Abstract
BACKGROUND
Osteopontin affects several steps of the metastatic cascade. Despite direct correlation with metastasis in experimental systems and in patient studies, the extracellular and intracellular basis for these observations remains unsolved. We used human melanoma and sarcoma cell lines to evaluate the effects of soluble osteopontin on metastasis.
METHODS
Exogenous osteopontin or negative controls, including a site-directed mutant osteopontin, were used in functional assays in vitro, ex vivo, and in vivo designed to test extracellular and intracellular mechanisms involved in experimental metastasis.
RESULTS
In the extracellular environment, we confirm that soluble osteopontin is required for its pro-metastatic effects; this phenomenon is specific, RGD-dependent, and evident in experimental models of metastasis. In the intracellular environment, osteopontin initially induces rapid Tyr-418 dephosphorylation of c-Src, with decreases in actin stress fibers and increased binding to the vascular endothelium. This heretofore undescribed Tyr dephosphorylation is followed by a tandem c-Src phosphorylation after tumor cell attachment to the metastatic site.
CONCLUSION
Our results reveal a complex molecular interaction as well as a dual role for osteopontin in metastasis that is dependent on whether tumor cells are in circulation or attached. Such context-dependent functional insights may contribute to anti-metastasis strategies.
Keywords: c-Src, endothelium, metastasis, osteopontin
INTRODUCTION
Osteopontin (reviewed in 1–4) plays a central role in the metastatic potential of both human and experimental tumors.5–7 Clinically, elevated levels of circulating osteopontin in cancer patients correlate with increased metastasis and poor prognosis for many solid tumors, most notably documented in malignant tumors of breast6, 8, prostate9, 10, liver11, and head and neck.12 Since the original discovery of this matricellular protein by Oldberg et al.13, the precise extracellular and intracellular mechanisms through which osteopontin promotes metastasis and correlates with poor prognosis in cancer patients remain unresolved, although there is a recognized dependence on the arginine-glycine-aspartic acid (RGD) motif.5
Osteopontin (also termed secreted phosphoprotein-1, urinary stone protein, and early T lymphocyte activation-1) is an acidic, secreted, non-collagenous matricellular protein with cytokine- and chemokine-like functions.1, 14 It was first isolated as a major bone sialoprotein containing an RGD motif.13 Early work established that osteopontin binds to αvβ3 integrin on osteoclasts; later, it became clear that osteopontin also recognizes several other members of the αv family and β1 family of integrins (reviewed in 1–4). Such a broad integrin-binding profile indicates that osteopontin mediates cell adhesion, proliferation, migration, and survival. Therefore, it is likely to be relevant in the context of tumor progression, angiogenesis, and metastasis.
Herein, we have developed functional assays in vitro, in tumor cells, in mouse models, and ex vivo with patient-derived samples to gain mechanistic insight into the role of soluble osteopontin in metastasis. Together, the results indicate the existence of an RGD-dependent, osteopontin-triggered activation cascade involving the Src oncogene pathway in non-adherent tumor cells. Because we have also shown that human cancer cells express functional osteopontin cell-surface receptors regardless of tumor type, these findings have potential implications for therapeutic intervention.
MATERIALS AND METHODS
Cloning and production of wild-type osteopontin and mutant osteopontin-RGE
Total RNA was isolated from cultured human KS1767 Kaposi sarcoma cells with Trizol® (Invitrogen, Carlsbad, CA). After DNase (Promega, Madison, WI) treatment, 1 μg of total RNA was transcribed with oligo dT primers and SuperScript™ III Reverse Transcriptase (Invitrogen). One μl cDNA was amplified with forward primer 5′-ACTCGGATCCATGAGAATTGCAGTGATTTGCTTT-3′ and reverse primer 5′-TTTGCGGCCGCTTAATTGACCTCAGAAGATGCACTATCT-3′ containing BamHI and NotI restriction sites (nucleotides shown in italics). After digestion with BamHI and NotI (Roche, Basel, Switzerland), PCR products were purified by electrophoresis and cloned into pGEX-6P-1 expression vector (GE Healthcare, Piscataway, NJ). GST-osteopontin-RGE was generated by amplification of the cloned osteopontin with the following primers: 5-prime end forward primer 5′-CTCGGATCCATGAGAATTGCAGTGATTTGCTTT-3′ and reverse primer 5′-AAACCACACTTTCACCTCGGCCATCATATGTGTCT-3′, and 3-prime end forward primer 5′-ATGGCCGAGGTGAAAGTGTGGTTTATGGACTGAGGT-3′ and reverse primer 5′-TTTGCGGCCGCTTAATTGACCTCAGAAGATGCACTATCT-3′ that generate 5′- and 3′-ends containing the Asp→Glu mutation. The corresponding nucleotide for this single point mutation is shown in bold. The ends were purified, mixed in 1:1 molar ratio, and used to generate a full-length mutant osteopontin-RGE. The amplification primers used were the same as those for wild-type osteopontin. The integrity of constructs was verified by DNA sequencing and by restriction enzyme mapping.
Purified or recombinant proteins and synthetic peptides
The GST-osteopontin (wild-type or mutant) constructs were used to transform E. coli strain BL21 (Novagen, Madison, WI). Recombinant proteins were produced as described.15 Briefly, cells were grown to O.D. approximately 1.0, harvested, resuspended in phosphate-buffered saline (PBS) containing 1% Triton® X-100 and protease inhibitor cocktail (Roche), and lysed by sonication. The lysate was cleared by centrifugation, and the GST proteins were bound to glutathione-Sepharose (GE Healthcare). After three washing steps, the proteins were eluted with reduced glutathione (Sigma, St Louis, MO).
Fibronectin was purified by affinity chromatography on gelatin-Sepharose.16 A polymeric form of fibronectin (superfibronectin, sFN) was generated as described.17, 18 Fibrinogen was purchased from Enzyme Research Laboratories (South Bend, IN). Soluble peptides were synthesized and cyclized (if necessary) at AnaSpec (San Jose, CA) to our specifications.
Cell culture
Cell culture reagents were purchased from Invitrogen unless otherwise indicated. Human tumor cell lines C8161 (melanoma) and KRIB (osteosarcoma) were maintained in DMEM supplemented with 10% FCS, vitamins, non-essential amino acids, and antibiotics. Cells were grown to approximately 80% confluence at the time of use. After detachment with phosphate-buffered saline (PBS) containing 2.5 mM EDTA, cells were washed three times with DMEM, counted, and resuspended in DMEM prior to subsequent treatments. Cell viability was monitored before and after treatments by counting of Trypan blue-excluding cells.
In vivo assay
All animal experimentation was reviewed and approved by each respective Institutional Animal Care and Use Committee (IACUC) at the Burnham Institute and at the University of Texas M. D. Anderson Cancer Center (UTMDACC). Two-month-old nude mice (Harlan Sprague Dawley) were anesthetized with Avertin® (0.015 ml/g) and were injected intravenously (tail vein) with 106 tumor cells, which were previously treated with 200 μl DMEM containing either 1 μg/ml or 10 μg/ml of GST-osteopontin for 10 min at room temperature (RT). The RGE mutant of GST-osteopontin was used in the same manner and at the same concentrations. GRGDSP and RGD-4C peptides were used at 1 mg/ml (n = 10 for each group). These experiments were repeated three times.
Metastases were monitored during the study by the killing of sentinel tumor-bearing mice from different cohorts at different time points (12–16 weeks) and by determination of metastatic tumor loads.18 In selected cohorts, the number of metastatic foci was counted under a dissecting microscope, and a histological examination was performed after tissue fixation. Sentinel tumor-bearing mice served to estimate tumor burden prior to termination of each cohort. Experiments were terminated when the animals displayed signs of discomfort or weight loss. Actuarial survival is demonstrated in the Kaplan-Meier plot.19
Actin, integrin, and CD44 visualization
C8161 and KRIB cells were cultured on CultureWell™ cover glasses (Invitrogen). After several washes, the cells were stimulated with GST-osteopontin or GST-osteopontin-RGE for 10 min. Cells were washed three times with PBS, fixed with PBS containing 2% paraformaldehyde, rendered permeable with 0.2% Triton® X-100 for 5 min, and stained with AlexaFluor488 phalloidin (Invitrogen), anti-integrin αv (Chemicon, Temecula, CA), and anti-CD44 (clone IM7; AbCam, Cambridge, MA) antibodies. These experiments were repeated three times in duplicate.
Cell signaling
The cells were stimulated for 1, 10, 20, 30, and 60 min in suspension, after which they were centrifuged and lysed in RIPA buffer (50 mM Tris-HCl, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate; pH 7.4) containing Complete™ protease inhibitors (Roche) and phosphatase inhibitors (1 mM Na3VO4 and 1 mM NaF; Sigma). Lysates (30 μg per lane) were resolved on SDS-polyacrylamide gels, transferred onto nitrocellulose membranes, and probed with mouse anti-phosphotyrosine (clone 4G10) and rabbit anti-phospho-Src (Tyr-418) antibodies (Upstate, Temecula, CA). Total Src was detected with rabbit anti-Src IgG (Cell Signaling Technology, Danvers, MA). These experiments were repeated three times.
Ex vivo cell binding assay
For analysis of the binding tumor cells to vascular endothelium, aortas were dissected from mice and cut open longitudinally, after which they were attached on 4% agarose with staples. 105 tumor cells were treated in suspension for 10 minutes with osteopontin, with osteopontin-RGE, with osteopontin and phosphatase inhibitors (1 mM Na3VO4 and 1 mM NaF; inhibitors were administered 2 minutes before osteopontin), or DMEM. The cells were allowed to attach to the aortas (n = 3 for each group) for 10 minutes, after which they were gently washed three times with PBS and fixed with 2% paraformaldehyde. The number of cells bound to the vascular endothelium was determined under the light microscope. This experiment was repeated twice.
Statistical analysis
Given that there were no substantially skewed data points and metastatic foci were distributed evenly throughout the lung, the statistical significance of the differences in the extent of metastases between tumor-bearing mice cohorts was initially determined by a t-test. However, to expand statistical data to support the significance of our findings, we performed additional non-parametric tests (i.e., Kruskal-Wallis and Wilcoxon). In addition, summary statistics, such as mean, median and standard deviation, were calculated for lung weight and metastatic foci in the lung. Scatter plots were generated based on the results, with a bar denoting the mean of each group. Due to the number of samples in each cohort, non-parametric tests were carried out based on the ranks of each variable. Dunnett’s methodology was applied to adjust for the inflated type I error rate generated from multiple comparisons. This method provides a better power in the cases of comparing between a single control and each of the treatment groups, while holding the maximum experiment-wise error rate to a level not exceeding the stated type I error rate. All of the statistical methods used supported our conclusions.
RESULTS
Human melanoma and sarcoma cells express osteopontin receptors
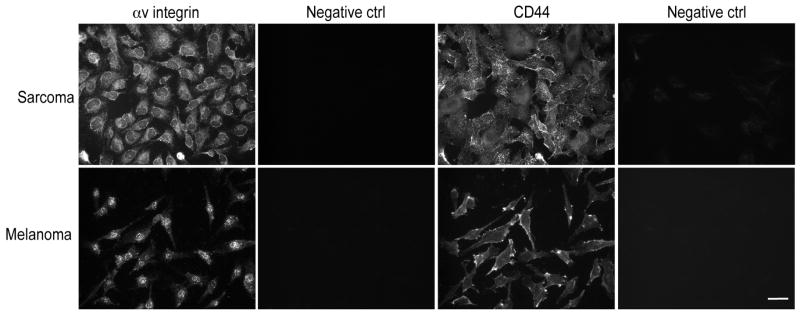
We selected well-established cell lines that are of human origin, from different solid tumor pathological types (i.e., melanomas and sarcomas), and with wide metastatic capability. C8161 (human melanoma) and KRIB (human osteosarcoma) cells contain the principal known osteopontin receptors that include αvβ3 integrin, αvβ5 integrin, and CD44. The CD44 receptor mediates attachment, homing, and aggregation of immune system cells.4 Immunofluorescence revealed that all human tumor cell types studied expressed αv integrins and CD44 (Fig. 1). Flow cytometric analysis confirmed high expression of these osteopontin receptors (data not shown).
FIGURE 1.
Osteopontin receptors are expressed on human cancer cells. Cultured human C8161 melanoma and KRIB osteosarcoma cells were immunostained for two of the major osteopontin receptors: αv integrins and CD44. Abundant punctuate αv integrin immunoreactivity was detected in all cell types. The same cells displayed also CD44 immunoreactivity. Negative staining controls with irrelevant isotype control plus secondary antibodies displayed no immunoreactivity. Scale bar, 20 μm.
Exogenous soluble osteopontin increases experimental metastasis in mouse models in an RGD-dependent manner
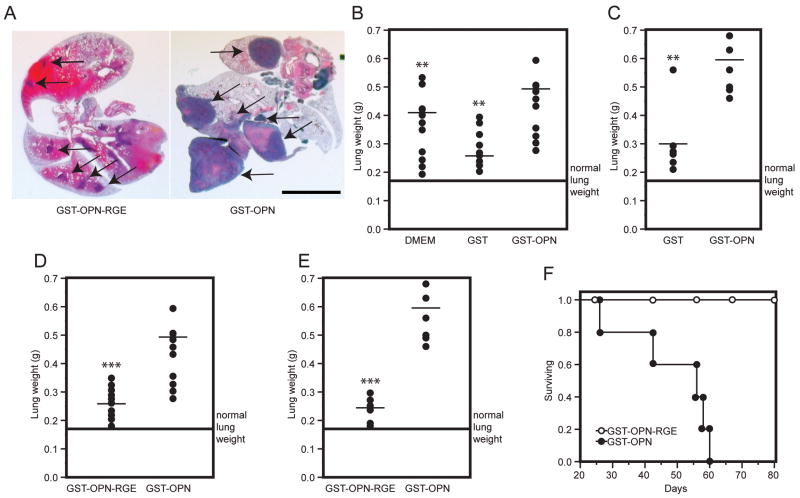
We reasoned that osteopontin likely functions at several sites in the multi-step metastatic cascade (e.g., the primary tumor, the circulation, and/or the metastasis site). Relatively little is known about the role of circulating osteopontin. We therefore asked whether exogenous administration of osteopontin--which would presumably mimic what cancer cells encounter in the bloodstream--might have an effect on tumor cell adhesion, spreading, and metastasis. After confirming that tumor cells exhibit osteopontin surface receptors, we performed experimental metastasis assays in vivo. Tumor cell burden in the lungs was determined by measuring lung mass, a methodology we have previously validated as a suitable surrogate.18 Treatment of cancer cells with recombinant osteopontin enhanced colony formation in the lungs (Fig. 2A). Treatment of C8161 melanoma cells with recombinant osteopontin was associated with a significant increase in lung weight (p<0.01; Fig. 2B) relative to negative controls that included mice treated with vehicle (DMEM) and control proteins. To exclude the possibility that the effect of exogenous osteopontin was restricted to a specific melanoma cell line, we treated the KRIB human osteosarcoma cell line in a manner identical to that of the C8161 cells. The metastatic enhancement effect of osteopontin on sarcoma cells was similar to that observed with melanoma cells. Consistently, osteopontin increased experimental lung metastasis of osteosarcoma cells, in comparison to negative controls (p<0.01; Fig. 2C).
FIGURE 2.
Tumor cell exposure to exogenous osteopontin increases experimental metastasis that is RGD-dependent. A, Typical gross appearance of lungs after intravenous administration of tumor cells. Arrows indicate tumor foci in the lungs. Scale bar, 0.5 cm. B, C8161 melanoma cells were pre-incubated for 10 min with DMEM, GST or GST-osteopontin and were subsequently intravenously injected into the tail vein of nude mice (106 cells/mouse). After 12–16 weeks, animals were killed and the lung weights were measured (18). The weight was significantly increased (p<0.01; asterisk) in nude mice receiving human melanoma cells previously incubated with GST-osteopontin relative to DMEM- or GST-treated cells. C, Lung weight of mice receiving human KRIB osteosarcoma cells, which were pretreated for 10 min with GST or GST-osteopontin. Average normal lung weight (0.175 g) is indicated by the line. A significant increase in lung metastasis was observed when tumor cells were treated with GST-osteopontin relative to GST (p<0.01). D and E, Tumor cells were pre-incubated for 10 min with GST-osteopontin-RGE or GST-osteopontin. The Asp→Glu (termed RGE) mutation, which disrupts the integrin-binding RGD-domain in osteopontin, abolished its effects on tumor cells as seen in a statistically significant difference in lung weights of mice receiving C8161 melanoma cells (p<0.001) or KRIB osteosarcoma cells (p<0.001). Average normal lung weight (0.175 g) is indicated by the line. F, In a related experiment, mice received KRIB osteosarcoma cells treated with either GST-osteopontin or GST-osteopontin-RGE prior to administration. Survival of tumor-bearing mice was monitored, and the experiment was terminated at day 80 as indicated.
After establishing that exogenous osteopontin increases experimental metastasis formation, we asked whether this action is integrin-mediated. It is well established that the tripeptide RGD is an essential integrin recognition site. Thus, to address this question, we replaced aspartic acid with glutamic acid (Asp→Glu) in the RGD motif of recombinant osteopontin (GST-osteopontin-RGE mutant protein) and treated human melanoma and osteosarcoma cells with it as was done with wild-type GST-osteopontin. Mutant GST-osteopontin-RGE treatment did not increase tumor metastasis to the lungs of either cell line (p<0.001 in each case), relative to tumor cells treated with vehicle only (Fig. 2D and E and data not shown). This effect was reflected in the actuarial survival of the tumor-bearing mice. Indeed, mice receiving osteosarcoma cells that were pre-incubated with wild-type osteopontin had a greatly reduced actuarial survival relative to mice receiving cells that were pre-incubated with mutant osteopontin-RGE (Fig. 2F).
Enhancement of experimental metastasis is specific for osteopontin
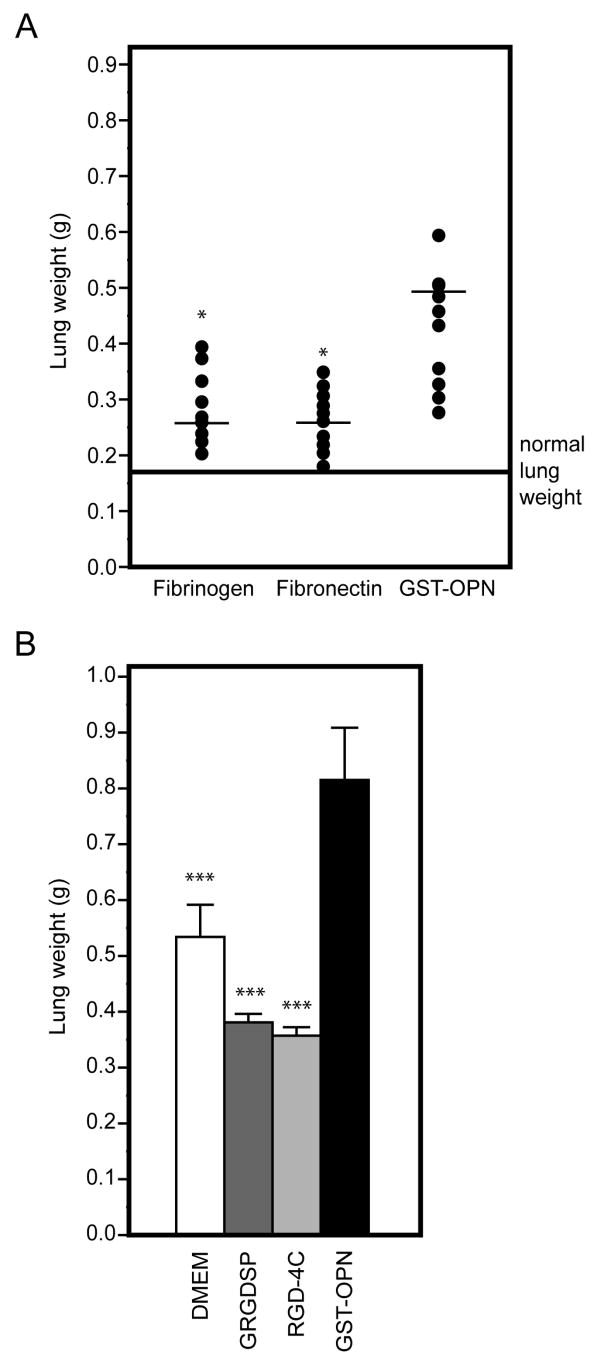
Because RGD is present in other adhesive extracellular proteins that bind to αv integrins, it is possible that the pro-metastatic effect of osteopontin might merely represent a non-specific effect of any RGD motif in circulating proteins. To exclude this possibility, we compared the effects of two RGD-containing circulating control proteins (fibronectin and fibrinogen) with that of osteopontin on experimental metastasis. Neither of these RGD-containing circulating proteins had detectable effects on lung colonization of C8161 melanoma cells (Fig. 3A). Finally, to analyze the role of soluble RGD-containing synthetic peptides in experimental metastasis formation, we also treated melanoma cells with the “classic” linear RGD peptide (sequence GRGDSP20, 21) or with the double-cyclic RGD-4C peptide (sequence ACDCRGDCFCG) that has binds selectively to αv integrins.22–26 In contrast to osteopontin, treatment with RGD synthetic peptides was associated only with a mild inhibitory effect on lung colonization (Fig. 3B). These data indicate that the osteopontin-mediated enhancement of experimental metastasis is relatively broad to different tumor cells but specific to osteopontin.
FIGURE 3.
The RGD-dependent pro-metastatic effect is specific for osteopontin. A, C8161 melanoma cells were pre-incubated for 10 min with fibrinogen and fibronectin, both of which contain the integrin-binding RGD motif, or GST-osteopontin. Treatment with GST-osteopontin significantly increased metastasis formation relative to fibrinogen (p<0.05; asterisk) or fibronectin (p<0.05). Average normal lung weight (0.175 g) is indicated by the line. B, In a similar experiment, mice received C8161 melanoma cells treated with DMEM, 1 mM linear GRGDSP peptide, 1 mM double-cyclic RGD-4C peptide, or GST-osteopontin. The cohort receiving cells treated with GST-osteopontin exhibited a significant increase in lung metastasis relative to the other groups (p<0.001). Bars indicate mean ± SD.
Soluble osteopontin modulates the actin cytoskeleton of tumor cells
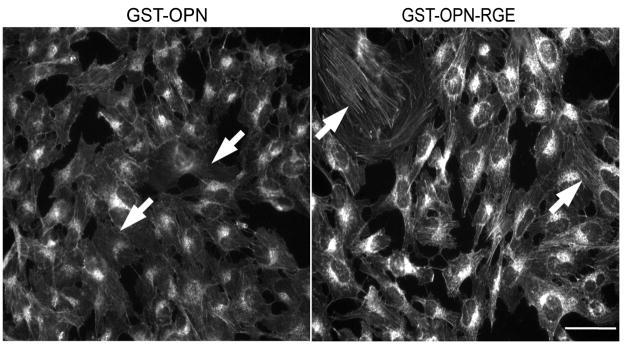
Effective antigen-presenting cell (APC) interaction requires decreased cell rigidity, which is achieved by disengagement of the cortical actin cytoskeleton from the membrane.27 To evaluate whether osteopontin would similarly induce relaxation of the actin cytoskeleton to facilitate binding of tumor cells to the endothelium, we stained adherent human cancer cells with Alexa Fluor 488-conjugated phalloidin to reveal cytoskeletal changes after osteopontin stimulation. Consistently, treatment with osteopontin for only ten minutes markedly altered the actin fiber network and reduced the density of transcytoplasmic actin cables, relative to those in cells treated with osteopontin-RGE (Fig. 4). These results indicate that cytoskeletal configuration could be relevant to tumor/endothelial cell functional interactions, as observed in other cell types such as certain specialized T-lymphocytes.27, 28
FIGURE 4.
Osteopontin reduces actin fibers in adherent tumor cells. Adherent KRIB osteosarcoma cells were treated with GST-osteopontin or GST-osteopontin-RGE for 10 min, after which the cells were fixed and stained for cytoskeletal actin. Tumor cells treated with GST-osteopontin displayed a reduced actin network, with a decrease in transcytoplasmic actin cables, in comparison with cells treated with GST-osteopontin-RGE (arrows). Scale bar, 20 μm.
Osteopontin activates tumor cells through a Src-mediated signal transduction pathway
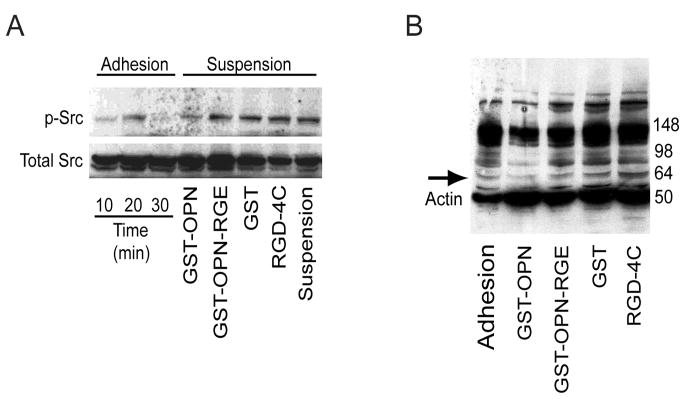
Osteopontin activates several intracellular signaling molecules after ligand-receptor binding to cell surface integrins.3 One such molecule is c-Src, a member of the non-receptor protein tyrosine kinase family that plays a central role in signaling downstream from integrins that regulate actin dynamics.29 We therefore investigated the activation of c-Src in osteosarcoma cells after osteopontin stimulation.30 Surprisingly, we found that rapid dephosphorylation of c-Src at Tyr-418 occurred when cells in suspension were stimulated with osteopontin for only one minute. When cells were treated with several protein or peptide controls (e.g., mutant osteopontin-RGE, GST, or RGD-4C peptide) or left unstimulated, such dephosphorylation did not occur (Fig. 5A). Moreover, this result was also observed when osteosarcoma lysates were analyzed for general tyrosine phosphorylation. Several proteins (predominantly between 50–98 kDa) were found to be rapidly dephosphorylated after exposure of cells to osteopontin, in comparison to proteins after exposure of cells to osteopontin-RGE or to those from unstimulated cells (Fig. 5B).
FIGURE 5.
Osteopontin treatment is associated with Src dephosphorylation of detached tumor cells. A, KRIB osteosarcoma cells were detached from tissue culture substrates. Some of the cells were allowed to adhere to the culture dish, while others were treated for 1 min with GST-osteopontin, GST-osteopontin-RGE, GST, RGD-4C peptide, or DMEM only. The cells were subsequently lysed, and 30 μg of the lysate was analyzed for the phosphorylation of Tyr-418 c-Src. Adhesion of the cells to plastic induced transient activation of c-Src. Treatment of the cells with GST-osteopontin induced dephosphorylation of c-Src. Analysis of total Src confirmed equal loading of the proteins. B, Analysis of the tyrosine phosphorylation of the same lysates (30 μg) revealed general dephosphorylation of tyrosine in proteins (note range 50–98 kDa proteins). Arrow indicates c-Src; cellular actin (40kDa) was detected at the same time to ensure equal loading of the proteins.
Osteopontin enhances tumor cell binding to vascular endothelium
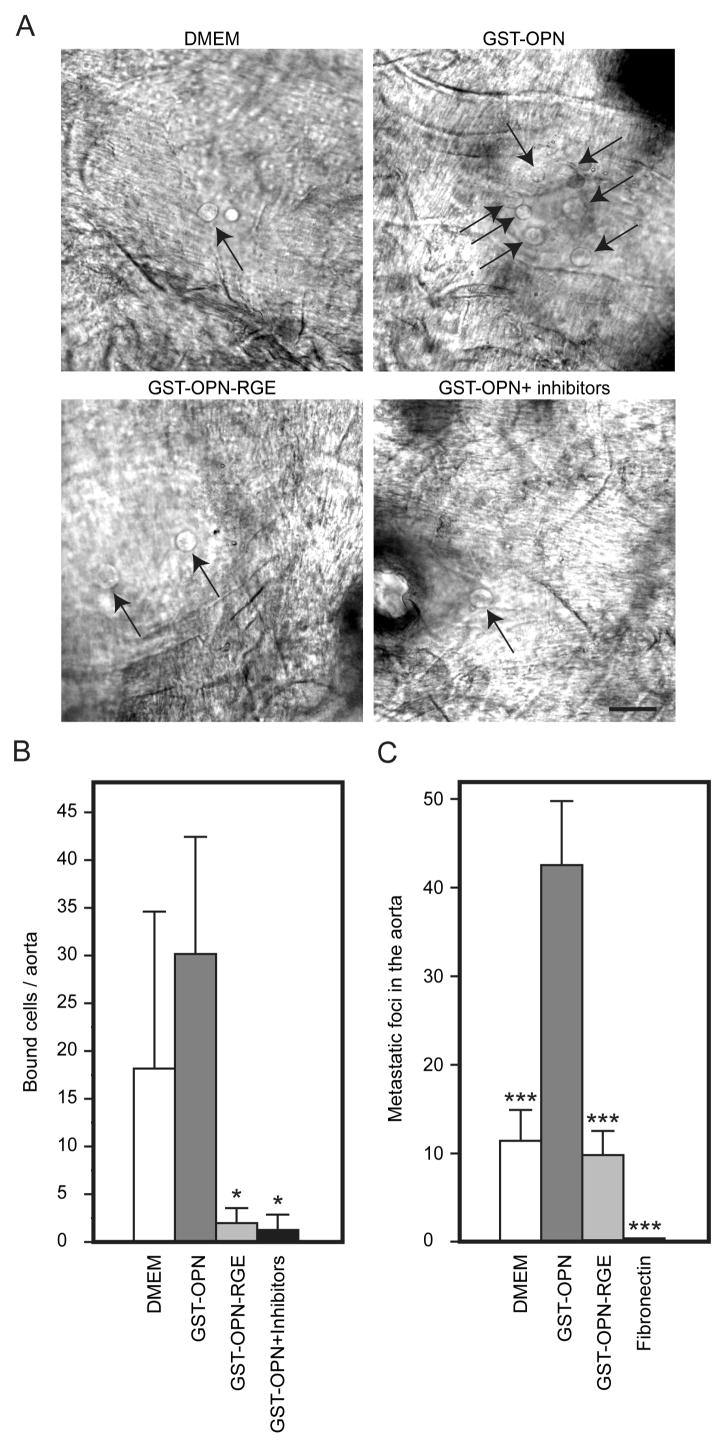
To ask whether the specific and rapid dephosphorylation of proteins such as we observed has biological relevance, we analyzed the binding of sarcoma cells to vascular endothelium after exposure to osteopontin but in the presence of phosphatase inhibitors (1 mM Na3VO4 and 1 mM NaF). Indeed, osteopontin enhanced tumor cell binding to vascular endothelium, and phosphatase inhibitors completely abolished this binding (Fig. 6A and B), data indicating that the interaction of osteopontin with disseminated tumor cells leading to a rapid dephosphorylation of proteins, including c-Src, has a significant impact on circulating tumor cell behavior. This effect was also seen in vivo: the hearts of the mice contained more tumors after they were injected with cells that were treated with osteopontin relative to those treated with DMEM (Fig. 6C).
FIGURE 6.
Promotion of tumor cell binding to vascular endothelium by osteopontin. A and B, KRIB osteosarcoma cells were treated with DMEM, GST-osteopontin, GST-osteopontin-RGE, or phosphatase inhibitors prior to GST-osteopontin and were subsequently incubated with the endothelium of the dorsal aorta. Relative to the GST-osteopontin-RGE and phosphatase inhibition groups, GST-osteopontin significantly increased cell binding (arrows) to the aortas (p<0.05; asterisk). Bars indicate mean ± SD. Scale bar, 20 μm. C, The number of tumor foci was increased in nude mice receiving human sarcoma cells previously incubated with GST-osteopontin relative to DMEM-treated cells.
DISCUSSION
Osteopontin has pleotropic roles in tumor cell biology. Although it is involved in angiogenesis, apoptosis, anchorage-independent cell growth, and metastasis, the mechanisms accounting for these functions have not been entirely explained.4 Many studies have demonstrated the effect of osteopontin in tumor growth and metastasis, with different types of solid tumors and sites of metastasis.5–7, 31–34 However, these reports have focused on the effect of endogenous osteopontin. Because osteopontin presumably functions at several sites in the multi-step metastatic cascade, altering the level of endogenous expression of osteopontin does not necessarily elucidate the role of circulating osteopontin in metastasis. We reasoned that direct stimulation of tumor cells with exogenous osteopontin would minimize systemic effects and gain insight on whether circulating osteopontin affects the metastasis of disseminated tumor cells. We decided to use recombinant osteopontin because cells adhere to recombinant and native osteopontin by similar mechanisms.15, 35 Our findings show that osteopontin has marked and reproducible pro-metastatic effects on human melanoma and sarcoma cells. Osteopontin often induced a doubling in experimental lung metastasis that was ultimately reflected in a severely-reduced actuarial survival rate of tumor-bearing mice. This result is particularly relevant because cancer patients with increased serum osteopontin also have more metastases and poor overall survival.2–4, 6, 8–12 Although the lung colony assay is indeed not a bone fide metastasis model and the findings might not apply generally to the metastasis of tumors in vivo, our functional results suggest that circulating osteopontin is not only a correlative biomarker for metastatic potential but also enhances the capacity of circulating tumor cells to establish metastases. Our functional results indicate that circulating osteopontin is not only a correlative biomarker for metastatic potential but also enhances the capacity of circulating tumor cells to establish metastases. Thus, the studies presented here provide a previously unrecognized function for circulating osteopontin.
The pro-metastatic effects of osteopontin require an intact RGD motif, an observation indicating that a molecular interaction with integrins is critical for enhancement of lung colonization. Although this possibility could perhaps have been anticipated because the RGD domain in osteopontin contributes to lymphatic metastases of breast cancer5, our work provides novel insights for this finding and for the role of osteopontin in metastatic activity. First, soluble exogenous osteopontin appears to function in a manner similar to that of endogenous osteopontin. Second, cells do not need to be adherent (i.e., they can be in circulation) to respond to osteopontin. Third, our results demonstrate that osteopontin promotes RGD-dependent endothelial adherence of disseminated tumor cells and thus explain the early advantage of osteopontin to metastatic spread. Fourth, a previous report on the functional role of RGD used an osteopontin construct in which the entire RGD-sequence was deleted5; in contrast, in this work we used the classic negative control (RGE) in this setting: a single point mutation (Asp→Glu) is less likely to generate unpredictable structural effects (i.e., steric hindrance) within the osteopontin molecule.
Finally, the effects of osteopontin on lung colonization contrast with the effects of other (control) RGD-containing proteins and peptides; serum proteins such as soluble fibrinogen and fibronectin have little or no discernible effects. As an additional positive control, a polymeric form of fibronectin blocked metastasis (data not shown), an effect that has been previously reported.18 Small RGD-containing peptides also exhibited a mild inhibitory effect on experimental metastasis; this result is consistent with several earlier reports showing that soluble RGD-peptides and RGD-containing snake venom proteins can reduce experimental metastasis.36–38 Therefore, osteopontin behaves quite differently from other RGD-containing molecules. Although the reason for this functional distinction is unclear, one possibility is that CD44 also functions as a receptor for osteopontin in our model.31, 39–41 Both CD44 and αv integrins are expressed on the tumor cells used in our studies, and we have confirmed that the isoforms v6/7 and v9 of CD44 that have been shown to bind osteopontin35, 42 are expressed in osteosarcoma cells (unpublished observations). However, our findings in in vitro, in vivo, and ex vivo assays consistently indicate that the RGD motif is required for the pro-metastatic function of osteopontin. Whether or not other receptors are involved and how they cooperate with αv (and perhaps other) integrins in the interaction between cancer cells and osteopontin remain to be determined.
It is possible, although unlikely, that the pro-metastatic effects of osteopontin are due to its direct effects on the host nude mice. First, mixture of tumor cells and osteopontin results in an approximately 10-fold dilution of osteopontin (with a total blood volume in mice of approximately 2 ml). This dilution reduces the concentration of osteopontin below integrin-binding levels. Second, attached tumor cells responded to exogenous soluble osteopontin with a reduction in cytoskeletal actin fibers and, in suspension, with a rapid inactivation of c-Src that led to enhanced binding to vascular endothelium. These in vitro events are host-independent and were osteopontin-specific. Thus, despite evidence that host-related factors can also influence metastasis43, the in vitro data presented here clearly indicate that osteopontin directly affects tumor cells (the “seed”) rather than the lung microenvironment (the “soil”). However, our results do not exclude the non-mutually exclusive possibility that locally produced osteopontin (by host or tumor cell) also affects either tumor or host cells after the tumor cells exit the bloodstream and establish metastases at distant sites; such possibility can be addressed in further studies.
The oncogene Src is important in the regulation of cytoskeletal structure and actin dynamics. Osteopontin affects osteoclast migration and function; notably, osteoclasts are extremely motile, bone-resorbing cells with high rates of cytoskeletal turnover29 through c-Src.44 Thus, we evaluated c-Src activation in cancer cells after exposure to osteopontin by determination of the state of phosphorylation of the conserved residue Tyr-418 within the activation loop. Phosphorylation of c-Src at that site enhances kinase activity.30 Unexpectedly, osteopontin induced rapid dephosphorylation of Tyr-418, data indicating inactivation of c-Src after osteopontin exposure. This new observation is in striking contrast to earlier reports describing the effects of osteopontin in the context of c-Src activation. However, all the previous studies were carried out with immobilized osteopontin and adherent cells45, or in different experimental settings.44, 46 Indeed, we were able to confirm similar c-Src activation through Tyr-418 phosphorylation when cells were allowed to adhere to plastic dishes. Arguably, the analysis of c-Src phosphorylation in a cell suspension is more relevant, because circulating tumor cells encounter osteopontin in plasma. Our experiments show that rapid changes in signaling take place after tumor cells interact with osteopontin. This result is further supported by the fact that addition of soluble osteopontin to adherent cells causes reduction of actin stress fibers. In addition, exposure to osteopontin induced tumor cell binding to vascular endothelium, which was abolished by phosphatase inhibitors. Aortic metastasis rarely (if ever) occurs in patients but one might speculate that the strong blood flow accompanied by shear forces within the aortic circulation could retard, or even prevent, metastasis. Thus, we believe that this “reductionist” aortic assay in vitro--while limited and restricted in scope relative to in vivo settings--might simulate at least some of the early general events that may occur when tumor cells encounter an intact vascular endothelium. Based on all our in vitro results, one might surmise that osteopontin induces similar cytoskeletal arrangements in tumor cells that occur during T-cell activation28, when interaction with APCs involves the formation of large areas of intimate cell-membrane contact. Faure et al. has reported that disanchoring of the cortical actin cytoskeleton from the plasma membrane decreases cellular rigidity and leads to more efficient T cell-APC complex formation; such disanchoring was achieved through rapid inactivation of ezrin-radixin-moesin proteins via a Vav1-Rac1 pathway.27 This type of cell relaxation could allow clustering of adhesion molecules on the cell membrane and thus facilitate tumor cell binding and penetration of the vascular endothelium. Although our results support this hypothesis, extensive further experimentation will be required to confirm and expand this interpretation.
In summary, we show that osteopontin has marked pro-metastatic effects in experimental models of metastasis. We also show that the RGD motif in osteopontin is functional and required for the effects. Finally, in non-adherent cells we observed Src dephosphorylation, and enhanced tumor cell binding to the vascular endothelium that was completely blocked by phosphatase inhibitors. This osteopontin-dependent biological phenomenon might be a molecular mechanism for tumor cells in suspension. If so, osteopontin could affect Src regulation and the release of adhesion molecules from the cytoskeleton, a reaction allowing their rearrangement on the cell membrane that facilitates enhanced cell binding to vascular endothelium. Presumably, tumor cells undergo two types of osteopontin-mediated sequential effects: (i) an initial rapid Src dephosphorylation accompanied by an increase in the compliance of the cell membrane while in circulation, and (ii) a later Src phosphorylation after cells attach to a metastasis site. If confirmed, these mechanistic data might lead to novel anti-metastatic strategies in human cancer.
Acknowledgments
We thank Dr. Emmanuel Dias-Neto for comments on the manuscript and Dr. Amin Hajitou for technical assistance.
This work was supported by grants CA69306 (JWS), CA30199 (RP and JWS), and CA90270 (RP and WA) from the National Institutes of Health, grant DAMD 17-98-1-8041 from the Department of Defense (RP) and grants from the California Breast Cancer Research Program (JWS and ECKL). The Army Breast Cancer Research Program (DDH) provided additional support. WA and RP received support from the Gillson-Longenbaugh Foundation. JM received support from the Helsingin Sanomat Centennial Foundation, the Emil Aaltonen Foundation, the Research and Science Foundation of Farmos, and the Maud Kuistila Memorial Foundation.
References
- 1.Denhardt DT, Noda M, O'Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furger KA, Menon RK, Tuck AB, Bramwell VH, Chambers AF. The functional and clinical roles of osteopontin in cancer and metastasis. Curr Mol Med. 2001;1:621–632. doi: 10.2174/1566524013363339. [DOI] [PubMed] [Google Scholar]
- 3.Rangaswami H, Bulbule A, Kundu GC. Osteopontin: role in cell signaling and cancer progression. Trends Cell Biol. 2006;16:79–87. doi: 10.1016/j.tcb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 4.Weber GF. The metastasis gene osteopontin: a candidate target for cancer therapy. Biochim Biophys Acta. 2001;1552:61–85. doi: 10.1016/s0304-419x(01)00037-3. [DOI] [PubMed] [Google Scholar]
- 5.Allan AL, George R, Vantyghem SA, et al. Role of the integrin-binding protein osteopontin in lymphatic metastasis of breast cancer. Am J Pathol. 2006;169:233–246. doi: 10.2353/ajpath.2006.051152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bramwell VH, Doig GS, Tuck AB, et al. Serial plasma osteopontin levels have prognostic value in metastatic breast cancer. Clin Cancer Res. 2006;12:3337–3343. doi: 10.1158/1078-0432.CCR-05-2354. [DOI] [PubMed] [Google Scholar]
- 7.Kang Y, Siegel PM, Shu W, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–549. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 8.Singhal H, Bautista DS, Tonkin KS, et al. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res. 1997;3:605–611. [PubMed] [Google Scholar]
- 9.Hotte SJ, Winquist EW, Stitt L, Wilson SM, Chambers AF. Plasma osteopontin: associations with survival and metastasis to bone in men with hormone-refractory prostate carcinoma. Cancer. 2002;95:506–512. doi: 10.1002/cncr.10709. [DOI] [PubMed] [Google Scholar]
- 10.Ramankulov A, Lein M, Kristiansen G, Loening SA, Jung K. Plasma osteopontin in comparison with bone markers as indicator of bone metastasis and survival outcome in patients with prostate cancer. Prostate. 2006;67:330–340. doi: 10.1002/pros.20540. [DOI] [PubMed] [Google Scholar]
- 11.Ye QH, Qin LX, Forgues M, et al. Predicting hepatitis B virus-positive metastatic hepatocellular carcinomas using gene expression profiling and supervised machine learning. Nat Med. 2003;9:416–423. doi: 10.1038/nm843. [DOI] [PubMed] [Google Scholar]
- 12.Le QT, Sutphin PD, Raychaudhuri S, et al. Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clin Cancer Res. 2003;9:59–67. [PMC free article] [PubMed] [Google Scholar]
- 13.Oldberg A, Franzen A, Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proc Natl Acad Sci U S A. 1986;83:8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bornstein P, Sage EH. Matricellular proteins: extracellular modulators of cell function. Curr Opin Cell Biol. 2002;14:608–616. doi: 10.1016/s0955-0674(02)00361-7. [DOI] [PubMed] [Google Scholar]
- 15.Xuan JW, Hota C, Chambers AF. Recombinant GST-human osteopontin fusion protein is functional in RGD-dependent cell adhesion. J Cell Biochem. 1994;54:247–255. doi: 10.1002/jcb.240540213. [DOI] [PubMed] [Google Scholar]
- 16.Engvall E, Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977;20:1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- 17.Morla A, Zhang Z, Ruoslahti E. Superfibronectin is a functionally distinct form of fibronectin. Nature. 1994;367:193–196. doi: 10.1038/367193a0. [DOI] [PubMed] [Google Scholar]
- 18.Pasqualini R, Bourdoulous S, Koivunen E, Woods VL, Jr, Ruoslahti E. A polymeric form of fibronectin has antimetastatic effects against multiple tumor types. Nat Med. 1996;2:1197–1203. doi: 10.1038/nm1196-1197. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:157–481. [Google Scholar]
- 20.Pierschbacher MD, Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci U S A. 1984;81:5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- 22.Arap W, Pasqualini R, Ruoslahti E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science. 1998;279:377–380. doi: 10.1126/science.279.5349.377. [DOI] [PubMed] [Google Scholar]
- 23.Assa-Munt N, Jia X, Laakkonen P, Ruoslahti E. Solution structures and integrin binding activities of an RGD peptide with two isomers. Biochemistry. 2001;40:2373–2378. doi: 10.1021/bi002101f. [DOI] [PubMed] [Google Scholar]
- 24.Hajitou A, Trepel M, Lilley CE, et al. A hybrid vector for ligand-directed tumor targeting and molecular imaging. Cell. 2006;125:385–398. doi: 10.1016/j.cell.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 25.Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology (N Y) 1995;13:265–270. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 26.Pasqualini R, Koivunen E, Ruoslahti E. Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol. 1997;15:542–546. doi: 10.1038/nbt0697-542. [DOI] [PubMed] [Google Scholar]
- 27.Faure S, Salazar-Fontana LI, Semichon M, et al. ERM proteins regulate cytoskeleton relaxation promoting T cell-APC conjugation. Nat Immunol. 2004;5:272–279. doi: 10.1038/ni1039. [DOI] [PubMed] [Google Scholar]
- 28.Dustin ML, Cooper JA. The immunological synapse and the actin cytoskeleton: molecular hardware for T cell signaling. Nat Immunol. 2000;1:23–29. doi: 10.1038/76877. [DOI] [PubMed] [Google Scholar]
- 29.Linder S, Aepfelbacher M. Podosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 2003;13:376–385. doi: 10.1016/s0962-8924(03)00128-4. [DOI] [PubMed] [Google Scholar]
- 30.Tang H, Hao Q, Rutherford SA, Low B, Zhao ZJ. Inactivation of SRC family tyrosine kinases by reactive oxygen species in vivo. J Biol Chem. 2005;280:23918–23925. doi: 10.1074/jbc.M503498200. [DOI] [PubMed] [Google Scholar]
- 31.Ito T, Hashimoto Y, Tanaka E, et al. An inducible short-hairpin RNA vector against osteopontin reduces metastatic potential of human esophageal squamous cell carcinoma in vitro and in vivo. Clin Cancer Res. 2006;15(12):1308–1316. doi: 10.1158/1078-0432.CCR-05-1611. [DOI] [PubMed] [Google Scholar]
- 32.Shevde LA, Samant RS, Paik JC, et al. Osteopontin knockdown suppresses tumorigenicity of human metastatic breast carcinoma, MDA-MB-435. Clin Exp Metastasis. 2006;23:123–133. doi: 10.1007/s10585-006-9013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki M, Mose E, Galloy C, Tarin D. Osteopontin gene expression determines spontaneous metastatic performance of orthotopic human breast cancer xenografts. Am J Pathol. 2007;171:682–692. doi: 10.2353/ajpath.2007.070232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAllister SS, Gifford AM, Greiner AL, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katagiri YU, Sleeman J, Fujii H, et al. CD44 variants but not CD44s cooperate with beta1-containing integrins to permit cells to bind to osteopontin independently of arginine-glycine-aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res. 1999;59:219–226. [PubMed] [Google Scholar]
- 36.Humphries MJ, Olden K, Yamada KM. A synthetic peptide from fibronectin inhibits experimental metastasis of murine melanoma cells. Science. 1986;233:467–470. doi: 10.1126/science.3726541. [DOI] [PubMed] [Google Scholar]
- 37.Morris VL, Schmidt EE, Koop S, et al. Effects of the disintegrin eristostatin on individual steps of hematogenous metastasis. Exp Cell Res. 1995;219:571–578. doi: 10.1006/excr.1995.1266. [DOI] [PubMed] [Google Scholar]
- 38.Trikha M, De Clerck YA, Markland FS. Contortrostatin, a snake venom disintegrin, inhibits beta 1 integrin-mediated human metastatic melanoma cell adhesion and blocks experimental metastasis. Cancer Res. 1994;54:4993–4998. [PubMed] [Google Scholar]
- 39.Lin YH, Yang-Yen HF. The osteopontin-CD44 survival signal involves activation of the phosphatidylinositol 3-kinase/Akt signaling pathway. J Biol Chem. 2001;276:46024–46030. doi: 10.1074/jbc.M105132200. [DOI] [PubMed] [Google Scholar]
- 40.Teramoto H, Castellone MD, Malek RL, et al. Autocrine activation of an osteopontin-CD44-Rac pathway enhances invasion and transformation by H-RasV12. Oncogene. 2005;24:489–501. doi: 10.1038/sj.onc.1208209. [DOI] [PubMed] [Google Scholar]
- 41.Weber GF, Ashkar S, Glimcher MJ, Cantor H. Receptor-ligand interaction between CD44 and osteopontin (Eta-1) Science. 1996;271:509–512. doi: 10.1126/science.271.5248.509. [DOI] [PubMed] [Google Scholar]
- 42.Khan SA, Cook AC, Kappil M, et al. Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: novel post-transcriptional, post-translational regulation. Clin Exp Metastasis. 2005;22:663–673. doi: 10.1007/s10585-006-9007-0. [DOI] [PubMed] [Google Scholar]
- 43.Crawford HC, Matrisian LM, Liaw L. Distinct roles of osteopontin in host defense activity and tumor survival during squamous cell carcinoma progression in vivo. Cancer Res. 1998;58:5206–5215. [PubMed] [Google Scholar]
- 44.Hruska KA, Rolnick F, Huskey M, Alvarez U, Cheresh D. Engagement of the osteoclast integrin alpha v beta 3 by osteopontin stimulates phosphatidylinositol 3-hydroxyl kinase activity. Endocrinology. 1995;136:2984–2992. doi: 10.1210/endo.136.7.7540546. [DOI] [PubMed] [Google Scholar]
- 45.Das R, Mahabeleshwar GH, Kundu GC. Osteopontin induces AP-1-mediated secretion of urokinase-type plasminogen activator through c-Src-dependent epidermal growth factor receptor transactivation in breast cancer cells. J Biol Chem. 2004;279:11051–11064. doi: 10.1074/jbc.M310256200. [DOI] [PubMed] [Google Scholar]
- 46.Chellaiah M, Fitzgerald C, Alvarez U, Hruska K. c-Src is required for stimulation of gelsolin-associated phosphatidylinositol 3-kinase. J Biol Chem. 1998;273:11908–11916. doi: 10.1074/jbc.273.19.11908. [DOI] [PubMed] [Google Scholar]