Abstract
In jawed vertebrates, the heterogeneous nonclassical MHC class Ib (class Ib) gene family encodes molecules structurally similar to classical MHC class Ia (class Ia) but with more limited tissue distribution and lower polymorphism. In mammals, class Ib gene products are involved in stress responses, malignancy and differentiation of intrathymic CD8 T cells. The frog Xenopus laevis possesses at least 20 class Ib genes (XNCs), and 9 subfamilies have been defined so far. We have characterized two novel subfamilies, XNC10 and XNC11. XNC10 is phylogenetically and structurally distinct from both class Ia and other XNC genes. Besides thymic lymphoid tumors, XNC10 is preferentially expressed by circulating T cells and thymocytes of the CD8 lineage both in adult and in larvae from the onset of thymus organogenesis. XNC11 is expressed only by thymocytes and upregulated by several thymic lymphoid tumors. These data provide the first evidence of the expression of any class Ib genes in Xenopus larvae, and suggests evolutionary relationships between certain class Ib genes, malignancy and CD8 T cell ontogeny.
Keywords: Xenopus, thymocytes, T cell ontogeny
1. Introduction
CD8 T cells recognize antigenic peptides presented on the surface of infected or malignant cells through their interactions with MHC class I molecules. MHC class I molecules are traditionally classified as either classical (class Ia) or nonclassical (class Ib). In contrast to class Ia molecules that are highly polymorphic, ubiquitously expressed and found at high densities on the surface of most cells, the class Ib gene family includes very heterogeneous genes that usually have a limited tissue distribution, a low polymorphism, and a lower level of cell surface expression. Class Ib molecules are hypothesized to be indicators of intracellular stress and malignancy (Gleimer & Parham, 2003) and have been shown to play a crucial role in immune responses when there is low or suboptimal expression of class Ia molecules, such as during neoplasmic transformations. Certain class Ib molecules are postulated to have specialized functions because of their limited tissue distribution and rapid rate of evolutionary change. In mammals, for example, some class Ib, such as CD1d and TL, are expressed within the thymus primarily by cortical thymocytes, and interact with developing NKT and γδ T cells, respectively, to promote their differentiation (Berg, 2007).
Although orthologous relationships are difficult to distinguish because of their rapid rate of evolution, class Ib genes have been identified throughout jawed vertebrates, from fish to man (reviewed in Flajnik & Kasahara, 2001). However, the number of class Ib genes and their expression pattern vary greatly from one species to another. In the frog, Xenopus laevis, there are at least 20 Xenopus nonclassical class Ib molecules (XNC) genes, and 9 subfamilies have been characterized based on sequence similarity (Flajnik et al, 1993). Interestingly, although X. laevis is tetraploid, both MHC and XNC gene complexes are located far apart on the same arm of only one pair of chromosomes, which indicates that these genes have been diploidized (Courtet et al, 2001). While some of these subfamilies are ubiquitously expressed, such as XNC7, others have a more limited tissue distribution, such as XNC6, which is found only in the lung (Salter-Cid et al, 1998). There is very little known about the function of any of these subfamily members. Additionally, no class Ib gene expression has been detected to date in Xenopus larvae, which also do not express cell surface class Ia proteins (Flajnik et al, 1986; 1993; Salter-Cid et al, 1998).
Several lymphoid tumor cell lines (named B3B7, ff-2 and 15/0) have been established from spontaneously occurring thymic tumors in Xenopus (Robert et al., 1994). We used the 15/0 tumor line to develop a non-mammalian tumor-immunity model (Robert et al. 1995). The 15/0 tumor is highly tumorigenic when transplanted into its MHC-defined isogenetic LG-15 X. laevis clone of origin. LG-15 is a hybrid between X. laevis and X. gilli species producing diploid eggs that can develop by gynogenesis (Kobel & Du Pasquier, 1975). Effector cells involved in immune response against the 15/0 tumor include both CD8 T and NK cells (Rau et al, 2002; Goyos et al, 2004; Maniero et al, 2004). While the 15/0 tumor does not express class Ia molecules, we have shown by RNA interference that class Ib gene products are critically involved in interactions between tumor cells and anti-tumor effector cells (Goyos et al, 2007). From these results we hypothesized that 15/0 tumor cells express particular class Ib gene products recognized by subsets of CD8 T and NK cells. To identify XNC genes expressed by the 15/0 tumor, we used a RACE PCR approach. We report here the characterization of two novel XNC subfamily members, XNC10 and XNC11, which in addition to the 15/0 tumor, are expressed mainly in lymphoid organs. Additionally, this is the first report documenting the expression of any class Ib genes in Xenopus larvae.
2. Material and Methods
2.1 Animals and tumor cell lines
Outbred X. laevis adults and larvae were obtained from our breeding colony (http://www.urmc.rochester.edu/smd/mbi/xenopus/index.htm). Partially inbred MHC homozygous X. laevis f, g, (Du Pasquier et al, 1975) and r stains (Flajnik 1983), and other Xenopus and Silurana species were from the University of Maryland (Baltimore, MD). Sublethal γ-irradiation (10 Gy) was performed on pre-metamorphic larvae stages 56–58, with a cobalt source. All animals were handled under strict laboratory and UCAR regulations (Approval number 2004-199), minimizing discomfort at all times. The 15/0, B3B7 and ff-2 lymphoid tumor lines were derived from spontaneously arising thymic tumors (Robert et al, 1994).
RNA Extraction, RACE-PCR and RT-PCR
Total RNA was isolated from Xenopus cell lines or tissues using Trizol reagent (Invitrogen). 5′-and 3′-RACE-Ready cDNA was synthesized using SMART cDNA amplification kit (Clonetech Laboratories, Inc.) according to the manufacturer’s protocol and RACE-PCR was performed using an XNC consensus primer specific for the α3 domain which does not cross react with class Ia (Table 1). Bands were then cloned into pGEM-T Easy (Promega Corp.) and sequenced. For RT-PCR, 500ng of total RNA were reverse transcribed using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories). Minus RT controls were included for every reaction and all primers span at least one intron (Table 1). cDNAs were diluted two times and 1μL was used as a template in all PCR reactions (Genescript Corp.).
Table 1.
Primer sequences and uses
| Primers names | Sequences | Used for |
|---|---|---|
| XNC_Cons_3′ | GAGTCAGTGTGGAGGTAACACCAGAG | 3′-RACE |
| XNC10_3′RACE_a1_137F | AACGCCATCGCATTCGTCTTTCG | |
| XNC11_3′RACE_a1_163F | GAACACTTGGAGATGCTGACGAAC | |
| XNC_Cons_5′ | CTCCACACTGACTCTGATCTGATAGG | 5′-RACE |
| XNC7_a1_F1 | CCGATGTGAGCCTTTGTTTCAG | Expression study |
| XNC7_a2_R | GTGCACTCATTTTCCATGAATAGT | |
| XNCZ-F | GGCAGTCACATTTTCCAATACAGC | |
| XNC10_a2_256R | AATAAGGAAGGTGCAGTTTC | |
| XNC11_a1_29F | CGATGACTTCTTCACCGACC | |
| XNC11_a2_187R | CCTTGCTCCACTCTGGTATG | |
| CD4_RTPCR_C2F | GGATAACGGTGGCGAATGTTC | |
| CD4_RTPCR_C2R | ACCGTGCCGCCAGAATAACGA | |
| CD8-fs-1F | ACAGACGTCCACGACTGCAG | |
| CD8-fs-R | ATTCCAGACCACAGCACTGC | |
| CD8a-253-1F | CCAACACTCCGCAGAATGTG | |
| CD8a-575-1R | TTGTCACTGGAAGCTGCCTC | |
| CTX-#434-F | GCAGCAGCGGTAATCGGAG | |
| CTX-#433-R | CTCAGCATGGTCATGGAATTG | |
| TCR-Vb3-#33-F | TGACGGTGAATCCTGGAGAC | |
| TCR-C-#504-R | CGATAGCCGTGACAATGAGC | |
| β2m-Cons-F1 | CCGGTGGTCAAGGTTTACACTG | |
| β 2m-Cons-58R | TAGAGATCAGTGATTGGATGA | |
| β-actin-ex2-F1 | GGTGTCATGGTTGGAATGG | |
| β-actin-ex2-R1 | TGGGTTACACCATCACCTGAG | |
| Rag1-#415-F | GCGCCAAGAATCTGTGTCACT | |
| Rag1-R | GTTCTGTTTCATGGTTGTCTACCA | |
Phylogenetic analysis
Deduced amino acid sequences of XNC α1, α2 and α3 domains were aligned with ClustalX. Phylogenetic analysis was performed using Molecular Evolutionary Genetics Analysis (MEGA, version 3.1). Phylogenetic trees were generated by the neighbor-joining method of Saitou and Nei (1987). Mean-character difference or Poisson correction was used to calculate genetic distances employing complete deletion or pairwise deletion of gaps (all four permutations yielded the same tree topology and support). Numbers on nodes represent percentages of 1,000 bootstrap replicates supporting each partition.
Northern and Southern blotting
Total RNA (10μg) from Xenopus cell lines or tissues, was separated on a 0.9% formaldehyde gel according to standard protocol, transferred to a Zeta-probe GT membrane (Bio-Rad), UV crosslinked, hybridized with XNC10 (α1-α2 domains) or XNC11 (α1 domain) 32P-labeled probe and washed under stringent conditions (0.2x SSC + 0.1% SDS at 65°C). The sizes of the mRNAs were determined using a 0.24- to 9.5-kb RNA ladder (Invitrogen). Genomic DNA from Xenopus erythrocytes was isolated as described (Wong et al., 1990) and digested to completion with restriction endonuclease. The digested DNA (10 μg/lane) was separated on 1% agarose gel and transferred onto nitrocellurose membranes (Shleicher & Schuell) by the capillary blotting technique in 10x SSC. Increasing amounts of DNA were loaded in higher ploidy animals according to the ploidy level.
FACS cell sorting of thymocytes
Adult and larval thymocytes (10 × 106 cells) were stained with anti-CD5 (2B1 supernatant), followed by FITC-conjugated goat anti-mouse secondary Ab, anti-CD8 (AM22-biotin) and APC-conjugated Streptavidin. Cells were sorted by FACS Vantage.
In situ hybridization of adult thymus
XNC10 α1-α2 domain (520bp), CD8β Leader-V domain-part of TM (555bp) and full-length class Ia (3.15kb; AF185580) probes were synthesized from a pBluescript vector. 2μg of linearized template was used to synthesize sense and antisense riboprobe using a RNA-labeling Mix (Roche). Tissues were fixed in 4% paraformaldehyde (PFA) followed by overnight 30% sucrose infiltration. Frozen sections were post-fixed in 4% PFA/PBS at RT, and digested with 20 μg/ml Proteinase K for 4 min at RT. Slides were then acetylated in 0.25% Acetic Acid in 0.1M Triethannolamine, and re-fixed in 4% PFA. Approximately 60ng of probe were denatured and hybridized to the slides at 67°C overnight. Sections were washed at high stringency (0.2x SSC at 72°C for 30 min ×2) and blocked with 10% heat-inactivated horse serum. DIG-labeled probes were detected using anti-DIG-alkaline phosphatase Fab fragments (Roche) and BCIP/NBT (Roche) substrate (Protocol adapted from Cai and Brown, 2003).
3. Results
3.1. Characterization of Novel Nonclassical MHC Class Ib Subfamily Members
We have recently shown that the potent immune response generated by gp96 against the MHC class Ianeg tumor 15/0 critically depends on the presence of Xenopus nonclassical MHC class Ib (XNC) proteins on the surface of the tumor cells (Goyos et al, 2004; Goyos et al 2007), and involves CD8 T and NK cells (Goyos et al, 2004). To determine which XNCs are expressed by the 15/0 tumor and possibly interact with anti-tumor effector cells, we employed a RACE-PCR approach using consensus α3 domain primers to amplify known XNCs and sequenced a total of 35 clones. We found that only four of the nine previously characterized XNC subfamilies were expressed, at different levels, by this tumor (Table 1). Importantly, two novel XNC subfamilies, XNC10 and XNC11, were identified. The latter is the most abundant member expressed in 15/0 tumor cells (11 of 35 clones).
Besides the 15/0 tumor cell line, two other tumor lines B3B7 (class Ia negative) and ff-2 (expressing class Ia) have been derived from two other spontaneously arising thymic lymphoid tumors in Xenopus (Robert et al, 1994). We therefore asked whether XNC10 and XNC11 were also expressed in ff-2 and B3B7 cells, and thus possibly represent a common tumor associated antigen. Both Northern blot and RT-PCR analysis demonstrate that XNC10 is expressed by 15/0 and ff-2, but not or weakly by B3B7, whereas XNC11 is expressed by all three tumor cell lines (Fig. 1A and 1B). The different transcript sizes detected on Northern blots with XNC10 and XNC11 probes suggest possible splice variants (Fig. 1A). The possibility that some of these transcripts are from X. gilli is unlikely since the same size bands are observed on a Northern Blot of X. laevis (Figs. 6A and S1). Additionally, XNC10 and XNC11 expression appears to be independent of class Ia, since it is expressed by ff-2 but not 15/0 tumors.
Figure 1. Expression of XNC10 and XNC11 in thymus-derived lymphoid tumor cell lines.
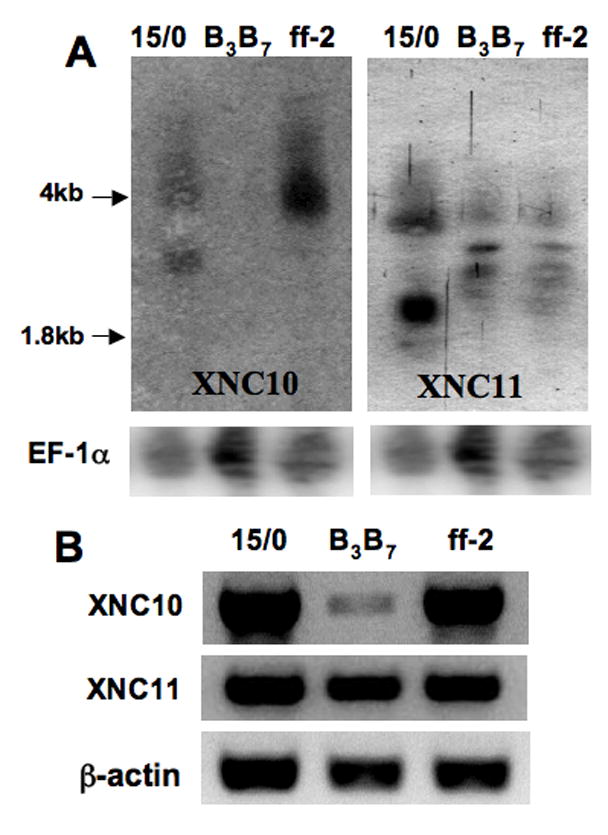
(A) Northern blot analysis of total RNA (15 μg/lane) from 15/0, B3B7 and ff-2 thymic tumor lines hybridized with either a 32P-labeled XNC10 (540bp, α1-α2 domain), a XNC11 α1 (255bp, α1 domain) or an Ef-1α cDNA fragment. (B) RT-PCR of the same tumor lines using XNC10 and XNC11 primers that sits in the α1 and α2 domains.
Figure 6. Expression pattern of XNC10 in adult thymus and spleen.
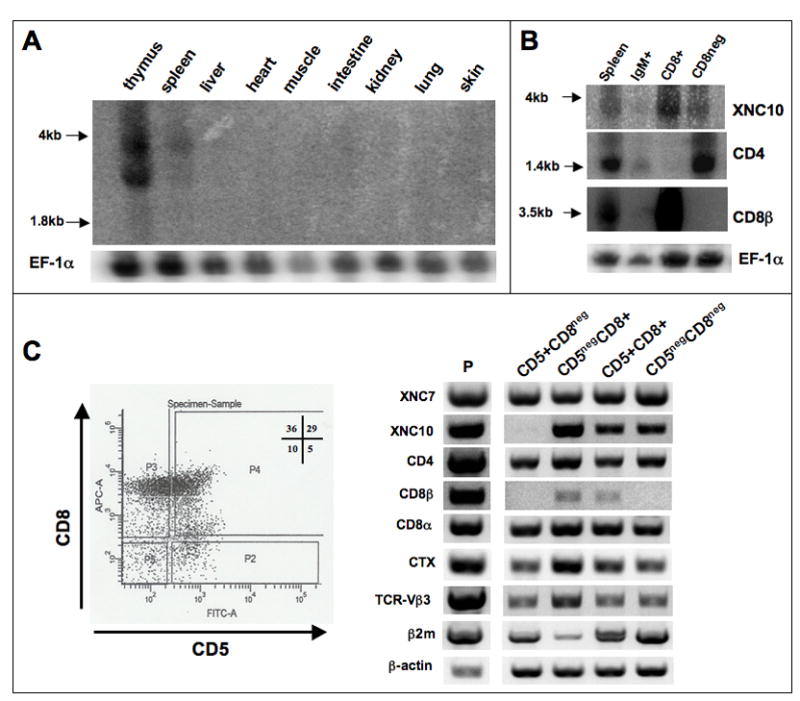
(A) Northern blot of total RNA (10μg) from 6 pooled adult outbred (OB) X. laevis tissues probed with a 540bp 32P-labeled XNC10 α1-α2 cDNA fragment. An Ef-1α probe was used for reference (lower panel). (B) Northern blot of total RNA from total and purified IgM+ B, CD8+ and CD8-depleted splenocytes hybridized successively with an XNC10 α1-α2, a CD4, CD8β and Ef-1α probes. (C) RT-PCR analysis of adult thymocyte populations sorted by FACS according to their expression of CD5 and CD8 surface markers. Left panel, dot plot of CD5 and CD8 expression with the gates used to sort each population: P2 (CD5+CD8neg), P3 (CD5negCD8+), P4 (CD5+CD8+) and P5 (CD5negCD8neg). Cells from each population and from unsorted sample (P) were used for RT-PCR using specific primers indicated in the left panel including XNC7, XNC10, CD4, CD8β, CTX, TCR-Vβ3, as well as beta2-microglobulin (β2m) and β-actin as controls (35 cycles for all genes except, CD8β: 40 cycles, β-actin: 25 cycles). Note that primers for XNC10 were designed in the α1 and α2 domains to ensure subfamily specificity while spanning an intron to avoid detection of possible genomic DNA contamination. Minus RT controls were also run with each primer set.
To address the relationship of XNC10 and XNC11 with other XNCs, we performed phylogenetic analysis. Neighbor Joining consensus trees from amino acid alignments of the three extracellular domains (α1, α2 and α3), either individually or all together, were constructed using 1000 bootstrap replicates to assess the robustness of the tree. We used multiple X. laevis class Ia sequences as outgroup in all four trees. As previously noted (Flajnik et al, 1993), analysis with the α1 and α2 domains of XNCs result in similar tree topologies, as these are the most divergent domains within XNCs, while the α3 domain is the most conserved (Fig. 2). The α3 domains of both XNC10 and XNC11 clearly group with other XNCs, which further support their classification as true class Ib sequences. However, in contrast to XNC11, whose α1 and α2 domains cluster tightly with XNC5, both XNC10 α1 and α2 domains branch apart from both XNCs and class Ia sequences. Importantly, the XNC10 node has a high statistical significance (over 99% bootstrap value; see arrow in Fig. 2), which strongly supports that XNC10 is a bona-fide but divergent XNC subfamily member distinct from class Ia. Because of the peculiar phylogenetic position of XNC10 relative to other XNCs and class Ia, we further characterized XNC10 genetically.
Figure 2. Phylogenetic analysis of Xenopus laevis class Ib.
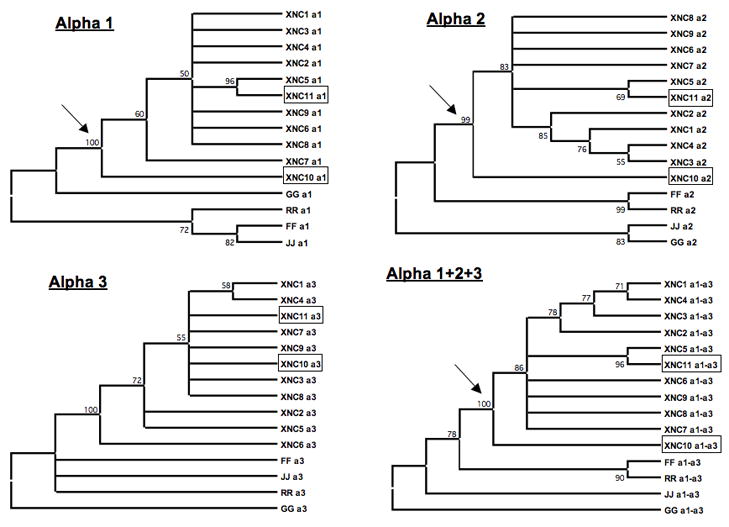
Sequences were aligned with ClustalX and phylogenetic analyses were performed using Molecular Evolutionary Genetics Analysis (MEGA; version 3.1). Phylogenetic trees were constructed using the neighbor-joining method with pairwise deletion of gaps using MEGA3.1 software (Kumar et al, 2001). Support for each node was assessed using 1000 bootstrap replicates. Accession numbers: XNC1 [GenBank:M58019], 2 [GenBank:L20725], 3 [GenBank:L20726], 4 [GenBank:L20727], 5 [GenBank:L20728], 6 [GenBank:L20729], 7 [GenBank:L20730], 8 [GenBank:L20731], 9 [GenBank:L20732], 10 [GenBank:bankit1167220], and 11 [GenBank:bankit1167224]; class Ia G [GenBank:AF185579], R [GenBank:AF185582], F [GenBank:AAF03402], and J [GenBank:AF185586].
The 15/0 tumor cell line was obtained from the LG-15 isogenetic clone that is a hybrid between X. laevis and X. gilli. To further establish that XNC10 is encoded by a gene present in the X. laevis genome and not an aberrant gene related to the genomic instability of the tumor, we analyzed by genomic Southern the progeny (Fig. 3 top panel, #s 1–20) of a cross between two inbred X. laevis MHC heterozygous (f/r × f/g) adults (Fig. 3 top panel, M and F). Hybridization with an XNC10 α1 domain probe reveals two bands per individual (Fig. 3, top panel). Moreover, the parental bands perfectly segregate into 4 Restriction Fragment Length Polymorphism (RFLP) classes, suggesting that XNC10 is either a single copy gene or a set of closely linked multiple genes. Therefore, we conclude that at least some XNC10 transcripts amplified from the 15/0 tumor are products of a gene present in the X. laevis genome.
Figure 3. XNC10 is genetically linked to the XNC locus.
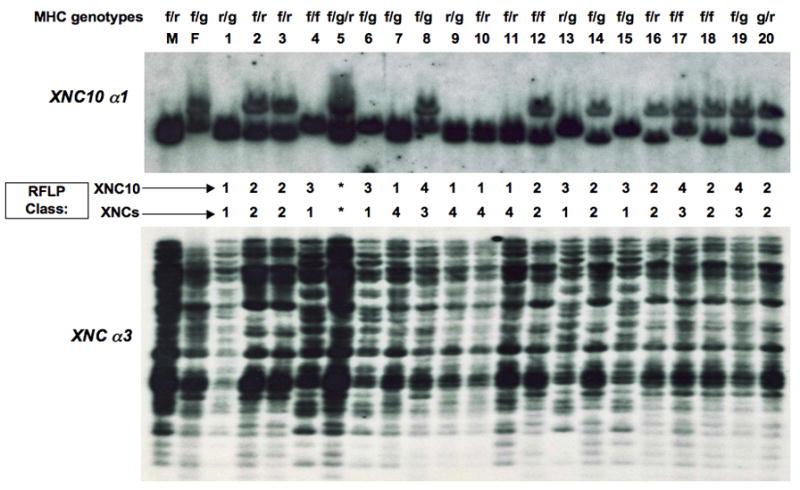
Genomic DNA from 20 progeny derived from a cross of two X. laevis adults of known MHC haplotypes (f/r × f/g) was digested with Eco RI. Hybridizations with 32P-labeled XNC10 α1 (top) or XNC α3 (bottom) domain-specific cDNA probes were done under low stringency conditions and high stringency washes. MHC genotypes, based on several genes in the MHC including class Ia, are listed above are listed above parents and progeny (Ohta et al 1999). (M) male; (F) female; sibling 5 is a natural triploid and marked as *.
In X. laevis, all XNC genes segregate as a single linkage group (Flajnik et al, 1993) and are located far apart from the MHC locus on the same chromosome (Courtet et al, 2001). Because of the peculiar phylogenetic relationship of XNC10 to class Ia and other XNCs (Fig. 2), we next asked whether XNC10 was linked to either the MHC or the XNC locus. We reprobed the membrane of Fig 3 with a fragment containing a XNC consensus α3 domain to identify all XNC genes. The MHC genotype of the siblings previously typed is indicated at the top of Fig 3. Individual #5 was left out of the study since it is a natural triploid. Polymorphisms, both with the XNC10 α1 (Fig. 3, top) and the XNC α3 domain (Fig. 3, bottom) probes, segregate the progenies into 4 different RFLP classes. Because the XNC10 α1 domain linkage groups do not correlate with the MHC haplotypes, XNC10 is not genetically linked to the MHC locus. Conversely, the segregation of RFLP classes obtained with both the XNC10 and XNC α3 consensus probes correlate for 18 out of 19 siblings. The lack of correlation for individual #1 indicates that it is a recombinant. We conclude from this analysis that XNC10 is linked to the XNC locus and not to the MHC proper, and taking into account the recombinant found among the siblings analyzed, XNC10 is likely located near the edge of XNC cluster.
Owing to the divergent nature of XNC10, we next asked whether the XNC10 gene is polymorphic. Southern blotting analysis of 27 outbred X. laevis adults with an XNC10 α1 domain probe reveals a maximum of two RFLPs, which shows that the XNC10 gene is polymorphic (Fig. 4A). To address whether these polymorphisms were confined to the coding regions of this gene, more particularly in the putative peptide binding domain as it is the case for class Ia genes, we PCR amplified the α1 domain from 4 individuals showing different RFLP patterns (Fig. 4A arrows). Multiple amino acid sequence alignments along with the sequences amplified from the 15/0 tumor (from Table 1) shows that the α1 domain of XNC10 is not very divergent (Fig 4B). There are only 4 amino acid substitutions and these are not confined to the putative peptide binding residues (pPBR). Similar sequence conservation is noted for the α2 domain. Interestingly, for both the α1 and α2 domains, the amino acid differences seem to lay in the loops in between the β-strands themselves and the α-helix. Therefore, we conclude that while the XNC10 gene is polymorphic by RFLP analysis, most of these polymorphisms is likely to lie outside of the pPBR, possibly in the untranslated regions of the gene.
Figure 4. Polymorphism of XNC10 Non-coding Regions.
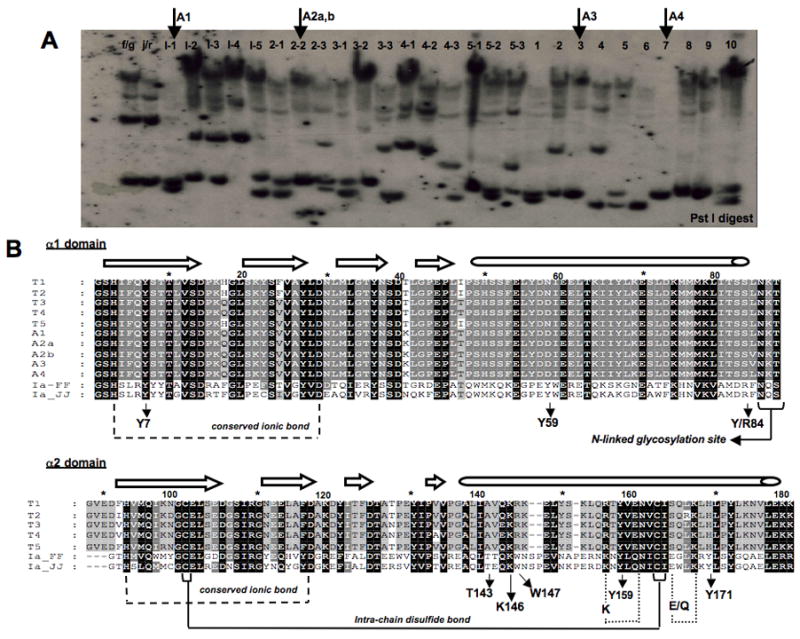
(A) Genomic Southern blot. DNA (10 μg) from individual outbred X. laevis adults were digested with Pst I, blotted and hybridized with a 32P-labeled XNC10 α1-domain cDNA probe. Arrows indicate individuals whose XNC10 α1 domain was amplified by PCR for sequence analysis in B. Note that the weaker signal of high molecular weight is most likely due to some non-specific cross-hybridization of the probe. (B) Alignment of the deduced amino acid sequences of the 5 XNC10 cDNA clones from the 15/0 tumor (T1-T5) obtained by RACE-PCR and 5 clones from normal outbred adult thymus (A1-A4) obtained by PCR. Small arrows under the alignments indicate residues and positions that align with HLA-A2 invariant peptide-binding residues within the pPBD. Larger arrows and long tubes above the alignments indicate the β strand and α helical regions of the protein, respectively.
Signature features of class I molecules present in almost all XNCs (Flajnik et al, 1993) are also present in XNC10 (Fig. 4B). For example, the intra-chain disulfide bonds in the α2 and α3 domains are conserved, as is the asparagine-linked glycosylation site at the C-terminus of the α1 domain (Fig. 4B and data not shown). Additionally, conserved ionic bonds between the first two β strands of both the α1 and α2 domains that help to stabilize the putative peptide-binding domain (pPBD; [Saper et al, 1991]), which are found in almost all XNCs, are also conserved in XNC10 (Fig. 4B). There are also two ionic bonds (K157-E161 and E166-K169) in the α2 domain that help to stabilize the α-helix of XNCs. The first of these contains a synonymous substitution from K157 to R157 in all XNC10 alleles and is likely to be preserved (Fig. 4B). Notably, this substitution is XNC10 specific since it is not found in any other XNC nor in any Xenopus class Ia molecules (data not shown). The second of these ionic bonds that promote α-helix stability in the α2 domain, however, has a mutation from E166 to S166 (Fig. 4B). This mutation is likely to eliminate the presence of this ionic interaction since Ser cannot form an ionic bond with Lys. Interestingly, this mutation is also XNC10 specific; all other XNCs and Xenopus class Ia have either Glu or Gln at this position (data not shown). Therefore, while the XNC10 α1 domain is conserved relative to other XNCs, the α-helix within the α2 domain of XNC10 is likely to be either less stable than other XNCs, or to form its own stabilizing interactions possibly altering the structural conformation of the XNC10 pPBD relative to other XNCs.
Since phylogenetic analysis indicates that XNC10 is more class Ia-like than any other XNC, we analyzed XNC10 residues that align with invariant amino acids of the PBD of mammalian class Ia proteins to see if there was any conservation in residues responsible for peptide binding. The side chains of these invariant residues point into the class I cleft and are essential for peptide anchoring through hydrogen or ionic bonding to backbone atoms of peptide N- and C-termini (Table 2 - modified from Flajnik et al, 1993; Madden et al, 1991). In mammalian class Ia molecules, both Tyr59 and Tyr171 residues help to anchor the N-terminal-most portion of the peptide within the class I cleft through hydrogen bonding (Matsumura et al, 1992). The putative peptide N-terminus of XNC10 contains two XNC10-specific residues: Asp/Asn59 and His171. Interestingly, residues at both of these positions are also capable of forming hydrogen bonds, which if bind peptides, will likely result in a distinct conformation from class Ia. The peptide C-terminus docking residues are also very different in XNC10 when compared to other XNCs. In XNC10, Lys146 is conserved with class Ia and Tyr/Arg84 is conservatively substituted to Ser, a residue that is still capable of forming hydrogen bonds. Therefore, XNC10 peptide docking residues are very different from other XNCs and have more conservation with class Ia than any other XNC. This suggests that if XNC10 is capable of binding and presenting antigens, it does so differently from other XNCs.
Table 2.
Summary of XNC subfamilies expressed by 15/0 tumor cells and their tissue distribution.
| XNC | Nb of clones | Tissue distribution |
|---|---|---|
| XNC11 | 1 | Thymus, spleen, skin and intestine2 |
| XNC41 | 4 | Thymus, spleen, and intestine2 |
| XNC61 | 10 | Lung and skin2 |
| XNC81 | 4 | Thymus, intestine and skin2 |
| XNC10 | 5 | Adult and larval thymus and spleen; 15/0 & ff-2 tumors |
| XNC11 | 11 | Adult thymus; 15/0, B3B7 & ff-2 tumors |
| Total | 35 |
from Flajnik et al., 1993
Because it has been proposed that class Ib genes are recent inventions that have evolved from class Ia genes (Parham, 1994), we wanted to address whether XNC10 was conserved in other species within the Xenopus genus. The subfamily Xenoponidae of the Pipidae family of frogs, containing the genera Xenopus and Silurana, is one of the few cases in vertebrates of speciation by genome duplication (allopolyploidization) (Kobel & Du Pasquier, 1986). The ploidy of different species within the Xenopus genus ranges from diploid to dodecaploid. In most cases, the functional MHC and XNC loci have been diploidized and only two loci are expressed regardless of how many chromosomes are present (Du Pasquier et al, 1977; Courtet et al, 2001). Hybridization of a genomic Southern blot with an XNC10 α1 domain probe, demonstrates that XNC10 is somewhat conserved in all species of the genus Xenopus (Fig. 5A). In contrast, only a faint band is seen in S. tropicalis that washes away upon increased stringency wash (arrow in Fig. 5A & 5B). No band is present in another Silurana species, S. epitropicalis, suggesting that the XNC10 gene is lost from the Silurana lineage. The weak hybridization to the S. tropicalis DNA may due to the cross-hybridization to other XNC genes. Interestingly, the lower X. borealis band also washes away upon increased stringency (Fig. 5B). Importantly, since there are only 2 bands, at most, in all Xenopus species examined in this study, regardless of their ploidy, XNC10 also appears to be silenced, most likely diploidized, in polyploid species.
Figure 5. Conservation and silencing of the XNC10 gene in species of the Genus Xenopidae.
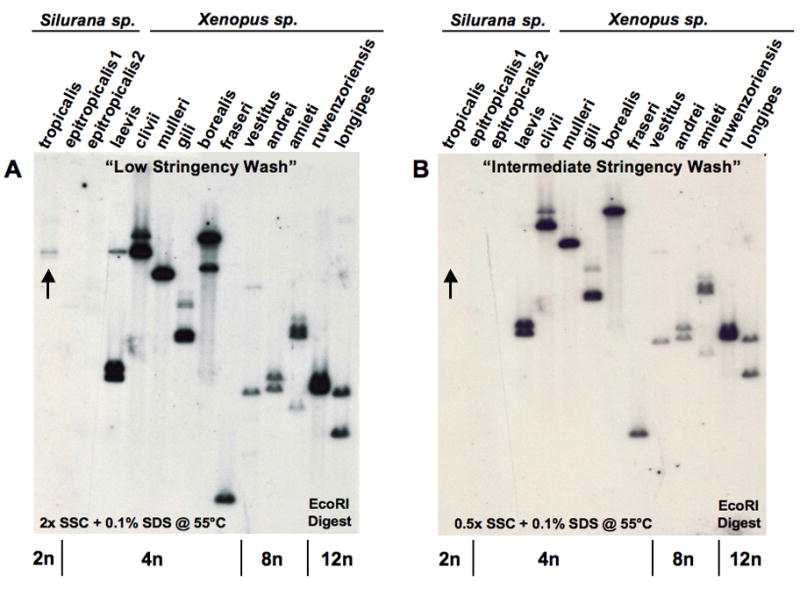
Equivalent amounts of genomic DNA (adjusted per ploidy) from a representative of each species within the genera Xenopus and Silurana digested with EcoRI, hybridized at 42°C with a 32P-labeled 264bp α1 XNC10 cDNA fragment, and washed under low (A) or intermediate (B) stringency. Arrows: cross-hybridizing band detected under low stringency conditions in S. tropicalis. Ploidy of each species is indicated at the bottom of the blot.
Overall, our analyses demonstrate that XNC10 is a very divergent XNC subfamily member with unique antigen-binding residues and structural conformation of its pPBD, which has been conserved and diploidized in all species of the genus Xenopus throughout 80 million years of evolution.
3.1. Expression pattern of XNC10 and XNC11
While Xenopus class Ia transcripts are detected in most adult tissues, the expression of XNC genes is much more restricted in terms of tissue distribution (Salter-Cid et al, 1998). Since XNC10 and XNC11 were characterized from a spontaneously arising thymic lymphoid tumor, we asked whether these subfamilies were present in normal adult thymus or other lymphoid organs. Northern analysis reveals that XNC10 is primarily expressed in the thymus and to a lesser extent in the spleen (Fig. 6A). XNC11 transcripts are also detected but to a much lower level than XNC10, and only in the thymus (Fig. S1). Interestingly, XNC10 and XNC11 are the first two class Ib molecules shown to have a lymphoid-specific pattern of expression in X. laevis adults. Since XNC11 expression in normal adult thymus is at best weak, we have focused expression analyses to XNC10.
To further determine which lymphocyte population expresses XNC10 in the spleen, splenic IgM+ B and CD8 T cells were purified by magnetic cell sorting and subjected to Northern analysis (Fig 6B). Purity of the sorted cell populations was controlled by reprobing the membrane successively with CD8β and with CD4 probes (Fig. 6B). Interestingly, a significantly higher amount of XNC10 mRNA was detected in the CD8 T cell fraction compared with CD8-depleted splenocytes, whereas specific signal was barely detectable for IgM+ B cells. These data suggest that in the periphery XNC10 is predominantly expressed by some CD8 T cells, but not by B cells.
In mammals, some class Ib (e.g., CD1d and TL) are expressed within the thymus primarily by cortical thymocytes, and interact with developing CD8 T cells to promote their differentiation [2]. Since XNC10 is expressed in the periphery by some CD8 T cells, we asked whether it could also be expressed by some thymocytes. To address this question, we sorted, by FACS, adult thymocytes based on their expression of the two surface markers we have available in Xenopus, CD5 (pan-T cell marker in Xenopus) and CD8, and determined the expression pattern of various genes by RT-PCR. A representative of two independent sorting experiments is depicted in Figure 6C. Sorted cells were gated first by their forward and side scatter profiles and then by their surface phenotype of CD5 and CD8 expression. In the absence of a Xenopus-specific CD4 antibody, putative CD4 single positive (SP) thymocytes can be distinguished by the lack of surface CD8 (CD5+CD8neg), from other CD8 expressing thymocytes (CD5+CD8+ and CD5negCD8+). This distinction is further supported by the expression pattern of CD4 and CD8β mRNA. We also monitored CTX (cortical thymocyte-specific antigen of Xenopus) expression to distinguish mature (CTXlow) vs more immature (CTXhigh) thymocytes (Chretien et al, 1996; Robert and Cohen, 1998b). As shown in Fig. 6C, XNC10 is mainly expressed by putative immature (CD5negCD8+ cells, expressing CD8β and high levels of CTX mRNA), and to a lesser extent by mature (CD5+CD8+ cells, expressing CD8β and low levels of CTX mRNA) CD8 thymocytes, but not by putative mature CD4 SP thymocytes (CD5+CD8neg). XNC10 is also expressed by the minor CD5negCD8neg cell population, but given the small number of these cells and the absence of specific markers the relevance of this observation is unclear. We have also ruled out the possibility that the residual XNC10 expression comes from contaminating red blood cells (RBCs). Northern blot analysis shows that while RBCs do express some XNC subfamily members (signal detected with an α3 probe), they do not express any XNC10 (α1 domain probe; data not shown). Therefore, for both circulating lymphocytes as well as lymphocytes within the thymus, there is a strong correlation between XNC10 and CD8β expression, which suggests an association of XNC10 with the CD8 T cell lineage.
To substantiate RT-PCR data and get a better spatial visualization of XNC10 expression we performed in situ hybridization on thymus frozen sections with digoxigenin-labeled RNA probes for XNC10, CD8β and MHC class Ia, as a positive control. Weak but significant XNC10 specific signal was detected in the thymic cortex with antisense but not sense probe (Fig. 7A, B). A similar but stronger specific staining pattern was obtained with CD8β anti-sense probe (Fig. 7C), whereas MHC class Ia message was primarily found in the medulla (Fig. 7D). The weaker intensity of XNC10 staining compared to both CD8β and MHC class Ia is not surprising since nonclassical MHC class Ib molecules, in general, tend to be expressed at a much lower level compared to their classical class Ia counterparts (Braud et al, 1999). In conclusion, Northern blotting and RT-PCR of purified thymocytes, as well as in situ hybridization on thymus frozen sections all point to a correlation between XNC10 and CD8β expression by thymocytes and splenic T cells.
Figure 7. Localization of XNC10 and CD8β expression in the thymic cortex by in situ hybridization.
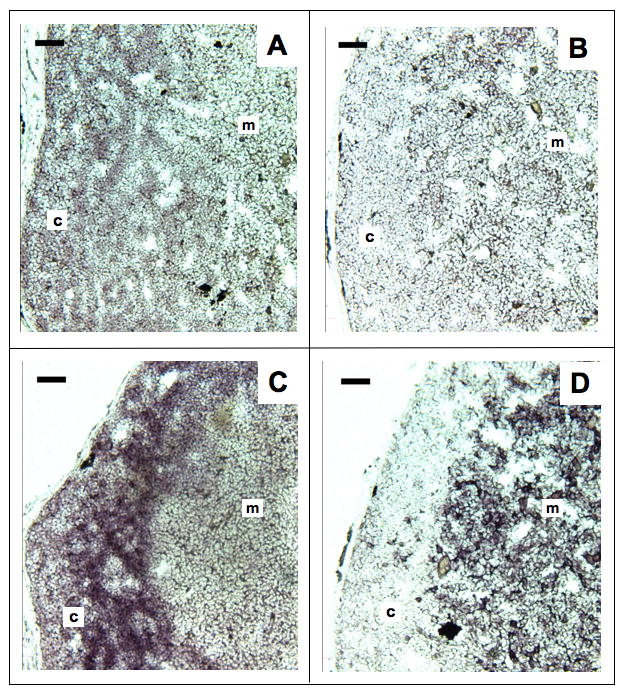
Frozen sections (8μm) of paraformaldehyde-fixed thymus from OB adult Xenopus were hybridized with XNC10 anti-sense (A), XNC10 sense control (B), CD8β anti-sense (C) and MHC class Ia anti-sense (D) DIG-conjugated riboprobes. Slides were developed with alkaline phosphatase substrate (purple color for positive staining). Scale bar = 50 μm; c, thymic cortex; m, thymic medulla.
3.3. Expression of XNC10 during early larval development
Since the 15/0 tumor presents multiple features of immature thymocytes (Robert et al, 1994), the possibility that XNC10 is expressed during early stages of T cell differentiation was investigated by monitoring its expression during early larval development. Northern blot analysis revealed that although similar predominant expression of XNC10 transcripts was found in the thymus of pre-metamorphic larvae, the amount was significantly lower when compared to adult thymus (Fig. 8A). Similar weak XNC10 specific signal was observed in the spleen irrespective of developmental stages, whereas signal was barely detectable in other tissues. Additional XNC10 specific bands were detected particularly in metamorphic stages. Whether these bands represent precursors or alternative spliced products is unknown. Moreover, in several independent experiments, XNC10 expression was detected by RT-PCR in larval thymus as early as stage 39 (3 days post-fertilization [dpf]), corresponding to the onset of thymic organogenesis before fully mature thymocytes have differentiated (Fig 8B). In addition to XNC10, we have also amplified transcripts of several other XNC subfamilies, including XNC1, XNC6 and XNC8, from larval thymus by RACE-PCR (data not shown). These data provide the first solid evidence that the thymus of Xenopus larvae expresses multiple XNC subfamily members.
Figure 8. Expression of XNC10 in larval thymus and spleen, at the onset of thymic organogenesis.
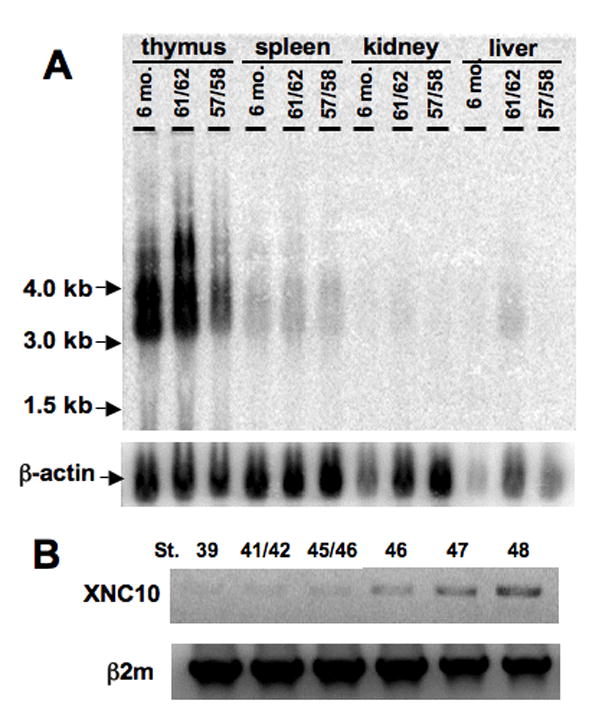
(A) Northern blot of total RNA (10μg) from tissues of pre-metarmorphic (st. 57/58), metamorphic (st. 61/62) and post-metamorphic OB X. laevis probed with a 540bp 32P-labeled XNC10 α1-α2 domain cDNA fragment. (B) RT-PCR of thymus (st. 47 and 48) or thymic anlage (st. 39, 41/42, 45/46 and 47) from early larval stages using primers specific for XNC10 and β2m as control.
To determine which larval thymocyte populations express XNC10, we sorted them, by FACS, according to their CD5 and CD8 surface expression pattern and performed RT-PCR. Figure 9A depict a representative of three independent sorting experiments. Compared to adult thymocytes, a higher fraction of larval thymocytes were CD5bright (70 versus 34%; Fig. 9A top and 6C left panel, respectively). The significance of this difference is not known. Similar to adults, XNC10 was highly expressed by immature (CD5negCD8+ cells, expressing CD8β and high levels of CTX mRNA) and more mature (CD5+CD8+ cells, expressing CD8β and low levels of CTX mRNA) CD8 thymocytes, but not by putative mature CD4 SP thymocytes (CD5+CD8neg). As in adult, the significance of XNC10 mRNA detected in the minor CD5negCD8neg population in the absence of markers remains to be determined. Therefore, in both adult and larvae, XNC10 is expressed primarily by subsets of thymocytes that co-express CD8β.
Figure 9. Expression of XNC10 by radiosensitive subsets of larval CD8+ thymocytes, but not by putative mature CD4+ thymocytes.
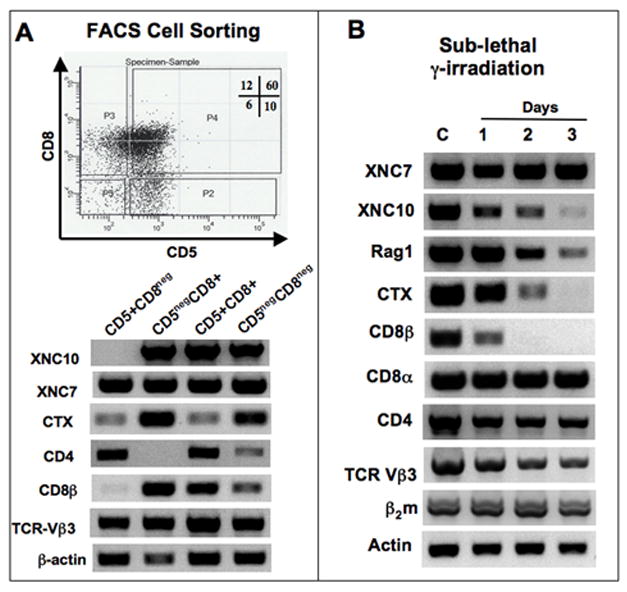
(A) RT-PCR analysis of thymocytes pooled from a population of 50 pre-metamorphic tadpoles and sorted by FACS according to their expression of CD5 and CD8 surface markers. Upper panel, dot plot of CD5 and CD8 expression with the gate used to sort each population: P2 (CD5+CD8neg), P3 (CD5 negCD8+), P4 (CD5+CD8+) and P5 (CD5negCD8neg). Cells from each population were used for RT-PCR using primers specific for XNC7, XNC10, CD4, CD8β, CTX, TCR-Vβ3, as well as β2m and β-actin as controls. (35 cycles for all genes except, CD8β: 40 cycles, β-actin: 25 cycles). (B) RT-PCR analysis of total thymocytes from pre-metamorphic tadpoles untreated, or 1 to 3 days following sub-lethal γ-irradiation (10 Gray).
To substantiate that XNC10 is expressed by larval thymocytes rather than by thymic stromal cells, we subjected larvae to sub-lethal γ-irradiation, which has been shown to mainly deplete thymocytes in Xenopus (Russ & Horton, 1987; Robert et al, 1995). As shown in a representative of two experiments in Fig. 9B, expression of some XNCs, such as XNC7, remains unchanged by sub-lethal γ-irradiation. In contrast, XNC10 is affected. Its level of expression decreases steadily from day 1 to day 3 post-irradiation, in parallel with the down regulation of Rag1 and CTX mRNA, two markers of differentiating thymocytes. Expression of TCRβ mRNA, monitored by the expression level of four different Vβ subfamilies (Fig. 9B and data not shown), is also downregulated following γ-irradiation, which further indicates that this treatment mainly targets thymocytes. Interestingly, CD8α is not affected by irradiation. This is not surprising, considering that in mammals CD8α is also expressed by other thymic cells, such as dendritic cells, which are more radio-resistant than thymocytes themselves (Vremec et al, 1992).
Collectively, these results demonstrate that while some XNCs are expressed by the thymic epithelia in Xenopus larvae, XNC10 is mainly expressed by radiosensitive subsets of immature and mature CD8+ thymocytes, but not by the thymic epithelia.
4. Discussion
In this study, we have identified, for the first time in a non-mammalian species, two sets of class Ib genes, XNC10 and XNC11, whose expression is restricted to lymphoid tissues. Whereas XNC10 expression is tightly associated with thymocyte differentiation of the CD8 T cell lineage from early in ontogeny, XNC11 expression is correlated with thymic lymphoid tumorigenesis. Although the rapid rate of evolution undergone by the class Ib gene family makes orthologous relationships difficult to establish, our findings are consistent with a conserved association of class Ib genes with T cell differentiation for more than 350 million years; the time which separates mammals and amphibians from a common ancestor. Additionally, our data demonstrate unambiguously that several XNC subfamilies, including XNC10, are expressed in larval thymus (Figs. 8, 9, & data not shown).
4.1. XNC11 as a possible tumor-associated antigen
The distinctive feature of the XNC11 subfamily worthy of interest concerns its very restricted expression pattern. Besides a low level of expression in adult thymus (Fig. S1), XNC11 is not detectable in other normal tissues. In contrast, XNC11 is highly expressed in 3 different thymic lymphoid tumor cell lines (Fig. 1). These tumor lines have been derived from 3 independent spontaneous thymic tumors that occurred in genetically distinct frogs (Robert et al, 1994). Overall, this unusual type of tumor has been observed on five separate occasions in genetically different adults in the Xenopus colony at the Basel Institute of Immunology (Du Pasquier & Robert, 1992; Robert et al, 1994; Goyos & Robert, 2008). Similar thymic tumors have also been reported by other laboratories (Earley et al, 1995). All available derived tumor cell lines (15/0, B3B7 and ff-2) display a unique immature dual T/B cell phenotype, which, in mammals, is encountered only in rare lymphocytic leukemias (Robert & Cohen, 1998a). The cell lines express specific T-cell lineage markers such as CD8 and CD5 (Du Pasquier et al, 1995), express T cell specific transcripts (CD8β, CD4) and the marker of cortical thymocytes CTX (Chretien et al, 1996). The high level of XNC11 expression is likely to constitute another common feature of this type of tumor. Whether XNC11 could represent a tumor associated antigen remains to be determined. There are several examples of class Ib genes in other species being upregulated during tumorigenesis including HLA-F, HLA-G and MIC (Lee and Geraghty, 2003; Noguchi et al, 2004; Seliger et al., 2003). It will be interesting to further determine the possible association of XNC11 with lymphoid tumors in Xenopus.
4.2. Unique phylogenetic, sequence and evolutionary characteristics of XNC10
The XNC10 subfamily also presents several unique characteristics. First, the fact that XNC10 forms an independent phylogenetic clade, intermediate between other XNCs and class Ia sequences, suggests an independent evolutionary origin of XNC10 relative to the 10 other XNC subfamilies (XNC1-9, XNC11) and implies that the encoded gene(s) might have become fixed to serve a specialized function early during Xenopus evolution. This is further supported by detection, under relatively stringent conditions, of cross-reacting sequences in several other species of the Xenopus genus. In contrast, the lack of cross-hybridizing sequences in S. tropicalis implies that the putative XNC10 ortholog in this species is missing, which is not surprising considering that S. tropicalis and X. laevis split from a common ancestor 80-100 MYA ago. Notably, lack of clear orthologous relationships among class Ib genes is not limited to Xenopus. With the exception of CD1, orthologs of class Ib genes are rare between humans and other primate species, and virtually non-existent between humans and rodents, whose most recent common ancestor dates back to 90 MYA (Adams & Parham, 2001). Therefore, the conservation of XNC10 genes in several Xenopus species over a period of more than 80 MYA is strongly suggestive of a critical function.
A second interesting feature of XNC10 concerns the primary structure of its putative peptide binding domain (pPBD). Although direct evidence that XNC10 presents peptides or other types of antigen is not yet available, the fact that it is expressed in a tumor for which CD8 T cells recognize some class Ib in an antigen dependent manner (Goyos et al, 2007), combined with the notion of its unique structural constraints within the α1 and α2 domains, suggests that it may indeed bind and present some kind of antigen. When compared to the class Ia pPBD, many of the residues critical for the interaction with a putative peptide in XNC10 are different from the other XNCs and are in fact more conserved with class Ia residues.
A third peculiarity of XNC10 is its limited degree of polymorphism. In addition to the polymorphism present in non-coding regions, sequence analysis of α1 and α2 domains indicate that a few potential alleles are maintained within the species. These alleles are not very divergent since they vary at only a few specific residues. It is noteworthy that most of the variability is confined to positions within the loops in between β-strands and the β-helices of the α1 and α2 domain. These variable residues do not correspond to the PBR characterized for class Ia molecules but that does not preclude the involvement of these positions in other functions. For example, recognition of polymorphic regions of MHC class I molecules by KIR receptors on NK cells in humans is well (Parham, 2005). It is therefore possible that the polymorphic residues within XNC10 are recognized by and interact with the products of KIR-like genes in Xenopus (Guselnikov et al, 2008).
4.3. Lymphoid tissue- and cell-specific expression pattern of XNC10
A final relevant feature of XNC10 is its lymphoid tissue-specific expression, which is mainly confined to circulating CD8 T cells and thymocytes in adult as well as larvae. In this regard, XNC10 presents some features reminiscent of the chicken CD1 ortholog (Salomonsen et al, 2005). Both Xenopus XNC10 and chicken CD1 are co-expressed by cells expressing CD8 molecules in the periphery, which include both T cells and NK cells. Furthermore, highest expression for both molecules is restricted to lymphoid tissues. Finally, there is minimal polymorphism within the coding region. CD1 has been proposed to represent a primordial type of MHC gene (Martin et al, 1986; Flajnik et al, 1991), and it is conserved among terrestrial vertebrates (Miller et al, 2005). Since the genome of X. laevis is not fully sequenced, we screened S. tropicalis genomic databases for the CD1 gene, but could not find a homolog. We also looked for the conservation of CD1 key functional motifs in XNC10, but did not find anything particular other than that which has already been noted between all XNC and CD1 α3 domains (Flajnik et al, 1993). Owing to the rapid rate of evolution and turnover undergone by class Ib genes, it is quite possible to conceive a convergent function fulfilled in different species by non-orthologous class Ib. In such a case, although XNC10 may not be a true ortholog of CD1, it may be an analogous gene, based on its lymphoid-specific pattern of expression.
4.4. Early expression of XNC10 in larval thymocytes
One intriguing problem that has not yet been fully elucidated is that although Xenopus larvae have thymus-dependent circulating mature CD8 T cells, no consistent expression, especially in the thymus, of class Ia molecules is detectable until the onset of metamorphosis (reviewed in Flajnik and Du Pasquier, 1990). Although earlier studies have not detected class Ia protein by Western or immunofluorescence microscopy, we have recently detected some expression of class Ia transcripts by RT-PCR (Goyos and Robert, unpublished). Whether, or not, a low level of class Ia protein is produced by these transcripts remains to be determined. However, it is possible that the relatively high level of XNC10 expressed in thymus, from early developmental stages, compensates for the lack or low level of class Ia expression. Therefore, it may be the case that in larvae, it is class Ib molecules that are primarily involved in regulating the differentiation of CD8 T cells. Support for this possibility comes from larval sublethal γ-irradiation experiments, which show that in the thymus XNC10 is predominantly expressed by the radiosensitive heamatopoietic population. In mice, there are several examples of thymic T cells being selected on class Ib molecules expressed by haematopoietic cells, in the absence of class Ia. For example, in bone marrow-chimeric mice, class Ib-restricted CD8 T cells are efficiently selected only when class Ib is expressed on hematopoietic cells (Urdahl et al, 2002). Additionally, our FACS cell sorting of larval thymocytes shows that XNC10 is expressed by subsets of immature and mature CD8 thymocytes. Certain class Ib proteins, such as CD1d and TL, expressed on CD4 and CD8 double positive (DP) cortical thymocytes, have been shown to be required for the selection of alternative T cell subsets, termed “innate” CD8 T cells (Berg, 2007). For example, the class Ib molecules TL and CD1d are expressed on cortical DP thymocytes and seem to be the key to the development of γδas well as NKT cells, respectively (Ladi et al, 2006). It will be interesting to explore the possibility that since space is limited in Xenopus larvae, they may preferentially generate class Ib-restricted ‘innate’ T cells, which have the intrinsic property of an activated phenotype thus requiring much less time to carry out effector functions.
Supplementary Material
Northern blot of total RNA (10μg) from 6 pooled adult OB Xenopus tissues probed with a 255bp 32P-labeled XNC11 α1 cDNA fragment. Blot was washed with 0.5x SSC + 0.1% SDS at 65°C and exposed for 21 days. The membrane was reprobed with EF-1α probe under stringent conditions for reference (lower panel).
Table 3.
Analysis of XNC10 putative antigen binding residues
| Docking | X. laevis Class Ia | XNC1-9 | XNC10 |
|---|---|---|---|
| Peptide N-terminus | |||
| Tyr7 | Tyr | 6 Tyr, 2 Phe | Tyr |
| Tyr59 | Tyr | 1 Tyr, 8 His | Asp/Asn* |
| Tyr159 | Tyr | 4 Tyr, 4 Phe | Tyr |
| Trp167 | Trp/Gly | 2 Gln, 6 His | Gln |
| Tyr171 | Tyr | 7 Tyr | His* |
| Peptide C-terminus | |||
| Tyr84 or Arg84 | Arg | 3 Tyr, 1 Arg, 3 Phe, 2 Val | Ser* |
| Thr143 | Thr | 4 Val, 3 Met, 1 Leu | Ala* |
| Lys146 | Lys | 3 Gln, 5 Leu | Lys* |
| Trp147 | Trp | 7 Trp, 1 Leu | Arg* |
Numbering based on HLA-A2; Table modified from Flajnik et al 1993
= XNC10-specific residues
Acknowledgments
We gratefully acknowledge Hristina Nedelkovska and Julie Sahler for their significant contribution in sequencing XNC10 and XNC11, and David Albright and Tina Martin for expert animal husbandry. We would also like to thank Dr. John Frelinger for critically reading the manuscript. Research was supported by NIH F31-AI068610 (A. G.), AI27877 (Y. O.), and R01-CA-108982-02, R24-AI-059830.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Ana Goyos, Email: Ana_Goyos@urmc.rochester.edu.
Yuko Ohta, Email: YOta@som.umaryland.edu.
Sergey Guselnikov, Email: sgus@bionet.nsc.ru.
Jacques Robert, Email: Jacques_Robert@urmc.rochester.edu.
References
- Adams EJ, Parham P. Species-specific evolution of MHC class I genes in the higher primates. Immunol Rev. 2001;183:41–64. doi: 10.1034/j.1600-065x.2001.1830104.x. [DOI] [PubMed] [Google Scholar]
- Berg LJ. Signaling through TEC kinases regulated conventional versus innate CD8+ T-cell development. Nat Rev Immunol. 2007;7:479–485. doi: 10.1038/nri2091. [DOI] [PubMed] [Google Scholar]
- Braud VM, Allan DJ, McMichael AJ. Functions of nonclassical MHC and non-MHC-encoded class I molecules. Curr Opin Immunol. 1999;11:100–108. doi: 10.1016/s0952-7915(99)80018-1. [DOI] [PubMed] [Google Scholar]
- Cai L, Brown DD. Expression of type II iodothyronine deiodinase marks the time that a tissue responds to thyroid hormone-induced metamorphosis in Xenopus laevis. Dev Biol. 2004;266:87–95. doi: 10.1016/j.ydbio.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Chretien I, Robert J, Marcuz A, Garcia-Sanz JA, Courtet M, Du Pasquier L. CTX, a novel molecule specifically expressed on the surface of cortical thymocytes in Xenopus. Eur J Immunol. 1996;26:780–791. doi: 10.1002/eji.1830260409. [DOI] [PubMed] [Google Scholar]
- Connolly JM, Hansen TH, Ingold AL, Potter TA. Recognition by CD8 on cytotoxic T lymphocytes is ablated by several substitutions in the class I a2 domain. Proc Natl Acad Sci. 1990;87:2137–2141. doi: 10.1073/pnas.87.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtet M, Flajnik M, Du Pasquier L. Major histocompatibility complex and immunoglobulin loci visualized by in situ hybridization on Xenopus chromosomes. Dev Comp Immunol. 2001;25:149–157. doi: 10.1016/s0145-305x(00)00045-8. [DOI] [PubMed] [Google Scholar]
- Du Pasquier L, Chardonnens X, Maggiano V. A major histocompatibility complex in the toad Xenopus laevis (Daudin) Immunogenetics. 1975;1:482. [Google Scholar]
- Du Pasquier L, Miggiano V, Kobel HR, Fischberg M. The genetic control of histocompatibility reactions in natural and laboratory-made polyploid individuals of the clawed toad Xenopus. Immunogenetics. 1977;5:129–141. [Google Scholar]
- Du Pasquier L, Courtet M, Robert J. A Xenopus lymphoid tumor cell line with complete Ig genes rearrangements and T-cell characteristics. Mol Immunol. 1995;32:583–593. doi: 10.1016/0161-5890(95)00002-v. [DOI] [PubMed] [Google Scholar]
- Flajnik MF. Doctoral dissertation. University of Rochester; Rochester, NY: 1983. The Xenopus major histocompatibility complex. [Google Scholar]
- Flajnik MF, Kaufman JF, Hsu E, Manes M, Parisot R, Du Pasquier L. Major histocompatibility complex-encoded class I molecules are absent in immunologically competent Xenopus before metamorphosis. J Immunol. 1986;137:3891–3899. [PubMed] [Google Scholar]
- Flajnik MF, Du Pasquier L. The major histocompatibility complex of frogs. Immunol Rev. 1990;113:47–63. doi: 10.1111/j.1600-065x.1990.tb00036.x. [DOI] [PubMed] [Google Scholar]
- Flajnik MF, Canel C, Kramer J, Kasahara M. Evolution of the major histocomopatibility complex: molecular cloning of major histocompatibility complex class I from the amphibian Xenopus. Proc Natl Acad Sci. 1991;88:537–541. doi: 10.1073/pnas.88.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, Kasahara M, Shum BP, Salter-Cid L, Taylor E, Du Pasquier L. A novel type of class I gene organization in vertebrates: a large family of non-MHC linked class I genes is expressed at the RNA level in the amphibian Xenopus. EMBO J. 1993;12:4385–4396. doi: 10.1002/j.1460-2075.1993.tb06123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flajnik MF, Kasahara M. Comparative Genomics of the MHC: Glimpses into the Evolution of the Adaptive Immune System. Immunity. 2001;15:351–362. doi: 10.1016/s1074-7613(01)00198-4. [DOI] [PubMed] [Google Scholar]
- Gao GF, Tormo J, Gerth UC, Wyer JR, McMichael AJ, Stuart DI, Bell JI, Jones Y, Jakobsen BD. Crystal structure of the complex between human CD8aa and HLA-A2. Nature. 1997;387:630–634. doi: 10.1038/42523. [DOI] [PubMed] [Google Scholar]
- Gleimer M, Parham P. Stress Management: MHC Class I and Class I-like Molecules as Reporters of Cellular Stress. Immunity. 2003;19:469–477. doi: 10.1016/s1074-7613(03)00272-3. [DOI] [PubMed] [Google Scholar]
- Goyos A, Cohen N, Gantress J, Robert J. Anti-tumor MHC class Ia-unrestricted CD8 T cell cytotoxicity elicited by the heat shock protein gp96. Eur J Immunol. 2004;34:2449–2458. doi: 10.1002/eji.200425105. [DOI] [PubMed] [Google Scholar]
- Goyos A, Guselnikov S, Chida AS, Sniderhan LF, Maggirwar SB, Nedelkovska H, Robert J. Involvement of nonclassical MHC class Ib molecules in heat shock protein-mediated anti-tumor responses. Eur J Immunol. 2007;37:1494–1501. doi: 10.1002/eji.200636570. [DOI] [PubMed] [Google Scholar]
- Goyos A, Robert J. Tumorigenesis and anti-tumor immune responses in Xenopus. Front Biosci. 2008 doi: 10.2741/3238. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guselnikov S, Ramanayaka T, Erilova AY, Mechetina LV, Najakshin AM, Robert J, Taranin AV. The Xenopus FcR-family demonstrates continually high diversification of paired receptors in vertebrate evolution. BMC Evo Biol. 2008;8:148. doi: 10.1186/1471-2148-8-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobel HR, Du Pasquier L. Genetics of polyploidy Xenopus. Trends Genet. 1986;2:310–315. [Google Scholar]
- Kobel HR, Du Pasquier L. Production of large clones of histocompatible, fully identical clawed toads (Xenopus) Immunogenetics. 1975;2:7–91. [Google Scholar]
- Kumar S, Tamura K, Jakobsen IB, Nei M. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics. 2001;17:1244–1245. doi: 10.1093/bioinformatics/17.12.1244. [DOI] [PubMed] [Google Scholar]
- Ladi L, Yin X, Chtanova T, Robey EA. Thymic microenvironments for T cell differentiation and selection. Nat Immunol. 2006;7:338–343. doi: 10.1038/ni1323. [DOI] [PubMed] [Google Scholar]
- Lee N, Geraghty DE. HLA-F surface expression on B cell and monocyte cell lines is partially independent from tapasin and completely independent from TAP. J Immunol. 2003;171:5264–5271. doi: 10.4049/jimmunol.171.10.5264. [DOI] [PubMed] [Google Scholar]
- Madden DR, Gorga JC, Strominger JL, Wiley DC. The structure of HLA-B27 reveals nonamer self-peptides bound in an extended conformation. Nature. 1991;353:321–325. doi: 10.1038/353321a0. [DOI] [PubMed] [Google Scholar]
- Maniero GD, Robert J. Phylogenetic conservation of gp96-mediated antigen-specific cellular immunity: new evidence from adoptive cell transfer in Xenopus. Transplantation. 2004;78:1415–1421. doi: 10.1097/01.tp.0000140846.73210.91. [DOI] [PubMed] [Google Scholar]
- Martin LH, Calabi F, Milstein C. Isolation of CD1 genes: a family of major histocomopatibility complex-related differentiation antigens. Proc Natl Acad Sci. 1986;83:9154–9158. doi: 10.1073/pnas.83.23.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura M, Fremont DH, Peterson PA, Wilson IA. Emerging principles for the recognition of peptide antigens by MHC class I molecules. Science. 1992;257:927–934. doi: 10.1126/science.1323878. [DOI] [PubMed] [Google Scholar]
- Miller MM, Wang C, Parisini E, Coletta RD, Goto RM, Lee SY, Barral DC, Townes M, Roura-Mir C, Ford HL, Brenner MB, Dascher CC. Characterization of two avian MHC-like genes reveals an ancient origin of the CD1 family. Proc Natl Acad Sci. 2005;102:8399–8400. doi: 10.1073/pnas.0500105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Isogai M, Kuwada E, Noguchi A, Goto S, Egawa K. Detection of anti-HLA-F antibodies in sera from cancer patients. Anticancer Res. 2004;24(5C):3387–3392. [PubMed] [Google Scholar]
- Ohta Y, Powis SJ, Coadwell WJ, Haliniewski DE, Liu Y, Li H, Flajnik MF. Identification and genetic mapping of Xenopus TAP2 genes. Immunogenetics. 1999;49:171–182. doi: 10.1007/s002510050478. [DOI] [PubMed] [Google Scholar]
- Parham P. The rise and fall of great class I genes. Semin Immunol. 1994;6:373–382. doi: 10.1006/smim.1994.1047. [DOI] [PubMed] [Google Scholar]
- Parham P. MHC Class I Molecules and KIRS in Human History, Health and Survival. Nat Rev Immunol. 2005;5:201– 214. doi: 10.1038/nri1570. [DOI] [PubMed] [Google Scholar]
- Rau L, Gantress J, Bell A, Stewart R, Horton T, Cohen N, Horton J, Robert J. Identification and characterization of Xenopus CD8+ T cells expressing an NK cell-associated molecule. Eur J Immunol. 2002;32:1574–1583. doi: 10.1002/1521-4141(200206)32:6<1574::AID-IMMU1574>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Robert J, Guiet C, Du Pasquier L. Lymphoid Tumors of Xenopus laevis with different capacities for growth in larvae and adults. Dev Immunol. 1994;3:297–307. doi: 10.1155/1994/37392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert J, Guiet C, Du Pasquier L. Ontogeny of the alloimmune response against a transplanted tumor in Xenopus laevis. Differentiation. 1995;59:135–144. doi: 10.1046/j.1432-0436.1995.5930135.x. [DOI] [PubMed] [Google Scholar]
- Robert J, Cohen N. Evolution of immune surveillance and tumor immunity: studies in Xenopus. Immunol Rev. 1998a;166:231–243. doi: 10.1111/j.1600-065x.1998.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Robert J, Cohen N. Ontogeny of CTX expression in Xenopus. Dev Comp Immunol. 1998b;22:605–612. doi: 10.1016/s0145-305x(98)00028-7. [DOI] [PubMed] [Google Scholar]
- Robert J, Gantress J, Rau L, Bell A, Cohen N. Minor histocompatibility antigen-specific MHC-restricted CD8 T cell responses elicited by heat shock proteins. J Immunol. 2002;168:1697–1703. doi: 10.4049/jimmunol.168.4.1697. [DOI] [PubMed] [Google Scholar]
- Russ JH, Horton JD. Cytoarchitecture of the Xenopus thymus following γ-irradtiation. Development. 1987;100:95–105. doi: 10.1242/dev.100.1.95. [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salomonsen J, Sorensen MR, Marston DA, Rogers SL, Collen T, van Hateren A, Smith AL, Beal RK, Skjodt K, Kaufman J. Two CD1 genes map to the chickem MHC, indicating that CD1 genes are ancient and likely to have been present in the primordial MHC. Proc Natl Acad Sci. 2005;102:8668–8673. doi: 10.1073/pnas.0409213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter RD, Benjamin RJ, Wesley PK, Buxton SE, Garrett TPJ, Clayberger C, Krensky AM, Norment AM, Littman DR, Parham P. A binding site for the T-cell co-receptor CD8 on the a3 domain of HLA-A2. Nature. 1990;345:41–46. doi: 10.1038/345041a0. [DOI] [PubMed] [Google Scholar]
- Salter-Cid L, Nonaka M, Flajnik MF. Expression of MHC Class Ia and Class Ib During Ontogeny: High Expression in Epithelia and Coregulation of Class Ia and lmp7 Genes. J Immunol. 1998;160:2853–2861. [PubMed] [Google Scholar]
- Saper MA, Bjorkman PJ, Wiley DC. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991;219:277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Seliger B, Abken H, Ferrone S. HLA-G and MIC expression in tumors and their role in anti-tumor immunity. Trends Immunol. 2003;24:82–87. doi: 10.1016/s1471-4906(02)00039-x. [DOI] [PubMed] [Google Scholar]
- Sun J, Leahy DJ, Kavathas PB. Interaction between CD8 and Major Histocompatibility Complex (MHC) Class I mediated by multiple contact surfaces that include the a2 and a3 domains of MHC class I. J Exp Med. 1995;182:1275–1280. doi: 10.1084/jem.182.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urdahl KB, Sun JC, Bevan MJ. Positive selection of MHC class Ib-restricted CD8+ T cells on hematopoietic cells. Nat Immunol. 2002;3:772–779. doi: 10.1038/ni814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vremec D, Zorbas M, Scollay R, Saunders DJ, Ardavin CF, Wu L, Shortman K. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992;176:47–58. doi: 10.1084/jem.176.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WM, Au DM, Lam VM, Tam JW, Cheng LY. A simplified and improved method for the efficient double-stranded sequencing of mini-prep plasmid DNA. Nucleic Acids Res. 1990;18:5573. doi: 10.1093/nar/18.18.5573. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Northern blot of total RNA (10μg) from 6 pooled adult OB Xenopus tissues probed with a 255bp 32P-labeled XNC11 α1 cDNA fragment. Blot was washed with 0.5x SSC + 0.1% SDS at 65°C and exposed for 21 days. The membrane was reprobed with EF-1α probe under stringent conditions for reference (lower panel).


