Abstract
Context
Depression occurs in more than half of patients who have experienced a stroke. Poststroke depression has been shown in numerous studies to be associated with both impaired recovery in activities of daily living and increased mortality. Prevention of depression thus represents a potentially important goal.
Objective
To determine whether treatment with escitalopram or problem-solving therapy over the first year following acute stroke will decrease the number of depression cases that develop compared with placebo medication.
Design, Setting, and Participants
A multisite randomized controlled trial for prevention of depression among 176 nondepressed patients was conducted within 3 months following acute stroke from July 9, 2003, to October 1, 2007. The 12-month trial included 3 groups: a double-blind placebo-controlled comparison of escitalopram (n=59) with placebo (n=58), and a nonblinded problem-solving therapy group (n=59).
Main Outcome Measures
The main outcome measure was the development of major or minor poststroke depression based on symptoms elicited by the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) (DSM-IV) and the diagnostic criteria from DSM-IV for depression due to stroke with major depressivelike episode or minor depression (ie, research criteria).
Results
Patients who received placebo were significantly more likely to develop depression than individuals who received escitalopram (11 major and 2 minor cases of depression [22.4%] vs 3 major and 2 min or cases of depression [8.5%], adjusted hazard ratio [HR], 4.5; 95% confidence interval [CI], 2.4–8.2; P<.001) and also more likely than individuals who received problem-solving therapy (5 major and 2 minor cases of depression [11.9%], adjusted HR, 2.2; 95% CI, 1.4–3.5; P<.001). These results were adjusted for history of mood disorders and remained significant after considering possible confounders such as age, sex, treatment site, and severity of impairment in the model. Using an intention-to-treat conservative method of analyzing the data, which assumed that all 27 patients who did not start randomized treatment would have developed depression, and controlling for prior history of mood disorders, escitalopram was superior to placebo (23.1% vs 34.5%; adjusted HR, 2.2; 95% CI, 1.2–3.9; P=.007), while problem-solving therapy was not significantly better than placebo (30.5% vs 34.5%; adjusted HR, 1.1; 95% CI, 0.8–1.5; P=.51). Adverse events, including all-cause hospitalizations, nausea, and adverse effects associated with escitalopram were not significantly different between the 3 groups.
Conclusions
In this study of nondepressed patients with recent stroke, the use of escitalopram or problem-solving therapy resulted in a significantly lower incidence of depression over 12 months of treatment compared with placebo, but problem-solving therapy did not achieve significant results over placebo using the intention-to-treat conservative method of analysis.
Trial Registration
clinicaltrials.gov Identifier: NCT00071643
Prevention is a goal to which every field of medicine aspires because it reduces morbidity, may alleviate suffering, and reduces the cost of health care. Although the Commission on Chronic Illness proposed the classification of primary, secondary, and tertiary prevention1 in 1957, the Institute of Medicine Committee on Prevention of Mental Disorders recommended a new terminology2 in 1995. According to the new terminology, preventive intervention is defined as an intervention before the patient receives a diagnosis. Alternatively, treatment is an intervention for patients already with a diagnosis, and maintenance is the care of patients with chronic illnesses including relapse prevention. Furthermore, preventive interventions are categorized as (1) indicated, addressing high-risk individuals with premorbid signs or symptoms; (2) selective, for select individuals with demonstrated increased risk of developing illness; and (3) universal, for a whole population in a group with all levels of risk.
Although the ultimate goal of preventive intervention in mental disorders is universal, the major problem is identifying a population at sufficiently high risk to justify the expense and risk of pharmacological or psychological intervention.
The annual incidence of stroke (ischemic and hemorrhagic cerebral infarction), which exceeds 700 000 in the United States,3 represents a major public health problem. Patients who survive acute stroke may constitute a particularly good population for preventive intervention for depression because of their high risk of developing depression. Among combined studies of 2178 patients hospitalized for acute stroke or rehabilitation, 22% were diagnosed with major depression and 17% were diagnosed with minor depression.4–9 Furthermore, among patients without depression during the acute stroke period, 14% developed major depression and 23% developed minor depression during the first 2 years following stroke.9 Thus, approximately 37% of stroke survivors developed depression after the acute stroke period and represent a sizeable population in which to assess a selective preventive intervention strategy.
Several investigators have previously attempted to prevent the development of poststroke depression without success.10–12 Palomäki et al10 conducted a randomized placebo-controlled study of 100 patients younger than 71 years who were admitted to the hospital for acute ischemic stroke. Patients received either mianserin (60 mg/d) or placebo for 1 year and were examined at 2, 6, 12, and 18 months follow-up. At no time during the 18-month follow-up did the prevalence rate for major depression according to the Diagnostic and Statistical Manual of Mental Disorders, (Third Edition, Revised) differ between treatment groups. Rasmussen et al,12 using double-blind methods and random assignment, treated 137 patients without depression over 12 months following acute stroke with ser-traline (mean dose 63 mg/d; n=70) or placebo (n=67). The depression occurrence rate over the 12 months, based on a Hamilton-17 Depression Rating Scale13 score of greater than 18, was 8.2% for sertraline (90% confidence interval [CI], 2.4%–13.9%; 3 of 35 completers) vs 22.8% for placebo (90% CI, 13.7%–32.0%; 7 of 32 completers). Based on the number of completers, the power of this study to find significant intergroup differences was 0.44, which indicates that the study was underpowered.
The most recent report was by Almeida et al11 in which 111 patients within 2 weeks following stroke were randomized to treatment with sertraline (50 mg/d; n=55) or placebo (n=56) in a 24-week double-blind clinical trial. Results demonstrated no significant intergroup difference in the prevalence of scores of 8 or greater on the Hospital Depression and Anxiety Scale during the 24 weeks of treatment. Among sertraline-treated patients, 8 out of 48 (16.7%) developed depression compared with 11 out of 51 placebo-treated patients (21.6%) (rate ratio, 0.8; 95% CI, 0.3–2.1; P=.59). A recent meta-analysis, however, reported significant effect of antidepressants across 10 studies with duration of treatment ranging from 4 to 52 weeks. Some studies, however, were designed for prophylaxis while other studies were acute treatment studies in which nondepressed patients were assessed for measurement of recovery.14
Although 3 double-blind trials failed to show a significant effect of active treatment in preventing poststroke depression, we undertook the current preventive treatment study to assess the efficacy of escitalopram or psychological problem-solving therapy. Our rationale included larger numbers of patients to increase statistical power, multisite enrollment to achieve a more varied sample, and comparison of both psychological and pharmacological interventions to prevent poststroke depression. Problem-solving therapy was selected because it had been developed for use in elderly patients with depression15 and cognitive behavioral therapy had been shown to be unsuccessful in treating poststroke depression.16 We hypothesized that both escitalopram and problem-solving therapy would constitute effective preventive interventions.
METHODS
Patients
Patients were enrolled between July 9, 2003, and October 1, 2007, at the Department of Neurology, University of Iowa, Iowa City; the University of Chicago, Chicago, Illinois; and Burke Rehabilitation Hospital, White Plains, New York. Patients were also recruited for enrollment through newspaper advertisements. All patients were also within 3 months of an index stroke. The protocols were approved by the institutional review boards at each study site and written informed consent was obtained from each participant. A total of 200 patients were enrolled 28 from Burke Rehabilitation Hospital, 24 from the University of Chicago, and 148 from the University of Iowa. Inclusion criteria included age older than 50 years and younger than 90 years with clinical and neuroradiological findings consistent with either hemispheric, brainstem, or cerebellar stroke.
Patients with either ischemic or hemorrhagic stroke were entered in the study. Patients were excluded if they met DSM-IV diagnostic criteria for major or minor (research criteria) depressive disorder or had a score greater than 11 on the Hamilton-17 Depression Rating Scale.13 Patients with severe comprehension deficits, as demonstrated by inability to complete part 1 of the Token Test17 or patients with neuropsychological testing who showed impaired decision-making capacity, were excluded. Other exclusionary criteria included occurrence of stroke secondary to complications from an intracranial aneurysm, arterial-venous malformation, intracranial tumor or neoplastic process, stroke during the course of myocardial infarction, aortic dissection, or revascularization surgery. General exclusionary criteria included life-threatening heart or respiratory failure, renal or hepatic failure, severely disabling musculoskeletal disorder, cancer, and neurodegenerative disorders such as idiopathic Parkinson disease or Alzheimer disease. Patients were excluded if they experienced stroke due to complications of an intracranial aneurysm, arteriovenous malformation, or neoplastic disease because there are significant differences in the demographic characteristics of these patients, as well as in the risk factors and pathophysiology of these medical conditions. Patients with acute coronary syndromes and patients with neurodegenerative disorders were excluded because of evidence that there might be qualitative differences in the mechanism of depressive disorders frequently observed among these patients. In addition, patients were excluded if they met DSM-IV18 criteria for alcohol or substance abuse or dependence within the past 12 months prior to enrollment or if they met DSM-IV criteria for a depressive disorder at the time of the index stroke.
Neurological and Neuroradiological Evaluations
A complete physical and neurological examination was performed at the time of intake. The neurological findings were recorded using the National Institutes of Health Stroke Scale (NIHSS).19 Vital signs and body weight were recorded at baseline, 12 weeks, 6 months, 9 months, and 12 months follow-up. A 12-lead electrocardiogram, as well as laboratory measures of hemoglobin, hematocrit, white blood cell count, serum sodium, potassium, chloride, calcium, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total protein, glucose, creatinine, blood urea nitrogen, and urinalysis were obtained at the beginning of the study. Neuroimaging scans were obtained from the treating hospital for analysis by a neuroradiologist who was blinded to the treatment assignment. There was no standardized imaging protocol.
Experimental Design and Treatment
Patients were centrally randomized at the University of Iowa study site by a team member who was not involved in any evaluation using a permuted blocks randomization scheme. Specifically, at the beginning of the study, the targeted sample size was divided randomly (200 patients) into block sizes of 3, 6, and 9 and within each block patients were randomly assigned 1 of the 3 treatments using computer-generated random numbers of 1, 2, or 3 to escitalopram (10 mg/d in the morning for patients <65 years and 5 mg/d for patients ≥65years), placebo (all pills were identical), or problem-solving therapy. Escitalopram was selected, rather thancitalopram, because of empirical evidence in the literature indicating that escitalopram may have greater efficacy and a faster onset of action than citalopram and successful patient tolerance over a 12-month administration.20,21 The problem-solving therapy used in this study is a manual-based intervention originally developed in England for treating medical patients with depression14 that has been elaborated for application in the United States.22 The problem-solving therapy was administered to patients at all sites (10 in Chicago, 41 in Iowa, and 8 in New York) and consisted of 6 treatment sessions over the first 12 weeks (weeks 1, 2, 3, 4, 6, and 10) and 6 reinforcement sessions (months 4, 5, 6, 8, 10, and 12). Prior to administering problem-solving therapy, therapists had to successfully complete 5 training cases under the supervision of one of the investigators (M.H.). All sessions were audiotaped or videorecorded for supervision and adherence ratings by 1 investigator (M.H.). In patients without depression, the principles of therapy are the same as in patients with depression. The patient selects a problem and then goes through 7 steps to arrive at a course of action. Five patients were assigned to problem-solving therapy because they refused pharmacological treatment. Of these, 2 withdrew before treatment began and 3 were treated. None of these patients were included in the analysis of randomized patients. Patients were seen for evaluation by raters who were blinded to drug assignment or not involved in the administration of problem-solving therapy at 3, 6, 9, and 12 months.
Assessment Instruments and Depression Diagnosis
Patients were administered the Structured Clinical Interview for DSM-IV23 at the initial evaluation and at 3, 6, 9, and 12 months follow-up. We have previously demonstrated reliability and validity of structured interviews like the Structured Clinical Interview for DSM-IV among patients with stroke.24,25 The diagnoses of “depression due to stroke with major depressive-like episode” and “minor depression” (research criteria) were based on symptoms elicited by the Structured Clinical Interview and DSM-IV criteria.18,23 At each follow-up interview or if a patient reported a problem, the patient was examined for depression. The examiners were unaware of each patient’s treatment assignment and double-blinded assessments were done for escitalopram and placebo. The patients receiving problem-solving therapy were aware of their treatment and, although they were asked not to tell the rater, all raters became aware of their problem-solving therapy treatment due to patients divulging their treatment. The diagnosis of depression was based on the patient meeting diagnostic criteria for major or minor depression (ie, symptom duration for 2 or more weeks) and having a Hamilton-17 Depression Rating Scale13 score of greater than 12. The Hamilton-17 Depression Rating Scale and the Hamilton Anxiety Rating Scale26 were administered at each of the initial and follow-up interviews. The Hamilton-17 Depression Rating Scale has been widely used and demonstrated to be a reliable and valid measure of depressive symptoms among patients with stroke.27 The Hamilton Anxiety Rating Scale is a 16-item instrument that measures severity of anxiety symptoms and has been shown to be valid and reliable in patients with stroke.28,29 Socioeconomic status was determined using the Hollingshead and Redlich classification.30
Secondary outcome measures included an assessment of activities of daily living using the Functional Independence Measure.31 The Functional Independence Measure is an 18-item, 7-level, activities of daily living scale developed to assess interpersonal, familial, and occupational functioning. The maximum score is 122 points and higher scores indicate less impairment. The Functional Independence Measure has been widely used in rehabilitation settings and has been shown to be valid and reliable among patients with stroke.31 The Social Functioning Exam32 is a 28-item scale that assesses patients’ satisfaction with their social functioning prior to the stroke or during the 2 weeks prior to each examination. Scores on the Social Functioning Exam range from 0 to 1 with higher scores indicating greater severity of social impairment. Reliability and validity of this instrument have been demonstrated in a previous publication.33
Neuropsychological assessment included administration of the Line Bisection and Token Test at baseline to rule out significant hemispatial neglect and impaired ability to follow oral instructions. The remainder of the neuropsychological battery, which was administered at baseline and at the close of study, included the Repeatable Battery for the Assessment of Neuropsychological Status,34 a self-contained battery that includes tests of immediate and delayed memory, language, visuospatial/constructional ability, and attention; and additional tests of executive function. Detailed analysis of these data are beyond the scope of the current report.
Adverse Events
Patients, family members, and primary care physicians were asked about adverse events such as physical illnesses or medication adverse effects at 3-month intervals or sooner if an individual reported an adverse event— using a standardized checklist developed for this study. A data and safety monitoring board made up of investigators not involved in this trial assessed adverse events related to the 3 treatments.
Statistical Analysis
The study design was based on our a priori assumption that 35% of patients given placebo would develop a depressive disorder over 1 year. This was based on previous findings that 37% of stroke survivors developed depression after the acute stroke period and the mean rate of depression among placebo-treated patients in prior prevention treatment trials.9,10,12 We also predicted that 10% of patients who were given escitalopram would develop depression. This was based on the rate of depression in the sertraline group in the prevention trial by Rasmussen et al.12 Completer group sizes of 40 including new-onset cases of depression would, using an α of .05, give a power of 0.80 to detect significant intergroup differences.
Categorical data were analyzed using the Fisher exact test and logistic regression. Means and SDs were calculated for continuous measures and analyses were conducted using 1-way analysis of variance. Proportions, odds ratios (ORs), and hazard ratios (HRs) were reported along with their respective 95% confidence intervals. Size of the effect was assessed by reporting the number needed-to-treat along with the success rate difference and its CI following the recommendations by Kraemer and Kupfer.35 We used Wilson score CIs for success rate difference as recommended by Newcombe36 and Bender.37
In order to analyze the time-to-depression onset data, we built a proportional hazards Cox regression model using time-to-depression onset as the dependent variable and treatment group, site, and various baseline characteristics (eg, age, sex, prior history of mood disorder; Functional Independence Measure, Social Functioning Exam, and Repeatable Battery for the Assessment of Neuropsychological Status scores; hemispheric lesion location; and physical impairment) as independent variables. Only predictors that were significant were kept in the model. We tested all the 2-way interactions between these covariates and the treatment group. We used a robust estimate of the variance to account for the correlation of each data point within each site. This analysis included all the patients who completed the study as well as those who dropped out after randomization (ie, 176 patients). The latter were considered in the model as censored observations at the time they stopped participating in the study. The proportional hazards assumption was tested and met.
To investigate the effect of treatment on the secondary outcomes (ie, Functional Independence Measure, Social Functioning Exam, and Repeatable Battery for the Assessment of Neuropsychological Status global scores), we used a linear mixed model that included secondary outcome scores as the dependent variable, and treatment group, time, and site (random effect) as dependent variables. Other possible confounders such as change in Hamilton-17 Depression Rating Scale score between baseline and close out, sex, educational level, prior history of mood disorders, physical impairment, and age were considered in the model. Only significant predictors and interactions with the treatment group were considered in the final models. The mixed-model analytical approach allowed us not only to model the correlation of observations within sites, but also the correlation of patient outcomes over time while including all the available data during the course of this study.
All analyses were performed using R 2.5.1 (R Foundation for Statistical Computing, Vienna, Austria) and SAS version 9.1.3 for Windows (SAS Institute Inc, Cary, North Carolina). All the P values reported are 2-tailed. Significance level was set at P<.05.
RESULTS
Participants
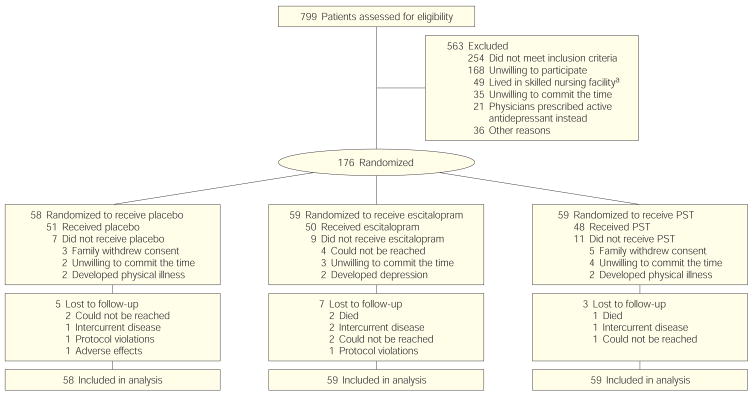
The patient flow diagram is shown in Figure 1. A total of 200 patients signed informed consent, 176 were randomized, and 149 began randomized treatment. After signing informed consent, 24 patients (12.0%) dropped out of the study before randomization and 27 (13.5%) dropped out before receiving the first drug dose or problem-solving therapy session. Reasons for withdrawal prior to beginning treatment included 9 participants who did not want to commit the time to the study, 4 who developed a physical illness, 8 whose families withdrew consent, 4 who could not be reached and 2 who developed depression. Out of the total 149 patients who began treatment, 15 patients (10.1%) dropped out of the study, 5 within 90 days, 4 between 91 and 180 days, and 6 between 181 and 365 days. Of these drop-out patients, 3 died (sepsis, lung cancer, and severe traumatic brain injury following a motor vehicle crash), 4 developed an intercurrent disease that was judged to be incompatible with study continuation, 1 experienced adverse effects that might have been related to the study medication and requested to be withdrawn, 1 patient in the placebo group and 1 in the escitalopram group were excluded because of protocol violations, and 5 could not be reached for follow-up. We compared demographic and baseline variables (eg, age, sex, marital status, baseline Hamilton-17 Depression Rating Scale and Functional Independence Measure scores) for the group of patients who were randomized to treatment with those for patients who dropped out. A logistic regression analysis found no demographic or baseline impairment variables related to postrandomization dropout.
Figure 1.
Patients Assessed and Randomized to Receive Escitalopram, Problem-Solving Therapy, or Placebo
PST indicates problem-solving therapy. Of 149 patients who received at least 1 dose of escitalopram or 1 session of PST, 89.9% reached the study end points.
aStudy participation was not allowed at skilled nursing facilities.
The demographic characteristics of the patients included in the intention-to-treat sample (n=176) are shown in Table 1. There were 58 patients randomized to placebo, 59 to escitalopram, and 59 to problem-solving therapy. There were no significant differences between the groups in age, sex, years of education, marital status, or socioeconomic status. The overall illness severity, as well as the existence of comorbid illness for the 3 groups, is shown in Table 1. There were no significant intergroup differences in overall cumulative illness scores, coronary artery disease, low-density lipoprotein cholesterol, atrial fibrillation, chronic obstructive pulmonary disease, or systolic blood pressure. The patients randomized to escitalopram, however, had a significantly greater frequency of diabetes mellitus when compared with the placebo group (Fisher exact test P value=.01).
Table 1.
Characteristics of Poststroke Patients Randomized to Receive Escitalopram, Problem-Solving Therapy, or Placebo
| No. (%) | |||
|---|---|---|---|
| Escitalopram (n = 59) | Problem-Solving Therapy (n = 59) | Placebo (n = 58) | |
| Age, mean (SD), y | 61.3 (13.7) | 67.3 (11.2) | 63.9 (13.3) |
| Male | 38 (64.4) | 30 (50.8) | 37 (63.8) |
| Married | 34 (57.6) | 27 (45.8) | 34 (58.6) |
| Education, mean (SD), y | 13.5 (3.2) | 14.2 (3.0) | 13.3 (2.8) |
| Socioeconomic class IV–Va | 15 (25.4) | 14 (23.7) | 17 (29.3) |
| Previous history of mood disorder | 3 (5.1) | 3 (5.1) | 3 (5.2) |
| Baseline HDRS score, mean (SD) | 7.1 (4.5) | 7.0 (3.7) | 7.2 (4.2) |
| Medical comorbidity Systolic blood pressure, mean (SD), mm Hg |
137.9 (24.9) | 147.2 (20.7) | 144.5 (23.5) |
| Low-density lipoprotein cholesterol, mean (SD), mg/dL | 117.4 (39.6) | 113.0 (40.4) | 120.6 (46.1) |
| Diabetes mellitusb | 25 (42.3) | 11 (18.6) | 11 (19.0) |
| Coronary artery disease | 16 (27.1) | 18 (30.5) | 11 (19.0) |
| Congestive heart failure | 10 (16.9) | 10 (16.9) | 3 (5.5) |
| Atrial fibrillation | 10 (16.9) | 14 (23.7) | 7 (12.1) |
| Chronic obstructive pulmonary disease | 7 (11.9) | 5 (8.5) | 3 (5.2) |
| Cumulative illness rating scale total scores, mean (SD) | 10.3 (5.1) | 9.7 (4.8) | 9.7 (4.8) |
| Stroke characteristics and functional impairment Ischemic stroke |
57 (96.6) | 52 (88.1) | 53 (91.4) |
| Location supratentorial lesions | 45 (76.3) | 43 (72.9) | 49 (84.5) |
| Large artery thrombosis | 36 (61.0) | 27 (45.8) | 23 (39.7) |
| Bilateral deep white matter lesions | 23 (39.0) | 24 (40.7) | 20 (34.5) |
| Side left lesions | 35 (59.3) | 22 (37.3) | 30 (51.7) |
| Lacunar infarctions | 14 (23.7) | 19 (32.2) | 24 (41.4) |
| NIHSS score, mean (SD) | 7.2 (2.9) | 5.9 (2.3) | 6.4 (2.0) |
| Baseline SFE score, mean (SD) | 0.11 (0.1) | 0.12 (0.1) | 0.14 (0.1) |
| Baseline FIM score, mean (SD) | 115.3 (15.3) | 110.5 (16.2) | 116.7 (14.4) |
| Baseline RBANS score, mean (SD) | 96.6 (14.3) | 87.4 (17.3) | 86.4 (14.4) |
Abbreviations: FIM, Functional Independence Measure; HDRS, Hamilton-17 Depression Rating Scale; NIHSS, National Institutes of Health Stroke Scale; RBANS, Repeatable Battery to Assess Neuropsychological Status; SFE, Social Functioning Exam. See the “Methods” section for descriptions and score ranges of these instruments.
SI conversion factor: to convert low-density lipoprotein cholesterol to mmol/L, multiply values by 0.0259.
Class IV indicates some high school, completion of high school, attainment of general educational development (GED), and employment at an unskilled trade; and class V indicates completion of an 8th-grade education or less and employment at an unskilled trade or unemployment per Hollingshead classification.30
Patients randomized to escitalopram had a greater frequency of diabetes mellitus when compared with the placebo group, Fisher exact test P = .01.
Stroke Characteristics and Impairment
The type, location, and severity of stroke as well as severity of impairment in activities of daily living are shown in Table 1. There were no significant differences among the escitalopram, placebo, and problem-solving therapy groups in stroke characteristics, severity of stroke as measured by the National Institutes of Health Stroke Scale, or severity of impairment in activities of daily living, as measured by the Functional Independence Measure score. We found no evidence that these characteristics were different across the 3 participating centers.
Effect of Preventive Intervention on Depression
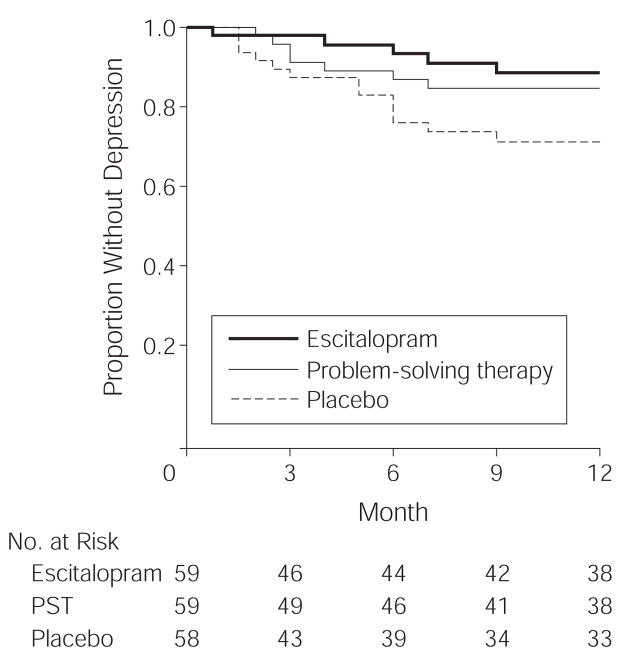
Using the 176 patients who were randomized to treatment, we built a Cox regression proportional hazards model using the time to depression onset as the dependent variable. Our final model included the type of treatment received and prior history of mood disorders as independent variables with data on the 27 patients who did not begin treatment censored at baseline (likelihood ratio test ; P=.03). The interaction between type of treatment received and a history of mood disorders was not statistically significant. After adjusting for the previous history of mood disorders, participants who received placebo (11 major and 2 minor cases of depression, total 22.4%) were 4.5 times more likely to develop depression than patients who received escitalopram (3 major and 2 minor cases of depression, total 8.5%; adjusted HR, 4.5; 95% CI, 2.4–8.2;P<.001) and 2.2 times more likely than patients who received problem-solving therapy (5 major and 2 minor cases of depression, total 11.9%; adjusted HR, 2.2; 95% CI, 1.4–3.5;P<.001;Figure 2). After adjusting for the treatment assignment, patients with a history of mood disorders were 5.2 times more likely to develop depression in the course of 1 year than patients without a previous history (adjusted HR, 5.2; 95% CI, 3.3–8.1; P<.001).
Figure 2.
Risk Comparison of Depression Onset for Patients Receiving Escitalopram, Problem-Solving Therapy (PST), or Placebo Over 1 Year
After adjusting for previous history of mood disorders, participants who received placebo were 4.5 times more likely to develop depression than participants who received escitalopram (adjusted hazard ratio, 4.5; 95% confidence interval, 2.4–8.2; P<.001) and 2.2 times more likely than participants who received problem-solving therapy (adjusted hazard ratio, 2.2; 95% confidence interval, 1.4–3.5; P<.001).
Based only on the frequency of depression onset during the 1 year of treatment, 7.2 acute stroke patients (success rate difference, 0.14; 95% CI, 0.01–0.27) would need to be treated with escitalopram to prevent 1 case of depression and 9.1 acute stroke patients (success rate difference, 0.11; 95% CI, −0.03 to 0.24) would need to be treated with problem-solving therapy to prevent 1 case of depression.36,37 This latter effect did not reach statistical significance.
An alternative intention-to-treat conservative method of analyzing our data is to assume that all 27 patients who did not start randomized treatment would have developed depression. Using this assumption and controlling for prior history of mood disorders, escitalopram was superior to placebo (23.1% vs 34.5%; adjusted HR, 2.2; 95% CI, 1.2–3.9; P=.007), while problem-solving therapy was not significantly better than placebo (30.5% vs 34.5%; adjusted HR, 1.1; 95% CI, 0.8–1.5; P=.51).
The majority of patients who developed depression during the study experienced major depression (19 of 25, 76%). The baseline Hamilton-17 Depression Rating Scale scores in the patients who developed depression were not significantly different from the scores of patients who did not develop depression.
Adverse Events and Adverse Effects
The frequency of adverse events or medication adverse effects is shown in Table 2. There were no significant differences between groups in the frequency of any of these events. Although this study was not designed to detect treatment-related differences in cardiovascular morbidity or other measures of morbidity related to the use of escitalopram, problem-solving therapy, or placebo, we did examine the number and cause of hospital admissions during the 1 year of treatment. A total of 5 patients receiving placebo and 5 patients receiving problem-solving therapy (8.6% of the combined group) and 2 receiving escitalopram (3.4%) sustained a cardiac event requiring hospitalization (OR, 2.7; 95% CI, 0.6–12.6; Fisher exact test P=.34). There was no evidence that patients receiving placebo or problem-solving therapy were any more or less likely than patients receiving escitalopram to be hospitalized with cardiovascular illness. Also, there were no significant differences between the groups in the frequency of hospital admissions resulting from gastrointestinal bleeding or falls, which have been previously described as complications of selective serotonin reuptake inhibitor treatment (Table 2).
Table 2.
Adverse Events in Poststroke Patients Randomized to Receive Escitalopram, Problem-Solving Therapy, or Placebo
| No. (%) | |||
|---|---|---|---|
| Escitalopram (n = 59) | Problem-Solving Therapy (n = 59) | Placebo (n = 58) | |
| Gastrointestinal adverse effects Dry mouth |
17 (29) | 23 (39) | 27 (47) |
| Constipation | 14 (24) | 16 (27) | 13 (22) |
| Indigestion | 13 (22) | 16 (27) | 11 (19) |
| Anorexia | 12 (20) | 9 (15) | 7 (12) |
| Diarrhea | 7 (12) | 14 (24) | 9 (16) |
| Abdominal pain | 5 (8) | 9 (15) | 9 (16) |
| Nausea | 4 (7) | 10 (17) | 4 (7) |
| Bleeding | 1 (2) | 1 (2) | 1 (2) |
| Sexual adverse effects Decreased libido |
12 (20) | 17 (29) | 9 (16) |
| Anorgasmia | 4 (7) | 3 (5) | 1 (2) |
| Ejaculation disorders | 3 (10) | 5 (23) | 2 (7) |
| Impotence | 3 (10) | 5 (23) | 2 (7) |
| Cardiovascular adverse effects Tachycardia |
38 (64) | 42 (71) | 36 (62) |
| Chest pain | 8 (14) | 12 (20) | 7 (12) |
| Palpitations | 5 (8) | 7 (13) | 3 (5) |
| Other adverse effects Dizziness |
37 (63) | 41 (69) | 34 (59) |
| Fatigue | 24 (41) | 21 (36) | 18 (31) |
| Increased sweating | 12 (20) | 17 (29) | 8 (14) |
| Falls | 1 (2) | 1 (2) | 1 (2) |
Effect on Secondary Outcome Variables
The effect of 1 year of treatment with escitalopram or problem-solving therapy or placebo on the longitudinal course of impairment inactivities of daily living, as assessed by the Functional Independence Measure, assessed the intention-to-treat sample(n=176). All patients experienced improvement in activities of daily living over time, particularly during the first 3 months. Scores on the Functional Independence Measure at 3-, 6-, 9-, and 12-month evaluations were significantly improved compared with baseline values for all treatment groups (F [4166]=9.49; P<.001). There was, however, no significant time × treatment interaction.
The assessment of social functioning over time revealed a significant effect of time on Social Functioning Exam scores (F [4167]=4.33; P=.002), but the time × treatment interaction was not significant, again indicating that all groups showed improvement. Assessment of neuropsychological testing revealed that the total score on the Repeatable Battery for the Assessment of Neuropsychological Status showed no significant intergroup differences at baseline. In comparing initial and end-of-study performance, there were no significant time, treatment, or time × treatment interactions across the 2 treatment groups.
COMMENT
Although universal preventive intervention has been a long-standing goal in psychiatry, there has not been a double-blind study that has demonstrated prevention of a psychiatric disorder. However, this study has demonstrated that depression can be significantly decreased in frequency by preventive use of escitalopram compared with placebo over the first year following an acute stroke, using double-blind methodology. In an open treatment group, we also found that problem-solving therapy significantly increased the time to onset of depression during the first year following stroke. Based on these results, we calculated that 7.2 stroke patients would need to be treated with escitalopram or 9.1 patients with problem-solving therapy to prevent 1 case of depression. This could be compared with preventive intervention in cardiology in which 40 male patients with hypercholesterolemia would need to be treated with pravastatin for 5 years to prevent 1 myocardial infarction.38
Before discussing the implications of these findings, the limitations of this study need to be acknowledged. First, the patients selected for the study did not include all patients with acute stroke. Patients with life-threatening comorbid physical illness, such as cancer or severe cardiac arrhythmia, were excluded. Similarly, patients with severe impairment in verbal comprehension or patients who had already developed a depressive disorder were excluded. Thus, our findings might not be applicable to all patients with stroke. However, as noted in Table 1, we included patients with multiple system illnesses and a representative range of stroke mechanisms and severity.
Second, the study included a relatively small sample size and the number of incident depression cases was also relatively small. Thus, further studies of prevention of poststroke depression are needed.
Third, our psychological treatment group could not be blinded except that the rater did not administer the treatment. Furthermore, there was no appropriate control for problem-solving therapy to account for the nonspecific effects of time and attention. It is possible, therefore, that some treatment bias may have influenced our findings with the problem-solving therapy group.
A fourth limitation is that the majority of patients were enrolled in Iowa (ie, 74%) and therefore the Iowa data had the greatest effect on outcomes. However, we found no statistically significant effect of treatment site on outcome.
A fifth limitation of this study is that a total of 51 patients out of the 200 who signed the informed consent form dropped out before receiving any treatment. Furthermore, following initiation of treatment, 15 of 149 patients (10.1%) dropped out during a 1-year treatment protocol. Thus, the loss of 33% of our study population may have influenced our results. This overall dropout rate, however, was lower than the 48.9% dropout rate in the study by Rasmussen et al12 and the 45.9% dropout rate in the study by Almeida et al11 and appears to have given us the statistical power needed to show a treatment effect.
Given these limitations, what are the implications of the current findings? Perhaps the first question is why this prevention trial worked while others failed to show a significant preventive effect. The mean age of the treated patients in the study by Rasmussen et al was 72 years (SD=9.1) and in the study by Almeida et al was 67 years (SD=13) while the mean age of our escitalopram-treated group was 62 years (SD=13). Although the measures of physical impairment in those studies were different from ours, the severity appears somewhat greater than ours. Thus, it is possible that our population differed from those of the other studies, which could explain the differences in prevention rates. Conversely, our increased sample size, as well as the medication selected and the dosage prescribed, may have led to successful preventive treatment in this population that was elderly and sometimes very frail. For example, Andersen et al39 demonstrated that citalopram was superior to placebo in the treatment of acute poststroke depression. Patients aged 65 years and older were given 10 mg of citalopram and those younger than 65 years were given 20 mg of citalopram. These relatively low doses of citalopram may have been particularly well suited for this patient population. Furthermore, in our previous study of treatment of poststroke depression,40 we used doses of as much as 40 mg of fluoxetine for all patients in that treatment group and found no effect of fluoxetine vs placebo for depression, but did find that fluoxetine produced a mean (SD) weight loss of 6.8 (3.6) kg, which was not seen with nortriptyline or placebo.40
The rates of gastrointestinal effects in the current study were not significantly different from placebo. It should also be acknowledged that the current study included a problem-solving therapy group that the other prevention studies did not have. It seems unlikely, however, that this influenced the overall study results. A recent 8-week nonblinded study of problem-solving therapy for preventing depression in older adults with macular degeneration also reported positive results for this treatment.41 The effects of problem-solving therapy, however, were lost over a 6-month follow-up period.41 In contrast to our study, the macular degeneration prevention protocol did not include reinforcement sessions. Thus, some form of maintenance problem-solving therapy may be necessary for long-term prevention.
The second logical question is what may be the consequence of prevention of poststroke depression? Since 1993, there have been at least 4 reports demonstrating an association of poststroke depression with increased mortality.42–45 For example, we reported in a 10-year follow-up of 103 patients with acute stroke that patients with acute-onset major or minor depression were more likely to die than patients who were without depression after the acute stroke, even after controlling for age, sex, marital status, socioeconomic status, cognitive impairment, activities of daily living impairment, previous and comorbid physical illness, stroke type, and stroke volume (adjusted OR, 3.7; 95% CI, 1.1–12.2; P=.03).42 Subsequently, House et al44 examined 448 patients with acute stroke and found that at 2 years follow-up, patients with 1 or more symptoms of depression on the General Health Questionnaire46 were significantly more likely to have died compared with patients with no depressive symptoms even after controlling for older age, lower Mini-Mental State Examination scores, lower Barthel score, prior stroke, and urinary incontinence (OR, 2.2; 95% CI, 1.2–4.0; P=.009). In a recent study of 104 acute stroke patients with or without depression, however, we found that 12 weeks of treatment with fluoxetine (20–40 mg) or nortriptyline (50–100 mg) significantly reduced the mortality rate at 7 to 9 years follow-up even after controlling for age, stroke type, comorbid physical illness, and diabetes mellitus.47 Whether prevention of post-stroke depression might lead to increased survival is an important issue for further investigation.
Although the mechanism by which escitalopram and problem-solving therapy were able to decrease the frequency of poststroke depression is unknown, it is possible that the mechanism for the treatment of acute depression may be different. Antidepressants have been associated with enhanced recovery in executive function,48 as well as recovery in activities of daily living49 independent of depression. In addition, it has been hypothesized that antidepressant effects on neuroplasticity50 mediate both recovery from depression as well as motor and cognitive recovery through different mechanisms.51 Perhaps prevention of depression may be another example of this alternative mode of action. Alternatively, one might also hypothesize, based on the study of macular degeneration, that regaining ability to facilitate valued activities may have led to a preventive effect on depression in patients receiving problem-solving therapy.41
In conclusion, this study has demonstrated that poststroke depression can be effectively prevented in a significant number of patients by administering escitalopram or problem-solving therapy during the first year following stroke. To our knowledge, this may be the first demonstration of selective prevention of a psychiatric disorder with adequate blinding for 2 of the 3 treatment groups. The 9 patients with prior history of depression clearly had maintenance intervention. However, the majority of patients had no prior history of mood disorder and this intervention protected them from depression. One might also question whether preventive intervention in all stroke survivors is superior to early detection and treatment. Although only a randomized controlled trial would answer this question, the major obstacle to early detection is that studies that have examined the detection of depression during standard stroke care have found that the vast majority of depression cases were overlooked by the treating physicians.52,53 The clinical implications of our findings are that patients who are given escitalopram or problem-solving therapy following acute stroke may be spared depression and perhaps its adverse consequences.
Acknowledgments
Funding/Support: This work was supported solely by National Institute of Mental Health (NIMH) grant R01 MH-65134. All of the study medications were purchased using NIMH grant funds.
Data and Safety Monitoring Board: Russell Noyes, MD, Harold Adams, MD, and Douglas Langbehn, MD, University of Iowa.
Role of the Sponsor: NIMH did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; or preparation, review, or approval of the manuscript.
Additional Contributions: We thank Stephanie Rosazza, BA, University of Iowa, for study coordination; Teresa Kopel, MA, University of Iowa, for administering problem-solving therapy; Jessica Thompson, BS, University of Chicago, for administering problem-solving therapy; and Mireya Montalvan, BA, Burke Rehabilitation Hospital, for performing neuropsychological assessment. None of these individuals received compensation for their work in association with this article.
Footnotes
Financial Disclosures: All of the authors received salary contributions from the grant supporting this study. Over the past 5 years, Dr Robinson reports serving as a consultant to the former Hamilton Pharmaceutical Company and Avanir Pharmaceutical Company; Dr Jorge reports receiving 2 travel awards to participate in national meetings from the former Hamilton Pharmaceutical Company and Avanir Pharmaceutical Company; and Dr Small reports that he conducted a research study funded by Northstar Neuroscience that was unrelated to this prevention study. The former Hamilton Pharmaceutical Company and Avanir Pharmaceutical Company had no financial interest in this prevention study. No other authors reported any financial disclosures.
Author Contributions: Dr Robinson had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Robinson, Jorge, Solodkin, Fonzetti, Hegel.
Acquisition of data: Robinson, Jorge, Solodkin, Small, Fonzetti.
Analysis and interpretation of data: Robinson, Jorge, Moser, Acion, Small, Arndt.
Drafting of the manuscript: Robinson, Jorge, Acion, Fonzetti, Hegel.
Critical revision of the manuscript for important intellectual content: Moser, Acion, Solodkin, Small, Hegel, Arndt.
Statistical analysis: Jorge, Acion, Arndt.
Obtained funding: Robinson, Solodkin.
Administrative, technical, or material support: Robinson, Solodkin, Small, Fonzetti.
Study supervision: Moser, Hegel.
References
- 1.Caplan GA. A Conceptual Model for Primary Prevention: Principles of Preventive Psychiatry. New York NY: Basic Books; 1964. [Google Scholar]
- 2.Mrazek PJ, Haggerty RJ, editors. Committee on Prevention of Mental Disorders; Division of Biobehavioral Sciences and Mental Disorders. Reducing Risks for Mental Disorders: Frontiers for Preventive Intervention Research. Washington, DC: National Academy Press; 1994. [PubMed] [Google Scholar]
- 3.Thom T, Haase N, Rosamond W, et al. Heart disease and stroke statistics—2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113(6):e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 4.Aström M, Adolfsson R, Asplund K. Major depression in stroke patients: a 3-year longitudinal study. Stroke. 1993;24(7):976–982. doi: 10.1161/01.str.24.7.976. [DOI] [PubMed] [Google Scholar]
- 5.Berg A, Psych L, Palomaki H, et al. Poststroke depression—an 18-month follow-up. Stroke. 2003;34(1):138–143. doi: 10.1161/01.str.0000048149.84268.07. [DOI] [PubMed] [Google Scholar]
- 6.Burvill PW, Johnson GA, Jamrozik KD, Anderson CS, Stewart-Wynne EG, Chakera TMH. Prevalence of depression after stroke: the Perth Community Stroke Study. Br J Psychiatry. 1995;166(3):320–327. doi: 10.1192/bjp.166.3.320. [DOI] [PubMed] [Google Scholar]
- 7.House A, Dennis M, Mogridge L, Warlow C, Hawton K, Jones L. Mood disorders in the year after first stroke. Br J Psychiatry. 1991;158:83–92. doi: 10.1192/bjp.158.1.83. [DOI] [PubMed] [Google Scholar]
- 8.Morris PLP, Robinson RG, Raphael B. Prevalence and course of depressive disorders in hospitalized stroke patients. Int J Psychiatry Med. 1990;20(4):349–364. doi: 10.2190/N8VU-6LWU-FLJN-XQKV. [DOI] [PubMed] [Google Scholar]
- 9.Robinson RG. The Clinical Neuropsychiatry of Stroke. 2. Cambridge, England: Cambridge University Press; 2006. [Google Scholar]
- 10.Palomäki H, Kaste M, Berg A, Lonqvisst R. Prevention of poststroke depression: 1 year randomised placebo controlled double blind trial of mainserin with 6 month followup after therapy. J Neurol Neurosurg Psychiatry. 1999;66(4):490–494. doi: 10.1136/jnnp.66.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Almeida OP, Waterreus A, Hankey GJ. Preventing depression after stroke: results from a randomized placebo-controlled trial. J Clin Psychiatry. 2006;67(7):1104–1109. doi: 10.4088/jcp.v67n0713. [DOI] [PubMed] [Google Scholar]
- 12.Rasmussen A, Lunde M, Poulsen DL, Sorensen K, Qvitzau S, Bech P. A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics. 2003;44(3):216–221. doi: 10.1176/appi.psy.44.3.216. [DOI] [PubMed] [Google Scholar]
- 13.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Patel NC, Guo JJ, Zhan S. Antidepressant prophylaxis for poststroke depression: a meta-analysis. Int Clin Psychopharmacol. 2007;22(3):159–166. doi: 10.1097/YIC.0b013e32807fb028. [DOI] [PubMed] [Google Scholar]
- 15.Mynors-Wallis LM, Gath DH, Lloyd-Thomas AR, Tomlinson D. Randomised controlled trial comparing problem solving treatment with amitryptyline and placebo for major depression in primary care. BMJ. 1995;310(6977):441–445. doi: 10.1136/bmj.310.6977.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lincoln NB, Flannaghan T. Cognitive behavioral psychotherapy for depression following stroke: a randomized controlled trial. Stroke. 2003;34(1):111–115. doi: 10.1161/01.str.0000044167.44670.55. [DOI] [PubMed] [Google Scholar]
- 17.De Renzi E, Vignolo LA. The Token Test: a sensitive test to detect disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- 18.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 19.Kunitz SC, Gross CR, Heyman A, et al. The pilot stroke data bank: definition, design and data. Stroke. 1984;15(4):740–746. doi: 10.1161/01.str.15.4.740. [DOI] [PubMed] [Google Scholar]
- 20.Tamminga CA, Nemeroff CB, Blakely RD, et al. Developing novel treatments for mood disorders: accelerating discovery. Biol Psychiatry. 2002;52(6):589–609. doi: 10.1016/s0006-3223(02)01470-1. [DOI] [PubMed] [Google Scholar]
- 21.Moore N, Verdoux H, Fantino B. Prospective, multicentre, randomized, double-blind study of the efficacy of escitalopram versus citalopram in outpatient treatment of major depressive disorder. Int Clin Psychopharmacol. 2005;20(3):131–137. doi: 10.1097/00004850-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Hegel MT, Barrett JE, Cornell JE, Oxman TE. Predictors of response to problem-solving treatment of depression in primary care. Behav Ther. 2002;33(4):511–527. [Google Scholar]
- 23.Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) New York, NY: Biometric Research, New York State Psychiatric Institute; 1995. [Google Scholar]
- 24.Fedoroff JP, Starkstein SE, Parikh RM, Price TR, Robinson RG. Are depressive symptoms non-specific in patients with acute stroke? Am J Psychiatry. 1991;148(9):1172–1176. doi: 10.1176/ajp.148.9.1172. [DOI] [PubMed] [Google Scholar]
- 25.Paradiso S, Ohkubo T, Robinson RG. Vegetative and psychological symptoms associated with depressed mood over the first two years after stroke. Int J Psychiatry Med. 1997;27(2):137–157. doi: 10.2190/BWJA-KQP3-7VUY-D06T. [DOI] [PubMed] [Google Scholar]
- 26.Hamilton M. The assessment of anxiety state of rating. Br J Med Psychol. 1959;32(1):50–55. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 27.Robinson RG, Kubos KL, Starr LB, Rao K, Price TR. Mood disorders in stroke patients: importance of location of lesion. Brain. 1984;107(pt 1):81–93. doi: 10.1093/brain/107.1.81. [DOI] [PubMed] [Google Scholar]
- 28.Shimoda K, Robinson RG. Effects of anxiety disorder in impairment and recovery from stroke. J Neuropsychiatry Clin Neurosci. 1998;10(1):34–40. doi: 10.1176/jnp.10.1.34. [DOI] [PubMed] [Google Scholar]
- 29.Kimura M, Robinson RG. Treatment of poststroke generalized anxiety disorder comorbid with poststroke depression: Merged analysis of nortriptyline trials. Am J Geriatr Psychiatry. 2003;11(3):320–327. [PubMed] [Google Scholar]
- 30.Hollingshead AB, Redlich FC. Social Class and Mental Illness: A Community Study. New York, NY: Wiley; 1958. [Google Scholar]
- 31.Forer S, Granger CV. Functional Independence Measure. Buffalo, NY: The Buffalo General Hospital, State University of New York at Buffalo; 1987. [Google Scholar]
- 32.Starr LB, Robinson RG, Price TR. The Social Functioning Exam: an assessment for stroke patients. Soc Work Res Abstr. 1982;18(4):28–33. doi: 10.1093/swra/18.4.28. [DOI] [PubMed] [Google Scholar]
- 33.Starr LB, Robinson RG, Price TR. Reliability, validity, and clinical utility of the social functioning exam in the assessment of stroke patients. Exp Aging Res. 1983;9(2):101–106. doi: 10.1080/03610738308258434. [DOI] [PubMed] [Google Scholar]
- 34.Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. New York, NY: The Psychological Corp; 1998. [Google Scholar]
- 35.Kraemer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59(11):990–996. doi: 10.1016/j.biopsych.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17(8):873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 37.Bender R. Calculating confidence intervals for the number needed to treat. Control Clin Trials. 2001;22(2):102–110. doi: 10.1016/s0197-2456(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 38.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia: West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333 (20):1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 39.Andersen G, Vestergaard K, Riis JO, Lauritzen L. Incidence of post-stroke depression during the first year in a large unselected stroke population determined using a valid standardized rating scale. Acta Psychiatr Scand. 1994;90(3):190–195. doi: 10.1111/j.1600-0447.1994.tb01576.x. [DOI] [PubMed] [Google Scholar]
- 40.Robinson RG, Schultz SK, Castillo C, Kopel T, Kosier T. Nortriptyline versus fluoxetine in the treatment of depression and in short term recovery after stroke: a placebo controlled, double-blind study. Am J Psychiatry. 2000;157(3):351–359. doi: 10.1176/appi.ajp.157.3.351. [DOI] [PubMed] [Google Scholar]
- 41.Rovner BW, Casten RJ, Hegel MT, Leiby BE, Tasman WS. Preventing depression in age-related macular degeneration. Arch Gen Psychiatry. 2007;64(8):886–892. doi: 10.1001/archpsyc.64.8.886. [DOI] [PubMed] [Google Scholar]
- 42.Morris PLP, Robinson RG, Andrezejewski P, Samuels J, Price TR. Association of depression with 10-year post-stroke mortality. Am J Psychiatry. 1993;150(1):124–129. doi: 10.1176/ajp.150.1.124. [DOI] [PubMed] [Google Scholar]
- 43.Morris PLP, Robinson RG, Samuels J. Depression, introversion and mortality following stroke. Aust N Z J Psychiatry. 1993;27(3):443–449. doi: 10.3109/00048679309075801. [DOI] [PubMed] [Google Scholar]
- 44.House A, Knapp P, Bamford J, Vail A. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke. 2001;32(3):696–701. doi: 10.1161/01.str.32.3.696. [DOI] [PubMed] [Google Scholar]
- 45.Williams LS, Ghose SS, Swindle RW. Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am J Psychiatry. 2004;161(6):1090–1095. doi: 10.1176/appi.ajp.161.6.1090. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg DP, Hiller VF. A scaled version of the General Health Questionnaire. Psychol Med. 1979;9(1):139–145. doi: 10.1017/s0033291700021644. [DOI] [PubMed] [Google Scholar]
- 47.Jorge RE, Robinson RG, Arndt S, Starkstein SE. Mortality and post-stroke depression: a placebo controlled trial of antidepressants. Am J Psychiatry. 2003;160(10):1823–1829. doi: 10.1176/appi.ajp.160.10.1823. [DOI] [PubMed] [Google Scholar]
- 48.Narushima K, Paradiso S, Moser DJ, Jorge R, Robinson RG. Effect of antidepressant therapy on executive function after stroke. Br J Psychiatry. 2007;190:260–265. doi: 10.1192/bjp.bp.106.025064. [DOI] [PubMed] [Google Scholar]
- 49.Narushima K, Robinson RG. The effect of early versus late antidepressant treatment on physical impairment associated with poststroke depression: is there a time-related therapeutic window? J Nerv Ment Dis. 2003;191(10):645–652. doi: 10.1097/01.nmd.0000092197.97693.d2. [DOI] [PubMed] [Google Scholar]
- 50.Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 51.Wong ML, Licinio J. Research and treatment approaches to depression. Nat Rev Neurosci. 2001;2(5):343–351. doi: 10.1038/35072566. [DOI] [PubMed] [Google Scholar]
- 52.Feibel JH, Springer CJ. Depression and failure to resume social activities after stroke. Arch Phys Med Rehabil. 1982;63(6):276–278. [PubMed] [Google Scholar]
- 53.Schubert DSP, Taylor C, Lee S, Mentari A, Tamaklo W. Detection of depression in the stroke patient. Psychosomatics. 1992;33(3):290–294. doi: 10.1016/S0033-3182(92)71967-7. [DOI] [PubMed] [Google Scholar]