Abstract
Clostridium perfringens type C isolates cause necrotizing enteritis in humans and domestic animals. In vitro, type C isolates often produce beta toxin (CPB), beta2 toxin (CPB2), alpha toxin (CPA), perfringolysin O (PFO), and TpeL during (or after) late log-phase growth. In contrast, the current study found that many type C isolates respond to close contact with enterocyte-like Caco-2 cells by producing all toxins, except TpeL, much more rapidly than occurs during in vitro growth. This in vivo effect involves rapid transcriptional upregulation of the cpb, cpb2, pfoA and plc toxin genes. Rapid Caco-2 cell-induced upregulation of CPB and PFO production involves the VirS/VirR two-component system, since upregulated in vivo transcription of the pfoA and cpb genes was blocked by inactivating the virR gene and was reversible by complementation to restore VirR expression. However, the luxS quorum sensing system is not required for the rapid upregulation of type C toxin production induced by contact with Caco-2 cells. These results provide the first indication of host cell:pathogen cross-talk affecting toxin production kinetics by any pathogenic Clostridium spp., identify in vivo vs. in vitro differences in C. perfringens toxin expression, and implicate VirS/VirR as a possible contributor to some C. perfringens enteric diseases.
Keywords: Clostridum perfringens, host:pathogen cross-talk, toxin gene regulation, two-component regulatory system, quorum sensing
Introduction
The anaerobic, spore-forming bacterium Clostridium perfringens is one of the most important Gram-positive pathogens of humans and animals (McClane, 2006). C. perfringens diseases include numerous gastrointestinal syndromes and enterotoxemias, as well as gas gangrene and other histotoxic infections. The virulence of this bacterium is directly related to its production of potent toxins. Differential production of four lethal typing toxins [α, β, ε and/or ι] is used to classify C. perfringens isolates into 5 pathogenic types (A–E). Each C. perfringens type is associated with certain human or animal diseases (McClane, 2006; Smedley et al., 2004).
By definition, C. perfringens type C isolates must produce both α toxin (CPA) and β toxin (CPB). Some type C isolates also make β2 toxin (CPB2), perfringolysin O (PFO) or enterotoxin (CPE) (Fisher et al., 2006; Smedley et al., 2004). Furthermore, many type C isolates also produce a newly discovered toxin named TpeL, which is a truncated homolog of C. difficile TcdA and other large clostridial toxins (Amimoto et al., 2007).
C. perfringens type C isolates cause fatal diseases ranging from necrotizing enteritis to enterotoxemia in virtually all livestock species. Those type C-mediated animal diseases result in serious economic losses for the agricultural industry (McClane, 2004). In severe type C enterotoxemia, toxins are made in the intestines and then absorbed into the circulation, where they can cause rapid death of the infected animal (Songer and Uzal, 2005; Songer, 1996). Piglets are most commonly affected by type C isolates, with herd mortality rates between 50–100% despite a clinical course usually lasting <24 h (Songer, 1996; Niilo, 1988).
In humans, C. perfringens type C isolates cause enteritis necroticans, an often rapidly fatal disease that involves vomiting, diarrhea, severe abdominal pain, and the presence of blood in the stools (McClane, 2004). This illness is associated with low intestinal trypsin levels due to diet or disease, implicating trypsin as an important host defense mechanism against enteritis necroticans. On histologic examination, blunted villi and numerous bacteria are seen on the mucosal surface of the necrotic tissue (Walker et al., 1980). Outbreaks of acute human enteritis necroticans caused by type C isolates were first recorded in post-war Germany, where the disease was known as Darmbrand. This illness, currently endemic throughout Southeast Asia, is most closely associated with New Guinea, where it is referred to as Pigbel. In the 1970´s, pigbel was the leading cause of death in children older than 1 year of age in the New Guinea highlands (Lawrence and Cooke, 1980). Although uncommon, human enteritis necroticans caused by type C isolates also occurs in developed countries (Matsuda et al., 2007; Sobel et al., 2005; Tonnellier et al., 2001; Petrillo et al., 2000). Importantly, diabetic patients infected with these bacteria often survive <48 h after the onset of symptoms (Tonnellier et al., 2001; Severin et al., 1984).
Several observations support CPB as the major cause of the clinical signs associated with type C disease (Sakurai and Duncan, 1977). First, immunohistochemistry studies detected CPB on the necrotic intestinal epithelium of patients suffering from type C infection (Matsuda et al., 2007). In addition, we constructed and virulence-tested several toxin null mutants of type C isolate CN3685, which showed that beta toxin is necessary for this isolate to damage rabbit ileal loops (Sayeed et al., 2008). We also showed that CPB is sufficient to cause enteric disease by experimentally reproducing necrotic enteritis in rabbit ileal, jejunal or duodenal loops (but not colonic loops) by injecting purified CPB, along with trypsin inhibitor to avoid CPB degradation by endogenous trypsin (Vidal et al., 2008).
In other work, we showed that CPB, CPB2, PFO and PLC are produced during late log-phase by type C isolates growing in TGY medium (Fisher et al., 2006). In that study, in vitro toxin production levels by type C isolates were found to vary using different bacterial culture media for growth, suggesting that environmental signals are important for regulating type C toxin production and, by extension, perhaps virulence.
Regulation of toxin production by C. perfringens vegetative cells has only been studied for gangrene-producing type A isolates growing in vitro. A two component regulatory system named VirS/VirR, comprised of (respectively) a membrane sensor and transcriptional regulator (Lyristis et al., 1994; Shimizu et al., 1994), is encoded by the virS and virR genes that form an operon transcribed as a single 2.1 kb mRNA molecule (Ba-Thein et al., 1996). The VirS/VirR two component regulatory system helps to govern in vitro transcription of the chromosomal plc, pfoA, and colA toxin genes (encoding CPA, PFO and collagenase, respectively) and the plasmid-borne cpb2 gene encoding CPB2 toxin (Ohtani et al., 2003; Ba-Thein et al., 1996; Lyristis et al., 1994; Shimizu et al., 1994). In gangrene-producing type A strain 13, PLC and PFO toxin regulation also involves the luxS quorum-sensing mechanism (Ohtani et al., 2002).
Whether contact with host cells during disease affects toxin gene transcription has not yet been studied for C. perfringens or any pathogenic Clostridium spp. This gap is significant since other pathogens regulate their virulence gene expression in response to stimuli from host cells. For example, upon host-cell contact, enteropathogenic E. coli upregulates transcription of genes involved in intimate adherence, pedestal formation (Leverton and Kaper, 2005), and EspC toxin secretion (Vidal and Navarro-Garcia, 2006). Contact with host cells induces H. pylori to produce surface appendages and activate invasion mechanisms (Rohde et al., 2003). The presence of host cells stimulates Salmonella and Shigella to produce a functional type III secretion system and translocate invasion proteins into host cells (Demers et al., 1998; Ginocchio et al., 1994).
Therefore, the current study investigated toxin production by C. perfringens type C isolates in the presence of intestinal Caco-2 cells, as a model for human enterocytes. Type C isolates were found to sense Caco-2 cells and respond by quickly upregulating toxin production to exert cytotoxic consequences on host cells. This effect was shown to involve, in part, the VirS/VirR two component regulatory system but not luxS-controlled quorum sensing.
Results
Infection of Caco-2 cell cultures with C. perfringens type C strains induces a rapid accumulation of extracellular proteins
Since secretion of protein toxins is considered essential for the pathogenesis of type C intestinal disease (Sayeed et al., 2008; Smedley et al., 2004; Petit et al., 1999), we first investigated by SDS-PAGE whether infection with type C isolates affects protein levels present in supernatants of Caco-2 cell cultures. After as little as 1 h post-infection with C. perfringens type C strains JGS1495 or CN3685 significant levels of proteins were detected in the supernatants of Caco-2 cultures (Figure S1A and S1C, respectively). The molecular size of those supernatant proteins ranged from 20 to <100 kDa. The levels of those supernatant proteins increased even further by 2 or 3 h post-infection. No secreted proteins were seen in the supernatant of C. perfringens type C strains grown similarly in either TGY or MEM without Caco-2 cells (Figures S1A and S1C), suggesting the protein secretion evident in Figures S1A and S1C had been stimulated by C. perfringens infection of Caco-2 cell cultures.
To evaluate whether these changes in supernatant protein patterns were specifically due to C. perfringens infection, SDS-PAGE was performed on the supernatants of non-infected Caco-2 cell cultures grown for 3 h in MEM without FBS (fetal bovine serum). A major band corresponding to a >60 kDa protein was detected in the supernatant of non-infected Caco-2 cells (Figures S1A and S1C, control lane). However these analyses also indicated that most proteins of <60 kDa present in the supernatants of infected Caco-2 cultures had resulted from C. perfringens type C infection. Since most toxins and proteolytic enzymes secreted by C. perfringens type C strains have a molecular mass of <60 kDa (i.e. CPB2, 28 kDa; CPB, 35 kDa; CPA, 47 kDa; and PFO, 54 kDa), the S1 figure results were consistent with the possibility of host cell-mediated stimulation of bacterial toxin secretion occurring early during infection.
To evaluate whether the enhanced protein secretion observed in Figures S1A and S1C was simply due to host cell stimulation of bacterial overgrowth, the colony forming units (CFU) of C. perfringens type C strains present under each culture condition were determined. After a 3 h infection of Caco-2 cells, the CFU of C. perfringens type C strain JGS1495 cultures were similar to, if not reduced from, the other two growing conditions, i.e., MEM alone (no Caco-2 cells) or TGY (Figure 1A). Similarly, after a 1.5 h infection of Caco-2 cell cultures, the CFU of C. perfringens type C strain CN3685 was similar to those obtained for the other two culture conditions, i.e., MEM (no Caco-2 cells) or TGY (Figure S1D). The CN3685 isolate was incubated for only 1.5 h because the presence of this strain was already inducing morphological damage in Caco-2 cells by 2 h post-infection (as described later). These results indicate that the increased protein secretion triggered by C. perfringens infection of Caco-2 cell cultures is not simply attributable to a stimulation of bacterial overgrowth.
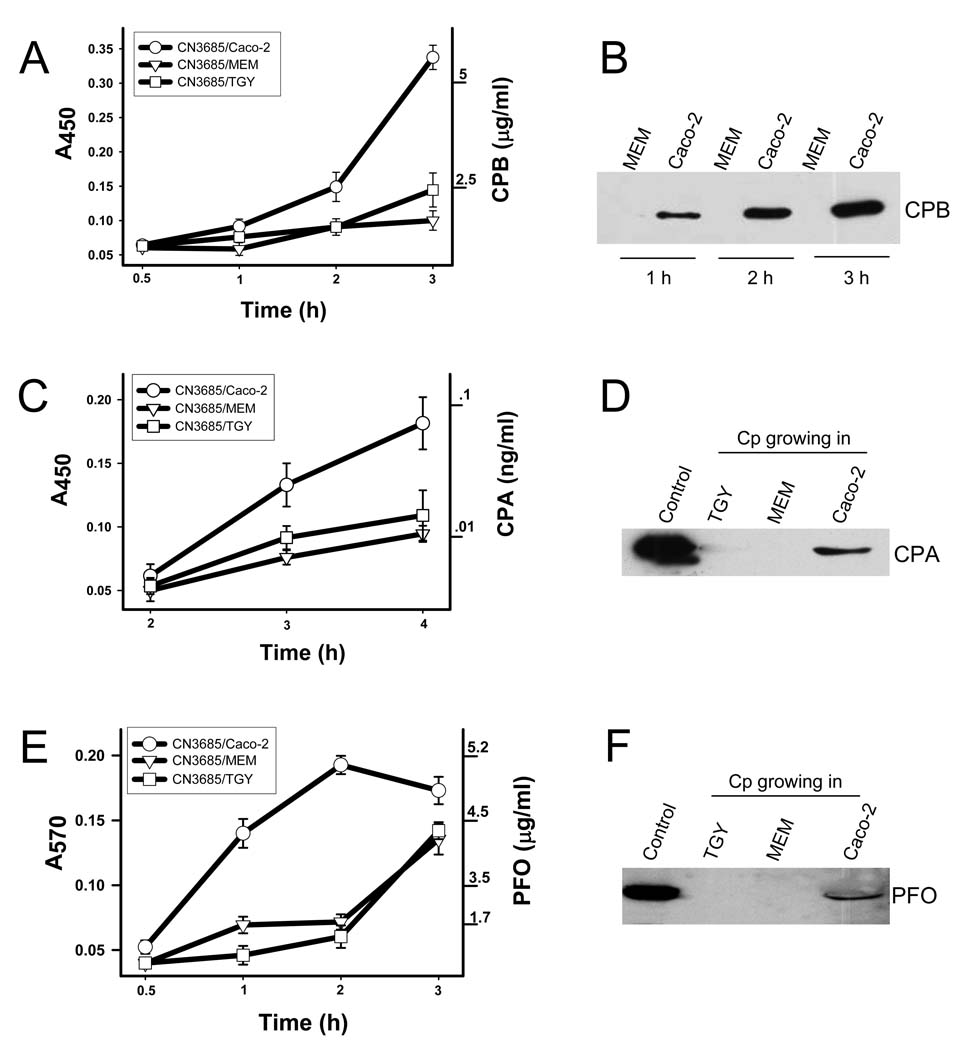
Figure 1. CPB secretion is upregulated during infection of mammalian Caco-2 cells.
(A) Cell culture dishes containing Caco-2 cell cultures, TGY or MEM were each inoculated with 1.5×107 CFU of C. perfringens (Cp) type C isolates JGS1495 and incubated for 3 h at 37°C (MOI=20). The culture supernatants containing bacteria (10 ml) were aspirated, serially diluted in BHI broth (10 ml final volume), and then plated (1 ml) onto BHI agar plates. The number of bacteria (cfu/ml) growing in each culture condition were recorded after a 24 h incubation under anaerobic conditions at 37°C. (B) Cell culture dishes containing FTG, TGY, MEM (no cells) or Caco-2 cells were inoculated with C. perfringens strain JGS1495 and then incubated for 3 h at 37°C. (C) Cell culture dishes containing MEM (no cells) or Caco-2 cells were infected with C. perfringens type C strains isolated from sheep (CN3685), pig (JGS1495) or human (CN5383) for 3 h at 37°C. Culture supernatant was then removed and filter-sterilized. Equal amounts of each supernatant were subjected to SDS-PAGE on a 12% acrylamide gel and proteins were then transferred to nitrocellulose membrane. Purified 35 kDa CPB (0.5 µg) was also included (β toxin lane). Membranes were blocked for 1 h before probing with a mouse monoclonal anti-CPB antibody. Bound antibody was detected with a horseradish peroxidase-conjugated secondary anti-species specific antibody, followed by incubation of blots with a chemiluminescent substrate. D) Caco-2 cells (Human), MDCK cells (Dog) or rat 1R-12 fibroblasts were infected with C. perfringens type C strain JGS1495 for 3 h at 37°C. Culture supernatants were then analyzed as described above. CPB molecular weight is shown at left of each figure in kDa. Figures shown are representative of at least 4 independent experiments.
The presence of Caco-2 cells and other mammalian cells causes rapid up-regulated secretion of C. perfringens beta toxin (CPB)
Since the S1 figure results indicated that some of the increased protein secretion stimulated by type C infection of Caco-2 cells included proteins matching the size of toxins, we next investigated whether CPB might be secreted rapidly into the supernatants of infected Caco-2 cell cultures. When those supernatants were analyzed by Western blotting using a monoclonal anti-CPB antibody, the presence of CPB was detected as early as 2 h post-infection in supernatants of Caco-2 cell cultures infected with C. perfringentype C strain JGS1495 (Figure S1B). In contrast, no CPB signal was detected in 2 h culture supernatants from MEM (no Caco-2 cells), TGY, or FTG (fluid thioglycolate medium) inoculated with JGS1495 (Figure S1B). CPB secretion into the supernatant of Caco-2 cells infected with JGS1495 increased further by 3 h (Figure 1B); in contrast, even after 3 h, no CPB signal was observed in the supernatant of the MEM, FTG or TGY cultures of JGS1495.
To evaluate the prevalence of this Caco-2 cell-induced upregulation of CPB secretion amongst type C isolates, Caco-2 cell cultures were separately infected with two other pathogenic C. perfringens type C strains that had been isolated from either a sheep with struck (CN3685) or a human pig-bel case (CN5383) (Fisher et al., 2006; Sakurai and Duncan, 1977). Western blot analyses showed those two strains resemble JGS1495 in rapidly secreting CPB when incubated in the presence of Caco-2 cells (Figure 1C and Figure 2B), confirming that this upregulated CPB secretion phenomenon is common among C. perfringens type C isolates. ELISA analysis indicated that, compared to 3 h growth in TGY or MEM without Caco-2 cells, >3-fold higher levels of CPB were present in supernatants of Caco-2 cultures infected with CN3685 (Fig. 2A).
Figure 2. Levels of CPB, CPA and PFO toxins secreted in the presence of Caco-2 cells.
ELISA analyses (A and C). Clostridium perfringens type C strain CN3685 was inoculated into Caco-2 cells (CN3685/Caco-2), MEM (CN3685/MEM) or TGY (CN3685/TGY) (MOI=20) and incubated for the indicated time at 37°C. Supernatants were removed and sterilized by filtration. Sterile supernatants and purified CPB (A) or purified CPA (C) were coated in separate wells of an ELISA microplate overnight at 4°C. The wells were incubated with a mouse monoclonal anti-CPB (A) or anti-CPA antibody (C) followed by a HRP-conjugated anti-mouse antibody. The bound antibody was detected with a TMB substrate solution and the color reaction stopped with sulphuric acid (0.18 M). Absorbance at 570 (A450) was determined using an ELISA reader. Western blot analyses (right panels). (B) CPB secretion. Caco-2 cells or MEM cultures were infected with CN3685 for 1, 2 or 3 h and incubated at 37°C. Equal amounts of sterile supernatants were analyzed by Western blot using a mouse monoclonal anti-CPB antibody. Secretion of 47 kDa CPA (D) and 54 kDa PFO (F) by Caco-2 cells grown in 100-mm culture dishes prior to infection for 3 h at 37°C with C. perfringens type C isolate JGS1495 (MOI=20). For comparison, culture dishes containing TGY or MEM (no cells) were similarly inoculated and then incubated under the same conditions. For D and F, supernatants were removed, filter sterilized and concentrated 200-fold (see material and methods). Control sample is an infected 8 h TGY culture that was similarly concentrated. The same amounts of each concentrated supernatant were subjected to SDS-PAGE on a 12% acrylamide gel and then analyzed by Western blotting using a mouse monoclonal anti-CPA (D) antibody or a rabbit-raised polyclonal anti-PFO antibody (F) as described. (E) Hemoglobin (Hb) release assay for PFO activity. Supernatants obtained as above or purified PFO were incubated (1:1) with a 1% suspension of horse red blood cells for 30 min at 37°C. PFO-induced Hb release was detected by obtaining the absorbance at 570 (A570).
To investigate whether the rapid upregulation of CPB secretion can also be stimulated by the presence of other host cells besides Caco-2 cells, MDCK and Rat-1/R12 cell lines were similarly infected with C. perfringens type C isolates. This experiment revealed that rapid upregulation of CPB secretion can be triggered by the presence of dog-, rat- or human-derived cell cultures of kidney, fibroblast or intestinal origin (Figure 1D), suggesting that many mammalian cells produce factor(s) that stimulate C. perfringens type C strains to rapidly secrete CPB.
Secretion of PFO, CPA and CPB2 toxins by type C isolates is also rapidly up-regulated by the presence of Caco-2 cells
In addition to CPB, C. perfringens type C isolates commonly secrete CPB2, CPA and PFO into the medium during late log-phase growth in TGY broth (Fisher et al., 2006; Smedley et al., 2004; Petit et al., 1999). C. perfringens alpha toxin (CPA) is a lethal toxin with phospholipase C and sphingomyelinase activities, while PFO is a lethal, cholesterol-dependent cytolysin (Titball et al., 1999; Tweten, 1988a); CPB2 action is unknown.
Therefore, studies were performed to evaluate whether the rapid host cell-induced CPB secretion noted in Fig. 1 also occurs for other toxins produced by type C isolates. Western blot analyses showed that CPA appears much earlier in the supernatant of Caco-2 cells infected with type C isolates compared to MEM or TGY cultures (Fig. 2D). An ELISA analysis revealed that, relative to equivalent MEM or TGY culture supernatants, CPA secretion is >4-fold higher in the supernatants of 3 h CN3685-infected Caco-2 cells (Fig. 2C).
Similarly, Western blot analyses demonstrated that, compared to MEM or TGY cultures, the presence of Caco-2 cells induces a more rapid secretion of PFO by type C isolates (Fig. 2F). The ability of C. perfringens supernatants to lyse horse erythrocytes and release hemoglobin (Hb) is specifically attributable to PFO activity (Lyristis et al., 1994), as supported by the inability of CN3685 pfoA mutant (Sayeed et al., 2008) to induce Hb release (data not shown). As shown in Fig. 2E, the horse erythrocyte lysis assay revealed that, compared to supernatants of 2 h MEM or TGY cultures of CN3685, there is a >4-fold increase in PFO levels present in supernatants of Caco-2 cell cultures infected for 2 h with C. perfringens type C strain CN3685.
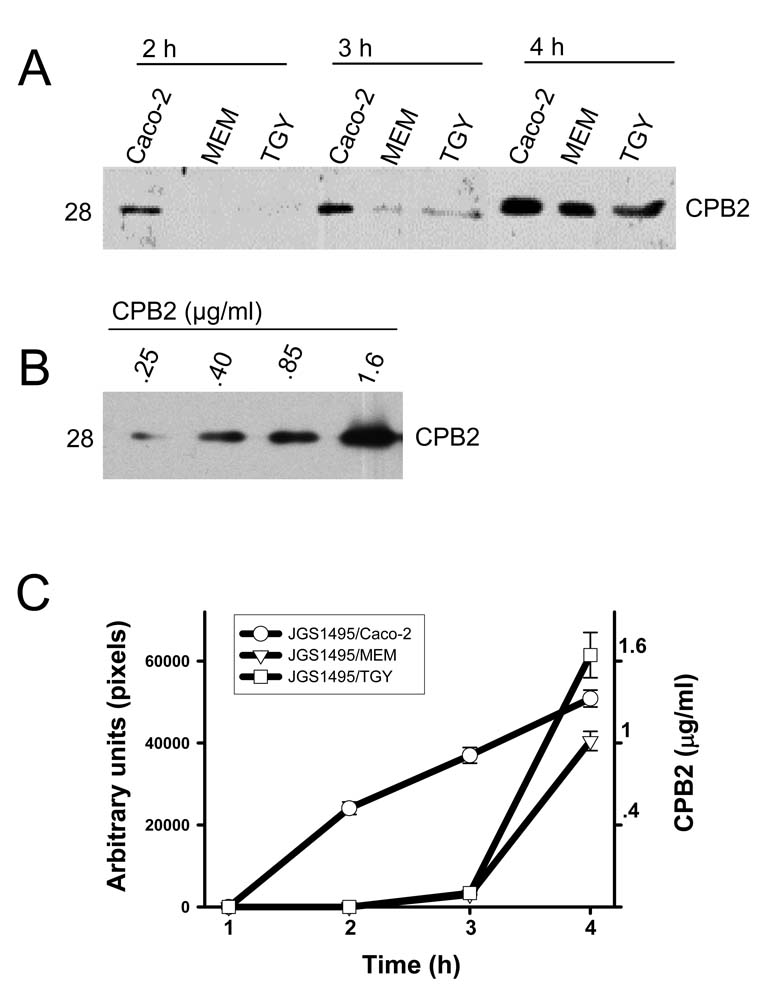
Finally, CPB2 secretion was also specifically stimulated by the presence of Caco-2 cells. Western blotting detected CPB2 in supernatants of Caco-2 cell cultures within 2 h post-infection by strain JGS1495, whereas no CPB2 signal was detected in the supernatant of 2 h FTG, TGY or MEM (no Caco-2 cells) cultures inoculated with a similar amount of this type C isolate (Fig. 3A). Densitometric analysis of these gels identified a >5-fold increase in CPB2 levels in supernatants from 3 h cultures of CN3685-infected Caco-2 cells compared to equivalent TGY or MEM cultures (Fig. 3C)
Figure 3. Levels of CPB2 secreted in the presence of enterocyte-like Caco-2 cells.
Clostridium perfringens type C strain JGS1495 was inoculated into Caco-2 cell cultures (JGS1495/Caco-2), MEM (JGS1495/MEM) or TGY (JGS1495/TGY) (MOI=20) and incubated for the indicated time at 37°C. Supernatants were removed and sterilized. (A) Equal amounts (25 µl) of each supernatant or different amount of purified CPB2 (B) were subjected to SDS-PAGE on a 12% acrylamide gel and proteins were then transferred to nitrocellulose membrane. Membranes were probed by Western blot using a polyclonal anti-CPB2 antibody. CPB2 molecular weight is shown at left of each figure in kDa. C) Quantification of CPB2 secretion. Experiments described above were repeated three times. Western blots were then scanned and their pixel intensity [(A) x-axis or (B) y-axis right] was quantified and graphically integrated against a standard curve of purified CPB2 to determine CPB2 levels.
The rapid host cell-mediated increase in C. perfringens type C toxin secretion follows early upregulated transcription of toxin genes
Collectively, the Figure 1–Figure 3 and S1 results suggested that a rapid global toxin up-regulation, likely part of a well-orchestrated virulence mechanism, is triggered when C. perfringens encounters its mammalian host and that this rapid host cell-induced toxin secretion is not attributable to stimulated bacterial growth. Therefore, RT-PCR analyses explored whether this effect might involve an early onset of toxin gene transcription.
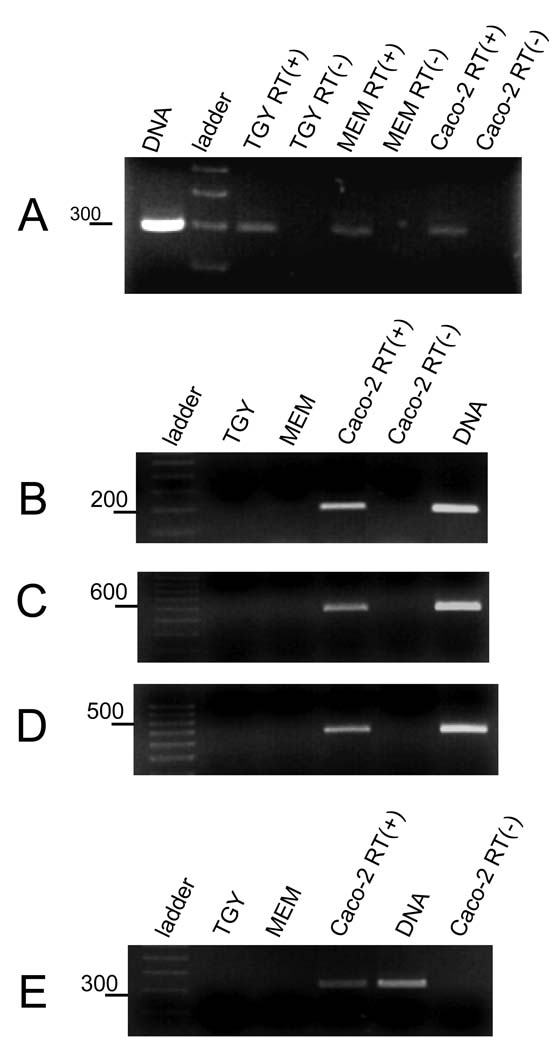
Using 20, 50 or 100 ng levels of RNA extracted from Caco-2 cell cultures that had been infected for only 2 h with type C strain JGS1495, RT-PCR studies detected transcripts encoding CPB, CPB2, PFO and CPA (Figs. 4B, 4C, 4D and 4E respectively). In contrast, no mRNA encoding any of those toxins could be detected by RT-PCR using the same levels of RNA extracted from JGS1495 grown for 2 h in either TGY or MEM (no Caco-2 cells). The type C isolate CN3685 also quickly upregulated transcription of the cpb gene in the presence of Caco-2 cells (Fig. S2A).
Figure 4. The transcription of cpb, cbp2, plc and pfoA toxin genes is quickly upregulated during Caco-2 infection.
Type C strain JGS1495 was inoculated into TGY, MEM or Caco-2 cell cultures (MOI=20) and then incubated for 2 h at 37°C. Bacteria were then collected and pelleted by centrifugation. Total RNA was extracted and treated with DNase I. R T-PCR reactions were performed with 20–100 ng of RNA (results shown are for 100 ng) and using a specific pair of primers to amplify (A) the house keeping polC gene, (B) cpb, (C) cpb2, (D) pfoA, or (E) plc toxin genes. Where indicated, reverse transcriptase (RT) was (+) or was not (−) added into reaction tubes as a control to confirm RT-PCR signals were from RNA rather than DNA. DNA from JGS1495 strain was also included, as a control reaction. Molecular markers shown are a 100 bp increment ladder with selected marker size, in bp, shown at left of each gel. For all panels, products were electrophoresed on a 2% agarose gel and then stained with ethidium bromide for visualization. Shown are representative figures of three independent experiments.
The Fig. 4 results indicated that the rapid upregulated secretion of toxins involves stimulation of toxin gene transcription soon after type C isolates encounter Caco-2 cells. To explore whether this stimulation of toxin gene expression simply reflects a general host cell-mediated increase in transcription of all C. perfringens genes, primers were designed to amplify the alpha subunit of the DNA polymerase III gene (polC), which is a C. perfringens type A housekeeping gene (Myers et al., 2006). As shown in Figure 4A, the polC gene could be PCR-amplified using template DNA extracted from either C. perfringens type C strain JGS1495 (Fig. 4A line 1) or CN3685 (result not shown). Moreover, RT-PCR detected mRNA from the polC gene in C. perfringens cultures growing for 2 h in either TGY, MEM or in the presence of Caco-2 cells (Fig. 4A).
Early transcription of the tpeL toxin gene is not stimulated by the presence of Caco-2 cells
TpeL, a recently described toxin that shares homology with TcdA and TcdB from C. difficile, is secreted by many C. perfringens type C isolates during the stationary phase of in vitro growth (Amimoto et al., 2007). Therefore, experiments were performed to determine whether tpeL toxin gene transcription is also rapidly up-regulated when C. perfringens type C isolates encounter Caco-2 cells. PCR first confirmed that the tpeL gene is present in the type C isolates used in this study and, as previously reported (Amimoto et al., 2007), that this gene in our type C isolates lacks the 3´fragment of the tcdA gene from C. difficile (Fig. 5A).
Figure 5. Transcription of the C. perfringens tpeL toxin gene is not upregulated early during infection of Caco-2 cell cultures.
(A) PCR was performed using primers to specifically amplify either the C. perfringens tpeL gene (lanes 2 and 4) or a 3´ fragment of the C. difficile tcdA gene (lanes 3 and 5) that is not present in the tpeL gene (Amimoto et al., 2007). As template, DNA extracted from C. difficile strain 00030 (C. diff) or C. perfringens strain JGS1495 (C. per) was used. Lane 1 contains 100 bp ladder, selected marker size is shown at left of each gel. (B) RT-PCR for tpeL or polC transcripts. Type C strain JGS1495 was inoculated into a TGY, MEM or a Caco-2 cell culture and then incubated for 2 h at 37°C, or inoculated in TY and incubated for 24 h. Bacteria were collected from growing conditions and pelleted by centrifugation. Total RNA was extracted from those pellets and treated with DNase I. RT-PCR reactions were then performed with 20–100 ng of RNA (results shown are for 100 ng) and using a specific pair of primers to amplify either tpeL or the house keeping polC gene. Where indicated, reverse transcriptase (RT) was (+) or was not (−) added into the reaction tubes. As a control, DNA from JGS1495 was added into a reaction tube. Molecular markers were increments of a 100 bp ladder, size of selected markers, in bp, is shown at the left of the gel.
In contrast to mRNA for other type C toxin genes, tpeL mRNA (Fig. 5B) was not detected by RT-PCR following a 2 h infection of Caco-2 cell cultures by JGS1495 or after 2 h growth of this isolate in TGY or MEM alone. However, tpeL message was detected in a 24 h TY culture of JGS1495, confirming that our RT-PCR assay could detect tpeL transcript, if present (Fig. 5B).
Rapid host cell-mediated up-regulation of C. perfringens type C toxin secretion requires close contact between bacteria and host cells
The results presented above indicate that rapid host cell-stimulated secretion of most toxins made by C. perfringens type C isolates is triggered by an unknown host factor present during infection. Since some host proteins (mostly with molecular masses >60 kDa) are present in the supernatant of non-infected Caco-2 cells (Figs. S1A and S1C, control lanes), we next evaluated whether supernatants of non-infected Caco-2 cell cultures, referred to here as conditioned medium (CM), are sufficient to stimulate the secretion of C. perfringens type C toxins.
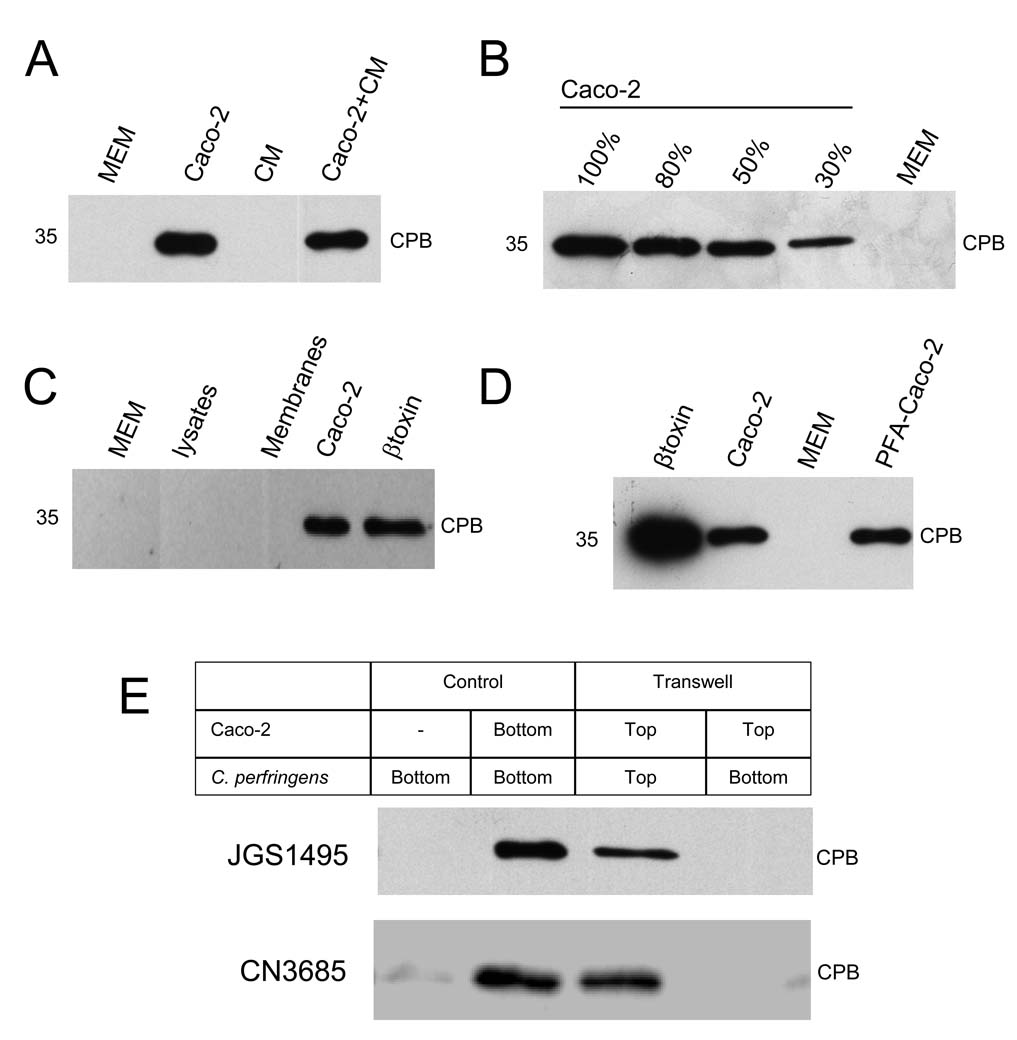
However, as shown in Figure 6A lane 3, CM (like MEM alone, Fig. 6A, lane 1) was unable to stimulate CPB secretion. Furthermore, when CM was added to the Caco-2 cell cultures before infection, CPB secretion remained similar to that of infected Caco-2 cells growing in fresh MEM (Figure 6A, compare line 2 and line 4). This negative result is less likely attributable to a soluble host cell factor being retained by the filter used for sterilizing supernatants since the same filter was freely permeable to a 70 kDa fluorescently-labeled dextran (data not shown). These results could suggest that a soluble factor secreted by Caco-2 cells is not responsible for stimulating rapid toxin secretion by C. perfringens type C isolates.
Figure 6. Upregulation of CPB secretion requires close contact between C. perfringens and host cells.
(A) Conditioned medium (CM) does not stimulate CPB secretion. C. perfringens type C strain JGS1495 was inoculated into a well of a 24-well tissue culture microplate containing; 1 ml of CM alone, 1 ml of MEM alone, Caco-2 cell cultures treated with 1 ml of CM, or Caco-2 cells in 1 ml of fresh (non-conditioned) MEM. After 3 h of incubation at 37°C, supernatants were analyzed by Western blot for CPB production using a mouse monoclonal anti-CPB antibody (expected migration of a 35 kDa protein indicated at left of each blot in panels A–D). (B) CPB secretion increases in proportion to the number of host cells in a culture. C. perfringens type C strain JGS1495 (1.5×107 CFU) was inoculated into Caco-2 cell cultures which were at the following confluency: 30, 50, 80 or 100% and then incubated for 3 h at 37°C. A mock infection without cells (MEM) was also included. Equal amounts of each sterilized supernatant were electrophoresed, transferred to nitrocellulose membranes and analyzed as described above. (C) Living cells, but not Caco-2 cell membranes or cell lysates, stimulated CPB secretion. JGS1495 was inoculated (2.5×108 CFU) into 100 mm tissue culture dishes containing 12 ml of MEM without additives, MEM without additives but with Caco-2 cell lysates, MEM without additives but with the Caco-2 cell membrane fraction or MEM plus confluent living Caco-2 cells. After 3 h of infection at 37°C, supernatants were analyzed by Western blot for CPB levels as described. (D) Fixed Caco-2 cells stimulate CPB secretion. JGS1495 was inoculated into MEM (no cells), Caco-2 cell cultures or plates with paraformaldehyde (PFA)-fixed Caco-2 cells and incubated for 3 h at 37°C. Supernatants were filter-sterilized and then analyzed by Western blot for CPB levels. Purified CPB (0.5 µg) was included (β toxin). (E) C. perfringens type C isolates JGS1495 or CN3685 was inoculated (3×107 CFU) into i) wells of a 12-well culture microplate containing MEM (no cells [-]) or a Caco-2 cell culture or ii) the Transwell filter chamber (Top) containing a confluent culture of Caco-2 cells or iii) the well (Bottom) in which a Transwell filter chamber containing Caco-2 cells was installed (i.e. bacteria and cells are present in the same well but physically separated). The infected cultures were incubated for 3 h at 37°C and the supernatants were collected and filter-sterilized. The sterile supernatants were analyzed by Western blot as above. Shown are representative results from at least four independent experiments.
Since Fig. 6A was consistent with a Caco-2 cell surface component triggering the enhanced toxin secretion observed in infected Caco-2 cultures, this possibility was further tested by varying the numbers of Caco-2 cells infected with a constant number of bacteria. Those studies revealed that as the number of eukaryotic cells in a culture increased, so too did CPB secretion levels (Fig. 6B), consistent with these bacteria sensing the concentration of host cells and then triggering a rapid toxin secretion in response. The host factor responsible for inducing rapid CPB production was trypsin- and phospholipase C (PLC)-resistant, since rapid host cell-induced CPB secretion was unaffected if Caco-2 cells were pretreated with trypsin or CPA (which is a PLC) prior to infection by type C isolates (Figs. 7A and 7B). Interestingly, pretreatment of Caco-2 cells with Pronase enhanced the rapid secretion of CPB (Fig. 7C).
Fig. 7. Treatment of Caco-2 cell cultures with pronase, but not trypsin or PLC, enhances CPB secretion.
(A) Caco-2 cells were treated with trypsin or EDTA for 15 min at 37°C. Detached cells were washed and resuspended in MEM to a final cell density of 70×105 cells/ml. Caco-2 cell cultures, MEM, trypsin-detached or EDTA-detached Caco-2 cells were then infected, in a 24-well microplate, with CN3685 for 1.5 h at 37°C. The supernatants were analyzed for CPB levels by Western blot. (B) Caco-2 cells were treated with 1×10−3 or 5×10−3 U/ml of phospholipase C (PLC) in the form of CPA for 1 h at 37°C. Cells were then washed and added with MEM. Untreated Caco-2 cell cultures, MEM or PLC-treated Caco-2 cells were infected with CN3685 for 1.5 h at 37°C. The supernatants were analyzed for CPB levels by Western blot. (C) Caco-2 cells were treated with pronase (100 µg/ml) for 20 min at 37°C. Detached cells were thoroughly washed and resuspended in MEM to a final cell density of 7×105 cells/ml. MEM, Caco-2 cell cultures or pronase-treated Caco-2 cells were then infected, in a 24-well microplate, with CN3685 for 45 min or 1.5 h at 37°C. The supernatants were analyzed for CPB levels by Western blot. Expected migration of a 35 kDa protein indicated at left of each blot.
To further explore whether Caco-2 cell-associated surface components are sufficient for stimulating toxin secretion, C. perfringens type C strain JGS1495 was incubated for 3 h with MEM containing either the insoluble membrane fraction of Caco-2 cells or Caco-2 cell lysates. Neither those isolated Caco-2 cell membranes nor Caco-2 cell lysates stimulated CPB secretion like intact, living Caco-2 cells (Fig. 6C), which could suggest that intact host cells, if not viable host cells, are needed to trigger rapid CPB secretion by type C isolates.
To evaluate whether viable host cells are needed to trigger rapid CPB secretion, Caco-2 cells were fixed using paraformaldehyde (PFA), which preserves the cell surface. CPB secretion was similar when C. perfringens type C strains JGS1495 or CN3685 were used to infect either living Caco-2 cells or the PFA prefixed-Caco-2 cells (Fig. 6D and Fig S2B, respectively). This experiment revealed that rapid toxin secretion by C. perfringens type C strains involves interactions with intact, but not necessarily viable, host cells. In addition, rapid CPB secretion in the presence of fixed cells was not seen when those PFA-fixed cells were incubated in PBS and that culture was then infected by CN3685, indicating a need for bacterial metabolism to upregulate Caco-2 cell-induced toxin production (Figure S2B).
To confirm definitively that close bacteria:host cell contact is a crucial step to trigger rapid toxin secretion, bacteria and Caco-2 cells were incubated in the same culture dish, but physically separated by a permeable 0.4 µm pore-size Transwell system membrane (see Materials and Methods). Consistent with our previous results, CPB secretion was stimulated when C. perfringens type C strain CN3685 or JGS1495 was allowed direct contact for 2 h or 3 h, respectively, with Caco-2 cells in the same chamber of the Transwell (Fig. 6E, line 3). However, rapid CPB secretion was abated when Caco-2 cells were similarly incubated with C. perfringens present in the same culture well but blocked for close physical contact by the Transwell membrane (Fig. 6E, line 4). This inhibition is less likely due to a soluble host cell factor being retained by the Transwell membrane since 70 kDa fluorescently-labeled dextran readily crossed this membrane (data not shown). These results support intimate bacteria:host cell contact as a key step for host cell-stimulated rapid toxin secretion by C. perfringens type C isolates.
Finally, the close physical contact needed to trigger rapid upregulation of toxin secretion by type C isolates does not involve tight bacterial adherence to Caco-2 cells, i.e., gentle washing displaced JGS1495 or CN3685 from the Caco-2 cell monolayer surface (not shown).
C. perfringens type C strains are rapidly cytotoxic for Caco-2 cells
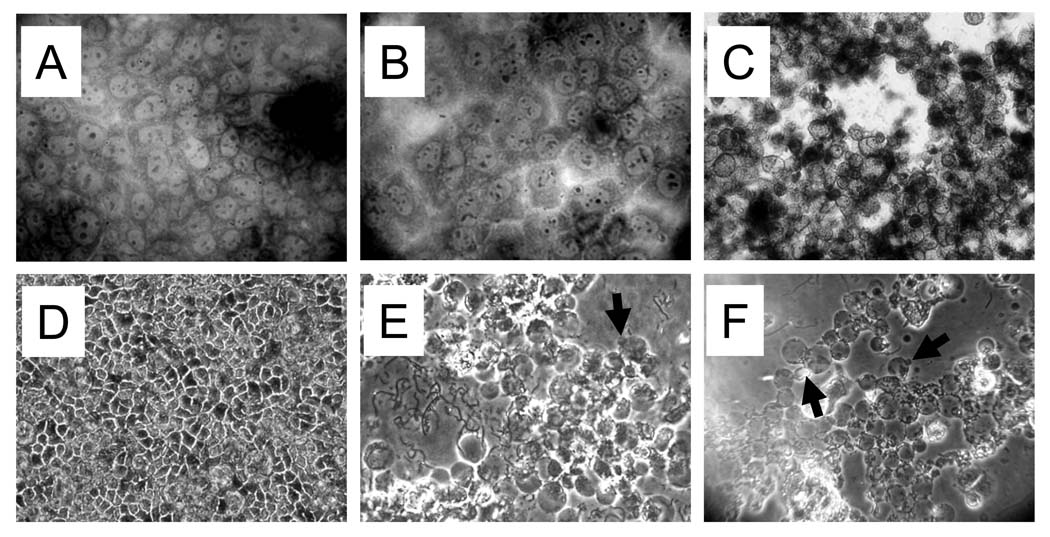
We next investigated whether the rapid host cell-induced stimulation of toxin production and secretion by C. perfringens type C isolates has cytotoxic consequences for Caco-2 cells. To test this, Caco-2 cells were infected with C. perfringens type C strain CN3685 for 1 h. As a control, bacteria were similarly grown for 1 h in MEM (no Caco-2 cells). Both supernatants containing secreted proteins were collected, filter-sterilized and added to fresh Caco-2 cell cultures for 2 h. As shown in Figure 8B, the supernatant from bacteria that had been incubated for 1 h in MEM (without Caco-2 cells) did not damage Caco-2 cells within 5 h. However, by that same time, the supernatant collected from infected Caco-2 cell cultures had induced morphological damage, which was characterized by cell rounding, cellular contraction, and membrane blebbing (Figure 8C). After 6 h of incubation, this treated Caco-2 cell monolayer had detached from the glass substratum.
Figure 8. C. perfringens type C strains are cytotoxic for Caco-2 cells.
(A–C) Cytotoxic consequences of host cell-induced stimulation of toxin secretion. C. perfringens type C strain CN3685 was inoculated into tissue culture wells containing MEM (no cells) or Caco-2 cells for 1 h. The culture supernatant was removed and filter-sterilized. The sterilized supernatant from inoculated MEM alone (no Caco-2 cells) (B) or infected Caco-2 cell cultures (C) was individually added to fresh confluent Caco-2 cells and incubated for 5 h at 37°C. Then, treated or untreated Caco-2 cells (A) were washed three times with pre-warmed PBS, fixed with 70% methanol and stained with Giemsa stain. Slides were analyzed at a magnification of 40X with standard bright-field light microscopy. (D–F) Cytotoxicity induced by C. perfringens type C infection. Phase-contrast microscopy of confluent Caco-2 cell cultures left uninfected (D) or infected with CN3685 (E) or JGS1495 (F) (MOI=20) for 5 h at 37°C. Arrows show membrane blebs.
To better characterize this cytotoxicity phenotype, a Caco-2 cell monolayer was infected with either C. perfringens type C strain CN3685 or JGS1495 at a MOI=20 and cell morphology changes were then followed (with cytotoxicity scored as described in Material and Methods). Interestingly, as early as 2 h post infection, strain CN3685 induced swelling of Caco-2 cells; by 3 h post-infection, this effect turned into a striking cytotoxic phenotype characterized by rounding, detachment of cells from the glass, and formation of some membrane blebs (Table 1 and Fig. 8E). A similar cytotoxic phenotype was induced by strains JGS1495 or CN5383, although those strains induced cytotoxicity more slowly than CN3685. Morphologic damage in JGS1495- or CN5383-infected Caco-2 cells cultures was observed by 3.5 h post-infection and rounding of the cell monolayer with membrane blebbing was seen by 5 h post infection (Table 1 and Fig. 8F). Together, these results indicate that C. perfringens type C strains produce toxins that are extremely cytotoxic for Caco-2 cells and that co-incubation of these bacteria with Caco-2 cells hastens the development of this cytotoxicity.
Table 1.
Morphological changes induced on Caco-2 cells by C. perfringens strains.
| Strain | Time (h) | Cytotoxicity* |
|---|---|---|
| CN3685 | 1.5 | − |
| 2 | + | |
| 2.5 | ++ | |
| 3 | +++ | |
| CPJV47 | 1.5 | − |
| 2 | − | |
| 2.5 | + | |
| 3 | + | |
| CPJV47(pJVRS3) | 1.5 | − |
| 2 | + | |
| 2.5 | ++ | |
| 3 | +++ | |
| JGS1495 | 1.5 | − |
| 2 | − | |
| 2.5 | − | |
| 3 | − | |
| 3.5 | + | |
| 4 | ++ | |
| 5 | +++ | |
| CN5383 | 1.5 | − |
| 2 | − | |
| 2.5 | − | |
| 3 | − | |
| 3.5 | + | |
| 4 | ++ | |
| 5 | +++ |
(+) >80% of Caco-2 cells were swollen, (++) <50% of Caco-2 cells were swollen and <50% of cells were rounded and (+++) >80% of Caco-2 cells were rounded and detached from the glass.
The luxS-controlled quorum sensing mechanism does not regulate rapid Caco-2 cell-induced CPB secretion
It was previously reported that a luxS-controlled quorum sensing mechanism partially regulates in vitro toxin production and toxin gene transcription for C. perfringens type A strain 13 (Ohtani et al., 2002). To evaluate whether luxS-mediated quorum sensing is required for the rapid Caco-2 cell-induced upregulation of toxin gene transcription and toxin secretion observed for type C strains, the luxS gene was insertionally-inactivated in CN3685 by using our previously described Targetron® technology (Li and McClane, 2008; Sayeed et al., 2008; Chen et al., 2005). A Group II intron (~ 900 bp) was inserted, in the sense orientation, between nucleotides 295|296 of the CN3685 luxS ORF. The presence of this intron insertion into the luxSgene of the mutant (CPJV19) was shown by PCR using two luxS-specific primers that supported PCR amplification of an ~300 bp product from the wild-type luxSgene, but (due to the intron insertion) amplified a larger ~1.2 kb product from CPJV19 (Fig. 9A). A Southern blot confirmed the presence of only a single intron insertion in the CPJV19 genome (Fig. S3A) and RT-PCR analyses showed that luxS mRNA was not made by CPJV19 (Fig. 9B).
Figure 9. luxS-controlled quorum sensing mechanism is not required for Caco-2 cell induced upregulation of toxin production by CN3685.
A) PCR showing an intron insertion in the luxS gene. Primers LuxS-L and LuxS-R amplified a 300 bp product of the luxS gene using DNA isolated from CN3685 (WT) or a 1.2 kb luxS-intron product using ΔluxS DNA (CPJV19). B) luxS mRNA is not produced by CPJV19. RNA (100 ng) isolated from an overnight TGY culture of CN3685 (WT) or CPJV19 was used as template for RT-PCR reactions using the LuxS-L and LuxS-R primers. Where indicated, retrotranscriptase (RT) was (+) or was not (−) added into the reaction tubes. As additional controls, reactions containing DNA from the WT or CPJV19 strain were included. Shown at left is a 100 bp ladder; selected marker size, in bp, are noted at left of the gel. C) CPB secretion is not affected in CPJV19. CN3685 (WT) or CPJV19 was infected in Caco-2 cell cultures for 2 h or 3 h (MOI=20). Sterile culture supernatants were obtained and analyzed by Western blot using a mouse monoclonal anti-CPB; expected migration of the 35 kDa CPB protein is noted on the left of the blot. D) Transcription of cpb, plc and pfoA toxin genes by CPJV19. Caco-2 cells were infected with CN3685 (WT) or CPJV19 for 2 h. Bacterial RNA (100 ng) was then isolated and used in RT-PCR reactions with primers to amplify the cpb, plc or pfoA genes. Where indicated, retrotranscriptase (RT) was (+) or was not (−) added into the reaction tubes. Reactions containing DNA from the WT was included. A 100 bp ladder is shown at left and selected marker size, in bp, are noted at left of the gel. Figures shown are representatives of at least three independent experiments.
Culture supernatants from Caco-2 cells infected for 2 or 3 h with CN3685 (WT) or CPJV19 showed a similar CPB signal, as detected by Western blot (Fig. 9C). As expected, no signal was detected in the supernatant of MEM cultures of CN3685 or CPJV19 at those same time points (data not shown). RT-PCR analyses detected cpb, plcand pfoA transcripts after a 2 h infection period of Caco-2 cell cultures with the WT strain or CPJV19 (Fig. 9D). To further evaluate the role of LuxS-controlled quorum sensing for in vitro toxin production, a kinetic analysis of CPB secretion was conducted. In this experiment, CPB secretion by, and growth rates for, the luxS mutant remained similar to WT levels during a 7 h culture in TGY, indicating that CPB secretion was not affected by the absence of the luxS-controlled quorum sensing system (Fig. S3B and S3C).
The virS/virR two-component regulatory system regulates the rapidly increased production and secretion of CPB and PFO in the presence of Caco-2 cells
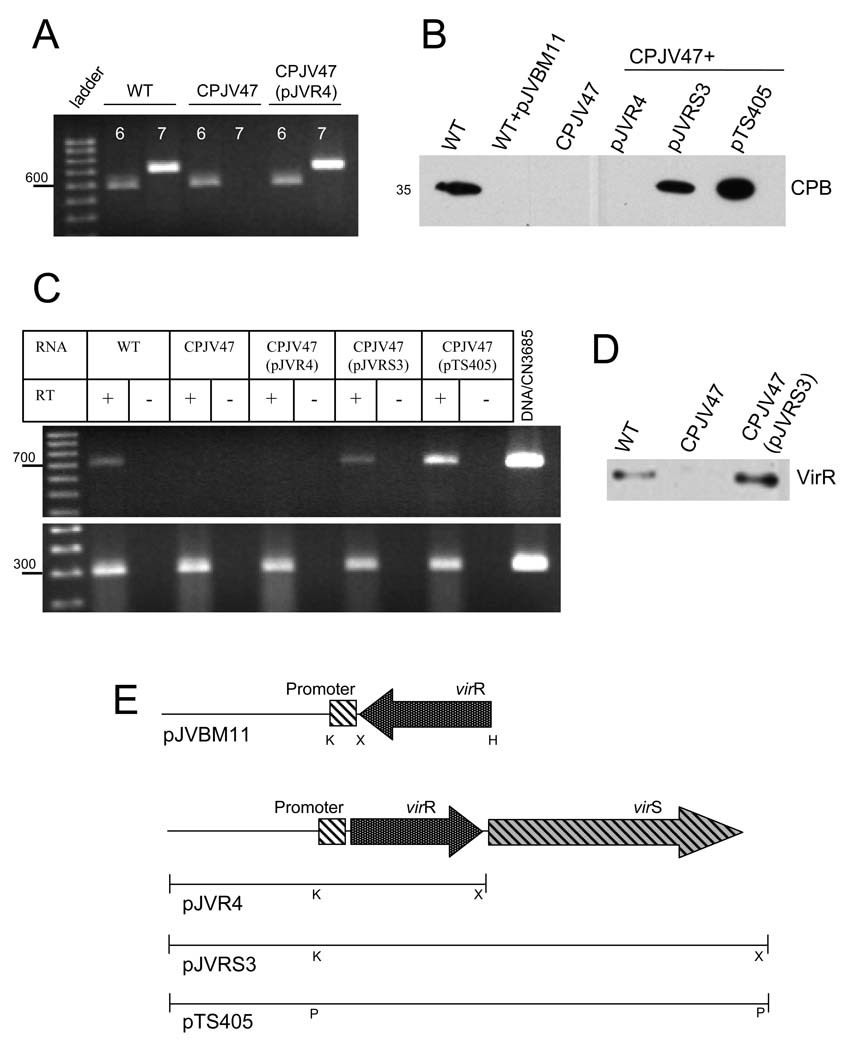
To address whether the VirS/VirR two-component regulatory system plays a role in Caco-2 cell-induced upregulation of toxin production and secretion by type C isolates, a CN3685 virR mutant (CPJV47) was constructed using an E. coli-based suicide plasmid (pKOR) that contains an ~590 bp fragment of the virR gene upstream of a tetracycline resistance gene (Shimizu et al., 1994). As shown by PCR, a ~590 bp virR fragment had replaced the ~710 bp wt virR gene in the mutant strain CPJV47 (Fig. 10A). RT-PCR analyses showed that CPJV47 does not produce virR mRNA (Fig. 10C) and Western blot analyses using an anti-VirR antibody confirmed that the VirR protein was not produced by CPJV47 (Fig. 10D).
Figure 10. The virS/virR two-component regulatory system regulates the Caco-2 cell-induced increase in CPB secretion.
A) Inactivation of the virR gene. PCR was performed with primers that amplify (6) a ~590 bp product or (7) ~710 bp virR wt gene using DNA from CN3685 (WT), CPJV47 or CPJV47(pJVR4). A 100 bp ladder is shown at left and migration of the 600 bp marker is noted. B) CPB secretion. Caco-2 cell cultures were infected with the indicated strain for 2 h at 37°C. Sterile culture supernatants were obtained and analyzed by Western blot using a mouse monoclonal anti-CPB (migration of the 35 kDa CPB protein is noted at left and right of the blot). C) Caco-2 cells were infected with CN3685 (WT), CPJV47, CPJV47(pJVR4), CPJV47(pJVRS3) or CPJV47(pTS405) for 1 h. RNA (50 ng) isolated from bacteria was used as template in RT-PCR reactions with primers that amplify (top panel) the virR gene or (bottom panel) the housekeeping polC gene. Where indicated, retrotranscriptase (RT) was (+) or was not (−) added into the reaction tubes. Reactions containing DNA from the WT strain was included. A 100 bp ladder is shown at left and the location of 700 bp (top panel) and 300 bp (bottom panel) markers is noted. Figures shown are representatives of at least three independent experiments. D) Production of the VirR protein. Cytoplasmic proteins (20 µg) from CN3685 (WT), CPJV47 or CPJV47(pJVR3) were electrophoresed and transferred to nitrocellulose membranes. The membranes were probed by Western blot using an anti-VirR antibody. E) Schematic diagrams showing the virR antisense vector (pJVBM11) or the plasmids pJVR4, pJVRS3 and pTS405. Note that pJVBM11 has the virR gene in the antisense orientation with respect to the virR promoter. pJVR3 only encodes the CN3685 virR gene, pJVRS3 encodes the virS/virR operon from CN3685, and pTS405 encodes the virS/virR operon from Strain 13. K, KpnI; X, XbaI; H, HindIII, P, PstI.
When CPJV47 was incubated in the presence of Caco-2 cells, no CPB secretion was observed after 2 h (Fig. 10B). In addition, RT-PCR analyses detected no cpbor pfoA mRNA using template RNA isolated from CPJV47 following a 2 h infection of Caco-2 cells (Fig. 11). Quantitative RT-PCR confirmed that, compared to the wt levels, CPJV47 had a significant decrease of cpb or pfoA mRNA levels. However, within 1 or 2 h post-infection, plc mRNA was produced by CPJV47 at the same levels as that of the wt strain, indicating that the rapid Caco-2 cell-induced increase in plc gene transcription can still occur in the absence of the virS/virR operon when this strain is grown in the presence of Caco-2 cells (Figs. 11C and 11D).
Figure 11. The virS/virR two-component regulatory system controls the Caco-2 induced increase in transcription of cpb and pfoA.
Caco-2 cells were infected with CN3685 (WT), CPJV47, CPJV47(pJVR4), CPJV47(pJVRS3) or CPJV47(pTS405) for 1 h at 37°C. Bacteria-containing supernatants were obtained to isolate the RNA as described in Material and Methods. Panels A–C, RT-PCR reactions were then conducted with 50 ng of RNA and primers that amplify the genes, (A) cpb, (B) pfoA or (C) plc/cpa. Where indicated, retrotranscriptase (RT) was (+) or was not (−) added into the reaction tubes. Reactions containing DNA from the WT was included. A 100 bp ladder is shown at left, with location of 200 bp (A), 700 bp (B) or 300 bp (C) markers is noted. Shown are representative figures of at least three independent experiments. Panel (D), Quantitative RT-PCR was then performed with 20 ng of the indicated RNA from infected Caco-2 cell cultures and primers that amplified the pfoA, cpb or plc/cpa gen. Average CT values were normalized to the housekeeping polC gene and the fold differences were calculated using the comparative CT method (2−ΔΔCT) (Livak and Schmittgen, 2001). Values below each bar indicate the calculated fold change relative to the wt strain CN3685. Shown is a representative graphic of three independent experiments.
To confirm a role for the VirS/VirR system in Caco-2 cell induced rapid transcriptional upregulation of some toxin genes, a virR antisense vector was constructed as described in Materials and Methods. The antisense virR mRNA produced from this vector inhibits protein production by mediating the catalytic degradation of the target virR mRNA or by binding to sites on virR mRNA essential for translation (Laursen et al., 2005). As shown in Figure 10B, CN3685 transformed with the virR antisense vector (WT+pJVBM11) did not rapidly secrete CPB into the supernatant of infected Caco-2 cell cultures. This strain was also non-hemolytic when grown on blood agar plates [CN3685 produces PFO to induce beta-hemolysis (Sayeed et al., 2008)] and RT-PCR analyses confirmed that the pfoA gene was not expressed after a 2 h infection of Caco-2 cell cultures. However, as also shown for CPJV47, plcmRNA was still produced within 2 h in the presence of Caco-2 cells (data not shown). Results presented above suggested that the virS/virR operon of CN3685 is required for regulating the early production of CPB and PFO, but not CPA, in the presence of enterocyte-like Caco-2 cells.
To prove that the loss of early CPB production by CPJV47 was specifically due to inactivation of its virR gene, either the wild-type virR gene alone or both the virR and virS genes together were amplified from type C strain CN3685 and ligated into the pJIR750 shuttle plasmid under the control of the virR promoter, to generate pJVR4 and pJVRS3, respectively (Fig. 10E). Those plasmids were then individually electroporated into the virR mutant strain CPJV47. In addition, the plasmid pTS405 (Okumura et al., 2008) which contains a PstI-digested fragment of Strain 13 chromosomal DNA that includes both the virR and virS genes, was also electroporated into CPJV47. The presence of the virR gene in CPJV47(pJVR4), CPJV47(pJVRS3) and CPJV47(pTS405) was confirmed by PCR (Fig. 10A and not shown). Consistent with previous studies indicating that the virR and virS genes are co-transcribed in an operon (Shimizu et al., 1994), RT-PCR analyses detected virR mRNA in both CPJV47 derivatives [i.e. CPJV47(pJVRS3) and CPJV47(pTS405)] complemented with the virS/virR operon, but not in CPJV47(pJVR4), which only encodes the virR gene (Fig. 10C). Western blot analyses using an anti-VirR antibody confirmed that the VirR protein had been produced by CPJV47(pJVRS3) (Fig. 10D).
This complementation restored CPB secretion by CPJV47(pJVRS3) and CPJV47(pTS405), but not by CPJV47(pJVR4) (Fig. 10B). RT-PCR detected transcription of cpb and pfoA mRNA using RNA isolated from CPJV47(pJVRS3) or CPJV47(pTS405) after a 2 h infection of Caco-2 cells (Fig. 11A and 11B). Moreover, qRT-PCR demonstrated the same levels of pfoA, cpb or cpa mRNA in the wt strain CN3685 and CPJV47(pJVRS3) after a 1 h infection of Caco-2 cells (Fig. 11D). Altogether, these results confirm that the virS/virR operon regulates the early transcription of cpb and pfoA by CN3685 in the presence of Caco-2 cells.
Finally, a Caco-2 cell cytotoxicity assay showed that the virR mutant CPJV47 strain was less cytotoxic than the WT strain for Caco-2 cells. However, complementing back the virS/virR operon completely restored the cytotoxic phenotype of this strain (Table 1).
Discussion
To our knowledge, this is the first study of clostridial toxin gene regulation in the presence of host cells, an important topic since pathogenic clostridia often rely upon in vivo production of potent toxins to cause animal and human disease (McClane, 2006; Voth and Ballard, 2005; Songer, 1996). With specific respect to C. perfringens type C isolates, previous in vitro studies had indicated these bacteria produce toxins mainly during the late-log or stationary growth phases (Amimoto et al., 2007; Fernandez-Miyakawa et al., 2007; Fisher et al., 2006; Sayeed et al., 2005; Voth and Ballard, 2005), consistent with the absence of early toxin production noted in the current study for type C isolates growing in TGY medium. In contrast to those in vitro data, the current research revealed that the presence of intestinal-like Caco-2 cells causes C. perfringens type C isolates to rapidly upregulate their toxin gene expression. These kinetic differences between in vivo vs. in vitro toxin regulation suggest some need for caution when extrapolating in vitro toxin regulation results to the in vivo situation.
More importantly, the discovery of rapid host cell-induced toxin upregulation suggests new insights into clostridial virulence. Many human and animal clostridial diseases begin in the intestines (McClane et al., 2006), including human necrotic enteritis caused by C. perfringens type C isolates. This necrotic enteritis can be fatal within 48 h, as toxins produced quickly in the intestines are then absorbed into the circulation to affect internal organs (Matsuda et al., 2007; Gui et al., 2002). The same explosive disease progression can also occur in animals, e.g. type C isolates cause “struck” in lambs, a disease named because the animal dies so suddenly that it appears to have been struck by lightning (Songer, 1996). Our current results suggest that the rapid disease and death associated with type C infections may involve pathogenic C. perfringens type C isolates sensing the presence of host cells to rapidly upregulate their secretion of potent toxins such as CPB, CPB2, CPA and PFO. Consistent with rapid host-cell mediated toxin secretion upregulation contributing to the virulence of type C infection, the current study showed that supernatants from infected Caco-2 cells cultures cause cytotoxic consequences in Caco-2 cells more rapidly than do supernatants from MEM (no Caco-2 cells) cultures.
To our knowledge, the rapid Caco-2 cell-induced toxin secretion reported in this study represents the first identification of cross-talk host:pathogen communication affecting production of classical exotoxins, which are the hallmark of clostridial infections. This phenomenon appears to be widespread among pathogenic C. perfringens type C isolates, as several different type C strains isolated from diseased humans or several domestic animal species were shown to rapidly upregulate their toxin production and secretion in the presence of Caco-2 cells. Nor is this phenomenon restricted to type C isolates, as two C. perfringens type D isolates also rapidly upregulated their production and secretion of epsilon toxin in the presence of Caco-2 cells (Fig. S4).
Toxin upregulation in type C isolates is not only induced by contact with human-derived intestinal Caco-2 cells, since similar effects can be triggered by exposure to dog kidney MDCK cells or rat fibroblast 1R-12 cells. The ability of all three surveyed mammalian cell lines to enhance toxin secretion suggests that the factor(s) triggering this effect is widely distributed among host cells, which may be important since it could indicate that host cell-induced toxin upregulation can also occur in extraintestinal C. perfringens infections, possibly including gas gangrene (which is mediated by CPA and PFO (Lyristis et al., 1994)).
To begin identifying the nature of the host cell factor(s) responsible for upregulated toxin secretion of CPB (and probably other toxins), we showed that toxin upregulation is dependent upon close contact, although not tight adherence, between C. perfringens and host cells. The inability of sterile Caco-2 cell supernatants to stimulate CPB secretion, and the sharp reduction noted when type C isolates and Caco-2 cells were physically separated by a Transwell filter, could suggest that this signaling does not involve a soluble factor derived from host cells (further discussion later). Similarly, Caco-2 cell membrane preparations or Caco-2 cell lysates also failed to cause type C isolates to upregulate their CPB secretion. However, enhanced CPB secretion was observed when these C. perfringens isolates were incubated in the presence of PFA-fixed Caco-2 cells. These results could suggest (further discussion later) that, to trigger maximal upregulation of toxin secretion, C. perfringens type C isolates must recognize some factor(s) present in a proper conformation on the host cell surface. This possibility is consistent with the increased CPB production observed after Caco-2 cells were pretreated with pronase, which might unmask a signaling molecule on the Caco-2 cell surface.
With respect to bacterial mechanisms, our study found that this host cell-induced toxin secretion does not simply involve host cell stimulation of bacterial growth, as C. perfringens CFU values were similar in the presence or absence of Caco-2 cells. Instead, the quick appearance of secreted toxins in infected Caco-2 cell cultures correlated with a rapid onset of toxin gene transcription, since RT-PCR showed rapidly upregulated transcription of cpb, cpb2, plc and pfoA toxin genes in the presence of Caco-2 cells. However, the presence of Caco-2 cells does not induce transcription of all type C toxin genes, since transcription of the tpeL gene was not detectable during a 2 h infection period of Caco-2 cells, TGY or MEM alone. This result indicates that tpeL expression is regulated by a different regulatory mechanism from that of other type C toxin genes, which is consistent with previous studies showing that, unlike other type C toxins, TpeL is made in vitro only during stationary phase (Amimoto et al., 2007).
Two component regulatory systems often mediate the responses of pathogenic bacteria to environmental alterations. In C. perfringens type A strains, a classic two component regulatory system named VirS/VirR was previously shown to control, at least in part, in vitro production of CPA, PFO, kappa-toxin (collagenase) and CPB2 (Ohtani et al., 2003; Ba-Thein et al., 1996; Lyristis et al., 1994). VirS, a histidine kinase located in the bacterial membrane, undergoes autophosphorylation after activation by a still unidentified external stimulus. VirS then phosphorylates VirR, which in turn directly activates the transcription of pfoA by binding to a VirR box located −40 to −80 bp upstream of the transcriptional start site for pfoA (Cheung et al., 2004). VirR also indirectly increases transcription of the plc, colA, cpb2 and other genes (Okumura et al., 2008; Cheung et al., 2004; Ohtani et al., 2003; Lyristis et al., 1994). However, VirR boxes are absent from the region upstream of those toxin genes; instead, in vitro transcription of those toxin genes is regulated by VirR binding to VirR boxes located upstream of the promoter controlling production of a regulatory RNA named VR-RNA (Okumura et al., 2008; Banu et al., 2000).
Our current results indicate that the early production of CPB and PFO (but not CPA) induced by the presence of Caco-2 cells is also dependent upon the VirS/VirR two-component regulatory system. For example, both CN3685 encoding a virR antisense plasmid (WT+pJVBM11) and an isogenic CN3685 virR mutant (CPJV47) did not secrete CPB or PFO after a 3 h infection of Caco-2 cell cultures (Figures 9B and not shown). Notably, CPJV47 was also less cytotoxic for Caco-2 cell cultures than the WT strain (Table 1). Rapid Caco-2 cell-induced CPB secretion, as well as Caco-2 cell cytotoxicity, was restored when CPJV47 was complemented with the virS/virR operon, indicating that a functional virS/virR operon is required for production of toxins and is also important for cytotoxicity in vivo. This discovery that a functional VirS/VirR system is necessary for the rapid Caco-2 cell-induced upregulation of cpb transcription (as well as pfoA transcription) is interesting since VirR boxes are not readily identifiable immediately upstream of the cpb promoter. This may suggest that cpb transcription is under the control of a regulatory RNA, which could be VR-RNA or another recently-identified C. perfringens regulatory RNA (Okumura et al., 2008). In contrast to the cpb results, rapid Caco-2 cell-induced upregulation of plc transcription occurred even in a virR null mutant of a type C isolate. Unfortunately, the involvement of VirS/VirR in regulating the in vivo transcription of cpb2 by type C isolates could not be assessed because our cpb2-positive type C isolates were not sufficiently transformable to construct a VirR mutant.
Despite several qualitative similarities between VirS/VirR-mediated toxin gene regulation during in vivo versus in vitro growth (e.g., pfoA transcription is completely dependent on a functional VirS/VirR system both in vivo and in vitro, while plc transcription is not), an obvious difference between in vitro vs. in vivo toxin gene regulation by VirS/VirR concerns transcriptional kinetics. Type C isolates slowly produce PFO, PLC, CPB2 and CPB in TGY medium (Fisher et al., 2006), but the presence of Caco-2 cells cause more rapid toxin production by wild-type type C isolate compared to an isogenic virR mutant (this study). This suggests that a major role for VirS/VirR during type C disease may be to mediate rapid host cell-induced upregulation of some toxins, including CPB. This in vivo VirS/VirR toxin regulation should have significant pathogenic consequences since CPB is required for type C isolate virulence (Sayeed et al., 2008). If future animal studies confirm the importance of VirS/VirR for type C virulence, this would provide the first linkage of VirS/VirR to some C. perfringens enteric diseases. VirS/VirR has already been shown to be important for C. perfringens-induced gas gangrene in the mouse model (Lyristis et al., 1994); as mentioned earlier, this requirement for VirS/VirR in C. perfringens-induced gas gangrene could involve, at least in part, mediating the upregulation of toxin production after type A isolates contact muscle or other host cells.
It is not yet clear why C. perfringens transcribe toxin genes more quickly in the presence of host cells. However, these differences could involve, in part, the signaling that activates the VirS sensor. VirS/VirR signaling molecules remain poorly understood, but this two-component system might become more rapidly activated in the in vivo environment because either, i) VirS responds to a different signal under in vivo vs. in vitro conditions (e.g. perhaps a Caco-2 cell surface molecule provides the in vivo signal?) or ii) the same signal is produced by C. perfringens under both in vivo and in vitro conditions, but the presence of Caco-2 cells directly or indirectly triggers greater or faster production of this signal.
Quorum sensing molecules often provide the signals that modulate two component regulatory systems to upregulate virulence factor expression during bacterial disease (Novick, 2003) (Clarke et al., 2006). In C. perfringens type A strain 13, the luxS gene product (which is involved in production of the AI-2 quorum sensing autoinducer) has been previously implicated in regulating the in vitro transcription of the plc and pfoA genes (Ohtani et al., 2002). However, the current study still observed rapid transcriptional upregulation of the cpb, pfoA and plc genes when a luxS mutant of CN3685 was used to infect Caco-2 cell cultures, indicating the AI-2 autoinducer is not required for rapid in vivo toxin gene transcription by this type C isolate.
Finally, further studies are clearly needed to better identify the mechanism by which contact with Caco-2 cells induces C. perfringens to rapidly upregulate production of several toxins. It is particularly interesting that this effect can involve VirS, which is located in the plasma membrane and thus buried under a peptidoglycan cell wall. Among several possibilities to explain how VirR could be activated upon close contact of C. perfringens with host cells, VirR activation might involve interactions between surface factors on the host and bacterial cells, with the C. perfringens surface factor then signaling VirS. This hypothesis would appear to conflict with the inability of isolated Caco-2 cell membranes to mediate upregulated toxin production; however that negative result might simply reflect the loss or inactivation of the necessary Caco-2 cell surface factor during membrane isolation. An alternative explanation is that a soluble host-derived factor mediates VirR activation. Although appearing inconsistent with the lack of toxin upregulation observed, i) using conditioned supernatants from infected cultures or ii) when bacteria and host cells are separated by Transwell membranes, this possibility should not yet be eliminated. For example, supernatants of infected cultures, or Transwell-separated bacteria and host cells, may not show toxin upregulation because a host cell-derived soluble factor might only induce signaling when present at locally high concentrations, as occurs when Caco-2 cells and C. perfringens are in close contact. If a soluble host cell-derived factor does mediate the signaling that triggers upregulation of toxin production, this factor could be present on the host surface and then released be by one of the many potent exoenzymes (e.g., proteases, sialidases, phospholipases) produced by C. perfringens. The increased toxin production observed after pronase pretreatment of host cells is consistent with signalling involving either direct host cell-bacterial cell surface factor interaction or a soluble host factor, i.e., pronase-induced removal of some host surface protein(s) might expose the signaling model for direct interactions with a bacterial surface factor or for cleavage by a C. perfringens exoenzyme. Further study of the signaling process behind upregulated toxin production is currently underway to better address these uncertainties.
Experimental procedures
Bacterial strains and culture conditions
C. difficile isolate CD00030 (tcdA+/tcdB+) was kindly provided by Dr. Scott Curry. The toxin genotypes of C. perfringens isolates used in this study are described in Table 2. C. perfringens toxin genotypes had been already determined using a previously described mutiplex PCR assay (Fisher et al., 2006); in addition, PCR amplification of tpeL or tcdA genes was performed using primers listed in Table 3. Frozen (−20°C) stock cultures of each isolate were prepared in cooked meat medium (Difco Laboratories). Those stock cultures were routinely used to inoculate 10 ml tubes of FTG (Difco Laboratories), which were then incubated overnight at 37°C. To obtain isolated colonies, each FTG culture was inoculated onto an TCS agar plate, (SFP agar [Difco Laboratories] containing 0.1% D-cycloserine [Sigma-Aldrich]) which was then incubated overnight at 37°C under anaerobic conditions. For experiments, a single bacterial colony from a TCS agar plate was inoculated into ~10 ml of FTG, which was grown for 12 h at 37°C. This culture was then centrifuged at 8000 × g for 20 min at 4°C. That bacterial pellet was washed twice with ice-cold phosphate-buffered saline (PBS, pH 7.4) before delicate resuspension in 10 ml of ice-cold PBS containing 0.1% of cysteine (Difco Laboratories). Those washed bacteria were then immediately used to infect cell cultures as described below.
Table 2.
C. perfringens type C isolates, mutants and plasmids used in this study.
| Strain | Description | Origen or reference |
|---|---|---|
| JGS1495 | cpb+, cpb2+, plc+, pfo+ and tpeL+ | Piglet with necrotic enteritis (Fisher et al.,2006). |
| CN3685 | cpb+, plc+, pfo+ and tpeL+ | Sheep with struck, (Fisheret al., 2006). |
| CN5383 | cpb+, plc+, pfo+ and tpeL+ | Human pig bel case, (Fisher et al., 2006). |
| C. difficile 00030 | tcdA+, tcdB+ | Human with colitis, (Curryet al., 2007) |
| NCTC8346 | etx+, plc+ and pfo + | Deceased Sheep, (Sayeedet al., 2005) |
| CN1634 | etx+, plc+ and pfo+ | Lamb with dysentery, (Sayeed et al., 2005) |
| CPJV19 | CN3685∷luxS | This work |
| CPJV47 | CN3685∷virR | This work |
| Plasmids | ||
| pKOR |
E. coli-based C. perfringens suicide plasmid with 590 bp of the virR gene. |
(Shimizu et al., 1994) |
| pJIR750 | E. coli-C. perfringens shuttle vector | (Bannam and Rood, 1993) |
| pJVBM11 | pJIR750 with virR promoter and the virR gene in antisense orientation, from CN3685. |
This work |
| pJVR4 | pJIR750 with virR promoter and the virR gene from CN3685. |
This work |
| pJVRS3 | pJIR750 with virR promoter, virR gene and virS gene from CN3685. |
This work |
| pTS405 | pJIR418 with a PstI fragment from strain 13 genomic DNA containing virR promoter, virR gene and virS gene. |
(Ba-Thein et al., 1996) |
| pJIR750ai | pJIR750 with plc targeted intron in antisense orientation. |
(Chen et al., 2005) |
| pJVLux | pJIR750ai with luxS targeted intron in antisense orientation. |
This work |
Table 3.
Primers used in this study.
| Primer | Sequence | Source or reference |
|---|---|---|
| cpbF | GCGAATATGCTGAATCATCTA | (Garmory et al., 2000) |
| cpbR | GCAGGAACATTAGTATATCTTC | |
| cpb2MPF | AGATTTTAAATATGATCCTAACC | (Fisher et al., 2005) |
| cpb2MPR | CAATACCCTTCACCAAATACTC | |
| cpaF | GCTAATGTTACTGCCGTTGA | (Garmory et al., 2000) |
| cpaR | CCTCTGATACATCGTGTAAG | |
| pfoAF1 | ATCCAACCTATGGAAAAGTTTCTGG | (Fisher et al., 2006) |
| pfoAR1 | CCTCCTAAAACTACTGCTGTGAAGG | |
| polCJVL | AATATATGATACTGAAGAGAGAGTAA | This study |
| polCJVR | TCTAAATTATCTAAATCTATGTCTACT | |
| tpeLJVL | TTTTGAAGTTCCACAAGCTCTAATATC | This study |
| tpeLJVR | CTCCTTTACTGTTAATGAAGCAAATC | |
| tcdAJVL | AATTTTTACTTTAGAAATGGTTTAC | This study |
| tcdAJVR | AGAAATATATAACACCATCAATCTC | |
| virR-L | ATGTTTAGTATTGCCTTATGTGAAGA | This study |
| virR-R | TTAACA TAT TAA ATC CCC TAA AAG GC | |
| virR590-L | TTGGATGAAATAGGAGTAGAGT | This study |
| virR590-R | TCTATGCTTACTTACAGGTA | |
| virRP-LKpnI | TTGGTACCTTATGTTCATAAAATAGAAAGTGG | This study |
| virRP-RXbaI | TTTCTAGATAAACATGTCTAATAATCTCCTTT | |
| virR-RXbaI | TTTCTAGATTAACATATTAAATCCCCTAAAAGGC | This study |
| virS-RXbaI | TTTCTAGACTAGGCTTCTTTTTCTTGATTTATA | This study |
| virRanti-L | TTAAGCTTTTTAGTATTGCCTTATGTGAAGAT | This study |
| virRanti-R | TTTCTAGATAACATATTAAATCCCCTAAAAG | |
| luxS-L | CCTAAAGGAGATATAGTTTCAAAG | This study |
| luxS-R | ATGACTCTTAGCTAATTCTAGTGA | |
| luxS-IBS | AAAAAAGCTTATAATTATCCTTATACTCCAAAGC TGTGCGCCCAGATAGGGTG |
This study |
| luxS-EBS1d | CAGATTGTACAAATGTGGTGATAACAGATAAGT CAAAGCTTCTAACTTACCTTTCTTTGT |
This study |
| luxS-EBS2 | TGAACGCAAGTTTCTAATTTCGGTTGAGTATCGA TAGAGGAAAGTGTCT |
This study |
Cell culture
Human-derived enterocyte-like Caco-2 cells were routinely cultured in Eagle’s Minimum Essential Medium (MEM) (Sigma) supplemented with heat-inactivated 10% fetal calf serum (FCS) (Mediatech Incorporated), 1% non-essential amino acids (Sigma), 1% glutamine (Sigma), penicillin (100 U/ml), and streptomycin (100 µg/ml). Madin-Darby canine kidney (MDCK) cells were cultured in a 50:50 mixture of Dulbeco´s Modified Eagle´s Media (DMEM) (Sigma) and Ham’s F12 (Sigma) supplemented with heat-inactivated 3% FCS, 1% non-essential amino acids, 1% glutamine, penicillin (100 U/ml), and streptomycin (100 µg/ml). Rat1-R12 fibroblasts were maintained in DMEM with 10% tet-off-certified fetal bovine serum, penicillin (100 U/ml), geneticin (100 µg/ml) and streptomycin (100 µg/ml). Each cell line was normally harvested with 0.25% trypsin (Gibco), resuspended in the cell culture medium, and incubated at 37°C in 5 % CO2 humidified atmosphere.
C. perfringens infection of Caco-2 cell cultures
For all experimental infections, Caco-2 cells were grown for 4–5 days until reaching confluency in either 100 mm tissue culture dishes (containing 1.2×107 cells/dish) or 24-well microplates (containing 7×105 cells/well). Prior to bacterial infection, the confluent Caco-2 cell cultures were washed three times with pre-warmed PBS (pH 7.4) and then incubated with 10 ml of MEM containing no additives, i.e. no serum or antibiotics (this was considered as a mock infection). Caco-2 cells were then infected with C. perfringens isolates at a multiplicity of infection of 20 (MOI=20) and incubated for indicated times at 37°C. Infection of MDCK cell cultures or Rat1-R12 fibroblast cultures with C. perfringens was performed similarly.
To compare the secretion of proteins and toxins by C. perfringens during in vitro growth versus growth in the presence of Caco-2 cells, the same number of bacteria were inoculated into tissue culture wells containing either TGY [3% tryptic soy broth (Difco), 2% glucose (Sigma), 1% yeast extract (Difco), 0.1% cysteine (Difco)], FTG or MEM and no Caco-2 cells. Those cultures were then incubated under the same conditions used for C. perfringens-infected Caco-2 cell cultures.
Determination of C. perfringens colony-forming units (CFU)
To determine the total number of bacteria present in each culture, Caco-2 cell cultures growing in a 24-well cell culture microplate (containing 7×105 cells/well) were infected with 1.5×107 CFU of C. perfringens strains (MOI=20) for 3 h at 37°C. The same number of bacteria were also inoculated into TGY or MEM added to a 24-well tissue culture plate lacking any Caco-2 cells. The supernatant containing bacteria (10 ml) was aspirated and those wells were extensively washed by pipetting up and down five times to detach all bacteria attached to the Caco-2 cell surface or to the bottom of the tissue culture microplate wells. Supernatants containing the harvested bacteria were then serially diluted in BHI broth (10 ml final volume) (brain heart infusion, Difco Laboratories) and 1 ml was plated onto BHI agar plates. After a 24 h incubation under anaerobic conditions at 37°C, CFU were determined. In addition, detachment of all bacteria from the Caco-2 cells was verified by scrapping these cells in cold PBS with a pipette tip and determining, as described above, CFU of the cell homogenate. Finally, to confirm that no bacteria were intracellular, Caco-2 cells were scraped into cold PBS containing 0.1% Triton X-100 (Sigma), vigorously vortexed for 30 s, and CFU were then recorded. No CFU differences were detected using any of these methods.
Analysis of protein and toxin secretion
To compare the secretion kinetics of proteins and toxins by C. perfringens isolates growing in the presence or absence of Caco-2 cells, confluent Caco-2 cell cultures grown in 100 mm tissue culture dishes (containing 1.2×107 cells/dish) were infected, for the indicated times, with 2.5 × 108 CFU of C. perfringens strains (MOI=20). For comparison, 100 mm tissue culture dishes containing an equivalent volume of TGY or MEM lacking Caco-2 cells were similarly infected. The supernatant of each culture, which contained C. perfringens secreted proteins and toxins, was aspirated and centrifuged 8000 × g at 4°C for 30 min. That supernatant was then filter sterilized using a 0.22 µm filter (Millipore) and the sterile filtrate was processed for the following analysis.
i) SDS-PAGE analysis of secreted proteins
The sterile supernatants prepared above were each concentrated 10-fold using an Amicon Ultra centrifugal filter device with a 10-kDa cutoff (Millipore, Bedford, Mass.). Equal volumes (25 µl) of the concentrated supernatants were then subjected to SDS-PAGE on a 12% polyacrylamide gel and stained with Coomassie blue to visualize total protein patterns in each supernatant sample (Laemmli, 1970). To evaluate whether the secreted protein patterns of Caco-2 cell cultures reflected C. perfringens infection, the supernatants of non-infected Caco-2 cells growing for 3 h in MEM without additives were similarly concentrated and evaluated by SDS-PAGE.
ii) Western blots of secreted toxins
To compare the secretion of CPB, CPB2 or ETX toxins in the presence or absence of Caco-2 cells, unconcentrated sterile supernatants, prepared as described above, were subjected to SDS-PAGE o n a 12% polyacrylamide gel and then transferred onto a nitrocellulose membrane. Those membranes were blocked with PBS-Tween 20 (0.05% v/v) and non fat dry milk (5% wt/v) for 1 h and then probed with either a rabbit polyclonal anti-CPB2 antibody (Fisher et al., 2005), a mouse monoclonal anti-CPB antibody, kindly provided by Dr. Paul Hauer, or with an monoclonal anti-ETX antibody (Sayeed et al., 2005). Bound antibody was then detected after incubation with a horseradish peroxidase-conjugated secondary anti-species specific antibody and addition of SuperSignal West Pico Chemiluminescent Substrate (Pierce). Where noted, purified CPB, CPB2 or ETX were added as a Western blot control; those purified toxins were obtained as previously described (Vidal et al., 2008; Sayeed et al., 2007; Fisher et al., 2005).
To quantify CPB2 secretion, different amounts of purified CPB2 or sterile supernatant from Caco-2 cells, MEM or TGY infected with JGS1495 (MOI=20) were run in a 12% SDS-PAGE and Western blotted. After scanning, the intensity of each band, in arbitrary units (pixels), was obtained using SigmaGel (Jandel Scientific) software and plotted graphically.
To analyze by Western blot the secretion of CPA and PFO toxins in the presence or absence of Caco-2 cells, the sterile culture supernatants were dialyzed overnight at 4°C versus 10 mM Tris, pH 7.5. The dialyzed supernatants were then lyophilized (using a Labconco freeze-dry system) and resuspended in ice-cold PBS (pH 7.4) to obtain a 200-fold concentrated supernatant. Identical amounts of lyophilized material were electrophoresed on a 10% acrylamide gel containing SDS. After transfer of separated proteins onto a nitrocellulose membrane, the membranes were blocked in PBS-Tween 20 (0.05% v/v) with non-fat dry milk (5% wt/v) for 1 h and probed with either a mouse monoclonal anti-CPA antibody (a kind gift of Dr. P. Hauer) or a rabbit polyclonal anti-PFO antibody (a kind gift of Dr. R. Tweten). Western blotting was then performed as described above.
iii) Hemoglobin (Hb) release assay to quantify secreted PFO activity
Horse red blood cell (RBC, Becton-Dickinson Laboratories) were washed three times with sterile phosphate-buffered saline (PBS) and resuspended in PBS to give a final 1% RBC suspension. Sterile Caco-2 cell supernatants, prepared as described above, were mixed with the erythrocyte suspension (1:1) and incubated at 37°C for 30 min. As controls, MEM, TGY or 0.1% saponin (positive control) were also incubated with the RBC suspension. PFO was purified from the supernatant of CN3685, essentially as described earlier (Tweten, 1988b) and different amounts were incubated with RBC as described. After incubation, the RBC suspension was centrifuged at 500 × g for 5 min at 4°C to pellet non-lysed erythrocytes and/or cell debris. Hb released into the supernatant (Abs570nm) was then measured using a microplate reader (Dinex technologies).
iv) ELISA to quantify secreted CPA and CPB levels
C. perfringens alpha toxin (Sigma Aldrich), purified CPB or sterile supernatants, were serially diluted with 1 M carbonate buffer (0.5 M Na2CO3 and 0.5 M NaHCO3; pH 9.5) in a 96-well ELISA microplate (Corning, Inc.). After overnight incubation at 4°C, plates were washed 3 times with PBS-Tween (PBST, 0.05% Tween 20) and blocked with bovine serum albumin (BSA, 1%) for 1 h at 37°C. The plates were then washed 10 more times and incubated with a mouse monoclonal anti-CPA antibody (1:5000) or monoclonal anti-CPB (1:5000) for 1 h at 37°C. After that incubation, the plates were washed ten times with PBST and incubated with a HRP-conjugated anti-mouse antibody (1:5000) for 1 h at 37°C. After 10 more washings with PBST, the bound HRP-conjugated antibody was detected using tetramethylbenzidine (TMB) substrate solution (Thermo scientific). After 15 min, the color reaction was stopped with sulphuric acid (0.18 M) and the sample absorbance at 450 nm (A450 nm) was determined using an ELISA reader (Dinex Technologies).
RT-PCR and qRT-PCR analyses
Supernatants containing bacteria were removed from infected Caco-2 cell cultures and pelleted at 8000 × g for 20 min at 4°C. MEM, FTG, TGY or TY [3% tryptic soy broth (Difco), 1% yeast extract (Difco), 0.1% cysteine (Difco)] cultures were similarly centrifuged. Total bacterial RNA was extracted from the centrifuged pellets using a RiboPure bacteria kit (Ambion), as described by the manufacturer. All RNA samples were treated with DNase I at 37°C for 30 min. RT-PCR reactions were then performed on those DNase-treated RNA samples using the AccesQuick RT-PCR system (Promega). Briefly, 20, 50 or 100 ng of each RNA sample were reverse-transcribed to cDNA at 45°C for 1 h and then used as template for PCR (denaturing at 94°C for 1 min, annealing at 55°C for 1 min and extension at 72°C for 1 min) with the toxin gene-specific primers listed in Table 3. RT-PCR reactions yielded the same results using any of the three tested RNA concentrations. Control RT-PCR reactions were similarly performed, except for the omission of reverse transcriptase. As an additional control, another reaction amplifying each toxin gene was performed by adding, into the RT-PCR reaction cocktail, DNA that had been extracted from C. perfringens type C isolates using the MasterPure Gram Positive DNA Purification kit (Epicentre Biotechnologies).
Quantitative RT-PCR (qRT-PCR) was performed using the iScript One-Step RT-PCR kit with SYBR Green (Bio-Rad) and the iCycler thermal cycler with a 96-well reaction module (Bio-Rad). qRT-PCR reactions were performed in triplicate with 20 ng of total RNA, 500 nM concentration of each primer (Table 3) and the following conditions; 1 cycle at 50°C for 30 min, 1 cycle at 95°C for 10 min and 40 cycles of 95°C for 15, 55°C for 1 min. Melting curves were generated by a cycle of 95°C for 1 min, 55°C for 1 min and 80 cycles of 55°C with 0.5°C increments. The relative quantitation of mRNA expression was normalized to the constitutive expression of the housekeeping polC gene and calculated by the comparative CT (2−ΔΔCT) method (Livak and Schmittgen, 2001).
Studies of Caco-2 cell mediated up-regulation of C. perfringens toxin expression
i) CPB secretion in conditioned medium (CM)
A confluent monolayer of Caco-2 cells was washed three times and then incubated for 3 h at 37°C in MEM without additives (serum or antibiotics). This medium containing secreted or released Caco-2 cells proteins (termed conditioned medium [CM]) was collected, centrifuged 7000 × g to remove cellular material, and filtered-sterilized.
C. perfringens type C strain JGS1495 or CN3685 was then inoculated (1.5×107 CFU) into 1 ml of CM alone, 1 ml of MEM alone (without additives), Caco-2 cell cultures treated with 1 ml of CM, or Caco-2 cells in fresh (non-conditioned) MEM in a 24-well tissue culture plate. After 3 h of incubation at 37°C, supernatants were centrifuged 8000 × g at 4°C for 30 min, filter-sterilized, and analyzed by Western blot for β-toxin production using a mouse monoclonal anti-CPB antibody, as described earlier.
ii) Effects of Caco-2 cell number on CPB secretion levels
Caco-2 cell cultures were grown for 3–4 days in 24-well microplates to the following confluency: 100% (containing 7 × 105 cells/well), 80% (containing 5.5 × 105 cells/well), 50% (containing 4 × 105 cells/well), 30% (containing 2.5 × 105 cells/well) or MEM alone (mock infection). Each well was then infected with 1.5×107 CFU of C. perfringens type C strain JGS1495 and then incubated for 3 h at 37°C. After this incubation, the culture supernatants were harvested and centrifuged 8000 × g at 4°C for 30 min. The supernatants were then filter-sterilized and analyzed by Western blot for CPB levels using a mouse monoclonal anti-CPB antibody, as described earlier.
iii) CPB secretion levels after incubation with Caco-2 cell lysates or Caco-2 cell membranes
Washed Caco-2 cells (1.2 × 107 cells) were scraped from a 100 mm tissue culture dish using a rubber policemen and homogenized in 500 µl of ice-cold PBS (pH 7.4). Cell homogenates were then lysed by sonication. Some of the lysates were centrifuged at 21 000 × g for 30 min at 4°C to obtain a Caco-2 membrane fraction.
C. perfringens type C strain JGS1495 (2.5 × 108 CFU) was inoculated into a 100 mm tissue culture dish containing 12 ml of MEM without additives, MEM without additives but with Caco-2 cell lysates, MEM without additives but with the Caco-2 cell membrane fraction, or MEM plus confluent living Caco-2 cells (MOI=20). After 3 h of infection at 37°C, supernatants were collected, centrifuged at 8000 × g at 4°C for 30 min, and analyzed by Western blot for CPB levels using a mouse monoclonal anti-CPB antibody, as described earlier.
iv) CPB secretion levels in the presence of fixed Caco-2 cells
Caco-2 cells were grown to confluency in 24-well microplates and washed twice with pre-warmed PBS (pH 7.4). The washed cultures were then fixed for 20 min with 2% paraformaldehyde (PFA), extensively washed with pre-warmed PBS (pH 7.4) and incubated with MEM without additives or with PBS. Those PFA-fixed cells were infected with C. perfringens (MOI=20), and incubated for the indicated time at 37°C. Supernatants were then collected, centrifuged 8000 × g at 4°C for 30 min and filter-sterilized. The sterile supernatants were analyzed for CPB levels by Western blotting using a mouse monoclonal anti-CPB antibody, as described earlier.
v) CPB secretion levels in the presence of trypsin, EDTA, PLC or pronase treated Caco-2 cells
Caco-2 cells growing in a 24-well microplate were treated with either; a) 0.25% trypsin or a 0.2% EDTA solution for 15 min; b) phospholipase C in the form of CPA (1×10−3 U/ml) or (1×10−5 U/ml) for 1 h or c) pronase (Roche) (100 µg/ml) for 20 min. All cell treatments were done at 37°C. Treated cells were washed three times with sterile PBS and resuspended in MEM (trypsin-, EDTA- or pronase-treated cells) or added with MEM (PLC-treated cells). The cell density was obtained by standard procedures using a cell counting chamber. Cell suspensions (7 × 105 cells/well) were infected with CN3685 or JGS1495 (MOI=20) and incubated for the indicated time at 37°C. The supernatant was obtained and analyzed by western blot for CPB levels as described earlier.
vi) CPB secretion levels in Transwell cultures of Caco-2 cells
Caco-2 cells were seeded into the membrane chamber of a Transwell-Col filter (0.4 µm pore size, Corning) installed in a 12-well cell culture plate. The cultures were then grown to confluency over four days. Before infection, the Caco-2 transwell cultures were carefully washed three times with pre-warmed PBS (pH 7.4) and incubated at 37°C in 2 ml of MEM (without additives) for 30 min. As a control, an uninoculated Transwell filter was sometimes installed in wells where Caco-2 cells (1.5 × 106 cells/well) were growing in the bottom of the culture well.
For infection, C. perfringens type C isolates were inoculated (3×107 CFU) directly into the Transwell filter chamber (that did or did not contain Caco-2 cells) and incubated for 3 h at 37°C. The supernatant from the Transwell filter chamber was collected, centrifuged at 8000 × g for 30 min, and filter-sterilized. The sterile supernatant was then analyzed by Western blot for CPB levels using a mouse monoclonal anti-CPB antibody, as described earlier.
Cytotoxicity of C. perfringens type C strains
To evaluate the cytotoxic consequences of Caco-2 cell-induced toxin up-regulation, we first inoculated Caco-2 cell cultures or MEM alone with C. perfringens type C strain CN3685 for 1 h. The culture supernatants were then removed and filtered-sterilized using a 0.22 µm filter (Millipore). Each sterilized supernatant was then added to fresh confluent Caco-2 cells and incubated for 2 h at 37°C. After this treatment, the Caco-2 cell cultures were washed three times with pre-warmed PBS (pH 7.4), fixed with 70% methanol and stained with Giemsa stain.
To directly observe cytotoxicity during infection, Caco-2 cells were infected with C. perfringens type C strains (MOI=20) and the development of morphological changes was then followed every 30 min using a Zeiss Axiovert 25 inverted microscope. Cytotoxicity induced by infection was scored as follows, (+) indicates that >80% of Caco-2 cells had swelled, (++) <50% of Caco-2 cells had swelled and <50% of cells had rounded and (+++) indicates that >80% of Caco-2 cells were rounded and had detached from the glass. Pictures of infected-Caco-2 cells were then taken using a Canon Powershot G5 fitted to the Zeiss Axiovert 25 microscope. We were unable to fix and stain infected Caco-2 cells since the development of morphological damage (i.e. swelling on >80% of cells) coincided with Caco-2 cell detachment from the glass after PBS washing.
Construction of a CN3685 luxS null mutant using TargeTron® technology
To inactivate the luxS gene, the wild-type luxS gene sequence was first entered into the Intron prediction program (www.sigma-genosys.com/targetron/). This program predicted a sense insertion site, with a low e-score (0.170), between the nucleotides 295|296 of the luxS gene. The primers, luxS-IBS, luxS-EBS1d and luxS-EBS2 (Table 3), were used to generate a 350 bp intron targeting sequence to this luxS gene. The amplified 350-bp fragment was then digested with HindIII and BsrGI and ligated into pJIR750ai, (a TargeTron®-carrying vector we created previously to construct a plc null mutant (Chen et al., 2005) in type A isolate ATCC3624), which had been similarly digested with the same two restriction enzymes. The resultant plasmid (pJVLux), carrying a luxS-targeted intron, was electroporated into wild-type C. perfringens type C isolate CN3685, as described previously (Chen et al., 2005). Transformants were plated onto BHI agar plates containing 15 µg/ml of chloramphenicol. Colonies were then PCR screened using luxS-specific primers that supported PCR amplification of an ~300 bp product from the wild-type luxS gene, but amplified a larger ~1.2 kb product from those mutants. The mutant PCJV19 was then shown by PCR to have the intron inserted in the luxS gene and by Southern blotting, to have a single intron insertion (Figure S3A). All PCR-screened reactions were done using Taq 2X Master Mix (New England Biolabs) and primers final concentration of 1 µM in a Techne (Burkhardtsdorf, Germany) thermocycler.
Inactivation of the virR gene in type C CN3685 isolate
To inactivate the virR gene, the E. coli-based C. perfringens suicide plasmid (pKOR) (Shimizu et al., 1994), which contains a ~590 bp fragment of the virR gene cloned upstream the tetracycline resistant gene, was electroporated into CN3685. Transformants were plated onto BHI agar plates containing 2.5 µg/ml of tetracycline. Only those bacteria with the ~590 bp fragment of the virR gene integrated in their genome were capable of growth in the presence of tetracycline. Tetracycline-resistant colonies were PCR screened using primers that supported the amplification of a ~590 bp product or primers that amplify the entire ~710 bp virR wt gene. A mutant, CPJV47, that had lost the ~710 bp PCR product (Figure 9A), was then grown in blood agar plates to test for PFO-induced beta hemolysis.
Complementation of virR mutant
To complement the virR mutant (CPJV47), the entire virS/virR operon from CN3685, (including the virR promoter), was PCR amplified using the primers virRP-LKpnI and virS-RXbaI. The product was then ligated into the E. coli-C. perfringens shuttle vector pJIR750 to generate pJVRS3 (Figure 9D). To construct pJVR4, the virR promoter and virR gene from CN3685 were PCR amplified using the primers virRP-LKpnI and virR-RXbaI and cloned into pJIR750 (Figure 9D). Plasmid pTS405, which encodes the virS/virR operon, was constructed by digesting Strain 13 DNA with PstI and cloned in E. coli-C. perfringens shuttle vector pJIR418. Those plasmids were electroporated into the virR mutant strain CPJV47, as described (Chen et al., 2005).
Construction of a virR antisense vector
To knockdown the virR gene with an antisense mRNA, a PCR product containing the virR promoter was cloned between the KpnI-XbaI sites of pJIR750. The virR gene was then amplified using the primers virRanti-L (HindIII) and virRanti-R (XbaI) and directionally cloned, in the antisense orientation, between the XbaI-HindIII sites of pJIR50, that lie upstream of the virR promoter (Figure 9D). The resulting plasmid, pJVBM11, was electroporated into the WT CN3685.
Southern blot hybridization
C. perfringens genomic DNA from wild type CN3685 or CPJV19 was obtained, as described earlier. This DNA was digested with EcoRI and run on a 0.8% agarose gel. After transfer to a nylon membrane (Roche), the blot was probed with a digoxigenin-labeled probe specific for the intron sequence. This probe was prepared using the primer pair luxS-IBS and luxS-EBS1d (Table 3) and labeled with a DIG labeling kit obtained from Roche Applied Science. CSPD substrate (Roche Applied Science) was used for detection of DIG-labeled hybridized probes, according to the manufacturer’s instruction.
Extraction of C. perfringens cytoplasmic proteins
An overnight FTG culture (1 ml) was inoculated in 20 ml of TGY and incubated for 4 h at 37°C and then centrifuged at 10 000 × g at 4°C for 30 min. The resulting pellet was washed twice with cold PBS and resuspended in 5 ml of PBS. Bacteria were lysed by passing trough a French Press (psi 1600) twice and the cytoplasmic fraction was obtained after centrifugation at 10 000 × g for 30 min. Cytoplasmic proteins (20 µg) were electrophoresed in a 12% SDS-PAGE, transferred to nitrocellulose membranes and probed by Western blot using rabbit polyclonal anti-VirR antibody (McGowan et al., 2002; Cheung and Rood, 2000).
Supplementary Material
Supplemental figure 1. Protein secretion and growth of C. perfringens type C isolates in the presence of Caco-2 cells. Panels A and C. Caco-2 cell cultures grown to confluency in 100 mm tissue culture dishes were infected with C. perfringens type C isolates JSG1495 (A) or CN3685 (C) for the indicated times at 37°C (MOI=20). For comparison, the same numbers of bacteria were inoculated into TGY or MEM (no Caco-2 cells) and then incubated under the same conditions. As a control, Caco-2 cells were left uninfected but incubated for 3 h in MEM without additives. At the conclusion of the experiment, culture supernatants were filter-sterilized and concentrated 10-fold using 10-kDa cutoff Amicon filter devices. Equal amounts of each concentrated supernatant containing C. perfringens secreted-proteins were electrophoresed on a 12% SDS-PAG and stained with Coomasie blue. Numbers at left are in kDa. Asterisks show the location of the 60 kDa major protein secreted by Caco-2 cells (Control lanes). (B) C. perfringens strain JGS1495 (1.5×107 CFU) was inoculated into FTG, TGY, MEM or Caco-2 cells and then incubated for 2 h at 37°C. Culture supernatant was removed and filter-sterilized. Equal amounts of each supernatant were electrophoresed on a 12% SDS-PAG and transferred to nitrocellulose membrane. Purified, 35 kDa CPB (0.5 µg) was also included (β toxin). Membranes were blocked for 1 h and probed with a mouse monoclonal anti-CPB antibody. (B) Caco-2 cell cultures, TGY or MEM were each inoculated with 1.5×107 CFU of C. perfringens (Cp) type C isolates CN3685 for 1.5 h, at 37°C (MOI=20). The culture supernatants containing bacteria (10 ml) were aspirated and serially diluted in BHI broth (10 ml final volume) and then plated (1 ml) onto BHI agar plates. The number of bacteria (cfu/ml) in each culture condition was recorded after 24 h of incubation under anaerobic conditions at 37°C. Figures shown are representative of at least 4 independent experiments.
Supplemental figure 2. cpb transcription by CN3685 and CPB secretion in the presence of paraformaldehyde-fixed Caco-2 cells. (A) cpb transcription. Caco-2 cells were infected with CN3685 for 1 h. RNA (50 ng) was isolated and used as template in RT-PCR reactions with primers that amplify a ~200 bp product from the cpb gene. Where indicated, retrotranscriptase (RT) was (+) or was not (−) added into the reaction tubes. A reaction containing DNA from CN3685 was included. A 100 bp ladder is shown at left, with migration of the 200 bp marker noted. (B) CPB secretion in the presence of paraformaldehyde (PFA)-fixed Caco-2 cells. MEM cultures, PFA-fixed Caco-2 cells, PBS alone or PFA-Caco-2 cells with PBS were infected with CN3685 during the indicated time at 37°C. Sterile supernatants were obtained and analyzed for CPB levels as described above. Migration of the 35 kDa CPB is noted on the blot.
Supplemental figure 3. A luxS-controlled quorum sensing mechanism is not required for CPB secretion in vitro. A) Southern blot showing a single intron insertion in CPJV19. Genomic DNA (3 µg) from CN3685 (WT) or CPJV19 was digested with EcoRI at 37°C and then electrophoresed in a 0.8 % agarose gel. After transferring to nylon membrane, the blot was probed with a DIG labeled probed that detects the sequence of the intron (see Material and Methods for details). Molecular size of markers, in kb, is shown at left. (B and C) CPB secretion and growth rates. WT or CPJV19 strain was inoculated in TGY and incubated during the indicated time. Sterile supernatants (B) or OD600 (C) were obtained in each time point. Supernatants were then electrophoresed on a 12% SDS-PAG and transferred to nitrocellulose membrane. Purified CPB (0.5 µg) was also included (β toxin). Membranes were blocked for 1 h and probed with a mouse monoclonal anti-CPB antibody as described before.
Supplemental figure 4. Caco-2 cells I nduce upregulated ETX secretion by C. perfringens type D. Caco-2 cells, MEM or TGY were infected with C. perfringens type D strain NCTC8346 (top) or CN1634 (bottom) for the indicated time and incubated at 37°C (MOI=20). Equal amounts of sterile supernatants were analyzed by Western blot using a mouse monoclonal anti-ETX antibody. Migration of the 33 kDa ETX (control) is noted on the blot.
Acknowledgements
This work was generously supported by grant R01 AI056177-04 (BAM) from National Institute of Allergy and Infectious Diseases. JEV also received generous support from the Mexican National Council of Science and Technology (CONACyT). The authors thank Dr. P. Hauer for supplying monoclonal antibodies against CPB, CPA and ETX, Dr. R. Tweten for the anti-PFO antibody and Dr. Julian I. Rood for supplying the anti-VirR antibody. Also thanks to Dr. Jackie Chueng for her technical advice and Dr. J. Carroll and Christy Nolder for their assistance and helpful advice with qRT-PCR experiments.
References
- Amimoto K, Noro T, Oishi E, Shimizu M. A novel toxin homologous to large clostridial cytotoxins found in culture supernatant of Clostridium perfringens type C. Microbiology. 2007;153:1198–1206. doi: 10.1099/mic.0.2006/002287-0. [DOI] [PubMed] [Google Scholar]
- Ba-Thein W, Lyristis M, Ohtani K, Nisbet IT, Hayashi H, Rood JI, Shimizu T. The virR/virS locus regulates the transcription of genes encoding extracellular toxin production in Clostridium perfringens. J Bacteriol. 1996;178:2514–2520. doi: 10.1128/jb.178.9.2514-2520.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannam TL, Rood JI. Clostridium perfringens-Escherichia coli shuttle vectors that carry single antibiotic resistance determinants. Plasmid. 1993;29:233–235. doi: 10.1006/plas.1993.1025. [DOI] [PubMed] [Google Scholar]
- Banu S, Ohtani K, Yaguchi H, Swe T, Cole ST, Hayashi H, Shimizu T. Identification of novel VirR/VirS-regulated genes in Clostridium perfringens. Mol Microbiol. 2000;35:854–864. doi: 10.1046/j.1365-2958.2000.01760.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, McClane BA, Fisher DJ, Rood JI, Gupta P. Construction of an alpha toxin gene knockout mutant of Clostridium perfringens type A by use of a mobile group II intron. Appl Environ Microbiol. 2005;71:7542–7547. doi: 10.1128/AEM.71.11.7542-7547.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JK, Rood JI. The VirR response regulator from Clostridium perfringens binds independently to two imperfect direct repeats located upstream of the pfoA promoter. J Bacteriol. 2000;182:57–66. doi: 10.1128/jb.182.1.57-66.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung JK, Dupuy B, Deveson DS, Rood JI. The spatial organization of the VirR boxes is critical for VirR-mediated expression of the perfringolysin O gene, pfoA, from Clostridium perfringens. J Bacteriol. 2004;186:3321–3330. doi: 10.1128/JB.186.11.3321-3330.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A. 2006;103:10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry SR, Marsh JW, Muto CA, O'Leary MM, Pasculle AW, Harrison LH. tcdC genotypes associated with severe TcdC truncation in an epidemic clone and other strains of Clostridium difficile. J Clin Microbiol. 2007;45:215–221. doi: 10.1128/JCM.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers B, Sansonetti PJ, Parsot C. Induction of type III secretion in Shigella flexneri is associated with differential control of transcription of genes encoding secreted proteins. Embo J. 1998;17:2894–2903. doi: 10.1093/emboj/17.10.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Miyakawa ME, Fisher DJ, Poon R, Sayeed S, Adams V, Rood JI, et al. Both epsilon-toxin and beta-toxin are important for the lethal properties of Clostridium perfringens type B isolates in the mouse intravenous injection model. Infect Immun. 2007;75:1443–1452. doi: 10.1128/IAI.01672-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher DJ, Miyamoto K, Harrison B, Akimoto S, Sarker MR, McClane BA. Association of beta2 toxin production with Clostridium perfringens type A human gastrointestinal disease isolates carrying a plasmid enterotoxin gene. Mol Microbiol. 2005;56:747–762. doi: 10.1111/j.1365-2958.2005.04573.x. [DOI] [PubMed] [Google Scholar]
- Fisher DJ, Fernandez-Miyakawa ME, Sayeed S, Poon R, Adams V, Rood JI, et al. Dissecting the Contributions of Clostridium perfringens Type C Toxins to Lethality in the Mouse Intravenous Injection Model. Infect Immun. 2006;74:5200–5210. doi: 10.1128/IAI.00534-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmory HS, Chanter N, French NP, Bueschel D, Songer JG, Titball RW. Occurrence of Clostridium perfringens beta2-toxin amongst animals, determined using genotyping and subtyping PCR assays. Epidemiol Infect. 2000;124:61–67. doi: 10.1017/s0950268899003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginocchio CC, Olmsted SB, Wells CL, Galan JE. Contact with epithelial cells induces the formation of surface appendages on Salmonella typhimurium. Cell. 1994;76:717–724. doi: 10.1016/0092-8674(94)90510-x. [DOI] [PubMed] [Google Scholar]
- Gui L, Subramony C, Fratkin J, Hughson MD. Fatal enteritis necroticans (pigbel) in a diabetic adult. Mod Pathol. 2002;15:66–70. doi: 10.1038/modpathol.3880491. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laursen BS, Sorensen HP, Mortensen KK, Sperling-Petersen HU. Initiation of protein synthesis in bacteria. Microbiol Mol Biol Rev. 2005;69:101–123. doi: 10.1128/MMBR.69.1.101-123.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence G, Cooke R. Experimental pigbel: the production and pathology of necrotizing enteritis due to Clostridium welchii type C in the guinea-pig. Br J Exp Pathol. 1980;61:261–271. [PMC free article] [PubMed] [Google Scholar]
- Leverton LQ, Kaper JB. Temporal expression of enteropathogenic Escherichia coli virulence genes in an in vitro model of infection. Infect Immun. 2005;73:1034–1043. doi: 10.1128/IAI.73.2.1034-1043.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McClane BA. A novel small acid soluble protein variant is important for spore resistance of most Clostridium perfringens food poisoning isolates. PLoS Pathog. 2008;4:e1000056. doi: 10.1371/journal.ppat.1000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lyristis M, Bryant AE, Sloan J, Awad MM, Nisbet IT, Stevens DL, Rood JI. Identification and molecular analysis of a locus that regulates extracellular toxin production in Clostridium perfringens. Mol Microbiol. 1994;12:761–777. doi: 10.1111/j.1365-2958.1994.tb01063.x. [DOI] [PubMed] [Google Scholar]
- Matsuda T, Okada Y, Inagi E, Tanabe Y, Shimizu Y, Nagashima K, et al. Enteritis necroticans 'pigbel' in a Japanese diabetic adult. Pathol Int. 2007;57:622–626. doi: 10.1111/j.1440-1827.2007.02149.x. [DOI] [PubMed] [Google Scholar]
- McClane BA, Lyerly DL, Wilkins TD. Enterotoxic clostridia: Clostridium perfringens type A and Clostridium difficile. In: Fischetti VA, R.P.N., Feretti JJ, Portnoy DA, Rood JI, editors. Gram positive pathogens. Washington DC: ASM Press; 2006. pp. 703–714. [Google Scholar]
- McClane BA, Uzal FA, Fernandez-Miyakawa M, Lyerly D, Wilkins TD. The Enterotoxigenic Clostridia. In: Dworkin M, S.F., Rosenburg E, Schleifer KF, Stackebrandt E, editors. The Prokaryotes. New York, NY: Springer-Verlag; 2004. pp. 698–752. [Google Scholar]
- McGowan S, Lucet IS, Cheung JK, Awad MM, Whisstock JC, Rood JI. The FxRxHrS motif: a conserved region essential for DNA binding of the VirR response regulator from Clostridium perfringens. J Mol Biol. 2002;322:997–1011. doi: 10.1016/s0022-2836(02)00850-1. [DOI] [PubMed] [Google Scholar]
- Myers GS, Rasko DA, Cheung JK, Ravel J, Seshadri R, DeBoy RT, et al. Skewed genomic variability in strains of the toxigenic bacterial pathogen, Clostridium perfringens. Genome Res. 2006;16:1031–1040. doi: 10.1101/gr.5238106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niilo L. Clostridium perfringens Type C Enterotoxemia. Can Vet J. 1988;29:658–664. [PMC free article] [PubMed] [Google Scholar]
- Novick RP. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol. 2003;48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- Ohtani K, Hayashi H, Shimizu T. The luxS gene is involved in cell-cell signaling for toxin production in Clostridium perfringens. Mol Microbiol. 2002;44:171–179. doi: 10.1046/j.1365-2958.2002.02863.x. [DOI] [PubMed] [Google Scholar]
- Ohtani K, Kawsar HI, Okumura K, Hayashi H, Shimizu T. The VirR/VirS regulatory cascade affects transcription of plasmid-encoded putative virulence genes in Clostridium perfringens strain 13. FEMS Microbiol Lett. 2003;222:137–141. doi: 10.1016/S0378-1097(03)00255-6. [DOI] [PubMed] [Google Scholar]
- Okumura K, Ohtani K, Hayashi H, Shimizu T. Characterization of genes regulated directly by the VirR/VirS system in Clostridium perfringens. J Bacteriol. 2008;190:7719–7727. doi: 10.1128/JB.01573-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit L, Gibert M, Popoff MR. Clostridium perfringens: toxinotype and genotype. Trends Microbiol. 1999;7:104–110. doi: 10.1016/s0966-842x(98)01430-9. [DOI] [PubMed] [Google Scholar]
- Petrillo TM, Beck-Sague CM, Songer JG, Abramowsky C, Fortenberry JD, Meacham L, et al. Enteritis necroticans (pigbel) in a diabetic child. N Engl J Med. 2000;342:1250–1253. doi: 10.1056/NEJM200004273421704. [DOI] [PubMed] [Google Scholar]
- Rohde M, Puls J, Buhrdorf R, Fischer W, Haas R. A novel sheathed surface organelle of the Helicobacter pylori cag type IV secretion system. Mol Microbiol. 2003;49:219–234. doi: 10.1046/j.1365-2958.2003.03549.x. [DOI] [PubMed] [Google Scholar]
- Sakurai J, Duncan CL. Purification of beta-toxin from Clostridium perfringens type C. Infect Immun. 1977;18:741–745. doi: 10.1128/iai.18.3.741-745.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed S, Li J, McClane BA. Virulence plasmid diversity in Clostridium perfringens type D isolates. Infect Immun. 2007;75:2391–2398. doi: 10.1128/IAI.02014-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed S, Fernandez-Miyakawa ME, Fisher DJ, Adams V, Poon R, Rood JI, et al. Epsilon-toxin is required for most Clostridium perfringens type D vegetative culture supernatants to cause lethality in the mouse intravenous injection model. Infect Immun. 2005;73:7413–7421. doi: 10.1128/IAI.73.11.7413-7421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayeed S, Uzal FA, Fisher DJ, Saputo J, Vidal JE, Chen Y, et al. Beta toxin is essential for the intestinal virulence of Clostridium perfringens type C disease isolate CN3685 in a rabbit ileal loop model. Mol Microbiol. 2008;67:15–30. doi: 10.1111/j.1365-2958.2007.06007.x. [DOI] [PubMed] [Google Scholar]
- Severin WP, de la Fuente AA, Stringer MF. Clostridium perfringens type C causing necrotising enteritis. J Clin Pathol. 1984;37:942–944. doi: 10.1136/jcp.37.8.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Ba-Thein W, Tamaki M, Hayashi H. The virR gene, a member of a class of two-component response regulators, regulates the production of perfringolysin O, collagenase, and hemagglutinin in Clostridium perfringens. J Bacteriol. 1994;176:1616–1623. doi: 10.1128/jb.176.6.1616-1623.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smedley JG, 3rd, Fisher DJ, Sayeed S, Chakrabarti G, McClane BA. The enteric toxins of Clostridium perfringens. Rev Physiol Biochem Pharmacol. 2004;152:183–204. doi: 10.1007/s10254-004-0036-2. [DOI] [PubMed] [Google Scholar]
- Sobel J, Mixter CG, Kolhe P, Gupta A, Guarner J, Zaki S, et al. Necrotizing enterocolitis associated with Clostridium perfringens type A in previously healthy north american adults. J Am Coll Surg. 2005;201:48–56. doi: 10.1016/j.jamcollsurg.2005.02.029. [DOI] [PubMed] [Google Scholar]
- Songer JG. Clostridial enteric diseases of domestic animals. Clin Microbiol Rev. 1996;9:216–234. doi: 10.1128/cmr.9.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Songer JG, Uzal FA. Clostridial enteric infections in pigs. J Vet Diagn Invest. 2005;17:528–536. doi: 10.1177/104063870501700602. [DOI] [PubMed] [Google Scholar]
- Titball RW, Naylor CE, Basak AK. The Clostridium perfringens alpha-toxin. Anaerobe. 1999;5:51–64. doi: 10.1006/anae.1999.0191. [DOI] [PubMed] [Google Scholar]
- Tonnellier M, Maury E, Guglielminotti J, Offenstadt G. A fatal sandwich. Lancet Infect Dis. 2001;1:202. doi: 10.1016/S1473-3099(01)00097-4. [DOI] [PubMed] [Google Scholar]
- Tweten RK. Nucleotide sequence of the gene for perfringolysin O (theta-toxin) from Clostridium perfringens: significant homology with the genes for streptolysin O and pneumolysin. Infect Immun. 1988a;56:3235–3240. doi: 10.1128/iai.56.12.3235-3240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tweten RK. Cloning and expression in Escherichia coli of the perfringolysin O (theta-toxin) gene from Clostridium perfringens and characterization of the gene product. Infect Immun. 1988b;56:3228–3234. doi: 10.1128/iai.56.12.3228-3234.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JE, Navarro-Garcia F. Efficient translocation of EspC into epithelial cells depends on enteropathogenic Escherichia coli and host cell contact. Infect Immun. 2006;74:2293–2303. doi: 10.1128/IAI.74.4.2293-2303.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JE, McClane BA, Saputo J, Parker J, Uzal FA. Effects of Clostridium perfringens beta-toxin on the rabbit small intestine and colon. Infect Immun. 2008;76:4396–4404. doi: 10.1128/IAI.00547-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voth DE, Ballard JD. Clostridium difficile toxins: mechanism of action and role in disease. Clin Microbiol Rev. 2005;18:247–263. doi: 10.1128/CMR.18.2.247-263.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker PD, Murrell TG, Nagy LK. Scanning electronmicroscopy of the jejunum in enteritis necroticans. J Med Microbiol. 1980;13:445–450. doi: 10.1099/00222615-13-3-445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. Protein secretion and growth of C. perfringens type C isolates in the presence of Caco-2 cells. Panels A and C. Caco-2 cell cultures grown to confluency in 100 mm tissue culture dishes were infected with C. perfringens type C isolates JSG1495 (A) or CN3685 (C) for the indicated times at 37°C (MOI=20). For comparison, the same numbers of bacteria were inoculated into TGY or MEM (no Caco-2 cells) and then incubated under the same conditions. As a control, Caco-2 cells were left uninfected but incubated for 3 h in MEM without additives. At the conclusion of the experiment, culture supernatants were filter-sterilized and concentrated 10-fold using 10-kDa cutoff Amicon filter devices. Equal amounts of each concentrated supernatant containing C. perfringens secreted-proteins were electrophoresed on a 12% SDS-PAG and stained with Coomasie blue. Numbers at left are in kDa. Asterisks show the location of the 60 kDa major protein secreted by Caco-2 cells (Control lanes). (B) C. perfringens strain JGS1495 (1.5×107 CFU) was inoculated into FTG, TGY, MEM or Caco-2 cells and then incubated for 2 h at 37°C. Culture supernatant was removed and filter-sterilized. Equal amounts of each supernatant were electrophoresed on a 12% SDS-PAG and transferred to nitrocellulose membrane. Purified, 35 kDa CPB (0.5 µg) was also included (β toxin). Membranes were blocked for 1 h and probed with a mouse monoclonal anti-CPB antibody. (B) Caco-2 cell cultures, TGY or MEM were each inoculated with 1.5×107 CFU of C. perfringens (Cp) type C isolates CN3685 for 1.5 h, at 37°C (MOI=20). The culture supernatants containing bacteria (10 ml) were aspirated and serially diluted in BHI broth (10 ml final volume) and then plated (1 ml) onto BHI agar plates. The number of bacteria (cfu/ml) in each culture condition was recorded after 24 h of incubation under anaerobic conditions at 37°C. Figures shown are representative of at least 4 independent experiments.
Supplemental figure 2. cpb transcription by CN3685 and CPB secretion in the presence of paraformaldehyde-fixed Caco-2 cells. (A) cpb transcription. Caco-2 cells were infected with CN3685 for 1 h. RNA (50 ng) was isolated and used as template in RT-PCR reactions with primers that amplify a ~200 bp product from the cpb gene. Where indicated, retrotranscriptase (RT) was (+) or was not (−) added into the reaction tubes. A reaction containing DNA from CN3685 was included. A 100 bp ladder is shown at left, with migration of the 200 bp marker noted. (B) CPB secretion in the presence of paraformaldehyde (PFA)-fixed Caco-2 cells. MEM cultures, PFA-fixed Caco-2 cells, PBS alone or PFA-Caco-2 cells with PBS were infected with CN3685 during the indicated time at 37°C. Sterile supernatants were obtained and analyzed for CPB levels as described above. Migration of the 35 kDa CPB is noted on the blot.
Supplemental figure 3. A luxS-controlled quorum sensing mechanism is not required for CPB secretion in vitro. A) Southern blot showing a single intron insertion in CPJV19. Genomic DNA (3 µg) from CN3685 (WT) or CPJV19 was digested with EcoRI at 37°C and then electrophoresed in a 0.8 % agarose gel. After transferring to nylon membrane, the blot was probed with a DIG labeled probed that detects the sequence of the intron (see Material and Methods for details). Molecular size of markers, in kb, is shown at left. (B and C) CPB secretion and growth rates. WT or CPJV19 strain was inoculated in TGY and incubated during the indicated time. Sterile supernatants (B) or OD600 (C) were obtained in each time point. Supernatants were then electrophoresed on a 12% SDS-PAG and transferred to nitrocellulose membrane. Purified CPB (0.5 µg) was also included (β toxin). Membranes were blocked for 1 h and probed with a mouse monoclonal anti-CPB antibody as described before.
Supplemental figure 4. Caco-2 cells I nduce upregulated ETX secretion by C. perfringens type D. Caco-2 cells, MEM or TGY were infected with C. perfringens type D strain NCTC8346 (top) or CN1634 (bottom) for the indicated time and incubated at 37°C (MOI=20). Equal amounts of sterile supernatants were analyzed by Western blot using a mouse monoclonal anti-ETX antibody. Migration of the 33 kDa ETX (control) is noted on the blot.