Abstract
The pKa’s of the 6–CH groups of N-methyl-2-pyridone and N-methyl-4-pyridone in aqueous solution were determined. No correlation between the stability of the carbanions and the rate of decarboxylation of corresponding carboxylic acids was found.
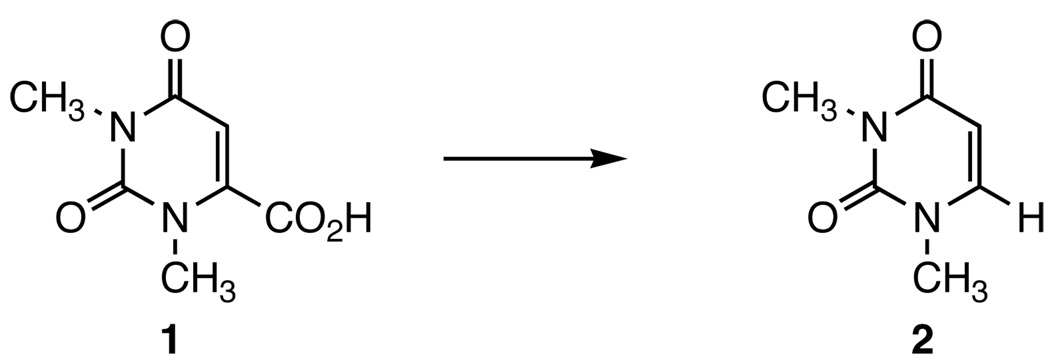
The decarboxylation of 1,3-dimethylorotic acid (1) and its analogues has been proven to be a useful model for the enzymatic decarboxylation catalyzed by orotidine-5’-monophosphate decarboxylase (ODCase).1–12 Most of the studies involve the investigation of the nature and stability of the intermediate. As shown in Scheme 1, acid 1 decarboxylates at elevated temperatures to give 1,3-dimethyluracil (2) as the sole product.
Scheme 1.
Decarboxylation of 1,3-Dimethylorotic Acid
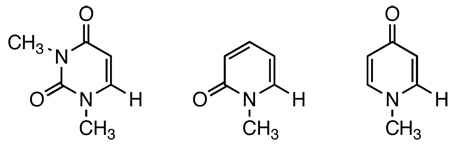
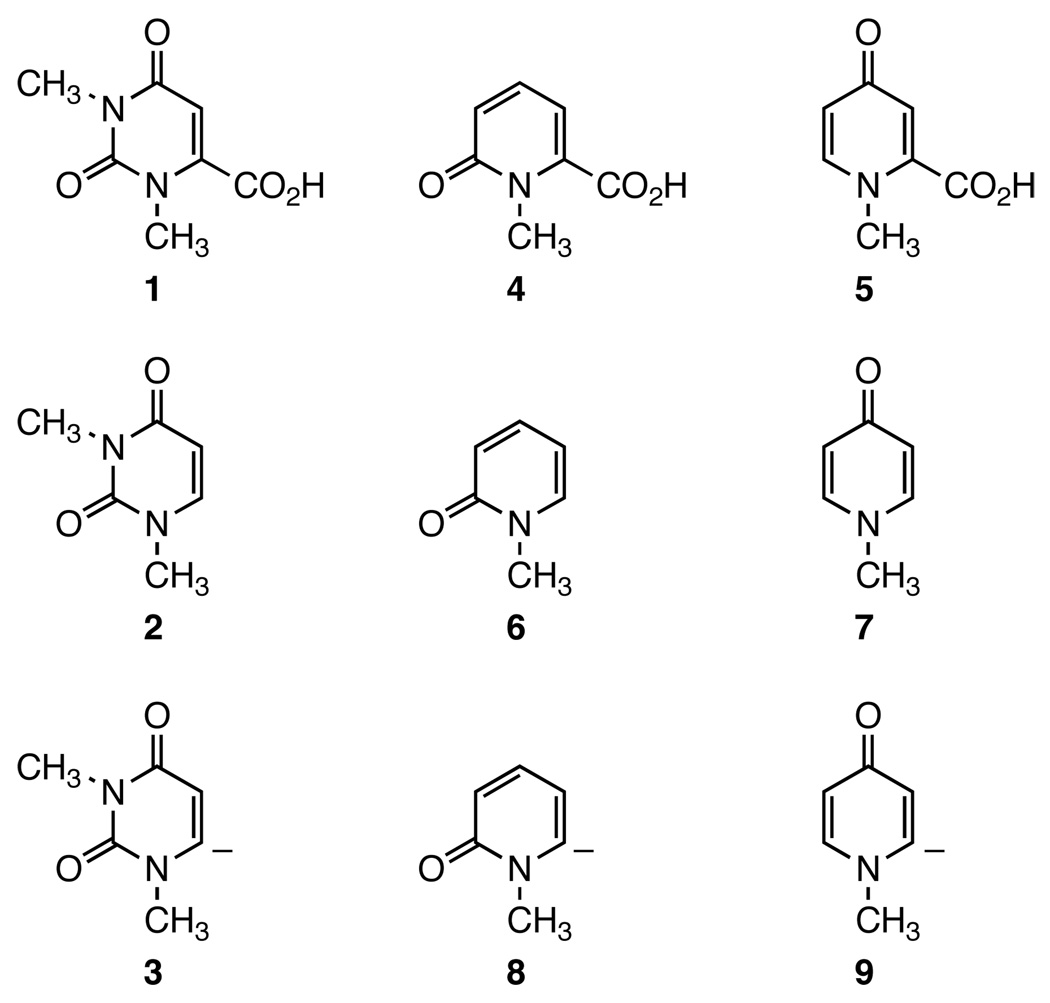
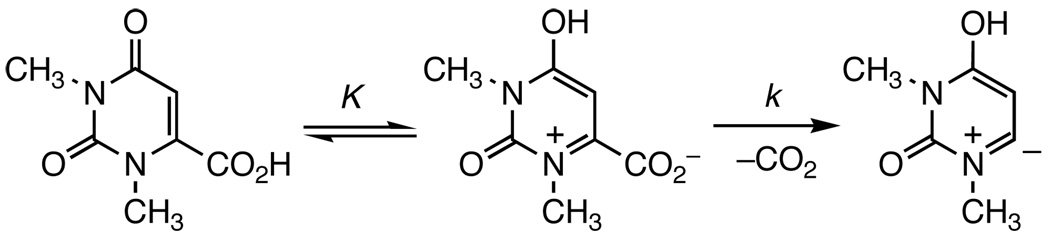
The decarboxylation of acids 1, 4 and 5 to uracil 2 and pyridones 6 and 7 (Figure 1), respectively, provides a unique opportunity to systematically investigate the mechanism of the reactions due to the large difference in their reaction rates despite their structural similarity.1,6,7 Acids 1 and 4 decarboxylate at the same rate, while acid 5 decarboxylates almost 3000 times faster.1,7 Studies on the gas-phase stability of the corresponding carbanions 3, 8, and 9 have established a lack of correlation between the rate of decarboxylation and the gas-phase stability of resulted carbanions.7 It was found that carbanion 3 is much more stable while carbanions 8 and 9 share the same stability.6,7 As a result, a two-step mechanism has been proposed to account for the large difference in rate constants measured for acids 1, 4, and 5 (Scheme 2).1,7 In this mechanism, the large differences in the equilibrium constants explain the differences in rate constants.
Figure 1.
Substrates, Products, and Carbanion Intermediates in the Decarboxylation Reactions
Scheme 2.
Proposed Mechanism of Decarboxylation
The pKa of uracil 2 in water has been determined to be 34 ± 2, which is suggested to be the evidence for the high reaction barrier for catalysis by ODCase.8 However, our gas-phase study has demonstrated that the stability of the carbanionic intermediates does not correlate with the rate of decarboxylation.7 One concern about gas-phase studies is that the results may not represent those in condensed phase. In condensed phase, solvation plays a major rule in the relative stability of species, especially ions. In this report, we have extended the study on the stability of carbanions 3, 8, and 9 to the aqueous solution.
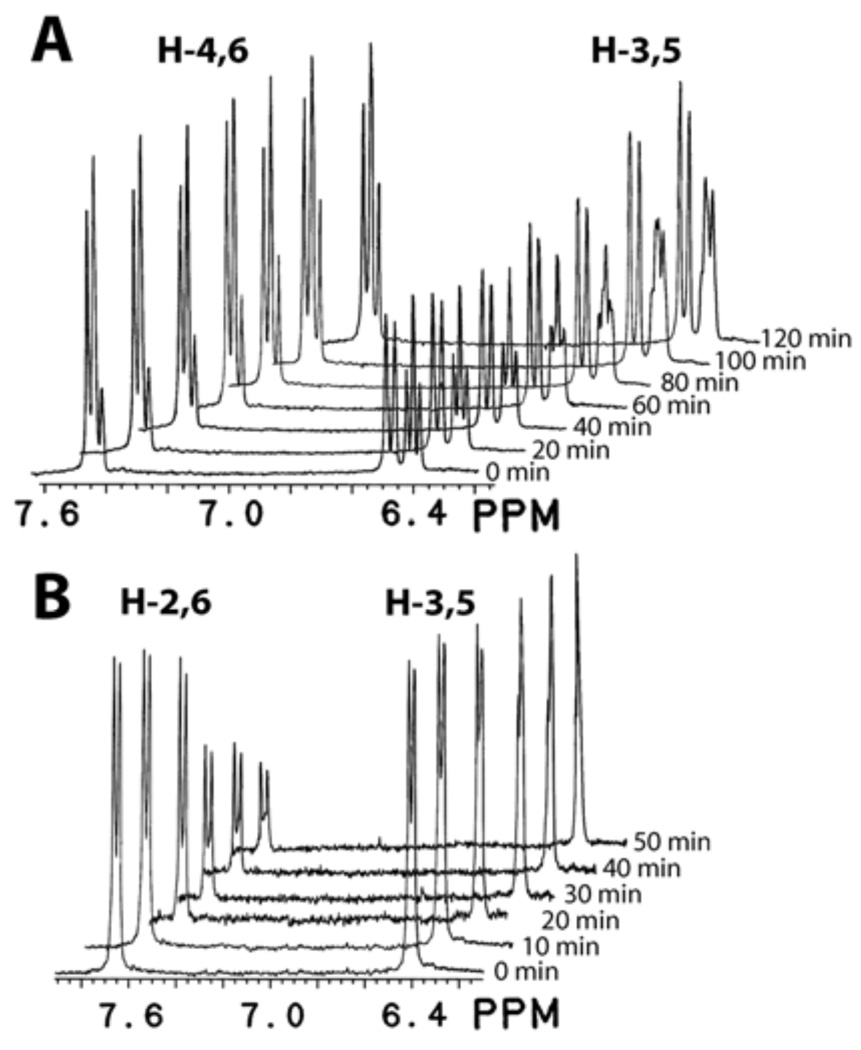
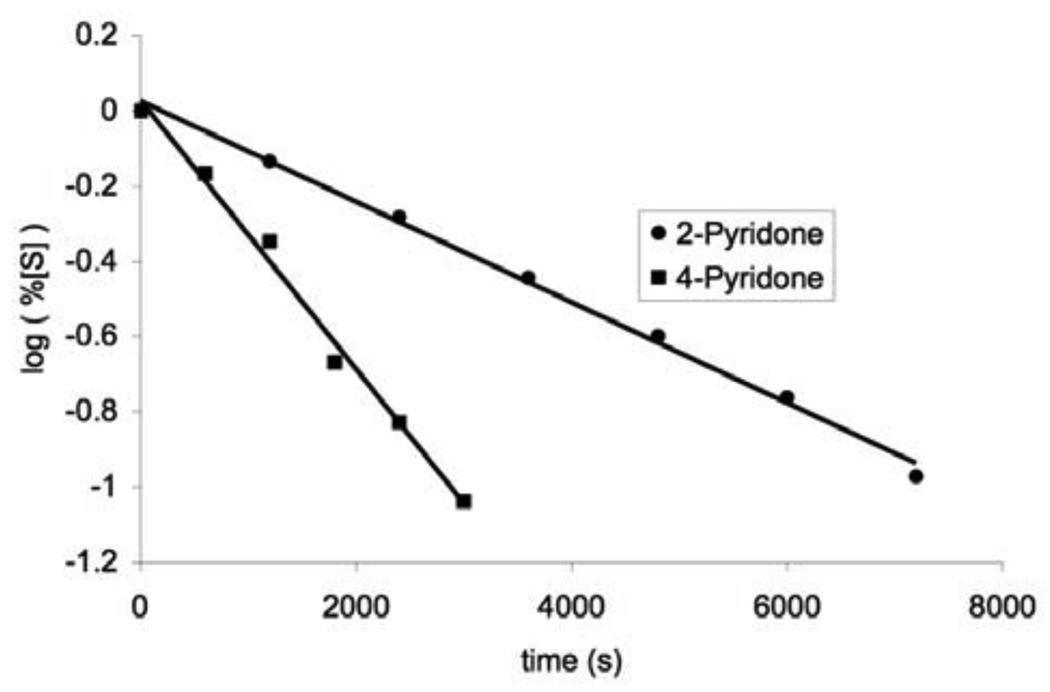
Richard and coworkers have determined pKa of weak carbon acids in aqueous solution by measuring the rate of proton-deuterium exchange on interested carbons using NMR spectroscopy.13,14 This method was employed by Sievers and Wolfenden in determining the pKa of 6–CH of uracil 2.8 However, when pyridones 6 and 7 were heated in acetate buffer in D2O as reported for 2, no proton-deuterium exchange was observed after 5 hrs. This observation indicates that pyridones 6 and 7 are less acidic than uracil 2 at carbon-6 and a much stronger base is required. Proton-deuterium exchange on carbon-6 of pyridones 6 and 7 has been reported in NaOD/D2O or NaOCH3/CH3OD solutions.15,16 We have therefore carried out the kinetic experiments in NaOD/D2O solution. It has been reported that the rate of proton-deuterium exchange on carbon-6 of pyridones 6 and 7 follows first-order kinetics on the concentration of substrate as well as NaOD.15,16 The specific rate constant at 1 N NaOD/D2O, kOD, was determined by following the disappearance of H–6 by NMR, as illustrated by representative runs at 100 °C shown in Figure 2.17 Excellent first-order kinetics was observed in our experiments as shown in Figure 3.
Figure 2.
NMR Monitoring of the disappearance of H–6 in pyridones 6 (A) and 7 (B) at 100 °C
Figure 3.
Rate plot for the disappearance of H–6 in pyridones 6 and 7 at 100 °C
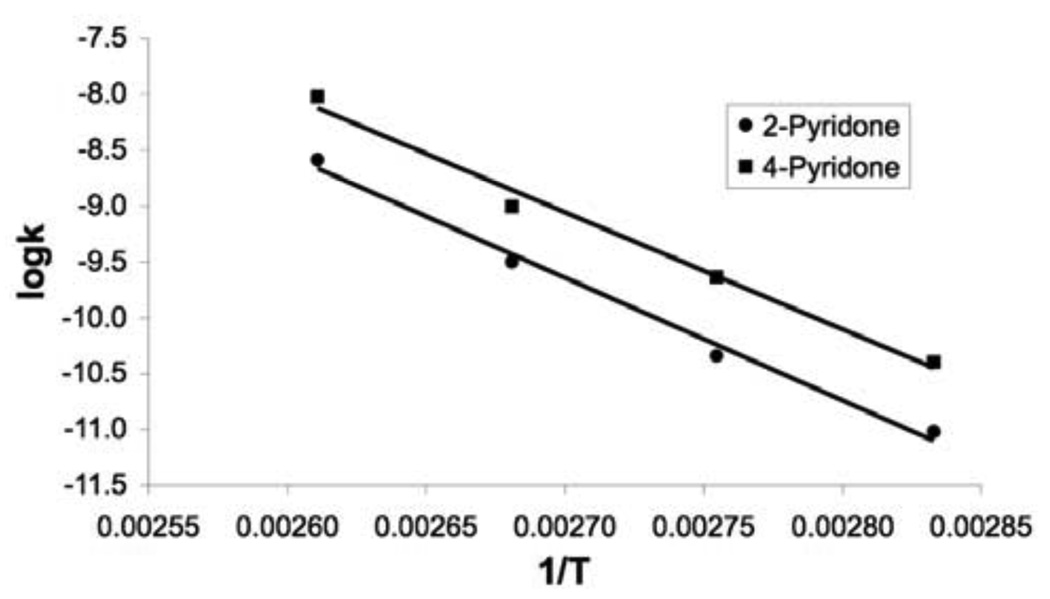
The rate of proton-deuterium exchange was determined over the temperatures of 80–110°C and was extrapolated to 25°C by Arrenhius plot as shown in Figure 4. Using the secondary solvent deuterium isotope effect of kDO/kHO = 1.46 as suggested by Richard and coworkers13, the rate constants for the hydroxide-catalyzed deprotonation, kOH, for pyridones 6 and 7 are 3.31 × 10−8 M−1 s−1 and 8.35 × 10−8 M−1 s−1, respectively. A rate constant of ~1011 s−1 was employed for the reaction between the carbanion and water, kHOH, as in the case of uracil 2.8 The pKa values of the 6–CH groups of both pyridones 6 and 7 are thus estimated to be 32 ± 2, using equation 1.
| (1) |
Figure 4.
Arrhenius plot for the rates of deuterium exchange in pyridones 6 and 7
The pKa value for uracil 2 should be even lower since our experiment has indicated that uracil 2 is more acidic than either pyridone 6 or 7 (vide infra). Unfortunately we were unable to carry out the same experiment with uracil 2 due to its rapid decomposition in alkaline solutions.18 However, it should be pointed out again that the same trend on carbanion stability was observed in the gas-phase, as discussed above.6,7
A difference of two or three pKa units between the 6–CH groups of uracil 2 and pyridones (6 and 7) is reasonable in light of our failure to observe any H–D exchange on 6 and 7 under the same reaction conditions reported for 2. This estimate will put the pKa of uracil 2 at around 30, which is lower than the reported value of 34.8 The same method were employed in the two studies. The rate constants in our study are extrapolated from a lower temperature range (80–110°C as compared to 175–217°C) and thus affected by uncertainties in measurement to a much lesser degree. Furthermore, no correction over temperature of the ionization constants of the buffers is required, eliminating another source of possible uncertainty. Several studies have suggested a quite stable carbanion 3. In a carbanion-carbon acid exchange experiment, it was found that carbanion 3 was less basic than the carbanion derived from triphenylmethane, placing the pKa of uracil 2 at less than 30.19 In another study, the pKa of 6–CH group of pyrimidine nucleotide bases has been estimated to be about 30.20 It has also been suggested that N–C=O group stabilizes adjacent carbanions, in general, and the 6-carbanion of uridine, in particular.14
The rate of H–D exchange in uracil 2 can also be estimated from the decomposition of 2 in alkaline solution. The rates of the disappearance of H–6 and N–CH3 are different and the difference (approximately 2.5% of the rate of decomposition) is consistent through different runs. Presumably the rate of disappearance of H–6 is greater because it is the combination of the rates of decomposition and exchange. If we use this difference in rates as an estimate for the rate of exchange, the rate constant, kOH, for uracil 2 can be estimated to be approximately 3 × 10−6 M−1 s−1. Therefore, the pKa of uracil 2 is estimated to be 30, consistent with the numbers reported as discussed above.
On the mechanistic front, acid 5 decarboxylates at a rate approximately 3000 times faster than acid 4; yet the stability of the 6-carbanions in aqueous solution is almost identical. The trend for the stability of the carbanions is the same as that found in gas-phase.6,7 This result has further illustrated the lack of correlation between the rate of decarboxylation and the stability of the resulting carbanions. On the other hand, the two-step mechanism shown in Scheme 2 was able to account for the large difference in the reaction rates.1,7 We are currently investigating each individual step in the proposed mechanism.
The mecchanism of ODCase remains unsolved despite the solution of its structures. The current study calls into questions how stabilization of the carbanion intermediate catalyzes the reaction. The formation of a zwitterionic intermediate through the direct protonation of one of the carbonyl groups on the pyrimidine ring is unlikely due to the lack of any basic residues adjacent to these groups. However, the enzyme might manipulate the environment of the active site to provide a pre-organized polar environment that is more conducive to the binding of the substrate in a reactive state that shares some structural features with the zwitterionic intermediate.
Acknowledgment
This investigation was supported by the National Institutes of Health, MBRS SCORE Program – Grant #5 S06 GM52588. C.C. was supported by the NIH MBRS RISE Program (5 R25-GM59298-08). We thank Wee Tam at SFSU for obtaining the NMR spectra. The NMR facility was funded by the National Science Foundation (DUE-9451624 and DBI 0521342). We also thank Professor Scott Gronert at SFSU for helpful discussions.
Reference and Footnotes
- 1.Beak P, Siegel B. J. Am. Chem. Soc. 1976;98:3601–3606. doi: 10.1021/ja00428a035. [DOI] [PubMed] [Google Scholar]
- 2.Radzicka A, Wolfenden R. Science. 1995;267:90–93. doi: 10.1126/science.7809611. [DOI] [PubMed] [Google Scholar]
- 3.Lee JK, Houk KN. Science. 1997;276:942–945. doi: 10.1126/science.276.5314.942. [DOI] [PubMed] [Google Scholar]
- 4.Wu W, Ley-han A, Wong FM, Austin TJ, Miller SM. Bioorg. Med. Chem. Lett. 1997;7:2623–2628. [Google Scholar]
- 5.Nakanishi MP, Wu W. Tetrahedron Lett. 1998;39:6271–6272. [Google Scholar]
- 6.Gronert S, Feng WY, Chew F, Wu W. Int. J. Mass Spectrom. 2000;195/196:251–258. [Google Scholar]
- 7.Feng WY, Austin TJ, Chew F, Gronert S, Wu W. Biochemistry. 2000;39:1778–1783. doi: 10.1021/bi992553w. [DOI] [PubMed] [Google Scholar]
- 8.Sievers A, Wolfenden R. J. Am. Chem. Soc. 2002;124:13986–13987. doi: 10.1021/ja021073g. [DOI] [PubMed] [Google Scholar]
- 9.Shem DL, Gronert S, Wu W. Bioorg. Chem. 2004;32:76–81. doi: 10.1016/j.bioorg.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Silverman RB, Groziak MP. J. Am. Chem. Soc. 1982;104:6434–6439. [Google Scholar]
- 11.Singleton DA, Merrigan SR, Kim BJ, Beak P, Phillips LM, Lee JK. J. Am. Chem. Soc. 2000;122:3296–3300. [Google Scholar]
- 12.Wong FM, Wu W. Bioorg. Chem. 2006;34:99–104. doi: 10.1016/j.bioorg.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Rios A, Amyes TL, Richard JP. J. Am. Chem. Soc. 2000;122:9373–9385. [Google Scholar]
- 14.Rios A, Richard JP, Amyes TL. J. Am. Chem. Soc. 2002;124:8251–8259. doi: 10.1021/ja026267a. [DOI] [PubMed] [Google Scholar]
- 15.Beak P, Bonham J. J. Am. Chem. Soc. 1965;87:3365–3371. [Google Scholar]
- 16.Beak P, Monroe EM. J. Org. Chem. 1969;34:589–596. [Google Scholar]
- 17.Kinetic Measurements. Compounds 2 and 6 are commercially available from Aldrich. Compound 7 was prepared according to procedures reported in reference 15. The progress of the proton-deuterium exchange reaction was followed by NMR spectrometry. A stock solution of 10 mg of substrate in 1 mL of NaOD/D2O was prepared. The concentration of NaOD was 1 M and was determined by titration with 0.1 N standard HCl solution. A series of NMR tubes containing the stock solution was heated in an oil-bath at a given temperature for time intervals of 10–120 minutes. The percentage of substrate remaining was calculated from the integration of corresponding peaks of the substrate and product. In the case of 2-pyridone 6, the disappearance of H–6 was followed by measuring the quantity (2x − 1), where x is the ratio of integration of the multiplet around 7.6 ppm (H–4 and H–6) to the integration of the multiplet around 6.6 ppm (H–3 and H–5) in the NMR spectra as discussed in reference 16. The term (2x − 1) thus refers to the ratio of H–6 to either H–3 or H–5. In the case of 4-pyridone 7, the amount of deuterium incorporation was simply measured by comparing the integration of H–2 and H–6 to that of H–3 and H–5. The observed rate constant obtained for 4-pyridone 7 was halved to give the value for kOD.
- 18.Lovett EG, Lipkin D. J. Org. Chem. 1977;42:2574–2580. doi: 10.1021/jo00435a009. [DOI] [PubMed] [Google Scholar]
- 19.Siegel B. Ph.D. Thesis. University of Illinois at Urbana/Champaign; 1974. Mechanistic Studies of the Decarboxylation Reaction of 1,3-Dimethylorotic Acid: A Possible Mechanism for Orotate Decarboxylase. [Google Scholar]
- 20.Wechter WJ. Collect. Czech. Chem. Commun. 1970;35:2003–2017. [Google Scholar]