Abstract
Background
Because pneumococcal serotype 6C was previously not distinguished from serotype 6A, the impact of the 7-valent pneumococcal conjugate vaccine (PCV7) on the carriage of serotype 6C is unknown.
Methods
The nasopharyngeal (NP) prevalence of the 6C serotype was determined using 1326 pneumococcal isolates collected from 7 cohorts of Massachusetts children between 1994 and 2007. Initially, the isolates were serotyped using the quellung reaction; subsequently, stored specimens of all putative 6A isolates were tested for 6C using monoclonal antibodies. The opsonophagocytic and antibiotic susceptibilities of the isolates were determined.
Results
The prevalence of 6A was 9.6% <y>(33/343) before 2001, 8.0% (18/226) in 2004, and 2.9% (12/416) in 2007. In contrast, the prevalence of 6C was 0.6% (2/343) before 2001, 2.2% (5/226) in 2004, and 8.7% (36/416) in 2007 (P < .001 for 2/343 vs. 36/416). 6C isolates from 2007 were more susceptible to antibiotics than were 6A isolates. PCV7 induced a low ability to opsonize different isolates of 6C.
Conclusions
Among NP isolates, the prevalence of 6C isolates has increased and the prevalence of 6A isolates has decreased since the introduction of PCV7 in Massachusetts in 2000. The observed increase in serotype 6C prevalence may be explained by the induction by PCV7 of low amounts of functional anti-6C antibody, compared with anti-6A and anti-6B antibodies.
Streptococcus pneumoniae is a significant human pathogen responsible for several invasive pneumococcal disease (IPD) syndromes, such as sepsis, meningitis, and pneumonia, as well as more common infections, such as otitis media. Pneumococci are present in the nasopharynges of many healthy children without causing any clinical symptoms [1, 2]. Because pneumococci are only rarely found in animals [3], the human nasopharynx is their dominant natural reservoir and is thus the source of pneumococci in children as well as adults. Consequently, the nasopharyngeal (NP) carriage of pneumococci in humans is central to their transmission.
To reduce the burden of pneumococcal infections in children, a 7-valent pneumococcal conjugate vaccine (PCV7) was licensed for clinical use in the United States in February 2000 [4]. PCV7 has significantly reduced the incidence of IPDs in young children as well as in older adults [5, 6]. It has also significantly reduced the frequency of pneumococcal carriage of vaccine serotypes (i.e., the serotypes included in the vaccine) in young children [1, 7]. This reduction in carriage of vaccine serotypes is thought to be responsible for the indirect protection of older adults through herd immunity [8, 9]. Because the conjugate vaccine provides serotype-specific protection, the reduction in IPD, the decrease in carriage, and herd immunity were restricted to vaccine serotypes as well as to some serotypes that are cross-reactive with the vaccine serotypes. However, the prevalence of NP carriage of many nonvaccine serotypes has increased after the introduction of PCV7 [1, 9, 10].
A new pneumococcal serotype, 6C, has recently been identified using monoclonal antibodies [11, 12]. The repeating unit of the 6C polysaccharide (PS) is → 2) glucose (1 → 3) glucose (1 → 3) rhamnose (1 → 3) ribitol (5 → phosphate [12]. In contrast, the 6A PS repeating unit is → 2) galactose (1 → 3) glucose (1 → 3) rhamnose (1 → 3) ribitol (5 → phosphate, and the 6B PS repeating unit is → 2) galactose (1 → 3) glucose (1 → 3) rhamnose (1 → 4) ribitol (5 → phosphate. Genetic studies have found that the galactosyl transferase gene at the capsule gene locus of the 6A serotype is replaced with a glucosyl transferase gene in the 6C serotype [13].
Serologically, serotype 6C is very similar to serotype 6A but is distinct from serotype 6B. Consequently, the 6C serotype has previously been identified by the quellung reaction as the 6A serotype [11]. Postmarketing studies of IPD in children have identified a decline in invasive disease due to serotype 6A [6, 9], suggesting that PCV7, which contains 6B PS, may provide cross-protection against 6A. However, no epidemiologic reports have specifically investigated changes in the prevalence of NP carriage of serotype 6C. If vaccination with PCV7 fails to elicit functional antibody against serotype 6C, it may selectively increase the prevalence of 6C, which was relatively uncommon before the introduction of PCV7 [11, 13]. To investigate this possibility, we examined the prevalence of NP carriage of 6C among 7 cohorts of children in Massachusetts over a 13-year period.
METHODS
Pneumococcal isolates
NP isolates of pneumococci were obtained from 7 cohorts of children in Massachusetts, all of which were enrolled in several separate studies of pneumococcal carriage as described below. Groups 1 and 7 were enrolled at Boston Medical Center, an urban hospital; groups 2 and 3 were recruited from Fall River and New Bedford, Massachusetts; subjects in groups 4 and 5 lived in 1 of 16 Massachusetts communities outside of metropolitan Boston; and those in group 6 were from 8 of these 16 communities.
Group 1 included children < 7 years old recruited from primary or urgent care, hematology, or infectious diseases/HIV clinics at the Boston Medical Center between March 1994 and April 1996. The children were attending the clinics for either well-child visits or acute medical problems. Hematology clinic patients had hemoglobinopathies and were receiving penicillin prophylaxis. Infectious diseases/HIV clinic patients had HIV infection and were receiving trimethoprim-sulfamethoxazole prophylaxis. In total, S. pneumoniae was recovered from 224 (18%) of the 1261 NP cultures, which were performed for 823 pediatric patients. Only the first pneumococcal isolate from each child was included for this analysis.
Group 2 included 196 children < 3 years of age with acute otitis media, enrolled between October 1999 and May 2000. Four NP cultures were obtained from each child, on days 0, 7, 30, and 90 after antibiotic treatment. S. pneumoniae was recovered from 119 (15%) of the 784 NP cultures (4 cultures/child). Only the first pneumococcal isolate from each child was included for this analysis.
Group 3 included 275 healthy children < 2 years old, recruited between October 2000 and September 2003 during well-child visits to 3 pediatric practices in Fall River and New Bedford, Massachusetts [14]. Of the 1278 cultures, 89% were from well-child visits and 11% were from visits during which acute otitis media was diagnosed; 145 children (53%) were culture positive for S. pneumoniae on at least 1 occasion [14].
For group 4, NP specimens were obtained between March and May 2001 from 742 children < 7 years old at 1 of 31 office practices in 16 Massachusetts communities. Twenty-seven percent of the children presented with acute respiratory tract illness (ARTI). S. pneumoniae was recovered from 190 (26%) of the 742 NP cultures (1 culture/child) [15].
For group 5, specimens were obtained between November 2003 and April 2004 from 994 children < 7 years old at 1 of 23 office practices in 16 Massachusetts communities. Twenty-seven percent of the children presented with ARTI. S. pneumoniae was recovered from 232 (23%) of the 994 NP cultures (1 culture/child) [15].
Specimens for group 6 were obtained between October 2006 and May 2007 from 972 children, between 3 months and 7 years of age, in physician offices in 8 of the 16 Massachusetts communities. Subjects were seen for well-child care or acute medical problems. Forty-six percent of the children presented with ARTI. S. pneumoniae was recovered from 294 (30%) of the 972 NP cultures (1 culture/child).
For group 7, 453 children < 7 years old were enrolled between October 2006 and May 2007. They had all been seen at the pediatric primary care clinic in the Boston Medical Center for either well-child visits or acute medical problems. S. pneumoniae was recovered from 122 (27%) of the 453 NP cultures (1 culture/child).
For groups 1–5, NP samples were obtained using a swab (Calgiswab). The swabs were then immediately placed into serum-tryptone-glucose-glycerol medium for transportation [16]. For groups 6 and 7, the Culturette system with Amies transport medium (Difco Laboratories) was used. All of the samples were transported to the Maxwell Finland Laboratory for Infectious Diseases once daily, where the swabs were streaked onto blood agar plates impregnated with gentamicin (5 μg/mL) and incubated overnight at 37°C in a candle jar. α-Hemolytic colonies with the typical morphology of S. pneumoniae were selected and tested for their optochin sensitivity and bile solubility. The colonies that were optochin sensitive and bile soluble were defined as pneumococci. Each pneumococcal isolate was subcultured on blood agar and frozen at −70°C.
Serotyping
Pneumococcal isolates were initially serotyped at the Maxwell Finland Laboratory, using the quellung reaction and typing serum samples from the Staten Serum Institut. Only 1 serotype was identified from each culture. (Carriage of multiple serotypes was not specifically assessed, and only 1 colony was generally selected from each culture for serotyping.) When sequential cultures were collected from the same cohort, only pneumococcal isolates from the first positive culture were used for this study (table 1). All isolates serotyped as 6A by the quel-lung reaction were sent to the University of Alabama at Birmingham (UAB) for retyping. (All serotype 6C isolates were originally typed as 6A by the quellung reaction.) At UAB, the isolates were typed as 6A or 6C on the basis of their ability to bind to 2 different monoclonal antibodies (Hyp6AG1 and Hyp6AM3), as described elsewhere [12].
Table 1.
Nasopharyngeal carriage among children.
| No. of pneumococcal isolates |
||||||
|---|---|---|---|---|---|---|
| Expressing serotypesa |
||||||
| Group | Study period | Obtained | Serotyped | 6A | 6C | 6B |
| 1 | March 1994–April 1996 | 224 | 224 | 20 | 0 | NA |
| 2 | October 1999–May 2000 | 119 | 119 | 13 | 2 | 8 |
| 3 | October 2000–September 2003 | 145 | 145 | 43 | 2 | 32 |
| 4 | March 2001–May 2001 | 190 | 153 | 22b | 3b | 14 |
| 5 | November 2003–April 2004 | 232 | 226 | 18b | 5b | 5 |
| 6 | October 2006–May 2007 | 294 | 294 | 6b | 26b | 2 |
| 7 | October 2006–May 2007 | 122 | 122 | 6 | 10 | 0 |
NOTE. Only the result from the first culture obtained per child was included in the table. NA, not available.
Serotypes 6A and 6C were determined using monoclonal antibodies. Serotype 6B was determined by the quellung method.
For groups 4, 5, and 6, respectively, 27, 24, and 34 isolates were originally typed as 6A by the quellung method; only 25, 23, and 32, respectively, were available for monoclonal antibody analysis of 6A vs. 6C. The 5 unavailable isolates were not included as 6A or 6B in our calculation.
Opsonophagocytosis assay
An adult serum pool was prepared by pooling serum from 4 adults who were immunized with PPV23. Two pools of serum samples obtained from 12 toddlers before and after immunization with PCV7 were provided by Wyeth Vaccines. The opsonic capacities of the serum samples were determined using the killing-type opsonization assay, which is currently accepted as the reference method [17] and has been described elsewhere in detail [18]. Differentiated HL-60 cells (ATCC) were used as phagocytes, and baby rabbit serum (Pel-Freez) was used as the complement source. Various pneumococcal isolates (five 6A, two 6B, and eight 6C isolates) were used as target bacteria for the assay. The target isolates included those used in the past [13] as well as three 6A and five 6C Massachusetts isolates obtained during this study. The lowest testable dilution of serum for this assay is 1:8.
Antibiotic sensitivity
Antimicrobial susceptibility was determined using the Etest from AB Biodisk [14]. Pneumococcal suspensions with 0.5 McFarland units were prepared in tryptic soy broth, and 100 μL of the suspension was spread on a Mueller-Hinton agar plate with blood and the Etest strips. After overnight incubation, the clear zone was measured. The susceptibility cutoffs of the Clinical and Laboratory Standards Institute were used to classify organisms as susceptible, intermediately resistant, or resistant.
Statistical methods
Fisher’s exact test was used to compare relevant proportions between the earlier and the more recent cohorts. These included the proportion of isolates typed as 6A by the quellung reaction and subsequently typed as 6C, the proportion of all pneumococcal isolates identified as 6C, and the proportion of isolates of each serotype susceptible to commonly used antibiotics. The calculation was performed using the following Web site: http://www.quantitativeskills.com/sisa/statistics/fisher.htm.
RESULTS
Increased NP carriage of 6C and decreased NP carriage of 6A
Pneumococci were isolated from the NP cultures for the children enrolled in the studies described above between 1994 and 2007 (table 1). Groups 1 and 2 were from studies conducted before the licensure of PCV7, and groups 4 –7 were from studies after its introduction. Study groups yielded 119 –294 pneumo-coccal isolates, with most of the isolates serotyped using the classic quellung method (table 1). The ~10%–30% of the isolates that were identified as 6A by the quellung reaction were further tested using monoclonal antibodies to determine whether they were the 6A or 6C serotype.
The relative fractions of 6A and 6C isolates among those previously identified as 6A by the quellung reaction changed significantly after the introduction of PCV7 in Massachusetts. In the 2 studies performed before 2001, only 2 isolates (5.7%) among the 35 pneumococci originally identified as 6A were instead typed as serotype 6C by monoclonal antibody testing, with almost all (94%) being 6A. However, the 6C percentage among isolates originally identified as 6A increased to 22% (5/23) by 2004 (group 5) (P < .11 for group 5 [5/23] vs. groups 1 and 2 [2/35]) and became 75% (36/48) by 2007 (group 6 and 7) (P < .001 for groups 6 and 7 vs. groups 1 and 2). If only groups 4 (2001), 5 (2004), and 6 (2007) are compared (all representing isolates from communities outside of Boston), the proportion of putative 6A isolates that were in fact 6C increased from 12% to 22% and then to 81% between 2001 and 2007. If only children at Boston Medical Center are considered, the proportion of putative 6A isolates that were in fact 6C increased from 0% in 1994 –1996 to 63% in 2007.
During the 13 years over which the included studies were conducted, the prevalence of isolates identified as 6A by the quellung method remained relatively constant, accounting for 8.9%, 12.6%, 17.6%, 10.6%, 11.6%, and 13.1% for groups 1, 2, 4, 5, 6, and 7, respectively. However, when the monoclonal antibody method was used for typing, the prevalence of 6A among all pneumococcal isolates decreased, and the prevalence of 6C significantly increased. Before the introduction of PCV7, serotype 6A accounted for~9.6% of all pneumococcal isolates (33/343 in groups 1 and 2). The prevalence of 6A changed little in groups 3 and 4 (i.e., years 2000–2003), but its prevalence decreased to 8.0% (18/226 in group 5) by 2004 and further declined to 2.9% (12/416) by 2007 (P <.001 for 33/343 vs. 12/416). In contrast, the prevalence of 6C among the pneumococcal isolates was rare in the prevaccine period (0.6% [2/343]) but increased to 2.2% (5/226) in 2004 and 8.7% (36/416) in 2007 (P < .001 for 2/343 vs. 36/416).
Sensitivity of 6C isolates to antibiotics
Because changes in serotype prevalence can be due to selective pressure from antimicrobial use, we evaluated the 48 isolates obtained in 2007 and originally classified by the quellung reaction as 6A for their susceptibility to 8 antibiotics (table 2). The antibiotics included amoxicillin, penicillin G, ceftriaxone, clindamycin, erythromycin, levofloxacin, rifampin, and trimethoprim-sulfamethoxazole. As seen in table 2, 6C isolates were much more susceptible than 6A isolates to penicillin G (P < .001) and amoxicillin (P < .002); 6C and 6A isolates were comparable in their susceptibilities to other antibiotics.
Table 2.
Susceptibility of 2007 pneumococcal isolates to various antibiotics.
| No. of pneumococcal isolates |
|||||||
|---|---|---|---|---|---|---|---|
| Serotype 6A |
Serotype 6C |
||||||
| Antibiotic | S | I | R | S | I | R | Pa |
| Amoxicillin | 2 | 3 | 7 | 25 | 8 | 3 | .002 |
| Penicillin G | 0 | 9 | 3 | 28 | 0 | 8 | < .001 |
| Ceftriaxone | 10 | 2 | 0 | 36 | 0 | 0 | .059 |
| Clindamycin | 12 | 0 | 0 | 35 | 1 | 0 | < .99 |
| Erythromycin | 7 | 0 | 5 | 30 | 0 | 6 | .12 |
| Levofloxacin | 10 | 2 | 0 | 33 | 2 | 1 | .59 |
| Rifampin | 12 | 0 | 0 | 35 | 1 | 0 | < .99 |
| Trimethoprim-sulfamethoxazole | 8 | 0 | 4 | 28 | 5 | 3 | .47 |
NOTE. Cutoff values (mg/L) were as follows for sensitivity and resistance, respectively: amoxicillin, ≥ 0.5 and ≤ 2; penicillin, ≥ 0.06 and ≤2.0; ceftriaxone, ≥0.5 and ≤2.0; clindamycin, ≥0.5 and ≤4; erythromycin, ≥0.25 and ≤1; levofloxacin, ≥2 and ≤8; rifampin, ≥1 and ≤4; and trimethoprim-sulfamethoxazole, ≥0.5 and ≤4. I, intermediately resistant; R, resistant; S, susceptible.
Fisher’s exact test was used to compare the fractions of 6A and 6C isolates that were susceptible to each antibiotic.
Conjugate vaccine and opsonization of 6C strains
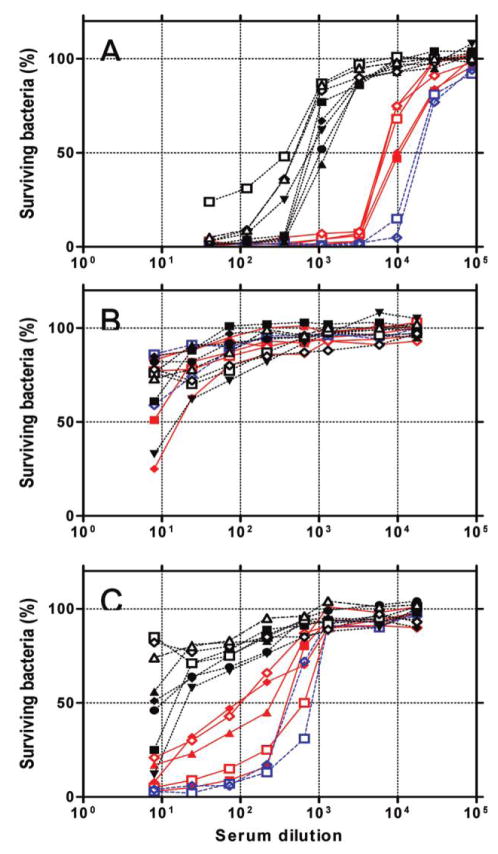
To investigate whether pneumococcal vaccines can provide immune protection against serotype 6C, we determined the ability of immune serum to opsonize pneumococci by means of serum pools obtained from toddlers and adults who were immunized with PCV7 or PPV23, respectively (figure 1). The adult serum pool readily killed 50% of target strains expressing serotypes 6B or 6A at dilutions of 1:20,000 or 1:7500, respectively (figure 1A). In contrast, the serum pool killed only 8 strains expressing serotype 6C at lower dilutions (1:400–1:1500) (figure 1A). Five 6C target strains ( black filled symbols in figure 1) were pneumococcal isolates obtained in Massachusetts during this study. This finding suggests that PPV23 would provide much less protection against serotype 6C than against serotypes 6A and 6B.
Figure 1.
No. of surviving bacteria after an opsonophagocytosis assay at various dilutions of serum pools from postimmune adults (A), preimmune children (B), and postimmune children (C). Target bacteria included serotypes 6A (solid red lines), 6B (dashed blue lines), and 6C (dotted black lines). Filled symbols indicate 6A and 6C strains obtained from Massachusetts during this study, and open symbols indicate strains used in another study [13]. The 6A strains used were LE4007 (red filled squares), FRV004H (red filled diamonds), 164S (red filled triangles), OREP6A (red open squares), and TriRep6A (red open diamonds) ; 6B strains were STREP6B (blue open squares) and SPEC6B (blue open diamonds) ; and 6C strains were CH2006 (black filled squares), ND6012 (black filled diamonds), 3041 (black filled triangles), FRV052H (black filled circles), 462–3–6 (black inverted triangles), CHPA037 (black open squares), KK177 (black open diamonds), and St745/04 (black open triangles).
When the opsonic capacities were examined with preimmune serum samples from toddlers, the serum pool did not kill 50% even at the lowest serum dilution (1:8) against all 6A, 6B, and 6C isolates, except for one 6A and one 6C target strain (figure 1B), which demonstrated a very low titer (< 1:20). When the post-PCV7 serum pool was examined (figure 1C), the pool killed 50% of 6B target bacteria at 1:500–1:1060 dilutions and 50% of 6A target bacteria at 1:100–1:660 dilutions. In contrast, the post-PCV7 pool did not kill even 50% of 5 target strains of serotype 6C even at the lowest dilution (1:8) and killed 50% of 3 6C target strains at a very low dilution (1:10–1:30). Thus, PCV7 failed to elicit opsonophagocytic activity against most 6C strains, suggesting that it provides little immune protection against 6C.
DISCUSSION
Several studies have shown that NP carriage of the 19A serotype has greatly increased (e.g., from ~3.5% in 2000 to 19% in 2004 [19]) since the introduction of PCV7 [15, 19]. Our study shows that the absolute prevalence of 6C among all pneumococcal isolates from the nasopharynx has also increased—from 0.6% before 2001 to 8.7% in 2007. Just as PCV7 provides little immunoprotection against the 19A serotype [20, 21], it also provides relatively little immune protection against 6C isolates compared with 6A isolates. However, serotype 19A isolates appear to have become antibiotic resistant before the introduction of PCV7 [22]. Consequently, the antibiotic resistance of 19A, not the poor immunoprotection of PCV7, may have been responsible for the increase of 19A isolates in the nasopharynx [23]. In the case of the 6C serotype, 6C isolates are generally more susceptible to antimicrobials than 6A isolates. Thus, the recent increase in the prevalence of 6C can be best explained by the serotype-selective immunoprotection caused by PCV7. Moreover, the susceptibility of 6C to antibiotics may have delayed its increase in prevalence compared with the 19A serotype.
NP colonization is the well-known initial event in the pathogenesis of IPD. Despite our findings showing a marked increase in the carriage of 6C serotype among children, the prevalence of IPD due to 6C among children appears to be low. The Centers for Disease Control and Prevention Active Bacterial Core surveillance (ABCs) studies [6, 24] have already demonstrated a major decline in IPD due to serotype 6A (as typed by the quellung reaction) in infants and toddlers [6, 8, 9, 25]. Perhaps the small degree of 6C-specific immunoprotection provided by PCV7 is sufficient to protect children against IPDs but is insufficient to reduce NP carriage. Alternatively, serotype 6C may be less virulent than serotype 6A in children. However, these proposals should be considered only as testable hypotheses, because the data on carriage are only from children in Massachusetts, whereas the data on IPD are from the nationwide ABCs network.
During the 13-year period of our study, NP carriage of sero-type 6C has replaced 6A carriage without much change in the overall prevalence of 6A isolates as identified by the quellung reaction. Because the pneumococci colonizing the nasopharynges of children are a well-known source of pathogens resulting in adult infections, the balanced replacement of NP 6A with 6C predicts no herd immunity against 6A isolates as identified by the quellung reaction. Indeed, PCV7 did not appear to provide herd immunity against serotype 6A disease (as identified by the quellung reaction) among older adults [8, 9]. Our study of the ABCs data set demonstrates an increased number of IPD cases caused by 6C in adults since 2000, which is approximately equal to the decreased number of 6A IPD cases in adults [25]. In future surveys, 6A and 6C serotypes must be distinguished.
Several limitations of our study should be taken into consideration. First, it is a retrospective analysis of 7 previously conducted studies. The children in the various study groups differ in several features that may alter their risk for carriage of S. pneumoniae. In addition, the study groups were composed of both asymptomatic children and those with ARTI in different proportions, with ARTI known to be related to the risk of colonization. Some of the cohorts included children through age 7 years, but others (groups 2 and 3) were limited to children < 3 years old. Although each of these variables may alter the risk for pneumococcal carriage, we are unaware that any of these demographic features would select for specific serotypes. Second, our study was performed in a single geographic region, which may not be representative of the country for a number of reasons, including the high penetration of PCV7 immunization in Massachusetts. These limitations should be transparent when our data are considered. Additional confirmation of these observations is needed to prove that the prevalence of serotype 6C has increased and that selective pressure from PCV7 is an important cause of these changes.
The prevalence of serotype 6C appears to vary among regions [11, 26], and it is possible that this variation may affect the findings of studies of cross-protection afforded by pneumococcal vaccines. Indeed, there are contrasting reports on cross-protection by pneumococcal conjugate vaccines against 6A isolates as identified by the quellung reaction [27, 28]. Furthermore, a serogroup may harbor new, as-yet unrecognized serotypes with different cross-reactivities and uneven geographic distributions. For instance, there is evidence for another new serotype within serogroup 11 [29]. Thus, when a pneumococcal vaccine is studied for its ability to induce protection against cross-reactive serotypes, it is necessary to consider its impact not only on the cross-reactive serotypes that are already known but also on those that have not yet been identified but that may nonetheless exist in the study population. Future surveillance of pneumococcal vaccines may reveal additional sero-types that were previously unrecognized.
Acknowledgments
We thank Dr. Phil Fernsten at Wyeth Vaccines for providing us with a pool of serum samples from children before and after vaccination with Prevnar.
Footnotes
Potential conflicts of interest: M.H.N. and J.L. are employed by the University of Alabama at Birmingham, which has applied for a patent covering the discovery of the 6C serotype. All other authors report no potential conflicts.
Financial support: National Institutes of Health (grants R01 AI-31473 to M.H.N. and R01 AI-66304 – 01A1 to J.A.F.); Wyeth Vaccines (investigator-initiated grant to S.I.P.).
References
- 1.Pelton SI, Huot H, Finkelstein JA, et al. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468 –72. doi: 10.1097/INF.0b013e31803df9ca. [DOI] [PubMed] [Google Scholar]
- 2.Hill PC, Cheung YB, Akisanya A, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian infants: a longitudinal study. Clin Infect Dis. 2008;46:807–14. doi: 10.1086/528688. [DOI] [PubMed] [Google Scholar]
- 3.Whatmore AM, King SJ, Doherty NC, Sturgeon D, Chanter N, Dowson CG. Molecular characterization of equine isolates of Streptococcus pneumoniae: natural disruption of genes encoding the virulence factors pneumolysin and autolysin. Infect Immun. 1999;67:2776 – 82. doi: 10.1128/iai.67.6.2776-2782.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Policy statement: recommendations for the prevention of pneumococcal infections, including the use of pneumococcal conjugate vaccine (Prevnar), pneumococcal polysaccharide vaccine, and antibiotic prophylaxis. Pediatrics. 2000;106:362– 6. doi: 10.1542/peds.106.2.362. [DOI] [PubMed] [Google Scholar]
- 5.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–95. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction: eight states, 1998 –2005. MMWR Morb Mortal Wkly Rep. 2008;57:144 – 8. [PubMed] [Google Scholar]
- 7.Dagan R, Melamed R, Muallem M, et al. Reduction of nasopharyngeal carriage of pneumococci during the second year of life by a heptavalent conjugate pneumococcal vaccine. J Infect Dis. 1996;174:1271– 8. doi: 10.1093/infdis/174.6.1271. [DOI] [PubMed] [Google Scholar]
- 8.Haber M, Barskey A, Baughman W, et al. Herd immunity and pneumococcal conjugate vaccine: a quantitative model. Vaccine. 2007;25:5390–8. doi: 10.1016/j.vaccine.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 9.Hicks LA, Harrison LH, Flannery B, et al. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998 –2004. J Infect Dis. 2007;196:1346 –54. doi: 10.1086/521626. [DOI] [PubMed] [Google Scholar]
- 10.Singleton RJ, Hennessy TW, Bulkow LR, et al. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska Native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297:1784 –92. doi: 10.1001/jama.297.16.1784. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Kaltoft MS, Brandao AP, et al. Validation of a multiplex pneumococcal serotyping assay with clinical samples. J Clin Microbiol. 2006;44:383– 8. doi: 10.1128/JCM.44.2.383-388.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45:1225–33. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park IH, Park S, Hollingshead SK, Nahm MH. Genetic basis for the new pneumococcal serotype, 6C. Infect Immun. 2007;75:4482–9. doi: 10.1128/IAI.00510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelton SI, Loughlin AM, Marchant CD. Seven valent pneumococcal conjugate vaccine immunization in two Boston communities: changes in serotypes and antimicrobial susceptibility among Streptococcus pneumoniae isolates. Pediatr Infect Dis J. 2004;23:1015–22. doi: 10.1097/01.inf.0000143645.58215.f0. [DOI] [PubMed] [Google Scholar]
- 15.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005;116:e408–13. doi: 10.1542/peds.2004-2338. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien KL, Nohynek H. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J. 2003;22:e1–11. doi: 10.1097/01.inf.0000049347.42983.77. [DOI] [PubMed] [Google Scholar]
- 17.Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. Use of opsonophagocytosis for the serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol. 2006;13:165–9. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton RL, Nahm MH. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol. 2006;13:1004 –9. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SY, Moore MR, Bruden DL, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr Infect Dis J. 2008;27:335–40. doi: 10.1097/INF.0b013e318161434d. [DOI] [PubMed] [Google Scholar]
- 20.Kim KH, Lee H, Burton R, Nahm M. Immune responses to vaccine-related serotypes, 6A, 6C, and 19A in infants immunized with 7-valent pneumococcal conjugate vaccine [abstract P3-057]. Program and abstracts of the 6th International Symposium on Pneumococci and Pneumococcal Diseases; Reykjavik, Iceland. 2008. [Google Scholar]
- 21.Yu X, Gray B, Chang SJ, Ward JI, Edwards KM, Nahm MH. Immunity to cross-reactive serotypes induced by pneumococcal conjugate vaccines in infants. J Infect Dis. 1999;180:1569 –76. doi: 10.1086/315096. [DOI] [PubMed] [Google Scholar]
- 22.Choi EH, Kim SH, Eun BW, et al. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis. 2008;14:275– 81. doi: 10.3201/eid1402.070807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore MR, Gertz RE, Jr, Woodbury RL, et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197:1016 –27. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 24.Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006;354:1455– 63. doi: 10.1056/NEJMoa051642. [DOI] [PubMed] [Google Scholar]
- 25.Park IH, Moore MR, Treanor JJ, et al. Differential effects of pneumococcal vaccine against serotypes 6A and 6C. J Infect Dis. 2008;198:1818–22. doi: 10.1086/593339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hermans PW, Blommaart M, Park IH, Nahm MH, Bogaert D. Low prevalence of recently discovered pneumococcal serotype 6C isolates among healthy Dutch children in the pre-vaccination era. Vaccine. 2008;26:449 –50. doi: 10.1016/j.vaccine.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 27.Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180:1171– 6. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 28.Dagan R, Givon-Lavi N, Zamir O, et al. Reduction of nasopharyngeal carriage of Streptococcus pneumoniae after administration of a 9-valent pneumococcal conjugate vaccine to toddlers attending day care centers. J Infect Dis. 2002;185:927–36. doi: 10.1086/339525. [DOI] [PubMed] [Google Scholar]
- 29.Yu J, Carvalho Mda G, Beall B, Nahm MH. A rapid pneumococcal serotyping system based on monoclonal antibodies and PCR. J Med Microbiol. 2008;57:171– 8. doi: 10.1099/jmm.0.47549-0. [DOI] [PubMed] [Google Scholar]