SUMMARY
CD4+CD25+FOXP3+ Treg cells require TCR engagement for suppressive function, thus ensuring that suppression only occurs in the presence of specific antigens, however, to date no studies have addressed the function of self antigen-specific Treg in humans. These studies were designed to determine whether peripheral generation and function of islet antigen-specific adaptive Treg are defective in human subjects with type 1 diabetes (T1D). Islet antigen-specific adaptive Treg were induced in vitro by activation of CD4+FOXP3− T cells with GAD and IGRP peptides in the context of T1D associated HLA-DRβ alleles. Antigen-specific Treg were characterized using flow cytometry for FOXP3 and class II tetramer (Tmr) and assessed for the ability to inhibit proliferation. These adaptive Treg were then compared to influenza-specific Treg from the same study population. The function of Tmr+ cells that expressed FOXP3 was similar for both influenza and islet antigens generated from control and T1D subjects. In fact, potency of suppression correlated with FOXP3 expression, not antigen specificity. Thus, these data suggest that development of functional adaptive Treg can occur in response to islet antigens and activation of islet-specific Treg may potentially be used as a targeted immunotherapy in T1D.
Keywords: autoimmunity, human subjects, type 1 diabetes, Treg
INTRODUCTION
CD4+CD25+FOXP3+ T regulatory cells are vital for immune regulation. The lack of Treg results in severe autoimmunity in both mouse and man [1–3]. The importance of Treg in type 1 diabetes (T1D) has been demonstrated in murine models by the acceleration of disease upon depletion of Treg as well as the cure of diabetes by transfer of Treg to animals prior to and after onset of disease [4–7]. Further, adoptive transfer is more effective when the transferred Treg are specific for an islet antigen [8]. In humans, defects in polyclonal Treg have been proposed as one mechanism by which individuals develop T1D and this defect appears to be in the function [9–11], as compared to the number of Treg [12]. The specificity of Treg in animal models and the role of Treg in regulating disease raises the possibility that islet-specific Treg are inadequate in number or function in individuals with T1D.
Although Treg were initially thought to originate only from the thymus [13], subsequent studies in mouse and man have shown that CD4+CD25+FOXP3+ Treg can develop in the periphery under a variety of conditions and these cells are referred to as adaptive Treg [14,15]. Other types of adaptive Treg have been described including Tr1 and Th3 cells, however, these adaptive Treg are not associated with FOXP3 expression. For this study we focus on CD4+CD25+FOXP3+ adaptive Treg and use the term adaptive Treg to refer to these cells in this manuscript. Adoptive transfer of polyclonal adaptive Treg can prevent development of and treat autoimmunity as evidenced in several animal models [16–18]. Moreover, these adoptively transferred murine adaptive Treg are long-lived and maintain FoxP3 expression suggesting that adaptive Treg may be used as immunotherapeutic agents to treat autoimmune and inflammatory disease.
The Treg of thymic origin are termed natural Treg and in mice are known to react with self antigens [19]. Similarly, in mice induction of adaptive self antigen-specific Treg may occur in vivo in the periphery under appropriate conditions and these antigen-specific adaptive Treg protect mice from developing autoimmunity [20,21]. In humans, induction of regulatory T cell responses has been observed in vivo where highly differentiated human CD4+CD45RO+CD25hiFOXP3+ T cells arose upon foreign antigen exposure [22]. Thus far, induction of adaptive self antigen-specific Treg has not been studied in humans. It is well established that self reactive T cells reside in the peripheral blood of healthy individuals [23,24] yet the cellular and molecular response to self antigen activation in these populations is less well understood, particularly due to the possibility that this population contains low affinity T cells [25,26]. Thus, it is important to address whether induction of a population of human self-reactive adaptive Treg occurs in humans in order to determine whether adaptive Treg may participate in regulation of autoreactivity in vivo, whether adaptive Treg are defective in individuals with autoimmunity and whether self antigen-specific adaptive Treg may be used as a therapeutic agent.
To date, neither the function of Treg specific to islet antigens nor the ability to generate self antigen-specific adaptive Treg has been examined in individuals with T1D. In these studies we measured induction of adaptive antigen-specific Treg by comparing the number and function of Treg generated following stimulation with well defined viral antigens and the representative islet autoantigens GAD and IGRP. Antigen-specific T cells were identified by use of HLA class II tetramers (Tmr) and regulatory cells were identified by use of intracellular FOXP3 staining and functional assays. We establish that Treg specific for the islet antigens, IGRP and GAD, can be induced from peripheral CD4+CD25−FOXP3− T cells of healthy individuals and that these self antigen-specific adaptive Treg function in a similar manner as those specific to the foreign antigen, influenza. When these same studies were performed with T1D subjects, we found a similar capacity to generate functional influenza and islet-specific adaptive Treg and that irrespective of the disease state, potency of suppression correlated with FOXP3 expression.
RESULTS
Islet-Specific CD4+CD25+FOXP3+ Adaptive Treg can be generated from CD4+CD25− T cells of Healthy Controls
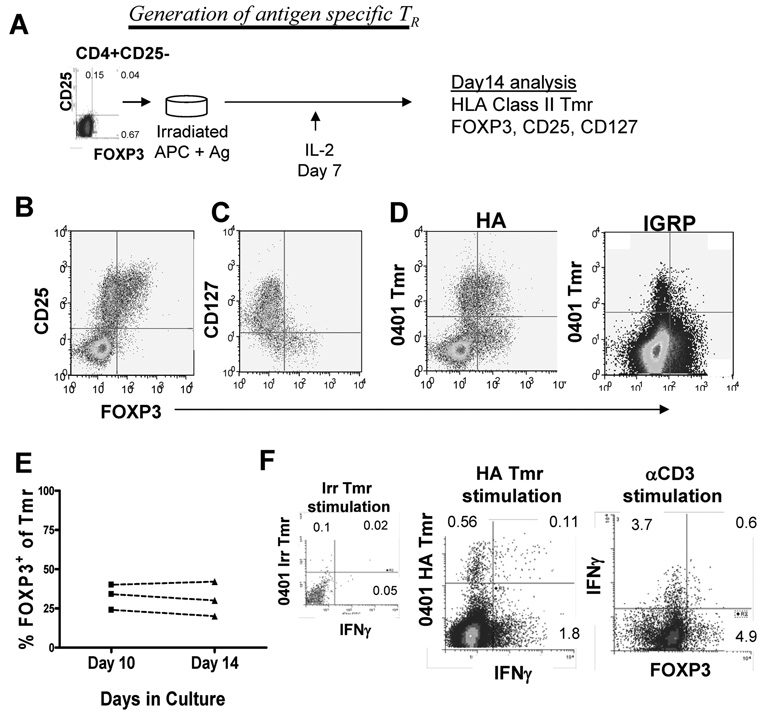
To determine whether adaptive Treg specific to self antigens can be generated in the periphery in humans, we compared generation of foreign antigen-specific influenza reactive Treg to that of islet-specific Treg in a group of healthy subjects. We examined specificity in the context of three T1D associated HLA class II alleles, HLA-DRβ1*0401, HLA-DRβ1*0404, and HLA-DRβ1*0301 [27]. A set of islet and influenza peptides was used to probe TCR specificity in the HLA class II Tmr assays based on HLA type of the subjects (Supporting Information Table 1). These peptides have been previously identified as important epitopes in healthy and T1D subjects [28–31]. Low affinity, self antigen-specific T cells are not found at a high enough frequency to be detected directly in peripheral blood using HLA class II Tmr. Since detection of CD4+ antigen-specific T cells by Tmr and induction of FOXP3 expression both require in vitro activation, we used an in vitro induction protocol for these studies (Figure 1A). CD4+CD25− T cells were isolated from peripheral blood and then activated with irradiated APC plus peptide in media containing human serum. Cells were kept in culture, receiving IL-2 (200 IU/ml) on day 7, but were otherwise left undisturbed. After 14 days, the CD4+ T cells were characterized for expression of FOXP3 and the ability to bind an HLA class II Tmr complexed to the peptide antigen with which these cells were activated. In these cultures, the FOXP3+ cells were present in the CD25brightCD127lo T cell population (Figure 1B and C). Among the Tmr+ populations both FOXP3+ and FOXP3− cells were observed (Figure 1D). FOXP3 expression was also found among a subset of Tmr negative cells. Previous studies have shown that this population of cells, Tmr−CD25+ cells, are not specific for the antigen used to stimulate the culture [32].
Figure 1. Antigen-specific CD25+FOXP3+ adaptive Treg can be generated from CD4+CD25− T cells of control subjects.
(A) Peripheral blood CD4+CD25− T cells were selected and activated with irradiated APC and 5 µg/ml peptide antigen. Fourteen days following activation, cells were assayed by flow cytometry for expression of CD25, CD127 and FOXP3 and binding of HLA class II Tmr as shown in a schematic. Representative data for a control subject sample assessed on day 14 for (B) CD25, (C) CD127 and (D) influenza and islet Tmr with respect to FOXP3 expression is shown. (E) Three representative samples were stained for Tmr and FOXP3 on day 10 and day 14 of culture. (F) Peripheral blood CD4+CD25− T cells from a control HLA-DRB1*0401 subject cultured with HA peptide for 14 days and were then re-stimulated with 0401-irrelevant or HA Tmr for 16 hours or with 5 µg/ml soluble αCD3 and irradiated APC for 6 hours in the presence of 1 nM BrefeldinA. IFN-γ secretion and FOXP3 expression were assessed using intracellular flow cytometry as described in Materials and methods. One representative sample of three is shown.
To confirm that Tmr+ adaptive Treg detected in our induction cultures arose from a CD25−FOXP3− population, two distinct populations of CD4+ T cells defined by CD25 and CD127 expression levels were sorted and activated with peptide antigen. These populations included CD25−CD127hi CD4+ T cells and CD25+CD127lo CD4+ T cells which are enriched for FOXP3 expression. Following selection and activation with HA or GAD peptide, FOXP3 expression was induced in the CD25− T cell populations regardless of the antigenic stimulation (Supporting Information Figure 1). Consistent with the observation that FOXP3+ T cells are relatively anergic, T cells in the CD25+CD127lo population did not proliferate enough in response to peptide stimulation to detect HA or GAD-specific T cells by Tmr staining on day14 but these cultures did contain a high frequency of FOXP3+ T cells. CD25hi T cells sorted at the end of culture of CD25−CD127hiCD4+ T cells and CD25+CD127lo CD4+ T cells were both capable of suppressing proliferation of autologous T cells (Supporting Information Figure 1).
Persistent expression of FOXP3 is associated with regulatory function [33] while transient expression of FOXP3 is associated with activation as demonstrated by co-expression of IFN-γ [34,35]. To determine whether we were measuring persistent expression of FOXP3, we analyzed the expression of FOXP3 over time. Between day 10 and day 14 of our induction culture, the frequency of FOXP3 within the Tmr+ population was constant (Figure 1E). We also found that although a subpopulation of influenza-specific Tmr+ cells secreted IFN-γ when re-stimulated with HLA matched Tmr or anti CD3 antibody, the FOXP3+ cells did not secrete IFN-γ (Figure 1F). These data suggest that FOXP3 is a marker of these adaptive Treg using this culture method.
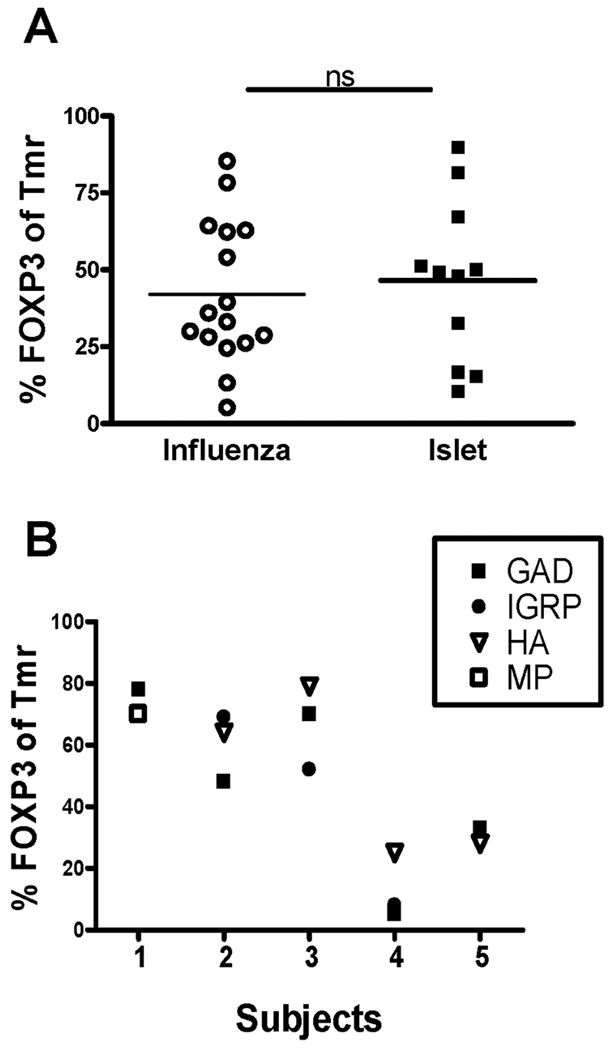
Using this induction culture, we first compared the percentage of Tmr+ cells which co-expressed FOXP3 following in vitro activation with the influenza and islet peptides. Tmr+ cells ranged in frequency from 0.39 – 6.63 (median 1.26). When analyzing induction of antigen-specific Tmr+FOXP3+ cells, we found wide variation in the percentage of FOXP3+ cells within the influenza and islet-specific Tmr+ populations but no difference between each antigen type studied (Figure 2A). Variation in these cultures may be due to differences in the frequency of activated cells, however, we found no correlation between the frequency of Tmr+ cells and the percentage of FOXP3+ cells (data not shown). Alternatively, variation in these induction cultures may be due to differences between individuals. Thus, we compared the frequency of FOXP3+ cells in influenza and islet-specific T cells in individuals who reproducibly have Tmr+ T cells to both antigen types. As shown in Figure 2B, the frequency of FOXP3+ cells within the Tmr+ populations were similar for both antigen types and, in each individual tested, variation was associated with different individuals not the antigen used for stimulation. Taken together, our data demonstrate that antigen-specific Tmr+ cells that persistently express FOXP3 can be generated from CD25−FOXP3− T cells of control subjects in response to antigenic stimulation by either foreign or self peptides.
Figure 2. Similar frequencies of adaptive influenza and islet-specific Treg can be generated from healthy controls.
(A) Peripheral blood CD4+CD25− T cells were isolated from control subjects and activated with either influenza- (○, open circle) or islet-specific (▪, closed square) peptide as defined in Table 1. Fourteen days following activation, the percentage of Tmr+ T cells that express FOXP3 was assessed by flow cytometry. Statistical significance was analyzed using an independent students t-test. (B) The percentage FOXP3 of Tmr+ cells for different Tmr specificities is shown for 5 control subjects. Solid symbols are islet-specific and open symbols are influenza-specific peptides. Control subjects 1 and 5 are HLA-DRβ1*0404 and control subjects 2–4 are HLA-DRB1*0401.
Potency of Influenza and Islet-Specific CD4+CD25+FOXP3+ Adaptive Treg are Similar in Healthy Controls
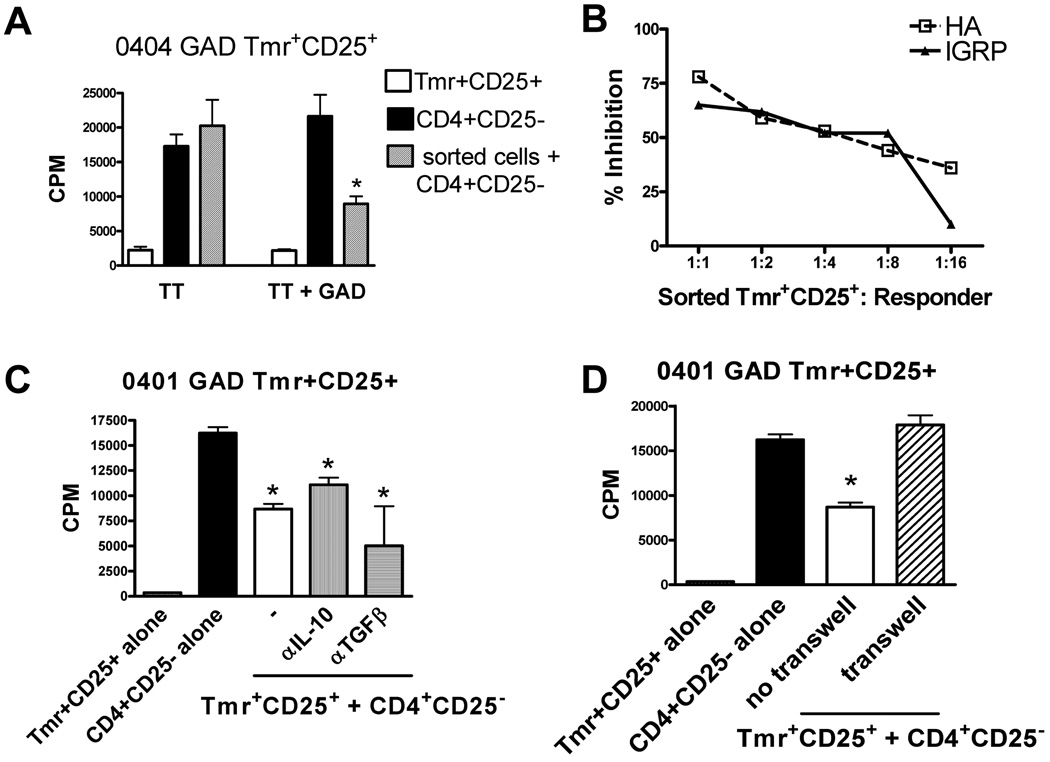
We have previously shown that HLA 0401 HA TMR+CD25+ T cells isolated from induction cultures are suppressive [36]. These cells require antigen specific activation, but are capable of suppressing bystander proliferation. We utilized the same assay to address the question of whether adaptive Treg specific for self antigen are also capable of suppressing CD4+ T cell proliferation. Islet-specific Tmr+CD25+ T cells were isolated from induction cultures on day 14, co-incubated with autologous CD4+CD25− T cells and activated with tetanus toxoid (TT) alone or in combination with the antigen for which the CD25+Tmr+ cells were specific. These sorted GAD and IGRP Tmr+CD25+ cells were capable of suppressing CD4+CD25− T cell proliferation in an antigen specific manner. This is evidenced by the fact that proliferation of CD4+CD25− T cells was not suppressed when cultures containing CD4+CD25− and sorted cells were stimulated with TT alone, but when activated with GAD or IGRP and TT, sorted Tmr+CD25+ T cells were capable of suppressing TT-specific proliferation of CD4+CD25− T cells in a bystander manner (Figure 3A). To measure the relative potency of islet-specific Treg we utilized foreign and self antigen-specific adaptive Treg generated from the same individual. The potency of suppression was similar for both antigen specificities over a range of CD25+Tmr+ and CD4+CD25− T cell ratios (Figure 3B). We used HLA 0401 GAD Tmr+CD25+ sorted T cells to determine whether islet-specific T cells require cytokine or cell contact for function. Several mechanisms of suppression have been described for FOXP3+ nTreg, adaptive Tr1 and Th3 cells, and adaptive FOXP3+ Treg in vitro including secretion of suppressive cytokines. Like HA-specific adaptive Treg [36], GAD-specific adaptive Treg suppress proliferation in an IL-10 and TGF-β independent (Figure 3C) and contact dependent manner (Figure 3D) when tested in vitro. Thus, influenza and islet antigen-specific Tmr+FOXP3+ adaptive Treg generated from healthy controls display similar potency.
Figure 3. Adaptive influenza and islet-specific Treg generated from healthy controls display similar potency.
(A) Fourteen days following activation with irradiated APC and peptide, CD4+CD25+Tmr+ cells were sorted and assayed for specificity and suppressive activity as described in Materials and methods. Specificity and suppressive activity of one representative of ten islet antigen-specific Treg generated from control subjects is shown. (B) Peripheral blood CD4+CD25− T cells were selected and activated in parallel with HLA matched IGRP or HA peptides. Fourteen days following activation, HA- and IGRP-specific CD4+CD25+Tmr+ cells were sorted and assayed for specificity and suppressive activity as described in Material and methods. HA (□, open squares) and IGRP (▴, closed triangle) reactivity of one representative HLA-DRβ*0401 control subject of four is shown. Potency of specific Treg was measured by calculating the percent inhibition of proliferation with Tmr+CD25+ sorted cells added at different ratios in the presence of TT+Ag. (C) Mechanism of function was assessed by addition of neutralizing anti-IL-10 antibody, neutralizing anti-TGF-β antibody, and (D) culture in a transwell. One representative control sample of 3 is shown. *p<0.05 from CD4+CD25− cells alone in A, C and D as determined by an independent student’s t-test.
Function of Islet-specific Adaptive Treg are Similar to Influenza-specific Adaptive Treg in T1D subjects
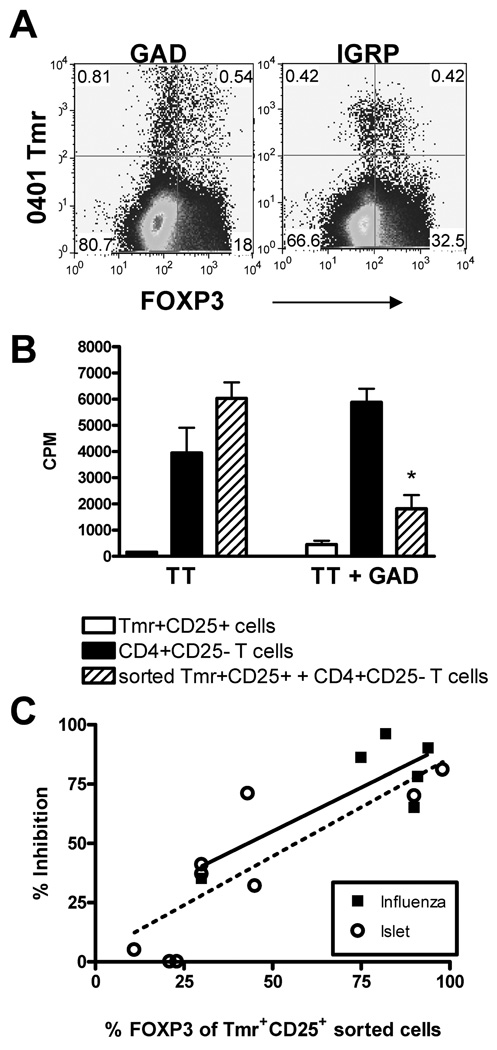
To address the question of whether islet-specific Treg are capable of being generated in T1D subjects, we activated CD4+CD25− T cells with islet-specific peptide as described in Figure 1A and found that FOXP3 expression could be induced in GAD-and IGRP-specific Tmr+ populations generated from CD4+CD25− T cells of T1D subjects (Figure 4A). This indicates that a unique defect in the induction of islet-specific FOXP3+ cells was not present in subjects with T1D.
Figure 4. Frequency and function of adaptive influenza and islet-specific Treg generated from T1D subjects do not differ.
(A) Peripheral blood CD4+CD25− T cells were isolated from T1D subjects and activated and stained as described in Figure 1A. Flow cytometric plots shown were derived from data gated on live CD4+ cells. Quadrants were drawn based on stains with HLA matched Tmr loaded with irrelevant peptide and stains with FOXP3 isotype control with specific Tmr. One representative stain for HLA-DRβ 0401 GAD and IGRP is shown. (B) Fourteen days following activation, CD4+CD25+Tmr+ cells were sorted and assayed for specificity and suppressive activity as performed in Figure 3A. Specificity and suppressive activity of one representative sample of nine islet antigen-specific Treg generated from T1D subjects is shown. *p<0.05 from CD4+CD25− cells alone as determined by an independent student’s t-test (C) Potency of the function of influenza- (▪, closed squares, n=6) and islet-specific (○, open circle, n=9) adaptive Treg generated from CD4+CD25− T cells of T1D subjects was compared using a nested linear model. Antigen specificity of the sorted Tmr+ population did not significantly influence the relationship between percent FOXP3 and percent inhibition.
We next evaluated the function of islet-specific adaptive Treg generated from T1D subjects. We found that both GAD and IGRP Tmr+CD25+ T cells generated from T1D subjects were capable of suppressing CD4+CD25− T cell proliferation in response to TT in a by-stander manner when the autoantigen was present but not with TT stimulation alone (Figure 4B). To evaluate whether the function of islet-specific adaptive Treg is impaired in T1D, we compared the level of suppression conferred by adaptive Treg specific for influenza and islet antigens. As shown with the healthy controls (Figure 2B), we noted a broad range in the number of FOXP3+ cells in the CD25+Tmr+ population. Based on this observation and previous work in our lab that demonstrates a strong correlation between the percentage of FOXP3+ cells and suppression [37], we analyzed suppression with respect to the percentage of FOXP3+ cells among the sorted Tmr+CD25+ population. Regardless of antigen specificity, we found a strong linear, and statistically significant (p<0.0001) relationship between the percent FOXP3+ cells and the degree of suppression. Including antigen specificity in the multivariant analysis did not significantly influence this relationship suggesting that the function of islet-specific adaptive Treg do not differ from influenza-specific adaptive Treg generated from T1D subjects (Figure 4C). Thus, we conclude that a specific defect in the induction or function of islet-specific adaptive Treg is not present in T1D subjects under these culture conditions.
Function of Influenza and Islet Antigen-Specific Adaptive Treg do not differ between Control and T1D Subjects
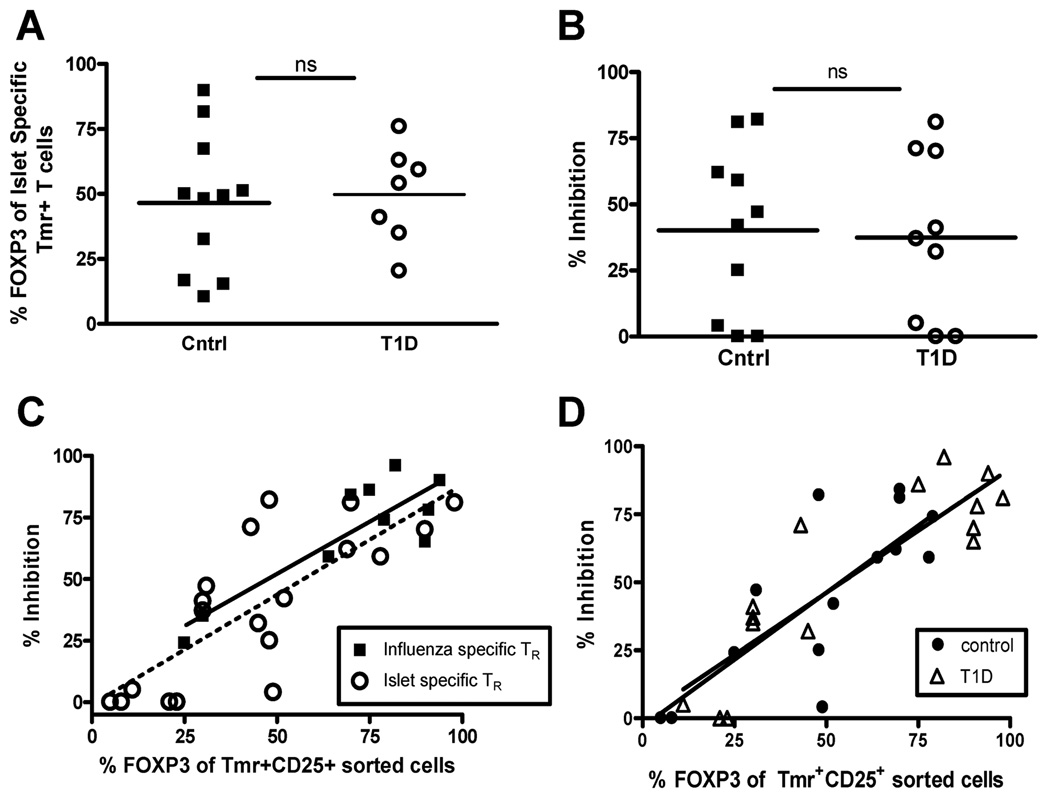
To determine whether self antigen-specific Tmr+ adaptive Treg are defective in T1D subjects, we directly compared the frequency and function of islet-specific adaptive Treg generated from CD4+CD25− T cells of control and T1D subjects and found no differences (Figure 5A and B). However, we did observe large variability in the frequency of FOXP3 in the Tmr+ population, so we performed further analysis taking the frequency of FOXP3 in the sorted Tmr+ population into account. For these further analyses, we compared the potency of suppression of foreign and self antigen-specific adaptive Treg generated from T1D and control subjects, using nested linear models. As seen in Figure 4C, we found a strong linear, and statistically significant (p<0.0001) relationship between the percent FOXP3+ cells and the percent of Tmr+CD25+ sorted cells with an associated R2 value of 0.0696. We determined that including disease status and antigen specificity did not significantly improve model fit (p = 0.5882, F test). Specifically, neither antigen specificity (Figure 5C) nor disease state (Figure 5D) significantly altered the model fit. These data indicate that islet-specific Treg can be induced from CD4+CD25− T cells of subjects with T1D and the function of these cells appear to be similar to that seen in healthy subjects.
Figure 5. FOXP3 expression in antigen-specific adaptive Treg correlates with function in both control and T1D subjects.
(A) Peripheral blood CD4+CD25− T cells were isolated from control (▪, closed square) and T1D (○, open circle) subjects and activated with islet antigen-specific peptides. Fourteen days following activation, flow cytometry was performed to determine the percentage of Tmr+ T cells that express FOXP3. Statistical significance was analyzed using an independent students t-test. (B) Fourteen days following activation of CD4+CD25− T cells of controls subjects, CD4+CD25+Tmr+ cells were sorted and assayed for specificity and suppressive activity as performed in Figure 3A. Statistical significance was analyzed using an independent students t-test. (C) Potency of influenza- (▪, closed squares) and islet-specific (○, open circles) Treg was assessed using a nested linear model. Antigen specificity did not significantly influence the relationship between percent FOXP3 and percent inhibition. (D) Potency of antigen-specific adaptive Treg in controls (●, closed circles) versus T1D (Δ, open triangle) subjects was assessed by combining influenza and islet antigen-specific responses for each study population. Disease status did not significantly influence the relationship between percent FOXP3 and percent inhibition.
DISCUSSION
Determining the role played by Treg in the development and progression of disease as well as the therapeutic potential of Treg are key unresolved questions in T1D. Previous studies of Treg in T1D subjects have focused on the number and function of the polyclonal CD4+CD25bright Treg found in peripheral blood [9–12]. The issue of antigen specificity has not been addressed – particularly the question of whether islet-specific adaptive Treg are impaired in T1D subjects. Here, we establish that CD4+CD25+FOXP3+ Treg can be generated in response to GAD- and IGRP islet-specific self antigens in the context of three different HLA class II molecules. The potency of islet-specific Treg is similar to that of foreign antigen-specific adaptive Treg, and do not differ between controls and T1D subjects. Thus, CD4+CD25−FOXP3− T cells of individuals with T1D have the capacity to become functional islet-specific adaptive Treg. Although the in vitro studies described in this paper cannot determine whether there is an impairment of islet-specific adaptive Treg generation in vivo, the ability to induce functional GAD- and IGRP-specific Treg in vitro does raise the possibility that enhancement of this process may be feasible for therapeutic purposes.
Our data provides the first evidence that functional islet antigen-specific CD25+FOXP3+ adaptive Treg can be generated from human peripheral CD4+CD25− T cells. Studies in mice have suggested that specificity to self antigens is a unique characteristic of natural Treg [8]. Moreover, natural Treg isolated directly from the blood are thought to be high avidity T cells that have escaped negative selection in the thymus [38]. In this paradigm, the peripheral generation of regulatory T cells would regulate immune responses to high affinity foreign antigens, not low affinity self antigens. In humans, although it is well established that self reactive T cells reside in the peripheral blood of healthy individuals [23,24] there are distinct differences between T cells specific for self as compared to foreign antigens, particularly in TCR affinity [25,26]. Despite these differences in affinity, we demonstrate that GAD- and IGRP-specific adaptive Treg can arise from the same CD4+CD25− population as seen with adaptive Treg specific to higher affinity influenza antigens. Moreover, the level of expression of Tmr, as measured by mean fluorescence intensity, was similar in the FOXP3+ and FOXP3− populations regardless of the antigen used to stimulate the culture (data not shown). These findings are consistent with the recent observation in mice that the TCR repertoire of FOXP3+ and FOXP3− T cells is overlapping and includes T cells specific for both foreign and self antigens [39].
We find that expression of FOXP3 in the antigen-specific adaptive Treg population correlates with suppression. Likewise, there is a direct link between stable FOXP3 expression with suppressive function [1,33,40,41]. However, for peripherally generated Treg, correlation of FOXP3 expression and function remains controversial. Using non-specific activation, others have observed that induction of FOXP3 is transient and depends on the level of activation [33–35,41]. Suppressive function depends on the frequency of cells expressing FOXP3 and the assay used to measure inhibition. We find that stimulation of CD4+CD25− T cells with influenza or islet antigen induces a subpopulation of cells with stable, persistent high expression levels of FOXP3 following long-term culture (14 days) which contains functionally active Treg. The persistent FOXP3 expression and lack of correlation with IFN-γ secretion as well as the suppressive function of these cells in vitro indicate that FOXP3 is a marker for Tmr+CD25hi adaptive Treg in our culture system. In fact, we show here that the frequency of Tmr+ T cells expressing FOXP3 directly correlates with suppressive potency regardless of the specificity of the adaptive Treg.
Unlike several studies which found the function of Treg isolated from T1D subjects to be impaired [9–11], we found that adaptive GAD- and IGRP-specific Treg generated from CD4+FOXP3− T cells of T1D subjects and controls function comparably. The in vitro assays used in this paper differ from those used in analysis of T1D polyclonal Treg in that APC and peptide are used to stimulate CD4+CD25− T cells and Treg, not anti-CD3 antibodies. It has previously been suggested that differences in signal strength alone contribute to differences in functional studies of Treg isolated from T1D subjects [10]. Antigenic stimulation likely results in a lower level of TCR stimulation than anti CD3 antibody stimulation. Alternatively, natural Treg isolated from T1D subjects may differ from adaptive Treg in their source and maturation, resulting in functional differences [42].
The efficacy of preventing or ameliorating autoimmunity through adoptive cellular therapy using ex vivo activated and expanded Treg has been well demonstrated in mice not only with natural Treg, [7,8,43] but also with adaptive Treg [16–18]. Several challenges remain in translating these findings into human therapies including the frequency and availability of antigen-specific T cells. Here, we demonstrate that fully functional islet-specific Tmr+FOXP3+ cells can be induced from human peripheral blood T cells. While challenges still remain in identifying and isolating pure populations of islet-specific FOXP3+ adaptive Treg, these data provide conceptual support for the potential development of a strategy for producing clinically useful numbers of functional self antigen-specific FOXP3+ T cells.
MATERIALS AND METHODS
Human Subjects
PBMC were derived from subjects (n=27) participating in studies under the auspices of the BRI-JDRF Center for Translational Research registry. Informed consent was obtained from all subjects according to IRB approved protocols at Benaroya Research Institute and Children’s Hospital and Regional Medical Center, Seattle. Control participants (n=19) were selected based on lack of personal or family history of autoimmunity or asthma. All subjects included in this study demonstrated a Tmr positive response to at least one of the peptides used in these studies (Supporting Information Table 1).
Antibodies and reagents
Antibodies used included FITC CD25, PE CD25 and CD127, PerCP CD4, and APC CD4 and CD25 (BD Pharmingen), AlexaFlour® 647 FOXP3 (clone 259D) and matching isotype control (BioLegend), purified anti-CD3 (UCHT1) and anti-CD28 (CD28.2)(BD Bioscience).
HLA-DRβ1*0401 Tmr loaded with influenza hemagglutinin antigen - HA (306–318), islet-specific glutamic acid decarboxylase - GAD (555–567I), or glucose-6-phosphate catalytic subunit-related protein - IGRP (247–259) peptides, HLA-DRβ1*0404 Tmr loaded with influenza matrix protein - MP (60–73) or GAD (555–567) peptides, and HLA-DRβ1*0301 Tmr loaded with influenza non-structural protein – NS-1 (32–45) or IGRP (226–238) peptides were produced as described previously [24,32].
Cell preparation and phenotyping
Fresh PBMC were prepared by centrifugation over Ficoll-Hyplaque gradients. CD4+ T cells were purified with a CD4+ no-touch T cell isolation kit (Miltenyi) followed by negative selection with Miltenyi CD25 microbeads. Antigen presenting cells (APC) were obtained from the positive fraction of the CD4+ no-touch selection. FOXP3 expression in CD4+CD25− cells was 0.1–1.2%. For some experiments, enrichment was performed by sorting based on CD25 and CD127 expression.
CD4+CD25− T cells were activated with peptide in the presence of irradiated (5000 rad) APC at a 1:2 ratio with 6 × 106 total cells/well in a 24 well plate. IL-2 (Chiron) (200 IU/ml) was added at day 7. Cells were cultured for 14 days and stained for expression of Tmr as described previously [32]. CD25 and FOXP3 staining was performed as per manufacturer’s instructions (BioLegend). When assaying co-expression of FOXP3 and IFN-γ secretion, cells were activated overnight with matching Tmr or irrelevant Tmr as a control. For non-specific activation cells were stimulated for 6 hours with 5µg/ml αCD3 plus irradiated APC in the presence of 1nM Brefeldin A and stained using the BioLegend FOXP3 staining protocol. FOXP3 isotype in conjunction with CD25 and CD127 expression on activated T cells were used as FOXP3 staining controls. Tmr staining controls utilized Tmr of the matched HLA class II type bound to an irrelevant peptide. Positive Tmr staining was determined to be responses at least 4-fold greater than control Tmr stains. An example of these staining controls in seen in Supporting Information Figure 2.
Functional Assays
Sorted Tmr+CD25hi cells (2.5 × 104), thawed CD4+CD25− cells (2.5 × 104) isolated from autologous PBMC, or both were incubated with irradiated APC (2.5 × 104), tetanus toxin (TT) and 5 µg/ml peptide antigen in a 96-well round-bottom plate as described previously [36]. 1 µCi 3H thymidine was added during the final 16 hrs of a 6–7 day assay and proliferation was measured by a scintillation counter. Cytokine blocking experiments were performed by adding 10 µg/ml anti IL-10 or TGF-β to cultures in a 96-well plate. For transwell experiments, cells were cultured with or without a 4 µm transwell separating CD4+CD25+ (2.5 × 105 cells/ well) cells from CD4+CD25− (2.5 × 105 / well) in a 48 well plate. All culture conditions were performed in triplicate.
Statistics
For analysis of experiments comparing a single variable, statistical significance was analyzed using an independent students t-test, unless otherwise noted, requiring a p value of <0.05 for the data to be significantly different. For experiments involving multiple variables comparing percentage FOXP3 of the sorted Tmr+ population and percentage suppression, regression analysis was performed and statistical significance between experimental groups was determined by measuring the improvement in goodness of fit, as defined by the residual sum of squares across nested linear models using F-tests.
Supplementary Material
ACKNOWLEDGEMENTS
The authors would like to thank K. Arugamanathan for cell sorting. We wish to acknowledge the investigators and staff of the BRI-JDRF Center for Translational Research at Children’s Hospital and Regional Medical Center, Seattle and the BRI Diabetes Clinical Research Program for subject recruitment as well as the BRI Translational Research Clinical Core for sample processing and handling. This work was supported by grants from the NIH (DK07245), JDRF (The Center for Translational Research at BRI), NCRR (M01-RR-00037) and NCRR (1UL1RR025014).
Abbreviations
- T1D
Type 1 Diabetes
- GAD
Glutamic acid decarboxylase
- IGRP
islet-specific glucose-6-phosphate catalytic subunit-related protein
- HA
Hemagglutinin
- NS-1
non-structural protein
- MP
matrix protein
- Tmr
Tetramer
- TT
Tetanus Toxoid
Footnotes
Conflict of interest: The authors declare no financial or commercial conflict of interest.
References
- 1.Bacchetta R, Passerini L, Gambineri E, Dai M, Allan SE, Perroni L, Dagna-Bricarelli F, Sartirana C, Matthes-Martin S, Lawitschka A, Azzari C, Ziegler SF, Levings MK, Roncarolo MG. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J.Clin.Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity. 2005;22:329–341. doi: 10.1016/j.immuni.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Lan RY, Ansari AA, Lian ZX, Gershwin ME. Regulatory T cells: development, function and role in autoimmunity. Autoimmun.Rev. 2005;4:351–363. doi: 10.1016/j.autrev.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone JA, Tang Q. Therapeutic vaccination using CD4+CD25+ antigen-specific regulatory T cells. Proc.Natl.Acad.Sci.U.S.A. 2004;101 Suppl 2:14622–14626. doi: 10.1073/pnas.0405234101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lundsgaard D, Holm TL, Hornum L, Markholst H. In vivo control of diabetogenic T-cells by regulatory CD4+CD25+ T-cells expressing Foxp3. Diabetes. 2005;54:1040–1047. doi: 10.2337/diabetes.54.4.1040. [DOI] [PubMed] [Google Scholar]
- 6.Tarbell KV, Yamazaki S, Olson K, Toy P, Steinman RM. CD25+ CD4+ T Cells, Expanded with Dendritic Cells Presenting a Single Autoantigenic Peptide, Suppress Autoimmune Diabetes. J.Exp.Med. 2004;199:1467–1477. doi: 10.1084/jem.20040180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tarbell KV, Petit L, Zuo X, Toy P, Luo X, Mqadmi A, Yang H, Suthanthiran M, Mojsov S, Steinman RM. Dendritic cell-expanded, islet-specific CD4+ CD25+ CD62L+ regulatory T cells restore normoglycemia in diabetic NOD mice. J.Exp.Med. 2007;204:191–201. doi: 10.1084/jem.20061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In Vitro-expanded Antigen-specific Regulatory T Cells Suppress Autoimmune Diabetes. J.Exp.Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brusko TM, Wasserfall CH, Clare-Salzler MJ, Schatz DA, Atkinson MA. Functional defects and the influence of age on the frequency of CD4+ CD25+ T-cells in type 1 diabetes. Diabetes. 2005;54:1407–1414. doi: 10.2337/diabetes.54.5.1407. [DOI] [PubMed] [Google Scholar]
- 10.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–99. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 11.Tritt M, Sgouroudis E, d'Hennezel E, Albanese A, Piccirillo CA. Functional waning of naturally-occurring CD4+ regulatory T cells contributes to the onset of autoimmune diabetes. Diabetes. 2008;57:113–123. doi: 10.2337/db06-1700. [DOI] [PubMed] [Google Scholar]
- 12.Brusko T, Wasserfall C, McGrail K, Schatz R, Viener HL, Schatz D, Haller M, Rockell J, Gottlieb P, Clare-Salzler M, Atkinson M. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007;56:604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- 13.Sakaguchi S. The origin of FOXP3-expressing CD4+ regulatory T cells: thymus or periphery. J.Clin.Invest. 2003;112:1310–1312. doi: 10.1172/JCI20274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taams LS, Akbar AN. Peripheral generation and function of CD4+CD25+ regulatory T cells. Curr.Top.Microbiol.Immunol. 2005;293:115–131. doi: 10.1007/3-540-27702-1_6. [DOI] [PubMed] [Google Scholar]
- 15.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat.Rev.Immunol. 2003;3:253–257. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 16.Selvaraj RK, Geiger TL. Mitigation of experimental allergic encephalomyelitis by TGF-beta induced Foxp3+ regulatory T lymphocytes through the induction of anergy and infectious tolerance. J.Immunol. 2008;180:2830–2838. doi: 10.4049/jimmunol.180.5.2830. [DOI] [PubMed] [Google Scholar]
- 17.Huter EN, Punkosdy GA, Glass DD, Cheng LI, Ward JM, Shevach EM. TGF-beta-induced Foxp3(+) regulatory T cells rescue scurfy mice. Eur.J.Immunol. 2008;38:1814–1821. doi: 10.1002/eji.200838346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Godebu E, Summers-Torres D, Lin MM, Baaten BJ, Bradley LM. Polyclonal adaptive regulatory CD4 cells that can reverse type I diabetes become oligoclonal long-term protective memory cells. J.Immunol. 2008;181:1798–1805. doi: 10.4049/jimmunol.181.3.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randolph DA, Fathman CG. Cd4+Cd25+ regulatory T cells and their therapeutic potential. Annu.Rev.Med. 2006;57:381–402. doi: 10.1146/annurev.med.57.121304.131337. [DOI] [PubMed] [Google Scholar]
- 20.Knoechel B, Lohr J, Kahn E, Bluestone JA, Abbas AK. Sequential development of interleukin 2-dependent effector and regulatory T cells in response to endogenous systemic antigen. J.Exp.Med. 2005;202:1375–1386. doi: 10.1084/jem.20050855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang HK, Michaels MA, Berner BR, Datta SK. Very low-dose tolerance with nucleosomal peptides controls lupus and induces potent regulatory T cell subsets. J.Immunol. 2005;174:3247–3255. doi: 10.4049/jimmunol.174.6.3247. [DOI] [PubMed] [Google Scholar]
- 22.Vukmanovic-Stejic M, Zhang Y, Cook JE, Fletcher JM, McQuaid A, Masters JE, Rustin MH, Taams LS, Beverley PC, Macallan DC, Akbar AN. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J.Clin.Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danke NA, Koelle DM, Yee C, Beheray S, Kwok WW. Autoreactive T cells in healthy individuals. J.Immunol. 2004;172:5967–5972. doi: 10.4049/jimmunol.172.10.5967. [DOI] [PubMed] [Google Scholar]
- 24.Yang J, James EA, Huston L, Danke NA, Liu AW, Kwok WW. Multiplex mapping of CD4 T cell epitopes using class II tetramers. Clin.Immunol. 2006;120:21–32. doi: 10.1016/j.clim.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 25.Gebe JA, Falk BA, Rock KA, Kochik SA, Heninger AK, Reijonen H, Kwok WW, Nepom GT. Low-avidity recognition by CD4+ T cells directed to self-antigens. Eur.J.Immunol. 2003;33:1409–1417. doi: 10.1002/eji.200323871. [DOI] [PubMed] [Google Scholar]
- 26.Mallone R, Kochik SA, Laughlin EM, Gersuk VH, Reijonen H, Kwok WW, Nepom GT. Differential recognition and activation thresholds in human autoreactive GAD-specific T-cells. Diabetes. 2004;53:971–977. doi: 10.2337/diabetes.53.4.971. [DOI] [PubMed] [Google Scholar]
- 27.Nepom GT, Erlich H. MHC class-II molecules and autoimmunity. Annu.Rev.Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- 28.Nepom GT, Lippolis JD, White FM, Masewicz S, Marto JA, Herman A, Luckey CJ, Falk B, Shabanowitz J, Hunt DF, Engelhard VH, Nepom BS. Identification and modulation of a naturally processed T cell epitope from the diabetes-associated autoantigen human glutamic acid decarboxylase 65 (hGAD65) Proc.Natl.Acad.Sci.U.S.A. 2001;98:1763–1768. doi: 10.1073/pnas.98.4.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reijonen H, Novak EJ, Kochik S, Heninger A, Liu AW, Kwok WW, Nepom GT. Detection of GAD65-specific T-cells by major histocompatibility complex class II tetramers in type 1 diabetic patients and at-risk subjects. Diabetes. 2002;51:1375–1382. doi: 10.2337/diabetes.51.5.1375. [DOI] [PubMed] [Google Scholar]
- 30.Danke NA, Yang J, Greenbaum C, Kwok WW. Comparative study of GAD65-specific CD4+ T cells in healthy and type 1 diabetic subjects. J.Autoimmun. 2005;25:303–311. doi: 10.1016/j.jaut.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Danke NA, Berger D, Reichstetter S, Reijonen H, Greenbaum C, Pihoker C, James EA, Kwok WW. Islet-specific glucose-6-phosphatase catalytic subunit-related protein-reactive CD4+ T cells in human subjects. J.Immunol. 2006;176:2781–2789. doi: 10.4049/jimmunol.176.5.2781. [DOI] [PubMed] [Google Scholar]
- 32.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J.Clin.Invest. 1999;104:R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allan SE, Alstad AN, Merindol N, Crellin NK, Amendola M, Bacchetta R, Naldini L, Roncarolo MG, Soudeyns H, Levings MK. Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol.Ther. 2008;16:194–202. doi: 10.1038/sj.mt.6300341. [DOI] [PubMed] [Google Scholar]
- 34.Allan SE, Crome SQ, Crellin NK, Passerini L, Steiner TS, Bacchetta R, Roncarolo MG, Levings MK. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int.Immunol. 2007;19:345–354. doi: 10.1093/intimm/dxm014. [DOI] [PubMed] [Google Scholar]
- 35.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4(+) T cells. Eur.J.Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 36.Walker MR, Carson BD, Nepom GT, Ziegler SF, Buckner JH. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25- T cells. Proc.Natl.Acad.Sci.U.S.A. 2005;102:4103–4108. doi: 10.1073/pnas.0407691102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Long SA, Buckner JH. Combination of rapamycin and IL-2 increases de novo induction of human CD4(+)CD25(+)FOXP3(+) T cells. J.Autoimmun. 2008;30:293–302. doi: 10.1016/j.jaut.2007.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakaguchi S, Sakaguchi N. Thymus and autoimmunity: capacity of the normal thymus to produce pathogenic self-reactive T cells and conditions required for their induction of autoimmune disease. J.Exp.Med. 1990;172:537–545. doi: 10.1084/jem.172.2.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pacholczyk R, Kern J, Singh N, Iwashima M, Kraj P, Ignatowicz L. Nonself-antigens are the cognate specificities of Foxp3+ regulatory T cells. Immunity. 2007;27:493–504. doi: 10.1016/j.immuni.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin W, Haribhai D, Relland L, Truong N, Carlson M, Williams C, Chatila T. Regulatory T cell development in the absence of functional Foxp3. Nat.Immunol. 2007;8:359–368. doi: 10.1038/ni1445. [DOI] [PubMed] [Google Scholar]
- 41.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc.Natl.Acad.Sci.U.S.A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St GB, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J.Exp.Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weber SE, Harbertson J, Godebu E, Mros GA, Padrick RC, Carson BD, Ziegler SF, Bradley LM. Adaptive islet-specific regulatory CD4 T cells control autoimmune diabetes and mediate the disappearance of pathogenic Th1 cells in vivo. J.Immunol. 2006;176:4730–4739. doi: 10.4049/jimmunol.176.8.4730. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.