Abstract
It has become clear that complex interactions underlie the relationship between the skeletal and immune systems. This is particularly true for the development of immune cells in the bone marrow as well as the functions of bone cells in skeletal homeostasis and pathologies. Because these two disciplines developed independently, investigators with an interest in either often do not fully appreciate the influence of the other system on the functions of the tissue that they are studying. With these issues in mind, this review will focus on several key areas that are mediated by crosstalk between the bone and immune systems. A more complete appreciation of the interactions between immune and bone cells should lead to better therapeutic strategies for diseases that affect either or both systems.
Keywords: Immune system, Osteoblast, Osteoclast, Osteoimmunology
INTRODUCTION
Recent reviews have highlighted the interactions between bone and immune cells as well as their overlapping regulatory mechanisms (1, 2). For example, cells related to osteoblasts-the bone-forming cells of the body-regulate hematopoietic stem cell niches from which all blood and immune cells are derived. On the other hand, osteoclasts, which function to resorb bone, are derived from the same myeloid precursor cells that give rise to macrophages and myeloid dendritic cells. Furthermore, many of the soluble mediators of immune cells, including cytokines, chemokines, and growth factors, regulate the activities of osteoblasts and osteoclasts. This increased recognition of the complex interactions between the immune system and bone led to the development of the interdisciplinary osteoimmunology field, which seeks to translate an understanding of the mechanisms governing the interface between the skeletal and immune systems into therapeutic strategies for the treatment of disorders characterized by bone loss (1, 3).
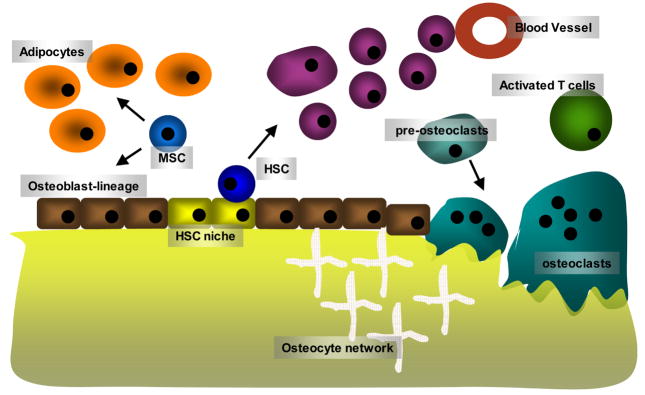
The regulation of bone by hematopoietic and immune cells produces a variety of physiologic and pathologic effects. It is likely that developing hematopoietic cells regulate bone turnover and maintain the marrow cavity by interacting with osteoblasts and osteoclasts. Conversely, during inflammatory states induced by cytokines, activated immune cells mediate increased bone turnover and the bone pathologies associated with diseases such as rheumatoid arthritis and inflammatory bowel disease. Indeed, if one views bone marrow spaces as “loosely compartmentalized lymphoid organ”, it won’t be difficult to conceptualize how intense immune cells and bone cells interact and influence each other (Fig. 1) (1). We are only beginning to understand the breadth of these interactions and this review is by no means complete. Examining the interface between these systems, however, should contribute to a scientific foundation for novel therapeutic strategies to treat disease states mediated by both systems.
Fig. 1.
A schematic diagram of the bone microenvironment. Hematopoietic stem cells (HSC) are maintained in the stem cell niche that is suggested to be provided by osteoblast lineage cells. Mesenchymal stem cells give rise to adipocytes and osteoblast-lineage cells. Cells derived from HSC differentiate, mature and migrate to the periphery though the vascular system. Memory T and B cells or activated T cells come back to the bone microenvironment, and provide factors influencing the bone cells such as osteoblasts and osteoclasts. Osteoclasts are derived from monocyte lineage cells, and resorb bone. Although not depicted in the diagram, there are numerous interactions among the cells in the bone microenvironment; hence it can be viewed as a loosely compartmentalized secondary lymphoid organ.
Here, we will provide a brief description of the current understanding of the interactions underlying the development of components of both the skeletal and immune systems. We will then focus on the cytokines that regulate bone cells and affect bone metabolism.
Osteoclasts and osteoblasts
Osteoclasts, which form as multinucleated cells following fusion between mononuclear precursor cells, are unique in their capacity to efficiently resorb bone (4, 5). In the 10 years since the osteoclast differentiation factor receptor activator of NF-κB ligand (RANKL) was discovered, a number of reviews have described the molecular pathways underlying the maturation of osteoclasts from bone marrow precursors (1, 2, 4, 5). Therefore, we will concentrate here on recent progress in the identification of the bone marrow cell population that serves as osteoclast precursors.
Bone marrow, peripheral blood, and spleen cell populations can form osteoclast-like cells (OCLs) in various in vitro culture systems (4, 5). Cultures with ST2 stromal cells, RANKL, and macrophage colony-stimulating factor (M-CSF) were used to demonstrate that murine bone marrow cell populations expressing c-kit can form OCLs (6). The authors concluded that the identified c-kit+c-fms+CD11blow bone marrow cells included multipotential progenitor cells that frequently gave rise to osteoclasts. These RANK− (the receptor for RANKL) progenitor cells expressed RANK in response to M-CSF. Interestingly, the precursors were not restricted to osteoclastogenesis; in methylcellulose cultures, they differentiated into macrophages and mononuclear TRAP+ cells. We later found that the osteoclast precursor cells were negative for CD3 and CD45R, and confirmed they did not or only weakly expressed the monocytic marker CD11b/Mac-1 (7). c-kit expression further separated this population into cells that rapidly formed OCLs in vitro when cultured with M-CSF and RANKL (c-kithigh cells) and cells that formed OCLs more slowly in vitro (c-kitlow or c-kit− cells). Although we initially found that the most efficient osteoclast precursors were CD11b− or CD11blow, culture with M-CSF and RANKL induced the mononuclear precursor cells to transiently express high levels of CD11b.
The relationship between osteoclasts and antigen-presenting dendritic cells has also been detailed. Human and murine cells expressing early markers for the myeloid dendritic cell lineage can differentiate into osteoclasts in vitro (8). Additionally, murine bone marrow cells that were able to present antigen to T lymphocytes following cytokine treatment formed OCLs in culture when they were treated with M-CSF and RANKL (9). Speziani et al., however, found that neither mature myeloid dendritic cells generated in vitro nor plasmacytoid dendritic cells generated in vivo formed OCLs in culture (10).
Although investigators previously suggested that common progenitor cells can differentiate into macrophages, osteoclasts, and myeloid dendritic cells (8), single cell clones from murine bone marrow were only recently isolated and differentiated into macrophages and dendritic cells (11). There is good evidence that these myeloid precursor cells can also differentiate into OCLs in vitro (J. A. L. unpublished data). Interestingly, commitment of the common precursors to the osteoclast lineage occurs relatively quickly (within 24 hours) after the cells are treated with RANKL (12).
Outside of the bone marrow, expression of the myeloid-specific antigen CD11b as well as Gr-1 was used to identify circulating osteoclast precursor cells, the cell number of which is regulated by the inflammatory state of the organism and in particular tumor necrosis factor-α (TNF-α) (13, 14). In human peripheral blood, RANK+ osteoclast precursors were identified using CD14 expression and the lack of CD16 expression (15–17). Migration and adhesion of these human CD14+ monocytes to sites of inflammation may be mediated through activation of microvascular endothelial cells by proinflammatory cytokines (18).
Interestingly, cells with cell-surface phenotypes similar to those of bone marrow osteoclast precursors were identified in the spleen, even though splenic osteoclastogenesis does not occur under any known condition, possibly because the splenic cells lack factors that are required for osteoclast differentiation. This hypothesis, however, seems unlikely because multiple investigators have established the osteoclastogenic potential of splenocytes in vitro. Another possibility is that the splenic microenvironment lacks critical signaling molecules that define an adherent condition for osteoclastogenesis (19). This hypothesis is supported by the recent findings that osteoclast differentiation and activation require various costimulatory molecules, which act in concert with M-CSF and RANKL (1, 2). These signals are transduced through immunoreceptor tyrosine-based activation motif domain-containing adaptor proteins, such as DAP12 and FcRγ, and at least four receptors have been found to associate with either FcRγ (OSCAR and PIR-A) or DAP12 (TREM-2 and SIRP β1) (20, 21). The ligands for these receptors are currently unknown.
Osteoblasts are derived from multipotential mesenchymal progenitor cells that also differentiate into marrow stromal cells and adipocytes (22). The regulatory signals that drive the progenitor cells to an osteoblast fate have not been fully elucidated. A number of critical paracrine signals and cell autonomous transcription factors, however, have been identified, including the transcription factors Runx2 and osterix as well as bone morphogenic proteins (BMPs), which initiate osteoblast differentiation (23–25). Wnt signaling also contributes to mesenchymal progenitor cells becoming either adipocytes or osteoblasts (26–28).
Cytokines and local immune cell factors as regulators of bone cells
In this section, we describe the current understanding of the roles of various inflammatory cytokines in bone metabolism.
RANKL
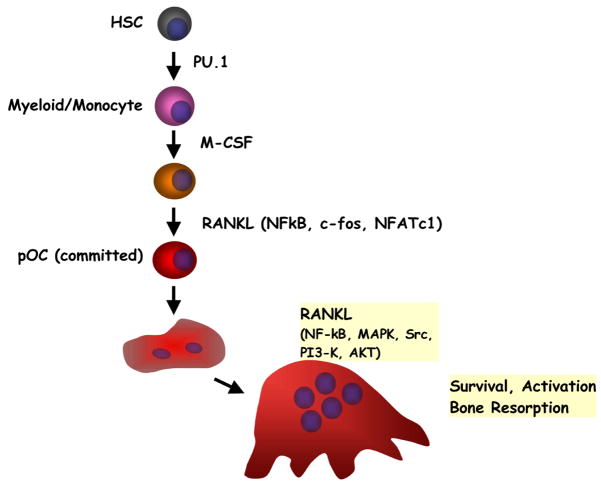
Characterizations of the functions of RANKL and its receptors (RANK and osteoprotegerin [OPG]) have elucidated the interplay between active immunity and bone homeostasis. A number of recent reviews about the diverse physiologic functions of the RANKL-RANK-OPG signaling axis in bone and it central roles in osteoimmunology have been published (1, 2, 4, 5). Therefore, we will briefly summarize the RANKL axis in Fig. 2.
Fig. 2.
A simplified view of osteoclast differentiation. RANKL produced by osteoblasts induces the differentiation of osteoclasts, and further regulates the activation and survival of mature osteoclasts. Some of the key transcription factors (PU.1, NF-κB, c-Fos, NFATc1) and signaling molecules (MAPK, c-Src, PI3K, AKT) are also shown in the diagram. HSC, hematopoietic stem cells.
TNF-α
TNF-α was shown to stimulate osteoclast formation and bone resorption in vivo, and enhance the formation of OCLs in bone marrow cultures (1, 2, 4, 5). The ability of TNF-α to stimulate osteoclast formation in mixed stromal cell/osteoclast precursor cell cultures was shown to be IL-1-dependent (29), whereas TNF-α-induced osteolysis was found to be dependent on M-CSF (30). Cultures of cells from RANK-deficient mice suggested that TNF-α directly stimulated osteoclast formation independent of RANK (31), although TNF-α administration produced only a few osteoclasts in RANK-deficient mice (32). TNF-α also inhibits osteoblast differentiation and collagen synthesis (33–35). Additionally, TNF-α is potently pro-apoptotic for osteoblasts (36), possibly through Fas-Fas ligand (FasL) signaling (37). Interestingly, however, mice deficient for both TNF receptor 1 and TNF receptor 2 do not have abnormal bone phenotypes, suggesting that TNF-α affects bone during inflammatory states, rather than during normal development.
Other members of the TNF superfamily
Precursor and mature osteoclasts express Fas and FasL (38). Treatment of mature osteoclasts with Fas induced apoptosis, whereas FasL treatment of cultured M-CSF/RANKL-treated osteoclast precursor cells increased osteoclast formation (39). In contrast, RANKL and FasL have counterregulatory roles in the apoptosis of mature osteoclasts; at high concentrations, RANKL inhibited the ability of FasL to induce this response (40). Additionally, the effects of FasL deficiency on bone mass are controversial; different studies found this index to increase or decrease in FasL-deficient mice (39, 41). Studying bone mass in Fas- or FasL-deficient mice, however, is difficult, because these models have generalized lymphroliferative disorders, which activate a wide variety of immune responses that affect bone. Interestingly, estrogen receptor-α was found to regulate FasL production in murine osteoclasts, which, in turn, mediated the bone loss induced by estrogen withdrawal (42).
Treatment of osteoclasts with TNF-related apoptosis inducing ligand (TRAIL) induces apoptosis (43). Accordingly, injection of young murine bone with TRAIL increased bone mass, which was associated with increased levels of the cyclin-dependent kinase inhibitor p27Kip1 (44). TRAIL may also contribute to the effects of myelomas on osteoblasts (45).
CD40 ligand (CD40L) is involved in the differentiation of TH1 effector cells. In humans, CD40L deficiency results in X-linked hyper IgM syndrome, which causes spontaneous bone fractures and osteopenia, possibly via activated T lymphocytes that are defective for interferon (INF)-γ production (46). Additionally, CD40L expression in the synovial cells of rheumatoid arthritis patients induces RANKL expression in these cells and enhances their osteoclastogenic activity (47).
M-CSF
Mutant op/op mice, which lack osteoclasts and show defective macrophage/monocyte formation, were found to be deficient for M-CSF. M-CSF injections or osteoblast-specific M-CSF expression in op/op mice rescued the osteoclast formation and bone resorption defects (1, 2, 4, 5). Consistently, bone resorption stimulators increase M-CSF production in bone (48, 49). Interestingly, membrane-bound M-CSF facilitated osteoclast differentiation (48, 50), whereas soluble M-CSF inhibited 1,25-dihydroxyvitamin D3-stimulated OCL formation in bone marrow cultures (51). In osteoclast precursor cells, M-CSF is a potent stimulator of RANK expression and proliferation (6, 7).
M-CSF also regulates osteoclast apoptosis as the addition of M-CSF to mature osteoclast cultures prolongs their survival (52). This may contribute to the osteopetrotic phenotype of op/op mice, because transgenic expression of apoptosis-inhibiting Bcl-2 in myeloid cells partially rescued the osteoclast and macrophage developmental defects in these animals (53).
Additional colony stimulating factors
GM-CSF and IL-3 also affect osteoclast differentiation (51, 54). Both factors inhibit RANKL-mediated osteoclastogenesis (55, 56), whereas they promote osteoclast precursor development (57); these proteins drive myeloid precursor cells to lineages other than osteoclasts (55), and inhibit TNF receptor expression on myeloid precursor cells (58). IL-3 also inhibits osteoblast differentiation, which may contribute to the effects of multiple myelomas on bone (59).
Systemic G-CSF injections decrease rodent bone mass, likely because of increased osteoclast formation and decreased osteoblast activity (60, 61). G-CSF also mobilizes hematopoietic precursor cells from bone marrow (62) and increases the number of circulating osteoclast precursors (63), which are likely related to increased osteoclast activity. Accordingly, G-CSF overexpression in mice inhibited the ability of osteoblasts to respond to BMP and increased bone resorption (64, 65).
IL-1
IL-1-a potent stimulator of bone resorption-is produced in bone and acts on osteoclasts directly and indirectly via enhanced RANKL production and activity (1, 2, 4, 5). Additionally, both RANKL- and 1,25-dihydroxyvitamin D3-stimulated osteoclast formation in vitro is mediated in part by IL-1 (66, 67). IL-1 also increases prostaglandin synthesis in bone (68, 69), which may account for some of its resorptive activity (70). Stimulation of osteoclastogenesis by IL-1 in mixed murine stromal cell/hematopoietic cell cultures was dependent on RANKL but not TNF-α (71). IL-1-mediated RANKL production in osteoblasts and osteoclasts survival depends on myeloid differentiation factor 88 (MyD88), PI3-kinase/AKT, and ERK, but not Toll/interleukin-1 receptor domain-containing adaptor inducing inter-feron-β (TRIF) (72, 73). A recent report found decreased bone mass in mice deficient for bioactive type I IL-1 receptor (IL-1R) (74), although our experiments did not support this result (75).
IL-6 family cytokines
IL-6-a multipotent cytokine with a wide variety of activities-is produced by osteoblastic cells and BMSCs (76, 77). Although IL-6-mediated bone resorption varies depending on the in vitro assay system (78, 79), it has been shown to regulate the development of mature osteoclasts (80), and directly stimulate the production RANKL and OPG mRNA as well as prostaglandins in bone (81, 82). IL-6 appears to mediate the increased bone resorption and pathologies that characterize various clinical syndromes, including Paget’s disease (83), hypercalcemia associated with malignancy (84), fibrous dysplasia (85), giant cell tumors of bone (86), and Gorham-Stout disease (87). Finally, there is conflicting data about the role of IL-6 in the PTH-mediated responses of bone (88, 89).
In response to resorptive stimuli, bone cells produce IL-11, which stimulates osteoclast formation and bone resorption in vitro (90–93). Interestingly, it has no effect on isolated mature osteoclasts. Mice deficient for the IL-11 receptor showed increased trabecular bone mass, which may reflect the decreased bone turnover, osteoclast formation, and resorption activity observed in vitro (94).
LIF is produced by bone cells in response resorption stimuli (95), resulting in variable effects on bone resorption. In some in vitro systems, LIF stimulated prostaglandin-dependent resorption (96), whereas, in others, it produced inhibitory effects (97, 98). In neonatal murine calvaria cultures, LIF stimulated both RANKL and OPG expression (82). Furthermore, local injections of LIF augmented parameters of bone resorption and formation, as well as the thickness of the treated bones (99). Mice lacking specific LIF receptors showed reduced bone volume and increased osteoclast numbers (100).
Although oncostatin M stimulates multinuclear cell formation in bone marrow cultures, these cells appeared to be macrophage polykaryons and not osteoclasts (101). On the other hand, oncostatin M inhibited 1,25-dihydroxyvitamin D3-stimulated OCL formation in human bone marrow cultures (101), and decreased bone resorption rates in fetal mouse long bone cultures (102). Moreover, oncostatin M overexpression in transgenic mice induced a osteopetrotic phenotype (103). Hence, oncostatin M likely inhibits osteoclast formation and bone resorption.
The roles of the IL-6 cytokine family in osteoclast formation should be examined based on data demonstrating that mice lacking the gp130 activator protein have increased osteoclast numbers (104). Because gp130 transduces signalling for all of the IL-6 family members, this result argues that at least some of these factors inhibit osteoclast formation and bone resorption. The available data implicate oncostatin M (102) and possibly LIF (97, 98) for this function.
IL-7
IL-7, which plays nonredundant roles in B- and T-cell lymphopoiesis, also regulates bone homeostasis (105); the mechanisms by which IL-7 affects bone cells, however, are controversial. Systemic IL-7 administration enhances osteoclast formation in peripheral blood by increasing osteoclastogenic cytokine production in T cells (106). Moreover, IL-7 did not induce bone loss in T-cell-deficient nude mice (107). Additionally, ovariectomy enhances T-cell development through IL-7, which may underlie ovariectomy-induced bone loss (108). Thus, interpreting the effects of IL-7 treatment is complicated by secondary effects in vivo, which result from T cells producing bone-resorbing cytokines (106, 107).
In contrast, we observed that IL-7 inhibited osteoclast formation in murine bone marrow cells cultured with M-CSF and RANKL (109). Moreover, the osteoclast number markedly increased and trabecular bone mass decreased in IL-7-deficient mice (110). Additionally, trabecular bone loss after ovariectomy was similar in wild-type and IL-7-deficient mice (110). Curiously, IL-7 mRNA levels in bone increased following ovariectomy, which may result from altered osteoblast function after estrogen withdrawal (105, 111). IL-7 also inhibited bone formation in newborn murine calvaria in vivo and in vitro (105). When IL-7 was locally overexpressed by osteoblasts, however, trabecular bone mass increased (112). Furthermore, targeted IL-7 overexpression in IL-7-knockout mice rescued their osteoporotic bone phenotype (113). These studies indicated that the effects of IL-7 on bone cells depend on whether IL-7 is delivered systemically or locally.
IL-8 and chemokines
Chemokines, which can be divided into the CXC, CC, C, and CX3C subtypes based on the sequence motif containing the first cysteine residue, act through G-protein-coupled receptors to initiate cytoskeletal rearrangement, adhesion, and directional migration (114). IL-8, a CXC chemokine produced by osteoclasts, stimulates osteoclastogenesis and bone resorption independent of the RANKL pathway (115, 116). Additionally, IL-8 produced by certain cancers stimulates lytic bone lesions in metastatic disease (115, 116). The effects of IL-8 on bone may be partly mediated by upregulated osteoclast nitric oxide synthase expression (117).
CCL3 (macrophage inflammatory protein-1α), which is expressed in bone and bone marrow cells, directly stimulates osteoclastogenesis through the receptors CCR1 and CCR5 (118–120). CCL3 also mediates the osteolytic activity of multiple myelomas (121, 122). Interestingly, CCL3 and IL-8 stimulate the motility but suppress the resorption activity of mature osteoclasts (123).
Expression of CCL9 (macrophage inflammatory peptide-1γ) and its receptor CCR1 in osteoclasts is induced by RANKL (124). Moreover, CCL9 and other chemokines that bind CCR1 (CCL3, CCL5, and CCL7) are produced by osteoclasts, osteoblasts, and their precursors in bone; expression of these chemokines in differentiating osteoblasts is induced by proinflammatory cytokines (IL-1 and TNF-α) (125). Additional chemokine receptors that are produced by osteoclasts include CCR3, CCR5, and CX3CR1 (118, 126).
Injections of M-CSF into osteopetrotic tl/tl rats, which lack M-CSF, induce osteoclastogenesis, bone resorption, and a rapid upregulation of CCR1 and CCL9 expression in bone (127). Furthermore, anti-CCL9 antibodies ameliorated M-CSF-stimulated osteoclastogenesis in these rats. Inhibiting CCR1 expression suppressed the migration of RAW 264.7 osteoclast precursor cells and murine bone marrow cells (128). Furthermore, blocking ligand binding by CCR1 in murine bone marrow cultures prevented OCL formation (128), and neutralizing antibodies against CCL9 inhibited RANKL-induced osteoclastogenesis (124).
CXCL12 (stromal cell derived factor-1) and its receptor CXCR4 contribute to hematopoietic cell homeostasis and immune responses. Osteoclast precursor cells express CXCR4 (129), which is downregulated as these cells differentiate to the osteoclast lineage (130). Treatment of RAW 264.7 cells with CXCL12 induces matrix metalloproteinase 9 (MMP9) expression, which may contribute to the migration of precursor cells to bone (129). In human osteoclast precursor cells, CXCL12 enhanced the migration and osteoclastogenesis induced by RANKL and M-CSF (129, 130). CXCL12 expression is upregulated in osteoclasts differentiating on a calcium phosphate matrix (130). CXCL12 may also be involved in precursor cell recruitment to giant cell tumors in bone (131) and the increased osteolysis associated with multiple myelomas (132).
CCL2 (monocyte chemoattractant protein-1)-the ligand for CCR2-is highly expressed in osteoblasts associated with induced inflammatory lesions (133), an effect that is mediated by proinflammatory cytokines (134). CCL2 may also contribute to the eruption of teeth via its expression in dental follicle cells (135), although CCL2-deficient mice demonstrated that CCL2 is not required for tooth eruption (136). In mononuclear precursor cells, CCL2 expression is induced by RANKL and enhances OCL formation (137). It was also recently shown that treating osteoblasts with PTH increased CCL2 expression and enhanced the fusion of preosteoclasts (138).
IL-10
IL-10 produced by activated T and B lymphocytes directly inhibits osteoclastogenesis and osteoblastogenesis via increased tyrosine phosphorylation of a variety of proteins in osteoclast precursor cells, suppressed production of osteoblastic proteins, and inhibition of the onset of mineralization (139–141). The direct effects of IL-10 on RANKL-stimulated osteoclastogenesis are associated with decreased NFATc1 expression and nuclear translocation (142) as well as suppressed c-Fos and c-Jun expression (143). Thus, IL-10 may be therapeutically useful to control wear-induced osteolysis (144). IL-10 also appears to indirectly affect osteoclastogenesis, because in vitro IL-10 treatment inhibited and enhanced RANKL and OPG production, respectively, in dental follicle cells (145). Recently reported results suggest that some effects of IL-10 on osteoclasts are mediated by interactions between the inducible T-cell costimulatory molecule 4-1BB its ligand (146).
IL-12
Although IL-12 is thought to inhibit osteoclastogenesis, the underlying mechanism is controversial. One study demonstrated that IL-12 directly inhibited RANKL-stimulated osteoclastogenesis in purified primary osteoclast precursors and RAW 264.7 cells, which was associated with suppressed NFATc1 expression (147). Interestingly, IL-12 did not inhibit osteoclastogenesis in cells pretreated with RANKL. In contrast, others have found that the inhibitory effects of IL-12 on osteoclastogenesis are indirect; one group showed that IL-12 inhibition was mediated by T lymphocytes and did not involve INF-γ (148), whereas a second group found that IL-12 inhibited osteoclastogenesis in T lymphocyte-deficient cultures or nude mice (149). The second study also reported that anti-INF-γ antibodies partially blocked IL-12-mediated inhibition of RANKL-stimulated osteoclastogenesis.
IL-15
IL-15, a member of the IL-2 superfamily, has been shown to increase osteoclast progenitor cell numbers in culture (150). Moreover, IL-15 production by T lymphocytes is involved in the increased osteoclastogenesis and bone destruction seen patients with rheumatoid arthritis (151).
IL-17 and IL-23
The IL-17 cytokines constitute a six-member family (IL-17A-F) that are central for adaptive immune responses (152). They are products of the TH17 subset of CD4+ T lymphocytes, which have a high IL-17-dependent osteoclastogenic activity (153). IL-17A was initially found to stimulate osteoclastogenesis in mixed hematopoietic cell/osteoblasts cultures via prostaglandin synthesis and RANKL expression (154). Moreover, IL-17A mediates the activation of osteoclasts and bone destruction in joints affected by rheumatoid arthritis (154), effects that are enhanced by TNF-α (155). IL-17A inhibition in an antigen-induced arthritis model inhibited joint and bone destruction and decreased the levels of RANKL, IL-1-β, and TNF-α in the pathologic lesions (156).
IL-23 is an IL-12-related cytokine that, along with TGF-β and IL-6, is critical for TH17 differentiation and proliferation (157). LPS-induced inflammatory bone destruction was markedly attenuated in mice deficient for either IL-17 or IL-23 (153). The authors also observed IL-23 mRNA expression in the synovial tissues of patients with rheumatoid arthritis, which suggests that similar mechanisms underlie rheumatoid arthritis-induced bone loss.
IL-18
The levels of IL-18, a member of the IL-1 superfamily, increase at sites of inflammation, such as those associated with rheumatoid arthritis (158). It is expressed by osteoblastic cells and inhibits osteoclast formation through a variety of mechanisms, including enhanced GM-CSF expression in T cells (159). IL-18, which is also a mitogen for osteoblastic cells in vitro (160), stimulates INF-γ production in bone (161) and its inhibition of osteoclastogenesis is enhanced by cotreatment with IL-12 (162). Additionally, it has been shown to increase OPG production (163). In IL-18-overexpressing transgenic mice, the number of osteoclasts decreased, although so did bone mass, suggesting that IL-18 may also affect bone growth (161). Surprisingly, IL-18 has also been shown to indirectly stimulate osteoclastogenesis through its effects on T lymphocytes (164).
Interferons
INF-γ is a type II INF that inhibits bone resorption in vitro as a result of its effects on osteoclast progenitors (165). INF-γ also inhibits the abilities of 1,25-dihydroxyvitamin D3, PTH, and IL-1 to stimulate OCL formation in bone marrow cultures (166). INF-γ inhibits RANK signalling by accelerating the ubiquitin/proteasome-mediated degradation of TRAF6 (167); it, however, does not directly inhibit resorption by mature osteoclasts (168). INF-γ is also reported to stimulate resorption through enhanced RANKL and TNF-α production in T lymphocytes (169). Moreover, it inhibits osteoblast proliferation and has variable effects on osteoblast differentiation (35, 160, 170). The in vivo effects of INF-γ on bone are different from its actions in vitro. In rats, intraperitoneal INF-γ injections induced osteopenia (171), whereas administration of INF-γ stimulated bone resorption and appeared to partially reverse the course of osteopetrosis in patients, which may be due to INF-γ stimulation of osteoclast superoxide synthesis or osteoclast formation (172, 173).
As for the type I INFs (INF-α and INF-β), mice deficient for the INF-α/β. receptor component IFNAR1 show reduced trabecular bone mass and increased osteoclast numbers (174). RANKL induces INF-β expression in osteoclasts, which, in turn, inhibits RANKL-mediated osteoclastogenesis by decreasing c-fos expression (174). For osteoclast development, therefore, osteoclast precursors upregulate the expression of the cytokine signaling regulator SOCS3 to suppress the effects of IFNs (175, 176). INF-α has also been shown to inhibit bone resorption, although the mechanism of action is not well understood (177). INF-α had no effect on bone turnover in vivo (178).
Additional cytokines
IL-4 and IL-13 are locally acting inhibitory cytokines that have related effects on osteoblasts and osteoclasts. Transgenic IL-4 overexpression causes osteoporosis due to inhibited osteoclast formation, osteoclast activity, and bone formation (179–181). IL-13 and IL-4 inhibited IL-1-stimulated bone resorption by decreasing prostaglandin levels and cyclooxygenase-2 activity (182). These cytokines also induce osteoblastic cell migration (183) and influence the ability of osteoblasts to regulate osteoclast formation and activity through increased and decreased OPG and RANKL production, respectively (184). Direct IL-4 inhibition of osteoclast precursor cell maturation, which is mediated by STAT6, NF-κB, peroxisome proliferator-activated receptor γ1, mitogen-activated protein kinase signalling, Ca2+ signalling, NFATc1, and c-Fos, is stronger than that of IL-13 (185–189).
Macrophage migration inhibitory factor (MIF) is produced by T lymphocytes, pituitary cells, and activated macrophages (190). MIF overexpression causes high-turnover osteoporosis in mice (191). Interestingly, MIF-deficient mice did not lose bone mass or have increased osteoblast or osteoclast numbers following ovariectomy (192), suggesting MIF mediates the effects of estrogen withdrawal on bone. Estrogen downregulates MIF expression in activated macrophages (193), which may explain bone and bone marrow responses following ovariectomy. MIF is also made by osteoblasts, in which its production is upregulated by various growth factors, including TGF-β, FGF-2, IGF-II, and fetal calf serum (194). Finally, MIF increases MMP9 and MMP13 expression in osteoblasts (195) and inhibits RANKL- stimulated osteoclastogenesis in vitro (196).
Factors stimulating Toll-like receptors (TLRs)
TLRs are critical activators of innate immune responses that are highly expressed on antigen-presenting cells, such as dendritic cells and macrophages (197). Receptor ligation by microbial molecules or endogenous “danger” factors results in upregulated expression of costimulatory molecules and inflammatory cytokines. Because macrophages, dendritic cells, and osteoclasts have common progenitors, it is not surprising that TLRs are also detected in bone cells (198). Direct signaling via TLRs on osteoclast precursors, including TLR4, inhibits RANKL- mediated osteoclastogenesis (198), which appears counterintuitive because bacterial infections can cause inflammatory diseases characterized by reduced bone mineral density, such as periodontitis, osteomyelitis, and bacterial arthritis (199). Additionally, LPS may stimulate bone loss in mice via increased osteoclast numbers, and TLR activation can enhance osteoclast differentiation through RANKL and TNF-α expression in osteoblasts (200, 201). Our recent data suggest that TLRs inhibit osteoclast differentiation partly via the expression of type I IFNs; INF-β receptor-deficient monocytes are resistant to the TLR-mediated suppression of osteoclastogenesis (Y. C., unpublished data).
The basis for how TLR stimulation negatively regulates osteoclastogenesis, while at the same time bacterial infections are associated with excessive bone resorption by osteoclasts remains unclear. As described earlier, Gram-negative bacterial infections lead to alveolar bone destruction in periodontitis as a result of T-cell responses, upregulation of RANKL expression, and enhanced osteoclastogenesis (202). In the same study, bacterial infections in immunodeficient mice did not cause significant levels of alveolar bone loss, suggesting that bone loss associated with bacterial infections may be an indirect outcome of exacerbated T cell responses.
Similar to macrophages and dendritic cells, osteoclast precursors produce proinflammatory cytokines in response to TLR ligands (198). Moreover, although TLR stimulation inhibits osteoclast differentiation, osteoclast precursors treated with TLR ligands retain marked phagocytic activities. Therefore, TLR stimulation of osteoclast precursors likely results in a net enhancement of immune responses, which can be achieved by increased cytokine production and inhibiting their differentiation into nonimmune cells, such as mature osteoclasts. Because these cells can differentiate into mature osteoclasts if TLR ligands are removed (198), however, residual activated T cells present after a microbial infection is cleared can drive the phagocytic precursors to differentiate into bone-resorbing osteoclasts. Additionally, TNF-α produced by osteoclast precursors following TLR stimulation enhances bone resorption.
Conversely, the RANKL axis may regulate the inflammatory effects of TLR signaling. For example, a recent report suggested that LPS-induced production of proinflammatory cytokines was reduced in OPG-deficient mice, whereas it increased in RANKL-knockout mice, which increased the lethality if LPS injections (203). Moreover, mice pretreated with RANKL were partially protected from LPS-induced death. These results suggest that RANKL may suppress the cytokine response to LPS or other TLR ligands.
TLRs are thus likely to regulate the balance between immune responses and bone metabolism during acute attacks by various microbes. in vivo stimulation of TLRs, however, may have different effects on bone metabolism depending on the nature of the immune response. For example, continual stimulation of TLRs by commensal bacteria may affect bone metabolism, which is supported by recent data showing that mice deficient for mediators of TLR/IL-1R signaling pathways exhibit altered bone metabolism, although the precise signaling defects are unclear (72, 204).
Conclusion
Key cellular and molecular mechanisms governing homeostasis in the immune and skeletal systems have been described. Despite extensive cross-regulation between bone metabolism and the immune system, however, the mechanisms by which these systems regulate each other are poorly understood, due in part to the challenges typically associated with crossing disciplinary boundaries. While it is difficult for scientists and physicians to keep abreast of advances in their own field, it is even harder to develop the knowledge base and materials necessary to address issues that touch on multiple disciplines. Therefore, it is critical to create environments conducive to the study of multidisciplinary challenges. Awareness of intersystem crosstalk will no doubt contribute to our understanding of how bone and the immune system are physiologically regulated. Moreover, this endeavor may lead to better treatments for pathologies involving both systems, including inflammatory and metabolic bone diseases as well as tumor-induced bone lysis. Many of these processes are being targeted with therapeutics in the absence of a solid scientific understanding of the underlying molecular and cellular processes.
A report from the US Surgeon General on bone health suggested one in two Americans more than 50 years old will be at risk for fractures related to osteoporosis or low bone mass by 2020. These secondary health concerns are becoming more prominent as people live longer and remain more active as they age. Future preventative treatments for chronic bone-related diseases that are often associated with inflammation and impact patients’ quality of life will require a high degree of specificity, especially in populations already suffering from or vulnerable to other age-related ailments. We believe these issues place osteoimmunology in a position of unique clinical significance.
Acknowledgments
This work was supported in part by the grants from the Public Health Service of the United States, National Institutes of Health. The authors are grateful for advises and encouragement from many collaborators and colleagues. Due to the space constraints, we apologize for the inability to cite all relevant references.
References
- 1.Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 2.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev Immunol. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 3.Arron JR, Choi Y. Bone versus immune system. Nature. 2000;408:535–536. doi: 10.1038/35046196. [DOI] [PubMed] [Google Scholar]
- 4.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 5.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 6.Arai F, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-Fms and receptor activator of nuclear factor kappaB (RANK) receptors. J Exp Med. 1999;190:1741–1754. doi: 10.1084/jem.190.12.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jacquin C, Gran DE, Lee SK, Lorenzo JA, Aguila HL. Identification of multiple osteoclast precursor populations in murine bone marrow. J Bone Min Res. 2006;21:67–77. doi: 10.1359/JBMR.051007. [DOI] [PubMed] [Google Scholar]
- 8.Miyamoto T, Ohneda O, Arai F, Iwamoto K, Okada S, Takagi K, Anderson DM, Suda T. Bifurcation of osteoclasts and dendritic cells from common progenitors. Blood. 2001;98:2544–2554. doi: 10.1182/blood.v98.8.2544. [DOI] [PubMed] [Google Scholar]
- 9.Alnaeeli M, Penninger JM, Teng YT. Immune Interactions with CD4+ T Cells Promote the Development of Functional Osteoclasts from Murine CD11c+ Dendritic Cells. J Immunol. 2006;177:3314–3326. doi: 10.4049/jimmunol.177.5.3314. [DOI] [PubMed] [Google Scholar]
- 10.Speziani C, Rivollier A, Gallois A, Coury F, Mazzorana M, Azocar O, Flacher M, Bella C, Tebib J, Jurdic P, Rabourdin-Combe C, Delprat C. Murine dendritic cell transdifferentiation into osteoclasts is differentially regulated by innate and adaptive cytokines. Eur J Immunol. 2007;37:747–757. doi: 10.1002/eji.200636534. [DOI] [PubMed] [Google Scholar]
- 11.Fogg DK, Sibon C, Miled C, Jung S, Aucouturier P, Littman DR, Cumano A, Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 12.Mochizuki A, Takami M, Kawawa T, Suzumoto R, Sasaki T, Shiba A, Tsukasaki H, Zhao B, Yasuhara R, Suzawa T, Miyamoto Y, Choi Y, Kamijo R. Identification and characterization of the precursors committed to osteoclasts induced by TNF related activation-induced cytokine/receptor activator of NF-kappa B ligand. J Immunol. 2006;177:4360–4368. doi: 10.4049/jimmunol.177.7.4360. [DOI] [PubMed] [Google Scholar]
- 13.Li P, Schwarz EM, O’Keefe RJ, Ma L, Looney RJ, Ritchlin CT, Boyce BF, Xing L. Systemic tumor necrosis factor alpha mediates an increase in peripheral CD11bhigh osteoclast precursors in tumor necrosis factor alpha-transgenic mice. Arthritis Rheum. 2004;50:265–276. doi: 10.1002/art.11419. [DOI] [PubMed] [Google Scholar]
- 14.Yao Z, Li P, Zhang Q, Schwarz EM, Keng P, Arbini A, Boyce BF, Xing L. Tnf increases circulating osteoclast precursor numbers by promoting their proliferation and differentiation in the bone marrow through upregulation of c-fms expression. J Biol Chem. 2006;281:11846–11855. doi: 10.1074/jbc.M512624200. [DOI] [PubMed] [Google Scholar]
- 15.Atkins GJ, Kostakis P, Vincent C, Farrugia AN, Houchins JP, Findlay DM, Evdokiou A, Zannettino AC. RANK Expression as a cell surface marker of human osteoclast precursors in peripheral blood, bone marrow, and giant cell tumors of bone. J Bone Min Res. 2006;21:1339–1349. doi: 10.1359/jbmr.060604. [DOI] [PubMed] [Google Scholar]
- 16.Komano Y, Nanki T, Hayashida K, Taniguchi K, Miyasaka N. Identification of a human peripheral blood monocyte subset that differentiates into osteoclasts. Arthritis Res Ther. 2006;8:R152. doi: 10.1186/ar2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sorensen MG, Henriksen K, Schaller S, Henriksen DB, Nielsen FC, Dziegiel MH, Karsdal MA. Characterization of osteoclasts derived from CD14+ monocytes isolated from peripheral blood. J Bone Min Res. 2007;25:36–45. doi: 10.1007/s00774-006-0725-9. [DOI] [PubMed] [Google Scholar]
- 18.Kindle L, Rothe L, Kriss M, Osdoby P, Collin-Osdoby P. Human microvascular endothelial cell activation by IL-1 and TNF-alpha stimulates the adhesion and transendothelial migration of circulating human CD14+ monocytes that develop with RANKL into functional osteoclasts. J Bone Min Res. 2006;21:193–206. doi: 10.1359/JBMR.051027. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto T, Arai F, Ohneda O, Takagi K, Anderson DM, Suda T. An adherent condition is required for formation of multinuclear osteoclasts in the presence of macrophage colony-stimulating factor and receptor activator of nuclear factor kappa B ligand. Blood. 2000;96:4335–4343. [PubMed] [Google Scholar]
- 20.Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 22.Aubin JE. Regulation of osteoblast formation and function. Rev Endocr Metab Disord. 2001;2:81–94. doi: 10.1023/a:1010011209064. [DOI] [PubMed] [Google Scholar]
- 23.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 24.Celeste AJ, Iannazzi JA, Taylor RC, Hewick RM, Rosen V, Wang EA, Wozney JM. Identification of transforming growth factor beta family members present in bone-inductive protein purified from bovine bone. Proc Natl Acad Sci U S A. 1990;87:9843–9847. doi: 10.1073/pnas.87.24.9843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 26.Bennett CN, Longo KA, Wright WS, Suva LJ, Lane TF, Hankenson KD, MacDougald OA. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102:3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Jüppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML Osteoporosis- Pseudoglioma Syndrome Collaborative Group. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 28.Kato M, Patel MS, Levasseur R, Lobov I, Chang BH, Glass DA, 2nd, Hartmann C, Li L, Hwang TH, Brayton CF, Lang RA, Karsenty G, Chan L. Cbfa1- independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157:303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitaura H, Zhou P, Kim HJ, Novack DV, Ross FP, Teitelbaum SL. M-CSF mediates TNF-induced inflammatory osteolysis. J Clin Invest. 2005;115:3418– 3427. doi: 10.1172/JCI26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, McCabe S, Elliott R, Scully S, Van G, Kaufman S, Juan SC, Sun Y, Tarpley J, Martin L, Christensen K, McCabe J, Kostenuik P, Hsu H, Fletcher F, Dunstan CR, Lacey DL, Boyle WJ. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000;97:1566–1571. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, Nanes MS. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141:3956. doi: 10.1210/endo.141.11.7739. [DOI] [PubMed] [Google Scholar]
- 34.Centrella M, McCarthy TL, Canalis E. Tumor necrosis factor-alpha inhibits collagen synthesis and alkaline phosphatase activity independently of its effect on deoxyribonucleic acid synthesis in osteoblast-enriched bone cell cultures. Endocrinology. 1988;123:1442–1448. doi: 10.1210/endo-123-3-1442. [DOI] [PubMed] [Google Scholar]
- 35.Smith DD, Gowen M, Mundy GR. Effects of interferon-gamma and other cytokines on collagen synthesis in fetal rat bone cultures. Endocrinology. 1987;120:2494–2499. doi: 10.1210/endo-120-6-2494. [DOI] [PubMed] [Google Scholar]
- 36.Jilka RL, Weinstein RS, Bellido T, Parfitt AM, Manolagas SC. Osteoblast programmed cell death (apoptosis): modulation by growth factors and cytokines. J Bone Miner Res. 1998;13:793–802. doi: 10.1359/jbmr.1998.13.5.793. [DOI] [PubMed] [Google Scholar]
- 37.Kawakami A, Eguchi K, Matsuoka N, Tsuboi M, Koji T, Urayama S, Fujiyama K, Kiriyama T, Nakashima T, Nakane PK, Nagataki S. Fas and Fas ligand interaction is necessary for human osteoblast apoptosis. J Bone Miner Res. 1997;12:1637–1647. doi: 10.1359/jbmr.1997.12.10.1637. [DOI] [PubMed] [Google Scholar]
- 38.Park H, Jung YK, Park OJ, Lee YJ, Choi JY, Choi Y. Interaction of fas ligand and fas expressed on osteoclast precursors increases osteoclastogenesis. J Immunol. 2005;175:7193–7201. doi: 10.4049/jimmunol.175.11.7193. [DOI] [PubMed] [Google Scholar]
- 39.Wu X, McKenna MA, Feng X, Nagy TR, McDonald JM. Osteoclast Apoptosis: The role of Fas in vivo and in vitro. Endocrinology. 2003;144:5545–5555. doi: 10.1210/en.2003-0296. [DOI] [PubMed] [Google Scholar]
- 40.Wu X, Pan G, McKenna MA, Zayzafoon M, Xiong WC, McDonald JM. RANKL regulates Fas expression and Fas-mediated apoptosis in osteoclasts. J Bone Min Res. 2005;20:107–116. doi: 10.1359/JBMR.041022. [DOI] [PubMed] [Google Scholar]
- 41.Katavic V, Lukic IK, Kovacic N, Grcevic D, Lorenzo JA, Marusic A. Increased Bone Mass Is a Part of the Generalized Lymphoproliferative Disorder phenotype in the mouse. J Immunol. 2003;170:1540–1547. doi: 10.4049/jimmunol.170.3.1540. [DOI] [PubMed] [Google Scholar]
- 42.Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K, Harada Y, Azuma Y, Krust A, Yamamoto Y, Nishina H, Takeda S, Takayanagi H, Metzger D, Kanno J, Takaoka K, Martin TJ, Chambon P, Kato S. Estrogen prevents bone loss via estrogen receptor alpha and induction of Fas ligand in osteoclasts. Cell. 2007;130:811–823. doi: 10.1016/j.cell.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 43.Roux S, Lambert-Comeau P, Saint-Pierre C, Lepine M, Sawan B, Parent JL. Death receptors, Fas and TRAIL receptors, are involved in human osteoclast apoptosis. Biochem Biophys Res Commun. 2005;333:42–50. doi: 10.1016/j.bbrc.2005.05.092. [DOI] [PubMed] [Google Scholar]
- 44.Zauli G, Rimondi E, Stea S, Baruffaldi F, Stebel M, Zerbinati C, Corallini F, Secchiero P. TRAIL inhibits osteoclastic differentiation by counteracting RANKL-dependent p27(Kip1) accumulation in pre-osteoclast precursors. J Cell Physiol. 2008;214:117–125. doi: 10.1002/jcp.21165. [DOI] [PubMed] [Google Scholar]
- 45.Tinhofer I, Biedermann R, Krismer M, Crazzolara R, Greil R. A role of TRAIL in killing osteoblasts by myeloma cells. FASEB J. 2006;20:759–761. doi: 10.1096/fj.05-4329fje. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Granados E, Temmerman ST, Wu L, Reynolds JC, Follmann D, Liu S, Nelson DL, Rauch F, Jain A. Osteopenia in X-linked hyper-IgM syndrome reveals a regulatory role for CD40 ligand in osteoclastogenesis. Proc Natl Acad Sci U S A. 2007;104:5056–5061. doi: 10.1073/pnas.0605715104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HY, Jeon HS, Song EK, Han MK, Park SI, Lee SI, Yun HJ, Kim JR, Kim JS, Lee YC, Kim SI, Kim HR, Choi JY, Kang I, Kim HY, Yoo WH. CD40 ligation of rheumatoid synovial fibro-blasts regulates RANKL-medicated osteoclastogenesis: evidence of NF-kappaB-dependent, CD40-mediated bone destruction in rheumatoid arthritis. Arthritis Rheum. 2006;54:1747–1758. doi: 10.1002/art.21873. [DOI] [PubMed] [Google Scholar]
- 48.Rubin J, Fan X, Thornton D, Bryant R, Biskobing D. Regulation of murine osteoblast macrophage colony-stimulating factor production by 1, 25(OH)2D3. Calcif Tissue Int. 1996;59:291–296. doi: 10.1007/s002239900125. [DOI] [PubMed] [Google Scholar]
- 49.Weir EC, Horowitz MC, Baron R, Centrella M, Kacinski BM, Insogna KL. Macrophage colony-stimulating factor release and receptor expression in bone cells. J Bone Miner Res. 1993;8:1507–1518. doi: 10.1002/jbmr.5650081214. [DOI] [PubMed] [Google Scholar]
- 50.Yao GQ, Sun BH, Hammond EE, Spencer EN, Horowitz MC, Insogna KL, Weir EC. The cell-surface form of colony-stimulating factor-1 is regulated by osteotropic agents and supports formation of multinucleated osteoclast-like cells. J Biol Chem. 1998;273:4119–4128. doi: 10.1074/jbc.273.7.4119. [DOI] [PubMed] [Google Scholar]
- 51.Shinar DM, Sato M, Rodan GA. The effect of hemopoietic growth factors on the generation of osteoclast-like cells in mouse bone marrow cultures. Endocrinology. 1990;126:1728–1735. doi: 10.1210/endo-126-3-1728. [DOI] [PubMed] [Google Scholar]
- 52.Fuller K, Owens JM, Jagger CJ, Wilson A, Moss R, Chambers TJ. Macrophage colony-stimulating factor stimulates survival and chemotactic behavior in isolated osteoclasts. J Exp Med. 1993;178:1733–1744. doi: 10.1084/jem.178.5.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lagasse E, Weissman IL. Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell. 1997;89:1021–1031. doi: 10.1016/s0092-8674(00)80290-1. [DOI] [PubMed] [Google Scholar]
- 54.Lorenzo JA, Sousa SL, Fonseca JM, Hock JM, Medlock ES. Colony-stimulating factors regulate the development of multinucleated osteoclasts from recently replicated cells in vitro. J Clin Invest. 1987;80:160–164. doi: 10.1172/JCI113042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Khapli SM, Mangashetti LS, Yogesha SD, Wani MR. IL-3 Acts Directly on Osteoclast Precursors and Irreversibly Inhibits Receptor Activator of NF-kappaB Ligand-Induced Osteoclast Differentiation by Diverting the Cells to Macrophage Lineage. J Immunol. 2003;171:142–151. doi: 10.4049/jimmunol.171.1.142. [DOI] [PubMed] [Google Scholar]
- 56.Udagawa N, Horwood NJ, Elliott J, Mackay A, Owens J, Okamura H, Kurimoto M, Chambers TJ, Martin TJ, Gillespie MT. Interleukin-18 (interferon-gamma-inducing factor) is produced by osteo-blasts and acts via granulocyte/macrophage colony-stimulating factor and not via interferon-gamma to inhibit osteoclast formation. J Exp Med. 1997;185:1005–1012. doi: 10.1084/jem.185.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takahashi N, Udagawa N, Akatsu T, Tanaka H, Shionome M, Suda T. Role of colony-stimulating factors in osteoclast development. J Bone Miner Res. 1991;6:97785. doi: 10.1002/jbmr.5650060912. [DOI] [PubMed] [Google Scholar]
- 58.Yogesha SD, Khapli SM, Wani MR. Interleukin-3 and granulocyte-macrophage colony-stimulating factor inhibits tumor necrosis factor (TNF)-alpha-induced osteoclast differentiation by down-regulation of expression of TNF receptors 1 and 2. J Biol Chem. 2005;280:11759–11769. doi: 10.1074/jbc.M410828200. [DOI] [PubMed] [Google Scholar]
- 59.Ehrlich LA, Chung HY, Ghobrial I, Choi SJ, Morandi F, Colla S, Rizzoli V, Roodman GD, Giuliani N. IL-3 is a potential inhibitor of osteoblast differentiation in multiple myeloma. Blood. 2005;106:1407–1414. doi: 10.1182/blood-2005-03-1080. [DOI] [PubMed] [Google Scholar]
- 60.Soshi S, Takahashi HE, Tanizawa T, Endo N, Fujimoto R, Murota K. Effect of recombinant human granulocyte colony-stimulating factor (rh G-CSF) on rat bone: inhibition of bone formation at the endosteal surface of vertebra and tibia. Calcif Tissue Int. 1996;58:337–340. doi: 10.1007/BF02509382. [DOI] [PubMed] [Google Scholar]
- 61.Takamatsu Y, Simmons PJ, Moore RJ, Morris HA, To LB, Levesque JP. Osteoclast-mediated bone resorption is stimulated during short-term administration of granulocyte colony-stimulating factor but is not responsible for hematopoietic progenitor cell mobilization. Blood. 1998;92:3465–3473. [PubMed] [Google Scholar]
- 62.Lapidot T, Petit I. Current understanding of stem cell mobilization: the roles of chemokines, proteolytic enzymes, adhesion molecules, cytokines, and stromal cells. Exp Hematol. 2002;30:973–981. doi: 10.1016/s0301-472x(02)00883-4. [DOI] [PubMed] [Google Scholar]
- 63.Purton LE, Lee MY, Torok-Storb B. Normal human peripheral blood mononuclear cells mobilized with granulocyte colony-stimulating factor have increased osteoclastogenic potential compared to non-mobilized blood. Blood. 1996;87:1802–1808. [PubMed] [Google Scholar]
- 64.Kuwabara H, Wada T, Oda T, Yoshikawa H, Sawada N, Kokai Y, Ishii S. Overexpression of the granulocyte colony-stimulating factor gene impairs bone morphogenetic protein responsiveness in mice. Lab Invest. 2001;81:1133–1141. doi: 10.1038/labinvest.3780325. [DOI] [PubMed] [Google Scholar]
- 65.Oda T, Wada T, Kuwabara H, Sawada N, Yamashita T, Kokai Y. Ovariectomy fails to augment bone resorption and marrow B lymphopoiesis in granulocyte colony-stimulating factor transgenic mice. J Orthop Sci. 2005;10:70–76. doi: 10.1007/s00776-004-0851-y. [DOI] [PubMed] [Google Scholar]
- 66.Lee SK, Gardner AE, Kalinowski JF, Jastrzebski SL, Lorenzo JA. RANKL-stimulated osteoclast- like cell formation in vitro is partially dependent on endogenous interleukin-1 production. Bone. 2005;38:678–685. doi: 10.1016/j.bone.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 67.Lee SK, Kalinowski J, Jastrzebski S, Lorenzo JA. 1, 25 (OH)(2) vitamin D(3)-stimulated osteoclast formation in spleen-osteoblast cocultures is mediated in part by enhanced IL-1alpha and receptor activator of NF-kappa B ligand production in osteoblasts. J Immunol. 2002;169:2374–2380. doi: 10.4049/jimmunol.169.5.2374. [DOI] [PubMed] [Google Scholar]
- 68.Lorenzo JA, Sousa S, Alander C, Raisz LG, Dinarello CA. Comparison of the bone-resorbing activity in the supernatants from phytohemagglutinin- stimulated human peripheral blood mononuclear cells with that of cytokines through the use of an antiserum to interleukin 1. Endocrinology. 1987;121:1164–1170. doi: 10.1210/endo-121-3-1164. [DOI] [PubMed] [Google Scholar]
- 69.Sato K, Fujii Y, Asano S, Ohtsuki T, Kawakami M, Kasono K, Tsushima T, Shizume K. Recombinant human interleukin 1 alpha and beta stimulate mouse osteoblast-like cells (MC3T3-E1) to produce macrophage-colony stimulating activity and prostaglandin E2. Biochem Biophy Res Comm. 1986;141:285–291. doi: 10.1016/s0006-291x(86)80366-7. [DOI] [PubMed] [Google Scholar]
- 70.Klein DC, Raisz LG. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology. 1970;86:1436–1440. doi: 10.1210/endo-86-6-1436. [DOI] [PubMed] [Google Scholar]
- 71.Ma T, Miyanishi K, Suen A, Epstein NJ, Tomita T, Smith RL, Goodman SB. Human inter-leukin-1-induced murine osteoclastogenesis is dependent on RANKL, but independent of TNF-alpha. Cytokine. 2004;26:138–144. doi: 10.1016/j.cyto.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Sato N, Takahashi N, Suda K, Nakamura M, Yamaki M, Ninomiya T, Kobayashi Y, Takada H, Shibata K, Yamamoto M, Takeda K, Akira S, Noguchi T, Udagawa N. MyD88 but not TRIF is essential for osteoclastogenesis induced by lipopolysaccharide, diacyl lipopeptide, and IL-1alpha. J Exp Med. 2004;200:601–611. doi: 10.1084/jem.20040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee ZH, Lee SE, Kim CW, Lee SH, Kim SW, Kwack K, Walsh K, Kim HH. IL-1alpha stimulation of osteoclast survival through the PI 3-kinase/Akt and ERK pathways. J Biochem. 2002;131:161–166. doi: 10.1093/oxfordjournals.jbchem.a003071. [DOI] [PubMed] [Google Scholar]
- 74.Bajayo A, Goshen I, Feldman S, Csernus V, Iverfeldt K, Shohami E, Yirmiya R, Bab I. Central IL-1 receptor signaling regulates bone growth and mass. Proc Natl Acad Sci U S A. 2005;102:12956–12961. doi: 10.1073/pnas.0502562102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vargas SJ, Naprta A, Glaccum M, Lee SK, Kalinowski J, Lorenzo JA. Interleukin-6 expression and histomorphometry of bones from mice deficient for receptors for interleukin-1 or tumor necrosis factor. J Bone Min Res. 1996;11:1736–1740. doi: 10.1002/jbmr.5650111117. [DOI] [PubMed] [Google Scholar]
- 76.Lowik CW, Van der PG, Bloys H, Hoekman K, Bijvoet OL, Aarden LA, Papapoulos SE. Parathyroid hormone (PTH) and PTH-like protein (PLP) stimulate interleukin-6 production by osteogenic cells: a possible role of interleukin-6 in osteoclastogeneis. Biochem Biophys Res Commun. 1989;162:1546–1552. doi: 10.1016/0006-291x(89)90851-6. [DOI] [PubMed] [Google Scholar]
- 77.Girasole G, Jilka RL, Passeri G, Boswell S, Boder G, Williams DC, Manolagas SC. 17á-Estradiol inhibits interleukin-6 production by bone marrow- derived stromal cells and osteoblasts in vitro: A potential mechanism for the antiosteoporotic effect of estrogens. J Clin Invest. 1992;89:883–891. doi: 10.1172/JCI115668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Al-Humidan A, Ralston SH, Hughes DE, Chapman K, Aarden L, Russell RGG, Gowen M. Interleukin-6 does not stimulate bone resorption in neonatal mouse calvariae. J Bone Min Res. 1991;6:3–7. doi: 10.1002/jbmr.5650060103. [DOI] [PubMed] [Google Scholar]
- 79.Ishimi Y, Miyaura C, Jin CH, Akatsu T, Abe E, Nakamura Y, Yamaguchi A, Yoshiki S, Matsuda T, Hirano T, Kishimoto T, Suda T. IL-6 is produced by osteoblasts and induces bone resorption. J Immunol. 1990;145:3297–3303. [PubMed] [Google Scholar]
- 80.Manolagas SC, Jilka RL. Mechanisms of disease: Bone marrow, cytokines, and bone remodeling- Emerging insights into the pathophysiology of osteoporosis. N Eng J Med. 1995;332:305–311. doi: 10.1056/NEJM199502023320506. [DOI] [PubMed] [Google Scholar]
- 81.Liu XH, Kirschenbaum A, Yao S, Levine AC. Cross-talk between the interleukin-6 and prostaglandin E(2) signaling systems results in enhancement of osteoclastogenesis through effects on the osteoproteger-in/receptor activator of nuclear factor-{kappa}B (RANK) ligand/RANK system. Endocrinology. 2005;146:1991–1998. doi: 10.1210/en.2004-1167. [DOI] [PubMed] [Google Scholar]
- 82.Palmqvist P, Persson E, Conaway HH, Lerner UH. IL-6, Leukemia Inhibitory Factor, and Oncostatin M Stimulate Bone Resorption and Regulate the Expression of Receptor Activator of NF-kappaB Ligand, Osteoprotegerin, and Receptor Activator of NF-kappaB in Mouse Calvariae. J Immunol. 2002;169:3353–3362. doi: 10.4049/jimmunol.169.6.3353. [DOI] [PubMed] [Google Scholar]
- 83.Whyte MP, Mumm S. Heritable disorders of the RANKL/OPG/RANK signaling pathway. J Musculoskelet Neuronal Interact. 2004;4:254–267. [PubMed] [Google Scholar]
- 84.Guise TA, Mundy GR. Cancer and bone. Endcr Rev. 1998;19:18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 85.Yamamoto T, Ozono K, Kasayama S, Yoh K, Hiroshima K, Takagi M, Matsumoto S, Michigami T, Yamaoka K, Kishimoto T, Okada S. Increased IL-6-production by cells isolated from the fibrous bone dysplasia tissues in patients with McCune-Albright syndrome. J Clin Invest. 1996;98:30–35. doi: 10.1172/JCI118773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reddy SV, Takahashi S, Dallas M, Williams RE, Neckers L, Roodman GD. Interleukin-6 anti-sense deoxyoligonucleotides inhibit bone resorption by giant cells from human giant cell tumors of bone. J Bone Miner Res. 1994;9:753–757. doi: 10.1002/jbmr.5650090522. [DOI] [PubMed] [Google Scholar]
- 87.Devlin RD, Bone HG, III, Roodman GD. Interleukin-6: A potential mediator of the massive osteolysis in patientswith Gorham-Stout disease. J Clin Endocrinol Metab. 1996;81:1893–1897. doi: 10.1210/jcem.81.5.8626854. [DOI] [PubMed] [Google Scholar]
- 88.Grey A, Mitnick MA, Masiukiewicz U, Sun BH, Rudikoff S, Jilka RL, Manolagas SC, Insogna K. A role for interleukin-6 in parathyroid hormone-induced bone resorption in vivo. Endocrinology. 1999;140:4683– 4690. doi: 10.1210/endo.140.10.7036. [DOI] [PubMed] [Google Scholar]
- 89.O’Brien CA, Jilka RL, Fu Q, Stewart S, Weinstein RS, Manolagas SC. IL-6 is not required for parathyroid hormone stimulation of RANKL expression, osteoclast formation, and bone loss in mice. Am J Physiol Endocrinol Metab. 2005;289:E784–793. doi: 10.1152/ajpendo.00029.2005. [DOI] [PubMed] [Google Scholar]
- 90.Elias JA, Tang W, Horowitz MC. Cytokine and hormonal stimulation of human osteosarcoma inter-leukin-11 production. Endocrinology. 1995;136:489–498. doi: 10.1210/endo.136.2.7835281. [DOI] [PubMed] [Google Scholar]
- 91.Girasole G, Passeri G, Jilka RL, Manolagas SC. Interleukin-11: A new cytokine critical for osteoclast development. J Clin Invest. 1994;93:1516–1524. doi: 10.1172/JCI117130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hill PA, Tumber A, Papaioannou S, Meikle MC. The cellular actions of interleukin-11 on bone resorption in vitro. Endocrinology. 1998;139:1564–1572. doi: 10.1210/endo.139.4.5946. [DOI] [PubMed] [Google Scholar]
- 93.Morinaga Y, Fujita N, Ohishi K, Zhang Y, Tsuruo T. Suppression of interleukin-11-mediated bone resorption by cyclooxygenases inhibitors. J Cell Physiol. 1998;175:247–254. doi: 10.1002/(SICI)1097-4652(199806)175:3<247::AID-JCP2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 94.Sims NA, Jenkins BJ, Nakamura A, Quinn JM, Li R, Gillespie MT, Ernst M, Robb L, Martin TJ. Interleukin-11 receptor signaling is required for normal bone remodeling. J Bone Min Res. 2005;20:1093–1102. doi: 10.1359/JBMR.050209. [DOI] [PubMed] [Google Scholar]
- 95.Greenfield EM, Horowitz MC, Lavish SA. Stimulation by parathyroid hormone of interleukin-6 and leukemia inhibitory factor expression in osteoblasts is an immediate-early gene response induced by cAMP signal transduction. J Biol Chem. 1996;271:10984–10989. doi: 10.1074/jbc.271.18.10984. [DOI] [PubMed] [Google Scholar]
- 96.Reid IR, Lowe C, Cornish J, Skinner SJ, Hilton DJ, Willson TA, Gearing DP, Martin TJ. Leukemia inhibitory factor: a novel bone-active cytokine. Endocinology. 1990;126:1416–1420. doi: 10.1210/endo-126-3-1416. [DOI] [PubMed] [Google Scholar]
- 97.Lorenzo JA, Sousa SL, Leahy CL. Leukemia inhibitory factor (LIF) inhibits basal bone resorption in fetal rat long bone cultures. Cytokine. 1990;2:266–271. doi: 10.1016/1043-4666(90)90027-q. [DOI] [PubMed] [Google Scholar]
- 98.Van Beek E, Van der Wee-Pals L, van de Ruit M, Nijweide P, Papapoulos S, Lowik C. Leukemia inhibitory factor inhibits osteoclastic resorption, growth, mineralization, and alkaline phosphatase activity in fetal mouse metacarpal bones in culture. J Bone Min Res. 1993;8:191–198. doi: 10.1002/jbmr.5650080210. [DOI] [PubMed] [Google Scholar]
- 99.Cornish J, Callon K, King A, Edgar S, Reid IR. The effect of leukemia inhibitory factor on bone in vivo. Endocrinology. 1993;132:1359–1366. doi: 10.1210/endo.132.3.8440191. [DOI] [PubMed] [Google Scholar]
- 100.Ware CB, Horowitz MC, Renshaw BR, Hunt JS, Liggitt D, Koblar SA, Gliniak BC, McKenna HJ, Papayannopoulou T, Thoma B, Cheng L, Donovan PJ, Peschon JJ, Bartlett PF, Willis CR, Wright BD, Carpenter MK, Davison BL, Gearing DP. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development. 1995;121:1283–1299. doi: 10.1242/dev.121.5.1283. [DOI] [PubMed] [Google Scholar]
- 101.Heymann D, Guicheux J, Gouin F, Cottrel M, Daculsi G. Oncostatin M stimulates macrophage- polykaryon formation in long-term human bone-marrow cultures. Cytokine. 1998;10:98–109. doi: 10.1006/cyto.1997.0258. [DOI] [PubMed] [Google Scholar]
- 102.Jay PR, Centrella M, Lorenzo J, Bruce AG, Horowitz MC. Oncostatin-M: A new bone active cytokine that activates osteoblasts and inhibits bone resorption. Endocrinology. 1996;137:1151–1158. doi: 10.1210/endo.137.4.8625883. [DOI] [PubMed] [Google Scholar]
- 103.Malik N, Haugen HS, Modrell B, Shoyab M, Clegg CH. Developmental abnormalities in mice transgenic for bovine oncostatin M. Mol Cell Biol. 1995;15:2349–2358. doi: 10.1128/mcb.15.5.2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kawasaki K, Gao YH, Yokose S, Kaji Y, Nakamura T, Suda T, Yoshida K, Taga T, Kishimoto T, Kataoka H, Yuasa T, Norimatsu H, Yamaguchi A. Osteoclasts are present in gp130-deficient mice. Endocrinology. 1997;138:4959–4965. doi: 10.1210/endo.138.11.5534. [DOI] [PubMed] [Google Scholar]
- 105.Weitzmann MN, Roggia C, Toraldo G, Weitzmann L, Pacifici R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J Clin Invest. 2002;110:1643–1650. doi: 10.1172/JCI15687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Weitzmann MN, Cenci S, Rifas L, Brown C, Pacifici R. Interleukin-7 stimulates osteoclast formation by up-regulating the T- cell production of soluble osteoclastogenic cytokines. Blood. 2000;96:1873–1878. [PubMed] [Google Scholar]
- 107.Toraldo G, Roggia C, Qian WP, Pacifici R, Weitzmann MN. IL-7 induces bone loss in vivo by induction of receptor activator of nuclear factor kappa B ligand and tumor necrosis factor alpha from T cells. Proc Natl Acad Sci U S A. 2003;100:125–130. doi: 10.1073/pnas.0136772100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ryan MR, Shepherd R, Leavey JK, Gao YH, Grassi F, Schnell FJ, Qian WP, Kersh GJ, Weitzmann MN, Pacifici R. An IL-7-dependent rebound in thymic T cell output contributes to the bone loss induced by estrogen deficiency. Proc Natl Acad Sci U S A. 2005;102:16735–16740. doi: 10.1073/pnas.0505168102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee SK, Kalinowski JF, Jastrzebski SL, Puddington L, Lorenzo JA. Interleukin-7 is a direct inhibitor of in vitro osteoclastogenesis. Endocrinology. 2003;144:524–3531. doi: 10.1210/en.2002-221057. [DOI] [PubMed] [Google Scholar]
- 110.Lee SK, Kalinowski JF, Jacquin C, Adams DJ, Gronowicz G, Lorenzo JA. Interleukin-7 influences osteoclast function in vivo but is not a critical factor in ovariectomy-induced bone loss. J Bone Miner Res. 2006;21:695–702. doi: 10.1359/jbmr.060117. [DOI] [PubMed] [Google Scholar]
- 111.Sato T, Watanabe K, Masuhara M, Hada N, Hakeda Y. Production of IL-7 is increased in ovariectomized mice, but not RANKL mRNA expression by osteoblasts/stromal cells in bone, and IL-7 enhances generation of osteoclast precursors in vitro. J Bone Miner Res. 2007;25:19–27. doi: 10.1007/s00774-006-0723-y. [DOI] [PubMed] [Google Scholar]
- 112.Lee S, Kalinowski JF, Adams DJ, Aguila HL, Lorenzo JA. Osteoblast specific overexpression of human interleukin-7 increases femoral trabecular bone mass in female mice and inhibits in vitro osteoclastogenesis. J Bone Miner Res. 2004;19:S410. doi: 10.1002/jbmr.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lee S, Kalinowski J, Adams DJ, Aguila HL, Lorenzo JA. Osteoblast specific overexpression of human interlukin-7 rescues the bone phenotype of interleukin-7 deficient female mice. J Bone Miner Res. 2005;20:S48. doi: 10.1002/jbmr.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;121:336–341. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 115.Bendre MS, Margulies AG, Walser B, Akel NS, Bhattacharrya S, Skinner RA, Swain F, Ramani V, Mohammad KS, Wessner LL, Martinez A, Guise TA, Chirgwin JM, Gaddy D, Suva LJ. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor- kappaB ligand pathway. Cancer Res. 2005;65:11001–11009. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- 116.Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 117.Sunyer T, Rothe L, Jiang XS, Osdoby P, Collin-Osdoby P. Proinflammatory agents, IL-8 and IL-10, upregulate inducible nitric oxide synthase expression and nitric oxide production in avian osteoclast-like cells. J Cell Biochem. 1996;60:469–483. doi: 10.1002/(sici)1097-4644(19960315)60:4<469::aid-jcb4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 118.Choi J, Oba Y, Jelinek D, Ehrlich L, Lee W, Roodman D. Blocking CCR1 or CCR5 inhibits both osteoclast formation and increased alpha1-integrin expression induced by MIP-1alpha. Eur J Haematol. 2003;70:272–278. [Google Scholar]
- 119.Kukita T, Nomiyama H, Ohmoto Y, Kukita A, Shuto T, Hotokebuchi T, Sugioka Y, Miura R, Iijima T. Macrophage inflammatory protein-1 alpha (LD78) expressed in human bone marrow: its role in regulation of hematopoiesis and osteoclast recruitment. Lab Invest. 1997;76:399–406. [PubMed] [Google Scholar]
- 120.Watanabe T, Kukita T, Kukita A, Wada N, Toh K, Nagata K, Nomiyama H, Iijima T. Direct stimulation of osteoclastogenesis by MIP-1alpha: evidence obtained from studies using RAW264 cell clone highly responsive to RANKL. J Endocrinol. 2004;180:193–201. doi: 10.1677/joe.0.1800193. [DOI] [PubMed] [Google Scholar]
- 121.Abe M, Hiura K, Wilde J, Moriyama K, Hashimoto T, Ozaki S, Wakatsuki S, Kosaka M, Kido S, Inoue D, Matsumoto T. Role for macrophage inflammatory protein (MIP)-1alpha and MIP-1beta in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100:2195–2202. [PubMed] [Google Scholar]
- 122.Choi SJ, Oba Y, Gazitt Y, Alsina M, Cruz J, Anderson J, Roodman GD. Antisense inhibition of macrophage inflammatory protein 1-alpha blocks bone destruction in a model of myeloma bone disease. J Clin Invest. 2001;108:1833–1841. doi: 10.1172/JCI13116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fuller K, Owens JM, Chambers TJ. Macrophage inflammatory protein-1à and IL-8 stimulate the motility but suppress the resorption of isolated rat osteoclasts. J Immunol. 1995;154:6065–6072. [PubMed] [Google Scholar]
- 124.Okamatsu Y, Kim D, Battaglino R, Sasaki H, Spate U, Stashenko P. MIP-1{gamma} Promotes Receptor Activator of NF-{kappa}B Ligand-Induced Osteoclast Formation and Survival. J Immunol. 2004;173:2084–2090. doi: 10.4049/jimmunol.173.3.2084. [DOI] [PubMed] [Google Scholar]
- 125.Yu X, Huang Y, Collin-Osdoby P, Osdoby P. CCR1 chemokines promote the chemotactic recruitment, RANKL development, and motility of osteoclasts and are induced by inflammatory cytokines in osteoblasts. J Bone Min Res. 2004;19:2065–2077. doi: 10.1359/JBMR.040910. [DOI] [PubMed] [Google Scholar]
- 126.Lean JM, Murphy C, Fuller K, Chambers TJ. CCL9/MIP-1gamma and its receptor CCR1 are the major chemokine ligand/receptor species expressed by osteoclasts. J Cell Biochem. 2002;87:386–393. doi: 10.1002/jcb.10319. [DOI] [PubMed] [Google Scholar]
- 127.Yang M, Mailhot G, MacKay CA, Mason-Savas A, Aubin J, Odgren PR. Chemokine and chemokine receptor expression during colony stimulating factor-1-induced osteoclast differentiation in the toothless osteopetrotic rat: a key role for CCL9 (MIP-1gamma) in osteoclastogenesis in vivo and in vitro. Blood. 2006;107:2262–2270. doi: 10.1182/blood-2005-08-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ishida N, Hayashi K, Hattori A, Yogo K, Kimura T, Takeya T. CCR1 Acts Downstream of NFAT2 in Osteoclastogenesis and Enhances Cell Migration. J Bone Min Res. 2006;21:48–57. doi: 10.1359/JBMR.051001. [DOI] [PubMed] [Google Scholar]
- 129.Wright LM, Maloney W, Yu X, Kindle L, Collin-Osdoby P, Osdoby P. Stromal cell-derived factor-1 binding to its chemokine receptor CXCR4 on precursor cells promotes the chemotactic recruitment, development and survival of human osteoclasts. Bone. 2005;36:840–853. doi: 10.1016/j.bone.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 130.Grassi F, Piacentini A, Cristino S, Toneguzzi S, Cavallo C, Facchini A, Lisignoli G. Human osteoclasts express different CXC chemokines depending on cell culture substrate: molecular and immunocytochemical evidence of high levels of CXCL10 and CXCL12. Histochem Cell Biol. 2003;120:391–400. doi: 10.1007/s00418-003-0587-3. [DOI] [PubMed] [Google Scholar]
- 131.Liao TS, Yurgelun MB, Chang SS, Zhang HZ, Murakami K, Blaine TA, Parisien MV, Kim W, Winchester RJ, Lee FY. Recruitment of osteoclast precursors by stromal cell derived factor-1 (SDF-1) in giant cell tumor of bone. J Orthop Res. 2005;23:203–209. doi: 10.1016/j.orthres.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 132.Zannettino AC, Farrugia AN, Kortesidis A, Manavis J, To LB, Martin SK, Diamond P, Tamamura H, Lapidot T, Fujii N, Gronthos S. Elevated serum levels of stromal-derived factor-1alpha are associated with increased osteoclast activity and osteolytic bone disease in multiple myeloma patients. Cancer Res. 2005;65:1700–1709. doi: 10.1158/0008-5472.CAN-04-1687. [DOI] [PubMed] [Google Scholar]
- 133.Rahimi P, Wang C, Stashenko P, Lee SK, Lorenzo JA, Graves DT. Monocyte chemoattractant protein-1 Expression and monocyte recruitment in osseous inflammation in the mouse. Endocrinology. 1995;136:2752–2759. doi: 10.1210/endo.136.6.7750500. [DOI] [PubMed] [Google Scholar]
- 134.Zhu JF, Valente AJ, Lorenzo JA, Carnes D, Graves DT. Expression of monocyte chemo-attractant protein 1 in human osteoblastic cells stimulated by proinflammatory mediators. J Bone Min Res. 1994;9:1123–1130. doi: 10.1002/jbmr.5650090721. [DOI] [PubMed] [Google Scholar]
- 135.Wise GE, Huang H, Que BG. Gene expression of potential tooth eruption molecules in the dental follicle of the mouse. Eur J Oral Sci. 1999;107:482–486. doi: 10.1046/j.0909-8836.1999.eos107610.x. [DOI] [PubMed] [Google Scholar]
- 136.Graves DT, Alsulaimani F, Ding Y, Marks SC., Jr Developmentally regulated monocyte recruitment and bone resorption are modulated by functional deletion of the monocytic chemoattractant protein-1 gene. Bone. 2002;31:282–287. doi: 10.1016/s8756-3282(02)00829-3. [DOI] [PubMed] [Google Scholar]
- 137.Kim MS, Day CJ, Morrison NA. MCP-1 is induced by RANKL, promotes osteoclast fusion and rescues GM-CSF suppression of osteoclast formation. J Biol Chem. 2005;280:16163–16169. doi: 10.1074/jbc.M412713200. [DOI] [PubMed] [Google Scholar]
- 138.Li X, Qin L, Bergenstock M, Bevelock LM, Novack DV, Partridge NC. Parathyroid hormone stimulates osteoblastic expression of MCP-1 to recruit and increase the fusion of pre/osteoclasts. J Biol Chem. 2007;282:33098–33106. doi: 10.1074/jbc.M611781200. [DOI] [PubMed] [Google Scholar]
- 139.Owens JM, Gallagher AC, Chambers TJ. IL-1O modulates formation of osteoclasts in murine hemopoietic cultures. J Immunol. 1996;157:936–940. [PubMed] [Google Scholar]
- 140.Hong MH, Williams H, Jin CH, Pike JW. The inhibitory effect of interleukin-10 on mouse osteoclast formation involves novel tyrosine-phosphorylated proteins. J Bone Min Res. 2000;15:911–918. doi: 10.1359/jbmr.2000.15.5.911. [DOI] [PubMed] [Google Scholar]
- 141.Van Vlasselaer P, Borremans B, Van Der Heuvel R, Van Gorp U, De Waal Malefyt R. Interleukin- 10 inhibits the osteogenic activity of mouse bone marrow. Blood. 1993;82:2361–2370. [PubMed] [Google Scholar]
- 142.Evans KE, Fox SW. Interleukin-10 inhibits osteoclastogenesis by reducing NFATc1 expression and preventing its translocation to the nucleus. BMC Cell Biol. 2007;19:4. doi: 10.1186/1471-2121-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mohamed SG, Sugiyama E, Shinoda K, Taki H, Hounoki H, bdel-Aziz HO, Maruyama M, Kobayashi M, Ogawa H, Miyahara T. Interleukin-10 inhibits RANKL-mediated expression of NFATc1 in part via suppression of c-Fos and c-Jun in RAW264.7 cells and mouse bone marrow cells. Bone. 2007;41:592–602. doi: 10.1016/j.bone.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 144.Carmody EE, Schwarz EM, Puzas JE, Rosier RN, O’Keefe RJ. Viral interleukin-10 gene inhibition of inflammation, osteoclastogenesis, and bone resorption in response to titanium particles. Arthritis Rheum. 2002;46:1298–1308. doi: 10.1002/art.10227. [DOI] [PubMed] [Google Scholar]
- 145.Liu D, Yao S, Wise GE. Effect of interleukin-10 on gene expression of osteoclastogenic regulatory molecules in the rat dental follicle. Eur J Oral Sci. 2006;114:42–49. doi: 10.1111/j.1600-0722.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- 146.Shin HH, Lee JE, Lee EA, Kwon BS, Choi HS. Enhanced Osteoclastogenesis in 4–1BB-Deficient Mice Caused by Reduced Interleukin-10. J Bone Min Res. 2006;21:1907–1912. doi: 10.1359/jbmr.060813. [DOI] [PubMed] [Google Scholar]
- 147.Amcheslavsky A, Bar-Shavit Z. Interleukin (IL)-12 mediates the anti-osteoclastogenic activity of CpG-oligodeoxynucleotides. J Cell Physiol. 2006;207:244–250. doi: 10.1002/jcp.20563. [DOI] [PubMed] [Google Scholar]
- 148.Horwood NJ, Elliott J, Martin TJ, Gillespie MT. IL-12 Alone and in synergy with IL-18 inhibits osteoclast formation in vitro. J Immunol. 2001;166:4915–4921. doi: 10.4049/jimmunol.166.8.4915. [DOI] [PubMed] [Google Scholar]
- 149.Nagata N, Kitaura H, Yoshida N, Nakayama K. Inhibition of RANKL-induced osteoclast formation in mouse bone marrow cells by IL-12: involvement of IFN- gamma possibly induced from non-T cell population. Bone. 2003;33:721–732. doi: 10.1016/s8756-3282(03)00213-8. [DOI] [PubMed] [Google Scholar]
- 150.Ogata Y, Kukita A, Kukita T, Komine M, Miyahara A, Miyazaki S, Kohashi O. A Novel Role of IL-15 in the Development of Osteoclasts: Inability to replace its activity with IL-2. J Immunol. 1999;162:2754–2760. [PubMed] [Google Scholar]
- 151.Miranda-Carus ME, ito-Miguel M, Balsa A, Cobo- Ibanez T, Perez De AC, Pascual-Salcedo D, Martin-Mola E. Peripheral blood T lymphocytes from patients with early rheumatoid arthritis express RANKL and interleukin-15 on the cell surface and promote osteoclastogenesis in autologous monocytes. Arthritis Rheum. 2006;54:1151–1164. doi: 10.1002/art.21731. [DOI] [PubMed] [Google Scholar]
- 152.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 153.Sato K, Suematsu A, Okamoto K, Yamaguchi A, Morishita Y, Kadono Y, Tanaka S, Kodama T, Akira S, Iwakura Y, Cua DJ, Takayanagi H. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J Exp Med. 2006;203:2673–2682. doi: 10.1084/jem.20061775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kotake S, Udagawa N, Takahashi N, Matsuzaki K, Itoh K, Ishiyama S, Saito S, Inoue K, Kamatani N, Gillespie MT, Martin TJ, Suda T. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Van bezooijen RL, Farih-Sips HC, Papapoulos SE, Lowik CW. Interleukin-17: A new bone acting cytokine in vitro. J Bone Min Res. 1999;14:1513–1521. doi: 10.1359/jbmr.1999.14.9.1513. [DOI] [PubMed] [Google Scholar]
- 156.Koenders MI, Lubberts E, Oppers-Walgreen B, Van Den BL, Helsen MM, Di Padova FE, Boots AM, Gram H, Joosten LA, Van den Berg WB. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am J Pathol. 2005;167:141–149. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 158.Yamamura M, Kawashima M, Taniai M, Yamauchi H, Tanimoto T, Kurimoto M, Morita Y, Ohmoto Y, Makino H. Interferon-gamma-inducing activity of interleukin-18 in the joint with rheumatoid arthritis. Arthritis Rheum. 2001;44:275–285. doi: 10.1002/1529-0131(200102)44:2<275::AID-ANR44>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 159.Horwood NJ, Udagawa N, Elliott J, Grail D, Okamura H, Kurimoto M, Dunn AR, Martin T, Gillespie MT. Interleukin 18 inhibits osteoclast formation via T cell production of granulocyte macrophage colony-stimulating factor. J Clin Invest. 1998;101:595–603. doi: 10.1172/JCI1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cornish J, Gillespie MT, Callon KE, Horwood NJ, Moseley JM, Reid IR. Interleukin-18 is a novel mitogen of osteogenic and chondrogenic cells. Endocrinology. 2003;144:1194–1201. doi: 10.1210/en.2002-220936. [DOI] [PubMed] [Google Scholar]
- 161.Kawase Y, Hoshino T, Yokota K, Kuzuhara A, Nakamura M, Maeda Y, Nishiwaki E, Zenmyo M, Hiraoka K, Aizawa H, Yoshino K. Bone malformations in interleukin-18 transgenic mice. J Bone Min Res. 2003;18:975–983. doi: 10.1359/jbmr.2003.18.6.975. [DOI] [PubMed] [Google Scholar]
- 162.Yamada N, Niwa S, Tsujimura T, Iwasaki T, Sugihara A, Futani H, Hayashi S, Okamura H, Akedo H, Terada N. Interleukin-18 and interleukin-12 synergistically inhibit osteoclastic bone-resorbing activity. Bone. 2002;30:901–908. doi: 10.1016/s8756-3282(02)00722-6. [DOI] [PubMed] [Google Scholar]
- 163.Makiishi-Shimobayashi C, Tsujimura T, Iwasaki T, Yamada N, Sugihara A, Okamura H, Hayashi SS, Terada N. Interleukin-18 Up-Regulates Osteoprotegerin Expression in Stromal/Osteoblastic Cells. Biochem Biophy Res Comm. 2001;281:361–366. doi: 10.1006/bbrc.2001.4380. [DOI] [PubMed] [Google Scholar]
- 164.Dai SM, Nishioka K, Yudoh K. Interleukin (IL) 18 stimulates osteoclast formation through synovial T cells in rheumatoid arthritis: comparison with IL1 beta and tumour necrosis factor alpha. Ann Rheum Dis. 2004;63:1379–1386. doi: 10.1136/ard.2003.018481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gowen M, Mundy GR. Actions of recombinant interleukin 1, interleukin 2, and interferon- gamma on bone resorption in vitro. J Immunol. 1986;136:2478–2482. [PubMed] [Google Scholar]
- 166.Takahashi N, Mundy GR, Roodman GD. Recombinant human interferon-gamma inhibits formation of human osteoclast-like cells. J Immunol. 1986;137:3544–3549. [PubMed] [Google Scholar]
- 167.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, Takaoka A, Yokochi T, Oda H, Tanaka K, Nakamura K, Taniguchi T. T-cell- mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 168.Hattersley G, Dorey E, Horton MA, Chambers TJ. Human macrophage colony-stimulating factor inhibits bone resorption by osteoclasts disaggregated from rat bone. J Cell Physiol. 1988;137:199–203. doi: 10.1002/jcp.1041370125. [DOI] [PubMed] [Google Scholar]
- 169.Gao Y, Grassi F, Ryan MR, Terauchi M, Page K, Yang X, Weitzmann MN, Pacifici R. IFN-gamma stimulates osteoclast formation and bone loss in vivo via antigen-driven T cell activation. J Clin Invest. 2007;117:122–132. doi: 10.1172/JCI30074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Gowen M, MacDonald BR, Russell GG. Actions of recombinant human gamma-interferon and tumor necrosis factor alpha on the proliferation and osteoblastic characteristics of human trabecular bone cells in vitro. Arthritis Rheum. 1988;31:1500–1507. doi: 10.1002/art.1780311206. [DOI] [PubMed] [Google Scholar]
- 171.Mann GN, Jacobs TW, Buchinsky FJ, Armstrong EC, Li M, Ke HZ, Ma YF, Jee WSS, Epstein S. Interferon-gamma causes loss of bone volume in vivo and fails to ameliorate cyclosporin A-induced osteopenia. Endocrinology. 1994;135:1077–1083. doi: 10.1210/endo.135.3.8070349. [DOI] [PubMed] [Google Scholar]
- 172.Key LL, Jr, Rodriguiz RM, Willi SM, Wright NM, Hatcher HC, Eyre DR, Cure JK, Griffin PP, Ries WL. Long-term treatment of osteopetrosis with recombinant human interferon gamma. N Eng J Med. 1995;332:1594–1599. doi: 10.1056/NEJM199506153322402. [DOI] [PubMed] [Google Scholar]
- 173.Vignery A, Niven-Fairchild T, Shepard MH. Recombinant murine interferon-gamma inhibits the fusion of mouse alveolar macrophages in vitro but stimulates the formation of osteoclast-like cells on implanted syngenic bone particles in mice in vivo. J Bone Min Res. 1990;5:637–644. doi: 10.1002/jbmr.5650050613. [DOI] [PubMed] [Google Scholar]
- 174.Takayanagi H, Kim S, Matsuo K, Suzuki H, Suzuki T, Sato K, Yokochi T, Oda H, Nakamura K, Ida N, Wagner EF, Taniguchi T. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- 175.Fox SW, Haque SJ, Lovibond AC, Chambers TJ. The possible role of TGF-beta-induced suppressors of cytokine signaling expression in osteoclast/macrophage lineage commitment in vitro. J Immunol. 2003;170:3679–3687. doi: 10.4049/jimmunol.170.7.3679. [DOI] [PubMed] [Google Scholar]
- 176.Hayashi T, Kaneda T, Toyama Y, Kumegawa M, Hakeda Y. Regulation of receptor activator of NF-kappa B ligand-induced osteoclastogenesis by endogenous interferon-beta (INF-beta ) and suppressors of cytokine signaling (SOCS). The possible counteracting role of SOCSs- in IFN-beta-inhibited osteoclast formation. J Biol Chem. 2002;277:27880–27886. doi: 10.1074/jbc.M203836200. [DOI] [PubMed] [Google Scholar]
- 177.Avnet S, Cenni E, Perut F, Granchi D, Brandi ML, Giunti A, Baldini N. Interferon-alpha inhibits in vitro osteoclast differentiation and renal cell carcinoma-induced angiogenesis. Int J Oncol. 2007;30:469–476. [PubMed] [Google Scholar]
- 178.Goodman GR, Dissanayake IR, Gorodetsky E, Zhou H, Ma YF, Jee WS, Epstein S. Interferon-alpha, unlike interferon-gamma, does not cause bone loss in the rat. Bone. 1999;25:459–463. doi: 10.1016/s8756-3282(99)00182-9. [DOI] [PubMed] [Google Scholar]
- 179.Lewis DB, Liggitt HD, Effmann EL, Motley ST, Teitelbaum SL, Jepsen KJ, Goldstein SA, Bonadio J, Carpenter J, Perlmutter RM. Osteoporosis induced in mice by overproduction of interleukin 4. Proc Natl Acad Sci U S A. 1993;90:11618–11622. doi: 10.1073/pnas.90.24.11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shioi A, Teitelbaum SL, Ross FP, Welgus HG, Suzuki H, Ohara J, Lacey DL. Interleukin 4 inhibits murine osteoclast formation in vitro. J Cell Biochem. 1991;47:272–277. doi: 10.1002/jcb.240470313. [DOI] [PubMed] [Google Scholar]
- 181.Okada Y, Morimoto I, Ura K, Nakano Y, Tanaka Y, Nishida S, Nakamura T, Eto S. Short-term treatment of recombinant murine interleukin-4 rapidly inhibits bone formation in normal and ovariectomized mice. Bone. 1998;22:361–365. doi: 10.1016/s8756-3282(97)00296-2. [DOI] [PubMed] [Google Scholar]
- 182.Onoe Y, Miyaura C, Kaminakayashiki T, Nagai Y, Noguchi K, Chen QR, Seo H, Ohta H, Nozawa S, Kudo I, Suda T. IL-13 and IL-4 inhibit bone resorption by suppressing cyclooxygenase- 2-dependent prostaglandin synthesis in osteoblasts. J Immunol. 1996;156:758–764. [PubMed] [Google Scholar]
- 183.Lind M, Deleuran B, Yssel H, Fink-Eriksen E, Thestrup-Pedersen K. IL-4 and IL-13, but not IL-10, are chemotactic factors for human osteoblasts. Cytokine. 1995;7:78–82. doi: 10.1006/cyto.1995.1010. [DOI] [PubMed] [Google Scholar]
- 184.Palmqvist P, Lundberg P, Persson E, Johansson A, Lundgren I, Lie A, Conaway HH, Lerner UH. Inhibition of hormone and cytokine-stimulated osteoclastogenesis and bone resorption by interleukin-4 and interleukin-13 is associated with increased osteoprotegerin and decreased RANKL and RANK in a STAT6- dependent pathway. J Biol Chem. 2006;281:2414–2429. doi: 10.1074/jbc.M510160200. [DOI] [PubMed] [Google Scholar]
- 185.Bendixen AC, Shevde NK, Dienger KM, Willson TM, Funk CD, Pike JW. IL-4 inhibits osteoclast formation through a direct action on osteoclast precursors via peroxisome proliferator-activated receptor gamma 1. Proc Natl Acad Sci U S A. 2001;98:2443–2448. doi: 10.1073/pnas.041493198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kamel Mohamed SG, Sugiyama E, Shinoda K, Hounoki H, Taki H, Maruyama M, Miyahara T, Kobayashi M. Interleukin-4 inhibits RANKL-induced expression of NFATc1 and c-Fos: A possible mechanism for downregulation of osteoclastogenesis. Biochem Biophy Res Comm. 2005;329:839–845. doi: 10.1016/j.bbrc.2005.02.049. [DOI] [PubMed] [Google Scholar]
- 187.Mangashetti LS, Khapli SM, Wani MR. IL-4 inhibits bone-resorbing activity of mature osteoclasts by affecting NF-kappa B and Ca2+ signaling. J Immunol. 2005;175:917–925. doi: 10.4049/jimmunol.175.2.917. [DOI] [PubMed] [Google Scholar]
- 188.Moreno JL, Kaczmareck M, Keegan AD, Tondravi M. IL-4 suppresses both osteoclast development and mature osteoclast function by a STAT6- dependent mechanism: irreversible inhibition of the differentiation program activated by RANKL. Blood. 2003;102:1078–1086. doi: 10.1182/blood-2002-11-3437. [DOI] [PubMed] [Google Scholar]
- 189.Yamada A, Takami M, Kawawa T, Yasuhara R, Zhao B, Mochizuki A, Miyamoto Y, Eto T, Yasuda H, Nakamichi Y, Kim N, Katagiri T, Suda T, Kamijo R. Interleukin-4 inhibition of osteoclast differentiation is stronger than that of interleukin-13 and they are equivalent for induction of osteoprotegerin production from osteoblasts. Immunology. 2007;120:573–579. doi: 10.1111/j.1365-2567.2006.02538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Baugh JA, Bucala R. Macrophage migration inhibitory factor. Crit Care Med. 2002;30:S27–S35. [PubMed] [Google Scholar]
- 191.Onodera S, Sasaki S, Ohshima S, Amizuka N, Li M, Udagawa N, Irie K, Nishihira J, Koyama Y, Shiraishi A, Tohyama H, Yasudam K. Transgenic mice overexpressing macrophage migration inhibitory factor (MIF) exhibit high-turnover osteoporosis. J Bone Min Res. 2006;21:876–885. doi: 10.1359/jbmr.060310. [DOI] [PubMed] [Google Scholar]
- 192.Oshima S, Onodera S, Amizuka N, Li M, Irie K, Watanabe S, Koyama Y, Nishihira J, Yasuda K, Minami A. Macrophage migration inhibitory factor-deficient mice are resistant to ovariectomy-induced bone loss. FEBS Lett. 2006;580:1251–1256. doi: 10.1016/j.febslet.2006.01.038. [DOI] [PubMed] [Google Scholar]
- 193.Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J Clin Invest. 2003;111:1309–1318. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Onodera S, Suzuki K, Kaneda K, Fujinaga M, Nishihira J. Growth factor-induced expression of macrophage migration inhibitory factor in osteoblasts: relevance to the plasminogen activator system. Semin Thromb Hemost. 1999;25:563–568. doi: 10.1055/s-2007-994966. [DOI] [PubMed] [Google Scholar]
- 195.Onodera S, Nishihira J, Iwabuchi K, Koyama Y, Yoshida K, Tanaka S, Minami A. Macrophage migration inhibitory factor up-regulates matrix metalloproteinase-9 and −13 in rat osteoblasts. Relevance to intracellular signaling pathways. J Biol Chem. 2002;277:7865–7874. doi: 10.1074/jbc.M106020200. [DOI] [PubMed] [Google Scholar]
- 196.Lee S, Jacquin C, Leng L, Bucala R, Kuchel G. Macrophage migration inhibitory factor is an inhibitor of osteoclastogenesis in vitro and in vivo. J Bone Min Res. 2006;21:S162. doi: 10.1016/j.bone.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 197.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 198.Takami M, Kim N, Rho J, Choi Y. Stimulation by toll-like receptors inhibits osteoclast differentiation. J Immunol. 2002;169:1516–1523. doi: 10.4049/jimmunol.169.3.1516. [DOI] [PubMed] [Google Scholar]
- 199.Nair SP, Meghji S, Wilson M, Reddi K, White P, Henderson B. Bacterially induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64:2371–2380. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Kikuchi T, Matsuguchi T, Tsuboi N, Mitani A, Tanaka S, Matsuoka M, Yamamoto G, Hishikawa T, Noguchi T, Yoshikai Y. Gene expression of osteoclast differentiation factor is induced by lipopolysaccharide in mouse osteoblasts via Toll-like receptors. J Immunol. 2001;166:3574–3579. doi: 10.4049/jimmunol.166.5.3574. [DOI] [PubMed] [Google Scholar]
- 201.Hayashi S, Yamada T, Tsuneto M, Yamane T, Takahashi M, Shultz LD, Yamazaki H. Distinct osteoclast precursors in the bone marrow and extramedullary organs characterized by responsiveness to Toll-like receptor ligands and TNF-alpha. J Immunol. 2003;171:5130–5139. doi: 10.4049/jimmunol.171.10.5130. [DOI] [PubMed] [Google Scholar]
- 202.Teng YT, Nguyen H, Gao X, Kong YY, Gorczynski RM, Singh B, Ellen RP, Penninger JM. Functional human T-cell immunity and osteoprotegerin ligand control alveolar bone destruction in periodontal infection. J Clin Invest. 2000;106:R59–67. doi: 10.1172/jci10763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Maruyama K, Takada Y, Ray N, Kishimoto Y, Penninger JM, Yasuda H, Matsuo K. Receptor activator of NF-kappa B ligand and osteoprotegerin regulate proinflammatory cytokine production in mice. J Immunol. 2006;177:3799–3805. doi: 10.4049/jimmunol.177.6.3799. [DOI] [PubMed] [Google Scholar]
- 204.Li H, Cuartas E, Cui W, Choi Y, Crawford TD, Ke HZ, Kobayashi KS, Flavell RA, Vignery A. IL-1 receptor-associated kinase M is a central regulator of osteoclast differentiation and activation. J Exp Med. 2005;201:1169–1177. doi: 10.1084/jem.20041444. [DOI] [PMC free article] [PubMed] [Google Scholar]