Abstract
Disabled-2 (Dab2) is expressed in primitive endoderm cells as they are differentiating from the inner cell mass and dab2 deficiency in mice results in lethality at E5.5-E6.5 due to the disorganization of the endoderm layers. Here we show that Dab2 suppresses c-Fos expression in endoderm cells. A morphological normal primitive endoderm layer was observed in putative E5.5 dab2 (−/−):c-fos (−/−) embryos, indicating that the primitive endoderm defect due to the loss of Dab2 is rescued by deletion of c-fos gene. The lethality of the double knockout embryos delayed until E9.5-E10.5 and the defective embryos failed to undergo organogenesis. We conclude that Dab2 plays a role in epithelial organization by suppression of c-Fos expression and suggest that unsuppressed c-Fos can lead to disruption of primitive endoderm epithelial organization, yet an additional dab2 function is required for later organogenesis.
Keywords: Disabled-2 (Dab2), c-Fos, primitive endoderm, cell surface positioning, cell positioning/organization, epithelial polarity, MAPK, mouse embryos
INTRODUCTION
Disabled-2 (Dab2) is one of the two mammalian orthologs of the drosophila Disabled (Gertler et al., 1989; Xu et al., 1995; Howell et al., 1997). Mouse dab1 is expressed mainly in the brain and studies of mutant and knockout mice suggest that Dab1 functions in brain cell positioning organization (Howell et al., 1997a,b; Sheldon et al., 1997). Dab2 was isolated as a signaling phosphoprotein and found to act as a putative tumor suppressor (Xu et al., 1995; Mok et al., 1998; Fazili et al., 1999). The cellular function of Dab2 is established to be an adaptor protein in endocytosis (Mishra et al., 2002; Bonifacino et al., 2003). Dab2 associates with endocytic cargos by binding to adaptin and clathrin (Morris and Cooper 2001; Mishra et al., 2002), and also to cargo passengers including trans-membrane glycoproteins such as low-density lipoprotein receptor family members (Trommsdorff et al., 1999; Oleinikov et al., 2000; Morris and Cooper 2001) and integrins (Wang et al., 2001; Huang et al., 2004). The C-terminus of Dab2 protein binds to the actin-based, minus end-directed motor protein myosin VI (Inoue et al., 2002; Morris et al., 2002a; Hasson, 2003); thus Dab2 mediates the attachment of cargos to motor proteins.
Dab2 is expressed in many epithelial cell types and was suggested to have a role in epithelial organization as a mode of action in tumor suppression (Fazili et al., 1999; Sheng et al., 2000; Yang et al., 2002a), paralleling the role of dab1 in neuronal cell positioning and organization (Howell et al., 1997b; Sheldon et al., 1997). The role of dab2 gene in epithelial organization is supported by the primitive endoderm disorganization in the early embryonic lethal phenotype of dab2 (−/−) mouse embryos (Yang et al., 2002b, 2007). In E5.5 dab2 homozygous deficient embryos, cells of the visceral endoderm, the epithelial cell type of the early embryos, mix within the interior rather than align as a layer covering the inner cell mass (Yang et al., 2002b, 2007). The role of Dab2 in mediating directional transporting of endocytis cargos to establish apical polarity is suggested to be one mechanism for surface positioning of the endoderm cells (Yang et al., 2007).
In conditional targeted knockout mice using Meox2-cre (Tallquist and Soriano, 2000; Morris et al., 2002b), the dab2 gene was not deleted in the extraembryonic endoderm, but was deleted in a mosaic fashion in cells of the embryo proper. These dab2 mosaic mutant mice completed development. Defects in kidney function, however, were noted (Morris et al., 2002b). Dab2 is expressed in several additional tissues (Fazili et al., 1999), but the potential developmental and biological roles of Dab2 in these tissues are not yet well studied.
In cultured cells, transfection/ expression of Dab2 resulted in suppression of c-Fos expression, uncoupling it from MAPK activation (He et al., 2001; Smith et al., 2001a). Dab2 may do so by limiting the entry of the activated MAPK into the nucleus (Smith et al., 2001a, 2004), where c-Fos transcriptional activation is a key target of the kinase. The role of Dab2 in regulating c-Fos expression has been shown in the retinoic acid-induced endoderm differentiation of embryonic carcinoma and stem cells (Smith et al., 2001a,b, 2004). c-fos, an immediate early gene, is a component of the AP-1 transcription complex (Curran and Franza, 1988). c-fos is dispensable for cell growth and development in mice, although c-fos deficient mice develop severe osteopetrosis such as foreshortening of the long bones, ossification of the marrow space, and absence of tooth eruption shortly after birth (Johnson et al., 1992; Hu et al., 1994). c-fos is required for malignant tumor growth (Saez et al., 1995), and overexpression of c-Fos can disrupt mammary epithelial organization (Reichmann et al., 1992). In a genetic analysis of anchor cell invasion of vulval epithelium in C. elegans, a key role for Fos in the disruption of epithelial basement membrane and invasion was demonstrated (Montell, 2005; Sherwood et al., 2005). The genetic approach also identified three Fos targets: a matrix metalloproteinase; an extracellular matrix component (hemicentin); and a protocadherin. These proteins were shown also to be critical for epithelial invasion and disruption of basement membrane in the formation of vulval structure (Montell, 2005; Sherwood et al., 2005).
In the current study, we sought to verify the in vivo relevance of the observation in cell culture that Dab2 suppresses c-Fos expression. Thus, by crossing dab2 and c-fos knockout mice, we investigated the influence of c-fos deletion on the visceral endoderm organization of Dab2 null embryos. The result confirmed the importance and in vivo activity of Dab2 in regulating c-Fos expression in the organization of visceral endoderm of early mouse embryos.
RESULTS
Dab2 Down Regulation Enhances c-Fos Expression in Differentiated Embryonic Stem Cells
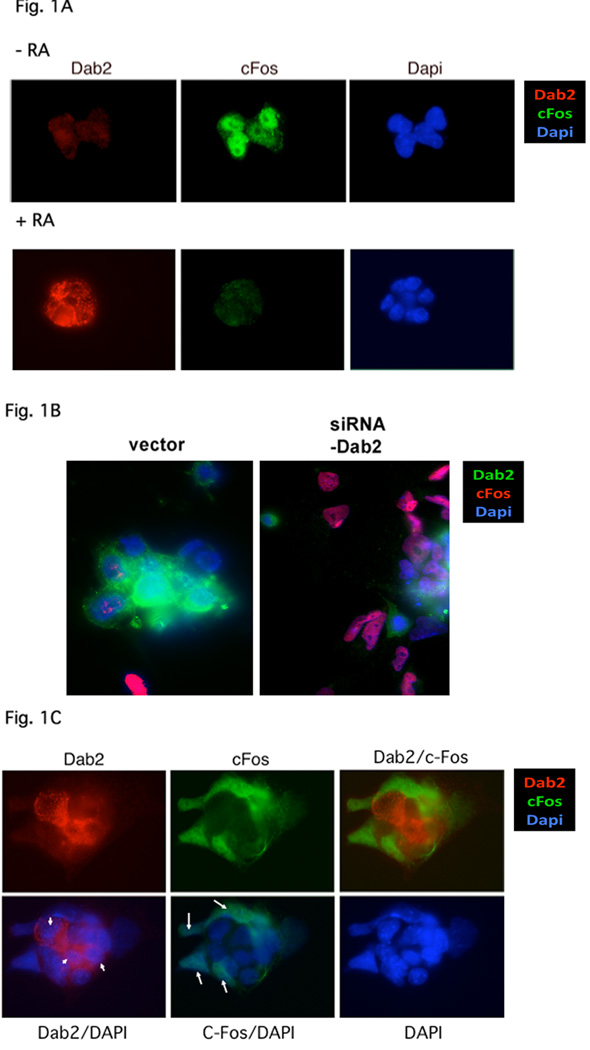
First, we examined whether Dab2 is essential for suppression of c-Fos expression in cultured mouse embryonic stem (ES) cells. Proliferating ES cells are negative for Dab2 and express c-Fos (Fig. 1A). Following treatment with retinoic acid for 4 days, the ES cells differentiated into primitive endoderm-like cells (Smith et al., 2004). In the differentiated cells, Dab2 expression is induced, and c-Fos expression is suppressed (Fig. 1A), consistent with previous studies in ES and F9 embryonic carcinoma cells (Smith et al., 2001a,b, 2004). When Dab2 expression is suppressed by siRNA in the differentiated cells, c-Fos expression is recovered (Fig. 1B,C), indicating Dab2 is essential for suppression of c-Fos expression in ES-derived primitive endoderm cells. In empty vector-transfected cells, retinoic acid induced endoderm differentiation and Dab2 expression is positive in the majority of cells (Fig. 1B), and only less than 15% of cells were undifferentiated (Dab2-negative) and expressing c-Fos (Fig. 1B, left panel). In parallel experiments, about 60% of the siRNA-Dab2 suppressing vector-transfected cells were Dab2-negative and showed high c-Fos expression (Fig. 1B, right panel). We consider that Dab2 was suppressed by siRNA in most of these cells, since the transfection efficiency was estimated to be 50% to 60%. The Dab2-siRNA vector was shown to be highly efficient in suppressing Dab2 expression in a previous report (Yang et al., 2007).
Fig. 1.
Dab2 is required for suppression of c-Fos expression in endoderm cells derived from differentiation of ES cells. (A) ES cells were treated with or without 1 µM retinoic acid (RA) for 3 days to induce endoderm differentiation. The cells were then cultured without serum for 18 hours +/− RA, stimulated with 15% serum for 90 min, and fixed for immunostaining for Dab2 (red), c-Fos (green), or DAPI staining (blue). (B) On day 1, ES cells were transfected with siRNA-Dab2 vector and simultaneously treated with or without RA for 3 more days. The cells were cultured in medium containing low serum (1%) over night and were then stimulated with 10% serum for 90 min and fixed for immunostaining for Dab2 (green), c-Fos (red), or for DAPI (blue). The cell numbers with various combinations of Dab2 and C-Fos expression were counted based on these images, and were used for statistical analysis as summarized in Table 1. (C) Dab2 (red) and c-Fos (green) expression in RA-treated ES cells transfected with the Dab2-siRNA suppressing vector. The cells are a mixture of with (arrow) or without (arrowhead) transfection/Dab2 suppression.
The close correlation between c-Fos expression and Dab2 suppression is shown by another example (Fig. 1C), of differentiated culture containing both Dab2-positive/c-Fos-negative (arrowhead), and Dab2-negative but c-Fos-positive (arrow) cells. Thus, c-Fos expression is suppressed in ES cells upon differentiation as report previously (Smith et al., 2001, 2004), and Dab2 is required for c-Fos suppression in these differentiated ES cells.
A simple statistical analysis was performed on the immunofluorescence images of the fractions cells with positive or negative Dab2 and c-Fos staining to compare vector and siRNA-Dab2-plasmid transfected cells (Table 1). The fraction of cells of either positive or negative for both Dab2 and c-Fos staining is in the range of 6% to 18%, and it is not statistically significant for difference between vector control or Dab2-suppressed cells. However, the shift from Dab2-positive/c-Fos negative in control (around 70%) to c-Fos-positive/Dab2-negative (around 60%) in Dab2-suppressed cells, is statistically significant (Table 1). Thus, we conclude that Dab2 expression has a negative impact on c-Fos expression in ES cells, consistent with previous reports (He et al., 2001; Smith et al., 2001a).
Table 1.
Fluorescence image analysis of the relationship between c-Fos and Dab2
| Expression | vector-transfected |
siDab2-transfected |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| dab2 | c-fos | Area | #1 | #2 | #3 | #1 | #2 | #3 | p-value |
| + | + | 2 (12%) | 0 (0%) | 2 (6%) | 4 (18%) | 2 (6%) | 3 (10%) | 0.34 | |
| − | + | 1 (6%) | 0 (0%) | 3 (9%) | 12 (54%) | 20 (62%) | 22 (73%) | 0.031 | |
| + | − | 12 (70%) | 6 (86%) | 23 (72%) | 4 (18%) | 5 (16%) | 2 (7%) | 0.005 | |
| − | − | 2 (12%) | 1 (14%) | 4 (12%) | 2 (9%) | 5 (16%) | 3 (10%) | 0.68 | |
| Total cell# | 17 | 7 | 32 | 22 | 32 | 30 | |||
The retinoic acid-differentiated ES cells were transfected with empty vector or siRNA Dab2-suppressing vector and stained for Dab2 and c-Fos. Three fields each of vector- or siDAb2 suppression plasmid-transfected cells were counted for c-Fos+ Dab2+; c-Fos+ Dab2−; c-Fos− Dab2+; and c-Fos− Dab2− cells. The staining was called for either positive or negative, ignoring relative intensity. The percentage of cells in each category was calculated and used for statistical analysis. The p-value for the difference between each group was calculated by student T-test (http://www.physics.csbsju.edu/stats/t-test_bulk_form.html). P < 0.05 is considered statistically significant.
c-fos Gene Deletion Rescues Endoderm Development in Dab2 Null Embryos
To examine the in vivo and biological relevance of the regulation of c-Fos expression by Dab2 that observed in cultured cells, we tested the genetic relationship of dab2 and c-fos genes in mice. Specifically, we determined if elimination of c-fos gene could rescue the embryonic lethal phenotype of dab2 knockout (Yang et al., 2002b, 2007) by crossing c-fos and dab2 heterozygous mice and examining the presence of offspring with dab2 (−/−):c-fos (−/−) genotype. In 181 progenies obtained from intercrosses between dab2 (+/−):c-fos (+/−) littermates examined, we found no newborn pups with the dab2 (−/−) genotype (Table 2). It is predicted that 1 in 16, or about 11 of the offspring among the total of 181 should be such genotype if the mutant mice could complete development. In this relatively small number of progenies analyzed, an under-representation of c-fos (−/−) pups was observed either in the dab2 wildtype (0.62/16 instead of 1/16 expected) or heterozygous (1.1/16 instead of 2/16 expected) background, consistent with the reported reduction of embryonic viability of the c-fos knockout (Johnson et al., 1992). Instead, proportion of the progenies with c-fos (+/−) genotypes was increased.
Table 2.
PCR genotyping of progenies from intercrosses between dab2 (+/−): c-fos (+/−) littermates
| Genotypes dab2 : c-fos | expected ratio | actual progeny # | observed ratio | |
|---|---|---|---|---|
| +/+ | +/+ | 1/16 | 10 | 0.88/16 |
| +/+ | +/− | 2/16 | 43 | 3.8/16 |
| +/+ | −/− | 1/16 | 7 | 0.62/16 |
| +/− | +/+ | 2/16 | 30 | 2.6/16 |
| +/− | +/− | 4/16 | 79 | 7.0/16 |
| +/− | −/− | 2/16 | 12 | 1.1/16 |
| −/− | +/+ | 1/16 | 0 | 0/16 |
| −/− | +/− | 2/16 | 0 | 0/16 |
| −/− | −/− | 1/16 | 0 | 0/16 |
| Total | 181 | |||
Thus, deletion of c-fos is not sufficient to rescue the complete development of dab2 (−/−) mutants. Nevertheless, when examine prenatal embryos (Table 3), we identified the presence of dab2 (−/−):c-fos (−/−) embryos that showed abnormal morphology at E8.5 to E10.5 stages in a ratio approximating the Mendelian prediction (1/16 is predicted to be double knockouts) (Table 3). However, no dab2 (−/−) embryos of c-fos (+/−) or (−/−) embryos were found in these developmental stages. dab2 (−/−) embryos normally do not exist beyond E6.5 (Yang et al, 2002, 2007). Thus, it appears that the c-fos knockout background delays the embryonic lethality phenotype of dab2 (−/−) mice.
Table 3.
PCR genotyping of embryos from intercrosses between dab2(+/−): c-fos (+/−) littermates
| Stages | Litter # | Total Embryo # | (mean litter size) | Abnormal embryos dab2 (−/−):c-fos (−/−) | ratio |
|---|---|---|---|---|---|
| E8.5 | 9 | 73 | (8.1) | 4 | 0.88/16 |
| E9.5 | 8 | 59 | (7.4) | 3 | 0.81/16 |
| E10.5 | 6 | 45 | (7.5) | 2 | 0.71/16 |
| Pups | 25 | 181 | (7.2) | 0 | 0/16 |
Note: The abnormal embryos were genotyped to be dab2:c-fos (−/−). The fraction of embryos expected to be dab2 (−/−):c-fos (−/−) is 1/16.
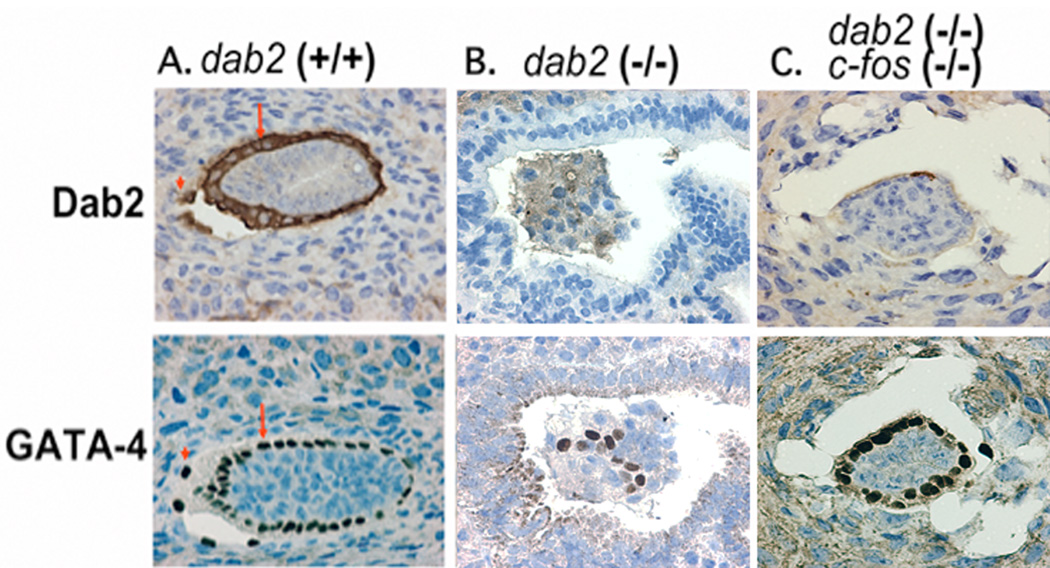
The E5.5 embryos from crosses between dab2 (+/−):c-fos (+/−) mice were analyzed by immunostaining for Dab2 to identify genotypes as dab2 (−/−) or dab2 (+/+) and dab2 (+/−). The Dab2-positive embryos exhibit the GATA4-positive visceral (arrow) and parietal (arrowhead) cells (Fig. 2A). Most Dab2-negative embryos showed the disorganization of GATA-4-positive endoderm cells (Fig. 2B), as reported previously for dab2 knockout phenotype (Yang et al, 2002, 2007). Several embryos were identified as dab2-negative but maintained organization and surface positioning of GATA4-positive endoderm cells (Fig. 2C). Such embryos were observed only from dab2 (+/−):c-fos (+/−) parents but were never detected in embryos from dab2 (+/−):c-fos (+/+) parents. We interpret that these embryos are dab2 (−/−):c-fos (−/−) genotype (Though such small embryos were not suitable for genotyping of c-fos status directly by PCR. Also the available anti-c-Fos antibody is not suitable for staining of fixed and paraffin-embedded tissues). The existence of dab2 (−/−):c-fos (−/−) embryos in later developing stages was confirmed by direct genotyping using PCR of dab2 and c-fos mutations (Table 3). The presence of later double mutant embryos supports the interpretation that of Dab2-nagative but morphological normal E5.5 embryos have dab2 (−/−):c-fos (−/−) genotype. Thus, we conclude that deletion of c-fos rescues the endoderm developmental defect of dab2 (−/−) embryos and postpones the lethality to a later stage.
Fig. 2.
c-fos gene deletion rescues the endoderm disorganization in E5.5 dab2 (−/−) embryos. E5.5 embryos of various genotypes were stained with Dab2 or GATA-4 to determine protein expression (for dab2 genotyping) and organization of the visceral endoderm cells. (A) An example of a wildtype embryo: the visceral endoderm cells are positive for GATA-4 and Dab2, and are organized into an epithelium covering the embryos. (B) An example of dab2 (−/−) embryo from a timed-mating between dab2 (+/−) mice. Lack of Dab2 immunostaining indicates it is a dab2 (−/−) embryo. Note that the GATA-4-positive endoderm cells are disorganized and fail to form a surface endoderm layer. (C) An example of a presumed dab2 (−/−):c-fos (−/−) embryo. Dab2 staining is negative indicating a dab2 (−/−) genotype, while GATA-4-positive visceral endoderm cells appear to organize into an epithelium.
Dynamic Dab2 Expression Pattern in Early Embryonic Development
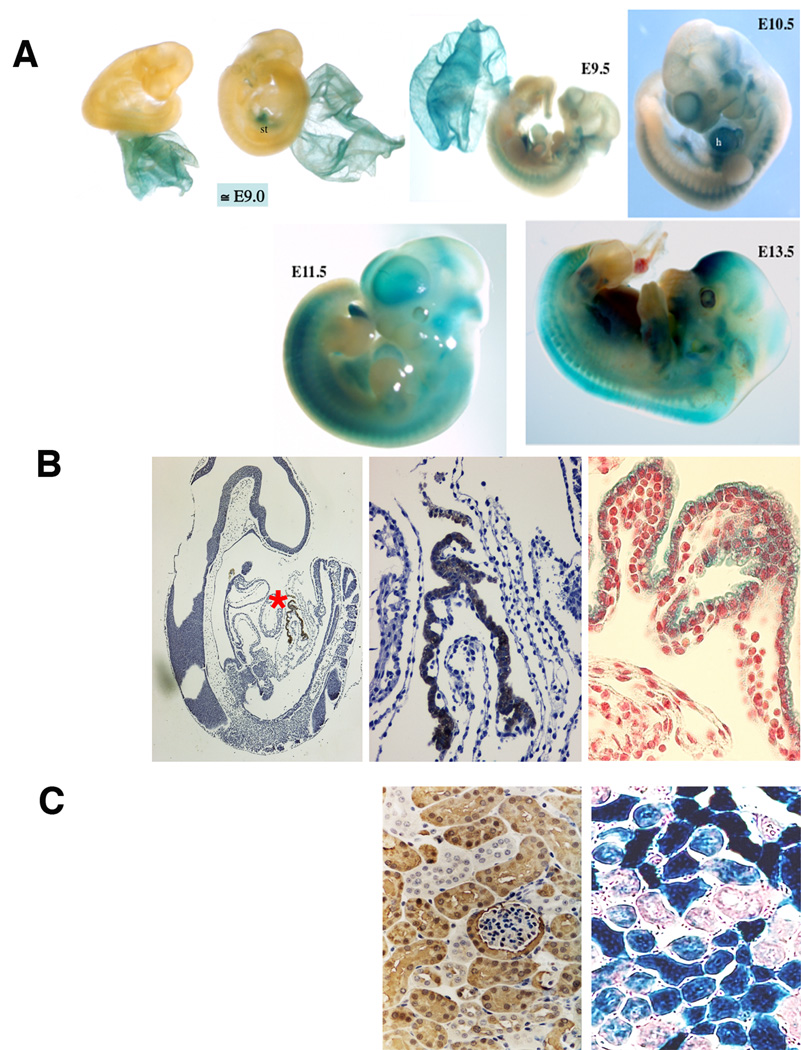
During mouse development, Dab2 is first expressed at E4.5 in the primitive endoderm cells and is then expressed only in extraembryonic tissues but not embryo proper until E8.5 to E9.0 stages (Yang et al, 2002). The dab2 mutation was made by an in-frame insertion of lacZ, allowing us to follow beta-galactosidase activity as a reporter for Dab2 expression in dab2 (+/−) mice (Yang et al, 2002). Essentially no beta-galactosidase staining was observed in embryos (only in extraembryonic yolk sac) prior to E9.0 (Fig. 3A). At a stage around E9.0, some of the dab2 (+/−) embryos express beta-galactosidase only in the yolk sac endoderm (Fig. 3A, left), while some littermates express beta-galactosidase in discrete sites within the embryo proper (for example, the septum transversum) (Fig. 3A, right) in addition to the yolk sac.
Fig. 3.
Dab2 expression in mouse embryos traced by lacZ reporter. dab2 (+/−) mouse embryos and tissues, in which an in-frame fusion of lacZ cDNA after the first 27 amino acids of the dab2 gene replaced the rest of the Dab2 protein, were used to trace Dab2 expression by staining for beta-galactosidase activity. (A) Representative embryos following beta-galactosidase activity staining are shown. Two representative E9.0 dab2 (+/−) embryos from the same litter are shown, that the extraembryonic tissues were stained. One embryo shows no staining in the definitive embryonic tissue and the other embryo shows a unique site of staining. Additional examples of beta-galactosidase staining in dab2 (+/−) E9.0, E9.5, E10.5, E11.5, and E13.5 embryos are shown. (B) Immunostaining of Dab2 in a section of an E9.0 embryo shows positivity only in extraembryonic yolk sac (left panel). The area indicated by a red “*” is shown in a higher magnification (middle panel). A section shows the positive beta-galactosidase staining of the extraembryonic yolk sac from an E9.0 embryo (right panel). The section was counterstained with fast red. (C) The patterns of Dab2 immunostaining and beta-galactosidase activity staining of a kidney from 3-week old dab2 (+/−) mouse are shown for comparison.
Starting from E9.5, beta-galactosidase staining becomes more widely distributed, including the strong staining of the cardiac tissue in E9.5 and E10.5 stages (Fig. 3A). At E11.5 and E13.5 (Fig. 3A), beta-galactosidase activity is present in most parts of the embryos. Sectioning of the embryos provides more precise localization of the staining (Fig. 3B). As shown in an E9.0 embryo, only extraembryonic yolk sac shows strong Dab2 immunostaining (Fig. 3B, indicated by a red “*” in the left panel and shown in a higher magnification in the middle panel). The beta-galactosidase staining of E9.0 yolk sac (Fig. 3A) is shown in a higher magnification of a section for comparison with Dab2 immunostaining (Fig. 3B, right panel). In mice, Dab2 mRNA expression is high in kidney (Fazili et al., 1999). The kidney tubule epithelia are strongly positive for Dab2 or beta-galactosidase activity (Fig. 3C), indicating the pattern of beta-galactosidase staining in the dab2 (+/−) mice is consistent with the presence of Dab2 protein.
Thus, these observations suggest that Dab2 is first expressed in the embryo proper at around E9.0, initially in the hepatic primordial region, next in the cardiac area, and then becomes widely distributed throughout the embryos.
c-fos Elimination Delays Embryonic Lethality of Dab2 Null Embryos
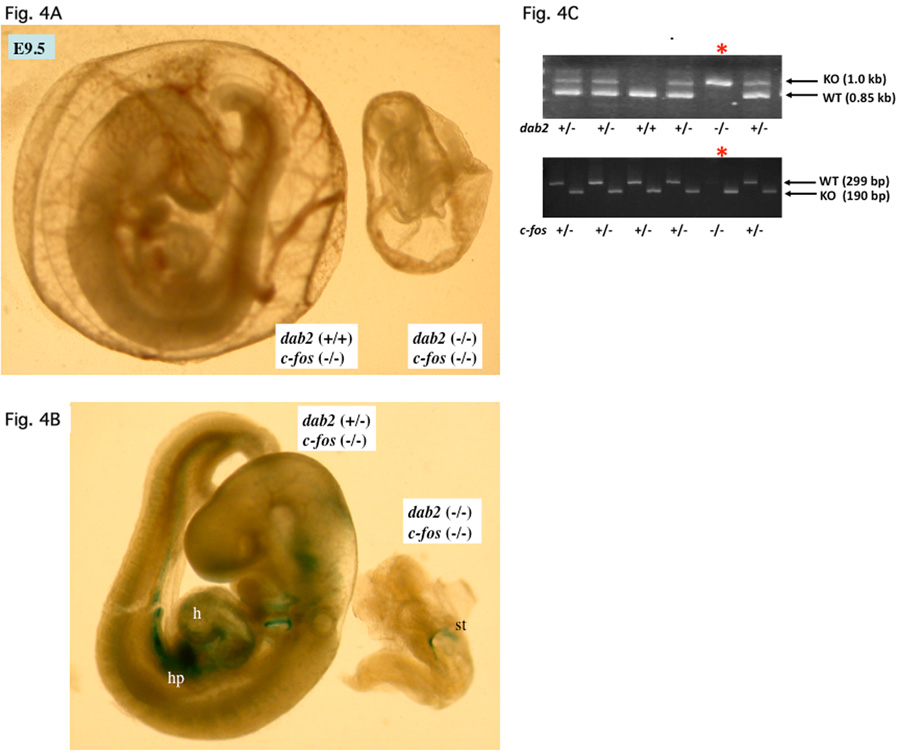
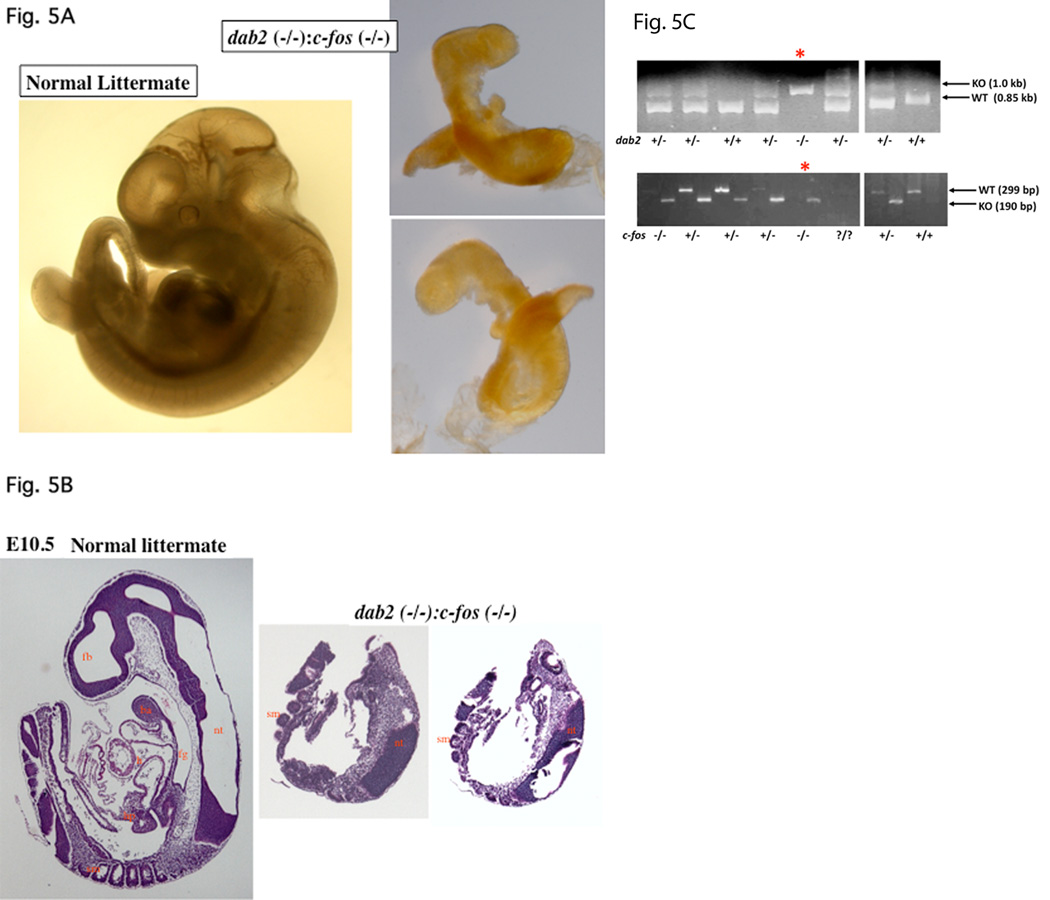
Overcoming extraembryonic endoderm disorganization and escaping lethality at E5.5-E6.5 stages, the dab2 (−/−):c-fos (−/−) embryos persisted to E9.5 to E10.5 stages (Table 3). Nevertheless, the double mutant embryos were found to have greatly disturbed morphology. Over the period of about 2 years when we continually produced and analyzed embryos from mating between dab2 (+/−):c-fos (+/−) mice, a large range of variable morphology and phenotypes of the double mutant embryos at E9.5 to E10.5 stages were observed. In the example of an E9.5 mutant embryo shown in Figure 4A and B, the abnormal embryo identified as dab2 (−/−):c-fos (−/−) by PCR (Fig. 4C) was much smaller than a dab2 (+/+) littermate. The endoderm area of the abnormal embryo stained positive for beta-galactosidase/Dab2 (Fig. 4B). In another striking example of an E10.5 dab2 (−/−):c-fos (−/−) embryo (Fig. 5), the abnormal embryo appeared unusually trim and lacked an apparent heart or other organs, which was confirmed by sectioning through the entire embryo (Fig. 5B). The dab2 (−/−):c-fos (−/−) genotype of this embryo was identified by PCR (Fig. 5C). This phenotype resembles a reported acardia embryo when both GATA4 and GATA6 are deleted (Zhao et al, 2008). Thus, deletion of c-fos delays the embryonic lethality of dab2 (−/−) genotype, which in turn reveals a role of dab2 in organogenesis when Dab2 is started to be expressed in hepatic primordial region at around E9.0.
Fig. 4.
Examples of E9.5 dab2 (−/−) :c-fos (−/−) embryos. Embryos from timed-matings between dab2 (+/−):c-fos (+/−) mice were analyzed. Following photographing under a microscope, part of the embryonic tissues (often the extraembryonic tissues) was used for genotyping by PCR. (A) Comparison of E9.5 embryos enclosed in extraembryonic tissue from a timed-mating of mice. A dab2 (+/+):c-fos (−/−) and a dab2 (−/−):c-fos (+/−) embryos are shown. (B) The embryos were dissected from the extraembryonic tissue and stained with beta-galactosidase. (C) Genotyping by PCR to identify the morphologically abnormal embryo, indicated by “*”, as dab2 (−/−): c-fos (−/−).
Fig. 5.
Delay of lethality to E10.5 of dab2 (−/−) embryos in the c-fos null background. Embryos from timed-mating between dab2 (+/−):c-fos (+/−) mice were analyzed. Following photographing under a microscope, part of the embryonic tissues (often the extraembryonic tissues, the yolk sac) was used for genotyping by PCR. (A) A pair of normal and abnormal (identified as dab2 (−/−):c-fos (−/−)) embryos is shown. The abnormal embryo was photographed on both sides to show a lack of apparent organs. (B) The abnormal E10.5 embryo was sectioned and stained with H&E to compare with a wildtype littermate control. The abnormal embryo was sectioned through and two representative sections around the midpoint are shown. (C) A small fragment of each embryo was used in PCR amplification of dab2 (wildtype, 1.0 kb; knockout, 0.85 kb) and c-fos (wildtype, 299 base pair; knockout, 190 base pair) genes for genotyping. The morphologically abnormal embryo, indicated by “*”, was identified as dab2 (−/−): c-fos (−/−) genotype. Abbreviations in Figures: ba, branchial arch; ca, cardiac mesoderm; en, endoderm; fg, foregut diverticulum; h, heart; hp, hepatic primordial; nt, neural tube; ov, optic vesicle; sm, somites; st, septum transversum.
This analysis indicates that Dab2 has a critical role in suppressing c-Fos expression in vivo, and loss of Dab2-mediated regulation of c-Fos expression may be a cause of extraembryonic disorganization in Dab2-null embryos. Another activity of Dab2 in addition to c-Fos suppression is required for organogenesis at a later stage (E9.0) when Dab2 expression is initiated in the embryo proper, coincident with the morphogenesis of heart and liver.
DISCUSSION
Previously, Dab2 was found to uncouple MAPK activation and c-Fos expression in differentiated ES and embryonic carcinoma cells in cultures (Smith et al, 2001a). In this study we examined the genetic relationship between dab2 and c-fos in early mouse development and the result suggests that the regulatory relationship between Dab2 and c-Fos exists in vivo. We found that absence of c-fos can partially rescue the early embryonic lethality of Dab2 null embryos. Thus, suppression of c-Fos expression by Dab2 is required for the formation and maintenance of extraembryonic endoderm organization in early embryos. However, dab2 (−/−):c-fos (−/−) embryos still die around E9.5 to E10.5, suggesting that Dab2 has additional, c-Fos-independent requirement in organ development in embryogenesis.
Role of Dab2 and c-Fos in Cell Mobility and Epithelial Organization
In cell culture studies, c-fos expression was suppressed by Dab2 expression (He et al, 2001; Smith et al, 2001a). The current study confirms that elimination of Dab2 can dis-regulate c-Fos expression in differentiated ES cells in culture. Additionally, elimination of c-Fos can rescue endoderm disorganization of Dab2-null embryos, suggesting that c-Fos contributes to the disorganization of endoderm in Dab2-null embryos. This conclusion on the biological function of c-Fos is consistent with earlier studies that c-Fos over-expression contributes to disorganization of mammary epithelium (Reichmann et al, 1992) and that c-Fos contributes to tumor malignancy (Saez et al, 1995).
Dab2 has a role in the establishment and maintenance of epithelial polarity through its cellular function in directional cargo endocytic trafficking. Previously, the role of Dab2 in establishing and maintaining cell polarity is also suggested to be critical in endoderm epithelial positioning and organization (Yang et al, 2007). Likely, Dab2 may function in endoderm epithelial organization through by two avenues: the establishment and maintenance of apical polarity to position the cells and the suppression of c-Fos to reduce mobility of the endoderm epithelial cells.
Mechanism for Regulation of c-Fos Expression by Dab2
The current study focus on verifying the in vivo relevance and the genetic relationship of Dab2 and c-Fos expression and has not provided direct insight into the mechanism of how Dab2 may regulate c-Fos. One likely hypothesis is that Dab2 controls c-Fos expression by regulating the nuclear entry of the activated MAPK. Although in cell culture studies, MAPK seems to readily enter nucleus upon activation, nuclear entry of the activated MAPK is regulated in vivo (Kumar et al, 2003). In Drosophila, activated MAPK is restricted to cytoplasm, termed as “cytoplasmic hold”, until certain event triggers the entry of the activated MAPK into the nucleus to induce cell fate determination (Kumar et al, 2003; Marenda et al, 2006). The “cytoplasmic hold” of MAPK in Drosophila was accounted for by the localization of importin 7/Moleskin to the cytoplasmic instead of nuclear (Vrailas et al, 2006). Importin 7/Moleskin is required for MAPK nuclear entry, and its relocalization to the nuclear pores concurs with nuclear entry of MAPK (Vrailas et al, 2006).
Thus, based on the understanding of nuclear entry of MAPK in Drosophila, a possible model in mammalian cells is that Dab2 may restrict MAPK nuclear entry by mediating the trafficking of cargos containing importin 7 (or other importin member in mammalian cells required for MAPK import) away from the nuclear pores, and thus, preventing the efficient entry of the activated MAPK into nucleus to activate the expression of c-Fos. These possibilities await experimental examination.
In sum, the current study demonstrates the in vivo and biological relevance of Dab2 in regulating c-Fos expression and function, and suggests presence of additional c-Fos-independent role of Dab2 in organogenesis in embryonic development.
EXPERIMENTAL PROCEDURES
dab2-, and c-fos-deficient Mice and Embryo Analysis
Two lines of dab2 knockout mice were established previously (Yang et al, 2002b) and the colonies were maintained by inbreeding in the Animal Facility of Fox Chase Cancer Center. The two lines have given identical phenotypes and will not be distinguished here. For embryo analysis, timed-matings were set up between 10 pairs of dab2 (−/−) mice of around 3 months old. The next day, vaginal plugs were examined as an indication of pregnancy. At scheduled days after conception, the pregnant females were sacrificed to harvest uteri for fixing and paraffin embedding. At earlier stages (earlier than E6.5), dab2 genotyping was performed by morphological examination and/or Dab2 immunostaining of the embryos. Dab2-positive littermates in the same uterus were used as positive controls.
The founder pair of c-fos mice (B6.129×1-Fostm1Pa/J) (Johnson et al, 1992) was purchased from the Jackson Laboratory and the mouse colony was maintained in C57/B6 background. The genotyping protocol by PCR amplification of tail genomic DNA of from the Jackson Lab (http://jaxmice.jax.org/pub-cgi/protocols/protocols.sh?objtype=protocol&protocol_id=1699) was followed.
Mating pairs were used to established a dab2 (+/−):c-fos (+/−) colony and were studied over a 2-year period. Embryos from timed mating of dab2 (+/−):c-fos (+/−) parents were harvested for analysis.
Histology and Immunohistochemistry
Uteri with embryos at different developmental stages were collected. The tissue samples were formalin-fixed and paraffin-embedded. Sections (5 µm) were cut and adhered to positively charged slides. Routine H&E staining was applied. Immunohistochemistry using a primary antibody to Dab2 (BD Biosciences) diluted 1:400 was performed as previously described (Yang et al, 2002b, 2007). For GATA4 staining, the sections were subjected to antigen retrieval by steaming for 20 min in citrate buffer (10 mM). Rabbit polyclonal anti-GATA4 antibodies (Santa Cruz Biotechnology, Inc.) were used (1:800 dilution).
Immunofluorescence Microscopy
Cells were plated on 22 × 40 mm cover slides in 6 well dishes and fixed with 4% paraformaldehyde when they had reached 60% confluence. Cells were permeabilized with 0.1% Triton X-100 in PBS for 5 min, washed with PBS and blocked with 3% BSA in PBS containing 0.1% Tween-20 (room temperature for 30 min). Dab2 antibodies were used at 1:200 dilution in 1% BSA in PBS containing 0.1% Tween-20 and incubated for 2 hours. c-Fos polyclonal antibodies (Santa Cruz Biotechnology, Inc.) were used at a 1:200 dilution also. The cellular localization of the antigens was revealed by fluorescein or Texas Red conjugated secondary antibodies (Jackson Immuno Research lab, West Grove, PA) at 1:200 dilution. The secondary antibodies were: donkey anti-mouse IgG conjugated with Texas Red and donkey anti-rabbit IgG conjugated with Fluorescein. Nuclei were marked by DAPI staining. The Nikon Eclipse E 800 epifluorescence microscope with 60 × oil immersion objective linked to a Roper Quantix CCD (charged coupled device) camera were used for observation and image acquisition. A Nikon Eclipse E800 fluorescence microscope with 60 × water immersion objective linked to a Bio-Rad Radiance 2000 LSCM (laser scanning confocal microscope) camera was also used to examine the slides. Images were overlaid using Adobe Photoshop.
Acknowledgments
We thank the excellent technical assistance from Cory Staub, Jennifer Smedberg, and Malgorzata Rula in the lab. We thank Drs. Myung Shin and Kimberly Tremblay for their advice and input in the analysis of embryo morphology during the course of the work. We appreciate the assistance and contribution of Xiang Hua at the Fox Chase Cancer Center transgenic mouse facility, Tony Lerro and Jackie Valvardi at the Fox Chase Cancer Center animal facility, Cass Renner and Fangping Chen of the Fox Chase Cancer Center pathology facility, and Dr. Sandra Jablonski of the Imaging Facility. We are also very grateful for the reading and comments by Dr. Robert Moore in the preparation of the manuscript. These studies were supported by funds from grants R01 CA095071, CA79716 and CA75389 to X.X. Xu from NCI, NIH.
The abbreviations used are
- Dab2
Disabled-2, protein
- dab2
Disabled-2 gene
- ES cells
embryonic stem cells
- RA
retinoic acid
REFERENCES
- Bonifacino JS, Traub LM. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem. 2003;72:395–447. doi: 10.1146/annurev.biochem.72.121801.161800. [DOI] [PubMed] [Google Scholar]
- Curran T, Franza BR., Jr Fos and Jun: the AP-1 connection. Cell. 1988;55:395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Fazili Z, Sun W, Mittelstaedt S, Cohen C, Xu XX. Disabled-2 inactivation is an early step in ovarian tumorigenicity. Oncogene. 1999;18:3104–3113. doi: 10.1038/sj.onc.1202649. [DOI] [PubMed] [Google Scholar]
- Gertler FB, Bennett RL, Clark MJ, Hoffmann FM. Drosophila abl tyrosine kinase in embryonic CNS axons: a role in axonogenesis is revealed through dosage-sensitive interactions with disabled. Cell. 1989;58:103–113. doi: 10.1016/0092-8674(89)90407-8. [DOI] [PubMed] [Google Scholar]
- Hasson T. Myosin VI: two distinct roles in endocytosis. J Cell Sci. 2003;116:3453–3461. doi: 10.1242/jcs.00669. [DOI] [PubMed] [Google Scholar]
- He J, Smith ER, Xu XX. Disabled-2 exerts its tumor suppressor activity by uncoupling c-Fos expression and MAP kinase activation. J Biol Chem. 2001;276:26814–26818. doi: 10.1074/jbc.M101820200. [DOI] [PubMed] [Google Scholar]
- Howell BW, Gertler FB, Cooper JA. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 1997a;16:121–132. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997b;389:733–737. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- Hu E, Mueller E, Oliviero S, Papaioannou VE, Johnson R, Spiegelman BM. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Cheng JC, Liao CH, Stern A, Hsieh JT, Wang CH, Hsu HL, Tseng CP. Disabled-2 is a negative regulator of integrin alpha(IIb)beta(3)-mediated fibrinogen adhesion and cell signaling. J Biol Chem. 2004;279:42279–42289. doi: 10.1074/jbc.M402540200. [DOI] [PubMed] [Google Scholar]
- Inoue A, Sato O, Homma K, Ikebe M. DOC-2/DAB2 is the binding partner of myosin VI. Biochem Biophys Res Commun. 2002;292:300–307. doi: 10.1006/bbrc.2002.6636. [DOI] [PubMed] [Google Scholar]
- Johnson RS, Spiegelman BM, Papaioannou V. Pleiotropic effects of a null mutation in the c-fos proto-oncogene. Cell. 1992;71:577–586. doi: 10.1016/0092-8674(92)90592-z. [DOI] [PubMed] [Google Scholar]
- Kumar JP, Hsiung F, Powers MA, Moses K. Nuclear translocation of activated MAP kinase is developmentally regulated in the developing Drosophila eye. Development. 2003;130:3703–3714. doi: 10.1242/dev.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marenda DR, Vrailas AD, Rodrigues AB, Cook SE, Powers MA, Lorenzen JA, Perkins LA, Moses K. MAP kinase subcellular localization controls both pattern and proliferation in the developing Drosophila wing. Development. 2006;133:43–51. doi: 10.1242/dev.02168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002;21:4915–4926. doi: 10.1093/emboj/cdf487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok SC, Chan WY, Wong KK, Cheung KK, Lau CC, Ng SW, Baldini A, Colitti CV, Rock CO, Berkowitz RS. DOC-2, a candidate tumor suppressor gene in human epithelial ovarian cancer. Oncogene. 1998;16:2381–2387. doi: 10.1038/sj.onc.1201769. [DOI] [PubMed] [Google Scholar]
- Montell DJ. Anchors away! Fos fosters anchor-cell invasion. Cell. 2005;121:816–817. doi: 10.1016/j.cell.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Morris SM, Cooper JA. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic. 2001;2:111–123. doi: 10.1034/j.1600-0854.2001.020206.x. Erratum in: 2002. Traffic 3:236. [DOI] [PubMed] [Google Scholar]
- Morris SM, Arden SD, Roberts RC, Kendrick-Jones J, Cooper JA, Luzio JP, Buss F. Myosin VI binds to and localises with Dab2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic. 2002a;3:331–341. doi: 10.1034/j.1600-0854.2002.30503.x. [DOI] [PubMed] [Google Scholar]
- Morris SM, Tallquist MD, Rock CO, Cooper JA. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J. 2002b;21:1555–1564. doi: 10.1093/emboj/21.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleinikov AV, Zhao J, Makker SP. Cytosolic adaptor protein Dab2 is an intracellular ligand of endocytic receptor gp600/megalin. Biochem J. 2000;3:613–621. [PMC free article] [PubMed] [Google Scholar]
- Reichmann E, Schwarz H, Deiner EM, Leitner I, Eilers M, Berger J, Busslinger M, Beug H. Activation of an inducible c-FosER fusion protein causes loss of epithelial polarity and triggers epithelial-fibroblastoid cell conversion. Cell. 1992;71:1103–1116. doi: 10.1016/s0092-8674(05)80060-1. [DOI] [PubMed] [Google Scholar]
- Saez E, Rutberg SE, Mueller E, Oppenheim H, Smoluk J, Yuspa SH, Spiegelman BM. c-fos is required for malignant progression of skin tumors. Cell. 1995;82:721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- Sheldon M, Rice DS, D'Arcangelo G, Yoneshima H, Nakajima K, Mikoshiba K, Howell BW, Cooper JA, Goldowitz D, Curran T. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature. 1997;389:730–733. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- Sheng Z, Sun W, Smith ER, Cohen C, Sheng Z, Xu XX. Restoration of positioning control following Disabled-2 expression in ovarian and breast tumor cells. Oncogene. 2000;19:4847–4854. doi: 10.1038/sj.onc.1203853. [DOI] [PubMed] [Google Scholar]
- Sherwood DR, Butler JA, Kramer JM, Sternberg PW. FOS-1 promotes basement-membrane removal during anchor-cell invasion in C. elegans. Cell. 2005;121:951–962. doi: 10.1016/j.cell.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Smith ER, Capo-chichi CD, He J, Smedberg JL, Yang DH, Prowse AH, Godwin AK, Hamilton TC, Xu XX. Disabled-2 mediates c-Fos suppression and the cell growth regulatory activity of retinoic acid in embryonic carcinoma cells. J Biol Chem. 2001a;276:47303–47310. doi: 10.1074/jbc.M106158200. [DOI] [PubMed] [Google Scholar]
- Smith ER, Smedberg JL, Rula ME, Hamilton TC, Xu XX. Disassociation of MAPK activation and c-Fos expression in F9 embryonic carcinoma cells following retinoic acid-induced endoderm differentiation. J Biol Chem. 2001b;276:32094–32100. doi: 10.1074/jbc.M105009200. [DOI] [PubMed] [Google Scholar]
- Smith ER, Smedberg JL, Rula ME, Xu XX. Regulation of Ras-MAPK pathway mitogenic activity by restricting nuclear entry of activated MAPK in endoderm differentiation of embryonic carcinoma and stem cells. J Cell Biol. 2004;164:689–699. doi: 10.1083/jcb.200312028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Epiblast-restricted cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Trommsdorff M, Gotthardt M, Hiesberger T, Shelton J, Stockinger W, Nimpf J, Hammer RE, Richardson JA, Herz J. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Vrailas AD, Marenda DR, Cook SE, Powers MA, Lorenzen JA, Perkins LA, Moses K. smoothened and thickveins regulate Moleskin/Importin 7-mediated MAP kinase signaling in the developing Drosophila eye. Development. 2006;133:1485–1494. doi: 10.1242/dev.02334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Makino K, Xia W, Kim JS, Im SA, Peng H, Mok SC, Singletary SE, Hung MC. DOC-2/hDab-2 inhibits ILK activity and induces anoikis in breast cancer cells through an Akt-independent pathway. Oncogene. 2001;20:6960–6964. doi: 10.1038/sj.onc.1204873. [DOI] [PubMed] [Google Scholar]
- Xu XX, Yang W, Jackowski S, Rock CO. Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J Biol Chem. 1995;270:14184–14191. doi: 10.1074/jbc.270.23.14184. [DOI] [PubMed] [Google Scholar]
- Yang DH, Smith ER, Cohen C, Patriotis C, Godwin AK, Hamilton TC, Xu XX. Molecular events associated with dysplastic morphological transformation and initiation of ovarian tumorigenicity. Cancer. 2002a;94:2380–2392. doi: 10.1002/cncr.10497. [DOI] [PubMed] [Google Scholar]
- Yang DH, Smith ER, Roland IH, Sheng Z, He J, Martin WD, Hamilton TC, Lambeth JD, Xu XX. Disabled-2 is essential for endodermal cell positioning and structure formation during early extraembryonic development. Dev Biol. 2002b;251:27–44. doi: 10.1006/dbio.2002.0810. [DOI] [PubMed] [Google Scholar]
- Yang DH, Cai KQ, Roland IH, Smith ER, Xu XX. Disabled-2 is an epithelial surface positioning gene. J Biol Chem. 2007;282:13114–13122. doi: 10.1074/jbc.M611356200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Watt AJ, Battle MA, Li J, Bondow BJ, Duncan SA. Loss of both GATA4 and GATA6 blocks cardiac myocyte differentiation and results in acardia in mice. Dev Biol. 2008;317 doi: 10.1016/j.ydbio.2008.03.013. 614-419. [DOI] [PMC free article] [PubMed] [Google Scholar]