center in the US have an undetermined etiology but an overall low risk of clinical complications and mortality. When blood cultures, collected at admission in automated, continuously-read blood culture systems utilizing enriched media become positive, they do so within 24 hours of culture incubation. Our observation of viridans streptococci as the most common gram-positive pathogen requires further follow-up and supports vancomycin use as part of initial empiric FN therapy in high risk patients such as those receiving high dose cytarabine.
Our reported findings combined with information about duration of hospitalization can help in the design of studies that examine alternative routes of antimicrobial therapy (oral versus intravenous) and site of care (inpatient versus outpatient) for management of FN in pediatric cancer patients; approaches for which currently there is much more acceptance and evidence-based practice for management of adult patients, rather than for children.
Acknowledgements
The authors would like to acknowledge Jawwad Yusuf, MBBS and Jenine Worley for chart review and data abstraction, Wally Bitar, MS and Anil Thridandapani, BS for constructing the study database and data QA and Dr. Jerry Shenep for his helpful comments about the manuscript.
This work was supported by a National Institutes of Health Grant CA21765 and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Disclaimers: All authors report no conflict of interest.
Part of this work was presented as a poster at the Seventh Annual St.Jude/Pediatric Infectious Disease Society (PIDS) Research Conference held in Memphis, TN in February 2008.
REFERENCES
- 1.Viscoli C, Varnier O, Machetti M. Infections in patients with febrile neutropenia: epidemiology, microbiology, and risk stratification. Clin Infect Dis. 2005 Apr 1;40(Suppl 4):S240–245. doi: 10.1086/427329. [DOI] [PubMed] [Google Scholar]
- 2.Hughes WT, Armstrong D, Bodey GP, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Clin Infect Dis. 2002 Mar 15;34(6):730–751. doi: 10.1086/339215. [DOI] [PubMed] [Google Scholar]
- 3.Zinner SH. Changing epidemiology of infections in patients with neutropenia and cancer: emphasis on gram-positive and resistant bacteria. Clin Infect Dis. 1999 Sep;29(3):490–494. doi: 10.1086/598620. [DOI] [PubMed] [Google Scholar]
- 4.Wisplinghoff H, Seifert H, Wenzel RP, Edmond MB. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin Infect Dis. 2003 May 1;36(9):1103–1110. doi: 10.1086/374339. [DOI] [PubMed] [Google Scholar]
- 5.Yadegarynia D, Tarrand J, Raad I, Rolston K. Current spectrum of bacterial infections in patients with cancer. Clin Infect Dis. 2003 Oct 15;37(8):1144–1145. doi: 10.1086/378305. [DOI] [PubMed] [Google Scholar]
- 6.Koll BS, Brown AE. The changing epidemiology of infections at cancer hospitals. Clin Infect Dis. 1993 Nov;17(Suppl 2):S322–328. doi: 10.1093/clinids/17.supplement_2.s322. [DOI] [PubMed] [Google Scholar]
- 7.Gaur AH, Giannini MA, Flynn PM, et al. Optimizing blood culture practices in pediatric immunocompromised patients: evaluation of media types and blood culture volume. Pediatr Infect Dis J. 2003 Jun;22(6):545–552. doi: 10.1097/01.inf.0000069762.44241.0d. [DOI] [PubMed] [Google Scholar]
- 8.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clin Infect Dis. 2002 Jan 1;34(1):7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 9.Ariffin H, Navaratnam P, Lin HP. Surveillance study of bacteraemic episodes in febrile neutropenic children. Int J Clin Pract. 2002 May;56(4):237–240. [PubMed] [Google Scholar]
- 10.Castagnola E, Fontana V, Caviglia I, et al. A prospective study on the epidemiology of febrile episodes during chemotherapy-induced neutropenia in children with cancer or after hemopoietic stem cell transplantation. Clin Infect Dis. 2007 Nov 15;45(10):1296–1304. doi: 10.1086/522533. [DOI] [PubMed] [Google Scholar]
- 11.Lehrnbecher T, Varwig D, Kaiser J, Reinhardt D, Klingebiel T, Creutzig U. Infectious complications in pediatric acute myeloid leukemia: analysis of the prospective multi-institutional clinical trial AML-BFM 93. Leukemia. 2004 Jan;18(1):72–77. doi: 10.1038/sj.leu.2403188. [DOI] [PubMed] [Google Scholar]
- 12.Mullen CA, Buchanan GR. Early hospital discharge of children with cancer treated for fever and neutropenia: identification and management of the low-risk patient. J Clin Oncol. 1990 Dec;8(12):1998–2004. doi: 10.1200/JCO.1990.8.12.1998. [DOI] [PubMed] [Google Scholar]
- 13.Lawson RD, Gentry LO, Bodey GP, Keating MJ, Smith TL. A randomized study of tobramycin plus ticarcillin, tobramycin plus cephalothin and ticarcillin, or tobramycin plus mezlocillin in the treatment of infection in neutropenic patients with malignancies. Am J Med Sci. 1984 Jan-Feb;287(1):16–23. doi: 10.1097/00000441-198401000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Santolaya ME, Alvarez AM, Becker A, et al. Prospective, multicenter evaluation of risk factors associated with invasive bacterial infection in children with cancer, neutropenia, and fever. J Clin Oncol. 2001 Jul 15;19(14):3415–3421. doi: 10.1200/JCO.2001.19.14.3415. [DOI] [PubMed] [Google Scholar]
- 15.Klastersky J, Ameye L, Maertens J, et al. Bacteraemia in febrile neutropenic cancer patients. Int J Antimicrob Agents. 2007 Nov;30(Suppl 1):S51–59. doi: 10.1016/j.ijantimicag.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Klastersky J, Paesmans M, Rubenstein EB, et al. The Multinational Association for Supportive Care in Cancer risk index: A multinational scoring system for identifying low-risk febrile neutropenic cancer patients. J Clin Oncol. 2000 Aug;18(16):3038–3051. doi: 10.1200/JCO.2000.18.16.3038. [DOI] [PubMed] [Google Scholar]
- 17.Kamana M, Escalante C, Mullen CA, Frisbee-Hume S, Rolston KV. Bacterial infections in low-risk, febrile neutropenic patients. Cancer. 2005 Jul 15;104(2):422–426. doi: 10.1002/cncr.21144. [DOI] [PubMed] [Google Scholar]
- 18.Hann I, Viscoli C, Paesmans M, Gaya H, Glauser M. A comparison of outcome from febrile neutropenic episodes in children compared with adults: results from four EORTC studies. International Antimicrobial Therapy Cooperative Group (IATCG) of the European Organization for Research and Treatment of Cancer (EORTC) Br J Haematol. 1997 Dec;99(3):580–588. doi: 10.1046/j.1365-2141.1997.4453255.x. [DOI] [PubMed] [Google Scholar]
- 19.Velasco E, Byington R, Martins CS, Schirmer M, Dias LC, Goncalves VM. Bloodstream infection surveillance in a cancer centre: a prospective look at clinical microbiology aspects. Clin Microbiol Infect. 2004 Jun;10(6):542–549. doi: 10.1111/j.1469-0691.2004.00874.x. [DOI] [PubMed] [Google Scholar]
- 20.Paul M, Gafter-Gvili A, Leibovici L, et al. The epidemiology of bacteremia with febrile neutropenia: experience from a single center, 1988-2004. Isr Med Assoc J. 2007 Jun;9(6):424–429. [PubMed] [Google Scholar]
- 21.Greenberg D, Moser A, Yagupsky P, et al. Microbiological spectrum and susceptibility patterns of pathogens causing bacteraemia in paediatric febrile neutropenic oncology patients: comparison between two consecutive time periods with use of different antibiotic treatment protocols. Int J Antimicrob Agents. 2005 Jun;25(6):469–473. doi: 10.1016/j.ijantimicag.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 22.Gaytan-Martinez J, Mateos-Garcia E, Sanchez-Cortes E, Gonzalez-Llaven J, Casanova-Cardiel LJ, Fuentes-Allen JL. Microbiological findings in febrile neutropenia. Arch Med Res. 2000 Jul-Aug;31(4):388–392. doi: 10.1016/s0188-4409(00)00080-1. [DOI] [PubMed] [Google Scholar]
- 23.Kanafani ZA, Dakdouki GK, El-Chammas KI, Eid S, Araj GF, Kanj SS. Bloodstream infections in febrile neutropenic patients at a tertiary care center in Lebanon: a view of the past decade. Int J Infect Dis. 2007 Sep;11(5):450–453. doi: 10.1016/j.ijid.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Reilly AF, Lange BJ. Infections with viridans group streptococci in children with cancer. Pediatr Blood Cancer. 2007 Nov;49(6):774–780. doi: 10.1002/pbc.21250. [DOI] [PubMed] [Google Scholar]
- 25.Okamoto Y, Ribeiro RC, Srivastava DK, Shenep JL, Pui CH, Razzouk BI. Viridans streptococcal sepsis: clinical features and complications in childhood acute myeloid leukemia. J Pediatr Hematol Oncol. 2003 Sep;25(9):696–703. doi: 10.1097/00043426-200309000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Paganini H, Staffolani V, Zubizarreta P, Casimir L, Lopardo H, Luppino V. Viridans streptococci bacteraemia in children with fever and neutropenia: a case-control study of predisposing factors. Eur J Cancer. 2003 Jun;39(9):1284–1289. doi: 10.1016/s0959-8049(03)00272-7. [DOI] [PubMed] [Google Scholar]
- 27.Shenep JL. Viridans-group streptococcal infections in immunocompromised hosts. Int J Antimicrob Agents. 2000 Mar;14(2):129–135. doi: 10.1016/s0924-8579(99)00172-7. [DOI] [PubMed] [Google Scholar]
- 28.Rossetti F, Cesaro S, Putti MC, Zanesco L. High-dose cytosine arabinoside and viridans streptococcus sepsis in children with leukemia. Pediatr Hematol Oncol. 1995 Jul-Aug;12(4):387–392. doi: 10.3109/08880019509029589. [DOI] [PubMed] [Google Scholar]
- 29.Kurt B, Flynn P, Shenep JL, et al. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer. 2008 May 5; doi: 10.1002/cncr.23563. [DOI] [PubMed] [Google Scholar]
- 30.Santolaya ME, Alvarez AM, Aviles CL, et al. Early hospital discharge followed by outpatient management versus continued hospitalization of children with cancer, fever, and neutropenia at low risk for invasive bacterial infection. J Clin Oncol. 2004 Sep 15;22(18):3784–3789. doi: 10.1200/JCO.2004.01.078. [DOI] [PubMed] [Google Scholar]
- 31.Klaassen RJ, Allen U, Doyle JJ. Randomized placebo-controlled trial of oral antibiotics in pediatric oncology patients at low-risk with fever and neutropenia. J Pediatr Hematol Oncol. 2000 Sep-Oct;22(5):405–411. doi: 10.1097/00043426-200009000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Innes HE, Smith DB, O'Reilly SM, Clark PI, Kelly V, Marshall E. Oral antibiotics with early hospital discharge compared with in-patient intravenous antibiotics for low-risk febrile neutropenia in patients with cancer: a prospective randomised controlled single centre study. Br J Cancer. 2003 Jul 7;89(1):43–49. doi: 10.1038/sj.bjc.6600993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klastersky J, Paesmans M, Georgala A, et al. Outpatient oral antibiotics for febrile neutropenic cancer patients using a score predictive for complications. J Clin Oncol. 2006 Sep 1;24(25):4129–4134. doi: 10.1200/JCO.2005.03.9909. [DOI] [PubMed] [Google Scholar]
- 34.Shenep JL, Flynn PM, Baker DK, et al. Oral cefixime is similar to continued intravenous antibiotics in the empirical treatment of febrile neutropenic children with cancer. Clin Infect Dis. 2001 Jan;32(1):36–43. doi: 10.1086/317552. [DOI] [PubMed] [Google Scholar]
- 35.Stabell N, Nordal E, Stensvold E, et al. Febrile neutropenia in children with cancer: A retrospective Norwegian multicentre study of clinical and microbiological outcome. Scand J Infect Dis. 2007 Oct 4;:1–7. doi: 10.1080/00365540701670436. [DOI] [PubMed] [Google Scholar]
- 36.Imhof A, Balajee SA, Fredricks DN, Englund JA, Marr KA. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis. 2004 Sep 1;39(5):743–746. doi: 10.1086/423274. [DOI] [PubMed] [Google Scholar]
- 37.Marty FM, Cosimi LA, Baden LR. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N Engl J Med. 2004 Feb 26;350(9):950–952. doi: 10.1056/NEJM200402263500923. [DOI] [PubMed] [Google Scholar]
- 38.Trifilio SM, Bennett CL, Yarnold PR, et al. Breakthrough zygomycosis after voriconazole administration among patients with hematologic malignancies who receive hematopoietic stem-cell transplants or intensive chemotherapy. Bone Marrow Transplant. 2007 Apr;39(7):425–429. doi: 10.1038/sj.bmt.1705614. [DOI] [PubMed] [Google Scholar]
- 39.Santolaya ME, Alvarez AM, Aviles CL, et al. Admission clinical and laboratory factors associated with death in children with cancer during a febrile neutropenic episode. Pediatr Infect Dis J. 2007 Sep;26(9):794–798. doi: 10.1097/INF.0b013e318124aa44. [DOI] [PubMed] [Google Scholar]
- 40.Klaassen RJ, Goodman TR, Pham B, Doyle JJ. “Low-risk” prediction rule for pediatric oncology patients presenting with fever and neutropenia. J Clin Oncol. 2000 Mar;18(5):1012–1019. doi: 10.1200/JCO.2000.18.5.1012. [DOI] [PubMed] [Google Scholar]
- 41.Rondinelli PI, Ribeiro Kde C, de Camargo B. A proposed score for predicting severe infection complications in children with chemotherapy-induced febrile neutropenia. J Pediatr Hematol Oncol. 2006 Oct;28(10):665–670. doi: 10.1097/01.mph.0000212996.94929.0b. [DOI] [PubMed] [Google Scholar]