Abstract
A hallmark of tissue injury in various models of ischemia/reperfusion (IR) is mitochondrial dysfunction and the release of mitochondrial pro-apoptotic proteins leading to cell death. Although IR-induced mitochondrial injury has been extensively studied and key mitochondrial functions affected by IR are chiefly characterized, the nature of the molecule that causes loss of mitochondrial integrity and function remains obscure. It has become increasingly clear that ceramide, a membrane sphingolipid and a key mediator of cell-stress responses could play a critical role in IR-induced mitochondrial damage. Emerging data point to excessive ceramide accumulation in tissue and, specifically, in mitochondria after IR. Exogenously added to isolated mitochondria, ceramide could mimic some of the mitochondrial dysfunctions occurring in IR. The recent identification and characterization of major enzymes in ceramide synthesis is expected to contribute to the understanding of molecular mechanisms of ceramide involvement in mitochondrial damage in IR. This review will examine the experimental evidence supporting the important role of ceramide in mitochondrial dysfunction in IR in order to highlight potential targets for pharmacological manipulation of ceramide levels.
Keywords: apoptosis, mitochondria, ceramide synthase, LASS (CerS), sphingomyelinase, cytochrome c, Bax, PP2A, cathepsin D, Bcl-2
Introduction
Sphingolipids are important structural components of cell membranes that are defined by the presence of a long-chain sphingoid backbone, generally sphingosine. Acylation of the sphingoid base, i.e. addition of a C14–C26 fatty acid on the amino group yields ceramide, which serves as a building block for many of the complex sphingolipids such as sphingomyelin (SM) or glycosphingolipids. In addition to their role as building blocks of cellular membranes, a wealth of reports unquestionably support a role of sphingolipids as pleotropic second-messengers in intracellular signaling pathways [1–3]. Basic organization and specific principles of sphingolipid-mediated cell regulation have been recently reviewed by Hannun and Obeid [4].
After more than a decade of extensive investigations, it has become clear that ceramide is a key sphingolipid messenger regulating a diverse range of cell-stress responses, including apoptosis and cell senescence [1, 3, 5–7]. Ceramide is tightly regulated in the cells, and its participation in cell death signaling pathways is controlled by rapid conversion of ceramide into less dangerous sphingolipids. Thus, ceramide can be metabolized into complex sphingolipids by glucosylceramide synthase or into SM by SM synthase or into ceramide-1-phosphate by ceramide kinase [3, 8] or into sphingosine-1-phosphate by ceramidase and sphingosine kinase [4].
Ceramide Generation
Ceramide is a family of about 50 distinct molecular species that are characterized by various acyl chains, their desaturation, and hydroxylation. Highly hydrophobic ceramides are generated by membrane-associated enzymes, and exert their effects either in close proximity to the generation site or require specific transport mechanisms to reach their targets in another intracellular compartment [9]. Ceramide appears to be able to flip-flop across the membrane [10]; however, spontaneous inter-bilayer transfer is extremely slow [11]. Therefore, the transfer of ceramide between intracellular compartments is facilitated by vesicular transport pathways [12]. Alternatively, ceramide is transported by a non-vesicular pathway involving a transfer protein (CERT) from its generation site in the endoplasmic reticulum (ER) to the Golgi where it is required for SM synthesis [13]. Ceramide is mainly synthesized de novo and also generated via degradation of SM or glycosphingolipids.
De novo ceramide biosynthesis
Remarkable progress has been made toward identifying enzymes involved in ceramide biosynthesis [9]. (Dihydro) ceramide synthase (EC 2.3.1.24) is a key enzyme in de novo synthesis of ceramide, and it utilizes fatty acid acyl CoA for N-acylation of sphinganine (dihydrosphingosine) yielding dihydroceramide that is converted to ceramide by desaturase (Scheme 1). In yeast, longevity assurance gene 1 (LAG1) was identified as a component of ceramide synthase. Deletion of LAG1 in haploid cells resulted in a pronounced increase (~50%) in mean and maximum life spans [14].
Recently, mammalian homologs of LAG1, which belong to the LASS (longevity assurance gene homolog) family, were cloned and characterized [9]. Each of the 6 known LASS (also known as CerS) genes appears to regulate synthesis of a specific subset of ceramides, and displays a unique substrate specificity profile for chain-length and/or saturation in fatty acid acyl CoA. Over-expression of any LASS protein in mammalian cells resulted in increased levels of a specific subset of ceramide species. It has been demonstrated that LASS1 exhibits high specificity for C18:0-CoA generating C18:0-ceramide [15, 16]. LASS2, LASS4, and LASS3 appear to have broader specificity [17, 18]. LASS2 or LASS4 mainly synthesizes C20:0-, C22:0-, C24:1-, C24:0-, C26:1 and C26:0 ceramide, but is unable to synthesize C16:0- or C18:0-ceramide [16, 18]. LASS3 generates C18:0-, C20:0-, C22:0- and C24:0-ceramide [17]. It has been shown that LASS5 generates C14:0-, C16:0-, C18:0-, and C18:1-ceramide [16, 19]; and LASS6 produces C14:0-, C16:0-, and C18:0-ceramide [16].
Availability of certain fatty acyl-CoA species and the characteristic distribution pattern of LASS family members in tissues seem to regulate the tissue-specificity of the ceramide species. Northern blot and real-time RT-PCR analysis revealed broad expression of LASS5, LASS4, and LASS6 genes in mammalian tissues, but LASS1 expression was limited to the brain and skeletal muscle [16, 18]. Interestingly, LASS2 mRNA was found to be in the greatest abundance compared to other LASS family members and had the broadest tissue distribution [18]. Except for a weak display in skin, LASS3 mRNA expression is limited almost solely to testis, implying that LASS3 plays an important role in this gland [17].
LASS are integral membrane proteins, but the exact number of transmembrane domains and their topology has not been resolved experimentally. All of the LASS genes have a highly conserved stretch of 52 amino acids known as the Lag1p motif which is essential for enzyme activity [15]. Some of the LASS proteins are post-translationally modified, and, for instance, LASS6 is expressed as a native and an N-glycosylated form. The N-glycosylation site is conserved in LASS6, LASS2, and LASS5, but this post-translational modification is not required for ceramide synthase activity [16]. Intriguingly, all LASS except LASS1 contain a homeobox domain, suggesting involvement in developmental regulation [20]. De novo synthesis of ceramide is required for cell survival in vivo, and is widespread among cell types and tissues. Regulation of ceramide synthesis is only beginning to be understood. Regulation at the transcriptional level has been observed with a number of agents, including endotoxin and cytokines, UVB irradiation, and retinoic acid [21].
The de novo ceramide biosynthesis occurs in the ER where all the participating enzymes have been found [22–24]. Ceramide is synthesized at the cytosolic side of the ER [21, 24] and then serves as a precursor for the biosynthesis of glycosphingolipids and SM in the Golgi [25, 26]. Mitochondria arise as another important intracellular compartment of ceramide metabolism. It has been shown that mitochondria contain a variety of sphingolipids, including SM and ceramide [27, 28]. New evidence suggests a local action of ceramide on mitochondria in intact cells. Thus, selective hydrolysis of a mitochondrial pool of SM by overexpressed sphingomyelinase (bSMase) targeted to mitochondria resulted in apoptosis. Whereas generation of ceramide in the plasma membrane, ER, or Golgi apparatus by bSMase targeted to these compartments had no effect on cell viability [29].
Although several enzyme activities involved in ceramide metabolism have been demonstrated in mitochondria, the nature of enzymes of ceramide biosynthesis in this organelle is still a matter of debate [18]. Emerging evidence suggests ceramide synthase activity in mitochondria. Thus, ceramide synthase activity was first detected [30, 31] and partially purified from a bovine brain mitochondria-enriched fraction [32] which was not characterized in terms of marker enzyme activities. Mitochondrial enzymes showed almost 2-fold higher specific ceramide synthase activity than the ceramide synthase from the ER. The mitochondrial enzyme had a pH optimum around 7.5 and maximal catalytic efficiency with C16:0- or C18:0-acyl CoA. The addition of liposomes to the mitochondrial enzyme increased ceramide synthase activity (approx. 7.8-fold) [32]. Purification of ceramide synthase from bovine liver mitochondria yielded two major protein bands, 62 and 72 kDa on SDS-gel [33]. This enzyme had an apparent Km of 146 μM and Vmax of 11.1 nmol/min/mg protein with C18:0-acyl CoA, and corresponding values of Vmax 144 μM and 8.5 nmol/min/mg protein towards sphinganine.
Detailed analysis of ceramide synthase activity in highly purified mitochondria by Bionda et al. essentially confirmed previous findings [34]. Ceramide synthase activity was demonstrated in rat liver mitochondria and in the sub-compartment of the ER closely associated with mitochondria. Further sub-mitochondrial investigation of ceramide synthase activity revealed that both outer and inner mitochondrial membranes are able to synthesize ceramide [34]. A recent report describing several ceramide synthase isoforms, including LASS1, LASS2, and LASS6, in purified mouse brain mitochondria [35] supports the notion that one or several ceramide synthesizing enzymes could be localized in mitochondria [36]. The additional source of ceramide in mitochondria is a reverse reaction of a neutral ceramidase, e.g., formation of ceramide as a result of condensation of palmitate and sphingosine that is CoA-independent ceramide synthase [37]. On the basis of molecular cloning and confocal microscopy data, this activity was ascribed to mitochondria [38], and it was demonstrated in purified mitochondria [34]. A recent report suggests that ceramide could be also transported from the ER to mitochondria through the contact sites between the ER and mitochondria [39].
Sphingomyelin hydrolysis
SM hydrolysis by one of several sphingomyelinases (SMases) is another source of ceramide in the cell. Three groups of SMases, acid, neutral, and alkaline, are distinguished by their primary structure, catalytic pH optimum, and localization.
Acid SMase
A well-characterized enzyme, acid SMase contributes to the catabolism of SM and ceramide formation in lysosomes [40, 41]. Acid SMase could relocate from intracellular compartments to the plasma membrane where it plays an important role in SM hydrolysis and ceramide generation within lipid rafts [42]. Acid SMase is a soluble enzyme with no transmembrane domains, and the mechanism of acid SMase association with the membrane where its substrate SM is, remains unclear. Acid SMase is also secreted through the Golgi secretory pathway, and it is constitutively present in plasma [43] where it is involved in hydrolysis of the lipoprotein bound SM, the second most abundant phospholipid in human plasma. Intriguingly, acid SMase hydrolyzes SM bound to oxidized LDL more effectively than SM bound to intact LDL [44]. The accelerated hydrolysis of SM could enhance the LDL aggregation leading to macrophage foam cell formation, suggesting a role for secretory acid SMase in pathogenesis of arteriosclerosis [45].
Neutral SMase
Neutral SMase had a dynamic dual localization [46], having been found in the Golgi of sub-confluent cells and at the plasma membrane at the regions of cell-cell contact [47]. Furthermore, it appears that oxidative stress could induce neutral SMase trafficking to the plasma membrane, whereas antioxidant (glutathione) directed its translocation to the perinuclear region [48]. Alkaline SMase lacks homology to neutral or acid SMase and its mRNA is abundant in the intestine where the enzyme plays a major role in digestion of dietary SM [49].
Recycling or Salvage Pathway
Ceramide is also produced during the recycling of sphingosine in the process termed the “salvage pathway” [50]. In this process, complex sphingolipids are broken down to ceramide and then to sphingosine, which is then used by ceramide synthase yielding ceramide. SM is converted to ceramide by acid SMase. Ceramide accumulation via the salvage pathway requires ceramide synthase which plays a key role in de novo synthesis of ceramide. Complex sphingolipids undergo constitutive degradation in the late endosomes and the lysosomes yielding ceramide which does not leave the lysosomes [51] unless converted into sphingosine by acid ceramidase. Free sphingosine could be released from the lysosomes and re-acylated by ceramide synthase to form ceramide.
Ceramide Accumulation in Ischemia/Reperfusion (IR)
Ceramide accumulation has been demonstrated in various in vivo models of IR and it has been implicated as an important mediator of apoptosis in the injured tissue, but the mechanisms of ceramide generation are not well-defined and the downstream targets of ceramide remain unresolved. The identification and characterization of key proteins of ceramide synthesis is expected to contribute to further understanding of molecular mechanisms of ceramide involvement in tissue damage in IR.
The progress was certainly hampered by lack of appropriate techniques that would allow simultaneous analysis of multiple sphingolipid species. Thus, the most common method for quantification of ceramide, the diglyceride (DG) kinase assay [52] has significant disadvantages including a limited separation of ceramide from dihydroceramide and the inability to determine the individual molecular species of ceramide. Recent advances in the development of new mass spectroscopy-based methods for quantitative analysis of sphingolipid molecular species may allow further dissection of ceramide specific pathways [53, 54].
Increasing evidence suggests that the fatty acid chain of ceramide is an important determinant of the biological effect mediated by the individual ceramide species. Most of the experimental evidence indicating the important roles of ceramides containing distinct fatty acids is summarized in an excellent review by Futerman and his colleagues [55], and new studies further support the notion of distinct roles of ceramide species in cell metabolism. It has been demonstrated that generation of C18:0-ceramide, and not C16:0-ceramide resulted in repression of human telomerase reverse transcriptase promoter in lung carcinoma cells [56]. Activation of acid SMase in the salvage pathway brought about a selective accumulation of C16:0-ceramide [57, 58] due to the involvement of ceramide synthase LASS5 localized in mitochondria-associated membranes [58]. In another study, the effects of chronic hypoxia on selected ceramide species were examined in cardiac tissue in a neonatal mouse model [59]. The study revealed the differential involvement of the right ventricle with regard to the levels of C16:0-ceramide and it precursor dihydro-C16:0-ceramide. The decrease in C16:0-ceramide observed in both hypoxic and control right ventricles over time was paralleled by a significant increase in dihydro-C16:0-ceramide in hypoxic but not control tissues suggesting a role for dihydro-C16:0-ceramide in the adaptive tissue response to hypoxia. Although ceramide species could have different effects on biophysical properties of the membrane lipid bilayer [60], it remains unclear how ceramides containing different fatty acids exert their effects upon cell physiology.
Heart
In several studies, elevated ceramide has been reported in myocardium after ischemia and IR. In the rat heart left coronary artery occlusion model, ischemia with subsequent reperfusion, but not ischemia alone, induced apoptosis in myocardial cells indicated by DNA laddering and measurement of soluble chromatin degradation products [61]. The content of ceramide in ischemic myocardium was elevated to 155% baseline levels after 30 min ischemia, and was further increased to 250% after 3 h reperfusion. In the rabbit heart left coronary artery occlusion model, ceramide content was increased during the first minute of ischemia, peaking at 5 min with mean ceramide levels ~127% of the baseline. However, this peak was transient because ceramide content returned to near-baseline values as soon as 10 min into the sustained ischemia [62]. In another study with the rat heart left coronary artery occlusion model, ceramide content in reperfused myocardium was found to increase up to 50%. This increase was not associated with enhanced neutral or acid SMase activity, but rather with reduced ceramidase, ceramide-metabolizing enzyme (Figure 1), activity [63]. In a global rat heart ischemia model, ceramide content was elevated about 2-fold after 30 min ischemia/30 min reperfusion which was attributed to SM hydrolysis. Thus, there was about 50% less SM in reperfused myocardium after IR [64]. This finding was confirmed in recent studies by the same group showing an increased accumulation of the ceramide in ischemic myocardium after 30 min ischemia/2 h reperfusion [65, 66]. An inhibitor of acid SMase activity desipramine prevented ceramide accumulation and provided cardioprotection. Intriguingly, a significant amount of ceramide accumulated in the caveolin-1-rich membrane microdomains after IR that was abolished by pre-treatment with desipramine [66]. The ceramide caveolin-1 interaction is believed to occur within lipid raft microdomains in the membranes leading to rafts stabilization [67] and alteration of receptor tyrosine kinase signal transduction [68].
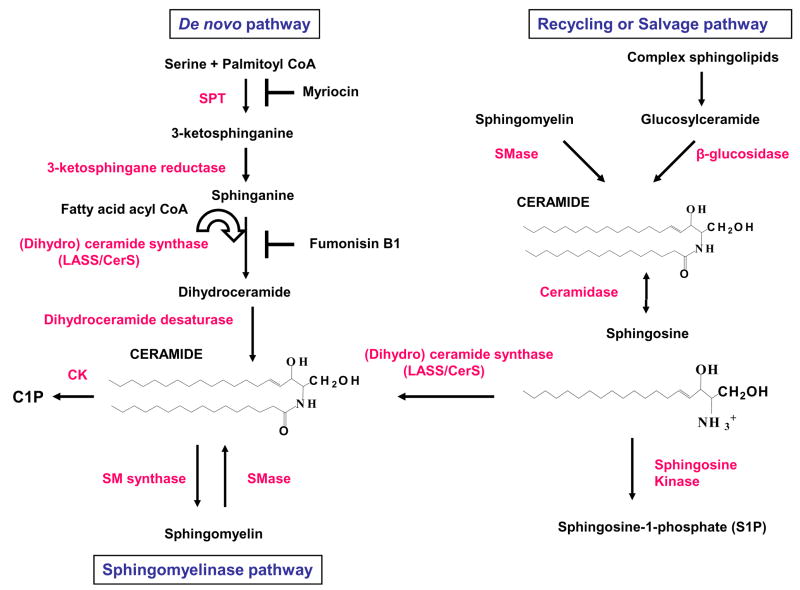
Figure 1. Biosynthesis of ceramide and its conversion into other bioactive sphingolipids.
De novo ceramide synthesis begins with the conversion of serine and fatty acyl CoA into 3-ketosphinganine by serine palmitoyl transferase (SPT), then 3-ketosphinganine is converted into dihydrosphingosine. Myriocin is a potent inhibitor of SPT activity. (Dihydro) ceramide synthase (LASS/CerS) acylates dihydrosphingosine to form dihydroceramide, which is then reduced to ceramide by dihydroceramide desaturase. Ceramide is also produced by sphingomyelinases (SMases) through sphingomyelin (SM) degradation in sphingomyelinase pathway. Ceramidase converts ceramide into sphingosine, which is phosphorylated by sphingosine kinase (SK) to generate sphingosine-1-phosphate. Ceramide is phosphorylated by ceramide kinase (CK) yielding ceramide-1-phosphate (C1P). In the salvage or recycling pathway, complex sphingolipids are broken down to ceramide by β-glucosidase and then by ceramidase to sphingosine, which is re-acylated to ceramide by LASS/CerS. Fumonisin B1 inhibits LASS/CerS activity.
In a very interesting study, analysis of cardiac tissues from mice subjected to IR revealed significant elevation of ceramide and inhibition of sphingosine kinase 1 activity (Figure 1) that could ultimately result in decreased sphingosine-1-phosphate levels [69]. Furthermore, sphingosine kinase 1 inhibition, ceramide accumulation, cardiomyocyte apoptosis, and infarct size were significantly decreased in mitochondrial monoamine oxidase (MAO-A)-deficient mice after IR. MAO-A appears to play an important role in reactive oxygen species-dependent cardiomyocytes apoptosis and postischemic cardiac damage [70]. The data imply that the upregulation of ceramide/sphingosine-1-phosphate ratio is a critical event in MAO-A-dependent cardiac cell apoptosis in IR.
Recently, increases of specific ceramide species in the rat heart were investigated after 30 min global ischemia/30 min reperfusion. IR increased accumulation of only 7 out of 14 ceramide species identified in the heart [71]. Of note, the relative magnitude of IR-induced myocardial accumulation of ceramide species was not proportional to their basal tissue concentrations. For instance, although C16:0-ceramide and C18:0-ceramide are the most abundant in rat heart (40% and 23% of total, respectively), IR increased their content by 48–54%. However, C18:2-ceramide, which contributes only 3.2% of total myocardial ceramide was increased by 281%. These findings suggest the role of specific ceramide species signaling in the mechanism of post-ischemic myocardial injury.
In vitro, hypoxia/reoxygenation activates a neutral SMase and ceramide accumulation in cardiomyocytes implicating the production of free radicals [72]. The neutral SMase activation could be abrogated by inhibition of a factor associated with neutral SMase activation (FAN) which is an adaptor protein connecting neutral SMase to the TNF receptor signaling pathway [73].
Brain
A few studies reported ceramide accumulation during cerebral ischemia [74, 75] and IR, and it appears that the mechanism of ceramide accumulation depends on the severity of the insult to the brain [76]. Thus, severe and lethal cerebral IR resulted in ceramide accumulation via activation of acid SMase and SM hydrolysis [74, 77] or inhibition of ceramide utilization by glucosylceramide synthase [78]. Consistent with these data, the extent of brain tissue damage was decreased in mice lacking acid SMase [79]. In a recent study, severe cerebral IR induced SM hydrolysis and increased ceramide and sphingosine levels in ischemic brain [57]. Similarly, chronic cerebral ischemia caused ceramide accumulation due to activation of SM degradation accompanied by reduced ceramide utilization via glucosylceramide synthase [80].
In mild IR, ceramide accumulation resulted from de novo ceramide biosynthesis rather from hydrolysis of SM [35]. There is apparent tissue specificity in the expression of individual ceramide species that might reflect the tissue specificity of the ceramide synthases. In brain, C18:0-, C18:1- and C24:1-ceramide are the major species expressed (39.5%, 34%, and 12.5% of total ceramide, respectively) whereas C16:0- ceramide contributes only 4% of total ceramide. All ceramide species were elevated in the ischemic brain about 1.5–2-fold. The enhanced accumulation of sphingolipids seems to occur during the reperfusion phase; there were no changes in sphingolipid content after ischemia without reperfusion. This finding is in line with data which show that both ischemia and the restoration of blood flow to ischemic tissue (reperfusion) causes cellular damage by different molecular mechanisms [81, 82]. Investigation of intracellular sites of ceramide accumulation revealed the elevation of ceramide species both in purified mitochondria and in the ER. In mitochondria, only C18:0-, C18:1- and C16:0-ceramides were increased, but all ceramide species increased in the ER suggesting activation of different ceramide synthases in these intracellular compartments. Indeed, several ceramide synthases were identified in mitochondria and the ER, including LASS1, LASS2, and LASS6, but LASS5 was localized only in the ER in the brain. Activity measurements indicated activation of LASS6 in ischemic mitochondria apparently via post-translational mechanisms, since IR did not affect the LASS6 protein expression levels [35]. This study illuminates a novel determinant in cerebral IR, mitochondrial ceramide synthase LASS6 which could be an important future target for neuroprotection.
In vitro, de novo synthesized ceramide increased after brief exposure of cultured brain cells to hypoxia, oxygen/glucose deprivation, or TNF [83, 84]. In neuronal precursor cells, hypoxia/reoxygenation triggered a robust elevation in C14:0- and C16:0-ceramides, and a small increase in C18:0-, C18:1- and C20:0-ceramides, and no increase in C24:0- and C24:1-ceramides [85]. The elevations in ceramides were primarily due to the actions of acid SMase and ceramide synthase LASS5, demonstrating the involvement of the salvage pathway. Interestingly, C2-ceramide infusion protected the brain against IR injury [86, 87]. However, this effect could be also attributed to the intracellular/extracellular conversion of ceramide into sphingosine-1-phosphate, which is known to protect cells from apoptosis [41, 88, 89].
Liver
Ceramide was elevated in injured liver tissue after cold ischemia and warm reperfusion during liver transplantation [90]. A critical role of acid SMase and ceramide accumulation was demonstrated in another study of hepatic IR injury [91]. Hepatic ceramide levels transiently increased after the reperfusion phase due to activation of acid SMase followed by acid ceramidase stimulation. Knocking down acid SMase by in vivo administration of siRNA decreased ceramide generation during IR, and attenuated hepatocellular necrosis, cytochrome c release, and caspase-3 activation. The study draws attention to an important role of ceramide in IR-induced liver damage and suggests that modulation of acid SMase could be of therapeutic relevance in liver transplantation.
Kidney
In the whole kidney IR model, ceramide content was increased about 1.8-fold in the injured tissue during the reperfusion phase [92] which was not accompanied by SM hydrolysis. In fact, there was no SM content change in post-IR tissue. Analysis of SMase activity revealed that ischemia induced declines (50%) in both acid and neutral SMase activity, and these persisted throughout the 24-h reperfusion period [93]. C16:0-, C22:0-, and C24:0-ceramides comprised 20%, 10%, and 70% of the total ceramide content in kidney tissue, respectively [94]. IR dramatically increased C16:0-ceramide (4-fold), and all other ceramides increased modestly. Interestingly, IR induced a striking shift towards unsaturated (vs. saturated) fatty acyl within C22:0- and C24:0- (but not C16:0-) ceramide pools.
Ceramide and Mitochondria in Cell Death
Irrespective of the type of IR, IR-related physiological events have a common final consequence: alteration of mitochondrial function and release of mitochondrial proteins, leading to cell death. Cells with hallmarks of necrosis or apoptosis have been detected in animal models of IR [95–98]. The mitochondrial changes appear to be one essential step in tissue damage in IR, and treatments that ameliorate tissue infarction were associated with better recovery of mitochondrial function [99, 100]. Multiple studies show intimate connections between ceramide signaling and functioning of mitochondria [6, 101], which play central role in integration of cellular signals to determine the outcome among apoptosis, necrosis, or proliferation [102–104].
Several lines of evidence have implicated changes in mitochondrial function as an intermediate step in transduction of ceramide signals that culminate in apoptotic or necrotic cell death [101, 105]. First, ceramide-induced apoptosis is accompanied by release of pro-apoptotic proteins from mitochondria [29, 106, 107], increased generation of mitochondrial reactive oxygen species (ROS) [108], and discharge of mitochondrial transmembrane potential, Δψ [106, 109–111]. Second, interventions that specifically prevent mitochondrial dysfunction suppress ceramide-induced apoptosis: inhibitors of the mitochondrial permeability transition pore (MPTP) bongkrekic acid [109, 112] and cyclosporin A [112–114]; and over-expression of Bcl-2 [109, 111, 115–117]. Third, TNF-α-, ischemia/reperfusion-, etoposide-, or UV-induced apoptosis is associated with simultaneous increase in mitochondrial ceramide [35, 118–120].
Depending on cell type and stimuli, ceramide can alter mitochondrial function indirectly or directly (Figure 2). Indirectly, ceramide modifies activity of pro-apoptotic and anti-apoptotic members of the Bcl-2 family of proteins that, in turn, alter the outer mitochondrial membrane permeability for cytochrome c and other pro-apoptotic molecules. Protein targets for ceramide in the cytoplasm include protein phosphatases PP1A and PP2A, protein kinases PKC ζ, raf-1, and kinase-suppressor Ras [121]. In the lysosomal compartment, ceramide activates aspartate protease cathepsin D [7, 122–124]. Among these targets, cathepsin D, PP2A, and PP1A could propagate a pro-apoptotic ceramide signal to the level of the mitochondria [7]. Interaction of ceramide with cathepsin D results in cleavage of Bid to active tBid with subsequent activation of caspase-9 and caspase-3 [122]. Activation by ceramides of serine/threonine protein phosphatase PP2A is involved in regulation of the apoptotic/anti-apoptotic activity of Bcl-2 family proteins by changing their phosphorylation status. Ceramide-activated PP2A increases the pro-apoptotic potential of Bcl-2 family proteins by dephosphorylation of Bax (activation) [125], or Bcl-2 (inactivation) [126]. An additional substrate for PP2A is serine/threonine kinase Akt/PKB [7]. Ceramide-dependent activation of PP2A leads to inactivation of Akt [7, 127, 128] that, in turn, results in dephosphorylation and activation of pro-apoptotic Bad, an Akt immediate substrate [129]. At the same time PP2A can directly dephosphorylate Bad, thus increasing its pro-apoptotic activity [130]. PP1A also can exert its effect on mitochondria by Bad dephosphorylation [127]. Interestingly, ceramide by itself can trigger transition of Bax into the active conformation, insertion in to the outer mitochondrial membrane with the subsequent release of cytochrome c and Smac in a cell free system [131]. Potentiation of Bax binding by ceramides to the outer mitochondrial membrane was shown by Birbes and colleagues [118] and in energized mitochondria ceramide-induced Bax-dependent MPTP opening [132]. Critical involvement of ceramide in triggering Bax translocation to the mitochondria was demonstrated during hypoxia/reoxygenation in neuronal cells [85]. Attenuation of Bax translocation by knockdown of ceramide synthase LASS5 or acid SMase suggests contribution of the activated salvage pathway in ceramide upregulation; however, the mechanisms by which ceramide exerts its effect remain unknown.
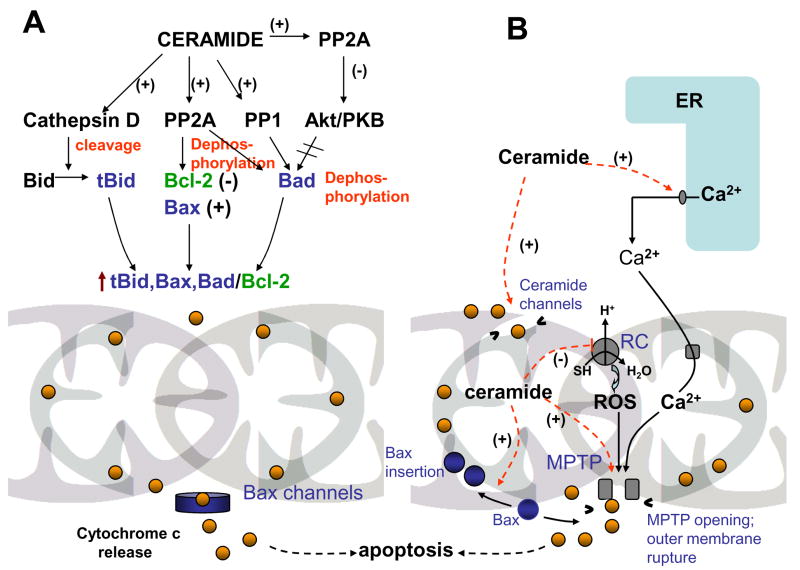
Figure 2. Ceramide modulates mitochondrial functions through direct and indirect mechanisms.
A. Indirect modulation of mitochondrial functions by ceramide occurs through the change in the ratio of pro-apoptotic/anti-apoptotic proteins of Bcl-2 family at the outer mitochondrial membrane. B. Direct modulation of mitochondrial functions by ceramide include a) formation of ceramide channels permeable for cytochrome c in the outer mitochondrial membrane; b) potentiation of mitochondrial permeability transition pore opening (MPTP) in the inner membrane in the presence of Ca2+ or Bax (ceramide-induced Ca2+ release from the endoplasmic reticulum (ER) can contribute to the processes; c) potentiation of Bax insertion (activation) in to the outer membrane, d) inhibition of the respiratory chain (RC) with a subsequent increase in reactive oxygen species (ROS) formation.
Less-defined, indirect mechanisms include interaction of ceramide with protein kinases PKC δ, p38 and JNK. Short-chain ceramides induce translocation of PKC δ from the cytoplasm to the mitochondria in LNCaP cells [133]. The translocation of PKC δ was accompanied by cytochrome c release. Mitochondrial translocation of PKC δ and activation of kinase activity was also evident when endogenous ceramides levels were raised by activation of de novo and neutral SMase-dependent pathways of ceramide production. Endogenous ceramide-induced PKC δ translocation similarly promoted release of cytochrome c and caspase-9 activation. A report by Huwiler et al. [134] indicates that ceramide can directly target PKC δ. Thus, increased ceramide levels during I/R can potentially contribute to mitochondrial translocation/activation of PKC δ, which enhances cytochrome c release in heart I/R [135]. Although potential mitochondrial PKC δ targets for which phosphorylation results in cytochrome c release remain illusive, PKC δ-dependant accumulation and dephosphorylation of Bad may contribute to the initiation of apoptotic program [135]. The member of the mitogen-activated protein kinase (MAPK) superfamily p38 MAPK was implicated in ceramide-induced apoptosis in cardiomyocytes [136]. Short-chain ceramide treatment induced phosphorylation/activation of p38 MAPK which was accompanied by release of cytochrome c from the mitochondria and by the discharge of mitochondrial membrane potential. P38 MAPK inhibitor, SB 202190, abrogated the effect of ceramide both on p38 MAPK phosphorylation and on mitochondrial dysfunction. An interesting aspect of the study was the phosphorylation of the mitochondria-associated p38 MAPK pool under the influence of ceramide. This observation might indicate local signaling in mitochondria-mediated cell death. Although mitochondria-related targets for ceramide-activated p38 MAPK are not well defined, a recent report by Capano and Crompton [137] demonstrates that activation of p38 MAPK during simulated ischemia in cardiomyocytes is a key regulatory point of Bax translocation from the cytosol to the mitochondria. The evidence of p38 MAPK-dependent phosphorylation of BimEL in apoptotic cell response has been provided [138]. Another member of the MAPK superfamily, JNK, was shown to be readily activated by both endogenous ceramide generation in liver I/R [91] and by addition of exogenous ceramide [139]. Activated JNK translocates to the mitochondria and initiates cytochrome c release and cell death by yet unidentified mechanisms [139, 140]; however, direct interaction of JNK with the mitochondrial pool of Bcl-xL was suggested [141]. Alternatively, activated JNK can induce mitochondrial dysfunction by phosphorylation of a pro-apoptotic member of the Bcl-2 family protein, Bim [91, 139, 142]. Translocation of activated Bim to mitochondria initiates Bax-dependent cytochrome c release and apoptosis [142]. Several other members of Bcl-2 family proteins have been proposed to mediate pro-apoptotic JNK signaling[143]. Overall, the increased ratio of pro-apoptotic/anti-apoptotic proteins bound to mitochondria is generally considered to trigger permeabilization of the outer mitochondrial membrane for cytochrome c and other mitochondrial inter-membrane resident proteins, initiators of apoptosis [104, 144]. The increases in cell ceramide species contents are expected to contribute to the induction of apoptosis by this mechanism.
Among non-protein indirect pathways, those associated with Ca2+ signaling attract special attention because of the well-known ability of these organelles both to respond to Ca2+ and to shape and propagate the Ca2+ signal within the cell [145, 146]. In this pathway, the Ca2+ pool of the ER is a target for ceramide [147, 148]. Ca2+ released by ceramide from the ER is readily accumulated in mitochondria that, in turn, results in MPTP opening, and cytochrome c release.
Evidence is also accruing to implicate a direct action of ceramide on mitochondria. In this context, modulation by ceramide of mitochondrial functions at the level of isolated organelles has provided further evidence in support of this mechanism. It has been reported that ceramides directly suppress respiratory chain activity at the level of respiratory chain Complex III and/or Complex I [35, 120, 149, 150]. Suppression of the respiratory chain by ceramides results in increased production of ROS [120, 150–152], well-known inducers of an apoptotic cell response [152, 153]. Increased ROS production by endothelial cells after hypoxia/reoxygenation was linked to the ceramide-induced suppression of the mitochondrial respiratory chain [154].
Moreover, current research is focused on the ability of ceramides to release cytochrome c or other pro-apoptotic proteins from the mitochondrial inter-membrane space. Within the model of Colombini and co-workers, pro-apoptotic protein release is due to formation of large pores in the outer mitochondrial membrane by ceramide itself, whereas the inner membrane is viewed as being ceramide-insensitive. This model is supported by extensive experimental material using isolated mitochondria [155–158] and artificial membranes (liposomes and black lipid membranes) [155, 159]. Importantly, it was recently shown that anti-apoptotic Bcl-2 can disassemble ceramide channels in the outer mitochondrial membrane and black lipid membranes [160], thus providing the mechanistic explanation for the original observation of Ghafourifar et al. [158] that Bcl-2 suppresses ceramide-induced cytochrome c release from isolated mitochondria. However, the formation of ceramide channels seems to be highly dependent on the conditions employed, and has been questioned in a number of publications [161–164]. Besides, a few reports suggest that the permeabilization of the inner mitochondrial membrane via the opening of the MPTP could be a primary event in initiation of cytochrome c release in the presence of ceramides [132, 164]. The switch between selective permeabilization of the outer membrane vs. permeabilization of the inner membrane in the presence of ceramide appears to depend on the composition of incubation medium and the nature of ceramide employed [157].
Additional direct effects of ceramide on mitochondria include modulation of the ionic permeability of the lipid component of the inner membrane [150] and displacement of cytochrome c from the inner membrane as a result of the direct interaction with the proteins in the respiratory chain Complex III [158, 161]. Emerging evidence suggests involvement of ceramides in reorganization of the mitochondrial network. Both exogenous and endogenously generated ceramides induce mitochondrial fission [165, 166], which may contribute to apoptotic cell death [167]. What particular effectors of mitochondrial fission (DRP1, Fis1, or Bax) or fusion (OPA-1, Mitofusins) machineries are the targets of ceramide in this process remains to be determined. However, in cardiomyocytes exposed to exogenous C2-ceramide, an increased expression of mitochondrial resident Fis1 and enhanced recruitment of cytosolic DRP1 to mitochondrial fission foci may contribute to disintegration of the mitochondrial network [166].
It should be appreciated that the ceramide/mitochondria interaction in the control of apoptosis should be considered in conjunction with the effects of its pro-apoptotic metabolites such as ganglioside GD3 and sphingosine. GD3 shares with ceramide the same properties with respect to its effects on isolated mitochondria and mitochondria in situ. In cells, it disrupts mitochondrial membrane potential in a Bcl-2-sensitive manner [168] and induces ROS production [169]. At the level of isolated mitochondria, it inhibits the mitochondrial respiratory chain at the level of Complex III [170], increases ROS production [171], opens the MPTP with the subsequent release of cytochrome c [162, 170, 172], and potentiates interaction of Bax with mitochondria [132]. Interestingly, while the effect of CD3 on ROS production is relatively nonspecific (lactosylceramide, GM1, GD1a and glucosylceramide produce a similar response [120]), the effect of GD3 in the induction of apoptosis and MPTP opening shows considerable specificity. GM3, GM1 GD1, GD1a, GT1 has no or a slight inhibitory effect [132, 162, 170]. In some instances, the effect of ceramide on mitochondria in cells can be explained by its conversion to GD3 [168, 173].
Another pro-apoptotic ceramide derivative, sphingosine, releases cytochrome c from mitochondria that could be inhibited by over-expression of anti-apoptotic Bcl-xL [174]. In contrast to ceramides and GD3, sphingosine suppresses the MPTP in isolated mitochondria, and thus MPTP-dependent cytochrome c release [175, 176]. It also inhibits ceramide channel formation in the outer mitochondrial membrane [177]. This indicates that indirect pathways of cytochrome c release, for example, by recruitment of Bax to mitochondria [178], are predominant in the action of sphingosine on mitochondria. At the same time, similar to ceramide, sphingosine suppresses respiratory chain activity [179] and increases ROS production by mitochondria, although at higher concentrations [120]. Interaction of ceramide, sphingosine, and ganglioside pathways in the control of mitochondrial functions in the time-course of apoptosis remains to be established. Thus, the proposed mechanisms by which ceramides may affect mitochondria vary, and the combination of direct and indirect mechanisms involved in propagation of ceramide signals to mitochondria depends on cell type and the nature of the stimuli employed.
Conclusion
Continued research efforts are required to better understand the pathophysiological mechanisms of IR injury, to identify and test new protective agents. Further studies of the molecular basis of ceramide’s role in the ischemic organs are warranted. Since many assumptions regarding ceramide functions in IR-induced tissue injury were based on in vitro studies employing artificial ceramides, it is of importance to critically evaluate the mitochondrial dysfunctions in IR-injured organs and define a possible role of natural ceramide as a cause of the mitochondrial impairment. This will allow the discovery of novel and groundbreaking therapeutic approaches to mitigate diseases that may result from an elevation in ceramide and its metabolites.
Acknowledgments
We apologize to colleagues for not citing all of the important publications owing to space constrains. We thank Dr. Jennifer G. Schnellmann for help with preparation of the manuscript. This work is supported by the NIH/NCCR COBRE in Lipidomics and Pathobiology P20 RR 17677-04 (TIG), VA Merit Award (TIG), and SAN was supported by NIH grant AG16583.
This work is supported by the NIH/NCCR COBRE in Lipidomics and Pathobiology P20 RR 17677-04 (TIG), VA Merit Award (TIG), and SAN was supported by NIH grant AG16583.
Abbreviations
- CK
ceramide kinase
- CerS
ceramide synthase
- ER
endoplasmic reticulum
- IR
ischemia/reperfusion
- JNK
c-Jun NH2-terminal kinase
- LAG1
longevity assurance gene 1
- LASS
gene longevity assurance gene
- MPTP
mitochondrial permeability transition pore
- PP1A
protein phosphatase 1A
- PP2A
protein phosphatase 2A
- RC
respiratory chain
- ROS
reactive oxygen species
- SM
sphingomyelin
- SMase
sphingomyelinase
- S1P
sphingosine-1-phosphate
- SK
sphingosine kinase
References
- 1.Hannun YA, Luberto C. Ceramide in the eukaryotic stress response. Trends Cell Biol. 2000;10(2):73–80. doi: 10.1016/s0962-8924(99)01694-3. [DOI] [PubMed] [Google Scholar]
- 2.Colombaioni L, Garcia-Gil M. Sphingolipid metabolites in neural signalling and function. Brain Res Brain Res Rev. 2004;46(3):328–55. doi: 10.1016/j.brainresrev.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Hannun YA, Obeid LM. The Ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J Biol Chem. 2002;277(29):25847–50. doi: 10.1074/jbc.R200008200. [DOI] [PubMed] [Google Scholar]
- 4.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9(2):139–50. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 5.Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annu Rev Physiol. 1998;60:643–65. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- 6.Mimeault M. New advances on structural and biological functions of ceramide in apoptotic/necrotic cell death and cancer. FEBS Lett. 2002;530(1–3):9–16. doi: 10.1016/s0014-5793(02)03432-4. [DOI] [PubMed] [Google Scholar]
- 7.Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochim Biophys Acta. 2002;1585(2–3):114–25. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- 8.Ogretmen B, Hannun YA. Biologically active sphingolipids in cancer pathogenesis and treatment. Nat Rev Cancer. 2004;4(8):604–16. doi: 10.1038/nrc1411. [DOI] [PubMed] [Google Scholar]
- 9.Futerman AH, Riezman H. The ins and outs of sphingolipid synthesis. Trends Cell Biol. 2005;15(6):312–8. doi: 10.1016/j.tcb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Lopez-Montero I, Rodriguez N, Cribier S, Pohl A, Velez M, Devaux PF. Rapid transbilayer movement of ceramides in phospholipid vesicles and in human erythrocytes. J Biol Chem. 2005;280(27):25811–9. doi: 10.1074/jbc.M412052200. [DOI] [PubMed] [Google Scholar]
- 11.Simon CG, Jr, Holloway PW, Gear AR. Exchange of C(16)-ceramide between phospholipid vesicles. Biochemistry. 1999;38(44):14676–82. doi: 10.1021/bi991537w. [DOI] [PubMed] [Google Scholar]
- 12.Perry RJ, Ridgway ND. Molecular mechanisms and regulation of ceramide transport. Biochim Biophys Acta. 2005;1734(3):220–34. doi: 10.1016/j.bbalip.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Hanada K, Kumagai K, Yasuda S, Miura Y, Kawano M, Fukasawa M, Nishijima M. Molecular machinery for non-vesicular trafficking of ceramide. Nature. 2003;426(6968):803–9. doi: 10.1038/nature02188. [DOI] [PubMed] [Google Scholar]
- 14.D’Mello NP, Childress AM, Franklin DS, Kale SP, Pinswasdi C, Jazwinski SM. Cloning and characterization of LAG1, a longevity-assurance gene in yeast. J Biol Chem. 1994;269(22):15451–9. [PubMed] [Google Scholar]
- 15.Spassieva S, Seo JG, Jiang JC, Bielawski J, Alvarez-Vasquez F, Jazwinski SM, Hannun YA, Obeid LM. Necessary role for the Lag1p motif in (dihydro)ceramide synthase activity. J Biol Chem. 2006 doi: 10.1074/jbc.M608092200. [DOI] [PubMed] [Google Scholar]
- 16.Mizutani Y, Kihara A, Igarashi Y. Mammalian Lass6 and its related family members regulate synthesis of specific ceramides. Biochem J. 2005;390(Pt 1):263–71. doi: 10.1042/BJ20050291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizutani Y, Kihara A, Igarashi Y. LASS3 (longevity assurance homologue 3) is a mainly testis-specific (dihydro)ceramide synthase with relatively broad substrate specificity. Biochem J. 2006;398(3):531–8. doi: 10.1042/BJ20060379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laviad EL, Albee L, Pankova-Kholmyansky I, Epstein S, Park H, Merrill AH, Jr, Futerman AH. Characterization of ceramide synthase 2: tissue distribution, substrate specificity, and inhibition by sphingosine 1-phosphate. J Biol Chem. 2008;283(9):5677–84. doi: 10.1074/jbc.M707386200. [DOI] [PubMed] [Google Scholar]
- 19.Lahiri S, Futerman AH. LASS5 is a bona fide dihydroceramide synthase that selectively utilizes palmitoyl-CoA as acyl donor. J Biol Chem. 2005;280(40):33735–8. doi: 10.1074/jbc.M506485200. [DOI] [PubMed] [Google Scholar]
- 20.Venkataraman K, Futerman AH. Do longevity assurance genes containing Hox domains regulate cell development via ceramide synthesis? FEBS Lett. 2002;528(1–3):3–4. doi: 10.1016/s0014-5793(02)03248-9. [DOI] [PubMed] [Google Scholar]
- 21.Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277(29):25843–6. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 22.Michel C, van Echten-Deckert G. Conversion of dihydroceramide to ceramide occurs at the cytosolic face of the endoplasmic reticulum. FEBS Lett. 1997;416(2):153–5. doi: 10.1016/s0014-5793(97)01187-3. [DOI] [PubMed] [Google Scholar]
- 23.Hirschberg K, Rodger J, Futerman AH. The long-chain sphingoid base of sphingolipids is acylated at the cytosolic surface of the endoplasmic reticulum in rat liver. Biochem J. 1993;290(Pt 3):751–7. doi: 10.1042/bj2900751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandon EC, Ehses I, Rother J, van Echten G, Sandhoff K. Subcellular localization and membrane topology of serine palmitoyltransferase, 3-dehydrosphinganine reductase, and sphinganine N-acyltransferase in mouse liver. J Biol Chem. 1992;267(16):11144–8. [PubMed] [Google Scholar]
- 25.Kolter T, Proia RL, Sandhoff K. Combinatorial ganglioside biosynthesis. J Biol Chem. 2002;277(29):25859–62. doi: 10.1074/jbc.R200001200. [DOI] [PubMed] [Google Scholar]
- 26.Futerman AH, Stieger B, Hubbard AL, Pagano RE. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990;265(15):8650–7. [PubMed] [Google Scholar]
- 27.Ardail D, Popa I, Alcantara K, Pons A, Zanetta JP, Louisot P, Thomas L, Portoukalian J. Occurrence of ceramides and neutral glycolipids with unusual long-chain base composition in purified rat liver mitochondria. FEBS Lett. 2001;488(3):160–4. doi: 10.1016/s0014-5793(00)02332-2. [DOI] [PubMed] [Google Scholar]
- 28.Tserng KY, Griffin R. Quantitation and molecular species determination of diacylglycerols, phosphatidylcholines, ceramides, and sphingomyelins with gas chromatography. Anal Biochem. 2003;323(1):84–93. doi: 10.1016/j.ab.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 29.Birbes H, El Bawab S, Hannun YA, Obeid LM. Selective hydrolysis of a mitochondrial pool of sphingomyelin induces apoptosis. Faseb J. 2001;15(14):2669–79. doi: 10.1096/fj.01-0539com. [DOI] [PubMed] [Google Scholar]
- 30.Morell P, Radin NS. Specificity in ceramide biosynthesis from long chain bases and various fatty acyl coenzyme A’s by brain microsomes. J Biol Chem. 1970;245(2):342–50. [PubMed] [Google Scholar]
- 31.Ullman MD, Radin NS. Enzymatic formation of hydroxy ceramides and comparison with enzymes forming nonhydroxy ceramides. Arch Biochem Biophys. 1972;152(2):767–77. doi: 10.1016/0003-9861(72)90272-x. [DOI] [PubMed] [Google Scholar]
- 32.Shimeno H, Soeda S, Yasukouchi M, Okamura N, Nagamatsu A. Fatty acyl-Co A: sphingosine acyltransferase in bovine brain mitochondria: its solubilization and reconstitution onto the membrane lipid liposomes. Biol Pharm Bull. 1995;18(10):1335–9. doi: 10.1248/bpb.18.1335. [DOI] [PubMed] [Google Scholar]
- 33.Shimeno H, Soeda S, Sakamoto M, Kouchi T, Kowakame T, Kihara T. Partial purification and characterization of sphingosine N-acyltransferase (ceramide synthase) from bovine liver mitochondrion-rich fraction. Lipids. 1998;33(6):601–5. doi: 10.1007/s11745-998-0246-2. [DOI] [PubMed] [Google Scholar]
- 34.Bionda C, Portoukalian J, Schmitt D, Rodriguez-Lafrasse C, Ardail D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem J. 2004;382(Pt 2):527–33. doi: 10.1042/BJ20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu J, Novgorodov SA, Chudakova D, Zhu H, Bielawska A, Bielawski J, Obeid LM, Kindy MS, Gudz TI. JNK3 signaling pathway activates ceramide synthase leading to mitochondrial dysfunction. J Biol Chem. 2007;282(35):25940–9. doi: 10.1074/jbc.M701812200. [DOI] [PubMed] [Google Scholar]
- 36.Futerman AH. Intracellular trafficking of sphingolipids: relationship to biosynthesis. Biochim Biophys Acta. 2006;1758(12):1885–92. doi: 10.1016/j.bbamem.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 37.El Bawab S, Birbes H, Roddy P, Szulc ZM, Bielawska A, Hannun YA. Biochemical characterization of the reverse activity of rat brain ceramidase. A CoA-independent and fumonisin B1-insensitive ceramide synthase. J Biol Chem. 2001;276(20):16758–66. doi: 10.1074/jbc.M009331200. [DOI] [PubMed] [Google Scholar]
- 38.El Bawab S, Roddy P, Qian T, Bielawska A, Lemasters JJ, Hannun YA. Molecular cloning and characterization of a human mitochondrial ceramidase. J Biol Chem. 2000;275(28):21508–13. doi: 10.1074/jbc.M002522200. [DOI] [PubMed] [Google Scholar]
- 39.Stiban J, Caputo L, Colombini M. Ceramide synthesis in the endoplasmic reticulum can permeabilize mitochondria to proapoptotic proteins. J Lipid Res. 2008;49(3):625–34. doi: 10.1194/jlr.M700480-JLR200. [DOI] [PubMed] [Google Scholar]
- 40.Tani M, Ito M, Igarashi Y. Ceramide/sphingosine/sphingosine 1-phosphate metabolism on the cell surface and in the extracellular space. Cell Signal. 2007;19(2):229–37. doi: 10.1016/j.cellsig.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 41.Tani M, Igarashi Y, Ito M. Involvement of neutral ceramidase in ceramide metabolism at the plasma membrane and in extracellular milieu. J Biol Chem. 2005;280(44):36592–600. doi: 10.1074/jbc.M506827200. [DOI] [PubMed] [Google Scholar]
- 42.Bollinger CR, Teichgraber V, Gulbins E. Ceramide-enriched membrane domains. Biochim Biophys Acta. 2005;1746(3):284–94. doi: 10.1016/j.bbamcr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 43.Spence MW, Byers DM, Palmer FB, Cook HW. A new Zn2+-stimulated sphingomyelinase in fetal bovine serum. J Biol Chem. 1989;264(10):5358–63. [PubMed] [Google Scholar]
- 44.Schissel SL, Jiang X, Tweedie-Hardman J, Jeong T, Camejo EH, Najib J, Rapp JH, Williams KJ, Tabas I. Secretory sphingomyelinase, a product of the acid sphingomyelinase gene, can hydrolyze atherogenic lipoproteins at neutral pH. Implications for atherosclerotic lesion development. J Biol Chem. 1998;273(5):2738–46. doi: 10.1074/jbc.273.5.2738. [DOI] [PubMed] [Google Scholar]
- 45.Tabas I. Secretory sphingomyelinase. Chem Phys Lipids. 1999;102(1–2):123–30. doi: 10.1016/s0009-3084(99)00080-8. [DOI] [PubMed] [Google Scholar]
- 46.Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45(38):11247–56. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- 47.Marchesini N, Osta W, Bielawski J, Luberto C, Obeid LM, Hannun YA. Role for mammalian neutral sphingomyelinase 2 in confluence-induced growth arrest of MCF7 cells. J Biol Chem. 2004;279(24):25101–11. doi: 10.1074/jbc.M313662200. [DOI] [PubMed] [Google Scholar]
- 48.Levy M, Castillo SS, Goldkorn T. nSMase2 activation and trafficking are modulated by oxidative stress to induce apoptosis. Biochem Biophys Res Commun. 2006;344(3):900–5. doi: 10.1016/j.bbrc.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nilsson A, Duan RD. Absorption and lipoprotein transport of sphingomyelin. J Lipid Res. 2006;47(1):154–71. doi: 10.1194/jlr.M500357-JLR200. [DOI] [PubMed] [Google Scholar]
- 50.Kitatani K, Idkowiak-Baldys J, Hannun YA. The sphingolipid salvage pathway in ceramide metabolism and signaling. Cell Signal. 2008;20(6):1010–8. doi: 10.1016/j.cellsig.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chatelut M, Leruth M, Harzer K, Dagan A, Marchesini S, Gatt S, Salvayre R, Courtoy P, Levade T. Natural ceramide is unable to escape the lysosome, in contrast to a fluorescent analogue. FEBS Lett. 1998;426(1):102–6. doi: 10.1016/s0014-5793(98)00325-1. [DOI] [PubMed] [Google Scholar]
- 52.Bielawska A, Perry DK, Hannun YA. Determination of ceramides and diglycerides by the diglyceride kinase assay. Anal Biochem. 2001;298(2):141–50. doi: 10.1006/abio.2001.5342. [DOI] [PubMed] [Google Scholar]
- 53.Sullards MC. Analysis of sphingomyelin, glucosylceramide, ceramide, sphingosine, and sphingosine 1-phosphate by tandem mass spectrometry. Methods Enzymol. 2000;312:32–45. doi: 10.1016/s0076-6879(00)12898-8. [DOI] [PubMed] [Google Scholar]
- 54.Pettus BJ, Kroesen BJ, Szulc ZM, Bielawska A, Bielawski J, Hannun YA, Busman M. Quantitative measurement of different ceramide species from crude cellular extracts by normal-phase high-performance liquid chromatography coupled to atmospheric pressure ionization mass spectrometry. Rapid Commun Mass Spectrom. 2004;18(5):577–83. doi: 10.1002/rcm.1373. [DOI] [PubMed] [Google Scholar]
- 55.Pewzner-Jung Y, Ben-Dor S, Futerman AH. When do Lasses (longevity assurance genes) become CerS (ceramide synthases)?: Insights into the regulation of ceramide synthesis. J Biol Chem. 2006;281(35):25001–5. doi: 10.1074/jbc.R600010200. [DOI] [PubMed] [Google Scholar]
- 56.Wooten-Blanks LG, Song P, Senkal CE, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. Faseb J. 2007;21(12):3386–97. doi: 10.1096/fj.07-8621com. [DOI] [PubMed] [Google Scholar]
- 57.Chudakova DA, Zeidan YH, Wheeler BW, Yu J, Novgorodov SA, Kindy MS, Hannun YA, Gudz TI. Integrin-associated Lyn kinase promotes cell survival by suppressing acid sphingomyelinase activity. J Biol Chem. 2008 doi: 10.1074/jbc.M803301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kitatani K, Idkowiak-Baldys J, Bielawski J, Taha TA, Jenkins RW, Senkal CE, Ogretmen B, Obeid LM, Hannun YA. Protein kinase C-induced activation of a ceramide/protein phosphatase 1 pathway leading to dephosphorylation of p38 MAPK. J Biol Chem. 2006;281(48):36793–802. doi: 10.1074/jbc.M608137200. [DOI] [PubMed] [Google Scholar]
- 59.Noureddine L, Azzam R, Nemer G, Bielawski J, Nasser M, Bitar F, Dbaibo GS. Modulation of total ceramide and constituent ceramide species in the acutely and chronically hypoxic mouse heart at different ages. Prostaglandins Other Lipid Mediat. 2008;86(1–4):49–55. doi: 10.1016/j.prostaglandins.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 60.Sot J, Aranda FJ, Collado MI, Goni FM, Alonso A. Different effects of long- and short-chain ceramides on the gel-fluid and lamellar-hexagonal transitions of phospholipids: a calorimetric, NMR, and x-ray diffraction study. Biophys J. 2005;88(5):3368–80. doi: 10.1529/biophysj.104.057851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bielawska AE, Shapiro JP, Jiang L, Melkonyan HS, Piot C, Wolfe CL, Tomei LD, Hannun YA, Umansky SR. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischemia and reperfusion. Am J Pathol. 1997;151(5):1257–63. [PMC free article] [PubMed] [Google Scholar]
- 62.Argaud L, Prigent AF, Chalabreysse L, Loufouat J, Lagarde M, Ovize M. Ceramide in the antiapoptotic effect of ischemic preconditioning. Am J Physiol Heart Circ Physiol. 2004;286(1):H246–51. doi: 10.1152/ajpheart.00638.2003. [DOI] [PubMed] [Google Scholar]
- 63.Zhang DX, Fryer RM, Hsu AK, Zou AP, Gross GJ, Campbell WB, Li PL. Production and metabolism of ceramide in normal and ischemic-reperfused myocardium of rats. Basic Res Cardiol. 2001;96(3):267–74. doi: 10.1007/s003950170057. [DOI] [PubMed] [Google Scholar]
- 64.Cordis GA, Yoshida T, Das DK. HPTLC analysis of sphingomylein, ceramide and sphingosine in ischemic/reperfused rat heart. J Pharm Biomed Anal. 1998;16(7):1189–93. doi: 10.1016/s0731-7085(97)00260-4. [DOI] [PubMed] [Google Scholar]
- 65.Cui J, Engelman RM, Maulik N, Das DK. Role of ceramide in ischemic preconditioning. J Am Coll Surg. 2004;198(5):770–7. doi: 10.1016/j.jamcollsurg.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 66.Der P, Cui J, Das DK. Role of lipid rafts in ceramide and nitric oxide signaling in the ischemic and preconditioned hearts. J Mol Cell Cardiol. 2006;40(2):313–20. doi: 10.1016/j.yjmcc.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Xu X, Bittman R, Duportail G, Heissler D, Vilcheze C, London E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). Comparison of cholesterol to plant, fungal, and disease-associated sterols and comparison of sphingomyelin, cerebrosides, and ceramide. J Biol Chem. 2001;276(36):33540–6. doi: 10.1074/jbc.M104776200. [DOI] [PubMed] [Google Scholar]
- 68.Zundel W, Swiersz LM, Giaccia A. Caveolin 1-mediated regulation of receptor tyrosine kinase-associated phosphatidylinositol 3-kinase activity by ceramide. Mol Cell Biol. 2000;20(5):1507–14. doi: 10.1128/mcb.20.5.1507-1514.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res. 2007;100(1):41–9. doi: 10.1161/01.RES.0000253900.66640.34. [DOI] [PubMed] [Google Scholar]
- 70.Bianchi P, Kunduzova O, Masini E, Cambon C, Bani D, Raimondi L, Seguelas MH, Nistri S, Colucci W, Leducq N, Parini A. Oxidative stress by monoamine oxidase mediates receptor-independent cardiomyocyte apoptosis by serotonin and postischemic myocardial injury. Circulation. 2005;112(21):3297–305. doi: 10.1161/CIRCULATIONAHA.104.528133. [DOI] [PubMed] [Google Scholar]
- 71.Beresewicz A, Dobrzyn A, Gorski J. Accumulation of specific ceramides in ischemic/reperfused rat heart; effect of ischemic preconditioning. J Physiol Pharmacol. 2002;53(3):371–82. [PubMed] [Google Scholar]
- 72.Hernandez OM, Discher DJ, Bishopric NH, Webster KA. Rapid activation of neutral sphingomyelinase by hypoxia-reoxygenation of cardiac myocytes. Circ Res. 2000;86(2):198–204. doi: 10.1161/01.res.86.2.198. [DOI] [PubMed] [Google Scholar]
- 73.O’Brien NW, Gellings NM, Guo M, Barlow SB, Glembotski CC, Sabbadini RA. Factor associated with neutral sphingomyelinase activation and its role in cardiac cell death. Circ Res. 2003;92(6):589–91. doi: 10.1161/01.RES.0000066290.29715.67. [DOI] [PubMed] [Google Scholar]
- 74.Nakane M, Kubota M, Nakagomi T, Tamura A, Hisaki H, Shimasaki H, Ueta N. Lethal forebrain ischemia stimulates sphingomyelin hydrolysis and ceramide generation in the gerbil hippocampus. Neurosci Lett. 2000;296(2–3):89–92. doi: 10.1016/s0304-3940(00)01655-4. [DOI] [PubMed] [Google Scholar]
- 75.Kubota M, Kitahara S, Shimasaki H, Ueta N. Accumulation of ceramide in ischemic human brain of an acute case of cerebral occlusion. Jpn J Exp Med. 1989;59(2):59–64. [PubMed] [Google Scholar]
- 76.Herr I, Martin-Villalba A, Kurz E, Roncaioli P, Schenkel J, Cifone MG, Debatin KM. FK506 prevents stroke-induced generation of ceramide and apoptosis signaling. Brain Res. 1999;826(2):210–9. doi: 10.1016/s0006-8993(99)01288-3. [DOI] [PubMed] [Google Scholar]
- 77.Kubota M, Narita K, Nakagomi T, Tamura A, Shimasaki H, Ueta N, Yoshida S. Sphingomyelin changes in rat cerebral cortex during focal ischemia. Neurol Res. 1996;18(4):337–41. doi: 10.1080/01616412.1996.11740432. [DOI] [PubMed] [Google Scholar]
- 78.Takahashi K, Ginis I, Nishioka R, Klimanis D, Barone FC, White RF, Chen Y, Hallenbeck JM. Glucosylceramide synthase activity and ceramide levels are modulated during cerebral ischemia after ischemic preconditioning. J Cereb Blood Flow Metab. 2004;24(6):623–7. doi: 10.1097/01.WCB.0000119990.06999.A9. [DOI] [PubMed] [Google Scholar]
- 79.Yu ZF, Nikolova-Karakashian M, Zhou D, Cheng G, Schuchman EH, Mattson MP. Pivotal role for acidic sphingomyelinase in cerebral ischemia-induced ceramide and cytokine production, and neuronal apoptosis. J Mol Neurosci. 2000;15(2):85–97. doi: 10.1385/JMN:15:2:85. [DOI] [PubMed] [Google Scholar]
- 80.Ohtani R, Tomimoto H, Kondo T, Wakita H, Akiguchi I, Shibasaki H, Okazaki T. Upregulation of ceramide and its regulating mechanism in a rat model of chronic cerebral ischemia. Brain Res. 2004;1023(1):31–40. doi: 10.1016/j.brainres.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 81.Chan PH. Future targets and cascades for neuroprotective strategies. Stroke. 2004;35(11 Suppl 1):2748–50. doi: 10.1161/01.STR.0000143325.25610.ac. [DOI] [PubMed] [Google Scholar]
- 82.Gustafsson AB, Gottlieb RA. Eat your heart out: Role of autophagy in myocardial ischemia/reperfusion. Autophagy. 2008;4(4):416–21. doi: 10.4161/auto.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ginis I, Schweizer U, Brenner M, Liu J, Azzam N, Spatz M, Hallenbeck JM. TNF-alpha pretreatment prevents subsequent activation of cultured brain cells with TNF-alpha and hypoxia via ceramide. Am J Physiol. 1999;276(5 Pt 1):C1171–83. doi: 10.1152/ajpcell.1999.276.5.C1171. [DOI] [PubMed] [Google Scholar]
- 84.Liu J, Ginis I, Spatz M, Hallenbeck JM. Hypoxic preconditioning protects cultured neurons against hypoxic stress via TNF-alpha and ceramide. Am J Physiol Cell Physiol. 2000;278(1):C144–53. doi: 10.1152/ajpcell.2000.278.1.C144. [DOI] [PubMed] [Google Scholar]
- 85.Jin J, Hou Q, Mullen TD, Zeidan YH, Bielawski J, Kraveka JM, Bielawska A, Obeid LM, Hannun YA, Hsu YT. Ceramide generated by sphingomyelin hydrolysis and the salvage pathway is involved in hypoxia/reoxygenation-induced Bax redistribution to mitochondria in NT-2 cells. J Biol Chem. 2008 doi: 10.1074/jbc.M801597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Ginis I, Hallenbeck JM. The protective effect of ceramide in immature rat brain hypoxia-ischemia involves up-regulation of bcl-2 and reduction of TUNEL-positive cells. J Cereb Blood Flow Metab. 2001;21(1):34–40. doi: 10.1097/00004647-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 87.Furuya K, Ginis I, Takeda H, Chen Y, Hallenbeck JM. Cell permeable exogenous ceramide reduces infarct size in spontaneously hypertensive rats supporting in vitro studies that have implicated ceramide in induction of tolerance to ischemia. J Cereb Blood Flow Metab. 2001;21(3):226–32. doi: 10.1097/00004647-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 88.Venkataraman K, Thangada S, Michaud J, Oo ML, Ai Y, Lee YM, Wu M, Parikh NS, Khan F, Proia RL, Hla T. Extracellular export of sphingosine kinase-1a contributes to the vascular S1P gradient. Biochem J. 2006;397(3):461–71. doi: 10.1042/BJ20060251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hait NC, Oskeritzian CA, Paugh SW, Milstien S, Spiegel S. Sphingosine kinases, sphingosine 1-phosphate, apoptosis and diseases. Biochim Biophys Acta. 2006;1758(12):2016–26. doi: 10.1016/j.bbamem.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 90.Bradham CA, Stachlewitz RF, Gao W, Qian T, Jayadev S, Jenkins G, Hannun Y, Lemasters JJ, Thurman RG, Brenner DA. Reperfusion after liver transplantation in rats differentially activates the mitogen-activated protein kinases. Hepatology. 1997;25(5):1128–35. doi: 10.1002/hep.510250514. [DOI] [PubMed] [Google Scholar]
- 91.Llacuna L, Mari M, Garcia-Ruiz C, Fernandez-Checa JC, Morales A. Critical role of acidic sphingomyelinase in murine hepatic ischemia-reperfusion injury. Hepatology. 2006;44(3):561–72. doi: 10.1002/hep.21285. [DOI] [PubMed] [Google Scholar]
- 92.Zager RA, Iwata M, Conrad DS, Burkhart KM, Igarashi Y. Altered ceramide and sphingosine expression during the induction phase of ischemic acute renal failure. Kidney Int. 1997;52(1):60–70. doi: 10.1038/ki.1997.304. [DOI] [PubMed] [Google Scholar]
- 93.Zager RA, Conrad S, Lochhead K, Sweeney EA, Igarashi Y, Burkhart KM. Altered sphingomyelinase and ceramide expression in the setting of ischemic and nephrotoxic acute renal failure. Kidney Int. 1998;53(3):573–82. doi: 10.1046/j.1523-1755.1998.00772.x. [DOI] [PubMed] [Google Scholar]
- 94.Kalhorn T, Zager RA. Renal cortical ceramide patterns during ischemic and toxic injury: assessments by HPLC-mass spectrometry. Am J Physiol. 1999;277(5 Pt 2):F723–33. doi: 10.1152/ajprenal.1999.277.5.F723. [DOI] [PubMed] [Google Scholar]
- 95.Nicotera P, Lipton SA. Excitotoxins in neuronal apoptosis and necrosis. J Cereb Blood Flow Metab. 1999;19(6):583–91. doi: 10.1097/00004647-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 96.Li Y, Powers C, Jiang N, Chopp M. Intact, injured, necrotic and apoptotic cells after focal cerebral ischemia in the rat. J Neurol Sci. 1998;156(2):119–32. doi: 10.1016/s0022-510x(98)00036-7. [DOI] [PubMed] [Google Scholar]
- 97.Li Y, Chopp M, Jiang N, Zaloga C. In situ detection of DNA fragmentation after focal cerebral ischemia in mice. Brain Res Mol Brain Res. 1995;28(1):164–8. doi: 10.1016/0169-328x(94)00220-9. [DOI] [PubMed] [Google Scholar]
- 98.Li Y, Chopp M, Jiang N, Zhang ZG, Zaloga C. Induction of DNA fragmentation after 10 to 120 minutes of focal cerebral ischemia in rats. Stroke. 1995;26(7):1252–7. doi: 10.1161/01.str.26.7.1252. discussion 1257–8. [DOI] [PubMed] [Google Scholar]
- 99.Kuroda S, Katsura K, Hillered L, Bates TE, Siesjo BK. Delayed treatment with alpha-phenyl-N-tert-butyl nitrone (PBN) attenuates secondary mitochondrial dysfunction after transient focal cerebral ischemia in the rat. Neurobiol Dis. 1996;3(2):149–57. doi: 10.1006/nbdi.1996.0015. [DOI] [PubMed] [Google Scholar]
- 100.Nakai A, Kuroda S, Kristian T, Siesjo BK. The immunosuppressant drug FK506 ameliorates secondary mitochondrial dysfunction following transient focal cerebral ischemia in the rat. Neurobiol Dis. 1997;4(3–4):288–300. doi: 10.1006/nbdi.1997.0146. [DOI] [PubMed] [Google Scholar]
- 101.Morales A, Lee H, Goni FM, Kolesnick R, Fernandez-Checa JC. Sphingolipids and cell death. Apoptosis. 2007;12(5):923–39. doi: 10.1007/s10495-007-0721-0. [DOI] [PubMed] [Google Scholar]
- 102.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3(11):E255–63. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 103.Brenner C, Kroemer G. Apoptosis. Mitochondria--the death signal integrators. Science. 2000;289(5482):1150–1. doi: 10.1126/science.289.5482.1150. [DOI] [PubMed] [Google Scholar]
- 104.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87(1):99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- 105.Taha TA, Mullen TD, Obeid LM. A house divided: Ceramide, sphingosine, and sphingosine-1-phosphate in programmed cell death. Biochim Biophys Acta. 2006;1758(12):2027–36. doi: 10.1016/j.bbamem.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hearps AC, Burrows J, Connor CE, Woods GM, Lowenthal RM, Ragg SJ. Mitochondrial cytochrome c release precedes transmembrane depolarisation and caspase-3 activation during ceramide-induced apoptosis of Jurkat T cells. Apoptosis. 2002;7(5):387–94. doi: 10.1023/a:1020034906200. [DOI] [PubMed] [Google Scholar]
- 107.Zhang X, Li B, Zhang Y, Liu J. Ceramide induces release of mitochondrial proapoptotic proteins in caspase-dependent and -independent manner in HT-29 cells. Sci China C Life Sci. 2008;51(1):66–71. doi: 10.1007/s11427-008-0015-y. [DOI] [PubMed] [Google Scholar]
- 108.Won JS, Singh I. Sphingolipid signaling and redox regulation. Free Radic Biol Med. 2006;40(11):1875–88. doi: 10.1016/j.freeradbiomed.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 109.Gendron MC, Schrantz N, Metivier D, Kroemer G, Maciorowska Z, Sureau F, Koester S, Petit PX. Oxidation of pyridine nucleotides during Fas- and ceramide-induced apoptosis in Jurkat cells: correlation with changes in mitochondria, glutathione depletion, intracellular acidification and caspase 3 activation. Biochem J. 2001;353(Pt 2):357–67. [PMC free article] [PubMed] [Google Scholar]
- 110.Lin CF, Chen CL, Chang WT, Jan MS, Hsu LJ, Wu RH, Tang MJ, Chang WC, Lin YS. Sequential caspase-2 and caspase-8 activation upstream of mitochondria during ceramideand etoposide-induced apoptosis. J Biol Chem. 2004;279(39):40755–61. doi: 10.1074/jbc.M404726200. [DOI] [PubMed] [Google Scholar]
- 111.Zamzami N, Marchetti P, Castedo M, Decaudin D, Macho A, Hirsch T, Susin SA, Petit PX, Mignotte B, Kroemer G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J Exp Med. 1995;182(2):367–77. doi: 10.1084/jem.182.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stoica BA, Movsesyan VA, Lea PMt, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22(3):365–82. doi: 10.1016/s1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- 113.Pastorino JG, Simbula G, Yamamoto K, Glascott PA, Jr, Rothman RJ, Farber JL. The cytotoxicity of tumor necrosis factor depends on induction of the mitochondrial permeability transition. J Biol Chem. 1996;271(47):29792–8. doi: 10.1074/jbc.271.47.29792. [DOI] [PubMed] [Google Scholar]
- 114.Pacher P, Hajnoczky G. Propagation of the apoptotic signal by mitochondrial waves. Embo J. 2001;20(15):4107–21. doi: 10.1093/emboj/20.15.4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Geley S, Hartmann BL, Kofler R. Ceramides induce a form of apoptosis in human acute lymphoblastic leukemia cells that is inhibited by Bcl-2, but not by CrmA. FEBS Lett. 1997;400(1):15–8. doi: 10.1016/s0014-5793(96)01284-7. [DOI] [PubMed] [Google Scholar]
- 116.Zhang J, Alter N, Reed JC, Borner C, Obeid LM, Hannun YA. Bcl-2 interrupts the ceramide-mediated pathway of cell death. Proc Natl Acad Sci U S A. 1996;93(11):5325–8. doi: 10.1073/pnas.93.11.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274(32):22532–8. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 118.Birbes H, Luberto C, Hsu YT, El Bawab S, Hannun YA, Obeid LM. A mitochondrial pool of sphingomyelin is involved in TNFalpha-induced Bax translocation to mitochondria. Biochem J. 2005;386(Pt 3):445–51. doi: 10.1042/BJ20041627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dai Q, Liu J, Chen J, Durrant D, McIntyre TM, Lee RM. Mitochondrial ceramide increases in UV-irradiated HeLa cells and is mainly derived from hydrolysis of sphingomyelin. Oncogene. 2004;23(20):3650–8. doi: 10.1038/sj.onc.1207430. [DOI] [PubMed] [Google Scholar]
- 120.Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272(17):11369–77. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- 121.Snook CF, Jones JA, Hannun YA. Sphingolipid-binding proteins. Biochim Biophys Acta. 2006;1761(8):927–46. doi: 10.1016/j.bbalip.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 122.Heinrich M, Neumeyer J, Jakob M, Hallas C, Tchikov V, Winoto-Morbach S, Wickel M, Schneider-Brachert W, Trauzold A, Hethke A, Schutze S. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11(5):550–63. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 123.Heinrich M, Wickel M, Schneider-Brachert W, Sandberg C, Gahr J, Schwandner R, Weber T, Saftig P, Peters C, Brunner J, Kronke M, Schutze S. Cathepsin D targeted by acid sphingomyelinase-derived ceramide. Embo J. 1999;18(19):5252–63. doi: 10.1093/emboj/18.19.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bidere N, Lorenzo HK, Carmona S, Laforge M, Harper F, Dumont C, Senik A. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J Biol Chem. 2003;278(33):31401–11. doi: 10.1074/jbc.M301911200. [DOI] [PubMed] [Google Scholar]
- 125.Xin M, Deng X. Protein phosphatase 2A enhances the proapoptotic function of Bax through dephosphorylation. J Biol Chem. 2006;281(27):18859–67. doi: 10.1074/jbc.M512543200. [DOI] [PubMed] [Google Scholar]
- 126.Ruvolo PP, Deng X, Ito T, Carr BK, May WS. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J Biol Chem. 1999;274(29):20296–300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]
- 127.Garcia A, Cayla X, Guergnon J, Dessauge F, Hospital V, Rebollo MP, Fleischer A, Rebollo A. Serine/threonine protein phosphatases PP1 and PP2A are key players in apoptosis. Biochimie. 2003;85(8):721–6. doi: 10.1016/j.biochi.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 128.Millward TA, Zolnierowicz S, Hemmings BA. Regulation of protein kinase cascades by protein phosphatase 2A. Trends Biochem Sci. 1999;24(5):186–91. doi: 10.1016/s0968-0004(99)01375-4. [DOI] [PubMed] [Google Scholar]
- 129.Datta SR, Dudek H, Tao X, Masters S, Fu H, Gotoh Y, Greenberg ME. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell. 1997;91(2):231–41. doi: 10.1016/s0092-8674(00)80405-5. [DOI] [PubMed] [Google Scholar]
- 130.Chiang CW, Kanies C, Kim KW, Fang WB, Parkhurst C, Xie M, Henry T, Yang E. Protein phosphatase 2A dephosphorylation of phosphoserine 112 plays the gatekeeper role for BAD-mediated apoptosis. Mol Cell Biol. 2003;23(18):6350–62. doi: 10.1128/MCB.23.18.6350-6362.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kashkar H, Wiegmann K, Yazdanpanah B, Haubert D, Kronke M. Acid sphingomyelinase is indispensable for UV light-induced Bax conformational change at the mitochondrial membrane. J Biol Chem. 2005;280(21):20804–13. doi: 10.1074/jbc.M410869200. [DOI] [PubMed] [Google Scholar]
- 132.Pastorino JG, Tafani M, Rothman RJ, Marcinkeviciute A, Hoek JB, Farber JL, Marcineviciute A. Functional consequences of the sustained or transient activation by Bax of the mitochondrial permeability transition pore. J Biol Chem. 1999;274(44):31734–9. doi: 10.1074/jbc.274.44.31734. [DOI] [PubMed] [Google Scholar]
- 133.Sumitomo M, Ohba M, Asakuma J, Asano T, Kuroki T, Hayakawa M. Protein kinase Cdelta amplifies ceramide formation via mitochondrial signaling in prostate cancer cells. J Clin Invest. 2002;109(6):827–36. doi: 10.1172/JCI14146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Huwiler A, Fabbro D, Pfeilschifter J. Selective ceramide binding to protein kinase C-alpha and -delta isoenzymes in renal mesangial cells. Biochemistry. 1998;37(41):14556–62. doi: 10.1021/bi981401i. [DOI] [PubMed] [Google Scholar]
- 135.Murriel CL, Churchill E, Inagaki K, Szweda LI, Mochly-Rosen D. Protein kinase Cdelta activation induces apoptosis in response to cardiac ischemia and reperfusion damage: a mechanism involving BAD and the mitochondria. J Biol Chem. 2004;279(46):47985–91. doi: 10.1074/jbc.M405071200. [DOI] [PubMed] [Google Scholar]
- 136.Kong JY, Klassen SS, Rabkin SW. Ceramide activates a mitochondrial p38 mitogen-activated protein kinase: a potential mechanism for loss of mitochondrial transmembrane potential and apoptosis. Mol Cell Biochem. 2005;278(1–2):39–51. doi: 10.1007/s11010-005-1979-6. [DOI] [PubMed] [Google Scholar]
- 137.Capano M, Crompton M. Bax translocates to mitochondria of heart cells during simulated ischaemia: involvement of AMP-activated and p38 mitogen-activated protein kinases. Biochem J. 2006;395(1):57–64. doi: 10.1042/BJ20051654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cai B, Chang SH, Becker EB, Bonni A, Xia Z. p38 MAP kinase mediates apoptosis through phosphorylation of BimEL at Ser-65. J Biol Chem. 2006;281(35):25215–22. doi: 10.1074/jbc.M512627200. [DOI] [PubMed] [Google Scholar]
- 139.Kurinna SM, Tsao CC, Nica AF, Jiffar T, Ruvolo PP. Ceramide promotes apoptosis in lung cancer-derived A549 cells by a mechanism involving c-Jun NH2-terminal kinase. Cancer Res. 2004;64(21):7852–6. doi: 10.1158/0008-5472.CAN-04-1552. [DOI] [PubMed] [Google Scholar]
- 140.Eminel S, Klettner A, Roemer L, Herdegen T, Waetzig V. JNK2 translocates to the mitochondria and mediates cytochrome c release in PC12 cells in response to 6-hydroxydopamine. J Biol Chem. 2004;279(53):55385–92. doi: 10.1074/jbc.M405858200. [DOI] [PubMed] [Google Scholar]
- 141.Kharbanda S, Saxena S, Yoshida K, Pandey P, Kaneki M, Wang Q, Cheng K, Chen YN, Campbell A, Sudha T, Yuan ZM, Narula J, Weichselbaum R, Nalin C, Kufe D. Translocation of SAPK/JNK to mitochondria and interaction with Bcl-x(L) in response to DNA damage. J Biol Chem. 2000;275(1):322–7. doi: 10.1074/jbc.275.1.322. [DOI] [PubMed] [Google Scholar]
- 142.Lei K, Davis RJ. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A. 2003;100(5):2432–7. doi: 10.1073/pnas.0438011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Weston CR, Davis RJ. The JNK signal transduction pathway. Curr Opin Cell Biol. 2007;19(2):142–9. doi: 10.1016/j.ceb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 144.Armstrong JS. Mitochondrial membrane permeabilization: the sine qua non for cell death. Bioessays. 2006;28(3):253–60. doi: 10.1002/bies.20370. [DOI] [PubMed] [Google Scholar]
- 145.Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- 146.Giorgi C, Romagnoli A, Pinton P, Rizzuto R. Ca2+ signaling, mitochondria and cell death. Curr Mol Med. 2008;8(2):119–30. doi: 10.2174/156652408783769571. [DOI] [PubMed] [Google Scholar]
- 147.Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science. 2003;300(5616):135–9. doi: 10.1126/science.1081208. [DOI] [PubMed] [Google Scholar]
- 148.Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. Embo J. 2001;20(11):2690–701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gudz TI, Tserng KY, Hoppel CL. Direct inhibition of mitochondrial respiratory chain complex III by cell-permeable ceramide. J Biol Chem. 1997;272(39):24154–8. doi: 10.1074/jbc.272.39.24154. [DOI] [PubMed] [Google Scholar]
- 150.Di Paola M, Cocco T, Lorusso M. Ceramide interaction with the respiratory chain of heart mitochondria. Biochemistry. 2000;39(22):6660–8. doi: 10.1021/bi9924415. [DOI] [PubMed] [Google Scholar]
- 151.Quillet-Mary A, Jaffrezou JP, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J Biol Chem. 1997;272(34):21388–95. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- 152.Andrieu-Abadie N, Gouaze V, Salvayre R, Levade T. Ceramide in apoptosis signaling: relationship with oxidative stress. Free Radic Biol Med. 2001;31(6):717–28. doi: 10.1016/s0891-5849(01)00655-4. [DOI] [PubMed] [Google Scholar]
- 153.Ott M, Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria, oxidative stress and cell death. Apoptosis. 2007;12(5):913–22. doi: 10.1007/s10495-007-0756-2. [DOI] [PubMed] [Google Scholar]
- 154.Therade-Matharan S, Laemmel E, Carpentier S, Obata Y, Levade T, Duranteau J, Vicaut E. Reactive oxygen species production by mitochondria in endothelial cells exposed to reoxygenation after hypoxia and glucose depletion is mediated by ceramide. Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1756–62. doi: 10.1152/ajpregu.00480.2004. [DOI] [PubMed] [Google Scholar]
- 155.Siskind LJ, Kolesnick RN, Colombini M. Ceramide channels increase the permeability of the mitochondrial outer membrane to small proteins. J Biol Chem. 2002;277(30):26796–803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6(3):118–25. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Di Paola M, Zaccagnino P, Montedoro G, Cocco T, Lorusso M. Ceramide induces release of pro-apoptotic proteins from mitochondria by either a Ca2+-dependent or a Ca2+-independent mechanism. J Bioenerg Biomembr. 2004;36(2):165–70. doi: 10.1023/b:jobb.0000023619.97392.0c. [DOI] [PubMed] [Google Scholar]
- 158.Ghafourifar P, Klein SD, Schucht O, Schenk U, Pruschy M, Rocha S, Richter C. Ceramide induces cytochrome c release from isolated mitochondria. Importance of mitochondrial redox state. J Biol Chem. 1999;274(10):6080–4. doi: 10.1074/jbc.274.10.6080. [DOI] [PubMed] [Google Scholar]
- 159.Montes LR, Ruiz-Arguello MB, Goni FM, Alonso A. Membrane restructuring via ceramide results in enhanced solute efflux. J Biol Chem. 2002;277(14):11788–94. doi: 10.1074/jbc.M111568200. [DOI] [PubMed] [Google Scholar]
- 160.Siskind LJ, Feinstein L, Yu T, Davis JS, Jones D, Choi J, Zuckerman JE, Tan W, Hill RB, Hardwick JM, Colombini M. Anti-apoptotic Bcl-2 Family Proteins Disassemble Ceramide Channels. J Biol Chem. 2008;283(11):6622–30. doi: 10.1074/jbc.M706115200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yuan HWS, Adachi S, Oltersdorf T, Gottlieb RA. Cytochrome c dissociation and release from mitochondria by trancated Bid and ceramide. Mitochondrion. 2003;2:237–244. doi: 10.1016/S1567-7249(02)00106-X. [DOI] [PubMed] [Google Scholar]
- 162.Kristal BS, Brown AM. Apoptogenic ganglioside GD3 directly induces the mitochondrial permeability transition. J Biol Chem. 1999;274(33):23169–75. doi: 10.1074/jbc.274.33.23169. [DOI] [PubMed] [Google Scholar]
- 163.Novgorodov SA, Szulc ZM, Luberto C, Jones JA, Bielawski J, Bielawska A, Hannun YA, Obeid LM. Positively charged ceramide is a potent inducer of mitochondrial permeabilization. J Biol Chem. 2005;280(16):16096–105. doi: 10.1074/jbc.M411707200. [DOI] [PubMed] [Google Scholar]
- 164.Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. Embo J. 1999;18(22):6349–61. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zeidan YH, Wu BX, Jenkins RW, Obeid LM, Hannun YA. A novel role for protein kinase Cdelta-mediated phosphorylation of acid sphingomyelinase in UV light-induced mitochondrial injury. Faseb J. 2008;22(1):183–93. doi: 10.1096/fj.07-8967com. [DOI] [PubMed] [Google Scholar]
- 166.Parra V, Eisner V, Chiong M, Criollo A, Moraga F, Garcia A, Hartel S, Jaimovich E, Zorzano A, Hidalgo C, Lavandero S. Changes in mitochondrial dynamics during ceramide-induced cardiomyocyte early apoptosis. Cardiovasc Res. 2008;77(2):387–97. doi: 10.1093/cvr/cvm029. [DOI] [PubMed] [Google Scholar]
- 167.Suen DF, Norris KL, Youle RJ. Mitochondrial dynamics and apoptosis. Genes Dev. 2008;22(12):1577–90. doi: 10.1101/gad.1658508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rippo MR, Malisan F, Ravagnan L, Tomassini B, Condo I, Costantini P, Susin SA, Rufini A, Todaro M, Kroemer G, Testi R. GD3 ganglioside directly targets mitochondria in a bcl-2-controlled fashion. Faseb J. 2000;14(13):2047–54. doi: 10.1096/fj.99-1028com. [DOI] [PubMed] [Google Scholar]
- 169.Colell A, Garcia-Ruiz C, Roman J, Ballesta A, Fernandez-Checa JC. Ganglioside GD3 enhances apoptosis by suppressing the nuclear factor-kappa B-dependent survival pathway. Faseb J. 2001;15(6):1068–70. doi: 10.1096/fj.00-0574fje. [DOI] [PubMed] [Google Scholar]
- 170.Scorrano L, Petronilli V, Di Lisa F, Bernardi P. Commitment to apoptosis by GD3 ganglioside depends on opening of the mitochondrial permeability transition pore. J Biol Chem. 1999;274(32):22581–5. doi: 10.1074/jbc.274.32.22581. [DOI] [PubMed] [Google Scholar]
- 171.Garcia-Ruiz C, Colell A, Paris R, Fernandez-Checa JC. Direct interaction of GD3 ganglioside with mitochondria generates reactive oxygen species followed by mitochondrial permeability transition, cytochrome c release, and caspase activation. Faseb J. 2000;14(7):847–58. doi: 10.1096/fasebj.14.7.847. [DOI] [PubMed] [Google Scholar]
- 172.Inoki Y, Miura T, Kajimoto T, Kawase M, Kawase Y, Yoshida Y, Tsuji S, Kinouchi T, Endo H, Kagawa Y, Hamamoto T. Ganglioside GD3 and its mimetics induce cytochrome c release from mitochondria. Biochem Biophys Res Commun. 2000;276(3):1210–6. doi: 10.1006/bbrc.2000.3601. [DOI] [PubMed] [Google Scholar]
- 173.De Maria R, Lenti L, Malisan F, d’Agostino F, Tomassini B, Zeuner A, Rippo MR, Testi R. Requirement for GD3 ganglioside in CD95- and ceramide-induced apoptosis. Science. 1997;277(5332):1652–5. doi: 10.1126/science.277.5332.1652. [DOI] [PubMed] [Google Scholar]
- 174.Cuvillier O, Edsall L, Spiegel S. Involvement of sphingosine in mitochondria-dependent Fas-induced apoptosis of type II Jurkat T cells. J Biol Chem. 2000;275(21):15691–700. doi: 10.1074/jbc.M000280200. [DOI] [PubMed] [Google Scholar]
- 175.Scorrano L, Penzo D, Petronilli V, Pagano F, Bernardi P. Arachidonic acid causes cell death through the mitochondrial permeability transition. Implications for tumor necrosis factor-alpha aopototic signaling. J Biol Chem. 2001;276(15):12035–40. doi: 10.1074/jbc.M010603200. [DOI] [PubMed] [Google Scholar]
- 176.Broekemeier KM, Pfeiffer DR. Inhibition of the mitochondrial permeability transition by cyclosporin A during long time frame experiments: relationship between pore opening and the activity of mitochondrial phospholipases. Biochemistry. 1995;34(50):16440–9. doi: 10.1021/bi00050a027. [DOI] [PubMed] [Google Scholar]
- 177.Elrick MJ, Fluss S, Colombini M. Sphingosine, a product of ceramide hydrolysis, influences the formation of ceramide channels. Biophys J. 2006;91(5):1749–56. doi: 10.1529/biophysj.106.088443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Phillips DC, Martin S, Doyle BT, Houghton JA. Sphingosine-induced apoptosis in rhabdomyosarcoma cell lines is dependent on pre-mitochondrial Bax activation and post-mitochondrial caspases. Cancer Res. 2007;67(2):756–64. doi: 10.1158/0008-5472.CAN-06-2374. [DOI] [PubMed] [Google Scholar]
- 179.Hassoun SM, Lancel S, Petillot P, Decoster B, Favory R, Marchetti P, Neviere R. Sphingosine impairs mitochondrial function by opening permeability transition pore. Mitochondrion. 2006;6(3):149–54. doi: 10.1016/j.mito.2006.05.001. [DOI] [PubMed] [Google Scholar]