Abstract
Background/Objective
Antiretroviral therapy (ART) adherence levels of ≥95% optimize outcomes and minimize HIV drug resistance. As such, identifying barriers to adherence is essential. We sought to assess travel to point-of-care for ART as a potential barrier to adherence in rural Zambia, within the context of patient demographics, perceived stigma, and selected clinical indices.
Methods
We studied 424 patients receiving ART from the Macha Mission Hospital (MMH). Interviews ascertained age, gender, education, perceived stigma, nearest rural health facility (RHF), and mode/cost/time of transport for each study participant. Motorcycle odometer and global positioning system way-points measured distance from the MMH to each of the RHFs, estimating patients’ home-to-MMH travel distances. Body mass index, World Health Organization HIV/AIDS stage, and pill counts were assessed from review of patients’ medical and pharmacy records.
Results
At least 95% adherence was documented for 83.7% of the patients in their first months of ART. Travel-related factors did not predict adherence. Adherence was higher for those on ART for a longer time (odds ratio = 1.04 per day; P = 0.002).
Conclusions
Patients in rural Zambia can achieve adherence rates compatible with good clinical outcomes despite long travel distances. The MMH was able to provide quality HIV/AIDS care by implementing programmatic features selecting for a highly adherent population in this resource-limited setting.
Keywords: access to health care, antiretroviral therapy, HIV, medically underserved area, patient compliance, Zambia
Zambia is a sub-Saharan African country of 11.7 million people, in which an estimated 17% of adults (age range: 15 to 49 years old) are infected with HIV.1 The potential for antiretroviral therapy (ART) to reduce morbidity and mortality attributable to HIV/AIDS is well documented.2–7 As we strive to prevent new cases, there is a compelling need to expand ART services. With increasing availability of ART under the auspices of the President’s Emergency Plan for AIDS Relief (PEPFAR); the Global Fund to Fight AIDS, Tuberculosis, and Malaria; and other sources, ongoing assessment of barriers to care is essential. Barriers to ART adherence are particularly important in that once initiated, adherence levels of ≥95% are required to optimize measures of patient outcomes8–17 and to prevent the emergence of antiretroviral drug-resistant strains of HIV.9,18 High levels of adherence to HIV/AIDS care have validated the benefits of ART in several resource-limited settings, although data on the effectiveness of ART delivery in rural areas are scarce.19–25 In Zambia, approximately half of the population lives in rural areas; thus, an assessment of barriers to care in the rural setting is germane.26
Health care facilities may be underutilized when they are distant or otherwise less accessible to patients. For example, duration of travel to health care is longer in rural areas as compared with urban areas.27–31 Longer duration of travel has been identified as a potential barrier to adherence in some studies32–36 but not in all.37 We conducted a study of HIV-infected persons receiving ART in rural Zambia, hypothesizing that lower ART adherence would correlate with longer patient travel duration and distance (home to point-of-care). Furthermore, we predicted that lower ART adherence would correlate with lack of access to transportation and higher travel costs.
METHODS
Background
We conducted this study in Zambia’s southern province at the Macha Mission Hospital (MMH), a 208-bed rural mission hospital that serves a population of approximately 160,000 people. In March 2005, antiretroviral medications (ARVs) became available to the MMH through the Zambian government, and in August 2005, the program was enhanced by means of AIDSRelief, a PEPFAR-supported consortium of faith-based institutions. ARVs are ordered from the Zambian government by means of a consortium partner (Catholic Relief Services), and other medications are procured through the government medical stores or directly from in-country pharmaceutic suppliers.
After testing positive for HIV, patients are eligible for enrollment into the MMH ART program. Before ARVs are initiated, however, patients must first participate in 2 group and 1 individual counseling sessions and they must demonstrate full adherence to non-ARV medications. On a patient’s first visit, clinical stage is assessed, blood is taken for screening, CD4 cell count is measured (if equipment and supplies allow at the time of the visit), and the first group counseling session takes place. Patients are given multivitamins and cotrimoxazole prophylaxis as treatment and as a tool to assess adherence. Two weeks later, at the second visit, the patient must bring a friend or family member (termed a treatment supporter), laboratory findings are evaluated, adherence is assessed by means of pill count for the multivitamins and cotrimoxazole, and a second group counseling session takes place. Based on clinician judgment, a decision is made concerning eligibility for ARVs. If ARVs are approved, the patient and his or her treatment supporter must return for a third clinic visit 2 weeks later. At this visit, the patient again has to demonstrate near-full adherence to multivitamins and cotrimoxazole. Each physician is given a degree of flexibility with regard to initiating ART; however, typically, treatment is delayed until these adherence requirements are met. The patient and treatment supporter have an individual counseling session the day the patient starts ARVs and at any later time as requested by clinic staff.
Population Sample
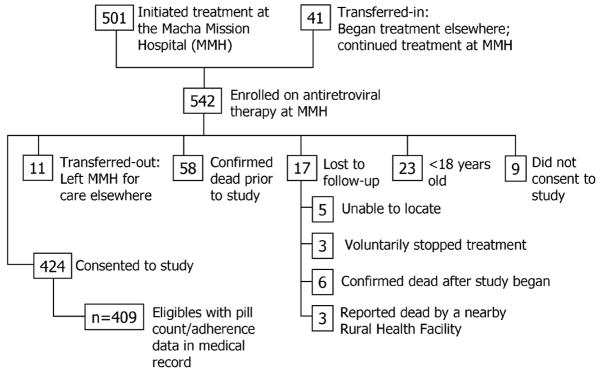
There were 542 people who had received ART from the MMH ART clinic as of June 6, 2006. Of these patients, 501 had initiated treatment at the MMH and 41 had initiated treatment elsewhere and then “transferred in” to continue their treatment at the MMH. We excluded persons who could not provide consent: those who had initiated care at the MMH and then “transferred out” to receive care elsewhere (n = 11); those who were confirmed dead before June 6, 2006 (n = 58); and those who had been lost to follow-up before June 6, 2006 (n = 17). Those who were <18 years old (n = 23) were also excluded. Nine patients did not consent to participation in the study. The remaining 424 patients (78.2% of the 542 patients placed on ARVs at the MMH) were recruited to the study during one of their regularly scheduled appointments from June to October 2006 (Fig. 1). Verbal informed consent was in the language of patient preference: Chitonga or English.
FIGURE 1.
Volunteers and population of origin for the rural Zambian study of predictors of adherence to ART (final study: n = 409).
Survey
Each patient was interviewed by 1 of 2 study staff members (using an interpreter when necessary) at one of his or her regularly scheduled appointments. A standardized survey was used to collect information on age, gender, tribal group/chiefdom, education, perceived sources of stigma, nearest rural health facility (RHF), mode and cost of transport, and home-to-MMH travel duration. Mode, cost, and duration of travel were queried for the dry and rainy seasons.
Chart Review
World Health Organization (WHO) stage, body mass index (BMI), and data used to calculate adherence to ART were extracted from each patient’s medical record.
BMI was calculated from heights and weights measured at patients’ initial clinic visits. WHO stage was assessed by ART clinic physicians at patients’ initial clinic visits.38 Thus, BMI and WHO stage at onset of treatment were considered proxy measures of baseline nutritional status and overall health, respectively.
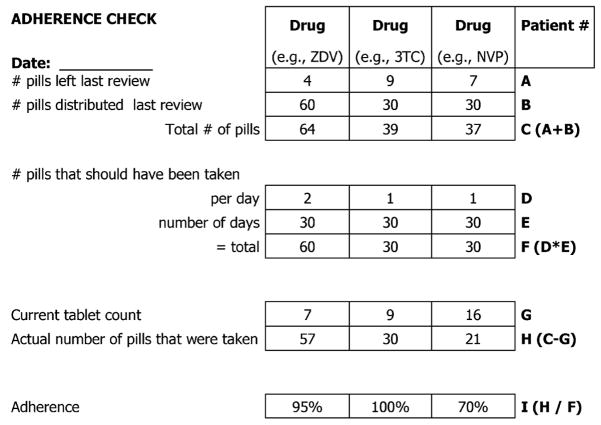
Adherence was defined as the number of doses taken out of the number of doses prescribed; optimal adherence was defined as ≥95% scheduled doses taken.8,11 Beginning in March 2006, the MMH began using pill counts in conjunction with pharmacy records to assess adherence at every clinic visit.39 Patients were required to bring their medications to all their ART clinic appointments. To determine the percent adherence to each ARV since the patient’s previous appointment, each ARV from a patient’s individualized multiple-drug regimen was counted and the number of remaining pills was compared with the number of pills that should have been remaining (Fig. 2). If adherence to any of the ARVs was <100%, a physician would probe into the frequency and reasons for the patient having missed doses and would counsel the patient about the ill effects of nonadherence. Calculations revealing adherence of >100% resulted occasionally when the number of remaining pills was less than the number of pills that should have been remaining based on pharmacy records. When this occurred, adherence was considered 100% and the patient was probed about the source of the discrepancy. When calculations revealed different adherence percentages for the various ARVs in an individual’s regimen, the drug to which the patient had the lowest percent adherence was used as a conservative estimate of the patient’s adherence for that interval of time over which the calculation was made. To determine overall percent adherence throughout the course of a patient’s treatment, an average was calculated using the most conservative estimates of percent adherence from each appointment and then adjusted for the total number of days over which the series of adherence calculations were made. We were unable to calculate adherence for 15 (3.5%) of the 424 patients because of insufficient pill count data. Patients were also asked to self-report missed doses; however, because of the paucity and subjectivity of the self-reported data, we decided to include only adherence as determined by pill counts in our final analyses.
FIGURE 2.
Form used to calculate patient adherence. The form utilizes pill counts (A and G) and pharmacy records (B) to determine the percentage of doses taken out of the number of doses prescribed for a given interval of time for a particular drug regimen. The lowest adherence percentage calculated for a single review was considered as a conservative estimate of a patient’s overall adherence. In this example, adherence was calculated for a 30-day period between clinic visits, and overall adherence for this period would be considered 70%. NVP indicates nevirapine; 3TC, lamivudine; ZDV, zidovudine.
The number of days over which adherence was measured was also considered for each of the 409 patients for whom we were able to calculate adherence. For patients initiating ART at the MMH after March 2006, the total days over which adherence was measured was the same as their total days on ART (n = 155). For patients initiating ART at the MMH before March 2006, the total days over which adherence was measured was less than their total days on ART; these patients had been receiving ART for ≥2 months at the onset of this study (n = 224). The remaining 30 patients transferred in to receive ART from the MMH, and as such, the total number of days over which their adherence was measured was at least as long as their total number of days on ART.
Mapping of Rural Health Facilities
We approximated the distance that a patient had to travel to the MMH by using the distance from the patient’s nearest RHF to the MMH. Two different approximations of this distance were recorded: the distance by road/trail between the RHF and the MMH (“actual distance”) and the straight-line global positioning system (GPS)–derived distance between the 2 facilities (“linear distance”).
Actual Distance
A total of 66 RHFs were identified in patient interviews. Fifty-five of the RHFs were within 60 km of the MMH. For these 55 RHFs, the distance between the MMH and RHF was measured by odometer using the shortest path accessible by motorcycle. The motorcycle odometer was verified by the GPS device odometer to ensure accuracy. The remaining 11 RHFs were too far away to be visited within the practical constraints of this study. Distances for 10 of them were estimated indirectly using existing maps belonging to the Malaria Institute at Macha (MIAM). The distance for 1 RHF was not measured because it could not be found on a detailed map or visited by motorcycle.
Linear Distance
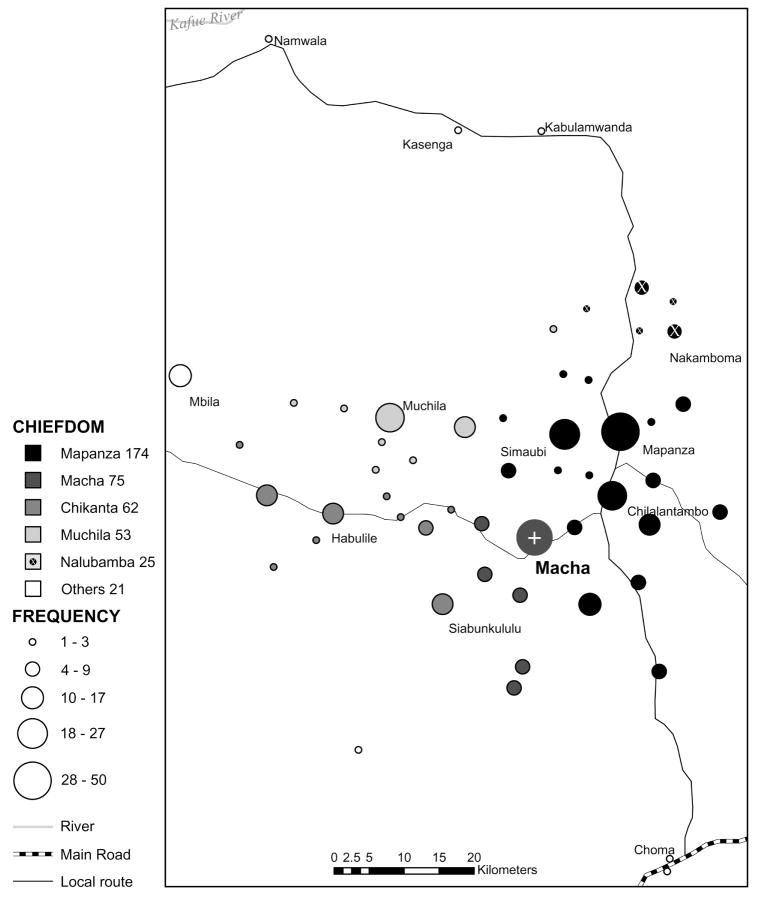
Garmin GPS 72 and Garmin 60CS (Garmin International, Olathe, KS) devices were used to collect way-points at each of the 55 RHFs accessible by motorcycle. Way-points for 2 of the 11 RHFs not visited during this study were obtained from prior MIAM staff visits to these sites. Way-points for the remaining identified RHFs were estimated using the nearest RHF (in the direction of the MMH) in relation to the RHF in question to estimate a patient’s linear distance from the MMH conservatively. Linear distances for 2 of the RHFs could not be measured: one was neither visited by motorcycle nor located on a map, whereas the other was visited by motorcycle; however, a GIS way-point was not collected for either, and we were not able to locate either on a map. ArcGIS Desktop 9.1 (Environmental Systems Research Institute, Redlands, CA) used the RHF way-points to construct a map of the Macha catchment, from which linear distances between each RHF and the MMH were extrapolated (Fig. 3), with chiefdoms superimposed.
FIGURE 3.
The number of patients per RHF per chiefdom. The map was created from GPS way-points of RHFs nearest to patients’ homes, as indicated by patient interviews.
Statistical Methods
Continuous variables, including age, actual distance, linear distance, home-to-MMH travel duration (dry and rainy seasons), days on ART, and BMI, were compared between optimal adherers (≥95%) and suboptimal adherers (<95%) using the Wilcoxon rank sum test. Possible associations between adherence and categoric variables, including gender, education, mode and cost of transport (dry and rainy seasons), stigma, and WHO stage, were tested using the Pearson χ2 test. Multivariable logistic regression was used to determine the effects (magnitude and direction) of these categoric and continuous variables on adherence.
Based on our qualitative interviews and preliminary data analysis, home-to-MMH travel duration, actual distance, and linear distance were all used in separate multivariable models. Home-to-MMH travel duration had the advantage of measuring the full effort required by a patient to reach the clinic, considering modes and availability of transportation, road obstacles, and distances.
RESULTS
Of the 409 patients for whom pill counts were available, 83.7% had optimal (≥95%) adherence (mean adherence = 96.6%, median adherence = 98.7%). In the complete cohort, no significant associations (P < 0.05) were found between adherence and any of the continuous or categoric variables tested. Among the 155 patients for whom complete adherence data were available for their total days on ART, those who were optimally adherent were more likely to have been on ART for more days than those who had suboptimal adherence (median: 73 vs. 56 days on ART; P = 0.005). None of the distance measures were associated with adherence (Table 1).
TABLE 1.
Characteristics of HIV-Infected Zambians Who Optimally Adhered (≥95% Adherence) to ART and Those Who Did Not
| n | <95% Adherence n = 54 | ≥95% Adherence n = 355 | Combined n = 409 | P* | |
|---|---|---|---|---|---|
| Age, y† | 409 | 39 (32 to 48) | 39 (32 to 47) | 39 (32 to 47) | 0.9 |
| Gender‡ | 409 | 1.0 | |||
| Male | 37% (20) | 37% (131) | 37% (151) | ||
| Female | 63% (34) | 63% (224) | 63% (258) | ||
| Formal education‡ | 409 | 0.5 | |||
| ≤Primary | 65% (35) | 60% (212) | 60% (247) | ||
| >Primary | 35% (19) | 40% (143) | 40% (162) | ||
| Actual distance, km† | 408 | 30 (19 to 52) | 26 (16 to 46) | 26 (16 to 47) | 0.1 |
| Linear distance, km† | 406 | 21 (15 to 30) | 19 (13 to 27) | 19 (13 to 29) | 0.1 |
| Home-to-MMH duration during dry season, h† | 409 | 3.0 (2.0 to 5.0) | 3.4 (2.0 to 5.0) | 3.0 (2.0 to 5.0) | 0.9 |
| Home-to-MMH duration during rainy season, h† | 409 | 4.0 (3.0 to 6.0) | 5.0 (3.0 to 8.0) | 5.0 (3.0 to 8.0) | 0.5 |
| Mode of transport in dry season‡ | 408 | 0.7 | |||
| Foot | 35% (19) | 43% (153) | 42% (172) | ||
| Bicycle or motorcycle | 24% (13) | 23% (81) | 23% (94) | ||
| Bicycle (with help) | 15% (8) | 11% (39) | 12% (47) | ||
| Group transport or other | 26% (14) | 23% (81) | 23% (95) | ||
| Mode of transport in rainy season‡ | 408 | 0.4 | |||
| Foot | 33% (18) | 44% (155) | 42% (173) | ||
| Bicycle or motorcycle | 20% (11) | 22% (78) | 22% (89) | ||
| Bicycle (with help) | 17% (9) | 12% (41) | 12% (50) | ||
| Group transport or other | 30% (16) | 23% (80) | 24% (96) | ||
| Cost of transport in dry season (Kwacha)‡ | 409 | 0.4 | |||
| No cost | 72% (39) | 77% (275) | 77% (314) | ||
| Some cost | 28% (15) | 23% (80) | 23% (95) | ||
| Cost of transport in rainy season (Kwacha)‡ | 409 | 0.2 | |||
| No cost | 69% (37) | 77% (275) | 76% (312) | ||
| Some cost | 31% (17) | 23% (80) | 24% (97) | ||
| Perceived stigma‡ | 409 | 0.9 | |||
| No | 65% (35) | 66% (233) | 66% (268) | ||
| Yes | 35% (19) | 34% (122) | 34% (141) | ||
| WHO stage at initial visit‡ | |||||
| 1 | 19% (10) | 9% (31) | 10% (41) | ||
| 2 | 22% (12) | 25% (88) | 25% (100) | ||
| 3 | 50% (27) | 56% (196) | 55% (223) | ||
| 4 | 403 | 9% (5) | 10% (34) | 10% (39) | 0.2 |
| BMI at initial visit† | 361 | 18 (17 to 20) | 18 (17 to 20) | 18 (17 to 20) | 0.4 |
| Days over which adherence was measured† | 409 | 64 (47 to 98) | 84 (57 to 98) | 84 (56 to 98) | 0.06 |
| Days on ART†§ | 155 | 56 (35 to 63) | 73 (56 to 84) | 69 (56 to 84) | 0.005 |
Wilcoxon test for continuous variables. χ2 test for categoric variables.
Continuous variables, including age, actual distance, linear distance, home-to-MMH travel duration (dry and rainy seasons), BMI, days over which adherence was measured, and days on ART, are reported as median (lower quartile to upper quartile).
Categoric variables, including gender, education, mode and cost of transport (dry and rainy seasons), stigma, and WHO stage, are reported as percent (number).
Patients initiating ART at the MMH after March 2006, for whom their total days over which adherence was measured was the same as their total days on ART.
The primary multivariable model, including home-to-MMH travel duration (dry season), WHO stage dichotomized into stages 1 and 2 versus stages 3 and 4, BMI, perceived stigma, and cost of transport (dry season) dichotomized into no cost or some cost, did not demonstrate any significant (P < 0.05) predictors of adherence (Table 2).
TABLE 2.
Primary Multivariable Model: Relation of Adherence to Home-to-MMH Travel Duration (Dry Season), WHO Stage Dichotomized Into Stages 1 and 2 Versus Stages 3 and 4, BMI, Patient-Perceived HIV Stigma, and Cost of Transport (Dry Season) Dichotomized Into No Cost or Some Cost
| Parameter | OR | 95% CI | P |
|---|---|---|---|
| Home-to-MMH travel duration, h (dry season) | 1.0 | (0.91 to 1.1) | 0.9 |
| WHO stage 3 or 4 | 1.9 | (0.98 to 3.7) | 0.06 |
| BMI | 1.1 | (0.96 to 1.3) | 0.2 |
| Stigma: had perceived vs. none | 1.1 | (0.55 to 2.1) | 0.8 |
| Cost of transport (dry season):some cost vs. none | 0.7 | (0.35 to 1.4) | 0.3 |
Home-to-MMH travel duration was correlated with linear and actual distance (Spearman rank correlation = 0.56 and P < 0.0001 for both). For completeness, identical models substituting the distance measures for travel duration were also analyzed. Neither linear distance (odds ratio [OR] = 0.96 per 10 km, 95% confidence interval [CI]: 0.80 to 1.15; P = 0.6) nor actual distance (OR = 0.97 per 10 km, 95% CI: 0.85 to 1.11; P = 0.7) was a predictor of adherence.
The primary model using home-to-MMH travel duration in the dry season was also fit, including age, gender (with male as the reference group), and the dichotomous education variable (with primary school or less as the reference group). None of these demographic variables added useful information to the model (OR = 0.87 per 10 years, 95% CI: 0.64 to 1.17; P = 0.4 [OR = 0.87 for females, 95% CI: 0.44 to 1.7; P = 0.7 and OR = 1.1 for more than primary school, 95% CI: 0.55 to 2.0; P = 0.9]).
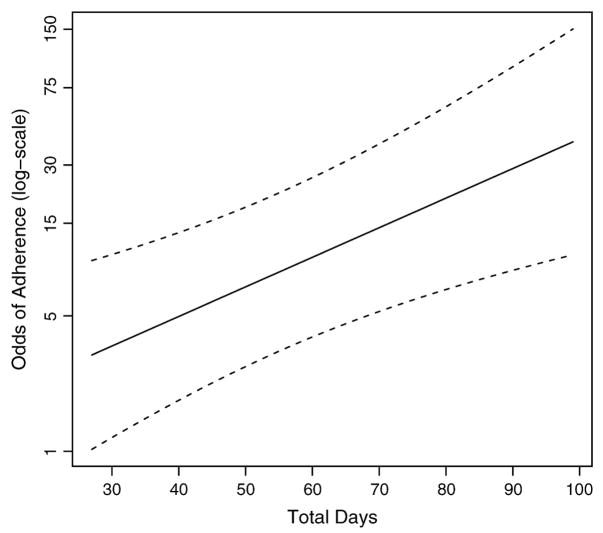
The primary model was also modified to include total days on ART, including only the 155 subjects for whom the total days over which adherence was measured equaled total days on ART. First, “total days on ART” was dichotomized at 30 days. When controlling for all other variables in the model, those patients observed >30 days had 3.1 times the odds of ≥95% adherence compared with those whose adherence had been measured for ≤30 days (95% CI: 1.0 to 9.4; P = 0.04). The model was refit with days on ART left as a continuous variable using restricted cubic splines in the event that the relation between days on ART and adherence was not linear. Results showed the nonlinear portion to be nonsignificant (P = 0.35). Therefore, the model was fit assuming linearity between days on ART and adherence. Odds of adherence increased with days on ART when controlling for all other variables in the model (OR = 1.04 per day, 95% CI: 1.01 to 1.06; P = 0.002; Fig. 4).
FIGURE 4.
Odds of adherence across the total days over which pill counts were measured (days on ART), adjusted for home-to-MMH travel time, WHO stage, BMI, perceived stigma, and cost of transportation at their median values.
DISCUSSIN
High Rates of Adherence
In the face of considerable challenges, including lengthy travel, variable modes and costs of transport, and advanced disease, patients in rural Zambia can achieve adherence rates to ART compatible with good clinical outcomes, despite dire predictions of the nonfeasibility of ART in rural settings.40 We believe that the MMH’s prescreening efforts succeeded in identifying highly adherent individuals; as such, we acknowledge that our results might not be generalizable to programs that are more permissive in ART initiation.
Predictors of Adherence
Travel duration and distance were not predictive of adherence. Most patients traveled 3 or more hours each way, often on foot; yet, most were able to achieve optimal adherence in their first months of therapy. It is likely that the rural and predominantly agricultural lifestyle of this population involves and allows burdensome travel, whether it is to sell products, obtain water, or facilitate access to health care. Those individuals who successfully fulfilled prescreening demands despite these travel burdens were likely to be highly motivated. In addition, there are no reasonable alternatives to securing HIV care in this region of rural Zambia. The MMH is in the process of expanding its field outreach for HIV care to reduce travel burden and make effective ART possible for persons unable to access care at present.
For at least the first several months of treatment, the number of days a patient was on ART was highly predictive of ART adherence (see Fig. 4). In this real-world setting, ART adherence was higher for people who had been on treatment longer. Programmatic strategies should focus on maintaining high levels of adherence through ongoing education and interventions to make programs more convenient for rural residents, especially within the first months on ART.
Programmatic Features
In settings in which material and human resources are limited, rationing of care continues to be a reality.41,42 In the context of HIV/AIDS care, rationing is often based on pragmatic considerations. Not only is there concern about individuals failing treatment as a result of poor adherence but there is the risk of poor adherence contributing to drug resistance. To diminish this possibility, the MMH program maintains strategies that promote adherence by selectively granting access to patients who have demonstrated adherence to appointments and non-ARV medications. Only patients demonstrating excellent adherence to a multiweek course of multivitamins and cotrimoxazole are initiated into an ARV regimen. The results of our study provide evidence for the utility of rationing care in this way. Only a fraction of persons infected with HIV in this rural area of Zambia were receiving ARVs at the time of this study, however. Even if only 8% of the people living within the MMH catchment area of 160,000 people are infected with HIV, the MMH ART clinic covered <4% of the infected population in 2006. Whether a program ensuring adherence through a “vetting” process that reduces the risk of emergence of drug-resistant HIV or whether a program with more permissive enrollment criteria that allows rapid benefit to numerous individuals is the better approach is a subject for further investigation.
Research Strengths and Limitations
Problems with adherence are not likely to be random events; individuals’ adherence rates correlate across visits.43 As such, a program that filters out nonadherers is likely to enhance adherence-dependent outcomes. Regarding these potential nonadherers, we, unfortunately, do not have data on the extent of unmet need (or mortality) among other HIV-infected persons. We also cannot comment on whether the MMH method of rationing care has led any specific groups—socioeconomic, geographic, age, gender, or otherwise—to have differential access to care. Ideally, increased supply (from increased programmatic resources) and decreased demand (from successful prevention programs) would eliminate the need for rationing care; however, until then, programmatic strategies must be used to maximize benefit and to minimize harm.
The major strengths of this study include the fact that we were able to enroll nearly four-fifths of the persons receiving ART in this rural Zambian setting. Also, we had multiple measures of travel burden, such that multiple approaches could be assessed in case one or another approach might be superior. Furthermore, our data set was remarkably complete, given its reliance on review of medical and pharmacy records.
A limitation is that we were unable to include those individuals who initiated ART at the MMH but died before the study.44 Such persons may have been so seriously ill that they died despite adhering to ART, or, in contrast, they might have been nonadherent and died as a consequence. In addition, we have no adherence data for the 17 patients lost to follow-up before the study. Hence, we may have slightly overestimated the actual adherence levels of this population. Furthermore, using pill counts and pharmacy records to measure adherence has limitations. This method likely overestimates adherence; there is no way to guarantee that missing pills were actually taken by the patient.39 Those patients with fewer pills in their bottles than they should have had (ie, had >100% adherence according to the calculation) were considered to have had 100% adherence; it is conceivable that some of them took extra doses to compensate for side effects (eg, vomiting), gave their ARVs to others, or may have discarded pills. The relatively short duration of this study is another limitation, and we hope to mount a follow-up study in 2008. It may be that highly adherent people need to be observed over a longer period to reveal whether distance has a long-term effect on adherence.
Implementation research is a vital component of the expansion of ARTwithin developing countries because there is little experience with lifelong administration of medications in the world’s poorest rural settings. As urban areas are covered with HIV care and treatment services, we need new models of care for rural areas that take into account serious travel, financial, cultural, and infrastructural challenges. In a few years, one hopes that African HIV programs may further inform care and adherence for other lifelong therapy needs.
Acknowledgments
Supported in part by a President’s Emergency Plan for AIDS Relief award to the Catholic Relief Services by the United States Agency for International Development (R. Redfield, PI), the Vanderbilt-Meharry Center for AIDS Research (National Institutes Health grant P30AI054999), the Vanderbilt University School of Medicine Emphasis Program, and the Overall Fellowship.
The authors thank Charles Banda for help with patient interviews and translation; Robert Redfield and Anthony Amoroso of the Institute for Human Virology at the University of Maryland for help in conceptualizing and initiating the project; Phil Thuma, Janneke VanDijk, Harry Hamapumbu, and Petros Moono of the MIAM for intellectual and material support; Robin Kraak, Iman VanDenBerge, and Hope Mkunte for help with the MMH patient database; Melvin Mabeta for help with survey translation and mapping; Peter Wright for support as the International Health Emphasis Area leader at Vanderbilt University School of Medicine; and the staff of the MMH, AIDS Relief, and Catholic Relief Services in Zambia for making the project possible.
References
- 1.World Bank. [Accessed January 27, 2007];World development indicators: Zambia data profile. Available at: http://devdata.worldbank.org.proxy.library.vanderbilt.edu/external/CPProfile.asp?PTYPE=CP&CCODE=ZMB.
- 2.Hogg RS, Yip B, Kully C, et al. Improved survival among HIV-infected patients after initiation of triple-drug antiretroviral regimens. CMAJ. 1999;160:659–665. [PMC free article] [PubMed] [Google Scholar]
- 3.Jerene D, Naess A, Lindtjorn B. Antiretroviral therapy at a district hospital in Ethiopia prevents death and tuberculosis in a cohort of HIV patients. AIDS Res Ther. 2006;3:1–10. doi: 10.1186/1742-6405-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy EL, Collier AC, Kalish LA, et al. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135:17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- 5.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 6.Begovac J, Lisic M, Lukas D, et al. Marked improvement in survival among adult Croatian AIDS patients after the introduction of highly active antiretroviral treatment. Coll Antropol. 2006;30:175–179. [PubMed] [Google Scholar]
- 7.Chan CW, Cheng LS, Chan WK, et al. Highly active antiretroviral therapy per se decreased mortality and morbidity of advanced human immuno-deficiency virus disease in Hong Kong. Chin Med J (Engl) 2005;118:1338–1345. [PubMed] [Google Scholar]
- 8.Alexander CS, Asselin JJ, Ting LS, et al. Antiretroviral concentrations in untimed plasma samples predict therapy outcome in a population with advanced disease. J Infect Dis. 2003;188:541–548. doi: 10.1086/376835. [DOI] [PubMed] [Google Scholar]
- 9.Harrigan PR, Hogg RS, Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191:339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 10.Howard AA, Arnsten JH, Lo Y, et al. A prospective study of adherence and viral load in a large multi-center cohort of HIV-infected women. AIDS. 2002;16:2175–2182. doi: 10.1097/00002030-200211080-00010. [DOI] [PubMed] [Google Scholar]
- 11.Paterson DL, Swindells S, Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 12.Press N, Tyndall MW, Wood E, et al. Virologic and immunologic response, clinical progression, and highly active antiretroviral therapy adherence. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S112–S117. doi: 10.1097/00126334-200212153-00005. [DOI] [PubMed] [Google Scholar]
- 13.Van Dyke RB, Lee S, Johnson GM, et al. Reported adherence as a determinant of response to highly active antiretroviral therapy in children who have human immunodeficiency virus infection. Pediatrics. 2002;109:e61. doi: 10.1542/peds.109.4.e61. [DOI] [PubMed] [Google Scholar]
- 14.Wood E, Hogg RS, Yip B, et al. Effect of medication adherence on survival of HIV-infected adults who start highly active antiretroviral therapy when the CD4+ cell count is 0.200 to 0.350 × 10(9) cells/L. Ann Intern Med. 2003;139:810–816. doi: 10.7326/0003-4819-139-10-200311180-00008. [DOI] [PubMed] [Google Scholar]
- 15.Wood E, Hogg RS, Yip B, et al. Impact of baseline viral load and adherence on survival of HIV-infected adults with baseline CD4 cell counts > or = 200 cells/μL. AIDS. 2006;20:1117–1123. doi: 10.1097/01.aids.0000226951.49353.ed. [DOI] [PubMed] [Google Scholar]
- 16.Gross R, Yip B, Re VL, 3rd, et al. A simple, dynamic measure of antiretroviral therapy adherence predicts failure to maintain HIV-1 suppression. J Infect Dis. 2006;194:1108–1114. doi: 10.1086/507680. [DOI] [PubMed] [Google Scholar]
- 17.Garcia de Olalla P, Knobel H, Carmona A, et al. Impact of adherence and highly active antiretroviral therapy on survival in HIV-infected patients. J Acquir Immune Defic Syndr. 2002;30:105–110. doi: 10.1097/00042560-200205010-00014. [DOI] [PubMed] [Google Scholar]
- 18.Pillay D. The emergence and epidemiology of resistance in the nucleoside-experienced HIV-infected population. Antivir Ther. 2001;6(Suppl 3):15–24. [PubMed] [Google Scholar]
- 19.Byakika-Tusiime J, Oyugi JH, Tumwikirize WA, et al. Adherence to HIV antiretroviral therapy in HIV+ Ugandan patients purchasing therapy. Int J STD AIDS. 2005;16:38–41. doi: 10.1258/0956462052932548. [DOI] [PubMed] [Google Scholar]
- 20.Laniece I, Ciss M, Desclaux A, et al. Adherence to HAART and its principal determinants in a cohort of Senegalese adults. AIDS. 2003;17(Suppl 3):S103–S108. doi: 10.1097/00002030-200317003-00014. [DOI] [PubMed] [Google Scholar]
- 21.Orrell C, Bangsberg DR, Badri M, et al. Adherence is not a barrier to successful antiretroviral therapy in South Africa. AIDS. 2003;17:1369–1375. doi: 10.1097/00002030-200306130-00011. [DOI] [PubMed] [Google Scholar]
- 22.Stringer JS, Zulu I, Levy J, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. JAMA. 2006;296:782–793. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 23.Weiser S, Wolfe W, Bangsberg D, et al. Barriers to antiretroviral adherence for patients living with HIV infection and AIDS in Botswana. J Acquir Immune Defic Syndr. 2003;34:281–288. doi: 10.1097/00126334-200311010-00004. [DOI] [PubMed] [Google Scholar]
- 24.Ferradini L, Jeannin A, Pinoges L, et al. Scaling up of highly active antiretroviral therapy in a rural district of Malawi: an effectiveness assessment. Lancet. 2006;367:1335–1342. doi: 10.1016/S0140-6736(06)68580-2. [DOI] [PubMed] [Google Scholar]
- 25.Mills EJ, Nachega JB, Buchan I, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296:679–690. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 26.Zambia Demographic and Health Survey 2001–2002. Calverton, MD: Central Statistical Office, Central Board of Health; 2003. Available at: http://www.measuredhs.com/pub_details.cfm?ID=403. [Google Scholar]
- 27.Attah EB. Underutilization of Public Sector Health Facilities in Imo State, Nigeria: A Study with Focus Groups. Washington, DC: World Bank; 1986. [Google Scholar]
- 28.Heller PS. A model of the demand for medical and health services in Peninsular Malaysia. Soc Sci Med. 1982;16:267–284. doi: 10.1016/0277-9536(82)90337-9. [DOI] [PubMed] [Google Scholar]
- 29.Lindelow M, Ward P, Zorzi N. Primary Health Care in Mozambique: Service Delivery in a Complex Hierarchy: World Bank. 2004 Available at: http://siteresources.worldbank.org/AFRICAEXT/Resources/ww11888final201.pdf.pdf.
- 30.Perry B, Gesler W. Physical access to primary health care in Andean Bolivia. Soc Sci Med. 2000;50:1177–1188. doi: 10.1016/s0277-9536(99)00364-0. [DOI] [PubMed] [Google Scholar]
- 31.Whetten R, Whetten K, Pence BW, et al. Does distance affect utilization of substance abuse and mental health services in the presence of transportation services? AIDS Care. 2006;18(Suppl 1):S27–S34. doi: 10.1080/09540120600839397. [DOI] [PubMed] [Google Scholar]
- 32.Reif S, Golin CE, Smith SR. Barriers to accessing HIV/AIDS care in North Carolina: rural and urban differences. AIDS Care. 2005;17:558–565. doi: 10.1080/09540120412331319750. [DOI] [PubMed] [Google Scholar]
- 33.Rowe KA, Makhubele B, Hargreaves JR, et al. Adherence to TB preventive therapy for HIV-positive patients in rural South Africa: implications for antiretroviral delivery in resource-poor settings? Int J Tuberc Lung Dis. 2005;9:263–269. [PubMed] [Google Scholar]
- 34.Stout BD, Leon MP, Niccolai LM. Nonadherence to antiretroviral therapy in HIV-positive patients in Costa Rica. AIDS Patient Care STDS. 2004;18:297–304. doi: 10.1089/108729104323076034. [DOI] [PubMed] [Google Scholar]
- 35.van der Werf TS, Dade GK, van der Mark TW. Patient compliance with tuberculosis treatment in Ghana: factors influencing adherence to therapy in a rural service programme. Tubercle. 1990;71:247–252. doi: 10.1016/0041-3879(90)90036-8. [DOI] [PubMed] [Google Scholar]
- 36.Whetten K, Reif S, Lowe K, et al. Gender differences in knowledge and perceptions of HIV resources among individuals living with HIV in the Southeast. South Med J. 2004;97:342–349. doi: 10.1097/01.SMJ.0000118902.64603.A5. [DOI] [PubMed] [Google Scholar]
- 37.Kimerling ME, Petri L. Tracing as part of tuberculosis control in a rural Cambodian district during 1992. Tuber Lung Dis. 1995;76:156–159. doi: 10.1016/0962-8479(95)90559-6. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. Interim proposal for a WHO Staging System for HIV infection and disease. Wkly Epidemiol Rec. 1990;65:221–224. [PubMed] [Google Scholar]
- 39.Gill CJ, Hamer DH, Simon JL, et al. No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. AIDS. 2005;19:1243–1249. doi: 10.1097/01.aids.0000180094.04652.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Attaran A. Adherence to HAART: Africans take medicines more faithfully than North Americans. PLoS Med. 2007;4:e83. doi: 10.1371/journal.pmed.0040083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rosen S, Sanne I, Collier A, et al. Hard choices: rationing antiretroviral therapy for HIV/AIDS in Africa. Lancet. 2005;365:354–356. doi: 10.1016/S0140-6736(05)17792-7. [DOI] [PubMed] [Google Scholar]
- 42.Bennett S, Chanfreau C. Approaches to rationing antiretroviral treatment: ethical and equity implications. Bull World Health Organ. 2005;83:541–547. [PMC free article] [PubMed] [Google Scholar]
- 43.Kleeberger CA, Buechner J, Palella F, et al. Changes in adherence to highly active antiretroviral therapy medications in the Multicenter AIDS Cohort Study. AIDS. 2004;18:683–688. doi: 10.1097/00002030-200403050-00013. [DOI] [PubMed] [Google Scholar]
- 44.Hoover DR, Munoz A, Carey V, et al. The unseen sample in cohort studies: estimation of its size and effect. Multicenter AIDS Cohort Study. Stat Med. 1991;10:1993–2003. doi: 10.1002/sim.4780101212. [DOI] [PubMed] [Google Scholar]