Abstract
The induction of inflammatory cytokines during respiratory viral infections contributes to both disease pathogenesis and resolution. The present studies investigated the role of the chemokine CxCL10 and its specific receptor, CxCR3, in the host response to pulmonary respiratory syncytial virus (RSV) infection. Antibody-mediated neutralization of CxCL10 resulted in a significant increase in disease pathogenesis, including airway hyperresponsiveness (AHR), mucus gene expression, and impaired viral clearance. When the pulmonary cytokine levels were examined, only type I IFN and IL-12p70 were significantly reduced. These latter observations were reflected in reduced dendritic cell (DC) numbers and DC maturation in the lungs of RSV-infected mice treated with anti-CxCL10. Neutralization of the only known receptor for CxCL10, CxCR3, resulted in similar increases in pathogenic responses. When bone marrow-derived DC (BMDC) were incubated with CxCL10 and RSV, an upregulation of type I IFN was observed. In addition, T lymphocytes were also examined and a significant decrease in the number of RSV M2 peptide-specific CD8+ T cells was identified. These findings highlight a previously unappreciated role for the CXCL10:CXCR3 signaling axis in RSV-infected animals by recruiting virus-specific T cells into the lung and promoting viral clearance.
Keywords: Rodent, Lung, Dendritic Cells, Chemokines, Cytokines
Introduction
Respiratory syncytial virus (RSV) infects nearly all infants by age 2 and is the leading cause of bronchiolitis in children worldwide [1]. It is estimated by the CDC that up to 125,000 pediatric hospitalizations in the United States each year are due to RSV, at an annual cost of over $300,000,000 [2]. Despite the generation of RSV-specific adaptive immune responses, RSV does not confer protective immunity and recurrent infections throughout life are common [3, 4]. While RSV is especially detrimental in very young infants whose airways are small and easily occluded, RSV is also widely becoming recognized as an important pathogen in transplant recipients, patients with chronic obstructive pulmonary disease (COPD), the elderly, as well as likely other patients with chronic lung disease, especially asthma. Recent data suggest that mortality for all ages combined have been approximately 30/100,000 from 1990–2000, with an annual average of over 17,000 in the US [5, 6]. These numbers are likely grossly underestimated, as RSV infection has not been examined in adults in a consistent manner. Thus, RSV not only causes significant exacerbated lung disease in young and old, but also is associated with significant mortality. Although anti-RSV antibodies are available and appear to alleviate severe disease, they perform best when given prior to infection, and few other options exist for combating the RSV infections in susceptible patient populations [7].
Clinical studies have suggested that the severity of RSV-induced disease correlates with the influx of leukocytes, resulting in damage to the airways [3]. Chemokines (chemotactic cytokines) have been shown to correlate directly to the intensity of the inflammatory response and are induced by viral infection. In particular, a number of studies have demonstrated that CxCL8 (IL-8), CCL3 (MIP-1alpha), and CCL5 (RANTES) released during the RSV infection correlate to the most severe cases of RSV infection in infants [8-10]. More recently, chemokine production has been linked directly to recognition of pathogen associated molecular patterns (PAMPs) that are expressed during the viral infection[11, 12]. In particular, the recognition of dsRNA by either TLR3 or RIG-I pathways induces the production of type I IFN and chemokines, especially CCL5 and CxCL10[12]. Blockade of CCL5-mediated responses in animal models of RSV appears to attenuate disease pathogenesis by reducing airway hyperreactivity and mucus overproduction[13]. Little information exists on the potential role of CxCL10 and its receptor CxCR3 in the development of pathogenesis during RSV infection. The results from previous studies, together with the one presented here, indicate that while both CCL5 and CxCL10 are expressed at significantly high levels in vitro and in vivo during RSV infection, CxCL10 appears to have a protective role to the host by reducing viral load and pathogenesis.
Results
Induction/ Neutralization of CxCL10 in vivo
Our previous studies have demonstrated the temporal production of CxCL10 during RSV infection [14]. Our first objective in the current studies was to determine the kinetics of CxCL10 induction during RSV infection, and evaluate the efficacy of our neutralizing antibody. Balb/c mice were treated with anti-CxCL10 or control antibodies, and infected with 105 pfu of RSV. The levels of CxCL10 in the lungs were assessed by ELISA assay of lung homogenates. RSV infection in Balb/c mice resulted in significant induction of CxCL10 in the lungs. As previously demonstrated, in control antibody treated mice, a dramatic induction of CxCL10 protein was observed beginning at day 3 post-infection, and persisting to day 8 post-infection (Table 1). Treatment of mice with neutralizing antibodies to CxCL10 significantly decreased CxCL10 levels in the lungs (Table 1). These data demonstrate that RSV infection results in the dramatic induction of CxCL10 in the lungs, which is significantly reduced via treatment with neutralizing antibodies.
Table 1.
Induction and neutralization of CXCL10 (pg/ml) in the lungs of RSV infected mice.
| Days Post-Infection | Ctrl RSV | αCxCL10 |
|---|---|---|
| 0 | 1.06 ± 0.09 | 0.98 ± 0.07 |
| 3 | 7.44 ± 1.1 | 3.92 ± 0.38* |
| 8 | 3.83 ± 0.59 | 1.83 ± 0.29* |
p < 0.05 versus Ctrl
RSV-induced pathophysiology
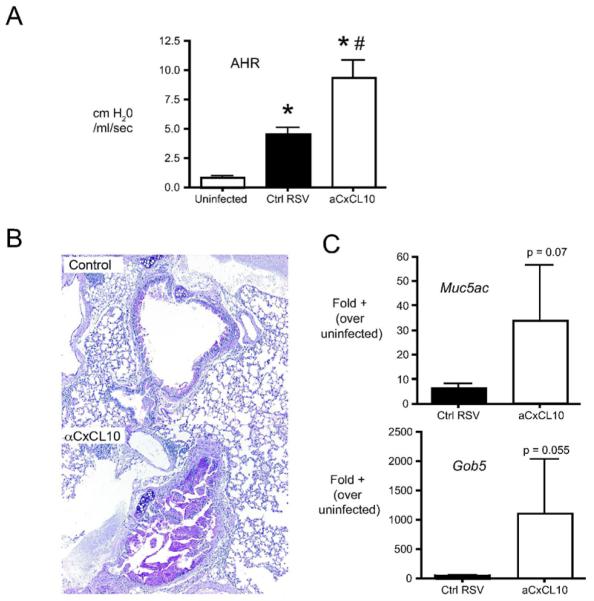
In our model, RSV infection in Balb/c mice results in an increase in airway hyperreactivity (AHR), as well as mucus hypersecretion [15]. To determine whether CxCL10 plays a role in mediating RSV-induced pathophysiology, mice were treated with control or anti-CxCL10 neutralizing antibodies, infected with RSV, and AHR and mucus production were assessed. An increase in airway resistance was observed in response to methacholine in RSV infected mice (Fig. 1A). Increased airway hyperresponsiveness was accompanied by increased transcription of the mucus associate genes Muc5ac and Gob5 (Fig. 1B). Anti-CxCL10 antibody treatment significantly increased airway hyperreactivity relative to control antibody treated mice (Fig. 1A). Similarly, CxCL10 neutralized animals exhibited increased PAS staining (Fig. 1B) and increased mucus associated gene expression (Fig. 1C). Taken together, these data demonstrate that neutralization of CxCL10 enhances RSV-induced pathophysiology.
Figure 1.
Effect of CxCL10 neutralization on RSV-induced pathophysiology. (A) Airway hyperreactivity (AHR) was assessed in uninfected, Ig-treated/RSV-infected (Ctrl RSV) and antiCxCL10-treated/RSV-infected (aCxCL10) mice by plethysmography on day 8 post-infection. Change in resistance represents the increase over baseline in response to methacholine challenge. Bars represent the mean of 10 mice per group ± SEM . *p< 0.05 vs. uninfected, #p<0.05 vs. control RSV infected. (B) PAS staining in lungs of control Ig-treated/RSV-infected (Ctrl RSV) and antiCxCL10-treated/RSV-infected (aCxCL10) mice at day 8 post RSV-infection. Histologic sections were stained with periodic acid Schiff (PAS). (C) The expression of the mucus-associated genes Muc5ac and Gob5 was determined by real-time PCR of whole lung RNA (day 8 post-infection).
Viral clearance
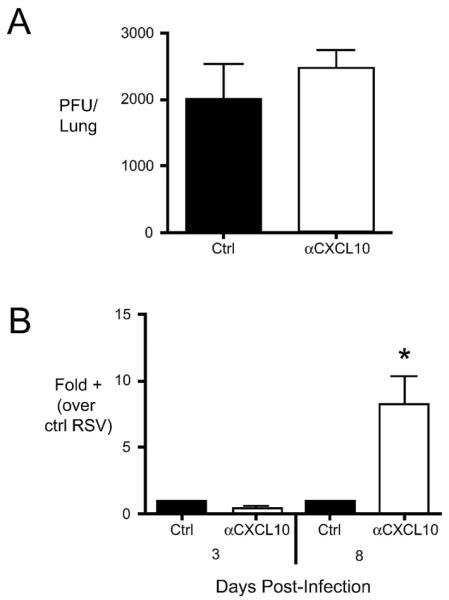
To determine whether CxCL10 plays a role in the clearance of RSV, we determined viral load via plaque assay and via quantitative PCR for RSV G protein transcript. The levels of infectious virus (PFU) were similar in the lungs of control Ig and anti-CxCL10 treated mice at day 3 post infection (Fig. 2A). At day 8 post-infection, PFU via plaque assay were below the level of detection. Similarly, RSV G expression in the lungs was similar in both groups at day 3 post-infection (Fig. 2B). At day eight, however, significantly more RSV G expression was present in the lungs of CxCL10 neutralized mice (Fig. 2B). These results suggest that neutralization of CxCL10 leads to impaired clearance of RSV from the lungs.
Figure 2.
Role of CxCL10 neutralization on the impaired clearance of RSV from the lungs. (A) The number of infectious RSV particles (plaque forming units, PFU) in the lungs was assessed by plaque assay at day 3 post-infection. PFU were below the limit of detection at day 8 post-infection. (B) As a complementary measure of viral load, expression of the RSV G protein transcript was assessed at day 3 and day 8 post-infection. The relative increase in RSV G protein expression was compared to lungs of control Ig-treated/RSV-infected (Ctrl) mice. Bars represent the mean of 5 mice per group ± SEM. *p< 0.05 vs. ctrl. Similar results were obtained in two independent experiments.
Pulmonary cytokines
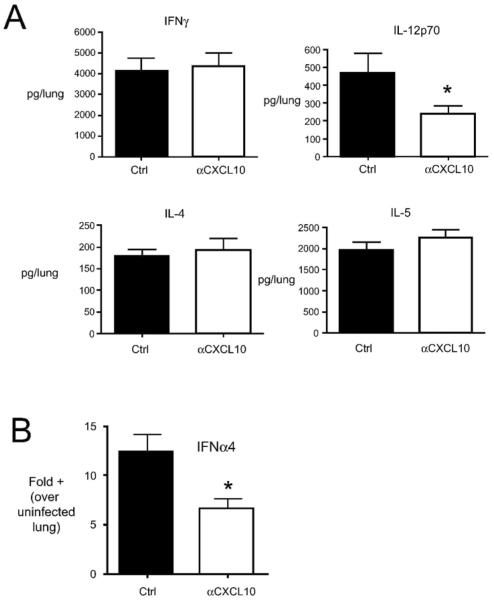
The expression of CxCR3 by T cells has generally been associated with Type 1 cell-mediated responses, Th1 and cytotoxic T1 (Tc1). One might predict that if Th1/Tc1 cells utilize CxCL10 in trafficking to the lungs, blockade of CxCL10 would likely result in a shift in the Th1/Th2 balance in favor of Th2. To determine whether CxCL10 neutralization would result in an enhanced Th2 response in the lungs, the levels of IL-4, IL-5, and IFNγ were assessed in control Ig treated and CxCL10 neutralized RSV infected mice, as described in the Methods. No alterations in IFNγ or Type 2 cytokines were observed in CxCL10 neutralized mice, relative to controls (Fig. 3A). However, a significant decrease in the Type 1 promoting cytokine IL-12p70 was observed in CxCL10 neutralized mice (Fig. 3A). Additionally, a decrease in Ifnα4 mRNA was observed in CxCL10 neutralized mice (Fig. 3B). Thus, neutralization of CxCL10 decreased IL-12 protein and Ifnα4 mRNA in the lungs, but these results were not associated with an increased Type 2 response in the lungs.
Figure 3.
Effect of CxCL10 neutralization on the production of cytokines in the lungs of RSV infected mice at day 8 post-infection. Mice were treated with anti-CxCL10 or control antibodies and infected with RSV. In (A), cytokine levels were assessed by ELISAs of homogenized lung samples. In (B), the expression of Ifnα4 was assessed by quantitative RT-PCR of lung RNA. Bars represent the mean of 5 mice per group ± SEM. *p< 0.05. Similar results were obtained in two independent experiments.
CXCR3 expression by pulmonary leukocytes
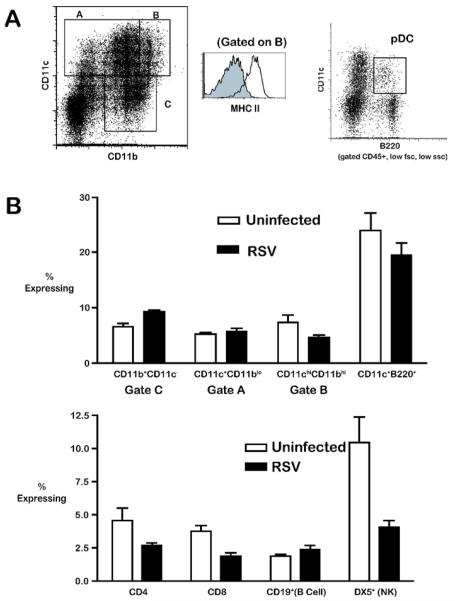
To determine which immune cells may be affected by CxCL10 neutralization, we characterized the expression of CxCR3 on a variety of leukocyte subsets from the lungs of uninfected and RSV-infected mice. Consistent with reports in the literature, CxCR3 expression was detectable on all cell types assayed, including CD8 T cells, CD4 T cells, B cells, NK cells, moncyte/macrophages, as well as both mDC and pDC (Fig. 4) [16-19]. The frequency of CxCR3 expression was highest on NK cells and pDCs (Fig. 4). Following RSV infection, however, only pDC maintained CxCR3 expression at high frequency (Fig 4). These data indicate that a variety of cell types in the lungs express CxCR3 prior to, and during RSV infection, but only pDCs maintained CxCR3 expression at high frequency.
Figure 4.
(A) Representative gating of dendritic cell populations from the lungs of RSV infected mice. Total lung leukocytes were isolated by enzymatic digest, and analysed without further purification by flow cytometry. Monocytes/macrophages (gates I and III) were defined as CD11b+CD11c lo and CD11bloCD11c+; mDC were defined by low auto fluorescence, CD45+, CD11bhi, CD11chi MHC-II+ (gate II); pDC were defined by low forward scatter, low side scatter, CD45R/B220+, CD11cint (right panel). The expression of MHC II by CD11chiCD11bhi mDC is shown in the histogram in the middle panel. (B) The frequency of CXCR3 expression by various leukocyte subsets from the lungs of uninfected and day 8 RSV infected mice. Macrophage/monocyte (gates I, III) and CD11chiCD11bhi mDC (gate II) were gated as shown in (A). Similar results were obtained in three independent experiments.
Lung dendritic cell trafficking/maturation
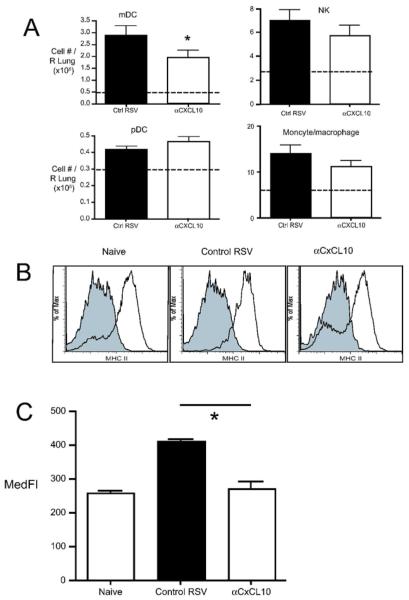
The decreased Ifnα4 and IL-12 in the lungs of CxCL10 neutralized mice led to a focus on lung dendritic cells. Resident lung dendritic cells (and macrophages) are among the first immune cells to encounter respiratory pathogens. Lung DC take up antigen and traffic to lung-draining lymph nodes, where they subsequently prime T cell responses. Pulmonary immunity to viruses and other pathogens also results in the accumulation of large numbers of dendritic cells in the lungs. To determine whether CxCL10 plays a role in the trafficking of these “inflammatory” dendritic cells to the lungs during pulmonary RSV infection, mice were treated with control or CxCL10 neutralizing antibodies and the numbers of myeloid (mDC) and plasmacytoid (pDC) dendritic cells in the lungs were assessed. In control IgG treated mice, RSV infection resulted in the dramatic recruitment of mDC to the lungs (Fig. 5A, approximately six-fold over uninfected controls - dashed line). Anti-CxCL10 treated mice had significantly fewer mDC in the lungs (Fig. 5A). A smaller, but significant increase in pDCs was found in the lungs of RSV infected mice, relative to uninfected lungs (Fig. 5A). CxCL10 neutralization had no effect on the accumulation of pDCs in the lungs of RSV infected mice. While the gate used to define pDC (CD45+, low FSC, low SSC, CD11cint, B220+) may include some NK DC (~20% DX5+ in both control and CxCL10 neutralized mice, Table 2), 50% of the CD11c+B220+ cells co-express Gr-1 (Table 2). These data confirm that these cells are predominantly pDC, and CxCL10 neutralization did not affect pDC trafficking to the lungs. This result was unexpected, however, as pDC in the lungs expressed CxCR3 at higher levels than did mDC (Fig. 4B). CxCL10 neutralization did not affect the recruitment of NK cells or monocyte/macrophages (Fig. 5A). These results demonstrate that CxCL10 promotes recruitment of mDC to the lungs during RSV infection, but since mDC expressed low levels of CxCR3, relative to pDC, this effect may be indirect.
Figure 5.
(A) Absolute numbers of myeloid dendritic cells (mDC), plasmacytoid dendritic cells (pDC), NK cells, and monocyte/macrophages in the lungs of RSV-infected, Ig-treated █ OK? █ control and RSV-infected, CxCL10 neutralized (aCxCL10) mice at day 8 post-infection. Frequencies were determined by flow cytometry, and absolute numbers were determined as described in the Materials and methods. Gating of myeloid cells was done as shown in Fig 4. Monocytes/macrophages represent gates I and III defined in Fig. 4. Bars represent the mean of 9–10 mice per group ± SEM. Dashed lines represent cell numbers in naive animals. *p < 0.05 (B) Representative histograms depict the expression of MHC II by mDC from the lungs of naïve mice, Ig-treated/RSV-infected controls and CxCL10-neturalized/RSV-infected mice at day 8 post-infection. (C) The median fluorescence intensity (MedFI) of MHC II staining is shown. Bars represent the mean of five mice ± SEM. *p<0.03. Similar results were obtained in 3 independent experiments.
Table 2.
Expression of pDC and NK associated markers by low FSC/SSC CD45+CD11c+B220+ in the lungs of RSV infected mice. Day 8 post-infection.
| Ctrl RSV | αCxCL10 | |
|---|---|---|
| Gr-1(%) | 52.0 ± 3.7% | 46.1 ± 5.2% |
| DX5 (%) | 19.2 ± 2.6% | 21.1 ± 2.9% |
Pathogen recognition by dendritic cells via TLRs and other pattern recognition receptors results in the up-regulation of MHC II and costimulatory molecules, collectively referred to as “maturation”. To determine whether CxCL10 plays a role in modulating dendritic cell maturation, we determined the expression MHC II and costimulatory molecules by dendritic cells from the lungs of anti-CxCL10 and control RSV-infected. The expression of MHC II was up-regulated in control RSV-infected mice, relative to controls (Fig. 5B,C). There was a trend toward increased CD80 and CD86 expression following RSV infection, but this did not reach statistical significance (data not shown). The up-regulation of MHC II by mDC was significantly impaired in CxCL10 neutralized mice (Fig. 5B,C). These results suggest that CxCL10 promotes pulmonary dendritic cell activation/maturation during RSV infection.
Pulmonary T cell numbers
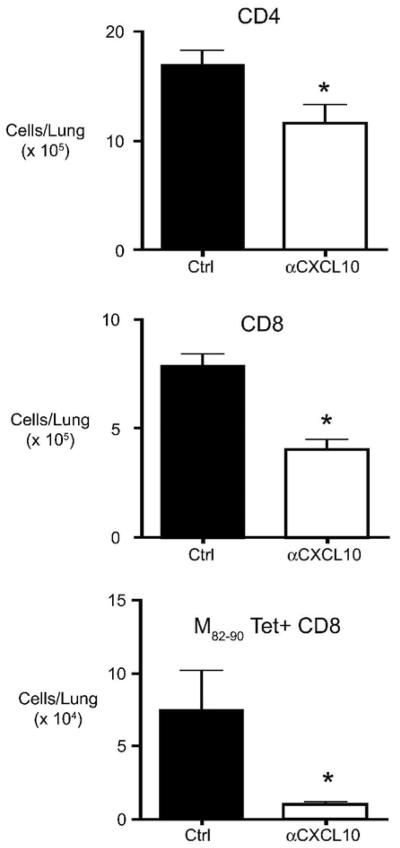
We next wanted to determine whether CxCL10 neutralization would impact T cell responses during RSV infection. To this end, RSV-infected mice were treated with control or CxCL10 neutralizing antibodies and the numbers of lung CD4, CD8, and RSV M82–90 Tetramer-specific CD8 T cells were determined from whole lungs. CxCL10 neutralization resulted in significantly decreased numbers of CD4, CD8, and RSV M82–90 specific CD8 T cells in the lungs of RSV infected mice (Fig. 6). These data demonstrate that CxCL10 promotes RSV-specific T cell responses in the lungs.
Figure 6.
Lung T cell numbers in anti-CxCL10/RSV-infected and control Ig-treated/RSV-infected mice. The absolute numbers of CD4, CD8, and RSV M82–90 tetramer+ T cells at day eight post-infection were determined by flow cytometric analysis of enzymatically digested lungs. *p < 0.05 versus control Ig treated mice. Similar results were obtained in two independent experiments.
CxCR3 blockade and RSV-induced pathophysiology
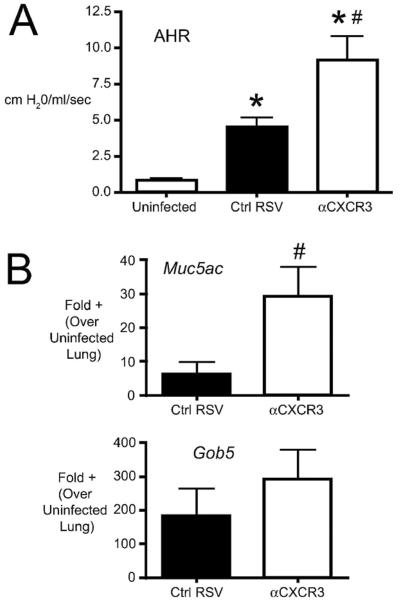
CxCR3 is the only known receptor for CxCL10 in mice. To determine whether blockade of CxCR3 would alter pathophysiology in a manner similar to CxCL10 neutralization, we administered anti-CxCR3 antibodies during RSV infection. Similar to anti CxCL10, neutralization of CxCR3 resulted in increased AHR (Fig. 7A), again suggesting that this receptor-ligand system has an important role in activation of the appropriate anti-viral response. Additionally, CxCR3 neutralized mice had significantly increased Muc5ac expression and a trend toward increased Gob5 (Fig. 7B), indicating that the overproduction of mucus corresponded with the changes in lung function (AHR). These data provide supporting evidence that the protective effects of CxCL10 during pulmonary RSV infection are mediated via CxCR3.
Figure 7.
Effect of CxCR3 neutralization on RSV-induced pathophysiology. (A) Airway hyperreactivity (AHR) was assessed in uninfected, Ig-treated/RSV-infected (Ctrl RSV) and antiCxCL10-treated/RSV-infected (aCxCL10) mice by plethysmography on day 8 post-infection. Change in resistance represents the increase over baseline in response to methacholine challenge. Bars represent the mean of 10 mice per group ± SEM . (B) The expression of the mucus-associated genes Muc5ac and Gob5 was determined by real-time PCR of whole lung RNA (day 8 post-infection). Bars represent the mean of 5 mice per group from one of two independent experiments. *p < 0.05 vs. uninfected, #p<0.05 vs. control Ig-treated/RSV infected.
CxCL10 promotes Ifnα4 induction and limits viral replication in CxCR3 expressing dendritic cells
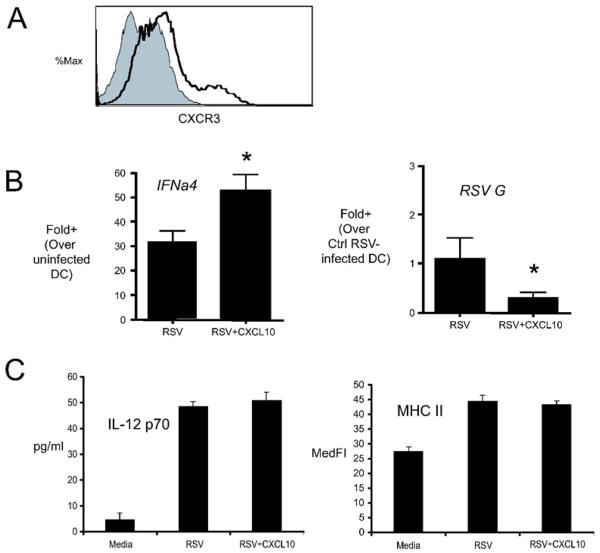
Our in vivo data demonstrated that CxCL10 neutralization altered lung dendritic cell numbers in the lungs of RSV infected mice. Our next objective was to determine whether CxCL10 plays a direct role in modulating dendritic cells. To this end, bone marrow derived dendritic cells (BMDC) were infected in vitro with RSV (MOI=1) and treated with media alone or CxCL10. BMDC were CD11b+ , consistent with published reports, and expressed moderate levels of CxCR3 (Fig. 8A). RSV infection of BMDC induced dramatic increases in Ifnα4, and CxCL10 treatment of RSV infected BMDC augmented IFNα4 mRNA expression (Fig. 8B). Interestingly, RSV replication/transcription, as assessed by RSV G protein expression, was significantly decreased in CxCL10 treated BMDC, compared to DC treated with media alone (Fig. 8B). The addtion of CxCL10 did not directly promote RSV-induced IL-12 production or MHC II up-regulation. These data suggest that CxCL10 promotes an “anti-viral state” in CxCR3 expressing DC, but does not directly influence DC maturation. Taken together with in vivo data, these results demonstrate that CxCL10 promotes Ifnα4.
Figure 8.
Effect of CxCL10 on type I IFN production and viral replication/transcription in vitro. BMDC were prepared as described in the Methods. (A) BMDC were stained with either anti-CxCR3 (shaded) or isotype control (hollow line) antibodies and analyzed by flow cytometry. One representative histogram is shown. Similar results were obtained in 2 independent experiments. (B) Effect of recombinant CxCL10 on RSV-induced expression of Ifna4 mRNA and RSV G transcript. BMDC were infected with RSV (MOI =1) alone, or in the presence of 100ng/ml CxCL10, and the levels of Ifnα4 mRNA and RSV G transcript were assessed by real time PCR 24 hours after infection. Bars represent the Mean of 3 replicates +/− SEM. *p<0.05 versus control. Similar results were obtained in two independent experiments. (C) Effect of CxCL10 on RSV-induced IL-12 p70 and MHC II expression. BMDC were cultured as in (B), IL-12p70 was measured in the supernatant at 24 hours post-infection. The expression of MHC II was determined by flow cytometry.
Discussion
The production of chemokines during the initial phases and throughout the infectious process in the lung provides a key role in immunity against and regulating pathogenesis of RSV. In the present study the role of CxCL10 and CxCR3 induced during RSV infection has been carefully explored using a murine model of disease. The neutralization of either CxCL10 or CxCR3 led to a similar exacerbation of disease pathogenesis with significant increases in AHR and mucus gene expression. These data correlated with the reduction in DC accumulation and viral specific CD8+ T cell numbers. The increased presence of virus gene expression after blocking this mediator cascade indicates that the activation event induced by this chemokine-receptor pair is critical to the function of the anti-viral responses. Furthermore, these results demonstrate that CxCL10 plays a role in modulating the host response to pulmonary RSV infection via promoting leukocyte recruitment and has a role in the trafficking and function of dendritic cells. The expression of CxCL10 has been observed in several models of respiratory virus infection, including Sendai, influenza, SARS-CoV, and adenovirus [20-23], as well as in neurogenic viral responses [24-28]. Thus, the responses induced by CxCL10 during the RSV-induced disease appear to be a general mechanism for responding to and/or clearing viral infections.
Neutralization of CxCL10 resulted in impaired type I IFN and dendritic cell maturation in the lungs of RSV infected mice. A number of studies have shown that type I IFN promotes dendritic cell maturation and costimulatory molecule expression [29-33]. Recent studies from our laboratory have shown that, in the absence of type I IFN signaling, RSV infected dendritic cells do not mature [34]. Thus, considerable evidence links type I IFN to dendritic cell maturation. Taken together, these results suggest that a likely mechanism for the dendritic cell maturation defect observed in CxCL10 neutralized mice (and consequently impaired T cell response) is a defect in the induction of type I IFN. We suggest that CxCL10 promotes type I IFN in CxCR3 expressing cells (likely pDC in vivo), which in turn promotes mDC maturation via type I IFN.
Clinical studies have suggested that the severity of RSV-induced disease correlates with the influx of leukocytes that lead to the damage in the airways [3]. In particular, studies demonstrated that a number of other chemokines released within the airways during RSV infection, including CxCL8 (IL-8), CCL3 (MIP-1alpha), and CCL5 (RANTES), correlate to the most severe cases of RSV infection in infants [8-10] [35, 36]. Two of these chemokines, CCL3 and CCL5, have also been shown to be associated with inflammatory responses in animal models of RSV infection. CCL3 has been linked to the severity of primary RSV-induced inflammation as well as with multiple infections with RSV. Furthermore, Domachowske et al [37] demonstrated that infection of mice with paramyxovirus induces increased CCL3 related to eosinophil and PMN recruitment. Likewise, studies examining CCL5 indicate a significant impact on the pathophysiologic responses in primary RSV infection as well as the pattern of leukocyte recruitment and leukotriene release in the lungs during RSV-induced allergen exacerbated disease [13, 38]. Previous studies have also found that the overproduction of mucus and development of airway hyperreactivity is directly related to the expression and activation of CxCR2 (an IL-8 receptor homolog) in mice [39]. At the same time the overproduction of CxCL10, a chemokine clearly associated with viral clearance and disease resolution, is also highly induced by RSV [14, 40, 41]. The overexpression of CxCR3 and CxCL10 ligand appears to mediate a protective response to RSV and therefore this represents a balance for the CCL5/CCR1-induced pathophysiology. In contrast, using a combination of reagents to block CCL5 or CCR1−/− mice numerous studies have shown that this highly expressed chemokine/receptor pathway corresponds to a detrimental pathologic consequence [13, 37, 38, 42, 43]. Thus, it appears that the appropriate activation and recruitment of CxCR3+ DC and T cell populations mediate a less pathogenic immune environment, while blocking the CCR1/CCL5-mediated pathway would provide a potential therapeutic strategy.
Several pathways may induce the production of CxCL10 in the airways during viral infection. One well-characterized mechanism via TLR-mediated activation of CxCL10 has been demonstrated in several viral systems including RSV [12, 44-46]. Initial studies using Poly-I:C to activate CxCL10 production demonstrated that it was one of the highest expressed cytokines during the activation of multiple cell populations including epithelial cells, fibroblasts and astrocytes [44, 47-51]. More recently, the activation of other dsRNA-mediated pathways have also been identified in the overproduction of CxCL10, as well as other IFN-inducible chemokines, including RIG-I and PKR [52-54]. Additionally, these pathways promote the activation of type I IFN production that provides additional increases in CxCL10 production, thus further augmenting its production within the system and enhancing the anti-viral environment.
The expression of CxCR3 on distinct immune cell populations may be key for understanding the mechanism of RSV clearance in the lung. Studies originally identified CxCR3 expression on Th1 CD4+ T cells but not on Th2 T cell populations, indicating a clear dichotomy in recruitment and function [55, 56]. Subsequent studies have identified that CD8+ cytotoxic T cells can also differentially express chemokine receptors, depending upon their state of activation [57, 58]. While there is still a paucity of data that fully define the receptor patterns on CD8+ T cells, CxCR3 appears to be defining for efficient recruitment of cytotoxic T cells for viral clearance in several infection models [25, 26, 59-62]. A mechanistic picture of the importance of CxCR3 in effective Th1 type anti-viral immunity continues to be developed but also includes the appropriate recruitment of DC populations [21, 63-66]. In particular, the migration of pDCs into the lymph node from the site of viral infection was found to depend upon CxCR3 providing activation signals for lymph node DC to properly activate efficient anti-HSV immunity [21]. The latter studies suggested that CxCR3 recruited pDC provided type I IFN signals in the lymph node that allowed the activation of the T cell populations. In the present studies we have also observed that pDC preferentially express CxCR3. Furthermore, previous studies have demonstrated a key role of pDC in the clearance of RSV and the maintenance of an effective, non-pathogenic response to RSV [67, 68]. Overall, our studies identify a key chemokine receptor system that initiates and maintains the proper immune environment for anti-viral responses.
Materials and Methods
Mice
Female Balb/c mice were obtained from the Jackson Laboratories (Bar Harbor, ME). Mice were housed under pathogen-free conditions in enclosed filter-topped cages. Clean food and water were given ad libitum. The mice were handled and maintained using microisolator techniques, with daily veterinarian monitoring. Bedding from the mice was transferred weekly to cages of uninfected sentinel mice that were subsequently bled at weekly intervals and found to be negative for antibodies to mouse hepatitis virus, Sendai virus, and Mycoplasma pulmonis. The University Committee on Use and Care of Animals (UCUCA) at the University of Michigan approved all studies involving mice.
Respiratory syncytial virus and infection
RSV A strain was derived from a clinical isolate at the University of Michigan and was propagated in Hep2 cells. After adsorption, medium was added to the flask, and the infection was allowed to proceed until syncytia were observed. The cells were frozen a −80°C overnight, and the supernatant was harvested, clarified, and aliquoted. Viral titers were determined by plaque assay. For infections, mice were anesthetized with a mixture of ketamine (100mg/kg) and xylazine (10mg/kg). An incision was made over the trachea and mice were infected with ~1×105 pfu of RSV in a volume of 40μl via intratracheal injection. The surgical wound was closed with surgical staples and mice recovered with minimal visible trauma.
Generation of CxCL10 and CxCR3 neutralizing antibodies
Rabbit anti-murine CxCL10 was generated by immunization of rabbits with recombinant protein. Rabbit anti-CxCR3 was generated by immunizing rabbits with a 16-mer peptide (PYDYGENESDFSDSPP) corresponding the NH2 terminus of murine CxCR3, and has been shown to neutralize CxCR3 in vivo [26, 69]. CxCR3 expressing T cells and dendritic cells were detected in anti-CxCR3 treated mice, demonstrating that these antibodies are neutralizing, but not depleting. Serum from immunized rabbits were titered by ELISA and verified for minimal cross-reactivity to a panel of other chemokines and cytokines. Antibodies were purified from total serum by protein A-based affinity columns and the concentration of antibody was determined by spectrophotometry. Neutralizing antibodies (3.5mg/mouse) were administered via intraperitoneal injection 1 day prior to RSV infection and then every 3 days. The efficacy of anti-CxCL10 antibody neutralization in the lungs is shown in Table 1.
Airway Hyperreactivity (AHR)
Airway Hyperreactivity was assessed as previously described[15]. Briefly, mice were anesthetized with sodium pentobarbital, intubated via cannulation of the trachea, and ventilated with a Harvard pump ventilator (~0.3ml tidal volume; 120 breaths per minute). Airway hyperreactivity was measured using a Buxco mouse plethysmograph and software for calculation of the measurements. Mice were administered a single optimized intravenous dose of methacholine (150mg/kg), and the peak airway resistance was recorded.
RNA and Protein Assays
The upper left lobe of mouse lungs was used for protein analysis and RNA was isolated from the lower left lobe. Lung samples were snap-frozen in liquid nitrogen and stored at −70°C until use. For protein assays, lungs were homogenized in buffered saline containing Complete™ protease inhibitors (Roche Applied Science, Indianapolis, IN). The concentration of protein was determined by sandwich ELISA using commercially available paired antibodies (R&D Systems, Minneapolis, MN) RNA was isolated from lower left lobes of lungs using Trizol (Invitrogen, Carlsbad, CA), and ~5μg of total RNA was reverse transcribed in a volume of 25μl. Real time PCR was performed on cDNA using commercially available primers for GAPDH, and custom designed primers for RSV G protein transcript, Gob-5, and Muc5ac, as previously described [39]. Ifnα4 expression was determined using SYBR green I dye, using the following primer sequences: mIFNα4 F: CCT GTG TGA TGC AGG AAC C Ifnα4 R: TCA CCT CCC AGG CAC AGA. Gene expression was normalized to GAPDH before the fold change was calculated.
Histology
Right lobes of the lungs were removed and immediately fixed in 10% neutral buffered formalin. Lung samples were subsequently processed, embedded in paraffin, sectioned, and placed on L-lysine-coated slides. Periodic-acid Schiff (PAS) staining was done to identify mucus and mucus-secreting cells.
Lung and Lymph Node Leukocyte Isolation
Lung leukocytes were isolated from enzyme dispersed lung tissue. Right lungs from each mouse were excised, washed in PBS, minced and digested enzymatically for 45 minutes in 15 ml/lung of digestion buffer (RPMI, 5% FCS, 1 mg/ml collagenase (Roche Applied Science), and 30 μg/ml DNase (Sigma-Aldrich, St Louis, MO)). Lung-associated lymph nodes (LALN) were dispersed similar to lungs, only 5mls of digestion buffer was used. Following erythrocyte lysis using ammonium chloride (NH4Cl) buffer, cells were washed, and resuspended in media (RPMI, 5% FCS). Total lung leukocyte numbers were assessed in the presence of trypan blue using a hemocytometer; viability was >85%.
Flow Cytometry
Leukocytes were washed and resuspended at a concentration of 107 cells/ml in Flow Buffer (PBS+ 1% FCS + 0.1% NaN3 (Sigma)), Fc receptors were blocked by the addition of anti-CD16/32 (Fc block™, BD Biosciences, San Jose, CA). Following Fc receptor blocking, 106 cells were stained, in a final volume of 120 μl in 12 × 75 polystyrene tubes (BD Biosciences) for 20 minutes at 4°C. Leukocytes were stained with the following monoclonal antibodies, per manufacturer's instructions: CD4 (RM4-5), CD8a (5H10-1), CD45 (30-F11), CD45R/B220 (RA3-6B2), CD11b (M1/70), CD11c (HL3), CD19 (1D3), NK (DX5), I-Ad (AMS-32.1), Gr-1 (RB6-8C5) (BD Biosciences), and CxCR3 (R&D Systems, Minneapolis, MN). Cells were washed twice with Flow buffer, resuspended in 100μl and 200μl of 4% formalin was added to fix the cells. A minimum of 75,000 events were acquired on a dual-laser Cytomics FC500™ flow cytometer (Beckman Coulter, Fullerton, CA) and analysed using Flowjo™ software (Treestar, Ashland, OR). Classical myeloid dendritic cells (mDCs) were defined by the following parameters: low auto fluorescence, CD45+, CD11bhi, CD11chi MHC-II+. Plasmacytoid dendritic cells (pDCs) were defined as: low forward scatter, low side scatter, CD45R/B220+, CD11cint. In some experiments, CD8+ T cells were stained with RSV M82–90 tetramer (NIH Tetramer Core, Emory University, Atlanta GA). RSV M82–90 has been previously shown to be the a dominant CD8+ epitope during RSV infection in Balb/c mice [70].
BMDC culture
Dendritic cells were grown from mouse bone marrow using minor modification of an establish method [71]. Bone marrow cells were flushed from the femurs and tibias of naïve Balb/c mice. Bone marrow cells were seeded at 2 × 105/ml in 30mls of BMDC media (RPMI+ 10% FCS, 1% Pen-Strep, 1% non-essential amino acids, 1% sodium pyrvuate, 1% L-glutamine, 5 × 10−5M 2-mercaptoethanol) with 10ng/ml recombinant GM-CSF (R&D) in T150 flasks. At day 2, another 15 mls of BMDC media + GM-CSF was added to the culture. At days 4, 6, and 8, half of the media was removed, centrifuged and the cell pellet added back to the culture with fresh media + GM-CSF. Non-adherent cells were harvested at day ten, and the resulting cells were >85% CD11c+. In some experiments, BMDC were infected with virus at an MOI = 1, and/or stimulated with recombinant CxCL10 (R&D).
Statistics
For airway responses and cytokine levels, p values were calculated using the two tailed student's t test, assuming equal or unequal variance, as dictated by F test. For quantitative real-time PCR, statistical comparisons were made by the same methods, except that p values were calculated based on cycle numbers normalized to GAPDH controls, before conversion to fold increases. P values < 0.05 were considered statistically significant.
Acknowledgments
This work was supported by National Institutes of Health Grants AI036302 (NWL), T32 HL07749 (DML), and NS041249 (TEL)
We thank Pamela Lincoln for technical assistance.
Abbreviations used in this manuscript
- RSV
respiratory syncytial virus
- AHR
airway hyperresponsiveness
- LALN
lung-associated lymph node
- Tc1
cytotoxic T cell type 1
- pDC
plasmacytoid dendritic cell
- mDC
myeloid dendritic cell
Footnotes
Conflict of Interest The authors have no competing interests.
References
- 1.Henderson FW, Collier AM, Clyde WA, Jr., Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530–534. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- 2.Openshaw PJ, Dean GS, Culley FJ. Links between respiratory syncytial virus bronchiolitis and childhood asthma: clinical and research approaches. Pediatr Infect Dis J. 2003;22:S58–64. doi: 10.1097/01.inf.0000053887.26571.eb. discussion S64–55. [DOI] [PubMed] [Google Scholar]
- 3.Welliver RC. Respiratory syncytial virus and other respiratory viruses. Pediatr Infect Dis J. 2003;22:S6–10. doi: 10.1097/01.inf.0000053880.92496.db. discussion S10–12. [DOI] [PubMed] [Google Scholar]
- 4.Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112–117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 5.Black CP. Systematic review of the biology and medical management of respiratory syncytial virus infection. Respir Care. 2003;48:209–231. discussion 231–203. [PubMed] [Google Scholar]
- 6.Stensballe LG, Devasundaram JK, Simoes EA. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22:S21–32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 7.Harkensee C, Brodlie M, Embleton ND, McKean M. Passive immunisation of preterm infants with palivizumab against RSV infection. J Infect. 2006;52:2–8. doi: 10.1016/j.jinf.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Bonville CA, Rosenberg HF, Domachowske JB. Macrophage inflammatory protein-1alpha and RANTES are present in nasal secretions during ongoing upper respiratory tract infection. Pediatr Allergy Immunol. 1999;10:39–44. doi: 10.1034/j.1399-3038.1999.101005.x. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Harb M, Bell F, Finn A, Rao WH, Nixon L, Shale D, Everard ML. IL-8 and neutrophil elastase levels in the respiratory tract of infants with RSV bronchiolitis. Eur Respir J. 1999;14:139–143. doi: 10.1034/j.1399-3003.1999.14a23.x. [DOI] [PubMed] [Google Scholar]
- 10.Gern JE, Martin MS, Anklam KA, Shen K, Roberg KA, Carlson-Dakes KT, Adler K, Gilbertson-White S, Hamilton R, Shult PA, Kirk CJ, Da Silva DF, Sund SA, Kosorok MR, Lemanske RF., Jr. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol. 2002;13:386–393. doi: 10.1034/j.1399-3038.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 11.Liu P, Jamaluddin M, Li K, Garofalo RP, Casola A, Brasier AR. Retinoic Acid-Inducible Gene I Mediates Early Antiviral Response and Toll-Like Receptor 3 Expression in Respiratory Syncytial Virus-Infected Airway Epithelial Cells. J. Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rudd BD, Burstein E, Duckett CS, Li X, Lukacs NW. Differential role for TLR3 in respiratory syncytial virus-induced chemokine expression. J Virol. 2005;79:3350–3357. doi: 10.1128/JVI.79.6.3350-3357.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John AE, Berlin AA, Lukacs NW. Respiratory syncytial virus-induced CCL5/RANTES contributes to exacerbation of allergic airway inflammation. Eur J Immunol. 2003;33:1677–1685. doi: 10.1002/eji.200323930. [DOI] [PubMed] [Google Scholar]
- 14.Miller AL, Bowlin TL, Lukacs NW. Respiratory syncytial virus-induced chemokine production: linking viral replication to chemokine production in vitro and in vivo. J Infect Dis. 2004;189:1419–1430. doi: 10.1086/382958. [DOI] [PubMed] [Google Scholar]
- 15.Tekkanat KK, Maassab HF, Cho DS, Lai JJ, John A, Berlin A, Kaplan MH, Lukacs NW. IL-13-induced airway hyperreactivity during respiratory syncytial virus infection is STAT6 dependent. J Immunol. 2001;166:3542–3548. doi: 10.4049/jimmunol.166.5.3542. [DOI] [PubMed] [Google Scholar]
- 16.Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Lopez MA, Sanchez-Madrid F, Rodriguez-Frade JM, Mellado M, Acevedo A, Garcia MI, Albar JP, Martinez C, Marazuela M. CXCR3 chemokine receptor distribution in normal and inflamed tissues: expression on activated lymphocytes, endothelial cells, and dendritic cells. Lab Invest. 2001;81:409–418. doi: 10.1038/labinvest.3780248. [DOI] [PubMed] [Google Scholar]
- 18.Kohrgruber N, Groger M, Meraner P, Kriehuber E, Petzelbauer P, Brandt S, Stingl G, Rot A, Maurer D. Plasmacytoid dendritic cell recruitment by immobilized CXCR3 ligands. J Immunol. 2004;173:6592–6602. doi: 10.4049/jimmunol.173.11.6592. [DOI] [PubMed] [Google Scholar]
- 19.Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol. 2004;5:1260–1265. doi: 10.1038/ni1138. [DOI] [PubMed] [Google Scholar]
- 20.Glass WG, Subbarao K, Murphy B, Murphy PM. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARSCoV) pulmonary infection of mice. J Immunol. 2004;173:4030–4039. doi: 10.4049/jimmunol.173.6.4030. [DOI] [PubMed] [Google Scholar]
- 21.Yoneyama H, Matsuno K, Toda E, Nishiwaki T, Matsuo N, Nakano A, Narumi S, Lu B, Gerard C, Ishikawa S, Matsushima K. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J Exp Med. 2005;202:425–435. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wareing MD, Lyon AB, Lu B, Gerard C, Sarawar SR. Chemokine expression during the development and resolution of a pulmonary leukocyte response to influenza A virus infection in mice. J Leukoc Biol. 2004;76:886–895. doi: 10.1189/jlb.1203644. [DOI] [PubMed] [Google Scholar]
- 23.Sarawar SR, Lee BJ, Anderson M, Teng YC, Zuberi R, Von Gesjen S. Chemokine induction and leukocyte trafficking to the lungs during murine gammaherpesvirus 68 (MHV-68) infection. Virology. 2002;293:54–62. doi: 10.1006/viro.2001.1221. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Gang Z, Yuling H, Luokun X, Jie X, Hao L, Li W, Chunsong H, Junyan L, Mingshen J, Youxin J, Feili G, Boquan J, Jinquan T. Different neurotropic pathogens elicit neurotoxic CCR9- or neurosupportive CXCR3-expressing microglia. J Immunol. 2006;177:3644–3656. doi: 10.4049/jimmunol.177.6.3644. [DOI] [PubMed] [Google Scholar]
- 25.Christensen JE, de Lemos C, Moos T, Christensen JP, Thomsen AR. CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system. J Immunol. 2006;176:4235–4243. doi: 10.4049/jimmunol.176.7.4235. [DOI] [PubMed] [Google Scholar]
- 26.Stiles LN, Hosking MP, Edwards RA, Strieter RM, Lane TE. Differential roles for CXCR3 in CD4+ and CD8+ T cell trafficking following viral infection of the CNS. Eur J Immunol. 2006;36:613–622. doi: 10.1002/eji.200535509. [DOI] [PubMed] [Google Scholar]
- 27.de Lemos C, Christensen JE, Nansen A, Moos T, Lu B, Gerard C, Christensen JP, Thomsen AR. Opposing effects of CXCR3 and CCR5 deficiency on CD8+ T cell-mediated inflammation in the central nervous system of virus-infected mice. J Immunol. 2005;175:1767–1775. doi: 10.4049/jimmunol.175.3.1767. [DOI] [PubMed] [Google Scholar]
- 28.Tsunoda I, Lane TE, Blackett J, Fujinami RS. Distinct roles for IP-10/CXCL10 in three animal models, Theiler's virus infection, EAE, and MHV infection, for multiple sclerosis: implication of differing roles for IP-10. Mult Scler. 2004;10:26–34. doi: 10.1191/1352458504ms982oa. [DOI] [PubMed] [Google Scholar]
- 29.Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: endogenous activators of dendritic cells. Nat Med. 1999;5:1249–1255. doi: 10.1038/15200. [DOI] [PubMed] [Google Scholar]
- 30.Ito T, Amakawa R, Inaba M, Ikehara S, Inaba K, Fukuhara S. Differential regulation of human blood dendritic cell subsets by IFNs. J Immunol. 2001;166:2961–2969. doi: 10.4049/jimmunol.166.5.2961. [DOI] [PubMed] [Google Scholar]
- 31.Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–1788. doi: 10.1084/jem.191.10.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, Seya T, Taniguchi T. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci U S A. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lopez CB, Garcia-Sastre A, Williams BR, Moran TM. Type I interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative-strand RNA viruses. J Infect Dis. 2003;187:1126–1136. doi: 10.1086/368381. [DOI] [PubMed] [Google Scholar]
- 34.Rudd BD, Luker GD, Luker KE, Peebles RS, Lukacs NW. Type I interferon regulates respiratory virus infected dendritic cell maturation and cytokine production. Viral Immunol. 2007;20:531–540. doi: 10.1089/vim.2007.0057. [DOI] [PubMed] [Google Scholar]
- 35.Noah TL, Becker S. Chemokines in Nasal Secretions of Normal Adults Experimentally Infected with Respiratory Syncytial Virus. Clin Immunol. 2000;97:43–49. doi: 10.1006/clim.2000.4914. [DOI] [PubMed] [Google Scholar]
- 36.Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. Macrophage inflammatory protein-1alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis. 2001;184:393–399. doi: 10.1086/322788. [DOI] [PubMed] [Google Scholar]
- 37.Domachowske JB, Bonville CA, Gao JL, Murphy PM, Easton AJ, Rosenberg HF. MIP-1alpha is produced but it does not control pulmonary inflammation in response to respiratory syncytial virus infection in mice. Cell Immunol. 2000;206:1–6. doi: 10.1006/cimm.2000.1730. [DOI] [PubMed] [Google Scholar]
- 38.Tekkanat KK, Maassab H, Miller A, Berlin AA, Kunkel SL, Lukacs NW. RANTES (CCL5) production during primary respiratory syncytial virus infection exacerbates airway disease. Eur J Immunol. 2002;32:3276–3284. doi: 10.1002/1521-4141(200211)32:11<3276::AID-IMMU3276>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 39.Miller AL, Strieter RM, Gruber AD, Ho SB, Lukacs NW. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J Immunol. 2003;170:3348–3356. doi: 10.4049/jimmunol.170.6.3348. [DOI] [PubMed] [Google Scholar]
- 40.Haeberle HA, Kuziel WA, Dieterich HJ, Casola A, Gatalica Z, Garofalo RP. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: role of MIP-1alpha in lung pathology. J Virol. 2001;75:878–890. doi: 10.1128/JVI.75.2.878-890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tripp RA, Jones L, Anderson LJ. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J Virol. 2000;74:6227–6229. doi: 10.1128/jvi.74.13.6227-6229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.John AE, Gerard CJ, Schaller M, Miller AL, Berlin AA, Humbles AA, Lukacs NW. Respiratory syncytial virus-induced exaggeration of allergic airway disease is dependent upon CCR1-associated immune responses. Eur J Immunol. 2005;35:108–116. doi: 10.1002/eji.200425439. [DOI] [PubMed] [Google Scholar]
- 43.Culley FJ, Pennycook AM, Tregoning JS, Dodd JS, Walzl G, Wells TN, Hussell T, Openshaw PJ. Role of CCL5 (RANTES) in viral lung disease. J Virol. 2006;80:8151–8157. doi: 10.1128/JVI.00496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loos T, Dekeyzer L, Struyf S, Schutyser E, Gijsbers K, Gouwy M, Fraeyman A, Put W, Ronsse I, Grillet B, Opdenakker G, Van Damme J, Proost P. TLR ligands and cytokines induce CXCR3 ligands in endothelial cells: enhanced CXCL9 in autoimmune arthritis. Lab Invest. 2006;86:902–916. doi: 10.1038/labinvest.3700453. [DOI] [PubMed] [Google Scholar]
- 45.Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, Oh SB, Park K, Kim JS, Lee SJ. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–256. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- 46.Proost P, Vynckier AK, Mahieu F, Put W, Grillet B, Struyf S, Wuyts A, Opdenakker G, Van Damme J. Microbial Toll-like receptor ligands differentially regulate CXCL10/IP-10 expression in fibroblasts and mononuclear leukocytes in synergy with IFN-gamma and provide a mechanism for enhanced synovial chemokine levels in septic arthritis. Eur J Immunol. 2003;33:3146–3153. doi: 10.1002/eji.200324136. [DOI] [PubMed] [Google Scholar]
- 47.Lebre MC, van der Aar AM, van Baarsen L, van Capel TM, Schuitemaker JH, Kapsenberg ML, de Jong EC. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J Invest Dermatol. 2007;127:331–341. doi: 10.1038/sj.jid.5700530. [DOI] [PubMed] [Google Scholar]
- 48.Morris GE, Parker LC, Ward JR, Jones EC, Whyte MK, Brightling CE, Bradding P, Dower SK, Sabroe I. Cooperative molecular and cellular networks regulate Toll-like receptor-dependent inflammatory responses. Faseb J. 2006;20:2153–2155. doi: 10.1096/fj.06-5910fje. [DOI] [PubMed] [Google Scholar]
- 49.Taima K, Imaizumi T, Yamashita K, Ishikawa A, Fujita T, Yoshida H, Takanashi S, Okumura K, Satoh K. Expression of IP-10/CXCL10 is upregulated by double-stranded RNA in BEAS-2B bronchial epithelial cells. Respiration. 2006;73:360–364. doi: 10.1159/000091646. [DOI] [PubMed] [Google Scholar]
- 50.Brentano F, Schorr O, Gay RE, Gay S, Kyburz D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 2005;52:2656–2665. doi: 10.1002/art.21273. [DOI] [PubMed] [Google Scholar]
- 51.Cheng G, Nazar AS, Shin HS, Vanguri P, Shin ML. IP-10 gene transcription by virus in astrocytes requires cooperation of ISRE with adjacent kappaB site but not IRF-1 or viral transcription. J Interferon Cytokine Res. 1998;18:987–997. doi: 10.1089/jir.1998.18.987. [DOI] [PubMed] [Google Scholar]
- 52.Berghall H, Siren J, Sarkar D, Julkunen I, Fisher PB, Vainionpaa R, Matikainen S. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 2006;8:2138–2144. doi: 10.1016/j.micinf.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Imaizumi T, Kumagai M, Taima K, Fujita T, Yoshida H, Satoh K. Involvement of retinoic acid-inducible gene-I in the IFN-{gamma}/STAT1 signalling pathway in BEAS-2B cells. Eur Respir J. 2005;25:1077–1083. doi: 10.1183/09031936.05.00102104. [DOI] [PubMed] [Google Scholar]
- 54.Carpentier PA, Williams BR, Miller SD. Distinct roles of protein kinase R and toll-like receptor 3 in the activation of astrocytes by viral stimuli. Glia. 2007;55:239–252. doi: 10.1002/glia.20450. [DOI] [PubMed] [Google Scholar]
- 55.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim CH, Rott L, Kunkel EJ, Genovese MC, Andrew DP, Wu L, Butcher EC. Rules of chemokine receptor association with T cell polarization in vivo. J Clin Invest. 2001;108:1331–1339. doi: 10.1172/JCI13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.D'Ambrosio D, Iellem A, Bonecchi R, Mazzeo D, Sozzani S, Mantovani A, Sinigaglia F. Selective up-regulation of chemokine receptors CCR4 and CCR8 upon activation of polarized human type 2 Th cells. J Immunol. 1998;161:5111–5115. [PubMed] [Google Scholar]
- 58.Wills MR, Okecha G, Weekes MP, Gandhi MK, Sissons PJ, Carmichael AJ. Identification of naive or antigen-experienced human CD8(+) T cells by expression of costimulation and chemokine receptors: analysis of the human cytomegalovirus-specific CD8(+) T cell response. J Immunol. 2002;168:5455–5464. doi: 10.4049/jimmunol.168.11.5455. [DOI] [PubMed] [Google Scholar]
- 59.Campbell JJ, Brightling CE, Symon FA, Qin S, Murphy KE, Hodge M, Andrew DP, Wu L, Butcher EC, Wardlaw AJ. Expression of chemokine receptors by lung T cells from normal and asthmatic subjects. J Immunol. 2001;166:2842–2848. doi: 10.4049/jimmunol.166.4.2842. [DOI] [PubMed] [Google Scholar]
- 60.Sorensen TL, Sellebjerg F. Selective suppression of chemokine receptor CXCR3 expression by interferon-beta1a in multiple sclerosis. Mult Scler. 2002;8:104–107. doi: 10.1191/1352458502ms781oa. [DOI] [PubMed] [Google Scholar]
- 61.Amoura Z, Combadiere C, Faure S, Parizot C, Miyara M, Raphael D, Ghillani P, Debre P, Piette JC, Gorochov G. Roles of CCR2 and CXCR3 in the T cell-mediated response occurring during lupus flares. Arthritis Rheum. 2003;48:3487–3496. doi: 10.1002/art.11350. [DOI] [PubMed] [Google Scholar]
- 62.Saetta M, Mariani M, Panina-Bordignon P, Turato G, Buonsanti C, Baraldo S, Bellettato CM, Papi A, Corbetta L, Zuin R, Sinigaglia F, Fabbri LM. Increased expression of the chemokine receptor CXCR3 and its ligand CXCL10 in peripheral airways of smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;165:1404–1409. doi: 10.1164/rccm.2107139. [DOI] [PubMed] [Google Scholar]
- 63.Asselin-Paturel C, Brizard G, Chemin K, Boonstra A, O'Garra A, Vicari A, Trinchieri G. Type I interferon dependence of plasmacytoid dendritic cell activation and migration. J Exp Med. 2005;201:1157–1167. doi: 10.1084/jem.20041930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoneyama H, Matsuno K, Zhang Y, Nishiwaki T, Kitabatake M, Ueha S, Narumi S, Morikawa S, Ezaki T, Lu B, Gerard C, Ishikawa S, Matsushima K. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol. 2004;16:915–928. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 65.Vanbervliet B, Bendriss-Vermare N, Massacrier C, Homey B, de Bouteiller O, Briere F, Trinchieri G, Caux C. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J Exp Med. 2003;198:823–830. doi: 10.1084/jem.20020437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Penna G, Vulcano M, Sozzani S, Adorini L. Differential migration behavior and chemokine production by myeloid and plasmacytoid dendritic cells. Hum Immunol. 2002;63:1164–1171. doi: 10.1016/s0198-8859(02)00755-3. [DOI] [PubMed] [Google Scholar]
- 67.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol. 2006;177:6263–6270. doi: 10.4049/jimmunol.177.9.6263. [DOI] [PubMed] [Google Scholar]
- 69.Belperio JA, Keane MP, Burdick MD, Lynch JP, 3rd, Xue YY, Li K, Ross DJ, Strieter RM. Critical role for CXCR3 chemokine biology in the pathogenesis of bronchiolitis obliterans syndrome. J Immunol. 2002;169:1037–1049. doi: 10.4049/jimmunol.169.2.1037. [DOI] [PubMed] [Google Scholar]
- 70.Kulkarni AB, Morse HC, 3rd, Bennink JR, Yewdell JW, Murphy BR. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J Virol. 1993;67:4086–4092. doi: 10.1128/jvi.67.7.4086-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]