Abstract
We have developed the Mycobacterium tuberculosis (Mtb) fusion protein (ID83), which contains the three Mtb proteins Rv1813, Rv3620 and Rv2608. We evaluated the immunogenicity and protective efficacy of ID83 in combination with several emulsion-formulated Toll-like receptor agonists. The ID83 subunit vaccines containing synthetic TLR4 or TLR9 agonists generated a T helper-1 immune response and protected mice against challenge with Mtb regardless of route. The ID83 vaccine formulated with gardiquimod (a TLR7 agonist) also resulted in a protective response when administered intradermally, whereas the same vaccine given subcutaneously failed to provide protection. This highlights the need to explore different routes of immunization based on the adjuvant formulations used.
Keywords: Mycobacterium tuberculosis, Subunit Vaccine, TLR agonists
1. Introduction
Approximately one-third of the world’s population has been exposed to Mycobacterium tuberculosis (Mtb), and 9.2 million new cases of tuberculosis (TB) were reported in 2006 [1]. The combination of increasing numbers of HIV-positive patients with combined TB infections, patients with multi-drug resistant TB (MDR-TB), the enormous expense and length of time it takes to treat individuals with either TB or MDR-TB and the breakdown of TB infrastructure for control of TB are all challenging obstacles to reducing Mtb infection [2, 3]. In high risk countries, BCG is given to infants as soon after birth as possible [4]. Although childhood BCG immunizations protect against serious forms of TB in most children, protection against adult pulmonary TB ranges from 0 to 80% [4]. Development of an effective vaccine that could either boost the existing TB vaccine Bacillus Calmette-Guerin (BCG), or that could be used as a therapeutic vaccine combined with an antibiotic regimen to shorten treatment, could contribute to the reduction of disease and decrease drug and medical care costs.
We have recently developed a vaccine called ID83, which is produced from the fusion of three individual tuberculosis genes: Rv1813, Rv3620, and Rv2608. Each of these individual proteins has recently been tested against aerosol challenge with Mtb [5]. In this paper we combine ID83 with different TLR agonists and test vaccine efficacy in the mouse model of tuberculosis.
A variety of factors have plagued efforts to develop vaccines against TB including safety, efficacy and longevity of the immune response generated by the vaccine. Vaccine protection against infection with Mtb in animal models has only been observed when a potent T helper (Th1)-type cell-mediated response is generated [4, 6, 7]. A protective role of cytotoxic CD8 T cells (CTL) has also been shown [8, 9]. While IFN-γ production alone is not a predictive factor for the positive outcome of a vaccine, the importance of IFN-γ has been shown in IFN-γ knock-out mice where the lack of IFN-γ results in uncontrolled growth of Mtb [10, 11]. Besides IFN-γ, TNF plays a critical role in the containment of Mtb following infection in the lung. TNF increases the production of chemokines involved in the recruitment of cells that keep Mtb-infected macrophages within the confines of granulomas in the lung [12]. The protective role of TNF in the immune response to Mtb has been demonstrated in mice with defects in genes for TNF and the 55 kDa receptor subunit TNF RII (55 kDa subunit) [13, 14]. The report of TB reactivation in rheumatoid arthritis patients who received long-term therapy with anti-TNF antibodies emphasizes the importance of this cytokine in protection against TB in humans [15].
TLR agonists are being investigated in our studies for use as vaccine adjuvants. Natural TLR ligands from microbe-derived antigens are able to induce dendritic cell (DC) maturation and can lead to the initiation of adaptive immune responses [16]. There are many mycobacterial-associated TLRs ligands including the glycolipid lipoarabinomannan (LAM) containing highly branched arabinofuranosyl side chains (AraLAM) (present in fast growing Mycobacteria), phosphatidyl-myo-inositol, and the 19-kDa lipoprotein which are all TLR2 ligands, a heat-sensitive cell wall component that can stimulate TLR4, and mycobacterial DNA which is recognized by TLR9 [17–20]. Interestingly, LAM from Mtb and M. bovis BCG that are terminally capped with mannose (ManLAM) are not able to activate cells in a TLR-dependent manner [19]. In this study we tested ID83 with TLR agonists formulated in an oil-in-water emulsion to determine which adjuvant systems were most efficacious. We characterized the immune responses and protective effects following delivery of these vaccines by either the subcutaneous (s.c.) or intradermal (i.d.) route. The ID83 vaccine in combination with any of the TLR agonist/adjuvants was capable of inducing IFN-γ, and ID83 plus either the TLR4 or TLR9 agonist was able to protect mice against Mtb infection regardless of the route of immunization. In contrast, adjuvants containing gardiquimod (TLR7 agonist) were only effective when the ID83 subunit vaccine was given i.d. Thus, we determined that protection against Mtb challenge in a mouse model with ID83 was route-dependent based on the TLR agonists that were included in the adjuvant formulation.
2. Materials and Methods
2.1. ID83 cloning strategy
ID83 was generated through a tandem fusion of the individual cloned and amplified genes of Rv1813, Rv3620, and Rv2608 using restriction site linkers. The recombinant pET28a plasmids (Novagen, Madison, WI) containing the individual Rv1813, Rv3620, and Rv2608 genes were previously described [5]. ID83 PCR primers were designed to incorporate specific restriction enzyme sites 5’ and 3’ of the gene of interest with primer sequences as follows: Rv1813-5’NdeI:CAATTACATATGGGTACCCATCTCGCCA-ACGGTTCGATG; Rvl813-3’SacI, CAATTAGAGCTCGTTGCACGCCCAGTTGAC-GAT; Rv3620-5’SacI, CAATTAGAGCTCATGACCTCGCGTTTTATGACG; Rv3620-3’SalI, CAATTAGTCGACGCTGCTGAGGATCTGCTGGGA; Rv2608-5’SalI, CAATT-AGTCGACATGAATTTCGCCGTTTTGCCG; and Rv2608-3’HindIII, CAATTAAAGC-TTTTAAGTACTGAAAAGTCGGGGTAGCGCCG. The DNA sequences were amplified from plasmid DNA templates using Pfx DNA polymerase (Invitrogen, Carlsbad, CA) with 30 cycles at 94°C for 15 sec, 58°C for 30 sec and 68°C for 1h 30 min. The Rv1813 PCR product was digested with NdeI/SacI restriction enzymes then cloned into the pET28a vector. Rv3620 and the Rv1813pET construct were digested with SacI/SalI and ligated. Rv2608 was digested with SalI/HindIII and ligated into the Sa1I/HindIII-cut pET28a-Rv1813–3620 vector. The sequence of the resulting plasmid containing the fusion gene construct Rv1813-Rv3620-Rv2608 was verified and named ID83 since it encodes an 83 kDa protein containing an N-terminal six-histidine tag followed by a thrombin cleavage site and the M. tuberculosis genes of interest separated by restriction site linkers.
2.2. Protein Expression and Purification
ID83 was expressed in E. coli host BL-21plysS grown in 2xYT media at 37°C. Expression was induced with 1mM isopropyl β-D-1-thiogalactopyranoside at an OD 0.6, and growth continued for 4 h. Cultures were centrifuged and cell pellets were resuspended in lysis buffer (20 mM Tris pH8, 100 mM NaCl, 2 mM PMSF) and stored at −20°C. Cell pellets were thawed on ice, lysed using sonication, and spun at 30,000 × g for 20 min. The ID83 fusion protein remained in the insoluble inclusion body fraction and was purified under denaturing conditions. The inclusion body was washed twice with 1% CHAPS, 20 mM Tris pH 8.0, centrifuged at 10000 × g, and then solubilized in 60 ml binding buffer (8 M urea, 20 mM Tris pH 8, 100 mM NaCl). ID83 protein was purified using Ni-nitrilotriacetic acid (NTA)-metal ion affinity chromatography according to the manufacturer’s instructions (QIAGEN, Valencia, CA). The NTA resin was washed sequentially with 10 column volumes of 0.5% deoxycholate, 20 mM Tris pH 8.0, 60% isopropanol, 20 mM Tris pH 8.0, and 0.5% deoxycholate, 20 mM Tris pH 8.0, and then re-equilibrated with binding buffer. ID83 protein fractions were eluted with an increasing imidazole gradient and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Affinity-purified protein fractions were combined and dialyzed against 20 mM Tris, pH 8.0, concentrated using Amicon Ultra 10 kDa molecular mass cut-off centrifugal filters (Millipore), and quantified using the BCA protein assay (Pierce, Rockford, IL). Residual LPS contamination was evaluated by the Limulus amoebocyte lysate assay (Cambrex Corp., East Rutherford, N.J.) and determined to be less than 5 EU/mg of protein.
2.3. Immunization, challenge & CFU
C57BL/6 female mice, 4–6 weeks of age, were purchased from Charles River Laboratories (Wilmington, MA). All mice were maintained in IDRI’s animal facility under specific pathogen-free conditions and were treated in accordance with the regulations and guidelines of the IDRI Animal Care and Use Committee. Adjuvant formulations were given as IDRI emulsions (EM) based on squalene-in-water emulsion (IDRI-EM001) to which various synthetic TLR agonists are added. The formulations tested were: TLR4-EM (IDRI-EM005), TLR7-EM (IDRI-EM006), TLR9-EM (IDRI-EM009), TLR4/7-EM (IDRI-EM011), TLR4/9-EM (IDRI-EM014), and TLR7/9-EM (IDRI-EM024). The formulations contained the following dose of agonist(s): TLR9-EM contained synthetic TLR9 oligonucleotide (25 µg of CpG ODN1826, Coley Pharmaceutical Group), TLR4-EM contained a synthetic TLR4 agonist (20 µg of agonist), and TLR7-EM contained gardiquimod (20 µg of gardiquimod [GDQ], InvivoGen, San Diego, CA) or combinations of the same quantities of these agonists in an oil-in-water formulation.
Two repeat experiments were performed in C57Bl/6 mice (n=10 per group per experiment). Mice were immunized either i.d. or s.c. three times, 3 wk apart with 8 µg of protein in PBS plus IDRI adjuvant emulsion systems containing the TLR agonists at the doses indicated above. Those mice immunized with BCG were given a single i.d. dose of 5 × 104 CFU (Pasteur Strain, Sanofi Pasteur). Four weeks after the last immunization, groups of mice (n=7 mice/experiment) were challenged with a low dose of aerosolized Mtb H37Rv (ATCC #35718; American Type Culture Collection, Manassas, VA) using a Glas-Col aerosol generator (Terre Haute, IN), calibrated to deliver 50–100 bacteria into the lungs. The amount of bacteria delivered into the lung was confirmed 24 hrs after exposure of three mice. Protection was determined 4 wk post-challenge by harvesting the lungs and spleens from the infected mice, homogenizing the tissues in 0.05% PBS-Tween 80, and plating five-fold serial dilutions on 7H10 agar plates (Molecular Toxicology, Inc. Boone, NC) for bacterial growth. Bacterial colonies were counted 2–3 wk later after incubation at 37°C. Values ranging between 10 and 100 colonies were used to calculate the bacterial burden per organ and were expressed as: the MeanLog10 CFUsaline-MeanLog10 CFUvaccine. The two replicate protection experiments (each including 7 mice/group) were combined to give a total of 14 mice/group for CFU analysis.
2.4. Antibody ELISAs
Animals (n=10 mice/group) were bled one week after the last immunization, and ID83-specific IgG1 and IgG2c antibodies were determined. Nunc Polysorb plates were coated with 2 µg/ml of recombinant protein (ID83) in 0.1 M bicarbonate buffer and blocked overnight at 4°C with 0.05% PBS- Tween 20/1% BSA. Plates were washed and developed using SureBlue tetramethylbenzidine (TMB) substrate (Kirkegaard & Perry Laboratories Inc., Gaithersburg MD). The enzymatic reaction was stopped with 1 N H2SO4, and plates were read within 30 min at 450 nm with a reference filter set at 650 nm using a microplate ELISA reader (Molecular Devices, Sunnyvale, CA) and Soft Max Pro5 software. Endpoint titers were determined with GraphPad Prism 4 (GraphPad Software, Inc. San Diego, CA) with a cutoff of 0.1 absorbance unit.
2.5. ELISPOT
Three weeks after the third immunization, spleens were harvested from 3 mice/group in order to perform an IFN-γ ELISPOT. A MultiScreen 96-well filtration plate (Millipore, Bedford MA) was coated with 10 µg/ml rat anti-mouse IFN-γ capture Ab (eBioscience) and incubated overnight at 4°C. Plates were washed with PBS, blocked with RPMI 1640 and 10% FBS for at least 1h at RT, and washed again. Splenocytes were plated in duplicate at 2 × 105 cells/well and stimulated with medium, ConA (3 µg/ml), PPD (3 µg/ml), or ID83 (10 µg/ml) for 48 hours at 37°C. The plates were then washed with 0.1% PBS-Tween 20 and incubated overnight with a biotin-conjugated rat anti-mouse IFN-γ secondary Ab (eBioscience) diluted 1:250 in 0.1% PBS-Tween 20/0.5% BSA. The filters were developed using the VectaStain ABC avidin peroxidase conjugate and Vectastain AEC substrate kits (Vector Laboratories, Burlingame, CA) according to the manufacturer’s protocol. The reaction was stopped by washing the plates with deionized water, plates were dried in the dark, and spots were counted on an automated ELISPOT reader (C.T.L. Serie3A Analyzer, Cellular Technology Ltd., Cleveland, OH) and analyzed with ImmunSpot® software (CTL Analyzer LLC).
2.6. Flow cytometry
Three weeks following the last immunization, splenocytes from 3 mice/group were pooled and plated at 1–2 × 106 cells/well in 96-well V bottom plates and were stimulated for 12 hours with anti-CD28/CD49d (eBioscience), each at 1 ng/ml, and either ID83 (10 µg/ml); a pool of 15-mer overlapping peptides from Rv2608, Rv1813, Rv3620; PPD; or PMA/Ionomycin (included as a positive control) in the presence of GolgiStop (eBioscience). The cells were then fixed for 10 min with Cytofix/Cytoperm (BD Biosciences, San Jose CA), washed in PBS BSA 0.1%, incubated with Fc Block (anti-CD16/CD32, eBioscience) for 15 minutes at 4°C. Cells were stained with fluorochrome-conjugated mAb anti-CD3, CD8, CD44, IFN-γ, TNF (eBioscience) and CD4 mAb (Invitrogen) in Perm/Wash buffer 1X (BD Biosciences) for 30 min at 4°C, washed twice in Perm/Wash buffer, suspended in PBS, and analyzed on a modified 3 laser LSRII flow cytometer (BD Biosciences). Viable lymphocytes were gated by forward and side scatter, and 20,000 CD3+CD8+ events were acquired for each sample and analyzed with BD FACSDiva software v5.0.1 (BD Biosciences).
2.7. Statistical analysis
Student’s t-Test and standard one-way ANOVA followed by Dunnett’s Multiple Comparison Test were used to determine statistical significance. Analysis was performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA). P values of 0.05 or less were considered significant.
3. Results
3.1. Immunogenicity of ID83
The three Mtb proteins Rv1813, Rv3620 and Rv2608 were connected in tandem by restriction site linkers to produce the fusion antigen ID83. The proteins were selected based on antigenicity studies using PBMC from healthy PPD+ individuals, protection data, and the nature of the family of proteins they represent [5]. Rv2608 is a member of the PE/PPE family, whereas Rv1813 is induced under the pressure of hypoxia and Rv3620 is a member of the EsX family. Each of the individual proteins included in ID83 has shown varying levels of protection in the mouse TB model, with Rv2608 conferring the highest efficacy of the three proteins against Mtb in the lung [5].
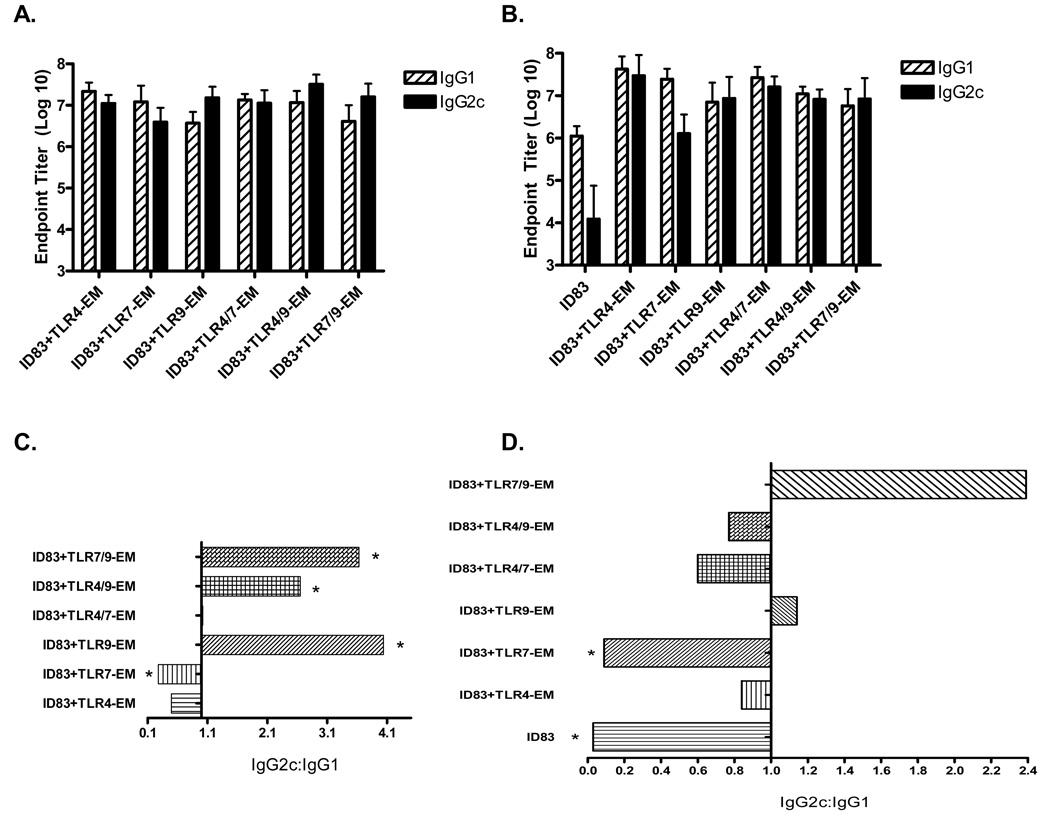
Vaccines were prepared using the ID83 antigen and three different TLR agonists. All of the agonists were formulated in an oil-in-water emulsion (EM). These agonist emulsions included a synthetic TLR4 agonist emulsion (TLR4-EM), one with the TLR7 agonist GDQ (TLR7-EM), a CpG TLR9 agonist-containing emulsion (TLR9-EM), and adjuvants with combinations of these ligands. Formulating the adjuvants in EM was done to increase the stability of the adjuvant and to potentially influence antigen presenting cell (APC) recruitment, activation, and antigen uptake at the site of immunization. MF59, a similar oil-in-water emulsion adjuvant, is currently licensed for use in Fluad®, an influenza vaccine, which is effective in the elderly population, and has been reported to significantly enhance the immune response to several antigens. Animals were immunized either i.d. or s.c. three times, 3 wk apart. Blood was collected 1 wk following the third immunization and antibody titers and IgG2c:IgG1 ratios are shown in Figure 1. All animals that received the ID83 vaccines responded to the immunizations by generating ID83-specific antibodies (Fig. 1A–B); saline controls did not produce antibodies (data not shown). In animals that received i.d. immunizations, the addition of emulsion-formulated TLR agonists clearly enhanced the level of specific antibody over protein alone (Fig. 1B). We did not include a group with the ID83 protein alone in the s.c. experiments. In the animals that received s.c. immunizations (Fig. 1C), all TLR9 agonist-containing adjuvants + ID83 (ID83 + TLR9-EM) resulted in a statistically significant response skewed towards IgG2c, indicative of a Th1-driven humoral response, while ID83 plus the TLR7-EM (containing GDQ) induced a statistically significant IgG1-skewed response (Fig. 1C). A balanced level of ID83-specific IgG1 and IgG2c antibodies were induced with the ID83 vaccine plus either TLR4-EM or TLR4/7-EM (Fig. 1C).
Fig. 1.
ID83-specific IgG1 and IgG2c endpoint antibody titers and IgG2c:IgG1 ratios from mice immunized via the s.c. route (A, C) and the i.d. route (B, D). Serum was collected 1 wk following the third immunization. Mean reciprocal dilutions are represented as the endpoint titer (Log10) ± SD. Asterisks represent statistical significance between IgG2c and IgG1 within each vaccine group, p<0.05.
Unlike the s.c.-injections noted above in which all of the ID83 vaccines containing CpG produced statistically significant Th1-biased IgG2c responses, the IgG2c to IgG1 ratio was increased only slightly when injections were given i.d. (Fig. 2D); none of the i.d. administered ID83 vaccines generated statistically significant levels of IgG2c compared to IgG1. Even though high levels of both IgG subclasses were generated, the IgG2c:IgG1 ratio was >1, indicating a Th1 influence. ID83, either alone or plus TLR7-EM, induced a statistically significant levels of ID83-specific IgG1 compared to IgG2c (Fig. 1D), similar to the results that were observed following s.c. immunization with ID83 + TLR7-EM. All of the other vaccines tested, including ID83/TLR4-EM, ID83/TLR9-EM, ID83/TLR4/7-EM and ID83/TLR4/9-EM, given i.d. induced balanced IgG2c:IgG1 ratios ranging from 0.6 to 1.2 (Fig. 1D).
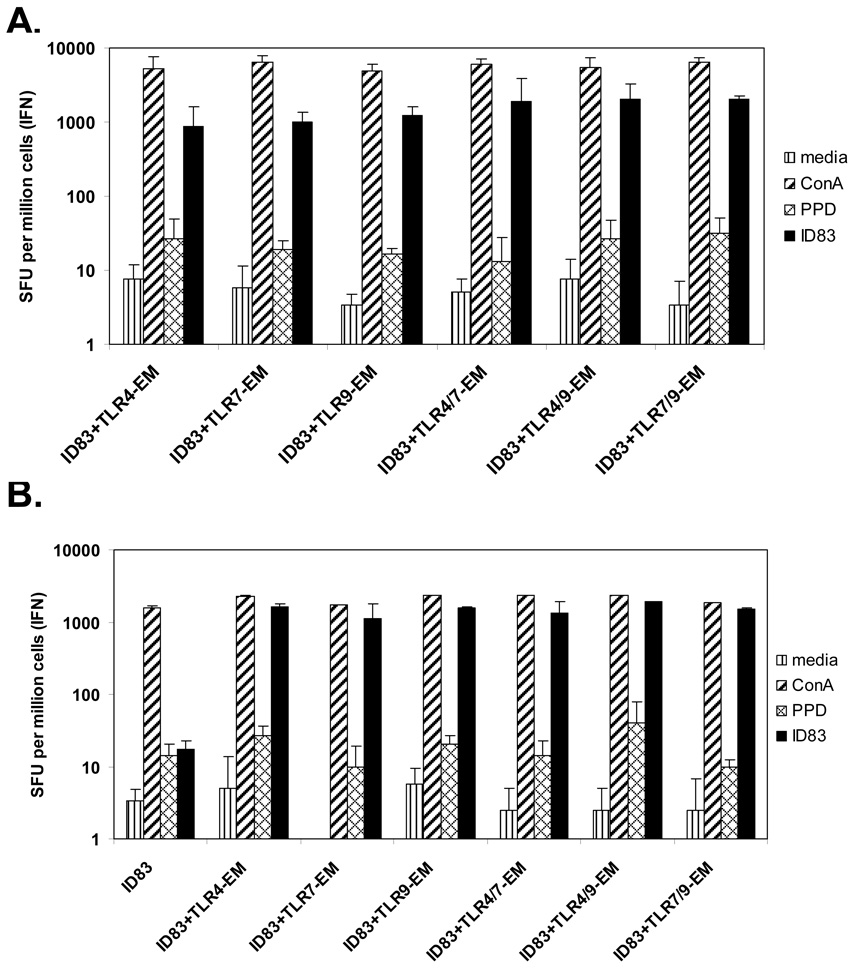
Fig. 2.
IFN-γ production from splenocytes following either (A) s.c. or (B) i.d. immunization. Mice were immunized three times, 3 wk apart and spleens were removed 4 wk after the last immunization. Splenocytes isolated from immunized animals were plated in duplicate at 2 × 105 cells/well and cultured with medium, PPD, ConA (3 µg/ml), or the recombinant fusion protein ID83 (10 µg/ml) for 48 hours. Frequencies of IFN-γ-secreting cells were determined by ELISPOT (e-Bioscience). Results are represented as the mean SFU (spot forming units) per 106 cells ± SD.
Considering the importance of IFN-γ in protection from Mtb infection the IFN-γ responses generated in these s.c. and i.d. immunization groups were also examined by ELISPOT. All of the vaccines with ID83 and TLR agonists generated similar orders of magnitude of T cells secreting IFN-γ after three immunizations (Fig. 2). Not surprisingly, the group that was given ID83 alone without adjuvant (Fig. 2B), and the saline control group (data not shown), failed to induce an IFN-γ response.
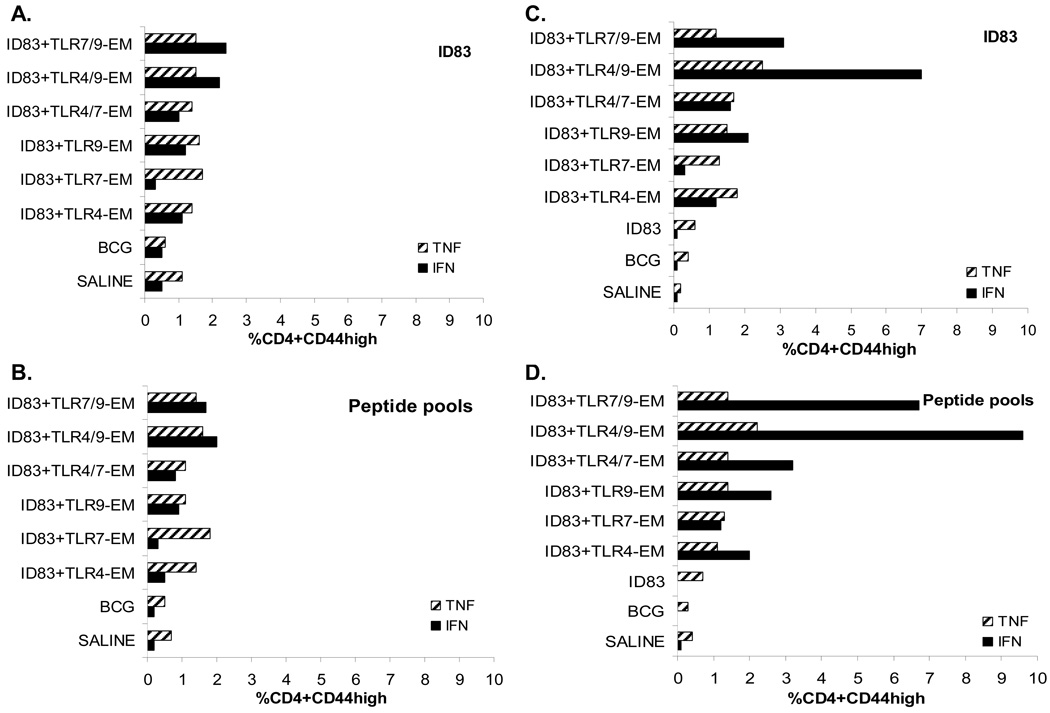
To more fully characterize the immune response following immunization with the ID83 vaccines, we performed intracellular staining for IFN-γ and TNF using flow cytometry. Splenocytes from the immunized mice were stimulated with ID83 or pooled peptides from the individual proteins that comprise ID83. Splenocytes were gated based on CD3, CD44hi and either CD4 or CD8 cell phenotypes. The percentage of ID83-stimulated or peptide-stimulated CD4 T cells producing either IFN-γ or TNF are shown in Figure 3. Intradermal immunization resulted in higher frequencies of ID83- or peptide-specific CD4 T cells expressing IFN-γ in all of the groups containing the TLR9 agonist compared to the same vaccines delivered s.c. (Fig. 3). Mice receiving ID83 protein alone failed to induce IFN-γ and resembled the saline group (Fig. 3C, D). For the adjuvanted groups, the ID83+TLR7-EM vaccine administered either s.c. or i.d. gave the lowest frequency of ID83-specific IFN-γ-producing cells. Overall, higher frequencies of CD4 T cells expressing IFN-γ were observed in the i.d. immunized groups whereas the frequencies of CD4 T cells expressing TNF were similar regardless of the route of immunization (Fig. 3). The mice immunized i.d. with ID83 plus more than one TLR agonist, either TLR4/9-EM or TLR7/9-EM, responded with the greatest frequency of IFN-γ (Fig. 3C, D) which appear additive. A similar additive trend with TLR4/9 and TLR7/9 agonists was also observed with the s.c. immunizations however the frequencies were lower with this route of immunization (Fig. 3A, B).
Fig. 3.
Cytokine production from ID83-specific CD4 T cells in immunized mice was measured by flow cytometry. Splenocytes from vaccinated mice were stimulated with ID83 or peptides for 12 hr in the presence of GolgiStop. ID83-stimulated or peptide-stimulated splenocytes were identified by ICS based on CD3 and CD4 expression and were further gated as CD44high (antigen experienced). The percent frequency of cells expressing either IFN-γ or TNF was determined from either s.c. immunized mice (A–B) or from i.d. immunized mice (C–D). The values from pooled splenocytes (n=3 mice/group) are shown.
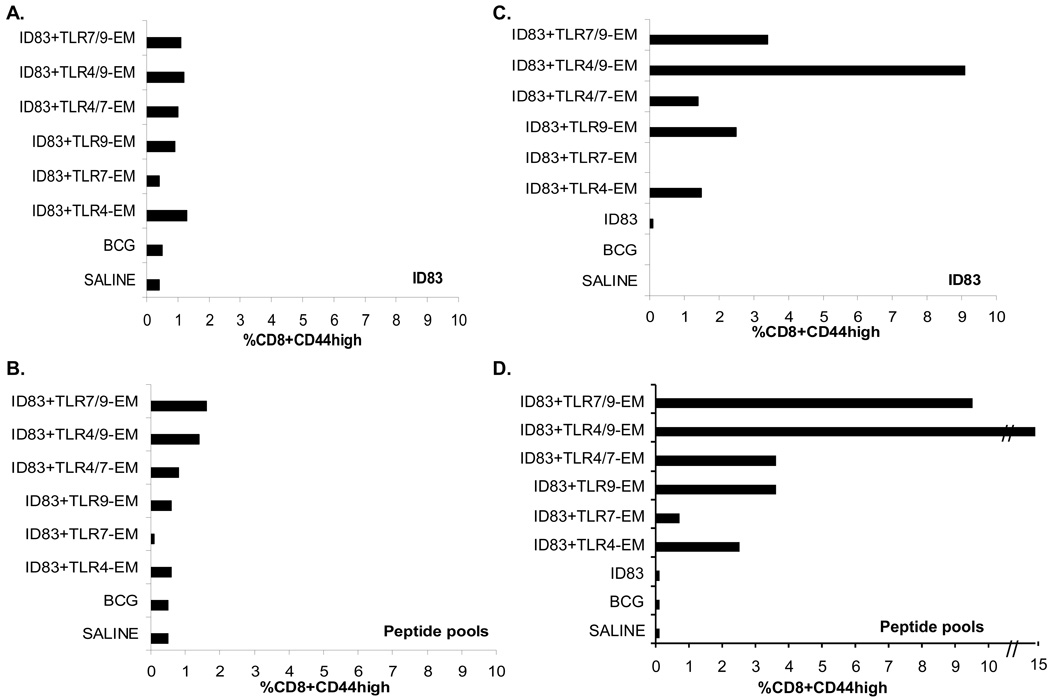
Differences in the CD8 T cell IFN-γ responses after s.c. versus i.d. injections were also seen (Fig. 4). The groups given i.d. immunizations had higher frequencies of CD8 T cells staining for IFN-γ compared to the s.c. immunized animals given the same vaccines (Fig. 4). Similar to the ID83-specific CD4 T cell responses, all of the ID83 vaccines containing TLR9-EM induced greater IFN-γ responses when delivered i.d. (Fig. 4C, D) compared to responses seen following s.c. immunization (Fig. 4A, B). Moreover, there was also an additive effect on the frequency of CD8 T cells secreting IFN-γ when TLR4 and TLR9 agonists were combined with ID83, and these populations were equivalently stimulated to produce cytokines by either intact protein or pooled peptides (Fig. 4C, D). The i.d. immunized groups given ID83 plus TLR4/9-EM induced the highest frequency of IFN-γ-secreting CD8 T cells; no response was generated to the ID83 protein alone (Fig. 4C, D). Minimal ID83-specific CD8+ TNF responses were observed following either immunization route (data not shown).
Fig. 4.
IFN-γ production from ID83-specific CD8 T cells in immunized mice was measured by flow cytometry. Splenocytes from vaccinated mice were stimulated with ID83 or peptides for 12 hr in the presence of GolgiStop. ID83-stimulated or peptide-stimulated splenocytes were identified by ICS based on CD3 and CD8 expression and were further gated as CD44high (antigen experienced). The percent frequency of cells expressing IFN-γ was determined from either s.c. immunized mice (A–B) or from i.d. immunized mice (C–D). The values from pooled splenocytes (n=3 mice/group) are shown.
3.2. Protection by ID83 adjuvanted with TLR agonists in oil-in-water emulsion following s.c. or i.d. immunization
We measured the efficacy of each of the vaccines given either s.c. or i.d. in parallel groups of mice by determining the protection in the lungs following a low dose aerosol challenge with M. tuberculosis H37Rv. BCG was included in the experiments as a positive control. BCG was protective in both the s.c. and i.d. experiments, providing 0.92 and 1.0 log10 protection respectively, in the lung (Table 1 and Table 2). In this experiment an ID83 protein alone group was included only in the i.d. experiment and this vaccine was not protective on its own (Table 1). In contrast, ID83 plus TLR4-EM was protective via either route. For this vaccine, the level of protection was highest in the i.d. immunized mice (log10 protection in the lung was 0.29, p<0.05 in the s.c. group and 0.45, p<0.01 in the i.d. group). ID83 plus TLR9-EM and ID83 plus TLR4/9-EM vaccines were also protective (p<0.01) when given by either of these routes. Greater differences in the two routes were observed for the ID83 plus TLR9-EM vaccine candidate than for the ID83+TLR4/9-EM vaccine (Table 1 and Table 2). In contrast to the other vaccine groups, GDQ-containing vaccines were protective only when given i.d. (Table 2); s.c. administration of GDQ vaccines did not significantly reduce the bacterial loads in the lungs of infected mice (Table 1). And even though the level of protection provided by TLR7-EM plus ID83 given i.d. was statistically significant, the mice in this group responded with the least amount of protection (log10 protection in the lung was 0.35) compared to the other i.d. vaccine groups aside from ID83 alone (Table 1).
Table 1.
Vaccine-induced protection in mice following s.c. immunization and aerosol infection with M. tuberculosis.
| Vaccine groupsa | CFUb Log10 | S.D. | Log10 Protection vs. Saline | P Valuec |
|---|---|---|---|---|
| Saline | 6.14 | 0.35 | NA | NA |
| BCG | 5.22 | 0.28 | 0.92 | <0.01 |
| ID83+TLR4-EM | 5.86 | 0.26 | 0.29 | <0.05 |
| ID83+TLR7-EM | 5.91 | 0.27 | 0.23 | >0.05 |
| ID83+TLR9-EM | 5.80 | 0.26 | 0.35 | <0.01 |
| ID83+TLR4/7-EM | 5.92 | 0.22 | 0.23 | >0.05 |
| ID83+TLR4/9-EM | 5.73 | 0.26 | 0.41 | <0.01 |
| ID83+TLR7/9-EM | 5.88 | 0.29 | 0.27 | >0.05 |
Mice were immunized by the s.c. route three times, 3 wk apart.
Viable colony forming units (CFU) of bacteria in the lungs of immunized mice compared to saline controls 4 wk after a low-dose aerosol challenge with M. tuberculosis H37Rv. Data represents the mean of two independent experiments (n=14 mice total).
One way ANOVA followed by Dunnett’s Multiple Comparison Test was used for statistical analysis. P values <0.05 are considered statistically significant.
Table 2.
Vaccine-induced protection in mice following i.d. immunization and aerosol infection with M. tuberculosis.
| Vaccine groupsa | CFUb Log10 | S.D. | Log10 Protection vs. Saline | P Valuec |
|---|---|---|---|---|
| Saline | 6.14 | 0.22 | NA | NA |
| BCG | 5.14 | 0.38 | 1.00 | <0.01 |
| ID83 | 5.90 | 0.28 | 0.24 | >0.05 |
| ID83+TLR4-EM | 5.69 | 0.28 | 0.45 | <0.01 |
| ID83+TLR7-EM | 5.79 | 0.16 | 0.35 | <0.01 |
| ID83+TLR9-EM | 5.54 | 0.25 | 0.60 | <0.01 |
| ID83+TLR4/7-EM | 5.59 | 0.20 | 0.56 | <0.01 |
| ID83+TLR4/9-EM | 5.61 | 0.22 | 0.54 | <0.01 |
| ID83+TLR7/9-EM | 5.67 | 0.19 | 0.47 | <0.01 |
Mice were immunized by the i.d. route three times, 3 wk apart.
Viable colony forming units (CFU) of bacteria in the lungs of immunized mice compared to saline controls 4 wk after a low-dose aerosol challenge with M. tuberculosis H37Rv. Data represents the mean of two independent experiments (n=14 mice total).
One way ANOVA followed by Dunnett’s Multiple Comparison Test was used for statistical analysis. P values <0.05 are considered statistically significant.
4. Discussion
We have recently shown that each of the three individual proteins that make up ID83 (Rv3620, Rv1813 and Rv2608) combined with CpG induced some level of protective against aerosol infection in the TB mouse model when delivered by the s.c. route [5]. The aim of this study was to determine whether a vaccine containing the ID83 fusion protein formulated with TLR agonist/adjuvants and administered either s.c. or i.d. was protective against an aerosol challenge of M. tuberculosis. The potential use of TLR agonists as adjuvants for TB subunit vaccines is extensive and could be tailored to induce Th1-biased immune responses since adaptive immunity is closely connected to responses that occur during innate immunity [21]. TLR engagement leading to induction of IL-12 may preferentially result in Th1-type responses while producing a MyD88-dependent signal that inhibits Th2-type responses [22]. We have previously determined that each of the TLR agonists included as adjuvants in the present studies induce IL-12p40 from bone marrow-derived murine DCs (unpublished data). Similar in vitro TLR studies have been reported using purified DCs. One such study showed synergistic levels of IL-12p70 following stimulation of mouse bone marrow-derived DCs and human monocyte-derived or peripheral blood-derived DCs with a combination of a TLR4 agonist (LPS) plus TLR7/8 agonist (Resiquimod, R848) [23]. Synergistic upregulation of IFN-γ and IL-12 has also been shown when TLR4 and TLR7/8 agonist combinations are added to human PBMCs [24]. Hence, these agonists are ideal for vaccines designed to generate a Th1-mediated immune response.
The use of TLR agonists as adjuvants for TB vaccines has been demonstrated both in animal models and in humans. The TLR4 agonist MPL is a component of the AS01B/AS02A adjuvant systems used with the Mtb subunit vaccine antigen Mtb72F currently in human clinical trials [25]. TLR agonists used with experimental Mtb vaccines have included a TB protein TLR2 agonist (acylated Rv1411) [26], TLR3 (poly(I:C)) and TLR9 agonists (plasmid DNA) bound to cationic liposomes [27], TLR4 agonist (MPL) [28], and a TLR9 agonist (CpG) [5].
We first measured the immunogenicity of ID83 combined with TLR agonists. The characterization of ID83-specific IgG2c:IgG1 antibody ratios following immunization with ID83 plus TLR agonists were included as a measure of Th1 versus Th2 balance. IFN-γ is involved in antibody class switching from IgM to IgG2a (or IgG2c in C57BL/6 mice used here) whereas IL-4 produced from Th2 cells leads to IgG1 and IgE [29]. All of the vaccines in this study produced ID83-specific IgG subclasses (either IgG1 or IgG2c) and in most cases a balanced IgG2c:IgG1 response was generated. Interestingly, s.c. injected ID83 vaccines containing CpG produced significantly greater IgG2c versus IgG1 responses whereas the same vaccine given i.d. resulted in a more balanced IgG2c:IgG1 response (even though the ratio was >1 suggesting a Th1 influence). A similar observation was made in another study where i.d. injection with a recombinant M type 6 protein from Streptococcus pyrogenes combined with CpG induced high levels of both IgG1 and IgG2a antibodies [30]. In this study neither antibody subclass was dominant but the ratios of IgG2a to IgG1 were >1. In contrast, the ID83/GDQ-EM vaccine, administered both s.c. and i.d., resulted in an antibody response skewed towards IgG1. It is not clear why dominant IgG2c responses with GDQ-EM were not observed, although this may have been a dose related effect. Fransen et al. have shown that IgG2a titers with imiquimod (another TLR7 agonist) in combination with a meningococcal vaccine are much greater with a higher adjuvant concentration (100µg) versus a lower adjuvant concentration (10µg) [31]. As expected, ID83 given as protein alone resulted in a dominant IgG1 response. All of the vaccines (except the ID83 vaccine without adjuvant) induced IFN-γ. Frequencies of cells producing IFN-γ (and TNF) were also examined by flow cytometry. Additive levels of ID83-specific T cells secreting IFN-γ were observed in both the s.c. and the i.d. immunized groups when a combination of TLR agonists were included in the vaccine (TLR7/9-EM and TLR4/9-EM). The i.d. vaccinated groups given ID83 + TLR4/9-EM responded with the highest frequency of both IFN-γ-secreting CD4 and CD8 T cells and thus were able to augment the immune response when the vaccines were given via this route. Napolitani et al. have shown that synergistic IL-12p70 responses are induced from both mouse and human bone marrow-derived DCs in the presence of both LPS (TLR4) and CpG (TLR9) agonists in vitro [23].
Once we determined that the vaccines were inducing protective Th1 immune responses, we tested each of the ID83 Mtb subunit vaccines in a mouse aerosol challenge model using M. tuberculosis H37Rv. All of the vaccines with agonists targeting TLR4, TLR7 and TLR9 and administered i.d. provided protection against M. tuberculosis in the lung. Subcutaneous vaccination with ID83 plus TLR4-EM, or TLR9-EM, or with a combination of TLR4/9-EM afforded protection in the lung.
In contrast, the ID83 vaccines containing GDQ (TLR7-EM) given s.c. failed to induce a protective response against Mtb. GDQ is a synthetic imidazoquinoline compound that signals through the TLR7, a receptor which also recognizes the natural ligand ssRNA [32]. Imiquimod (IMQ) is also an imidazoquinoline TLR7 agonist and is contained in Aldara cream (5%). Aldara cream is used as a topical skin treatment of human actinic keratosis, superficial basal cell carcinoma and external genital warts. TLR7 is expressed on both myeloid and plasmacytoid DCs of mice [33] and humans [34]. IMQ is able to induce the maturation of human epidermal Langerhans cells (LC) in vitro [35, 36] and topical application of IMQ to mice has been shown to increase FITC-induced LC migration from the skin to the draining lymph node [35, 36].
BCG, which is currently the only approved Mtb vaccine, is given i.d. The skin is an ideal site for vaccination due to the presence of APCs such as the LCs and dermal dendritic cells (DDC), that can endocytose injected antigen and recognize TLR agonists, migrate to the draining lymph node and present antigen to naïve T cells. LC and DDC are part of the Skin Associated Lymphoid Tissue (SALT) in both mice and humans [37]. Although our studies were performed in mice, the vaccine is ultimately being developed for use in humans. Similarities in the SALT exist in both human and mouse skin including many, but not all, of the surface markers on cutaneous DCs (see review [38]), in addition to LC function and migration of LC in response to TNF and IL-1β [39]. There are also interspecies differences that exist including lipid profiles and thickness of the skin. The thickness of viable epidermis in a rodent (rat) is 9.9 µm [40] for example, whereas the thickness of human epidermis on the front of the male forearm is 67.8 µm [41]. The overall thickness of the skin in humans (epidermis plus dermis) on the forearm is 1171.9 µm, although thickness of the skin varies even in humans depending on gender and race [41]. It is important to note that there are cellular and morphological differences in the skin of rodents and humans and that any advantages seen following vaccine injection in mouse skin may not translate into the same positive results observed in humans.
Recently, Zhang and Matlashewski showed that the route of immunization also played a role in protection when a TLR7 agonist was used in combination with a Leishmania vaccine [42]. When autoclaved L. major (ALM) was given s.c. with Resiquimod (R848), a TLR7/8 agonist, or with topical IMQ the vaccine was protective. However, when the ALM vaccine plus R848 was given intramuscularly (i.m.) it was not protective. It is possible that the low molecular weight and solubility of the TLR7 and TLR7/8 agonists make their action more sensitive to the site of administration, and they may function more effectively in sites where rapid clearance into the blood-stream is minimized (such as the dermis and s.c. layer). Although all of the immunized mice were capable of inducing IFN-γ, those given the vaccine by the s.c. route induced decreased Th2-type cytokines including reduced IL-4 and IL-10 synthesis, in contrast to the i.m. immunized mice that responded with high levels of IL-4 and IL-10. Thus, the predictive measure of protection in this experiment was a reduction in IL-4 and IL-10 rather than a shift to a Th1 response. We are currently examining the role of Th2-type cytokines as a negative predictor of vaccine protection. In the future we also plan to determine the long-term protective effects of the ID83 vaccine in combination with these TLR agonists. We also plan to characterize the role of these TLR agonists in the protection against pathology caused by undesirable host responses towards Mtb infection in the lungs and draining lymph nodes using the guinea pig model of tuberculosis, where detrimental responses lead to necrosis within the granulomas and contribute to loss of viable lung tissue [43]. In addition, studies are underway to determine whether multifunctional Th1 cells that simultaneously produce IFN-γ, TNF and IL-2 are generated in response to the ID83 vaccine in the presence of TLR agonists and when delivered by different routes. Multifunctional CD4+ Th1 cells have recently been described as a correlate of vaccine-mediated protection against leishmaniasis that, like TB, is a disease that relies on cell-mediated immunity for protection [44].
It may be possible to enhance Th1 immune responses and protective efficacy of TB subunit vaccines if formulated with TLR agonist/adjuvants. Depending on the TLR agonist formulation, the route may also play a role in the efficacy of the vaccine.
Acknowledgements
This work was supported in part by NIH Contract N01-AI-25479 and NIH Grants AI-044373 and AI-067251. The authors thank Elena Kristalinskaia, Valerie Reese, Tara Evers, and Kevin Durgan for their technical expertise and to Randall Howard for his valuable input in editing the manuscript. We also thank Dr. Martin Friede for reading this manuscript and for his helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dye C, Floyd F, Uplekar M. Global Tuberculosis Control: surveillance, planning, financing: WHO report: World Health Organization. 2008
- 2.Sander C, McShane H. Translational mini-review series on vaccines: Development and evaluation of improved vaccines against tuberculosis. Clinical and experimental immunology. 2007 Mar;147(3):401–411. doi: 10.1111/j.1365-2249.2006.03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dye C, Watt CJ, Bleed DM, Hosseini SM, Raviglione MC. Evolution of tuberculosis control and prospects for reducing tuberculosis incidence, prevalence, and deaths globally. Jama. 2005 Jun 8;293(22):2767–2775. doi: 10.1001/jama.293.22.2767. [DOI] [PubMed] [Google Scholar]
- 4.Andersen P, Doherty TM. The success and failure of BCG - implications for a novel tuberculosis vaccine. Nature reviews. 2005 Aug;3(8):656–662. doi: 10.1038/nrmicro1211. [DOI] [PubMed] [Google Scholar]
- 5.Bertholet S, Ireton GC, Kahn M, Guderian J, Mohamath R, Stride N, Laughlin EM, Baldwin SL, Vedvic TS, Coler RN, Reed SG. Identification of human T cell antigens for the development of vaccines agains Mycobacterium tuberculosis. The Journal of Immunology. 2008;181 doi: 10.4049/jimmunol.181.11.7948. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rook GA, Hernandez-Pando R, Dheda K, Teng SeahG. IL-4 in tuberculosis: implications for vaccine design. Trends in immunology. 2004 Sep;25(9):483–488. doi: 10.1016/j.it.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008 Apr;8(4):247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 8.Flynn JL, Goldstein MM, Triebold KJ, Koller B, Bloom BR. Major histocompatibility complex class I-restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proceedings of the National Academy of Sciences of the United States of America. 1992 Dec 15;89(24):12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. European journal of immunology. 2000 Dec;30(12):3689–3698. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. The Journal of experimental medicine. 1993 Dec 1;178(6):2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. The Journal of experimental medicine. 1993 Dec 1;178(6):2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell DG. Who puts the tubercle in tuberculosis? Nature reviews. 2007 Jan;5(1):39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 13.Bean AG, Roach DR, Briscoe H, France MP, Korner H, Sedgwick JD, et al. Structural deficiencies in granuloma formation in TNF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J Immunol. 1999 Mar 15;162(6):3504–3511. [PubMed] [Google Scholar]
- 14.Flynn JL, Goldstein MM, Chan J, Triebold KJ, Pfeffer K, Lowenstein CJ, et al. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995 Jun;2(6):561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 15.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. The New England journal of medicine. 2001 Oct 11;345(15):1098–1104. doi: 10.1056/NEJMoa011110. [DOI] [PubMed] [Google Scholar]
- 16.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes and infection / Institut Pasteur. 2004 Dec;6(15):1382–1387. doi: 10.1016/j.micinf.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 17.Hemmi H, Takeuchi O, Kawai T, Kaisho T, Sato S, Sanjo H, et al. A Toll-like receptor recognizes bacterial DNA. Nature. 2000 Dec 7;408(6813):740–745. doi: 10.1038/35047123. [DOI] [PubMed] [Google Scholar]
- 18.Kaisho T, Akira S. Toll-like receptors as adjuvant receptors. Biochimica et biophysica acta. 2002 Feb 13;1589(1):1–13. doi: 10.1016/s0167-4889(01)00182-3. [DOI] [PubMed] [Google Scholar]
- 19.Means TK, Wang S, Lien E, Yoshimura A, Golenbock DT, Fenton MJ. Human toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999 Oct 1;163(7):3920–3927. [PubMed] [Google Scholar]
- 20.Ferwerda G, Girardin SE, Kullberg BJ, Le BourhisL, de Jong DJ, Langenberg DM, et al. NOD2 and toll-like receptors are nonredundant recognition systems of Mycobacterium tuberculosis. PLoS pathogens. 2005 Nov;1(3):279–285. doi: 10.1371/journal.ppat.0010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nature immunology. 2004 Oct;5(10):987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 22.Sun J, Walsh M, Villarino AV, Cervi L, Hunter CA, Choi Y, et al. TLR ligands can activate dendritic cells to provide a MyD88-dependent negative signal for Th2 cell development. J Immunol. 2005 Jan 15;174(2):742–751. doi: 10.4049/jimmunol.174.2.742. [DOI] [PubMed] [Google Scholar]
- 23.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nature immunology. 2005 Aug;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh TK, Mickelson DJ, Solberg JC, Lipson KE, Inglefield JR, Alkan SS. TLR-TLR cross talk in human PBMC resulting in synergistic and antagonistic regulation of type-1 and 2 interferons, IL-12 and TNF-alpha. International immunopharmacology. 2007 Aug;7(8):1111–1121. doi: 10.1016/j.intimp.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Skeiky YA, Alderson MR, Ovendale PJ, Guderian JA, Brandt L, Dillon DC, et al. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J Immunol. 2004 Jun 15;172(12):7618–7628. doi: 10.4049/jimmunol.172.12.7618. [DOI] [PubMed] [Google Scholar]
- 26.Wang B, Henao-Tamayo M, Harton M, Ordway-Rodrigues D, Shanley C, Basaraba RJ, et al. A Toll-like Receptor-2 directed fusion protein vaccine against tuberculosis. Clin Vaccine Immunol. 2007 May 16; doi: 10.1128/CVI.00077-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaks K, Jordan M, Guth A, Sellins K, Kedl R, Izzo A, et al. Efficient immunization and cross-priming by vaccine adjuvants containing TLR3 or TLR9 agonists complexed to cationic liposomes. J Immunol. 2006 Jun 15;176(12):7335–7345. doi: 10.4049/jimmunol.176.12.7335. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin SL, D'Souza C, Roberts AD, Kelly BP, Frank AA, Lui MA, et al. Evaluation of new vaccines in the mouse and guinea pig model of tuberculosis. Infection and immunity. 1998 Jun;66(6):2951–2959. doi: 10.1128/iai.66.6.2951-2959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annual review of immunology. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 30.Teloni R, von Hunolstein C, Mariotti S, Donati S, Orefici G, Nisini R. Antibody classes & subclasses induced by mucosal immunization of mice with Streptococcus pyogenes M6 protein & oligodeoxynucleotides containing CpG motifs. The Indian journal of medical research. 2004 May;119 Suppl:126–130. [PubMed] [Google Scholar]
- 31.Fransen F, Boog CJ, van Putten JP, van der LeyP. Agonists of Toll-like receptors 3, 4, 7, and 9 are candidates for use as adjuvants in an outer membrane vaccine against Neisseria meningitidis serogroup B. Infection and immunity. 2007 Dec;75(12):5939–5946. doi: 10.1128/IAI.00846-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diebold SS, Kaisho T, Hemmi H, Akira S, Reis e Sousa C. Science. 5663. Vol. 303. New York, NY: 2004. Mar 5, Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA; pp. 1529–1531. [DOI] [PubMed] [Google Scholar]
- 33.Edwards AD, Diebold SS, Slack EM, Tomizawa H, Hemmi H, Kaisho T, et al. Toll-like receptor expression in murine DC subsets: lack of TLR7 expression by CD8 alpha+ DC correlates with unresponsiveness to imidazoquinolines. European journal of immunology. 2003 Apr;33(4):827–833. doi: 10.1002/eji.200323797. [DOI] [PubMed] [Google Scholar]
- 34.Ito T, Amakawa R, Kaisho T, Hemmi H, Tajima K, Uehira K, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. The Journal of experimental medicine. 2002 Jun 3;195(11):1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burns RP, Jr., Ferbel B, Tomai M, Miller R, Gaspari AA. Clinical immunology. 1. Vol. 94. Orlando, Fla: 2000. Jan, The imidazoquinolines, imiquimod and R-848, induce functional, but not phenotypic, maturation of human epidermal Langerhans' cells. 13–23.s. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki H, Wang B, Shivji GM, Toto P, Amerio P, Tomai MA, et al. Imiquimod, a topical immune response modifier, induces migration of Langerhans cells. The Journal of investigative dermatology. 2000 Jan;114(1):135–141. doi: 10.1046/j.1523-1747.2000.00833.x. [DOI] [PubMed] [Google Scholar]
- 37.Debenedictis C, Joubeh S, Zhang G, Barria M, Ghohestani RF. Immune functions of the skin. Clinics in dermatology. 2001 Sep-Oct;19(5):573–585. doi: 10.1016/s0738-081x(00)00173-5. [DOI] [PubMed] [Google Scholar]
- 38.Valladeau J, Saeland S. Cutaneous dendritic cells. Seminars in immunology. 2005 Aug;17(4):273–283. doi: 10.1016/j.smim.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Griffiths CE, Dearman RJ, Cumberbatch M, Kimber I. Cytokines and Langerhans cell mobilisation in mouse and man. Cytokine. 2005 Oct 21;32(2):67–70. doi: 10.1016/j.cyto.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 40.Stahl J, Niedorf F, Kietzmann M. Characterisation of epidermal lipid composition and skin morphology of animal skin ex vivo. Eur J Pharm Biopharm. 2008 Oct 4; doi: 10.1016/j.ejpb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y, Hwang K. Skin thickness of Korean adults. Surg Radiol Anat. 2002 Aug-Sep;24(3–4):183–189. doi: 10.1007/s00276-002-0034-5. [DOI] [PubMed] [Google Scholar]
- 42.Zhang WW, Matlashewski G. Immunization with a Toll-like receptor 7 and/or 8 agonist vaccine adjuvant increases protective immunity against Leishmania major in BALB/c mice. Infection and immunity. 2008 Aug;76(8):3777–3783. doi: 10.1128/IAI.01527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner OC, Basaraba RJ, Orme IM. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infection and immunity. 2003 Feb;71(2):864–871. doi: 10.1128/IAI.71.2.864-871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, et al. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature medicine. 2007 Jul;13(7):843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]