Abstract
Distinct IFN-gamma and IL-2 profiles of antigen-specific CD4+ T cells have recently been associated with different clinical disease states and antigen loads in viral infections. We assessed the kinetics and functional profile of Mycobacterium tuberculosis antigen-specific T cells secreting IFN-gamma and IL-2 in 23 patients with untreated active tuberculosis, when bacterial and antigen load are high, and after curative treatment when antigen load is reduced. Frequencies of M. tuberculosis antigen-specific IFN-gamma-secreting T cells declined during 28 months of follow up with an average percentage decline of 5.8% per year (P=0.005), whilst frequencies of antigen-specific IL-2-secreting T cells increased during treatment (P=0.02). These contrasting dynamics for the two cytokines led to a progressive convergence of the frequencies of IFN-gamma and IL-2-secreting cells over 28 months. Simultaneous measurement of IFN-gamma and IL-2 secretion at the single cell level revealed a co-dominance of IFN-gamma-only and IFN-gamma/IL-2 dual-secreting CD4+ T cells in active disease which shifted to dominance of IFN-gamma/IL-2-secreting CD4+ T cells and newly detectable IL-2-only-secreting CD4+ T cells during and after treatment. These distinct T cell functional signatures before and after treatment suggest a novel immunological marker of mycobacterial load and clinical status in tuberculosis, which now requires validation in larger prospective studies.
Keywords: human, memory, T cells, bacterial, cytokines
Introduction
Antigen-specific memory T cell responses are phenotypically and functionally heterogeneous (reviewed in Refs. (1-5)). Based on their phenotypic expression of CCR7, CD62L and CD45RA, and on their ability to produce cytokines and proliferate they have been subdivided into effector (TEM) and central (TCM) memory T cells. Interferon-gamma (IFN-γ) is predominantly produced by TEM while interleukin-2 (IL-2) is predominantly produced by TCM (6).
The IFN-γ and IL-2 cytokine profile of CD4+ T cells has been studied in a number of viral infections with different viral loads and persistence. High viral load in acute CMV and HIV-1 infection and in chronic progressive HIV-1 infection was associated with a dominance of CD4+ T cells secreting IFN-γ-only (7-10). Antigen persistence with low antigen load in chronic HCV, HSV, EBV, CMV and HIV-2 infections and in HIV-1 long-term nonprogressors, was associated with CD4+ T cells secreting IFN-γ-only, IFN-γ/IL-2 and IL-2-only (7, 8, 11, 12). Antigen clearance of past influenza infection was also associated with CD4+ T cells secreting IFN-γ-only, IFN-γ/IL-2 and IL-2-only but with a dominance of CD4+ T cells secreting IFN-γ/IL-2 (13). In contrast, in another model of antigen clearance, past tetanus toxoid vaccination, CD4+ T cells specific for tetanus toxoid predominantly secreted IL-2-only (8). When HIV-1 viral load was manipulated with administration of antiretroviral therapy or therapy interruptions a shift between these cytokine patterns was observed (8). These studies suggest that there is an association between distinct CD4+ T cell IFN-γ and IL-2 cytokine secretion patterns and viral load (14), with different antigen loads characterised by distinct functional signatures of CD4+ T cells.
The relationship between T cell functional signatures and antigen load has not hitherto been investigated in bacterial infections. We determined to assess the relationship between the IFN-γ and IL-2 cytokine secretion profile of antigen-specific T cells and bacterial load within a single disease entity, choosing tuberculosis as a model. Unlike in several chronic viral infections where viral load can be readily quantified, there is no direct quantitative measure of mycobacterial load. However, bacterial and antigen load are high in active disease and decline substantially after successful treatment when the individual has recovered. We hypothesised that the balance between frequencies of IFN-γ-secreting T cells and IL-2-secreting T cells would change after treatment of active tuberculosis and that patients with untreated active tuberculosis would have distinct T cell IFN-γ and IL-2 functional profiles from successfully treated patients. Although Mycobacterium tuberculosis-specific IFN-γ-secreting T cells have previously been enumerated during treatment of active tuberculosis (15-18) they have not been tracked beyond completion of therapy and the frequencies of IL-2-secreting T cells have not been reported.
Using enzyme-linked immunospot (ELISpot) assays for IFN-γ and IL-2 we tracked frequencies of T cells specific for two M. tuberculosis-specific antigens early secreted antigenic target protein (ESAT-6) and culture filtrate protein (CFP-10) encoded by the region of difference 1, an M. tuberculosis genomic segment that is absent from all strains of Mycobacterium bovis bacille Calmette-Guérin (BCG) vaccine (19). We studied 23 active tuberculosis cases during 28 months of follow up. To identify and quantify functional T cell subsets we simultaneously measured ESAT-6 and CFP-10-specific IFN-γ and IL-2 secretion at the single cell level using an ultra-sensitive cytokine secretion assay in patients with active tuberculosis before, during and after their anti-tuberculosis treatment.
Materials and Methods
Participants
Twenty-three consenting adult patients with active tuberculosis were prospectively recruited at Birmingham Heartlands Hospital, United Kingdom and longitudinally followed up for 28 months. After obtaining written informed consent the first heparinised blood sample was drawn before or within the first week of anti-tuberculosis treatment. Demographic data at screening, and clinical data at screening and all follow up timepoints (3, 6, 12, 18 and 28 months) were recorded on a standard questionnaire by the study research nurse. The 12 month follow up blood sample was drawn at 11 to 16 months following treatment initiation; the 18 month follow up blood sample was drawn at 17 to 21 months following treatment initiation and the 28 month sample was drawn at 25 to 32 months after treatment initiation. None of the patients we followed up reported contact with other tuberculosis cases after their diagnosis. Four additional patients with active tuberculosis were recruited at Birmingham Heartlands Hospital and Northwick Park Hospital, London to study cross-sectionally. Ethical approval was granted by the East Birmingham Local Research, Harrow Research and the Central Oxford Research Ethics Committees.
Of the patients tested for HIV infection, all were HIV antibody-negative. Of the remaining patients none had clinical or laboratory features specifically suggestive of HIV infection. TST was performed and read by an experienced tuberculosis nurse or doctor, using the Mantoux method with 0.1 mL (10 tuberculin units) of PPD (Evans Vaccines, Liverpool, U.K.). Cutaneous induration was measured after 72 h with a ruler. A response was scored as positive if the diameter of induration was 15 mm or greater, irrespective of BCG vaccination status. For logistical reasons in some cases the Heaf method was used instead, with the standard multiple puncture six-needle disposable-head Heaf gun (Bignall Surgical Instruments, Littlehampton, UK) and concentrated PPD (100,000 tuberculin units per mL, Evans Vaccines). Induration was measured one week later, as recommended for this application of the TST. A response was scored from grade 0 to 4 and grades 3 or 4 were recorded as positive irrespective of BCG vaccination status, in accordance with U.K. national guidelines.
Control participants with no known history of M. tuberculosis exposure were recruited from departmental immunology research laboratories.
Purification of lymphocytes from peripheral blood
30 mL blood was available at all of the timepoints except in active untreated tuberculosis where only 10 mL were available in half of the patients. Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized whole blood by Ficoll-PaquePLUS density gradient centrifugation (Amersham Biosciences, Bucks, UK), washed twice in RPMI (Sigma, St. Louis, MO) and suspended in R10 (RPMI 1640 (Sigma) supplemented with 1 mM sodium pyruvate (Invitrogen, Paisley, UK), 2 mM L-glutamine, and 10% heat-inactivated foetal calf serum (Sigma)). PBMC were cryopreserved in 90 % foetal calf serum (Sigma).
Ex vivo IFN-γ ELISpot
The in-house ex vivo IFN-γ ELISpot assays were performed as previously described (20). Briefly, pre-coated IFN-γ ELISpot plates (Mabtech AB, Stockholm, Sweden) were seeded with 2.5×105 PBMC per well: duplicate wells contained no peptide (negative control), 5 μg/mL phytohemagglutinin (positive control; MP Biomedicals, Aurora, OH, USA), 20 μg/mL tuberculin PPD (SSI, Copenhagen, Denmark), 100 U/mL streptokinase, 26 U/mL streptodornase (SKSD, Wyeth Farma SA, Spain) or one of six pools containing 15-mer peptides overlapping by 10 amino acids (10 μg/mL final concentration of each peptide, Core Labs LSUHSC, New Orleans, LA, USA) spanning the length of ESAT-6 (17 peptides) or CFP-10 (18 peptides) in 3 pools for each antigen. After 18 h incubation at 37°C in 5% carbon dioxide, plates were developed with ALP-conjugated IFN-γ detection antibody (Mabtech) followed by the chromogenic substrate BCIP/NBTPLUS (Moss, Pasadena, MD, USA). Spot forming cells (SFCs) were counted using an automated ELISpot reader (AID-GmbH, Strassberg, Germany). Settings for the intensity and size of a counted spot were predefined and the same settings used throughout. Responses were scored as positive if test wells contained a mean of at least five SFCs more than the mean of the negative control wells, and was at least twice the mean of the negative control wells. This threshold is similar to that used in the commercially available assay, T-SPOT.TB™ which is based on our ELISpot assay system, and which uses a threshold of 6 SFCs more than the negative control when the negative control has ≤5 SFCs. Positive ESAT-6 and CFP-10 peptide pool responses were summated for each participant to give the ESAT-6/CFP-10 response.
Ex vivo IL-2 ELISpot
The ex vivo IL-2 ELISpot was performed in the same manner as the ex vivo IFN-γ ELISpot with the following differences. The membrane of a MAIPS4510 plate (Millipore Corporation, Bedford, MA, USA) was pre-wet with 50 μL 70% ethanol per well for 2 min at room temperature. Wells were washed 5 times with 200 mL/well sterile water and incubated overnight at 4°C with 10 μg/mL coating IL-2 Ab (Mabtech). Wells were washed 5 times with 200 mL/well sterile PBS (Sigma) and blocked for 30 min at room temperature with R10. After incubation with stimulus for 18 h (see above) plates were developed with 1 μg/mL biotin-conjugated IL-2 detection Ab (Mabtech) for 2 h at room temperature and then streptavidin-ALP (Mabtech) diluted 1:1000 in PBS for 1 h at room temperature followed by the chromogenic substrate BCIP/NBTPLUS (Moss, Pasadena, MD, USA). SFCs were counted and interpreted as described above. Settings for the intensity and size of a counted spot were predefined and the same settings used throughout.
Ex vivo detection and magnetic bead enrichment of IFN-γ and IL-2 single and dual secreting antigen-specific cells
5 × 106 PBMC in a 48-well plate were rested at 37°C in 5% carbon dioxide overnight. Media alone, 1 μg/mL of staphylococcal enterotoxin B (positive control, Sigma) or 10 μg/mL of a peptide pool (ESAT-6 or CFP-10) were added to the cells for 5 h. Cells were transferred into a 15 mL falcon tube and washed with MACS buffer (PBS, 0.5% bovine serum albumin and 2 mM EDTA (Sigma)). After centrifugation at 1400rpm for 10 min at 4°C the cell pellet was resuspended in 80 μL cold RH5 (RPMI 1640 supplemented with 5% heat-inactivated human AB serum (BTS, Bristol, UK)). 10 μL of IFN-γ catch reagent and 10 μL of IL-2 catch reagent (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) were added per test condition and incubated on ice for 5 min. 5 mL warm RH5 was added and cells were slowly rotated for 45 min at 37°C in 5% carbon dioxide. An equal volume of cold MACS buffer was added and incubated on ice for 10 min. Cells were washed with MACS buffer and after centrifugation the cell pellet was resuspended in 80 μL cold MACS buffer and stained with 10 μL allophycocyanin (APC)-conjugated IFN-γ-specific detection antibody, 10 μL phycoerythrin (PE)-conjugated IL-2-specific detection antibody (Miltenyi Biotec), 5 μL fluorescein isothiocyanate (FITC)-conjugated anti-CD4+ antibody (eBioscience Inc, San Diego, CA, USA), 4 μL peridinin chlorophyll protein (PerCP)-conjugated anti-CD14 and 4 μL PerCP-conjugated anti-CD19 (BD PharMingen, San Diego, CA USA) for 15 min on ice. Cells were washed with MACS buffer and cell pellet resuspended in 80 μL cold MACS buffer. Superparamagnetic microbeads (20 μL) conjugated to monoclonal mouse anti-PE and anti-APC antibodies (Miltenyi Biotec) were added and incubated for 15 min at 4 to 8°C. Cells were washed in cold MACS buffer and 10% of the cell sample was removed for pre-enrichment flow cytometric analysis. The remainder of the cells were positively selected for by passing through two MS+ columns (Miltenyi Biotec). Cells bound to microbeads are uniformly retained in the column once a certain threshold of bound microbeads has been reached. Dead cells were excluded by staining with Viaprobe 7-AAD (BD PharMingen) just prior to acquisition. Flow cytometric acquisiton was performed on a FACSCalibur and analysis performed using FlowJo® v6.1 software (Tree Star Inc., Ashland, OR, USA). The frequency of CD4+ T cells secreting IFN-γ and/or IL-2 was calculated by dividing the number of post-enrichment CD4+ T cells secreting IFN-γ and/or IL-2 by the number of CD4+ T cells in the pre-enrichment sample multiplied by 9 (to account for the fact that 90% of the cells were used for the enrichment).
Statistical analysis
Average ESAT-6/CFP-10, ESAT-6, CFP-10 and SKSD-specific T cell responses were summarised as geometric mean counts of spot-forming cells (SFCs), expressed together with 95% confidence intervals. Rates of change in geometric means over the 28 months of follow up, were estimated by fitting a linear regression model allowing for repeated measurements on each individual, and the significance of change estimated (21). As estimation was undertaken on the log-scale, the exponentiated parameter estimates can be interpreted as symmetrical percentage changes (22), and are standardised to change per year. Comparison of T cell responses between contacts followed for 28 months and contact followed up for less than 28 months; and between ELISpot-positive and ELISpot-negative contacts at 28 months were compared using t-tests on log counts. Spearman’s rank correlation coefficient was used to test association between frequencies of IFN-γ and IL-2-secreting ESAT-6/CFP-10-specific T cells at corresponding timepoints. Differences in proportions were compared using the Chi-squared test. Comparisons of IFN-γ, IFN-γ/IL-2 and IL-2 secretion between active tuberculosis and during and after treatment were tested using the Mann-Whitney test. A P value of <0.05 was considered significant. Analyses were performed using Stata version 9.2 (Stata Corporation, College Station, TX, USA).
Results
Demographic and clinical characteristics of patients with active tuberculosis and control participants
Demographic and clinical characteristics of the 23 patients with active tuberculosis followed up are summarised in Table 1. All participants had clinical and radiological findings consistent with active tuberculosis (American Thoracic Society class 3 (23)). Diagnosis was confirmed by bacteriological isolation of M. tuberculosis in 12 participants and a further 8 cases had histological appearance of granulomas and clinical and radiological findings strongly suggestive of tuberculosis. Three participants were classified as highly probable tuberculosis on the basis of clinical and radiological features highly suggestive of tuberculosis which were unlikely to be caused by another disease, with a decision to initiate anti-tuberculosis chemotherapy made by the attending physician, and an appropriate response to therapy. The site of disease in the 23 participants represented a broad clinical spectrum (Table 1). All participants were treated in accordance with the British Thoracic Society guidelines and received therapy for 6 months or in the case of 2 patients with tuberculosis meningitis and 1 patient with joint tuberculosis, therapy for 12 months (24). Treatment was successful in all participants, as evidenced by no clinical or radiographic evidence of current disease, completion of anti-tuberculosis chemotherapy and sterile mycobacterial cultures. Nine participants were followed up for 6 timepoints, 5 participants for 5 timepoints, 6 participants for 4 timepoints and 3 participants for 3 timepoints of the study. IFN-γ and IL-2 ELISpot assays and clinical assessments were performed at each of these timepoints. However, it was only possible to do IL-2 ELISpot assays in approximately 50% of patients at the first study timepoint when diagnosed with active tuberculosis, due to limited blood sample volume (see Methods). One participant died during the study but cause of death was attributed to cancer and not tuberculosis, otherwise missed follow up timepoints were for logistical reasons only.
Table I.
Demographic and clinical characteristics of patients with active tuberculosis followed longitudinally n=23
| Demographic characteristics | |
| Age in years, median (range) | 35 (17, 83) |
| Gender, female (%) | 9 (39) |
| Ethnic Origina | |
| Indian subcontinent | 18 |
| Black African | 3 |
| Indonesian | 1 |
| White | 1 |
| Clinical characteristics | |
| Basis of diagnosisb | |
| M.tuberculosis culture-positive | 12 |
| Histological appearance of granulomas | 8 |
| Clinical & radiological findings highly suggestive of tuberculosis | 3 |
| Site of disease | |
| Lymphatic | 8 |
| Pulmonary | 7 |
| Pleural | 3 |
| Disseminatedc | 2 |
| Genitourinary | 1 |
| Joint | 1 |
| Meningeal | 1 |
| Tuberculin skin test | |
| Positived | 22 |
| ND | 1 |
All participants were born in their country of ethnic origin except one Paskistani that was born in UK
Diagnosis is equivalent to American Thoracic Society class 3 (23)
Meningeal & pleural n=1; pulmonary & colonic n=1
Mantoux ≥15mm n=10, Heaf grade ≥3 n=12
The 4 active tuberculosis patients studied cross-sectionally at the pre-treatment timepoint only had a median age of 29 (range 21 to 62) and all were male. Three patients were of African and 1 patient was of Indian ethnicity and birth. All 4 had smear and culture confirmed pulmonary tuberculosis. Three were TST positive (20, 22 and 24mm induration) and 2 were BCG-vaccinated.
The 13 control participants were healthy, laboratory personnel from regions with a low prevalence of tuberculosis (U.K., France, Canada, Israel and New Zealand) and with no known exposure to M. tuberculosis. Eleven were Caucasian and 2 were of Indian ethnicity. The median age was 29 years (range 20 to 47), 8 were female and 11 were BCG vaccinated. All tested negative by the ESAT-6/CFP-10-specific IFN-γ ELISpot assay indicating absence of M. tuberculosis infection (20, 25, 26).
Frequencies of ESAT-6 and CFP-10-specific IFN-γ and IL-2-secreting T cells during follow up
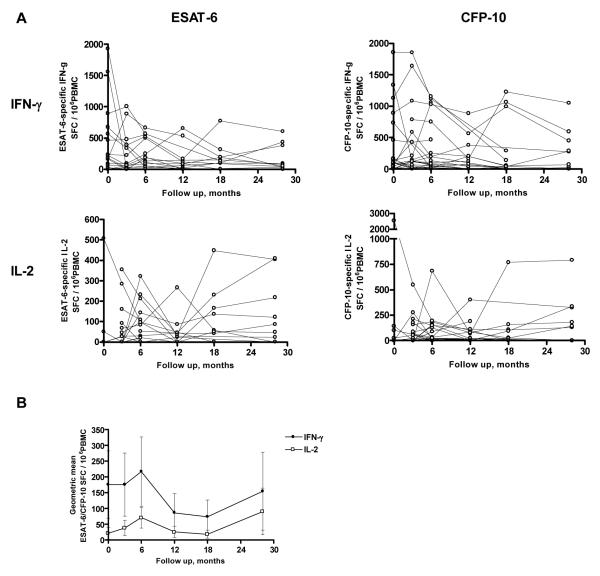
Frequencies of ESAT-6 and CFP-10-specific IFN-γ and IL-2-secreting T cells for each participant are shown in Figure 1A. Frequencies of ESAT-6/CFP-10-specific IFN-γ-secreting T cells declined during 28 months of follow up with an average percentage decline per year of 5.8% (95% CI −9.6% to −1.7% P=0.005; Table 2). Frequencies of IFN-γ-secreting T cells specific for CFP-10 declined more during 28 months of follow up than frequencies of IFN-γ-secreting T cells specific for ESAT-6 (an average decline of 8.2% (95% CI −13.0% to −3.1%) P=0.02 compared to 4.1% (95% CI −7.8% to −0.2%) P=0.04; Table 2). Four patients were ex vivo ESAT-6/CFP-10-specific IFN-γ ELISpot-negative when diagnosed with active tuberculosis. Excluding these 4 patients, frequencies of ESAT-6/CFP-10 peptide-specific IFN-γ-secreting T cells declined during 28 months of follow up with an average percentage decline per year of 8.1% (95% CI −11.6% to −4.4%; P<0.0001, n=19). Cases followed up for 28 months (n=12) and cases not followed up for 28 months (n=11) showed no significant difference in initial frequencies of ESAT-6/CFP-10-specific IFN-γ-secreting T cells (0 month: geometric means (95% CI) SFC / 106 peripheral blood mononuclear cells (PBMC) 142 (21, 956) versus 84 (12, 575), respectively P=0.67).
Figure 1.
Frequencies of ESAT-6 and CFP-10-specific IFN-γ and IL-2-secreting T cells in 23 patients with active tuberculosis followed up for 28 months during and after successful treatment. Frequencies of ESAT-6 and CFP-10-specific IFN-γ and IL-2-secreting T cells were enumerated by ex vivo ELISpot at 0, 3, 6, 12, 18 and 28 months after diagnosis of active tuberculosis disease (A); geometric means of ESAT-6/CFP-10-specific IFN-γ and IL-2 SFCs (B).
Table II.
Trends of ESAT-6, CFP-10, ESAT-6/CFP-10 and SKSD-specific IFN-γ-secreting T cells during follow up
| Geometric mean counts of spot forming cells (95% CI), n | Test for trend | Geometric mean counts of spot forming cells (95% CI), n | Test for trend | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 months | 3 months | 6 months | 0 to 6 months | 12 months | 18 months | 28 months | 0 to 28 months | |||||||
| ESAT-6 | ||||||||||||||
| IFN-γ | 24 (6 to 98) | 23 | 11 (3 to 44) | 22 | 21 (6 to 81) | 22 | P=0.77 | 8 (2 to 40) | 16 | 8 (2 to 40) | 17 | 27 (5 to 145) | 12 | P=0.04 |
| IL-2 | 2 (0 to 9) | 10 | 5 (2 to 20) | 19 | 11 (3 to 37) | 21 | P=0.06 | 4 (1 to 14) | 16 | 5 (1 to 20) | 17 | 12 (2 to 81) | 12 | P=0.83 |
| CFP-10 | ||||||||||||||
| IFN-γ | 60 (18 to 208) | 23 | 70 (21 to 227) | 22 | 76 (26 to 220) | 22 | P=0.75 | 1 (1 to 1) | 16 | 18 (4 to 85) | 17 | 18 (2 to 149) | 12 | P=0.002 |
| IL-2 | 5 (1 to 53) | 10 | 11 (3 to 43) | 19 | 30 (12 to 75) | 21 | P=0.06 | 6 (2 to 27) | 16 | 5 (1 to 20) | 17 | 11 (1 to 92) | 12 | P=0.41 |
| ESAT-6/CFP-10 | ||||||||||||||
| IFN-γ | 111 (32 to 384) | 23 | 119 (38 to 370) | 22 | 161 (60 to 430) | 22 | P=0.57 | 44 (9 to 206) | 16 | 36 (7 to 178) | 17 | 57 (8 to 399) | 12 | P=0.005 |
| IL-2 | 5 (1 to 58) | 10 | 22 (6 to 86) | 19 | 55 (23 to 134) | 21 | P=0.02 | 14 (3 to 60) | 16 | 9 (2 to 44) | 17 | 31 (4 to 237) | 12 | P=0.72 |
| SKSD | ||||||||||||||
| IFN-γ | 19 (8 to 44) | 21 | 22 (12 to 41) | 22 | 25 (12 to 51) | 22 | P=0.26 | 28 (10 to 81) | 16 | 23 (10 to 51) | 17 | 19 (7 to 54) | 12 | P=0.73 |
| IL-2 | 89 (0 to 8250569) | 2 | 24 (12 to 46) | 17 | 28 (13 to 61) | 18 | P=0.85 | 34 (14 to 85) | 14 | 35 (14 to 89) | 13 | 49 (25 to 95) | 12 | P=0.29 |
Frequencies of ESAT-6 and CFP-10-specific IL-2-secreting T cells were generally lower than IFN-γ-secreting T cells (Figure 1A, B), and showed a significant increase during treatment over 6 months (P=0.02, Table 2).
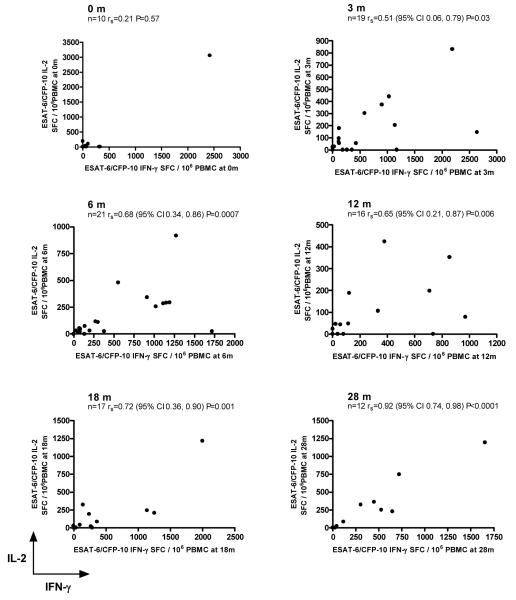
The pattern in the geometric mean frequencies of T cells secreting IFN-γ and IL-2 mirrored each other and became progressively closer to each other from 12 months onwards (Figure 1B). In addition, there was a statistically significant positive correlation between frequencies of IFN-γ-secreting and IL-2-secreting T cells after 3 months of treatment which became progressively stronger over time (Figure 2).
Figure 2.
Correlation between ESAT-6/CFP-10-specific IFN-γ and IL-2-secreting T cells measured separately with the ex vivo IFN-γ and IL-2 ELISpot assays at 0, 3, 6, 12, 18 and 28 months of follow up. There is an increasing positive association between the frequency of T cells secreting IFN-γ and the frequency of T cells secreting IL-2 at corresponding timepoints during follow up.
To determine if changes in the M. tuberculosis-specific T cell response during follow up were reflective of M. tuberculosis-specific immunity and not of generalised fluctuations in T cell recall response we quantified frequencies of IFN-γ-secreting and IL-2-secreting T cells specific for the non-tuberculosis antigen streptokinase streptodornase (SKSD) during follow up. There was no significant trend in frequencies of SKSD-specific IFN-γ-secreting or IL-2-secreting T cells during follow up (Table 2).
ESAT-6 and CFP-10-specific IFN-γ-secreting T cell responses have previously been demonstrated to be M. tuberculosis specific because they are absent in unexposed controls (15, 16, 25-27). Here we observed that ESAT-6 and CFP-10-specific IFN-γ-secreting and IL-2-secreting T cell responses are M. tuberculosis-specific, as none of the 13 healthy controls responded to these peptides in either the ex vivo IFN-γ ELISpot or in the IL-2 ELISpot (all responses were <20 spot forming cells (SFCs) per 106 PBMC; data not shown).
Proportion of patients with IFN-γ and IL-2 responses to ESAT-6 vs CFP-10 in active tuberculosis and during follow up
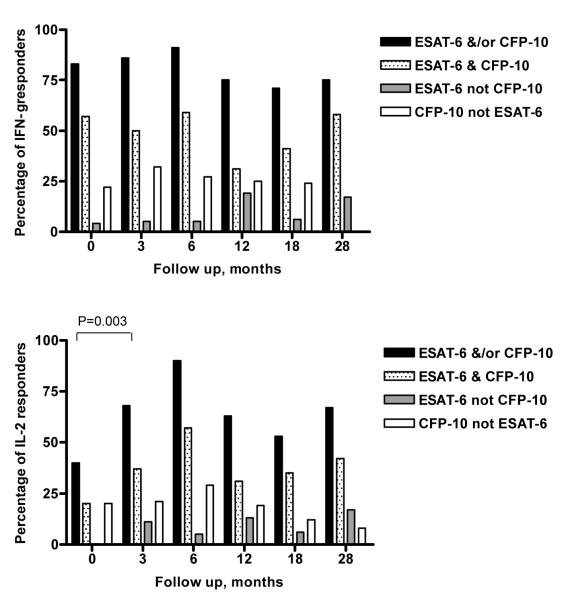
Four patients (pulmonary n=2, pleural n=1 and lymphatic n=1) were ESAT-6/CFP-10 IFN-γ ELISpot-negative when diagnosed with active tuberculosis but became positive in each case 3 months into treatment (Figure 3). Four patients turned ESAT-6/CFP-10 IFN-γ ELISpot-negative (at 3 months n=2, 6 months n=1 and 18 months n=1) i.e. the frequency of ESAT-6 and CFP-10-specific T cells decreased to levels below 20 SFCs per 106 PBMC which we defined as the threshold of detection of antigen-specific T cells by the ex vivo ELISpot, and remained ELISpot-negative for at least two consecutive timepoints and during the rest of their follow up. We also used the cytokine secretion assay with enrichment of IFN-γ and IL-2-secreting cells in 3 of the 4 participants at timepoints when the ex vivo IFN-γ ELISpot had turned negative. No CD4+ T cells specific for ESAT-6 or CFP-10 secreting either of these cytokines could be detected (data not shown). Two participants became ESAT-6/CFP-10 peptide IFN-γ ELISpot-negative at 3 and 12 months respectively, but became IFN-γ ELISpot-positive again 3 to 6 months later. One participant turned ESAT-6/CFP-10 peptide IFN-γ ELISpot-negative at 18 months but did not attend their 28 month timepoint.
Figure 3.
Proportion of patients with IFN-γ or IL-2-secreting T cells as defined by a positive ex vivo ELISpot response, in active tuberculosis and during follow up. The percentage of patients that responded to ESAT-6 and/or CFP-10, ESAT-6 and CFP-10, ESAT-6 alone or CFP-10 alone is shown.
Most patients were ex vivo ESAT-6/CFP-10 IFN-γ ELISpot-positive when diagnosed with active tuberculosis and during their follow up (Figure 3). Only 40% of patients with active tuberculosis were ex vivo ESAT-6/CFP-10 IL-2 ELISpot-positive at diagnosis; however, the proportion of ex vivo ESAT-6/CFP-10 IL-2 ELISpot-positive patients significantly increased to 90% after 6 months treatment (P=0.01). In general, more patients responded to CFP-10 alone in the IFN-γ and IL-2 ELISpot assays than to ESAT-6 alone during the first 6 months after treatment initiation; thereafter, the proportion of patients responding either to CFP-10 or ESAT-6 alone were similar (Figure 3).
Sensitive detection of CD4+ T cells secreting IFN-γ, IL-2 or both cytokines at the single cell level
Because the lower limit of detection of conventional flow cytometric techniques is approximately 0.02% to 0.05% (28, 29) detection of IFN-γ and IL-2 at the single cell level using these techniques would be difficult in several of our patients in whom frequencies of antigen-specific IFN-γ or IL-2-secreting T cells were less than 0.05%. However, the cytokine secretion assay with magnetic bead enrichment of cytokine secreting cells has demonstrated reliable detection of ultra-low frequencies of cytokine secreting T cells (13, 30, 31). As this technique specifically enriches for cytokine-secreting cells of interest it reduces background staining. We know from our previously published work that T cells responding to ESAT-6 and CFP-10 are predominantly CD4+ (16, 25, 32); thus, although CD4- T cells also contribute to the M. tb-specific IFN-γ response, we gated on live CD4+ lymphocytes.
To define an appropriate lower limit of detection for this assay in the tuberculosis model, we assessed the background level of staining of CD4+ T cells secreting IFN-γ and IL-2 following magnetic bead enrichment. We recruited 7 healthy unexposed controls and stimulated their PBMC with ESAT-6 and CFP-10 peptides. The median frequency of ESAT-6-specific CD4+ T cells secreting IFN-γ was 0% (range 0 to 0.009), IFN-γ/IL-2 0.0005% (range 0 to 0.002) and IL-2 0% (range 0 to 0.001). The median frequency of CFP-10-specific CD4+ T cells secreting IFN-γ was 0% (range 0 to 0.007), IFN-γ/IL-2 0% (range 0 to 0.002) and IL-2 0.001% (range 0 to 0.005). Based on the distribution of this data compared to the distribution of frequencies detected in the 4 active tuberculosis cases, we defined the lower limit of detection of ESAT-6 and CFP-10-specific CD4+ T cells using magnetic bead enrichment as 0.007% of CD4+ T cells.
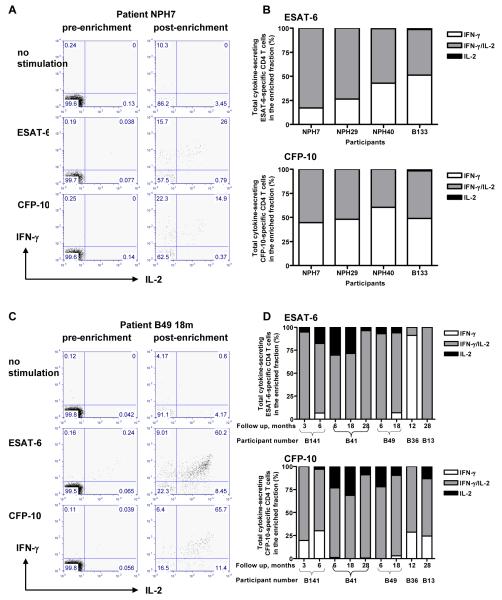
PBMC from 4 untreated patients with active tuberculosis were stimulated for 5 hours with either ESAT-6 peptides or CFP-10 peptides. After cell surface capture of secreted IFN-γ and/or IL-2, cells were magnetically enriched for IFN-γ and/or IL-2-positive cells. Representative dot plots are shown in Figure 4A. The relative proportions of CD4+ T cells producing IFN-γ and/or IL-2 in the post-enrichment fraction with background levels of non-specific cytokine production subtracted in all 4 active tuberculosis cases are shown in Figure 4B. CD4+ T cells secreting IFN-γ-only and IFN-γ/IL-2 co-dominated the response to both ESAT-6 and CFP-10. Nine follow up samples from 5 patients 3 months into treatment and thereafter are shown in Figures 4C and D. CD4+ T cells secreting IFN-γ/IL-2 dominated the response to both ESAT-6 and CFP-10 with a substantial decrease in IFN-γ-only-secreting CD4+ T cells and the appearance of IL-2-only-secreting CD4+ T cells.
Figure 4.
The IFN-γ and IL-2 cytokine profile of CD4+ T cells in active tuberculosis and during and after anti-tuberculosis therapy. Representative dot plots from an active tuberculosis patient (A) and from a different patient 18 months after initiation of treatment (C). Percentages plotted indicate the relative proportions of CD4+ cells producing IFN-γ and/or IL-2 in the enriched fraction with background levels of non-specific cytokine production subtracted in active tuberculosis (B) and during follow up (D). ESAT-6 and CFP-10-specific IFN-γ and IFN-γ/IL-2-secreting CD4+ T cells co-dominated in active tuberculosis, when bacterial load was high whilst IFN-γ/IL-2-secreting T cells dominated during and after treatment when viable bacterial load was reduced or cleared, with the loss of IFN-γ only secreting CD4+ T cells and the appearance of IL-2-secreting CD4+ T cells. This data was reproducibly observed in 4 active tuberculosis cases pre-treatment and in 5 patients at 9 follow up time points in total during and after their treatment. The 4 active tuberculosis cases and 3 follow up patients had pulmonary disease and the remaining follow up patients had lymphatic disease.
Quantitative comparison of functional T cell subsets before and after initiation of treatment
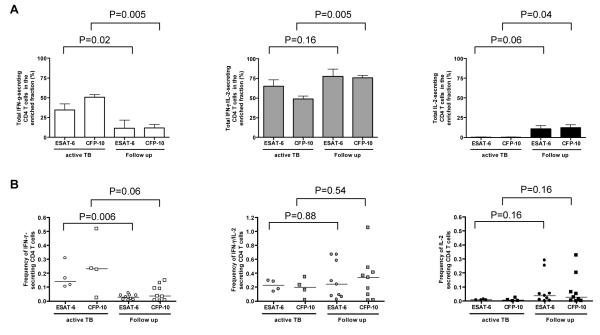
We compared the relative proportions and frequencies of ESAT-6 and CFP-10-specific IFN-γ, IFN-γ/IL-2 and IL-2-secreting CD4+ T cells between samples from active tuberculosis patients before initiation of treatment with samples after initiation of treatment (i.e. after at least three months of treatment). The relative proportion of ESAT-6 and CFP-10-specific CD4+ T cells secreting IFN-γ–only were significantly higher in untreated active tuberculosis compared to follow up during and after treatment (P=0.02 and P=0.005 respectively; Figure 5A). In contrast, the relative proportion of ESAT-6 and CFP-10-specific CD4+ T cells secreting IL-2 only were higher during and after treatment compared to pre-treatment (P=0.06 and P=0.04 respectively). The relative proportion of CD4+ T cells secreting both cytokines was significantly higher during follow up compared with untreated active tuberculosis for CFP-10-specific T cells (P=0.005) and non-significantly different for ESAT-6-specific T cells (P=0.16).
Figure 5.
The proportion and frequency of ESAT-6 and CFP-10-specific CD4+ T cell IFN-γ and/or IL-2 responses in active tuberculosis compared to follow up timepoints (during and after treatment). The proportion of ESAT-6 and CFP-10-specific CD4+ T cell secreting IFN-γ and/or IL-2 in the enriched fraction (A) and frequencies of these cells as determined after magnetic enrichment of IFN-γ and IL-2-secreting cells (B) where the horizontal lines indicate the median positive response. The P values indicate whether there is a statistical difference between active tuberculosis and follow up.
Similarly, absolute frequencies of ESAT-6 and CFP-10-specific IFN-γ-only secreting CD4+ T cells were higher in untreated active tuberculosis compared to during follow up (P=0.006 and P=0.06 respectively Figure 5B) whilst frequencies of ESAT-6 and CFP-10-specific IL-2-only secreting CD4+ T cells were higher during follow up compared to pre-treatment, although the difference did not reach statistical significance (P=0.16 for each antigen). Frequencies of CD4+ T cells secreting both IFN-γ and IL-2 did not change significantly between active tuberculosis and during follow up (Figure 5B). Some of the frequencies detected could also have been detected with conventional flow cytometric techniques; however, within every individual tested, frequencies of at least one of the functional subsets were below the detection limit of conventional flow cytometric techniques.
Discussion
The aim of this study was to assess the relationship between the frequency and functional signature of antigen-specific T cells secreting IFN-γ and IL-2 and antigen load in tuberculosis. Our statistical analysis revealed a significant decline in the frequency of ESAT-6/CFP-10-specific T cells secreting IFN-γ over 28 months and a significant increase in the number of ESAT-6/CFP-10-specific T cells secreting IL-2 during 6 months of treatment. These contrasting dynamics for the two cytokines led to a progressive convergence of the frequencies of IFN-γ and IL-2-secreting cells over 28 months, during and after treatment. From simultaneous analysis of IFN-γ and IL-2 secretion at the single cell level in a subset of our cohort using the ultra-sensitive cytokine secretion assay, we found this was due to a change in the functional signature of M. tuberculosis-specific T cells over time. There was a shift in the IFN-γ and IL-2 cytokine profile from a co-dominance of IFN-γ-only and IFN-γ/IL-2-secreting T cells in active tuberculosis to a dominance of IFN-γ/IL-2-secreting T cells and the appearance of IL-2 only secreting T cells during and after treatment. Thus, IFN-γ measured with the ex vivo ELISpot is secreted by two functional subsets of CD4+ T cells with different relative proportions in untreated active tuberculosis compared with during and after treatment.
The decline in ESAT-6 and CFP-10-specific IFN-γ T cell responses ex vivo is consistent with the decline observed during treatment of active tuberculosis in other studies (15-18, 33). However, in our study, which is the longest longitudinal study of antigen-specific T cells in tuberculosis to-date, the decline in ESAT-6 and CFP-10-specific IFN-γ-secreting T cells was only statistically significant over 28 months whereas in previous studies the decline in ESAT-6 or CFP-10-specific IFN-γ-secreting T cells was significant over 6 or 12 months using different statistical analyses (Table II; (16, 18, 33)). An increase in IFN-γ secretion from cultured PBMC during anti-tuberculosis therapy has been observed in other studies (34-38), where IFN-γ was measured after several days in vitro culture. These results reflect IFN-γ derived from in vitro antigen-stimulated proliferating memory T cells. The increased IFN-γ production after treatment with these assays likely reflect the fact that antigen-specific lymphoproliferation is inhibited by the non-specific immunosuppresion associated with active, untreated tuberculosis (16). This read-out is different to the direct ex vivo enumeration of IFN-γ-secreting T cells (18 hours), as performed in this study (28, 39-41).
Harari and colleagues showed that functional T cell heterogeneity is associated with changes in HIV antigen load (8) and we have now demonstrated that functional T cell heterogeneity is also associated with changes in M. tuberculosis antigen load. In active disease IFN-γ is secreted from two functional subsets of IFN-γ only and IFN-γ/IL-2 dual secreting T cells whereas after treatment IFN-γ is predominantly secreted from one subset of IFN-γ/IL-2 dual secreting T cells. These observations would not have been possible from the IFN-γ and IL-2 ELISpot assays alone. It is possible that IFN-γ/IL-2 functional profiles might correlate with specific clinical parameters such as disease severity or anatomical site of disease. However, the small numbers of patients within each clinical subgroup precluded statistically meaningful comparisons which would require a larger study population. Although our data are from cases of active tuberculosis not paired with follow up samples, the demographic and clinical characteristics of the untreated patients and the patients tested during and after treatment were similar.
The IFN-γ and IL-2 profile after treatment is similar to a model of antigen clearance, past influenza infection (13). This suggests that after anti-tuberculosis treatment viable bacilli and antigen may be cleared. This is consistent with the clinical observation that less than 10% of tuberculosis patients relapse within the first year after completing anti-tuberculosis treatment (42) and none of the patients we studied relapsed during 28 months of follow up. Interestingly in a different model of antigen clearance where infection with live organisms is not involved, past tetanus toxoid vaccination, IL-2-only secreting T cells persisted years after vaccination with minimal levels of IFN-γ/IL-2 dual-secreting T cells. Whilst detailed phenotyping of M. tuberculosis-specific IFN-γ and IL-2-secreting T cells was beyond the scope of this study, previous studies have identified a relationship between the function and phenotype of memory CD4+ T cells, and proposed that the IL-2-only-secreting cells that are typical TCM that persist after antigen clearance while the IFN-γ/IL-2 and IFN-γ-only secreting T cells are typical of TEM (6, 8, 43).
IFN-γ is an important mediator of macrophage activation and resistance to M. tuberculosis infection and is therefore crucial in the effector response to this intracellular pathogen (44-46). The paradoxical functions of IL-2 could explain why IL-2 is secreted from cells also secreting IFN-γ both in the early and in the later stages of infection and why IL-2 is secreted when antigen load has declined or been cleared by treatment (47). Dual IFN-γ/IL-2-secreting cells can support their own expansion as IL-2 is a potent T cell growth factor. The presence of these cells in active tuberculosis when antigen load is high may therefore suggest their involvement in the initiation phase of the immune response through the expansion of effector cells which may further differentiate into IFN-γ-only secreting cells. The relative increase in the proportion of CD4+ T cells secreting both cytokines during and after treatment may reflect the maintenance of a stable effector response. Secretion of IL-2 when antigen load is reduced or cleared may reflect its function in the termination of T cell responses. This proposed signalling function augments the growth and survival of regulatory T cells which control inflammatory responses (48). Regulatory T cells express high levels of CD25, the IL-2 receptor α-chain, and have recently been described in active tuberculosis where they suppressed IFN-γ-secreting ESAT-6 and CFP-10-specific T cells ex vivo (49).
As a quantitative measure of T cell function, the IFN-γ ELISpot assay for diagnosis of M. tuberculosis infection (15, 16, 20, 25, 26, 50) holds promise as a tool for tracking antigen load and monitoring disease activity (16, 17, 40). But T cell function defined solely by quantification of IFN-γ secretion may prove an insufficient biomarker of antigen load and clinical disease status and other measures of T cell function will probably be required in addition (14). Through simultaneous measurement of IFN-γ and IL-2 secretion we noted a shift in the cytokine profile of M. tuberculosis-specific T cells associated with the treatment-induced change from high to low antigen load. Our data thus suggest that IFN-γ and IL-2 functional signatures are associated with antigen load and clinical disease status.
We propose this dynamic functional signature could be used as an immunological marker of mycobacterial load. Existing clinical, radiological and microbiological parameters to monitor the response to treatment in active tuberculosis have several limitations. The IFN-γ and IL-2 functional signature could be used to evaluate new therapies for active tuberculosis entering clinical trials where new biomarkers are urgently needed. In latent tuberculosis infection, for which there are no clinical, radiological or microbiological parameters for assessing response to therapy, the IFN-γ and IL-2 functional signature could be used to monitor the impact of conventional and novel preventive treatments, as well as new vaccines. The IFN-γ and IL-2 functional signature could also be used to monitor individuals infected with M. tuberculosis at high risk of progression to active tuberculosis, e.g. patients with HIV co-infection or on anti-TNF therapy, to guide the early initiation of treatment as antigen load increases prior to clinical reactivation. Whether such changes in the M. tuberculosis antigen-specific T cell functional signature predict specific clinical outcomes is the subject of our ongoing prospective studies.
Acknowledgements
The authors thank all participants of this study. We thank Lemuel Mallari, Geoff Pasvol and Rob Davidson for sample collection at Northwick Park Hospital and Martina Sester for critical appraisal of the manuscript.
Nonstandard abbreviations
- TEM
effector memory T cells
- TCM
central memory T cells
- ESAT-6
early secreted antigenic target protein
- CFP-10
culture filtrate protein
- SFCs
spot forming cells
- TST
tuberculin skin test
- BCG
bacille Calmette-Guérin
Footnotes
Publisher's Disclaimer: This is an author-produced version of a manuscript accepted for publication in The Journal of Immunology (The JI). The American Association of Immunologists, Inc. (AAI), publisher of The JI, holds the copyright to this manuscript. This version of the manuscript has not yet been copyedited or subjected to editorial proofreading by The JI; hence, it may differ from the final version published in The JI (online and in print). AAI (The JI) is not liable for errors or omissions in this author-produced version of the manuscript or in any version derived from it by the U.S. National Institutes of Health or any other third party. The final, citable version of record can be found at www.jimmunol.org.
References
- 1.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 2.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]
- 4.Lanzavecchia A, Sallusto F. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science. 2000;290:92–97. doi: 10.1126/science.290.5489.92. [DOI] [PubMed] [Google Scholar]
- 5.Lanzavecchia A, Sallusto F. Progressive differentiation and selection of the fittest in the immune response. Nat Rev Immunol. 2002;2:982–987. doi: 10.1038/nri959. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 7.Emu B, Sinclair E, Favre D, Moretto WJ, Hsue P, Hoh R, Martin JN, Nixon DF, McCune JM, Deeks SG. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol. 2005;79:14169–14178. doi: 10.1128/JVI.79.22.14169-14178.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harari A, Vallelian F, Meylan PR, Pantaleo G. Functional heterogeneity of memory CD4 T cell responses in different conditions of antigen exposure and persistence. J Immunol. 2005;174:1037–1045. doi: 10.4049/jimmunol.174.2.1037. [DOI] [PubMed] [Google Scholar]
- 9.Younes SA, Yassine-Diab B, Dumont AR, Boulassel MR, Grossman Z, Routy JP, Sekaly RP. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J Exp Med. 2003;198:1909–1922. doi: 10.1084/jem.20031598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day CL, Walker BD. Progress in defining CD4 helper cell responses in chronic viral infections. J Exp Med. 2003;198:1773–1777. doi: 10.1084/jem.20031947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semmo N, Day CL, Ward SM, Lucas M, Harcourt G, Loughry A, Klenerman P. Preferential loss of IL-2-secreting CD4+ T helper cells in chronic HCV infection. Hepatology. 2005;41:1019–1028. doi: 10.1002/hep.20669. [DOI] [PubMed] [Google Scholar]
- 12.Duvall MG, Jaye A, Dong T, Brenchley JM, Alabi AS, Jeffries DJ, van der Sande M, Togun TO, McConkey SJ, Douek DC, McMichael AJ, Whittle HC, Koup RA, Rowland-Jones SL. Maintenance of HIV-specific CD4+ T cell help distinguishes HIV-2 from HIV-1 infection. J Immunol. 2006;176:6973–6981. doi: 10.4049/jimmunol.176.11.6973. [DOI] [PubMed] [Google Scholar]
- 13.Lucas M, Day CL, Wyer JR, Cunliffe SL, Loughry A, McMichael AJ, Klenerman P. Ex vivo phenotype and frequency of influenza virus-specific CD4 memory T cells. J Virol. 2004;78:7284–7287. doi: 10.1128/JVI.78.13.7284-7287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- 15.Lalvani A, Nagvenkar P, Udwadia Z, Pathan AA, Wilkinson KA, Shastri JS, Ewer K, Hill AV, Mehta A, Rodrigues C. Enumeration of T cells specific for RD1-encoded antigens suggests a high prevalence of latent Mycobacterium tuberculosis infection in healthy urban Indians. J Infect Dis. 2001;183:469–477. doi: 10.1086/318081. [DOI] [PubMed] [Google Scholar]
- 16.Pathan AA, Wilkinson KA, Klenerman P, McShane H, Davidson RN, Pasvol G, Hill AV, Lalvani A. Direct ex vivo analysis of antigen-specific IFN-gamma-secreting CD4 T cells in Mycobacterium tuberculosis-infected individuals: associations with clinical disease state and effect of treatment. J Immunol. 2001;167:5217–5225. doi: 10.4049/jimmunol.167.9.5217. [DOI] [PubMed] [Google Scholar]
- 17.Carrara S, Vincenti D, Petrosillo N, Amicosante M, Girardi E, Goletti D. Use of a T cell-based assay for monitoring efficacy of antituberculosis therapy. Clin Infect Dis. 2004;38:754–756. doi: 10.1086/381754. [DOI] [PubMed] [Google Scholar]
- 18.Nicol MP, Pienaar D, Wood K, Eley B, Wilkinson RJ, Henderson H, Smith L, Samodien S, Beatty D. Enzyme-linked immunospot assay responses to early secretory antigenic target 6, culture filtrate protein 10, and purified protein derivative among children with tuberculosis: implications for diagnosis and monitoring of therapy. Clin Infect Dis. 2005;40:1301–1308. doi: 10.1086/429245. [DOI] [PubMed] [Google Scholar]
- 19.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, Small PM. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284:1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 20.Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, Monk P, Lalvani A. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet. 2003;361:1168–1173. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 21.Twisk J. Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. Cambridge University Press; Cambridge: 2003. [Google Scholar]
- 22.Cole TJ. Sympercents: symmetric percentage differences on the 100 log(e) scale simplify the presentation of log transformed data. Stat Med. 2000;19:3109–3125. doi: 10.1002/1097-0258(20001130)19:22<3109::aid-sim558>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 23.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med. 2000;161:1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 24.Control and prevention of tuberculosis in the United Kingdom: code of practice 2000. Joint Tuberculosis Committee of the British Thoracic Society. Thorax. 2000;55:887–901. doi: 10.1136/thorax.55.11.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalvani A, Pathan AA, McShane H, Wilkinson RJ, Latif M, Conlon CP, Pasvol G, Hill AV. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am J Respir Crit Care Med. 2001;163:824–828. doi: 10.1164/ajrccm.163.4.2009100. [DOI] [PubMed] [Google Scholar]
- 26.Lalvani A, Pathan AA, Durkan H, Wilkinson KA, Whelan A, Deeks JJ, Reece WH, Latif M, Pasvol G, Hill AV. Enhanced contact tracing and spatial tracking of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Lancet. 2001;357:2017–2021. doi: 10.1016/S0140-6736(00)05115-1. [DOI] [PubMed] [Google Scholar]
- 27.Chapman AL, Munkanta M, Wilkinson KA, Pathan AA, Ewer K, Ayles H, Reece WH, Mwinga A, Godfrey-Faussett P, Lalvani A. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. Aids. 2002;16:2285–2293. doi: 10.1097/00002030-200211220-00008. [DOI] [PubMed] [Google Scholar]
- 28.Klenerman P, Cerundolo V, Dunbar PR. Tracking T cells with tetramers: new tales from new tools. Nat Rev Immunol. 2002;2:263–272. doi: 10.1038/nri777. [DOI] [PubMed] [Google Scholar]
- 29.Sester M, Sester U, Gartner B, Heine G, Girndt M, Mueller-Lantzsch N, Meyerhans A, Kohler H. Levels of virus-specific CD4 T cells correlate with cytomegalovirus control and predict virus-induced disease after renal transplantation. Transplantation. 2001;71:1287–1294. doi: 10.1097/00007890-200105150-00018. [DOI] [PubMed] [Google Scholar]
- 30.Brosterhus H, Brings S, Leyendeckers H, Manz RA, Miltenyi S, Radbruch A, Assenmacher M, Schmitz J. Enrichment and detection of live antigen-specific CD4(+) and CD8(+) T cells based on cytokine secretion. Eur J Immunol. 1999;29:4053–4059. doi: 10.1002/(SICI)1521-4141(199912)29:12<4053::AID-IMMU4053>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 31.Campbell JD. Detection and enrichment of antigen-specific CD4+ and CD8+ T cells based on cytokine secretion. Methods. 2003;31:150–159. doi: 10.1016/s1046-2023(03)00125-7. [DOI] [PubMed] [Google Scholar]
- 32.Shams H, Klucar P, Weis SE, Lalvani A, Moonan PK, Safi H, Wizel B, Ewer K, Nepom GT, Lewinsohn DM, Andersen P, Barnes PF. Characterization of a Mycobacterium tuberculosis peptide that is recognized by human CD4+ and CD8+ T cells in the context of multiple HLA alleles. J Immunol. 2004;173:1966–1977. doi: 10.4049/jimmunol.173.3.1966. [DOI] [PubMed] [Google Scholar]
- 33.Aiken AM, Hill PC, Fox A, McAdam KP, Jackson-Sillah DJ, Lugos MD, Donkor SA, Adegbola RA, Brookes RH. Reversion of the ELISPOT test after treatment in Gambian tuberculosis cases. BMC Infect Dis. 2006;6:66. doi: 10.1186/1471-2334-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson RJ, Vordermeier HM, Wilkinson KA, Sjolund A, Moreno C, Pasvol G, Ivanyi J. Peptide-specific T cell response to Mycobacterium tuberculosis: clinical spectrum, compartmentalization, and effect of chemotherapy. J Infect Dis. 1998;178:760–768. doi: 10.1086/515336. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch CS, Toossi Z, Othieno C, Johnson JL, Schwander SK, Robertson S, Wallis RS, Edmonds K, Okwera A, Mugerwa R, Peters P, Ellner JJ. Depressed T-cell interferon-gamma responses in pulmonary tuberculosis: analysis of underlying mechanisms and modulation with therapy. J Infect Dis. 1999;180:2069–2073. doi: 10.1086/315114. [DOI] [PubMed] [Google Scholar]
- 36.Vekemans J, Lienhardt C, Sillah JS, Wheeler JG, Lahai GP, Doherty MT, Corrah T, Andersen P, McAdam KP, Marchant A. Tuberculosis contacts but not patients have higher gamma interferon responses to ESAT-6 than do community controls in The Gambia. Infect Immun. 2001;69:6554–6557. doi: 10.1128/IAI.69.10.6554-6557.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Attiyah R, Mustafa AS, Abal AT, Madi NM, Andersen P. Restoration of mycobacterial antigen-induced proliferation and interferon-gamma responses in peripheral blood mononuclear cells of tuberculosis patients upon effective chemotherapy. FEMS Immunol Med Microbiol. 2003;38:249–256. doi: 10.1016/S0928-8244(03)00166-4. [DOI] [PubMed] [Google Scholar]
- 38.Ferrand RA, Bothamley GH, Whelan A, Dockrell HM. Interferon-gamma responses to ESAT-6 in tuberculosis patients early into and after anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2005;9:1034–1039. [PubMed] [Google Scholar]
- 39.Lalvani A, Brookes R, Hambleton S, Britton WJ, Hill AV, McMichael AJ. Rapid effector function in CD8+ memory T cells. J Exp Med. 1997;186:859–865. doi: 10.1084/jem.186.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lalvani A. Counting antigen-specific T cells: a new approach for monitoring response to tuberculosis treatment? Clin Infect Dis. 2004;38:757–759. doi: 10.1086/381763. [DOI] [PubMed] [Google Scholar]
- 41.Godkin AJ, Thomas HC, Openshaw PJ. Evolution of epitope-specific memory CD4(+) T cells after clearance of hepatitis C virus. J Immunol. 2002;169:2210–2214. doi: 10.4049/jimmunol.169.4.2210. [DOI] [PubMed] [Google Scholar]
- 42.Gelband H. Regimens of less than six months for treating tuberculosis. Cochrane Database Syst Rev. 2000 doi: 10.1002/14651858.CD001362. CD001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harari A, Vallelian F, Pantaleo G. Phenotypic heterogeneity of antigen-specific CD4 T cells under different conditions of antigen persistence and antigen load. Eur J Immunol. 2004;34:3525–3533. doi: 10.1002/eji.200425324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Flynn JL, Chan J, Triebold KJ, Dalton DK, Stewart TA, Bloom BR. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooper AM, Dalton DK, Stewart TA, Griffin JP, Russell DG, Orme IM. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ottenhoff TH, Kumararatne D, Casanova JL. Novel human immunodeficiencies reveal the essential role of type-I cytokines in immunity to intracellular bacteria. Immunol Today. 1998;19:491–494. doi: 10.1016/s0167-5699(98)01321-8. [DOI] [PubMed] [Google Scholar]
- 47.Malek TR, Bayer AL. Tolerance, not immunity, crucially depends on IL-2. Nat Rev Immunol. 2004;4:665–674. doi: 10.1038/nri1435. [DOI] [PubMed] [Google Scholar]
- 48.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 49.Guyot-Revol V, Innes JA, Hackforth S, Hinks T, Lalvani A. Regulatory T cells are expanded in blood and disease sites in patients with tuberculosis. Am J Respir Crit Care Med. 2006;173:803–810. doi: 10.1164/rccm.200508-1294OC. [DOI] [PubMed] [Google Scholar]
- 50.Richeldi L, Ewer K, Losi M, Bergamini BM, Roversi P, Deeks J, Fabbri LM, Lalvani A. T cell-based tracking of multidrug resistant tuberculosis infection after brief exposure. Am J Respir Crit Care Med. 2004;170:288–295. doi: 10.1164/rccm.200403-307OC. [DOI] [PubMed] [Google Scholar]