SUMMARY
The neuron-astrocyte synaptic complex is a fundamental operational unit of the nervous system. Astroglia play a central role in the regulation of synaptic glutamate, via neurotransmitter transport by GLT1/EAAT2. The astroglial mechanisms underlying this essential neuron-glial communication are not known. Here we show that presynaptic terminals are sufficient and necessary for GLT1/EAAT2 transcriptional activation and have identified the molecular pathway that regulates astroglial responses to presynaptic input. Presynaptic terminals regulate astroglial GLT1/EAAT2 via kappa B-motif binding phosphoprotein (KBBP), the mouse homologue of human heterogeneous nuclear ribonucleoprotein K (hnRNP K), which binds to an essential element of GLT1/EAAT2 promoter. This neuron-stimulated factor is required for GLT1/EATT2 transcriptional activation and is responsible for astroglial alterations in neural injury. Denervation of neuron-astrocyte signaling in vivo, by acute corticospinal tract transection, ricin-induced motor neuron death, or chronic neurodegeneration in amyotrophic lateral sclerosis (ALS) all result in reduced astroglial KBBP expression and transcriptional dysfunction of astroglial transporter expression. Our studies indicate that presynaptic elements dynamically coordinate normal astroglial function and also provide a fundamental signaling mechanism by which altered neuronal function and injury leads to dysregulated astroglia in CNS disease.
INTRODUCTION
The core elements of the central nervous system (CNS) functional unit include pre-synaptic axons, post-synaptic dendrites, along with the peri-synaptic astroglia which ensheathe the vast majority of all synapses. Astroglia play a vital and active role in synaptic transmission(Halassa et al., 2007), by regulating extracellular K+ concentration, by releasing gliotransmitters, and by modulating glutamate receptor (GluR) activation through glutamate transporter (GluT)-mediated control of synaptic and extra-synaptic glutamate clearance(Huang and Bergles, 2004). The astroglial specific plasma membrane glutamate transporter subtype GLT1 and its human homology, EAAT2 are the dominant functional/physiologically active transporter in the CNS (Danbolt, 2001; Regan et al., 2007; Rothstein et al., 1994b; Tanaka, 1997). GLT1/EAAT2 is typically concentrated in peri-synaptic membranes (Chaudhry et al., 1995; Danbolt, 2001). GluTs directly contend with GluRs for glutamate, modulating the intensity and duration of post-synaptic activation and preventing the interference (“spillover” or “spillout”) of neighboring synapses (Arnth-Jensen et al., 2002; Huang and Bergles, 2004). Importantly, astroglial GluTs also prevent accumulation of extracellular glutamate and subsequent over-stimulation of GluRs, thus preventing possible excitotoxic neuronal death (Rothstein et al., 1996; Tanaka, 1997). Experimental disruption of this astroglial function is neurotoxic and promotes neurodegeneration (Rothstein et al., 1996). In neurological diseases, the neuron-astroglia synaptic unit is often disrupted, as reflected by severe loss of synaptic astroglial GluTs in post-mortem human tissue and rodent models of neurodegeneration (Lauriat et al., 2007; Lievens et al., 2001; Rothstein et al., 1992).
Despite of the importance of astroglial GluTs in synaptic function, the normal molecular regulation of the neuron-astrocyte functional unit is not known. Induction of astroglial GluT expression occurs in parallel with synapse maturation in early post-natal development (Furuta et al., 1997). Multiple in vitro studies of mixed neuron glial cultures suggested that unknown neuronal secreted factors, added glutamate and/or growth factors can induce expression of astroglial glutamate transporters GLT1 and GLAST (Munir et al., 2000; Schlag et al., 1998; Swanson et al., 1997; Zelenaia et al., 2000) or regulate its cytoplasmic clustering (Nakagawa et al., 2008; Zhou and Sutherland, 2004), but downstream molecular mechanisms have not yet explored. In vivo studies demonstrate that alteration of sensory activity by whisker stimulation induces changes in astroglial synaptic coverage and expression levels of astroglial GluTs, suggesting that neuronal activity could be linked to astroglial GluTs genomic regulation(Genoud et al., 2006). With the recent identification of the GLT1/EAAT2 promoter(Su et al., 2003), it became possible to evaluate GluT transcriptional regulation and the contribution of synaptic elements to this astrocytic peri-synaptic protein. In this study, we have identified the transcriptional mechanisms that underlie normal neuronal/synaptic regulation of astroglial GLT1/EAAT2 and how in vivo disruption of this astroglial pathway may be common to subacute and chronic neuronal disease.
RESULTS
Axons are sufficient and necessary for transcriptional activation of astroglial GLT1
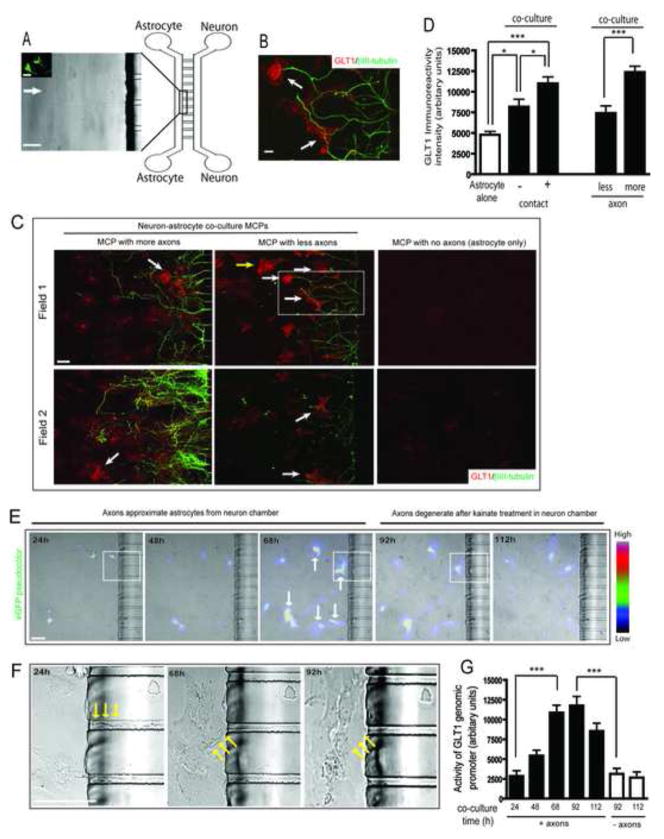
A microfluidic culture platform(Park et al., 2006) (MCP, Figure 1A) was used to develop a novel neuron and astrocyte co-culture system in which only axons penetrate and establish contact with astrocytes, thereby providing a system to study the role of neuronal processes on GLT1 activation at a single axon/astrocyte resolution. High-density neurons (1–3 ×106/ml) were first plated on the right side of the MCP. GDNF (10ng/ml) was added to the left side of the MCP to stimulate axon outgrowth 24h after seeding of neurons. Extensive neuronal processes grew through channels connecting both sides of MCP 3–5 days after adding GDNF (Figure 1A, 1B). A representative magnified image of axon and astrocyte is shown in Figure 1B. More than 90% of neuronal processes growing through the channels are axons, as confirmed by unique growth cone morphology, immunoreactivity for the growth cone marker 2G13 (Figure 1A), and for synapsin-I (Supplementary Figure 1A) and immunonegative for MAP2 (data not shown). The solution exchange between both sides of the MCP is minimal(Park et al., 2006), as no DiI and DiO double-labeled astrocytes were observed in each side of the MCP (Supplementary Figure 1B) nor was there leakage of 3H-glutamate between chambers (data not shown). To establish neuron astrocyte co-culture in the MCP, GDNF was removed when neuronal processes were still inside the connecting channel, and astrocytes were plated into the left chamber. As shown in Figure 1B and 1C, axon bundles, visualized by βIII-tubulin staining, passed through the channel, entering the astrocyte side of MCP. Intensity of GLT1 immunostaining (10990±791, arbitrary units) in astrocytes with the presence of axons was significantly increased 230% (Figure 1D) compared to that in astrocytes alone chamber (4781±385), indicating that axons are sufficient to induce GLT1 activation. Axonal activation of GLT1 expression is apparently axon number/length dependent, as the intensity of GLT1 staining (12350±732) in platforms with more axons (indicated by the total length, 7500–18889μm/mm2) is significantly higher than that (7409±872) in platforms with fewer axons (3700–7500μm/mm2) (Figure 1C, 1D). In this model paradigm, the vast majority of GLT1 activation was only seen with astrocytes contacted by axons (white arrows in Figure 1B, 1C). Highest GLT1 intensity was found in all astrocytes with axon contact. Only occasional astrocytes, with no obvious direct axon/terminal contacts, were also GLT1 activated (yellow arrow in Figure 1C). Subsequent treatment of cultured astrocytes with differential neuronal components, i.e., neuronal supernatant (NS), neuronal membrane (NM), or neuron conditional medium (NCM) were all sufficient to increase GLT1 expression, to a similar level (Supplementary Figure 1C, 1D). These results would suggest that presynaptic interactions with astroglia via direct contact might activate transcriptional pathways in astroglia.
Figure 1. Axon-dependent transcriptional activation of GLT1 promoter and GLT1 protein expression on a microfluidic culture platform (MCP).
A. Diagram of an astrocyte neuron co-culture system using a microfluidic culture platform (MCP). Growth cone is stained with 2G13 antibody. Scale bar, 20μm (insert scale bar, 10μm). B. Magnified view of the interaction between axons and astrocytes. Astrocytes were stained with GLT1 and axons were stained with βIII-tubulin antibody. Scale bar, 50μm. C. Axons are sufficient to induce GLT1 up-regulation in cultured astrocytes on MCP. Astrocytes were stained with GLT1 and axons were stained with βIII-tubulin antibody. White arrow: astrocyte with axon contact; yellow arrow; astrocyte without axon contact; Representative two fields were shown from each group. Scale bar, 50 μm. D. Quantitative analysis of axon-induced GLT1 expression (5–10 astrocytes per field, 2–3 field/MCP from total 5–7 MCPs). E. Time-lapse recording of astrocytic eGFP (from BAC GLT1 eGFP mice) fluorescence and outgrowth of axons within MCP. Confocal images were collected 24h to 112h after co-culture. eGFP fluorescence intensity in astrocyte using pseudocolor represention (Image J). Kainate (200 μM) was added to the right side of MCP after taking images at 68h to induce neural injury and axon degeneration. The exchange of solution between chambers is very minimal. Direct treatment of kainite (200 μM) to astrocyte did not change GLT1 expression and astrocyte viability. Scale bar, 50 μm. F. Magnified view of axon and astrocyte interaction in MCP. Examples of axons (yellow arrow) that extend through MCP channels (24h–68h) and axonal degeneration after kainite treatment (68h–92h). Scale bar, 50 μm. G. Quantitative analysis of axon-dependent change of eGFP fluorescence intensity from time-lapse images (5–8 astrocytes per field, 2–3 fields/MCP from total 3–5 MCPs (*P<0.05, *** P<0.001, mean ± SEM, one-way ANOVA and Bonferroni posthoc analysis).
To study molecular modulation of astroglial synaptic-relevant pathways, we utilized BAC GLT1 eGFP promoter reporter mice(Regan et al., 2007), which allow spatial and temporal in situ monitoring of single astrocyte GLT1 promoter activity by fluorescence reporter intensity. The expression of the reporter correlates with endogenous GLT1 promoter activation, protein expression and functional activity(Regan et al., 2007). To monitor dynamic changes of the GLT1 promoter to axon stimulation, astrocytes derived from this mouse were added on the left side of MCP after culturing neurons for 3–5d, prior to entry of axons into the astroglial chamber. Time-lapse images were collected from 24h to 112h after co-culture (Figure 1E). Intensity of eGFP fluorescence, the indicator of GLT1 genomic promoter activity, in single astrocytes, was detected and represented by pseudocolor (Figure 1E). In the absence of axons, no GLT1 appreciable gene activation was observed. Dramatic increase of eGFP intensity was observed (from 2846±682 to 10860±941) within 48 hrs after axons approach and/or make contact with the astroglia (24h–68hr, Figure 1G). The dependence of the axon-astrocyte interaction was validated by the decrease in astrocytic eGFP expression (68–112hr), following kainate induced (200μM; added to neuronal side) neural injury and subsequent axonal degeneration (Figure 1E, 1F). In contrast, eGFP intensity from MCP without application of kainite only decreased slightly from 68h to 112h (Figure 1G). Axon-dependent eGFP intensity changes, quantified from these time-lapse images, clearly indicate that axons induced transcriptional activation of GLT1. GLT1 mRNA levels were also increased after directly adding neurons to astrocyte cultures (Supplementary Figure 1E), providing additional evidence that synaptic interaction with astroglia induces transcription activation of GLT1.
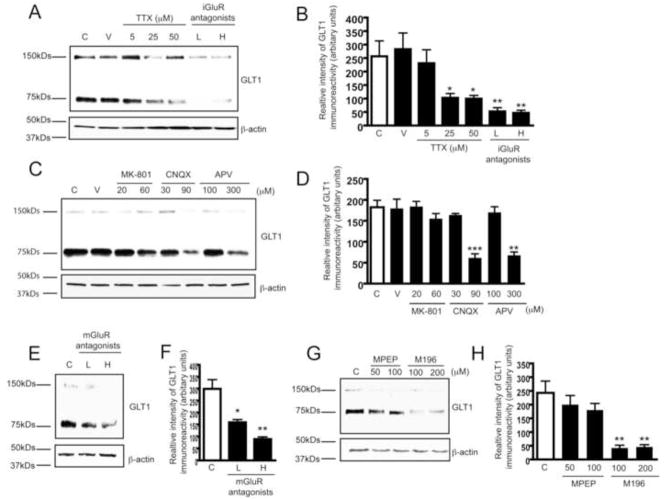
We further investigated whether glutamate receptor (GluR)-mediated neuron-astroglial signaling is involved in neuron-dependent GLT1 activation. P8 rat spinal cord organotypic slice cultures, a system faithfully mimicking the in vivo environment (Rothstein et al., 1993), were used to investigate altered neuron-astrocyte communication on GLT1 activation. As spinal cord slices are usually 350μm in thickness, relatively higher concentrations of pharmacological inhibitors were used in experiments. GLT1 expression levels in slice cultures were reduced in a dose-dependent manner following tetrodotoxin (TTX) treatment (Figure 2A,2B), suggesting that Na+ channel mediated synaptic transmission facilitate GLT1 activation in astrocytes. In addition, treatment of slice cultures with ionotropic or metabotropic GluR antagonist cocktails also resulted in a dose-dependent decrease of GLT1 expression (Figure 2A, 2B, 2E, 2F). Inhibition of astroglial iGluRs in astrocytes in MCP also leads to decrease of axon-induced GLT1 expression (Supplementary Figure 2A, 2B). Subsequent treatment of slices with individual glutamate receptor antagonists revealed that AMPA (antagonist CNQX) and mGluR 1/5 (antagonist m196) are involved in the neuron-dependent GLT1 activation (Figure 2C, 2D, 2G, 2H). NMDA antagonist MK801 had little effect, although high dose APV was inhibitory (Figure 2C, 2D). Overall, mGluR1/5 antagonists alone or in combination appeared to have the greatest effect on GLT1 expression in the slice culture paradigm (Figure 2E, 2F, 2G, 2H). GluRs are expressed on pre- and post-synaptic neurons as well as astrocytes, thus, inhibition of GluRs results in inhibition of synaptic transmission from neuron-to-neuron and neuron-to-astrocyte. The block of GLT1 activation by inhibition of neurotransmitter release (TTX) and by the GluR receptor antagonists suggests that synaptic activity is a strong component of pre-synaptic-based activation of astroglial GLT1.
Figure 2. Glutamate receptors are involved in neuron-dependent GLT1 expression.
A. Dose-dependent reduction of GLT1 expression levels induced by TTX (5–50μM) and iGluR antagonist cocktail treatment (7d) in rat organotypic spinal cord slice cultures. L: low dose of cocktail (20μM MK801 + 30μM CNQX + 100μM APV); H: high dose of cocktail (60μM MK801 + 90μM CNQX + 300μM APV). B. Densitometric analyses of GLT1 immunoreactivity from immunoblot following seven day TTX or iGluR antagonist cocktail treatment (n=3). C. Effect of individual iGluR antagonists on neuron-dependent GLT1 expression. Rat spinal cord cultures were treated with individual iGluR antagonist for 7d. D. Densitometric analyses of GLT1 immunoreactivity from immunoblot with individual iGluR antagonist (n=3). E. Dose-dependent reduction of GLT1 expression levels induced by mGluR antagonist cocktail treatment (7d) in rat spinal cord slice cultures. L: low dose of cocktail (100μM M196 + 50μM MPEP); H: high dose of cocktail (200μM M196 + 100μM MPEP). F. Densitometric analyses of GLT1 immunoreactivity from immunoblot with mGluR antagonists cocktail treatment (n=3). G. Effect of individual mGluR antagonists on neuron-dependent GLT1 expression. Rat spinal cord slice cultures were treated with individual mGluR antagonists for 7d. H. Densitometric analyses of GLT1 immunoreactivity from immunoblot with individual mGluR antagonists (n=3). All the immunoblots were quantified in Quantity One software and plotted in PRISM (***, P<0.001, **, P<0.01, *, P<0.05, error bars represent SEM, one-way ANOVA and Bonferroni test was used).
Previous in vitro preparations from mixed neuron-glial cultures had suggested that neuron conditioned medium can directly activate the expression of GLT1(Gegelashvili et al., 1997) (Schlag et al., 1998; Swanson et al., 1997), and that soluble factors may be secreted from neurons to stimulate the expression of astroglial glutamate transporter. Glutamate has long been speculated as one of the soluble factors that increase GLT1 expression, but direct exposure of high concentration of glutamate in vitro to astrocyte cultures fails to increase GLT1 expression, but does alter its cytoplasmic clustering (Nakagawa et al., 2008; Zhou and Sutherland, 2004). In fact, elevated levels of glutamate are closely associated with loss of EAAT2 in chronic neurological injuries including ALS, Huntington’s disease, and multiple sclerosis, suggesting that other released factors or altered membrane contact may play more important roles in mediating axon-induced GLT1 activation. For example, axon membrane contact can be a potent regulator of developmental astroglial Notch signaling(Eiraku et al., 2005). By using this novel isolated neuron astrocyte co-culture system, our results prove that astroglial activation is neuron dependant and that presynaptic interaction with astroglia including both secretion and direct membrane contact, transcriptionally activate astroglial GLT1.
Recruitment of kappa-B motif-binding phosphoprotein (KBBP) to the promoter is required for GLT1 transcriptional activation
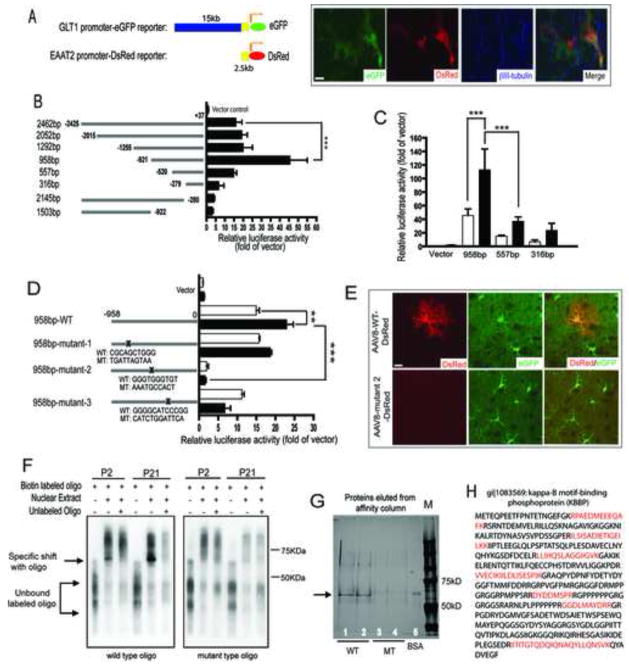
To evaluate how neurons regulate astroglial GLT1/EAAT2, we cloned a 2.5kb upstream promoter of human EAAT2 that shares high sequence homology (>70%) with GLT1 promoter from multiple species (Rothstein et al., 2005). To test whether this 2.5kb promoter behaves similarly as full-length (15kb) GLT1 genomic promoter in rodent astrocytes, a DsRed EAAT2 promoter reporter (2.5kb) (Figure 3A) was transfected into astrocyte cultures derived from BAC GLT1 eGFP transgenic mice (Regan et al., 2007). After transfection, newly prepared cortical neurons were plated on transfected astrocytes. As shown in Figure 3A, the presence of neurons markedly induced the expression of both DsRed and eGFP reporters in the same astrocyte, indicating that the 2.5kb promoter is functional in a rodent astrocyte environment and contains conserved cis-elements sufficient for neuron-derived activation signals.
Figure 3. Recruitment of Kappa B-motif binding phosphoprotein (KBBP) to GLT1 promoter is required for its activation.
A. A 2.5kb of human EAAT2 promoter is sufficient for neuronal stimulation in mouse astrocyte environment. The EAAT2 2.5kb promoter-DsRed reporter and GLT1 genomic promoter-eGFP reporter were both activated in astrocytes following co-culture with neurons identified by βIII-tubulin immunostaining. B. Identification of the regions essential for the basal activity of human EAAT2 promoter by serial deletion of human EAAT2 promoter (n=5–7). C. Promoter sequence from −958bp to −557bp is mainly responsible for neuron-dependent GLT1 promoter activation in primary astrocytes (n=4–8). □=astrocytes alone; ■=astrocytes and neurons. D. Mutagenesis of GGGTGGGTGT from −688bp to −679bp almost completely abolished basal and neuron-dependent GLT1 promoter activity in vitro (n=6–8). For luciferase assay, luciferase promoter reporters were first electroporated into cultured astrocytes and then freshly prepared neurons were plated on the top of transfected astrocytes. β-galactosidase was co-transfected for normalization. E. Mutagenesis of GGGTGGGTGT from −688bp to −679bp abolished GLT1 promoter activation (indicated by DsRed reporter expression) in astrocyte in vivo during early post-natal development. Astrocytes were identified by positive eGFP expression (20–30 astrocytes examined per serial section/mouse for total 4 mice each). F. Mutagenesis of cis-element GGGTGGGTGT abolished its specific binding to nuclear factors during GLT1 promoter activation in early post-natal development. G. Affinity purification by using wild type oligo GGGTGGGTGT but not the mutant oligo found unique nuclear protein from P21 mice cortex nuclear extracts. H. Trypsin digestion and LC/MS/MS identified Kappa B-motif binding phosphoprotein (KBBP) that specifically binds to GGGTGGGTGT. The red-colored amino acid sequences are small peptide sequences identified from LC/MS/MS that also match with KBBP sequence in NCBI.
To identify the major promoter regions responsible for neuron-dependent activation, luciferase promoter reporters were generated by serial deletion of the 2.5kb sequence and electroporated into P2 mouse primary astrocyte cultures. Sequence deletion from −921 to −279 (pGL958 vs. pGL316 in Figure 3B) sharply reduced both basal and neuron-dependent promoter activity (Figure 3B, 3C), with the most significant drop of activity from pGL958 to pGL557, indicating that the sequence between −921 to −520 contains primary elements for neuron-dependent GLT1 promoter activation. Multiple highly conserved transcription factor binding sites are present in the region from −921 to −520 by sequence analysis. Site-directed mutagenesis of pGL958 generated multiple mutant luciferase promoter reporters, which were examined in astrocyte cultures (Figure 3D). The 958bp-mutant 2 exhibited the greatest reduction in luciferase activity without altering co-transfected β-galactosidase activity, suggesting that the sequence mutated from −688 to −679 GGGTGGGTGT is essential for GLT1 promoter activity. Sequence alignment across ten species characterized GGGTGGGTGT as an evolutionally conserved site among mammals (supplementary Figure 3A). Notably, mutations of putative NF- B binding sites within this promoter region had no dramatic influence on neuron stimulated promoter activation (not shown).
The requirement of nucleotides −688 to −679 for GLT1 promoter activity was further tested in vivo. GLT1 mRNA transcription is strongly induced during early postnatal development in rodent brain (Schmitt et al., 1996; Sutherland et al., 1996), providing an in vivo model to test the GLT1 promoter activation. A DsRed reporter driven by the wild type 958bp or 958bp-mutant 2 EAAT2 promoters was delivered to brain of young mice using an adeno-associated virus (AAV) vector to examine in vivo reporter activation. AAV particles carrying the EAAT2 promoter-DsRed expression cassette were injected into the lateral cerebral ventricle of P0 pups of BAC GLT1 eGFP transgenic mice (Broekman et al., 2006). As shown in Figure 3E, confocal analysis of coronal brain sections of mice, 3 weeks after injection with 958bp-WT-DsRed containing AAV revealed expression of the DsRed reporter in astrocytes that also express eGFP reporter. However, no expression of DsRed was found from brain sections of mice injected with 958bp-mutant 2-DsRed containing AAV. Expression of the DsRed was also seen in nearby neurons for both AAV viruses (data not shown), suggesting that both viruses are effective in driving the DsRed reporter expression in vivo. The inability of 958bp-mutant 2 promoter to drive DsRed reporter expression in astrocytes in vivo confirmed the in vitro results that the sequence from −688 to −679, GGGTGGGTGT, is essential for neuron-dependant EAAT2 promoter activity.
The nuclear factors recruited to the GGGTGGGTGT sequence, were identified by performing a gel shift assay with nuclear extracts prepared from mouse cortex at developmental time points prior to GLT1 promoter activation (P2) and after strong promoter activation (P21). Wild type and mutant oligos (45mer) that only differ in the GGGTGGGTGT were synthesized and labeled with biotin. As shown in Figure 3F, increased specific binding with labeled WT oligo was found from P2 to P21 mice when the GLT1 promoter was strongly activated. The addition of extra-unlabeled WT oligo completely abolished the binding, suggesting that the binding is specific to this oligo. On the other hand, no specific binding with labeled mutant (MT) oligo was found, indicating the change of core sequence from GGGTGGGTGT to AAATGCCACT abolished the recruitment of specific nuclear proteins to this cis-element during GLT1 promoter activation. In addition, no other binding with labeled MT oligo was observed, suggesting that the replacement of GGGTGGGTGT with AAATGCCACT does not introduce non-specific binding with other nuclear proteins. This direct biochemical evidence of gel shift result, along with the genetic analysis in Figure 3D and 3E, demonstrates that the recruitment of nuclear factors to GGGTGGGTGT is essential for neuron-stimulated GLT1 promoter activity.
To identify the actual transcription factor binding to GLT1 regulatory site GGGTGGGTGT, nuclear extracts were affinity purified with the same oligos used in gel shift analysis. After purification, proteins bound to the WT or MT oligos were resolved on 4–12% PAGE gel and visualized by silver staining. A specific band with molecular weight between 50 to 75KDa was only found with labeled WT oligo but not with MT oligo (Figure 3G), confirming the results from gel shift analysis. Subsequent trypsin digestion and LC/MS/MS analysis of digested peptides (Figure 3H) revealed a nuclear protein, kappa-B motif-binding phosphoprotein (KBBP, gi|1083569), specifically binds to WT oligo.
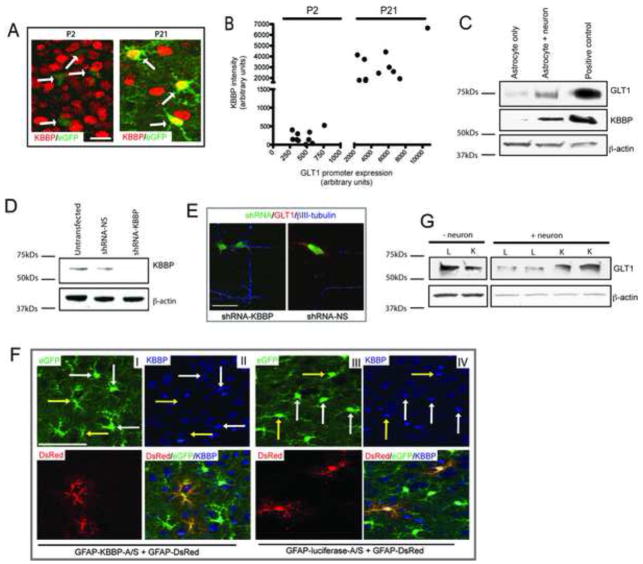
KBBP was first identified in a T lymphoma cell line that binds to kappa B enhancer element (GGGGACTTTCC)(Ostrowski et al., 1994). It is almost 100% homologous with human heterogeneous nuclear ribonucleoprotein K (hnRNP K) protein (Ostrowski et al., 1994). HnRNP K protein plays diverse roles in transcription, RNA splicing, and signal transduction through its interaction with many protein partners, including protein kinases, transcriptional factors and DNA/RNA(Bomsztyk et al., 2004). Notably, although the original kappa B enhancer element GGGGACTTTCC share partial homology with the KBBP binding sequence GGGTGGGTGT on GLT1 promoter, it is sufficient to compete out GGGTGGGTGT induced specific binding with KBBP in a dose dependent manner (supplementary Figure 3B). In addition, further mutation of GGG to AAA on KBBP binding sequence GGGTGGGTGT also abolished GLT1 promoter activity (supplementary Figure 3C), suggesting that these nucleotides are critical for KBBP binding and GLT1 promoter activity. As the mouse homolog of human hnRNP K, KBBP protein may also participate in transcription or RNA splicing. Expression of KBBP in astrocytes in vivo was first examined by using an antibody against human hnRNP K. Cortical immunoreactivity for KBBP in both P2 and P21 BAC GLT1 eGFP mice shows neuronal and astroglial immunolocalization. Notably, KBBP expression was barely detectable in P2 astrocytes and dramatically increased in astroglia from P2 to P21, which is identical to the very large increase in astroglial GLT1 promoter activity (indicated by eGFP reporter intensity) in the same astroglia (Figure 4A). The significant correlation between increased expression of astroglial KBBP and GLT1 promoter/protein (Figure 4B) during early postnatal development suggests that KBBP may play an important role in transcriptional regulation of GLT1. Interestingly, KBBP expression in cultured astrocytes was also induced by neurons, coupled to the increase of GLT1 (Figure 4C); the coupled increases of KBBP and GLT1 are consistent with the same changes in astrocytes observed in vivo.
Figure 4. KBBP is essential and sufficient for neuron-dependent transactivation of GLT1.
A. Expression of KBBP protein in astroglia in vivo. KBBP protein expression was minimal in P2 astroglia (BAC GLT1 eGFP reporter, white arrows) and was markedly induced in cortical astrocytes during early post-natal development, along with astroglial BAC-GLT1 eGFP expression (white arrows). Scale bar, 10μm B. Quantitative correlation of KBBP expression levels and GLT1 promoter activity in astroglia during early postnatal development. Positive linear relationship of KBBP expression levels and eGFP intensity was found. The KBBP immunoreactivity and eGFP fluorescence intensity was quantified in Image J (20 astrocytes examined per serial section/mouse for total 4 mice) C. KBBP expression in astrocytes is induced by the presence of neuron. Neurons and astrocytes were co-cultured for one week before sample collected. Positive control: mice brain lysate. D. Development of shRNA that specifically silences KBBP expression. shRNA against KBBP results in over 90% less of KBBP expression in 3T3 NIH cells. E. Silencing of KBBP expression by shRNA reduced GLT1 expression in astrocyte on neuron-astrocyte co-culture MCP. shRNA expressing astrocytes were identified by co-expressed eGFP from shRNA construct. GLT1 expression and neuronal processes were visualized by GLT1 or βIII-tubulin immunostaining, respectively. Scale bar, 20μm. F. AAV mediated expression of KBBP antisense in vivo reduced GLT1 promoter activity during early post-natal development. AAV8-GFAP-KBBP-A/S and AAV8-GFAP-DsRed particles were co-injected into cerebral lateral ventricle of P0 pups of BAC GLT1 eGFP transgenic mice. AAV8-GFAP-luciferase-A/S serves as antisense control for AAV8-GFAP-KBBP-A/S. Antisense expressing astrocytes were identified by DsRed reporter expression (20–30 astrocytes examined per serial section/mouse for total 4 mice each). Scale bar, 50μm (one-way ANOVA with Bonferroni posthoc analysis, **P<0.01, *** P<0.001; mean ± SEM) G. Overexpression of KBBP in astrocytes increases GLT1 expression with the presence of neurons. L: luciferase overexpression; K: KBBP overexpression. Neurons were plated on transfected astrocyte cultures 2d post transfection and kept for one week.
Small interference RNA (siRNA) specifically against KBBP was developed that effectively reduced KBBP expression level to 10% of that in control (Figure 4D) (Moumen et al., 2005), which results in significantly reduced GLT1 expression in astrocytes on neuron-astrocyte co-culture MCP (Figure 4E). We further tested whether a decrease of KBBP expression in astrocytes also reduces GLT1 promoter activity in vivo. To selectively silence astroglial KBBP, AAV particles that carried an expression cassette with the KBBP gene in the antisense orientation driven by the GFAP promoter, along with AAV particles that carried a DsRed reporter expression cassette driven by the same promoter, were intraventricularly injected into new born (P0) BAC GLT1 eGFP transgenic mice. AAV particles carrying luciferase in the antisense orientation served as a control. KBBP antisense was highly effective at silencing KBBP expression in cultured astrocytes (Supplementary Figure 4C). As shown in Figure 4F, KBBP antisense, but not luciferase antisense, effectively silenced KBBP expression in vivo. GLT1 promoter activity in KBBP antisense expressing astrocytes, identified by DsRed, was significantly reduced as indicated by reduced eGFP intensity (Figure 4F-I&II, shown by yellow arrow) compared to that in untransduced astrocytes (shown by white arrow) while GLT1 promoter activity in luciferase expressing astrocytes was unaltered (Figure 4F-III&IV, shown in yellow arrow). In aggregate, the loss-of-function analysis of KBBP by RNAi and antisense in astrocytes, along with the promoter mutagenesis analysis, indicate that recruitment of KBBP to the GLT1 promoter is required for GLT1 transcriptional activation in vivo.
We next determined if over-expression of KBBP could concomitantly alter GLT1 expression in astrocyte cultures. KBBP and luciferase overexpression constructs were prepared and electroporated into cultured astrocytes. As shown in Figure 4G, overexpression of KBBP, but not luciferase, was sufficient to increase GLT1 expression in the presence of neurons, though overexpression of KBBP itself was not sufficient to induce the GLT1 expression. In addition, direct treatment of cultured astrocytes with DHPG (50μM), the selective agonist for mGluR 1/5, increases both GLT1 and KBBP mRNA levels (supplementary Figure 4A, 4B), indicating that mGluR 1/5 may play more dominant role in neuron-depednent GLT1 up-regulation. Although direct treatment of physiological GluR agonist glutamate failed to increase the GLT1 expression, this could be due to the quick uptake of extracellular glutamate by GLAST in cultured astorcytes. These results are consistent with our observations that astroglial KBBP and GLT1 are expressed in parallel in vivo during early post-natal and synaptic maturation. This hints that the maturation of synapse might influence astroglial gene activation and expression of GLT1. Conversely, an alteration of this pathway due to the loss of pre-synaptic influence of neurons on astrocytes could be an important event linking neural injury to astroglial synaptic function.
In vivo neuron/axon denervation leads to GLT1 transcriptional dysregulation through reduction of KBBP expression
In order to evaluate the in vivo effect of altered synaptic coupling of neurons to astroglia, we investigated the alterations of astroglial GLT1 gene activation and regulation by KBBP in response to synaptic disruption by using several in vivo models including acute axon denervation induced by corticospinal tract lesion, ricin-induced acute motor neuron degeneration and the chronic neurodegeneration ALS mouse model employing SOD1 G93A mice.
Corticospinal Tract Transection
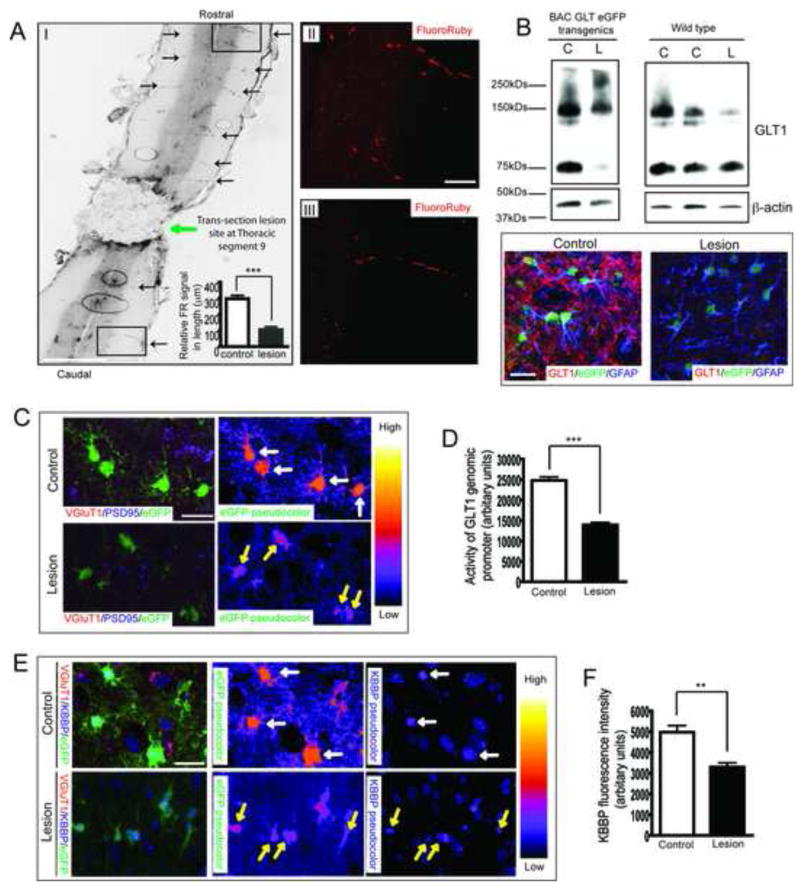
Corticospinal tracts originating from glutamatergic upper motor neurons possess excitatory presynaptic terminals on spinal cord interneurons and lower motor neurons that are modulated by perisynaptic astroglial GluTs. This pathway allows direct in vivo assessment of astroglial transporter expression in response to the loss of axonal signals induced by spinal cord transection. Thoracic cord (segment 9) transection was performed three weeks after mice received bilateral injection of Fluororuby (FR) into motor cortex to label corticospinal tracts by anterograde FR transport. One week after transection, thin horizontal sections of spinal cord were prepared and FR signals were examined to assess axon degeneration. Above the surgical lesion, abundant FR labeled axons, typical for corticospinal tract innervation, were found bilaterally in spinal cord white matter, projecting to grey matter neurons at each segment (Figure 5A–I). FR labeled neurons were also observed in grey matter (Figure 5A–II). In contrast, FR signals (including labeled axons and grey matter neurons) were reduced in the cord well below the lesion site in lumbar spinal cord (Figure 5A–III). Quantitative analysis revealed that more than 70% of FR labeled corticospinal axons in lumbar cord were depleted as early as 7 days following spinal transection (Figure 5A). GLT1 protein expression in lumbar cord of lesioned mice was markedly reduced compared to that of control mice (Figure 5B). A pronounced loss of GLT1 immunostaining was also observed in coronal sections of lesioned lumbar cords from BAC GLT1 eGFP mice. GLT1 promoter activity, as reflected by eGFP fluorescence intensity in coronal sections of lumbar cord of lesioned BAC GLT1 eGFP mice, was focally reduced (Figure 5C, 5D) in single astrocytes. These sharp astroglial changes were localized to regions with diminished synaptic terminals, as identified by the loss of pre-synaptic terminal marker vesicle glutamate transporter 1 (VGluT1), VGluT2 (supplementary Figure 5A) and preservation of post-synaptic marker PSD95.
Figure 5. Corticospinal tract presynaptic degeneration induced by spinal cord transection results in loss of GLT1 protein and promoter activity.
A. Thoracic transection induces massive degeneration of descending axons. Descending corticospinal tracts were shown by anterograde transport of FR from motor cortex. Abundant FR labelled axons/neurons (black arrow) were observed in white/grey matter of spinal cord above the lesion site (I, Scale bar, 0.5mm). Quantitative analysis of FR-labelled axon length showed that 70% of axons degenerated in lesioned mice compared to sham control mice (n=5). Representative images with FR labeled axons/neurons are shown in II&III, scale bar, 20μm. B. Thoracic spinal cord transection leads to loss of GLT1 protein in lumbar spinal cord by both immunoblotting (n=3) and immunostaining. Scale bar, 20μm. C. Single astrocyte analysis in vivo revealed loss of GLT1 promoter activity in astrocytes surrounding degenerated axons/pre-synaptic terminals of lesioned BAC GLT1 eGFP mice. Pre-synaptic complexes were identified by double staining for pre-synaptic VGluT1 and post-synaptic PSD95. Scale bar, 20μm. D. Quantitative analysis of the eGFP fluorescence intensity in lumbar cord astrocytes of lesioned mice (n≥93). E. Axon degeneration decreases KBBP immunostaining intensity in lumbar cord astrocytes of lesioned BAC GLT1 eGFP mice. Scale bar, 20μm. F. Quantitative analysis of the KBBP immunostaining intensity in lumbar cord astrocytes of lesioned mice (n≥45) (***P<0.001, mean ± SEM).
Overall, the loss of KBBP in individual astroglia (Figure 5E, yellow arrow) correlated with a decrease in GLT1 promoter-reporter eGFP intensity in same cells in lesioned lumbar cord (Figure 5E), along with loss of GLT1 protein (Figure 5B). The astroglial changes all followed the loss of presynaptic terminals. These in vivo studies demonstrate that presynaptic terminals act to maintain astroglial KBBP expression, which in turn activates GLT1 expression. Furthermore, these data provide a pathway by which abnormal axon/pre-synaptic signaling can alter astroglial synaptic integrity.
Neurotoxin Lesion
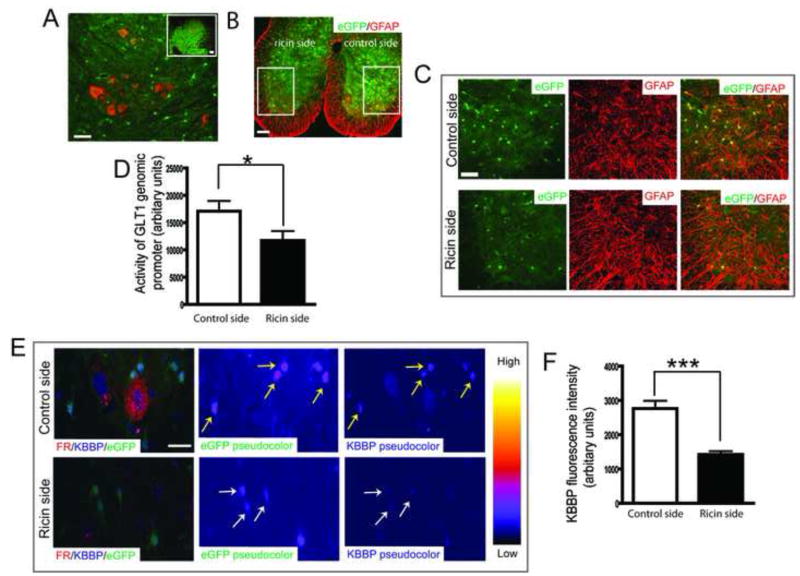
Neurodegeneration could alter the physiologic axon/astrocyte communication required for astroglial synaptic maintenance. The neurotoxin ricin, an extremely potent protein synthesis inhibitor, has been well established as a model for selectively lesioning discrete populations of motor neurons, through retrograde transport. In order to examine the response of individual astroglia in vivo, to the focal loss of spinal motor neurons, and their recurrent axon collaterals, we injected selected peripheral nerves with the toxin. Femoral nerves (originated from lumbar cord 2–4) of BAC GLT1 eGFP mice were dipped into fluororuby (FR) solution or mixture of ricin and FR solution unilaterally so that the change of GLT1 promoter activity in response to motor neuron death could be directly assessed at the same segment. As shown in Figure 6A, motor neurons were clearly labeled with FR in the control side of L2-L4 cord sections but not in the ricin injected side due to degeneration of motor neurons one week following injection (magnified view in Figure 6C). Mice received unilateral ricin injection also developed clear paralysis only in ricin side (Supplementary video), suggesting that retrograde ricin indeed induced dramatic motor neuron degeneration. The focal, selective loss of motor neurons and their recurrent collaterals was apparent on surrounding astroglia- eGFP fluorescence intensity was 30% reduced in ricin lesion side compared to that of control side (Figure 6B, 6C, 6D). This reduction of eGFP intensity was not due to astroglial death, as abundant GFAP immunoreactivity was still found in the astroglia with reduced eGFP on the lesioned side of the spinal cord (Figure 6C). Quantitative single astrocyte analysis of confocal images of thin section of L2-L4 cord from BAC GLT1 eGFP mice administrated with unilateral ricin also found 50% reduced KBBP immunoreactivity in astroglia with reduced eGFP intensity (Figure 6E, 6F). Ricin-induced motor neuron degeneration abolished its signaling to surrounding astroglia, likely mediated by both motor neuron dendrites and axon collaterals, which suggests that neuronal inputs are necessary to maintain KBBP expression levels and subsequent transactivation of the GLT1 promoter.
Figure 6. Motor neuron degeneration induced by neurotoxin ricin leads to reduced GLT1 promoter activity and KBBP expression.
A. Visualization of motor neurons in ventral horn of lumbar spinal cord by fluoro-ruby (FR). Femoral nerves were exposed and dipped into FR solution (10%) to allow retrograde transport of FR into motor neurons. Scale bar, 50μm (insert scale bar, 100μm). B. Unilateral administration of ricin induced reduction of eGFP intensity. C. Thin coronal sections of L2-L4 cord of BAC GLT1 eGFP mice was prepared and immunostained with GFAP antibody one week following unilateral administration of ricin. Reduced eGFP intensity but not the GFAP immunoreactivity was found in ricin side. Scale bar, 100μm (50μm for magnified image). D. Fluorescence intensity of eGFP in individual astroglial from ricin and control sides were measured in Image J and plotted using Prism (20–25 astrocytes per serial section/mouse for total 10 mice) (*, P<0.05, mean ± SEM, Student’s t-test). E. Single astrocyte analysis in vivo revealed reduction of KBBP immunoreactivity in astroglia with reduced eGFP intensity. Thin coronal sections of L2-L4 cord of BAC GLT1 eGFP mice was prepared and immunostained with KBBP antibody one week following unilateral administration of ricin. Motor neurons were identified by FR in control side. F. KBBP immunoreactivity in astroglia with reduced eGFP intensity was measured in Image J and plotted in Prism (20–25 astrocytes per serial section/mouse for total 10 mice). Scale bar, 20μm. Yellow arrow: astroglia in control side; White arrow: astroglia in ricin side. (Student’s t-test: ***, P<0.001, mean ± SEM).
G93A SOD1 ALS Mouse Neurodegeneration
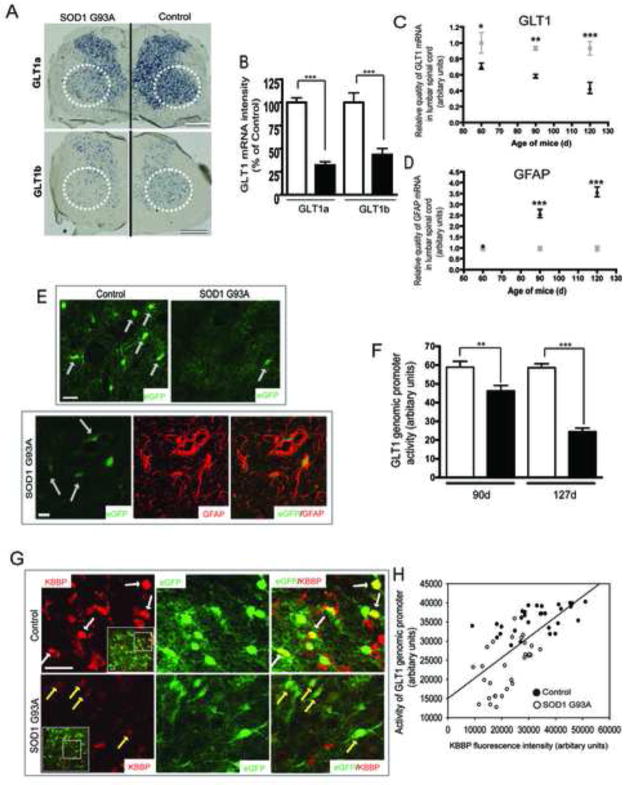
Severe loss of GLT1 protein has been observed in SOD1 G93A transgenic rodents (Howland et al., 2002), a model of familial ALS, and in the R6/2 mice model of Huntington’s disease (Lievens et al., 2001). The mechanism for this loss in these animal models remains unexplained. Total spinal cord EAAT2 mRNA levels were not found abnormal in prior ALS studies(Bristol and Rothstein, 1996). However, these past studies were technically limited due to post mortem tissue artifacts and the use of low-resolution, bulk tissue homogenization and non-in situ approaches-all which limit the ability to detect focal astroglial abnormalities known to occur in human and rodent ALS (Howland et al., 2002; Rothstein et al., 1994a). Similar to the acute corticospinal lesion experiments and ricin toxin lesion, the loss of GLT1 protein in rodent models of ALS is focal to the region of degenerating motor neurons and spreads along the lumbar spinal cord. To better detect focal GLT1 mRNA alterations in astroglia, in situ hybridization of GLT1a and GLT1b, the two major GLT1 transcripts was performed in lumbar spinal cord of SOD1 G93A transgenic rat (Figure 7A). GLT1a mRNA is far more abundant than GLT1b, which is consistent with previous studies(Berger et al., 2005). There was a pronounced focal loss of both GLT1a (70% loss compared to WT, Figure 7B) and GLT1b (50% loss compared to WT, Figure 7B) mRNA transcripts in the ventral horn of the SOD1 G93A mouse spinal cord, similar to reports of GLT1 protein(Howland et al., 2002). In addition, quantitative real time PCR analysis of GLT1 mRNA from lumbar spinal cord of SOD1 G93A mice at different stage of disease progression (60, 90, 120d) also showed that GLT1 mRNA was reduced to 65% of control at disease onset and further decreased to 40% of control as disease progressed to end stage (Figure 7C). In contrast, GFAP mRNA levels increased gradually as disease progressed to end-stage (Figure 7D). To better evaluate alterations of GLT1 regulation at the single cell level, the SOD1 G93A mice were mated to the GLT1 BAC eGFP reporter mice. The presence of the BAC-eGFP promoter reporter did not change the course of disease, with mice developing disease at approximately 90 days of age and reaching end stage at ~125 days of age (n=20). BAC GLT1 eGFPxSOD1 G93A mice were sacrificed at various time points and eGFP expression, as a reflection of GLT1 promoter activity(Regan et al., 2007), was examined and quantified by confocal microscopy of serial thin sections from lumbar cord (Figure 7E). A profound decrease of astroglial GLT1 promoter activity, indicated by a more than 60% loss of eGFP fluorescence intensity, was found in ventral gray matter of BAC GLT1 eGFPxSOD1 G93A mice, especially in ventral gray matter-localized astroglia near motor neurons from end stage (127d) animals (Figure 7F). The loss of GLT1 promoter activity/eGFP fluorescence was the result of transcriptional dysfunction in these spinal cord astroglia, rather than cell death as increased GFAP immunostaining signal was observed from astrocytes with decreased eGFP fluorescence (Figure 7E), as well as increased GFAP mRNA levels in lumbar spinal cord from end-stage SOD1 G93A mice (Figure 7D). Notably, there is a profound loss of pre-synaptic terminals in this transgenic model as a result of the loss of pre-synaptic corticospinal tract terminals, spinal interneurons and recurrent collaterals from motor neurons, as reflected by a decrease in synaptophysin expression (Supplementary Figure 4A)(Morrison et al., 1998).
Figure 7. GLT1 transcriptional dysfunction contributes to the loss of GLT1 protein in SOD1 G93A rodents.
A. In situ hybridization of major GLT1 transcripts in lumbar cord of end-stage (125d) SOD1 G93A rat. Scale bar, 0.5mm. B. Quantitative analysis of in situ signals of GLT1a and GLT1b (20–30 areas examined per serial section per rat, n=3 rats). C. QRT-PCR analysis of GLT1 mRNA levels in lumbar cord of SOD1 G93A mice. D. QRT-PCR analysis of GFAP mRNA levels in lumbar cord of SOD1 G93A mice. Total RNA from lumbar spinal cord of SOD1 G93A mice at different stages (60d, 90d, 120d) was prepared. The GLT1 and GFAP mRNA levels were determined by QRT-PCR with GLT1 or GFAP specific probe. The amount of RNA used in QRT-PCR was normalized by β-actin mRNA and 18s RNA. The GLT1 or GFAP mRNA levels of WT mice at 60d was used as calibrator for mRNA comparison (n=7–12) (2-way ANOVA with posthoc Bonferroni: *P<0.05, **P<0.01, ***P<0.001, mean±SEM). E. Loss of GLT1 promoter activity indicated by eGFP intensity in astrocytes of ventral lumbar cord of BAC GLT1 eGFP X SOD1 G93A mice. No apparent loss of astroglia was observed by GFAP immunostaining. F. Quantitative analysis of eGFP fluorescence intensity in astrocytes of BAC GLT1 eGFP X SOD1 G93A at different ages (35 astrocytes per serial section/mouse for total 20 mice). G. Decreased expression levels of KBBP in astrocytes with reduced eGFP fluorescence intensity in ventral lumbar cord of BAC GLT1 eGFP X SOD1 G93A mice. Scale bar, 20μm (insert scale bar, 50μm). H. Linear regression curve between KBBP immunoreactivity and eGFP intensity in astroglia. KBBP immunoreactivity and eGFP fluorescence intensity was measured in Image J and plotted in SigmaPlot. (n≥25) (Student’s t-test: ***, P<0.001, mean ± SEM).
To determine if there was a relationship between neural injury/synaptic loss and the alteration in regulation of astroglial synaptic control in this disease model, the expression level of astroglial KBBP in ventral lumbar cord of BAC GLT1 eGFPxSOD1 G93A mice (n=5) was also examined. As shown in Figure 7G, KBBP immunostaining and GLT1 promoter activity/eGFP reporter fluorescence was diminished in astrocytes from ventral lumbar cord of SOD1 G93A mice. The intensity of eGFP fluorescence and KBBP immunostaining in individual astrocytes (n≥25) were highly correlated (Figure 7H). Decreased KBBP expression levels (<20000, arbitrary units) were correlated with lower levels of eGFP fluorescence intensity (<25000, arbitrary units), which was also found in the corticospinal transection and ricin models described above. GLT1 transcriptional activation, KBBP immunoreactivity and eGFP expression levels were unaltered in dorsal horn astrocytes from the BAC GLT1 eGFPxSOD1 G93A mice (data not shown).
In summary, KBBP is a neuron-dependant downstream nuclear factor that regulates astroglial GLT1 transcription as determined by: 1) in vitro and in vivo genetic analysis of KBBP modulating GLT1 expression, 2) pharmacologic and synaptic activity regulated expression of GLT1 transcription, 3) correlation of developmental KBBP and GLT1 expression levels in astroglia, and 4) correlation of altered in vivo pre-synaptic inputs to astroglial KBBP and GLT1 promoter activation by employing models of synaptic denervation including corticospinal lesion, single neuron toxin lesion and an animal model of ALS. These studies provide a molecular mechanism for abnormal astroglia in neurodegeneration: alteration/loss of synaptic terminals decreases the astroglial expression of KBBP, which ultimately negatively regulates astroglial expression of the peri-synaptic GLT1.
DISCUSSION
In this study, we systematically dissected the mechanisms underlying neuronal regulation of astroglia, by examining the neurotransmitter transporter EAAT2/GLT1 in omplementary in vitro and in vivo models. Our results show that axonal interactions with astroglia, are sufficient and necessary for neuron-dependent astroglial GLT1 transcriptional activation. Physiologically, pre-synaptic-based activation of astroglial GLT1 transcription could serve as a neuron-glial coupled mechanism ultimately for the modulation of post-synaptic activation at excitatory synapses, by regulating the synthesis of GLT1 and its subsequent expression on astroglial membranes. The current study identified a critical downstream astroglial nuclear factor KBBP and demonstrated that the recruitment of KBBP to the GLT1 promoter is required for neuron-dependent GLT1 transcriptional activation. Although prior studies, of cultured fetal astrocytes and small promoter fragments, suggested that NF-κB could modulate this promoter (Sitcheran et al., 2005; Su et al., 2003), physiologically relevant neuron-stimulated promoter activation was not dependent upon that transcription factor in the current study. Normal developmental maturation of new synapses correlated with the activation of astroglial GLT1, via new KBBP expression. The loss of pre-synaptic signaling to astrocytes, as a result of acute axon transection or neuronal death in an acute or chronic neurodegeneration model, abolished signal transmission from neurons to astroglia leading to transcriptional dysregulation: decreased astroglial KBBP, subsequent diminished GLT1 promoter activation and loss of GLT1 protein expression.
Axon induced GLT1 activation is likely to be mediated by both presynaptic secretion and membrane contact. Previous in vitro preparations of simple mixed neuron-glial cultures had suggested that neuron secreted factors could activate astroglial differentiation and expression of GLT1 (or GLAST) (Gegelashvili et al., 1997; Munir et al., 2000; Schlag et al., 1998). The elements responsible for this neuron-glial interaction, e.g., dendrites, axons, synapses, receptor subtypes, are not known. In the current study, using the microfluidic culture platform, we were able to identify axon/pre-synaptic inputs as a distinct regulator of astroglial transporter activation, via KBBP. In parallel, we also identified comparable pre-synaptic inputs as a necessary component of KBBP activation of astroglial GLT1 in vivo. The mechanism of GLT1 regulation appears to involve both glutamate receptors as well as unspecified membrane-membrane interactions. For example, axon-glial membrane contact can be a potent regulator of developmental astroglial Notch signaling (Eiraku et al., 2005). In addition, neurons may also modulate the non-transcriptional processes in astrocyte-specific synaptic functions such as trafficking and membrane stabilization or clustering of GLT1 protein as suggested by in vitro studies (Nakagawa et al., 2008; Zhou and Sutherland, 2004).
Downstream signaling pathways between GluRs and KBBP recruitment to the GLT1 promoter are not yet understood. As KBBP can be phosphorylated by multiple upstream protein kinases(Bomsztyk et al., 2004), signals may be transmitted from GluRs to KBBP by protein kinase mediated phosphorylation. KBBP likely acts as a docking platform to aid in actions of other transcription factors with the GLT1 promoter. The interaction of KBBP with other astroglia-specific transcription factors remains to be characterized.
Expression of neurodegenerative disease proteins, including mutant SOD1, Huntington’s disease protein and spinocerebellar ataxia 7 protein have all implicated astroglial dysfunction in disease propagation(Lobsiger and Cleveland, 2007) with unclear mechanisms why astroglia becomes dysfunctional in diseases. Although multiple mechanisms likely contribute to motor- and inter-neuron death in ALS and its familial transgenic models, astroglial loss of GLT1/EAAT2 in both sporadic and familial ALS represents a common mechanism for disease propagation. Notably, a high percentage (≥50%) of motor/inter neuron degeneration has occurred at the time when symptoms start to develop (Martin et al., 2007), which is consistent with the loss of synaptophysin we observed. Synaptic stripping and loss of pre-synaptic inputs to neurons are described in animal models of neurodegeneration/neural injury (Blinzinger and Kreutzberg, 1968; Trapp et al., 2007) and in humans (Graeber et al., 1993). Our current studies suggest that disruption of the neuron-astroglial unit, specifically the loss of synaptic terminals/synaptic activity, alters normal astroglial function. It is likely that changes in synaptic terminals induce astroglial dysfunction- such as altered GLT1 expression and other synaptically relevant proteins which leads to additional neuronal injury and thereby acts as a feed forward mechanism to propagate neuronal disease/injury. A more complete understanding of the global transcriptional changes in astrocytes following neuronal injury may help identify other pathways by which astroglia contribute to normal and abnormal brain function.
Supplementary Material
Acknowledgments
Supported by NIH: NS33958 (JDR), NS36465 (MBR, JDR); the Robert Packard Center for ALS Research; Muscular Dystrophy Association (YY).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnth-Jensen N, Jabaudon D, Scanziani M. Cooperation between independent hippocampal synapses is controlled by glutamate uptake. Nat Neurosci. 2002;5:325–331. doi: 10.1038/nn825. [DOI] [PubMed] [Google Scholar]
- Berger UV, DeSilva TM, Chen W, Rosenberg PA. Cellular and subcellular mRNA localization of glutamate transporter isoforms GLT1a and GLT1b in rat brain by in situ hybridization. J Comp Neurol. 2005;492:78–89. doi: 10.1002/cne.20737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blinzinger K, Kreutzberg G. Displacement of synaptic terminals from regenerating motoneurons by microglial cells. Z Zellforsch Mikrosk Anat. 1968;85:145–157. doi: 10.1007/BF00325030. [DOI] [PubMed] [Google Scholar]
- Bomsztyk K, Denisenko O, Ostrowski J. hnRNP K: one protein multiple processes. Bioessays. 2004;26:629–638. doi: 10.1002/bies.20048. [DOI] [PubMed] [Google Scholar]
- Bristol LA, Rothstein JD. Glutamate transporter gene expression in amyotrophic lateral sclerosis motor cortex. Ann Neurol. 1996;39:676–679. doi: 10.1002/ana.410390519. [DOI] [PubMed] [Google Scholar]
- Broekman ML, Comer LA, Hyman BT, Sena-Esteves M. Adeno-associated virus vectors serotyped with AAV8 capsid are more efficient than AAV-1 or -2 serotypes for widespread gene delivery to the neonatal mouse brain. Neuroscience. 2006;138:501–510. doi: 10.1016/j.neuroscience.2005.11.057. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren CM, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Eiraku M, Tohgo A, Ono K, Kaneko M, Fujishima K, Hirano T, Kengaku M. DNER acts as a neuron-specific Notch ligand during Bergmann glial development. Nat Neurosci. 2005;8:873–880. doi: 10.1038/nn1492. [DOI] [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Danbolt NC, Schousboe A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem. 1997;69:2612–2615. doi: 10.1046/j.1471-4159.1997.69062612.x. [DOI] [PubMed] [Google Scholar]
- Genoud C, Quairiaux C, Steiner P, Hirling H, Welker E, Knott GW. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 2006;4:e343. doi: 10.1371/journal.pbio.0040343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB, Bise K, Mehraein P. Synaptic stripping in the human facial nucleus. Acta Neuropathol. 1993;86:179–181. doi: 10.1007/BF00334886. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med. 2007;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Howland DS, Liu J, She Y, Goad B, Maragakis NJ, Kim B, Erickson J, Kulik J, DeVito L, Psaltis G, et al. Focal loss of the glutamate transporter EAAT2 in a transgenic rat model of SOD1 mutant-mediated amyotrophic lateral sclerosis (ALS) Proc Natl Acad Sci U S A. 2002;99:1604–1609. doi: 10.1073/pnas.032539299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Lauriat TL, Richler E, McInnes LA. A quantitative regional expression profile of EAAT2 known and novel splice variants reopens the question of aberrant EAAT2 splicing in disease. Neurochem Int. 2007;50:271–280. doi: 10.1016/j.neuint.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Lievens JC, Woodman B, Mahal A, Spasic-Boscovic O, Samuel D, Kerkerian-Le Goff L, Bates GP. Impaired glutamate uptake in the R6 Huntington’s disease transgenic mice. Neurobiol Dis. 2001;8:807–821. doi: 10.1006/nbdi.2001.0430. [DOI] [PubMed] [Google Scholar]
- Lobsiger CS, Cleveland DW. Glial cells as intrinsic components of non-cell-autonomous neurodegenerative disease. Nat Neurosci. 2007;10:1355–1360. doi: 10.1038/nn1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, Golden WC. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007;500:20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- Morrison BM, Janssen WG, Gordon JW, Morrison JH. Time course of neuropathology in the spinal cord of G86R superoxide dismutase transgenic mice. J Comp Neurol. 1998;391:64–77. doi: 10.1002/(sici)1096-9861(19980202)391:1<64::aid-cne6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Moumen A, Masterson P, O’Connor MJ, Jackson SP. hnRNP K: an HDM2 target and transcriptional coactivator of p53 in response to DNA damage. Cell. 2005;123:1065–1078. doi: 10.1016/j.cell.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Munir M, Correale DM, Robinson MB. Substrate-induced up-regulation of Na(+)-dependent glutamate transport activity. Neurochem Int. 2000;37:147–162. doi: 10.1016/s0197-0186(00)00018-8. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Otsubo Y, Yatani Y, Shirakawa H, Kaneko S. Mechanisms of substrate transport-induced clustering of a glial glutamate transporter GLT-1 in astroglial-neuronal cultures. Eur J Neurosci. 2008;28:1719–1730. doi: 10.1111/j.1460-9568.2008.06494.x. [DOI] [PubMed] [Google Scholar]
- Ostrowski J, Van Seuningen I, Seger R, Rauch CT, Sleath PR, McMullen BA, Bomsztyk K. Purification, cloning, and expression of a murine phosphoprotein that binds the kappa B motif in vitro identifies it as the homolog of the human heterogeneous nuclear ribonucleoprotein K protein. Description of a novel DNA-dependent phosphorylation process. J Biol Chem. 1994;269:17626–17634. [PubMed] [Google Scholar]
- Park JW, Vahidi B, Taylor AM, Rhee SW, Jeon NL. Microfluidic culture platform for neuroscience research. Nat Protoc. 2006;1:2128–2136. doi: 10.1038/nprot.2006.316. [DOI] [PubMed] [Google Scholar]
- Regan MR, Huang YH, Kim YS, Dykes-Hoberg MI, Jin L, Watkins AM, Bergles DE, Rothstein JD. Variations in promoter activity reveal a differential expression and physiology of glutamate transporters by glia in the developing and mature CNS. J Neurosci. 2007;27:6607–6619. doi: 10.1523/JNEUROSCI.0790-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, Kanai Y, Hediger MA, Wang Y, Schielke JP, Welty DF. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Jin L, Dykes-Hoberg M, Kuncl RW. Chronic inhibition of glutamate uptake produces a model of slow neurotoxicity. Proc Natl Acad Sci U S A. 1993;90:6591–6595. doi: 10.1073/pnas.90.14.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Dykes-Hoberg M, Jin L, Levey A, Kuncl RW. Glutamate transporter subtypes: role in excitotoxicity and amyotrophic lateral sclerosis. Ann Neurol. 1994a;36:282. [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994b;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin LJ, Kuncl RW. Decreased glutamate transport by the brain and spinal cord in amyotrophic lateral sclerosis. N Engl J Med. 1992;326:1464–1468. doi: 10.1056/NEJM199205283262204. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, Robinson MB. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Asan E, Puschel B, Jons T, Kugler P. Expression of the glutamate transporter GLT1 in neural cells of the rat central nervous system: non-radioactive in situ hybridization and comparative immunocytochemistry. Neuroscience. 1996;71:989–1004. doi: 10.1016/0306-4522(95)00477-7. [DOI] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. Embo J. 2005;24:510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su ZZ, Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, Fisher PB. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proc Natl Acad Sci U S A. 2003;100:1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland ML, Delaney TA, Noebels JL. Glutamate transporter mRNA expression in proliferative zones of the developing and adult murine CNS. J Neurosci. 1996;16:2191–2207. doi: 10.1523/JNEUROSCI.16-07-02191.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Liu J, Miller JW, Rothstein JD, Farrell K, Stein BA, Longuemare MC. Neuronal regulation of glutamate transporter subtype expression in astrocytes. J Neurosci. 1997;17:932–940. doi: 10.1523/JNEUROSCI.17-03-00932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. Epilepsy and exacerbation of brain injury in mice lacking glutamate transporter GLT-1 (Vol 276, pg 1699, 1997) Science. 1997;278:21–21. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Trapp BD, Wujek JR, Criste GA, Jalabi W, Yin X, Kidd GJ, Stohlman S, Ransohoff R. Evidence for synaptic stripping by cortical microglia. Glia. 2007;55:360–368. doi: 10.1002/glia.20462. [DOI] [PubMed] [Google Scholar]
- Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, Grinspan JB, Rothstein JD, Robinson MB. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol. 2000;57:667–678. doi: 10.1124/mol.57.4.667. [DOI] [PubMed] [Google Scholar]
- Zhou J, Sutherland ML. Glutamate transporter cluster formation in astrocytic processes regulates glutamate uptake activity. J Neurosci. 2004;24:6301–6306. doi: 10.1523/JNEUROSCI.1404-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.