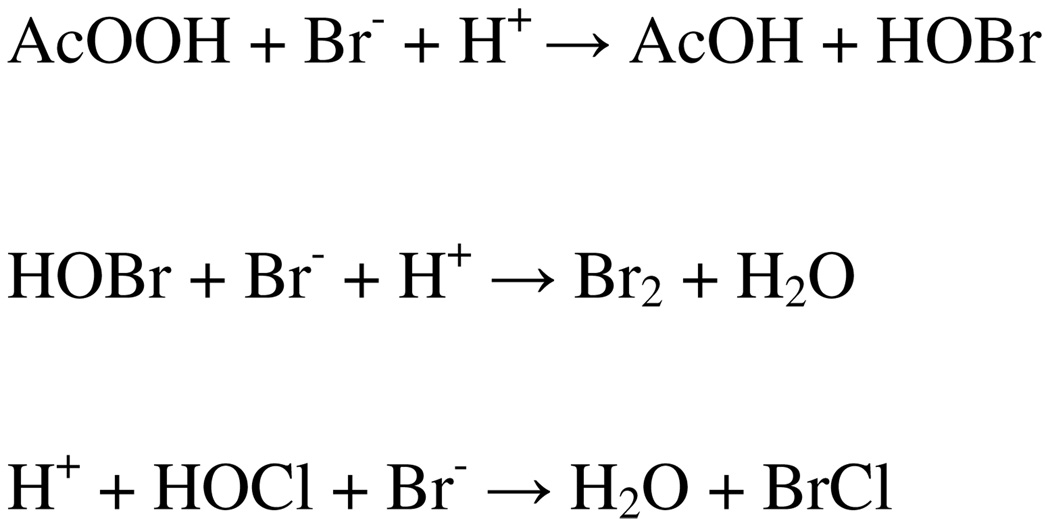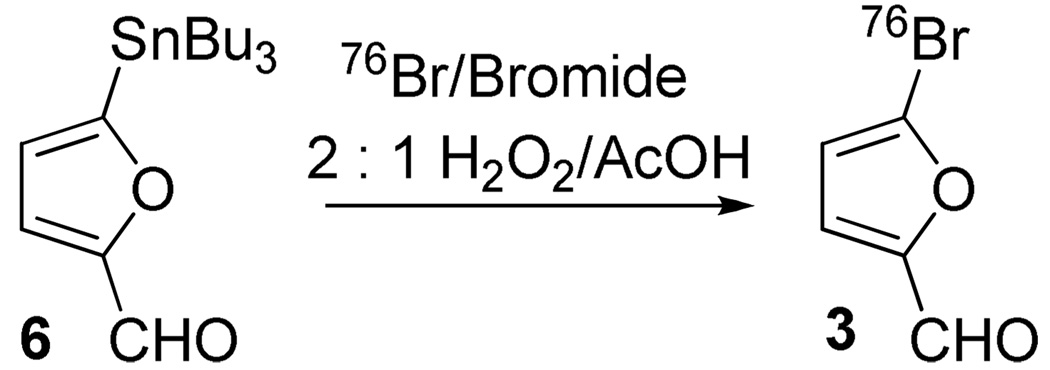Abstract
No-carrier-added (NCA) 76Br labeling of 4-(5-Acetoxy-7-bromobenzoxazol-2-yl)phenyl acetate, a diacetate-protected estrogen-receptor beta (ERβ) selective ligand, was carried out successfully using [76Br]bromide ion. The labeling was achieved via oxidative electrophilic destannylation of an organotin precursor molecule by modification of the leaving group (from Bu3Sn to Me3Sn) and the addition of methanol to the reaction mixture. The differences between the oxidative bromination reaction under small-scale macroscopic vs tracer level radiochemical conditions were explored in terms of effective brominating agents, which depend greatly on the nature of the solvent during the radiochemical bromination, and the potential interference by trace levels of highly reactive impurities in the reaction that compete for the desired bromination at the NCA level. Our observations, and our development of experimental protocols for successful radiobromination at the tracer NCA-scale, should be applicable to the synthesis of other radiobromine-labeled organic compounds of potential interest as PET radiopharmaceuticals and radiotherapy agents.
INTRODUCTION
Radiohalogenated organic compounds are commonly used in basic research and in the clinic as radiopharmaceutical probes for molecular imaging via positron emission tomography (PET1) or single photon emission computed tomography (SPECT). If labeled with a therapeutic nuclide, radiohalogenated compounds can be used for internal radiotherapy at the cellular level (1). Synthetic methods for radiofluorination and radioiodination have been extensively studied and reviewed (2–5). Radiobromination has been much less studied, especially regarding the effective brominating agents in radiobromination reactions (6, 7).
Bromine-labeled compounds have some advantages over iodine-labeled compounds: the stronger C-Br bond results in less dehalogenation, and unlike iodide, bromide does not accumulate in the thyroid (8). Over the past two decades, interest in radiobromine has shifted from 77Brand 82Br to 76Br (8). Radiopharmaceuticals labeled with 76Br (t1/2 = 16.2 h) are suitable for imaging slower physiologic processes than those labeled with 18F (t1/2 = 110 min). Furthermore, the decay scheme of 76Br (57% positron and 43% electron capture) (9) gives it potential both for PET imaging and for radiotherapy. Finally, the 76Br nuclide can be produced via the 76Se(p,n)76Br reaction on most medical cyclotrons (10).
Organic molecules often contain aromatic rings onto which 76Br can be introduced by using very high specific activity [76Br]bromide ion. Radiobromination at the tracer level produces products with the very high specific activities required for studying receptor-binding biological processes, but can differ from classical bromination and from radioiodination. Bromide is more difficult to oxidize than iodine; so harsher conditions are required for oxidative bromination. An effective brominating agent is also more reactive than an effective iodinating agent, so standard conditions for radioiodination might not be appropriate for radiobromination.
Major considerations for tracer-level synthesis are synthesis time (due to radioactive decay), specific activity (to achieve true tracer status), and relative stoichiometry. In classical electrophilic brominations, even those on a small scale, macroscopic amounts of the electrophilic brominating agent are used and are usually present in stoichiometric excess over the arene reactant. However in true tracer level, no-carrier-added electrophilic radiobromination reactions, the trace amount of “positive bromine” reacts with orders-of-magnitude excesses of a substrate in the presence of an excess of oxidizing agents. Because of this stoichiometric issue, the effective radiobrominating agent is likely to differ from that used in classical bromination. In addition, any highly reactive impurities present in tracer-scale brominations might preferentially react with the “positive bromine”, resulting in formation of bromine-substituted byproducts and reducing yield of the desired product. In contrast, in macroscopic scale reactions, the relatively tiny amounts of any highly reactive impurities compared to stoichiometric or excess bromine would produce chemically-ignorable bromine-substituted byproducts. The lack of understanding and published information regarding effective agents for radiobromination of organic moieties has impeded development of some radiopharmaceuticals.
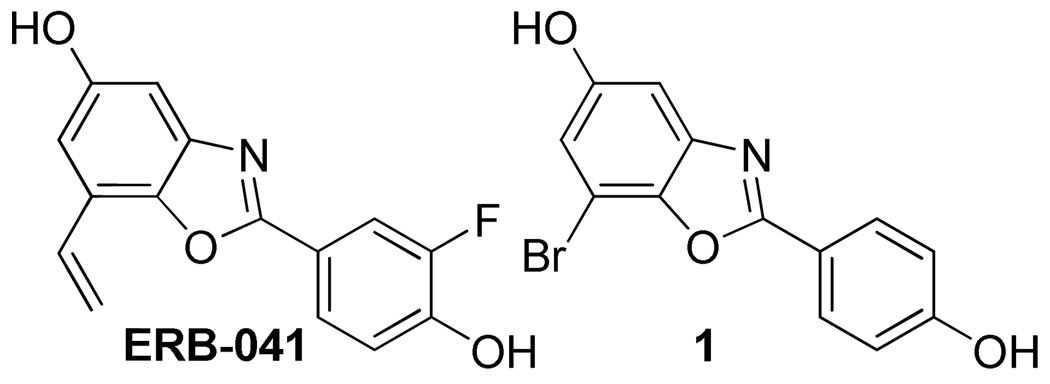
Compound 1 (Figure 1) (11), an analog of ERB-041 (11, 12), is an estrogen receptor beta (ERβ) selective ligand with good binding affinity and excellent selectivity over estrogen receptor alpha (ERα) (11, 12). Once labeled with 76Br, it is expected to have potential as a PET imaging agent for ERβ. Brominations of non-activated organic compounds via electrophilic destannylation have been successful using unlabeled bromide and carrier-added [76Br]bromide. However, no-carrier-added radiobrominations have been problematic. Difficulties in no-carrier-added radiolabeling of 1 with 76Br led us to explore radiobrominations in greater detail.
Figure 1.
Structures of ERB-041 and 1.
Here we report systematic exploration of reaction conditions leading to successful tracerscale, no-carrier-added 76Br radiobromination of aromatic compounds, most notably [76Br]2 (which can be deprotected to make [76Br]1) via electrophilic destannylation. We also report investigation of other issues, including the effect of solvents, on 76Br labeling of organic compounds including 5-bromo-2-furaldehyde 3. These observations and the development of experimental protocols for successful tracer-scale radiobromination should find application for the radiobromination of other organic compounds.
EXPERIMENTAL PROCEDURES
General Information
All chemicals and solvents were obtained from commercial sources and used without further purification unless noted. The synthesis of compounds 2, 4, and 5 will be described elsewhere. Compound 6 was synthesized according to the literature (13). Bromine-76 was produced at the Washington University cyclotron facility on the Japanese Steel Works 16/8 cyclotron by the 76Se(p,n)76Br nuclear reaction on a 76Se-enriched Cu2Se target. Bromine-76 was recovered via a modified dry distillation method (14). Reversed phase HPLC was performed with an ultraviolet detector and a radiation detector. Alltech Econosil or Altima C18 250 × 4.6 mm 10 urn or Agilent Zorbax SB-C18 250 × 4.6 mm 5 µm analytical columns were used for analysis. Acetonitrile and 0.1 M ammonium formate buffer (pH = 4.5) were used as the HPLC mobile phase. Ion Chromatography (IC) was performed using Dionex IonPac AS14A 250 × 4 mm column with UV at 210 nm and a radiation detector, with an elution solvent of 8 mM Na2CO3 and 1 mM NaHCO3 aqueous solution. RadioTLC was performed using a Bioscan System 2000 imaging scanner.
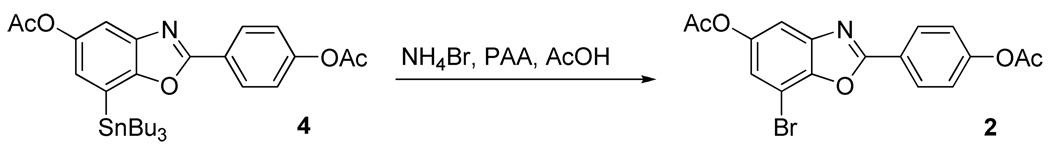
Typical Procedure for Non-radioactive Synthesis of 4-(5-Acetoxy-7-bromobenzoxazol-2-yl)phenyl Acetate (2) from Precursor 4
Into a solution of 4 (100 µg) in acetic acid (100 µL), NH4Br solution (3 µL, from a solution of 6 mg in 1000 µL water) and 3% peracetic acid in acetic acid (100 µL) were added. The reaction mixture was vortexed to homogeneity and allowed to react at ambient temperature. At 10 min, 10 µL of the reaction mixture was injected onto a reversed phase HPLC (Alltech Altima C18 250 × 4.6 mm 10 µm), eluting with 60% acetonitrile 40% 0.1 M ammonium formate buffer (pH = 4.5) at a flow rate of 2 mL/min and UV detection at 285 nm. Compound 2 eluted at 8 min as the only product according to HPLC, and it was confirmed by comparison with authentic standard 2.
Preparation of [76Br]Bromide
A solution of [76Br]bromide in 0.6 N ammonium hydroxide (up to 1 mL) was passed through a C18 SepPak (light) and a 0.45 µm Nylon filter, which were conditioned by methanol (10 mL) and water (10 mL) beforehand and washed with water (100 µL) afterwards. All collected activity was evaporated to dryness in a straight-sided shell vial (15 × 45 mm) in a heat block heated from ambient temperature to 130 °C and with a very gentle flow of N2 over the top of the vial, which did not result in any loss of radioactivity; high gas flow can result in a significant loss of radioactivity. When the radioactivity was completely dry (less than 10 min), it was reconstituted in water, methanol or acetic acid for the labeling reactions indicated below. Bromine-76 extracted in water or ethanol was used as is or dried directly at 130 °C.
Typical Procedure for Attempted No-carrier-added Synthesis of [76Br]2 from 4 and Control Reactions
A solution of [76Br]Bromide (20–100 µCi) in water, methanol or acetic acid (10–50 µL) was added to a solution of 4 (no 4 for control reactions) (100 µL), then acetic acid (100 µL) and a solution of peracetic acid in acetic acid or methanol (100 µL) were added to the solution. Reaction mixture was vortexed to achieve homogeneity and analyzed by radio-TLC.
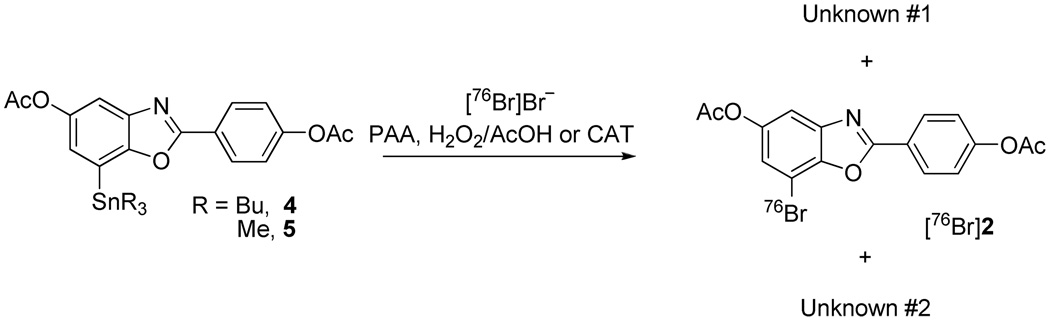
No-carrier-added Synthesis of [76Br]2 from Precursor 5
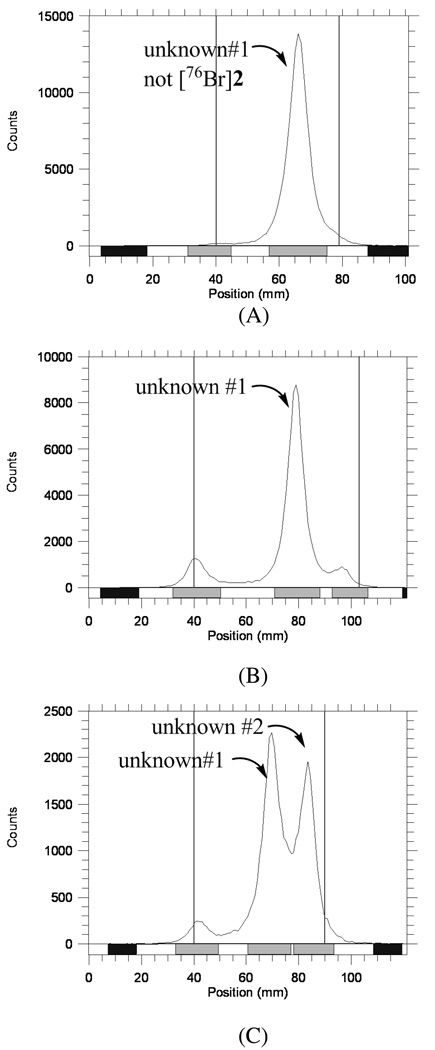
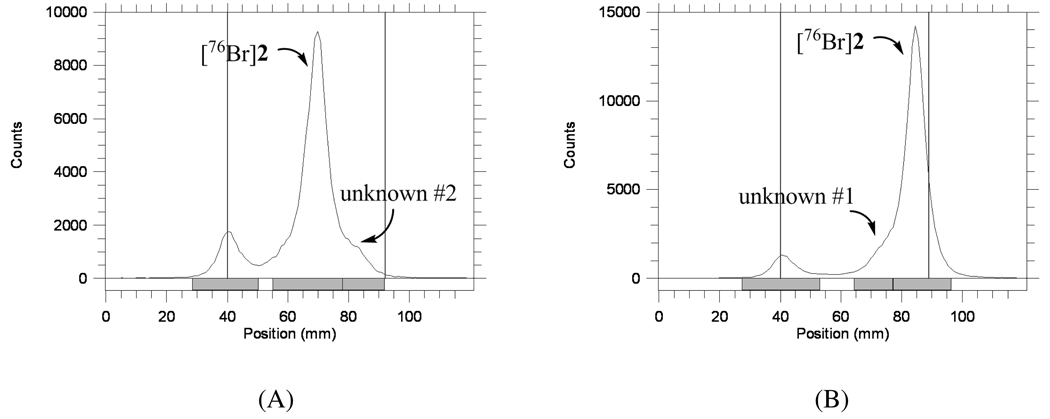
To [76Br]Bromide (1.5 mCi), dried as described above and reconstituted in MeOH (100 µL), was added a solution of trimethyltin precursor 5 (1 mg) in acetic acid (100 µL) and the reaction vessel was vortexed. After addition of 1% peracetic acid in methanol (100 µL), the reaction vessel was vortexed again and then allowed to react at ambient temperature. The reaction was checked by radio-TLC (silica); >80% [76Br]bromide was consumed and roughly 60% was obtained as [76Br]2 within 30 min. [76Br]2 was distinguished from unknown #1 by developing the plates in 1:1 ethyl acetate/hexanes and 10% methanol in dichloromethane (Figure 2 and Figure 3). Reversed phase HPLC purified [76Br]2 was confirmed by silica radio-TLC and HPLC co-injection with authentic standard 2 ([76Br]2: Rf = 0.55, 0.91 for 1:1 ethyl acetate/hexanes and 10% methanol in dichloromethane, respectively; HPLC: Agilent Zorbax SB-C18 250 × 4.6 mm 5 µm analytical column, 65% acetonitrile, 35% 0.1 M ammonium formate buffer (pH=4.5), 2 mL/min, 285 nm, tR = 5.2 min; Unknown #1, 0.54, 0.75 and unknown #2, 0.85, 0.99 on silica gel TLCs developed in 1:1 ethyl acetate/hexanes and in 10% methanol in dichloromethane, respectively. (For HPLC of unknown #1, see supporting information.)
Figure 2.
Radio-TLCs of attempted radiobrominations of 4 and a control reaction (performed in the absence of 4), showing formation of unknown #1 and unknown #2. (A) Attempted radiobromination of 4, using PAA; (B) Control reaction using PAA but no substrate 4; (C) Attempted radiobromination of 4, using CAT. Separations were on silica gel, 1:1 ethyl acetate/hexanes.
Figure 3.
Radio-TLCs of successful synthesis of [76Br]2 developed in two TLC solvent systems (Silica gel). (A) 1:1 ethyl acetate/hexanes. (B) 10% methanol in dichloromethane.
RESULTS AND DISCUSSION
Oxidizing Agents and Precursors
The most commonly used oxidizing agent for radioiodination and radiobromination is N-chloro-p-toluenesulfonamide sodium salt (Chloramine-T or CAT). Conditions have been optimized for radiobromination of activated phenol analogs using CAT (15), and these conditions have been used to label organic compounds, antibodies and nanoparticles, and for the direct and indirect labeling of peptides. Chloramine-T is not ideal for tracer-scale radiosyntheses, however, because simultaneous chlorination can reduce final effective specific activity if chlorinated by-products cannot be separated from the desired radiobrominated product (16). Furthermore, elevated temperatures may be necessary to promote labeling of electron-poor substrates. Premixed hydrogen peroxide/acetic acid (H2O2/AcOH), in which peracetic acid (PAA) is the effective oxidizing agent, also has been used to synthesize receptor-binding radiopharmaceuticals (17–19). Currently, the use of PAA as oxidizing agent is growing because reaction conditions are mild and labeling is cleaner compared to products obtained using CAT and H2O2. As a result, PAA was used for our radiobromination studies.
When small organic compounds of interest as potential radiopharmaceuticals do not contain an electron-rich aromatic ring activated for electrophilic aromatic substitution (e.g., tyrosine), electrophilic demetallation is an elegant approach to radiobromination (7). Among the metals used in these reactions (boron, silicon, germanium and tin), the tin precursors (SnMe3 and SnBu3 substituted) generally give the best results (20, 21). This method often allows an aromatic compound to be labeled regiospecifically, quickly and in high yields, and so the organic tin precursors 4, 5, and 6, were used for our investigations.
Non-Radioactive Bromination of Tributyltin Precursor 4
Non-radioactive electrophilic aromatic substitution of the tributyltin diacetate precursor 4 with bromine under classical macroscopic (but low-scale) reaction conditions was carried out in acetic acid using PAA as the oxidizing agent (Scheme 1; Table 1). Destannylation took place quickly, and the desired bromine-substituted compound 2 was the major product by HPLC analysis of the reaction mixture. The yield was not determined due to the small scale of the reactions and difficulty in determining the amount of starting material consumed, but investigations using non-radioactive bromine as the limiting reagent (see below and Table 3) suggest yields were high. When the PAA was reduced from 200 to 1 equivalent relative to NH4Br the reaction slowed, but a similar amount of 2 was obtained within 30 min (Table 1, entry 6), suggesting oxidation of bromide by PAA is highly efficient under these conditions.
Scheme 1.
Bromination of 4 Using NH4Br and PAA in Acetic Acid
Table 1.
Bromination of 4 Using NH4Br and Different Concentrations of PAA in Acetic Acida
| # | 4b (µmol) |
NH4Brc (µmol) |
PAAd (µmol) |
PAA:NH4Br (in µmol) |
Reaction Time (min) |
Relative Yielde 2 (%) |
|---|---|---|---|---|---|---|
| 1 | 0.17 | 0.18 | 39.4 | 219 : 1 | 10 | (100) |
| 2 | 0.17 | 0.18 | 13.1 | 73.0 : 1 | 10 | 84 |
| 3 | 0.17 | 0.18 | 4.4 | 24.3 : 1 | 10 25 |
80 97 |
| 4 | 0.17 | 0.18 | 1.5 | 8.1 : 1 | 20 | 94 |
| 5 | 0.17 | 0.18 | 0.49 | 2.7 : 1 | 20 | 87 |
| 6 | 0.17 | 0.18 | 0.16 | 0.9 : 1 | 20 | 95 |
Data from single experiment
4 (100 µg, 0.17 µmol) in AcOH (100 µL)
A solution of NH4Br (18 µg, 0.18 µmol) in H2O (3.0 µL)
PAA in AcOH (100 µL)
Yields are given relative to that obtained in reaction #1, based on HPLC integrations of 2.
Table 3.
| # | 4c (µmol) |
NH4Brd (mol/L) |
Yield of 2 (%)e |
|
|---|---|---|---|---|
| 10 min | 30 min | |||
| 1 | 0.33 | 6 × 10−4 | 67 | 79 |
| 2 | 0.33 | 6 × 10−5 | 74 | 76 |
| 3 | 0.33 | 6 × 10−6 | 24 | 25 |
| 4 | 0.33 | 6 × 10−7 | 5 | 5 |
Data from single experiment
Oxidant: PAA (3%, 39.4 µmol) in AcOH (100 µL).
4 (200 µg, 0.33 µmol) in AcOH (200 µL).
Concentration of NH4Br in the reaction mixture, added as a solution of NH4Br in H2O (3.0 µL).
Yields are based on bromide used as the limiting reagent and were determined by comparison with known concentrations of an authentic standard 2.
Chloramine-T and hydrogen peroxide/acetic acid were also investigated for their effectiveness in the classical oxidative bromination reaction (Table 2). In general, reactions were slower and yields lower with CAT, probably due to slower oxidation of bromide, but addition of acetic acid as a proton source significantly accelerated the reaction at room temperature. This is consistent with the reported oxidative bromination of tyrosine using CAT (15). At elevated temperatures, the amount of product obtained was similar to using 3% PAA. Hydrogen peroxide/acetic acid, which produces small amounts of PAA, did not give any detectable product by HPLC analysis. Hence for classical oxidative brominations, it appears that PAA is more efficient than CAT and H2O2/AcOH. The lack of reaction using H2O2/AcOH is not surprising, because H2O2 in the absence of strong acid is a much weaker oxidizing agent for bromide than PAA (22, 23). Apparently H2O2/AcOH does not produce enough PAA to result in detectable bromination products in classical, macroscopic oxidative brominations.
Table 2.
Bromination of 4 Using Different Oxidizing Agentsa
| # | 4b (µmol) |
NH4Brc (µmol) |
Oxidizer (µmol) |
Reaction Time (min) |
Temperature (°C) |
Relative Yieldd 2 (%) |
|---|---|---|---|---|---|---|
| 1 | 0.17 | 0.06 | PAA (39.4) AcOH (100 µL) |
15 | RT | (100) |
| 2 | 0.17 | 0.06 | CAT (0.44) MeOH (100 µL) |
15 | RT | 0.3 |
| 3 | 0.17 | 0.06 | 2:1 H2O2/AcOH (1960) (100 µL) |
15 | RT | 0 |
| 4 | 0.17 | 0.06 | CAT (0.44) MeOH/AcOH (100 µL/100 µL) |
15 40 |
RT RT |
40 60 |
| 5 | 0.17 | 0.06 | CAT (0.44) MeOH/AcOH (100 µL/100 µL) |
10 20 |
88 88 |
89 90 |
Data from single experiment
4 (100 µg, 0.17 µmol) in MeOH (100 µL)
a solution of NH4Br (6 µg, 0.06 µmol) in H2O (10 µL)
Yields are given relative to that obtained in reaction #1, based on HPLC integrations of 2.
When bromide (NH4Br) was the limiting reagent and its concentration was varied, the yield of 2 in classical oxidative brominations using PAA was determined by HPLC (Table 3). When bromide concentration was above 10−5M, the yield was high (74% after 10 min and 76% after 30 min). When bromide was reduced to 10−6 M, close to but still higher than in the NCA radiolabeling reaction, the yield of 2 was reduced dramatically to roughly 25% after 10 min or 30 min. For 10−7 M bromide, yields were just 5% after 10 min or 30 min. (Because the average specific activity of [76Br]bromide is more than 1000 Ci/mmol, the concentration of bromide is less than 10−6 mol/L for 1 mCi radioactivity in 1 mL solution.) In this series of reactions amounts of other brominated products were too small to allow their identification.
No-carrier-added Radiobromination of Precursor 4: Formation of Unexpected Byproducts (Scheme 2)
Scheme 2.
Synthesis of [76Br]2 from 4 or 5 Using [76Br]bromide and Various Oxidizing Agentsa
a Analyzed by radio-TLC.
Conditions were the same as those used for non-radioactive bromination reactions, shown in Table 1 and Table 2. When NH4[76Br]Br was used in 0.6 N ammonium hydroxide without drying, a single radioactive byproduct (unknown #1, Figure 2A) was formed within 15 min, according to radioactive detection using thin layer chromatography (Radio-TLC). Unfortunately, the Rf value of this peak is almost the same as that of the nonradioactive standard 2 in the developing solvents (1:1 ethyl acetate/hexanes), which caused some confusion. After the reaction mixture was diluted with water, however, this radioactive byproduct was converted completely to a more polar product. This initial radioactive byproduct (unknown #1) was also observed in the labeling of other compounds via electrophilic destannylation (data not shown).
It initially was assumed that unknown #1 was actually the desired radioactive product 2, and the more polar product formed after dilution with water was due to radiolysis from the high energy radiation of 76Br. However, unknown #1 also was found later in a control reaction, in which no precursor 4 was added (Figure 2B). Therefore, the initially observed radioactive byproduct clearly was not the desired product 2, nor was it any radioactive form of the tributyltin precursor 4. Unknown #1 was also observed when CAT (Figure 2C) or H2O2AcOH was used as the oxidizing agent, although its formation was slow with CAT. A second unknown, very lipophilic radioactive byproduct (unknown #2, Figure 2C) was observed with CAT and only in the presence of substrate 4 (Figure S1).
Effect of Carrier and Solvent on Radiobromination of Precursor 4
With carrier NH4Br in water added to the radiolabeling reaction ([bromide] = 5 × 10−4 M) and PAA as the oxidizing agent, desired product 2 was formed exclusively. Its identity was confirmed by HPLC analysis by co-eluting with non-radioactive standard 2. These results are consistent with our experiments conducting non-radioactive bromination with various bromide concentrations (Table 3). Thus, sufficient carrier results in efficient radiobromination of 4, but with no carrier added it would appear that [76Br]bromide in NH4OH reacts first with some unidentified, more reactive species present at trace levels, rather than replacing the tributyltin moiety in precursor 4. Thus at tracer scale, unknown #1 is produced rather than the desired product 2 under otherwise classical reaction conditions.
When [76Br]bromide was produced in water or ethanol, and either PAA or CAT as the oxidizing agent, unknown #1 was only a minor product. However, under these conditions, unknown #2 was the major product observed. With H2O2/AcOH as the oxidizing agent, no reaction was observed for [76Br]bromide produced in water or ethanol (Table 4).
Table 4.
Byproducts Produced from Attempted Radiobromination of Tributyltin Precursor 4 Using [76Br]bromide Produced in Ammonium Hydroxide and Water
| 76Br/Bromide | Oxidizing agent | Unknownsa | |
|---|---|---|---|
| #1 | #2 | ||
| Produced in 0.6N NH4OH or dried |
PAA | major | none |
| CAT | major | major | |
| 2:1 H2O2/AcOH | major | none | |
| Produced in H2O dried |
PAA | minor | major |
| CAT | minor | major | |
| 2:1 H2O2/AcOH | none | none | |
determined by radioTLCs (silica gel).
Exploration for the Source of the Radioactive Byproducts Unknowns #1 and #2
Efforts were taken to identify unknown #1 and to remove its source. We found that formation of unknown #1 was dominant and fast when 76Br in NH4OH was used without drying. This was true even with activated l-methoxy-4-tributylstannylbenzene analogs, where the desired radiolabeling reaction is expected to be prompt (data not shown). Ammonium ion is reported to react very rapidly (k = 107 M−1S−1) with HOBr to form NH2Br (24, 25), a species that has relatively feeble brominating activity (25). However, addition of NH4OH or NH4NO3 in solution to control reactions of PAA and [76Br]bromide ion (produced in water) produced no additional unknown #1.
A solution of [76Br]Bromide ion in 0.6 N NH4OH was analyzed by ion chromatography (IC) to evaluate the possibility that trace selenium could have catalyzed oxidative halogenations (26) to form unknown #1. It is possible that trace amounts of O2 leaked into the system during hourslong high temperature distillation could have oxidized trace selenium to form SeO2, which can be extracted as SeO32− in aqueous solution but can be separated from the bromide after IC purification. In these experiments, NO3− and SeO32− were observed, and identified by comparison to retention times of NH4NO3 and SeO32− (a solution of SeO2) in aqueous solutions (Figure S2). However, IC purified [76Br]bromide and crude [76Br]bromide in NH4OH produced radiolabeled unknown #1 exclusively in control reactions. Therefore, SeO32− cannot be responsible for the formation of unknown #1.
The high energy radiation of 76Br is likely to cause some radiolysis, and indeed formation of NO3− was observed in our reaction mixtures but not in plain 0.6 N NH4OH. We also observed that production of unknown #1 increased with the length of time [76Br]bromide remained in an NH4OH solution and with the amount of radioactivity present. Although it has not been possible to identify unknown #1 or its source, its formation can be avoided by producing [76Br]bromide in water or ethanol or by drying the activity immediately after its production in an NH4OH solution.
Unknown #2, a radioactive, highly lipophilic compound, was also observed in radiolabeling reactions with the tributyltin precursor 4 when CAT was used as the oxidant (Figure 2C, Table 4, Figure S1). With [76Br]bromide produced in water or ethanol, radiobromination of impurities present in the reaction mixture also contributed partially to production of unknown lipophilic compounds (Figure S1). Furthermore, a solvent-related radioactive product was observed exclusively both in radiobromination of 4 and in its control reaction (no substrate 4) when THF (inhibitor free), DMF or acetonitrile was used in the experiments (Figure S3). These solvents have been documented in the literature as suitable for radiobromination.
We are unable to determine the chemical form of unknown #2, formed from the reaction with the tributyltin precursor 4. It is more lipophilic than precursor 4 by silica gel TLC, and it is difficult to elute from a C18 Sep-Pak. It is resistant to 1 N LiOH treatment, but it can be converted to a polar product by treatment with trifluoroacetic acid (TFA). These characteristics suggest that unknown #2 still contains a tributyltin substituent.
Electrophilic substitution can take place at several sites on precursor 4 other than by ipso substitution of the tributyltin group, particularly on the aromatic rings which are activated by several electron donating substituents. Furthermore, the nitrogen of the benzoxazole could operate as a nucleophile to react with the electrophilic ‘positive bromine’. Two examples from the literature support these possibilities. A similar highly lipophilic radiobrominated byproduct was produced by 76Br radiobromination of 4-substituted-3,5-dimethoxytributylstannylbenzene (27), in which the benzene ring is activated by two methoxy groups. In that case, non-radioactive large-scale bromination produced the expected bromodestannylated product exclusively, while tracer-scale radiolabeling produced a radiobrominated product with the tributyltin substituent still present. In that reaction, the proton adjacent to the tributyltin on the benzene ring apparently was replaced by a radiobromine in preference to destannylation. Therefore, unknown #2 could be the product of electrophilic substitution of a proton on the activated benzene ring in 4. Also, others have reported N-bromination as a byproduct, along with the desired product, in no-carrier-added bromination of a phenol analog (16).
The low yield of the desired product 2 at low bromide concentrations (Table 3) is clearly due in part to competition with formation of unknowns #1 and #2, which are difficult to detect by HPLC in macroscopic-scale reactions using carrier-added or non-radiolabeled bromide but are readily apparent in tracer-scale radiobrominations.
Nature of the Active Electrophilic Brominating Agent in NCA Radiobromination Reactions: Dependence on Stoichiometry, Oxidant, and Solvent
If the reaction pathways for macroscopic non-radioactive (or carrier added) and tracer-scale NCA bromination are the same, both reactions should afford the same product. The differences we have observed could be due to differences in the nature of the effective brominating agents due to different levels of bromide in macroscopic versus tracer-scale reactions. Many effective brominating agents have been studied or suggested, including HOBr, H2OBr+, Br2, BrCl, MeOBr, AcOBr. Acetyl hypobromite was proposed as the effective brominating agent in acetic acid (28), and it is more reactive than Br2 in electrophilic substitutions (29). Molecular bromine is about 103 more reactive than HOBr in electrophilic substitution of p-xylene (30) and sodium p-anisate (31). Bromine chloride, about 2 × 103 more reactive than Br2 (30), has been proposed as an effective brominating agent under certain conditions (32), and is favored kinetically (33–34) and thermodynamically, especially in the presence of H+ and CI− (32). When PPA or H2O2/AcOH, is used as the oxidizing agent, HOBr is reported to form first (23). If the substrate does not react very rapidly with HOBr, HOBr will react further with excess of bromide to form Br2. Formation of Br2 is very fast (k = 1.6 × 1010M−2S−1) (35, 36) and thermodynamically favored (Kf(Br2) = 2.53 × 108 M−2) (Scheme 3) (37). However, formation of HOBr can be shifted depending on the solvent and pH of the solution, (22, 23, 30, 38). When CAT is the oxidizing agent, the mechanism is complicated, but BrCl has been proposed to be the effective brominating agent (Scheme 3) (6). However, BrCl can be converted to HOBr or its relatives easily by varying solvents and pH (32, 39).
Scheme 3.
Reactions that Form Active Electrophilic Bromination Species
In the non-radioactive and carrier-added bromination of 4 (bromide > 10−5 M), the effective agent is most likely Br2, which indeed was observed on TLC analysis (Rf = 0.34 in 1:1 ethyl acetate/hexanes, silica, visualized with I2). In the no-carrier-added radiobromination, oxidation of bromide by peracetic acid is efficient, but bromide concentration is so low that formation of Br2 is not likely. Therefore, at low bromide concentrations, the effective agent could be HOBr, AcOBr, H2OBr+, or MeOBr, depending on the solvent used and pH of the reaction solutions.
Molecular bromine and hypobromous acid may have different behavior in electrophilic substitution reactions. For example, HOBr has smaller steric requirements; HOBr afforded 38% ortho-derivative on t-butylbenzene compared to 10% for Br2 (40). Hypobromous acid and related sources of “positive bromine” can introduce a substituent adjacent to bulky groups in an aromatic ring (40). Trifluoroacetyl hypobromite (CF3COOBr), a relative of HOBr, also produced different electrophilic substituted products from those of Br2 on phenol analogs (41).
Unified Consideration of the Nature and Activity of the Bromination Reagent and the Reactivity of the Substrate
Outcomes of radiobromination likely can be determined by altering the effective brominating agent. In a tracer-scale radiolabeling reaction with an only moderately reactive substrate, the effective brominating agent can be affected by solvent and pH. If AcOH is the solvent, the effective brominating agent might be the very reactive AcOBr, resulting in low selectivity and creating undesired radioactive byproducts. This is quite likely with no-carrier-added radiobromination of 4. The effective brominating agent is also affected by concentration of bromine; in the carrier-added radiolabeling reactions, the effective brominating agent is Br2 and the desired product 2 is formed exclusively, just as in the classical bromination.
The rate of aromatic bromination varies from very slow to diffusion-controlled, depending on the activation and steric effects of substituents on the aromatic rings. When the substrate is very reactive towards electrophilic substitution (such as tyrosine), it will out-compete reactive impurities in the reaction medium, and the exact chemical nature of the effective bromination agent may be less important. However, electronic and/or steric effects can render a substrate less reactive than impurities in the reaction mixture. A bulky leaving group like the tributyltin of 4 could be changed to reduce the steric effect and perhaps shift a reaction toward production of the desired product, particularly if attention also is paid to the likely effective brominating agent.
Successful synthesis of [76Br]2
As a result of these observations, NCA radiobromination on the trimethyltin precursor 5 was attempted in acetic acid using peracetic acid as an oxidizing agent. The reaction afforded slightly better yields of 2 than the corresponding reaction with tributyltin precursor 4, but yields were still < 5% because of formation of unknowns #1 and #2. This result does support the idea that altering the reactivity of the precursor can increase production of the desired radiobromo-destannylation product.
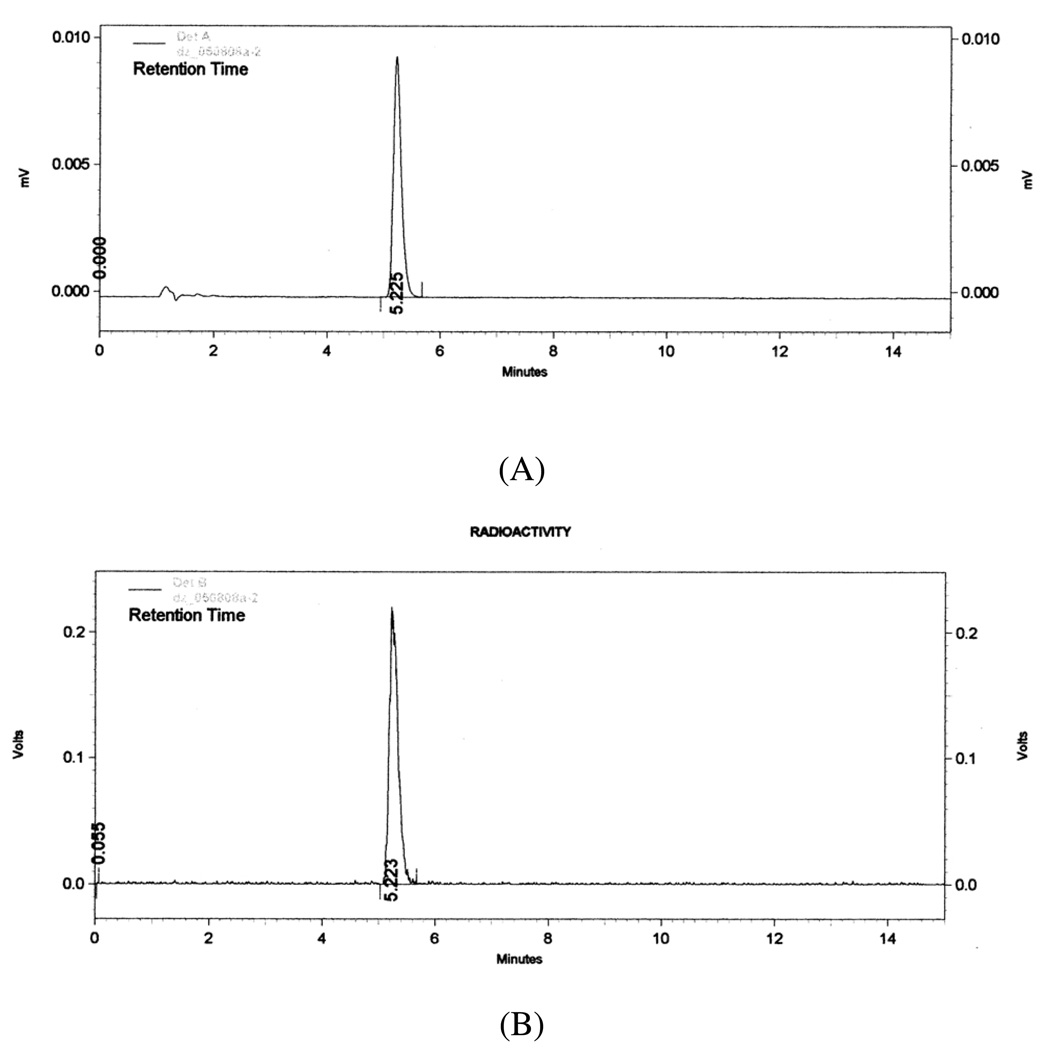
The solvent used also significantly affects the reaction. The reaction occurred quickly in acetic acid, but the most likely effective brominating agent, AcOBr, is very reactive and thus less selective. When radiobromination was carried out in a mixture of acetic acid and methanol, the reactivity of effective brominating agents was reduced by the addition of methanol. The desired bromination product 2 was the major radioactive product (61 ± 16 %, n = 5, by radio-TLC) (Figure 3). After purification by reversed phase HPLC, [76Br]2 was obtained in 30% yield (from 1.5 mCi [76Br]bromide) with good specific activity2. Reversed phase HPLC analysis confirmed that [76Br]2 coeluted with an authentic sample of unlabeled 2 and that the radiochemical purity was >99% (Figure 4). Therefore, by increasing the reactivity of the precursor toward the desired substitution and decreasing the reactivity of effective brominating agent, we were able to obtain the desired radiolabeled product [76Br]2 consistently and in good yields.
Figure 4.
HPLC chromatographs of purified [76Br]2 coinjected with authentic 2. (A) UV trace of 2 at 285 nm. (B) radioactive trace of [76Br]2. HPLC analysis was performed using Agilent Zorbax SB-C18 250 × 4.6 mm 5 µm analytical column and using 65% acetonitrile and 35% 0.1 M ammonium formate buffer (pH = 4.5) as mobile phase at a flow rate of 2 mL/min and UV at 285 nm.
Other Radiobromination Reactions
Peracetic acid is considered to be the effective oxidizing agent when a mixture of hydrogen peroxide and acetic acid is used, and it has been used in the radiobromination of steroid ligands (10). When premixed 2:1 H2O2/AcOH (up to 6 h) was used for radiobromination of precursors 4 or 5, however, no desired product was obtained, only rapid formation of unknown #1 (Table 4). This implies that the effective brominating agent from 2:1 H2O2/AcOH is not reactive enough to effect electrophilic substitution with these precursors.
Hydrogen peroxide/acetic acid (2:1) was also used in the radiobromination of a 5-tributyltin substituted furfural 6, in which the electron-rich furan ring is deactivated slightly by the aldehyde group, to make [76Br]3 (Scheme 4). [76Br]3 was used in the synthesis of a 76Br-labeled progestin 16α,17α-dioxolane for breast tumor imaging and radiotherapy (42). Four hours of premixing of 2:1 H2O2/ACOH was necessary to make enough PAA to afford a high yield of bromination product 3 (Table 5). However, when 1% PPA was used as the oxidizing agent in a mixture of acetic acid and methanol, no-carrier-added radiobromination of the tributyltin precursor 6 produced only a very lipophilic product, which likely retained the tributyltin group (Figure S4).
Scheme 4.
Radiobromination of 6
Table 5.
Radiobromination of 6 Using 2:1 H2O2s/AcOH
| # | Reaction Time |
Premixing Time (min) Yield of 3 (%)a |
||||
|---|---|---|---|---|---|---|
| 0 | 30 | 60 | 120 | 240 | ||
| 1 | 15 | 8 | 30 | 39 | 62 | 84 |
| 2 | 30 | 11 | 41 | 53 | 70 | 84 |
Radiochemical yield is determined by radio TLC.
As with unknown #2, this lipophilic product was not formed from side-reaction with solvents and is likely the product of electrophilic substitution other than by ipso substitution of the tributyltin on the furan ring under these reaction conditions, which provide a more reactive effective brominating agent than 2:1 H2O2/AcOH. When 1% PAA in freshly made 2:1 H2O2/AcOH was used, the radiobromo-destannylation product was formed exclusively, just as it was using premixed H2O2/AcOH. This observation is consistent with the proposed effective oxidizing agent PAA in the premixed H2O2/AcOH and suggests that solvent effects may contribute to the success of the radiobromination of 6.
When this radiobromination was repeated using 1% PAA in 2:1 or 1:1 water/acetic acid to study the solvent effect, the desired product [76Br]3 was formed exclusively. Under these aqueous conditions, HOBr is probably the effective brominating agent. It is less reactive and more selective than the effective brominating agents present in acetic acid/methanol or the AcOBr in acetic acid alone. The reactivity of the effective brominating agent in different solvents increases in the order of water, methanol and acetic acid.
In conclusion, tracer-scale NCA radiobromination differs from non-radioactive (or carrier-added) bromination and likely requires different effective brominating agents and careful attention to solvents. We suggest that the best results in tracer-scale NCA radiobromination can be achieved by considering the following:
Removing highly reactive impurities that might consume the radiobromine in preference to the substrate. [Avoiding ammonium ions, carefully drying [76Br]bromide and completely removing organic solvent residue from the substrate appeared to improve yields.]
Increasing the specific reactivity of the precursor when possible. [Switching from the tributyltin precursor 4 to the trimethyltin precursor 5 improved yields.]
Lowering the reactivity of the effective brominating agent. [Adding alcohols or water reduced side reactions and improved product yields.]
Considering opportunities to reduce the number of steps. [We have shown that PAA very efficiently oxidizes bromide, and unlike CAT, PAA and solvents can be removed easily from the reaction mixture under a flow of N2 or Ar (for non-volatile substrate and products). This avoids extraction and/or drying in multi-step reactions in which anhydrous conditions are needed, such as radiolabeling of steroids (10) and Bolton Hunter reagent (43).
SUMMARY AND CONCLUSION
Ipso substitution by electrophilic demetallation effectively introduces radiobromine regiospecifically into organic compounds containing aromatic rings, but competition can occur with other reactive sites, such as open positions on an activated aromatic ring and amine or amide groups. At the tracer level, low reactivity of the substrate can result in competitive reactions with reactive impurities present in reagents or solvents, and this competition can be modulated by solvent and pH, which alter the effective brominating agent. Therefore, a clear understanding of the nature of effective brominating agents and the reactivity of the substrate is important in developing methods for radiobromination of organic compounds.
By considering these factors, we were able to label 2 and 3 from the two trialkyltin precursors 5 and 6 with 76Br reliably and in good yield. We hope our strategy will help others achieve tracer-scale radiobromination of aromatic compounds of interest.
Supplementary Material
Synthesis of [76Br]3 using premixed 2:1 H2O2/AcOH and using PAA/H2O; radio-TLCs of radiobromination of 4 and its control reaction; ion chromatographs of [76Br]bromide extracted in 0.6 N NH4OH; radio-TLCs of control reactions using solvents THF, acetonitrile, and DMF; radio-TLCs of radiobromination of 6 and its control reaction; HPLC chromatograph of non-radioactive synthesis of 2 from 4 using NH4Br and PAA; and HPLC chromatographs of a reaction mixture from attempted synthesis of [76Br]2 from 4. This material is available free of charge via the Internet at http://pubs.acs.org.
ACKNOWLEDEGEMENT
The authors thank Bill Margenau, Pat Margenau, Rajendra Singh, Lucie Tang, Grainne Biddlecombe, and Tom Voller for the production of [76Br] isotope. In addition, special thanks go to Joanna B. Downer for excellent editorial assistance and helpful comments. This work was supported by grants from the National Cancer Institute (R24 CA086307 to M. J. W., and R01 CA25836 to J. A. K.).
Footnotes
Abbreviations: AcOH, acetic acid; CAT or Chloramine-T, N-chloro-p-toluenesulfonamide sodium salt; ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; IC, ion chromatography; NCA, no-carrier-added; PPA, peracetic acid; PET, positron emission tomography; SPECT, single photon emission computed tomography; TFA, Trifluoroacetic acid.
Once [76Br]2 was converted to [76Br]1 by the treatment of 1 M LiOH solution, the specific activity of [76Br]1 was determined by HPLC to be up to 1000 mCi/µmol.
LITERATURE CITED
- 1.Reviews see: Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals. New York: John Wiley & Sons Ltd.; 2003.
- 2.Lasne M-C, Perrio C, Rouden J, Barré L, Roeda D, Dolle F, Crouzel C. Chemistry of β+-emitting compounds based on fluorine-18. Top. Curr. Chem. 2002;222:201–258. [Google Scholar]
- 3.Bolton R. Radiohalogen incorporation into organic systems. J. Label. Compd. Radiopharm. 2002;45:485–528. [Google Scholar]
- 4.Seevers RH, Counsell RE. Radioiodination techniques for small organic molecules. Chem. Rev. 1982;82:575–590. [Google Scholar]
- 5.Eisenhut M, Mier W. Radioiodination Chemistry and Radioiodinated Compounds. In: Vértes A, Nagy S, Klencsár Z, editors. Handbook of Nuclear Chemistry. Volume 4: Radiochemistry and Radiopharmaceutical Chemistry in Life Sciences. New York: Springer; 2004. pp. 257–278. Chapter 7. [Google Scholar]
- 6.Coenen HH, Moerlein SM, Stoecklin G. No-carrier added radiohalogenation methods with heavy halogens. Radiochim. Acta. 1983;34:47–68. [Google Scholar]
- 7.Mazière B, Loc’h C. Radiopharmaceuticals labelled with bromine isotopes. Appl. Radiat. Isot. 1986;37:703–713. doi: 10.1016/0883-2889(86)90264-9. [DOI] [PubMed] [Google Scholar]
- 8.Rowland DJ, McCarthy TJ, Welch MJ. Radiobromine for imaging and therapy. In: Welch MJ, Redvanly CS, editors. Handbook of Radiopharmaceuticals. New York: John Wiley & Sons Ltd.; 2003. pp. 441–465. Chapter 14. [Google Scholar]
- 9.Stöcklin G, Pike VW, editors. Radiopharmaceuticals for Positron Emission Tomography. New York: Springer; 1993. p. 3. [Google Scholar]
- 10.Tolmachev V, Lövqvist A, Einarsson L, Schultz J, Lundqvist H. Production of 76Br by a low-energy cyclotron. Appl. Radiat. Isot. 1998;49:1537–1540. doi: 10.1016/s0969-8043(97)00235-2. [DOI] [PubMed] [Google Scholar]
- 11.Malamas MS, Manas ES, McDevitt RE, Gunawan I, Xu ZB, Collini MD, Miller CP, Dinh T, Henderson RA, Keith JC, Jr, Harris HA. Design and synthesis of aryl diphenolic azoles as potent and selective estrogen receptor ligands. J. Med. Chem. 2004;47:5021–5040. doi: 10.1021/jm049719y. [DOI] [PubMed] [Google Scholar]
- 12.Harris HA, Albert LM, Leathurby Y, Malamas MS, Mewshaw RE, Miller CP, Kharode YP, Marzolf J, Komm BS, Winneker RC, Frail DE, Henderson RA, Zhu Y, Keith JC., Jr Evaluation of an estrogen receptor-β agonist in animal models of human disease. Endocrinology. 2003;144:4241–4249. doi: 10.1210/en.2003-0550. [DOI] [PubMed] [Google Scholar]
- 13.Denat F, Gaspard-Iloughmane H, Dubac J. An easy one-pot synthesis of group 14 C-metallated 2(or 3)-furan-and thiophenecarbaldehydes. Synthesis. 1992;10:954–956. [Google Scholar]
- 14.Tang L. Radionuclide production and yields at Washington University School of Medicine. Q. J. Nucl. Med. Mol. Imag. 2008;52:121–133. [PubMed] [Google Scholar]
- 15.Petzold G, Coenen HH. Chloramine-T for no-carrier-added labelling of aromatic biomolecules with bromine-75, 77. J. Label. Compd. Radiopharm. 1981;18:1319–1336. [Google Scholar]
- 16.Suehiro M, Iwamoto M, Arai I, Nozaki T. Bromination, no-carrier-added radiobromination and simultaneously occurring chlorination by chloramine-T. Appl. Radiat. Isot. 1990;41:439–447. doi: 10.1016/0883-2889(90)90002-x. [DOI] [PubMed] [Google Scholar]
- 17.Katzenellenbogen JA, Senderoff SG, McElvany KD, O'Brien HA, Jr, Welch MJ. 16 alpha-[77Br]bromoestradiol-17 beta: a high specific-activity, gamma-emitting tracer with uptake in rat uterus and uterus and induced mammary tumors. J. Nucl. Med. 1981;22:42–47. [PubMed] [Google Scholar]
- 18.Senderoff SG, McElvany KD, Carlson KE, Heiman DF, Katzenellenbogen JA, Welch MJ. Methodology for the synthesis and specific activity determination of 16 alpha-[77Br]-bromoestradiol-17 beta and 16 alpha-[77Br]-11 beta-methoxyestradiol-17 beta, two estrogen receptor-binding radiopharmaceuticals. Int. J. Appl. Radiat. Isot. 1982;33:545–551. doi: 10.1016/0020-708x(82)90010-2. [DOI] [PubMed] [Google Scholar]
- 19.Katzenellenbogen JA, McElvany KD, Senderoff SG, Carlson KE, Landvatter SW, Welch MJ. 16 alpha-[77Br]bromo-11 beta-methoxyestradiol-17 beta: a gamma-emitting estrogen imaging agent with high uptake and retention by target organs. J. Nucl. Med. 1982;23:411–419. [PubMed] [Google Scholar]
- 20.Adam MJ, Ruth TJ, Homma Y, Pate BD. Radiobromination of aromatic compounds by cleavage of aryl-tin bonds. Int. J. Appl. Radiat. Isot. 1985;36:935–937. [Google Scholar]
- 21.Moerlein SM, Coenen HH. Regiospecific no-carrier-added radiobromination and radioiodination of aryltrimethyl group IVb organometallics. J. Chem. Soc. Perkin Trans. 1985:1941–1947. [Google Scholar]
- 22.Mohammad A, Liebhafsky HA. The kinetics of the reduction of hydrogen peroxide by the halides. J. Am. Chem. Soc. 1934;56:1680–1685. [Google Scholar]
- 23.Fortnum DH, Battaglia CJ, Cohen SR, Edwards JO. The kinetics of the oxidation of halide ions by monosubstituted peroxides. J. Am. Chem. Soc. 1960;82:778–782. [Google Scholar]
- 24.Wajon JE, Morris JC. Rates of formation of N-bromo amines in aqueous solution. Inorg. Chem. 1982;21:4258–4263. [Google Scholar]
- 25.Inman GW, Jr, Johnson JD. Kinetics of monobromamine disproportionation-dibromamine formation in aqueous ammonia solutions. Environ. Sci. Technol. 1984;18:219–224. doi: 10.1021/es00122a002. [DOI] [PubMed] [Google Scholar]
- 26.Mellegaard-Waetzig SR, Wang C, Tunge JA. Selenium-catalyzed oxidative halogenation. Tetrahedron. 2006;62:7191–7198. [Google Scholar]
- 27.Lang L, Ma Y, Jagoda EM, Kim BM, Rice KC, Szajek LP, Contoreggi C, Eckelman WC, Kiesewetter DO. [76Br]BMK-152: a PET ligand for corticotropin-releasing hormone (CRH) type 1 receptor. J. Label. Compd. Radiopharm. 2007;50:S56. [Google Scholar]
- 28.Ogata Y, Aoki K. Haloacyloxylation. II. addition of bromine or chlorine to propylene in the presence of peracetic acid. J. Org. Chem. 1966;31:4181–4183. [Google Scholar]
- 29.Barnett JR, Andrews LJ, Keefer RM. Trifluoroacetyl hypohalites as aromatic halogenating agents. J. Am. Chem. Soc. 1972;94:6129–6134. [Google Scholar]
- 30.Voudrias EA, Reinhard M. Reactivities of hypochlorous and hypobromous acid, chlorine monoxide, hypobromous acidium ion, chlorine, bromine, and bromine chloride in electrophilic aromatic substitution reactions with p-xylene in water. Environ. Sci. Technol. 1988;22:1049–1056. doi: 10.1021/es00174a009. [DOI] [PubMed] [Google Scholar]
- 31.Derbyshire DH, Waters WA. Significance of bromine cation in aromatic substitution. I. Kinetic evidence. J. Chem. Soc. 1950:564–573. [Google Scholar]
- 32.Wang TX, Kelley MD, Cooper JN, Beckwith RC, Margerum DW. Equilibrium, kinetic, and UV-spectral characteristics of aqueous bromine chloride, bromine, and chlorine species. Inorg. Chem. 1994;33:5872–5878. [Google Scholar]
- 33.Sander R, Crutzen PJ. Model study indicating halogen activation and ozone destruction in polluted air masses transported to the sea. J. Geophys. Res. 1996;101:9121–9138. [Google Scholar]
- 34.Farkas L, Lewin M, Bloch R. The reaction between hypochlorite and bromides. J. Am. Chem. Soc. 1949;71:1988–1991. [Google Scholar]
- 35.Bielski BHJ, Cabelli DE, Arudi RL, Ross AB. Reactivity of HO2/O2radicals in aquesous solution. J. Phys. Chem. Ref. Data. 1985;14:1041–1100. [Google Scholar]
- 36.Eigen M, Kustin K. The kinetics of halogen hydrolysis. J. Am. Chem. Soc. 1962;84:1355–1361. [Google Scholar]
- 37.Liebhafsky HA. The equilibrium constant of the bromine hydrolysis and its variation with temperature. J. Am. Chem. Soc. 1934;56:1500–1505. [Google Scholar]
- 38.Gilow HM, Ridd JH. Mechanism of aromatic bromination by hypobromous acid in aqueous perchloric acid. Kinetic evidence against the prior formation of ‘positive bromine’. J. Chem. Soc., Perkin Trans. 1973;2:1321–1327. [Google Scholar]
- 39.Kumar K, Margerum DW. Kinetics and mechanism of general-acid-assisted oxidation of bromide by hypochlorite and hypochlorous acid. Inorg. Chem. 1987;26:2706–2711. [Google Scholar]
- 40.De la Mare PBD. Electrophilic halogenation : reaction pathways involving attack by electrophilic halogens on unsaturated compounds. London: Cambridge University Press; 1976. p. 125. Chapter 7. [Google Scholar]
- 41.Langer O, Dollé F, Loc'h C, Halldin C, Vaufrey F, Coulon C, Crouzel C, Någren K, Mazière B. Preparation of 4- and 6-[76Br] bromometaraminol, two potential radiotracers for the study of the myocardial norepinephrine neuronal reuptake system with PET. J. Label. Compd. Radiopharm. 1997;39:803–816. [Google Scholar]
- 42.Zhou D, Sharp TL, Fettig NM, Lee H, Lewis JS, Katzenellenbogen JA, Welch MJ. Evaluation of a bromine-76-labeled progestin 16α,17α-dioxolane for breast tumor imaging and radiotherapy: in vivo biodistribution and metabolic stability studies. Nucl. Med. Bio. 2008;35:655–663. doi: 10.1016/j.nucmedbio.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bolton AE, Hunter WM. The labeling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem. J. 1973;133:529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthesis of [76Br]3 using premixed 2:1 H2O2/AcOH and using PAA/H2O; radio-TLCs of radiobromination of 4 and its control reaction; ion chromatographs of [76Br]bromide extracted in 0.6 N NH4OH; radio-TLCs of control reactions using solvents THF, acetonitrile, and DMF; radio-TLCs of radiobromination of 6 and its control reaction; HPLC chromatograph of non-radioactive synthesis of 2 from 4 using NH4Br and PAA; and HPLC chromatographs of a reaction mixture from attempted synthesis of [76Br]2 from 4. This material is available free of charge via the Internet at http://pubs.acs.org.