Abstract
Background
Systemic exposure to amphetamine (AMPH) leads to a number of long-lasting neuroadaptations including changes in dendritic morphology in rat forebrain. It remains unknown whether these changes relate to associative drug conditioning or to non-associative drug sensitization, two forms of plasticity produced by systemic exposure to AMPH.
Methods
We compared the behavioral, neuronal and morphological consequences of exposing rats to intraperitoneal (IP) AMPH to those of exposure to AMPH applied to the ventral tegmental area (VTA), infusions that sensitize AMPH-induced locomotion and nucleus accumbens (NAcc) DA overflow but do not produce drug conditioning.
Results
Both IP and VTA AMPH exposure sensitized locomotion and NAcc DA overflow but only IP AMPH exposure produced conditioned locomotion. Importantly, while IP AMPH exposure increased spine density, dendritic length and branching in the NAcc, exposure to VTA AMPH produced the opposite effects. A similar differentiation of effects was observed in cortical areas.
Conclusions
Together these findings suggest that the morphological changes seen following IP AMPH exposure reflect associative drug conditioning rather than non-associative drug sensitization. The decreases observed in the NAcc of VTA AMPH exposed rats may reflect the inability of these infusions to support conditioning.
Keywords: Conditioning, Dopamine Release, Dendritic Spines, Locomotion, Nucleus Accumbens, Sensitization
Introduction
Exposure to amphetamine (AMPH) leads to long-lasting sensitization of its psychomotor stimulant effects so that re-exposure to the drug weeks to months later produces enhanced locomotor responding as well as enhanced work output directed at obtaining and self-administering the drug (1, 2). These findings support the proposal that sensitization of the appetitive effects of AMPH and other psychostimulants promotes the pursuit and self-administration of these drugs and may underlie the transition from casual drug use to compulsive drug taking and abuse (2, 3).
A number of long-lasting neuroadaptations have been identified that provide neural correlates for the expression of these sensitized behaviors. A drug challenge administered weeks to months after psychostimulant exposure induces enhanced dopamine (DA) and glutamate overflow in the nucleus accumbens (NAcc) (2,4,5). Notably, terminals releasing these transmitters form synaptic contacts on the same dendritic spines of medium spiny neurons in the NAcc (6) and these spines have been reported to undergo long-lasting increases in density, an effect accompanied by increases in dendritic branching and length (7-9).
Although a number of findings support a critical role for the enhanced release of DA and glutamate in the NAcc in the expression of behavioral sensitization by psychostimulants (1,2,4,5,10), the contribution to sensitization of changes in dendritic morphology remains unclear and it was recently brought into question (11). Interestingly, structural changes have long been proposed to underlie learning and memory (12), and changes in spine morphology and density in particular have been linked to the formation of learned associations (13,14). The induction of drug-induced sensitization necessarily involves exposure to the drug in association with a complex of environmental stimuli, conditions that favor the formation of associations between the drug and these stimuli when the drug is administered systemically (15). Thus, it is possible that the increases in spine density, dendritic length and branching observed in the NAcc following systemic exposure to psychostimulants may reflect associative drug conditioning rather than non-associative drug sensitization (15). Indeed, we show here that exposure to amphetamine in the ventral tegmental area (VTA), a procedure that sensitizes drug-induced locomotion and NAcc DA overflow but does not produce drug conditioning (16-18), fails to increase spine density, dendritic length or branching in the NAcc.
Methods and Materials
Subjects
Male Sprague-Dawley rats (Harlan Sprague-Dawley, Madison, WI) weighing 250-300g on arrival were individually housed with food and water freely available in a reverse cycle room (12-h light/12-h dark). Animals were allowed to acclimate to these conditions for 3-4 days before the start of any procedures. All testing was conducted during the dark period of the light cycle. Female Sprague-Dawley rats were also used in some of the dendritic morphology experiments as the same effects have been reported in males and females (7,8).
In all experiments, rats were tested following exposure to AMPH or saline (SAL) administered either intraperitoneally (IP) or into the VTA using AMPH regimens known to produce locomotor and DA sensitization. Thus approximately half of all rats were surgically prepared with chronic bilateral guide cannulae aimed at the VTA (Supplement 1). Rats in different groups were subsequently tested in different experiments to assess the ability of exposure to IP or VTA AMPH to produce locomotor sensitization, sensitization of in vitro DA release, conditioned locomotion, and changes in dendritic morphology in forebrain sites.
Locomotor Sensitization
Rats in 4 groups were exposed to AMPH or SAL either IP or into the VTA. For systemic AMPH (3.0mg/kg, IP; n=6) or SAL (1.0ml/kg, IP; n=6), 5 injections were administered. For VTA AMPH (2.5μg/0.5μl/side; n=7) or SAL (0.5μl/side; n=6), 3 bilateral microinjections were made. In both cases, injections were made every third day. Testing was conducted 2-3 weeks following the last exposure injection. On the test, rats were placed in locomotor chambers (Supplement 2) and allowed to habituate for 1 hour. All rats were then administered AMPH (1.0mg/kg, IP) and their locomotor response measured for 2 hours. In all cases, AMPH (S(+)-amphetamine sulfate; Sigma Inc., St. Louis, MO) was dissolved in sterile saline. All doses refer to the weight of the salt.
Sensitization of In Vitro DA Release
Rats in 4 groups were exposed to AMPH or SAL either IP or into the VTA using the exposure regimens described for locomotor sensitization. Testing was conducted 2-3 weeks following the last exposure injection. On the test day, rats were sacrificed by decapitation, their brains quickly removed, and a 1-mm thick coronal section containing the NAcc and striatum obtained using an ice-cold brain matrix. The NAcc and striatum was dissected from each side (Figure 1G) and sections prepared for in vitro release (Supplement 3).
Figure 1.

VTA and IP AMPH similarly enhance AMPH-evoked locomotion and DA release from forebrain slice. Previous exposure to VTA AMPH enhanced AMPH-evoked locomotion (A) and DA release from NAcc (B) but not striatal (C) slice. Previous exposure to IP AMPH enhanced AMPH-induced locomotion (D) and DA release in both sites (E-F). Responding to AMPH was tested 2-3 weeks following exposure. Data are shown as mean (+SEM) 2-hr total locomotor counts and % of total DA released by AMPH. G, illustration of the NAcc and striatal regions dissected bilaterally in the release experiments. Line drawing is from (39) and depicts the caudal surface of a coronal hemi-section extending 1.2-2.2 mm from bregma. Numbers at the base of each bar indicate rats/group. **, p<0.01, ***, p<0.001, significantly greater than the SAL exposure condition.
Conditioned Locomotion
For each of the IP and VTA injection routes, rats in 3 groups were administered AMPH (1.0mg/kg, IP or 2.5μg/0.5μl/side in the VTA) or SAL. Rats in one group (Paired) received AMPH in the locomotor activity boxes and, the following day, SAL in their home cage. Rats in a second group (Unpaired) received SAL in the activity boxes and AMPH in their home cage. Rats in the third group (Control) received saline in both environments. All animals were left undisturbed on the third day. This procedure was repeated 4 times for VTA and 5 times for IP exposure. Testing for conditioned locomotion was conducted 2-3 weeks later. On this test, all rats were administered an IP saline injection and then immediately placed in an activity box where their locomotor response was measured for 1 hour. For VTA exposure, n/group was Paired, 7, Unpaired, 8, and Control, 4. For IP exposure, n/group was 8.
Dendritic Morphology
Rats in 4 groups were exposed to AMPH or SAL either IP or into the VTA. For systemic AMPH (1.0mg/kg, IP; n=9) or SAL (n=8), 7 injections were administered, 1 injection daily. For VTA AMPH (2.5μg/0.5μl/side; n=6) or SAL (0.5μl/side; n=5), 3 bilateral microinjections were made, one injection every third day. Rat brains were prepared for Golgi-Cox staining 2-3 weeks following the last exposure injection (Supplement 4). Morphological analyses focused on medium spiny neurons in the NAcc. For comparison, neurons in the striatum and three cortical regions were also analyzed: layer 5 pyramidal neurons in the prefrontal cortex (Cg3), layer 3 pyramidal neurons of the parietal cortex (Par1), and cells in the agranular insular dorsal aspect of the orbital cortex (AID). Dendritic morphology is altered in each of these regions by systemic AMPH exposure (7-9,21-23). Cells were selected and drawn using a camera lucida by an individual blind to treatment conditions. Three measures of dendritic morphology were obtained using methods described previously (7,9,24): spine density, dendritic length and branches (Supplement 4).
Histology
After completion of the experiments, rats with chronically implanted guide cannulae were deeply anesthetized and their brains processed for staining and verification of cannula tip placements. Only rats with injection cannula tips located bilaterally in the VTA were listed above and included in the data analyses (Supplement 5).
Data Analyses
Total test values obtained on the tests for locomotor sensitization and in vitro DA release were analyzed by 1-tailed t-tests for independent samples. The conditioned locomotion data were analyzed with 1-between 1-within ANOVA with groups as the between factor and time as the within factor. Post-hoc Scheffé comparisons were made according to Kirk (25). Dendritic morphology results were analyzed using 1-way ANOVA (26).
Results
Locomotor Sensitization
As expected, compared to SAL exposed controls, previous exposure to either VTA [t(11)=3.75; p<0.01] or IP [t(10)=4.52; p<.001] AMPH significantly enhanced locomotor responding to a systemic AMPH challenge administered 2-3 weeks later (Figure 1A and D).
Sensitization of In Vitro DA Release
In a manner paralleling the locomotor sensitization findings, compared to SAL exposed controls, AMPH-evoked DA release in the NAcc was significantly increased 2-3 weeks following exposure to either VTA [t(13)=3.26, p<0.01] or IP [t(16)=3.42, p<0.01] AMPH (Figure 1B and E). AMPH-evoked DA release was also significantly increased in the striatum following exposure to IP [t(17)=3.59, p<0.01] but not VTA AMPH [t(12)=0.89, ns] (Figure 1C and F). The lack of effect in striatum following VTA AMPH exposure is consistent with the fact that this forebrain region is not an A10 DA neuron projection field and supports the integrity and anatomical specificity of the VTA microinjections made.
Together with the locomotor sensitization results, these findings confirm and extend earlier reports showing that exposure to AMPH enhances its ability to produce locomotion and to release DA from DA terminals in forebrain (18,27,28). They also show that these effects are produced by either the IP or VTA route of AMPH exposure.
Conditioned Locomotion
It is well known that pairing systemically administered AMPH with a distinct environment will lead to the development of an association between that environment and the drug (15). Thus, Paired rats that received IP AMPH in the activity boxes 2-3 weeks earlier showed a conditioned locomotor response to IP saline in the drug-paired environment compared to Unpaired rats that received the same exposure to AMPH but unpaired with the activity boxes or Controls not previously exposed to the drug (Figure 2F). The ANOVA detected significant effects of group [F(2,21)=9.21, p<0.01], time [F(5,105)=72.38, p<0.001] and a significant group X time interaction [F(10,105)=2.25, p<0.05]. Post-hoc Scheffé tests revealed that Paired rats displayed significantly enhanced locomotion compared to the other two groups over much of the testing period (p<0.05-0.001). The latter two groups did not differ significantly from one another.
Figure 2.
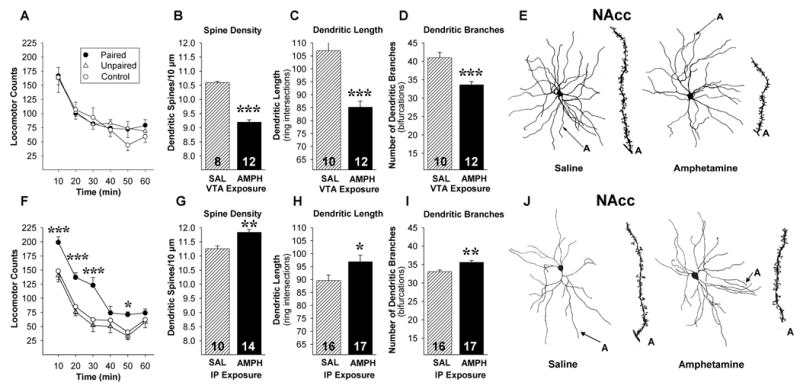
VTA and IP AMPH produce different effects on conditioned locomotion and dendritic morphology in the NAcc. VTA AMPH failed to produce conditioned locomotion (A) and decreased spine density, dendritic length and dendritic branching in the NAcc (B-E). IP AMPH produced conditioned locomotion (F) and increased spine density, dendritic length and dendritic branching in the NAcc (G-J). The Camera Lucida drawings are of representative medium spiny neurons from rats exposed to SAL or AMPH in the VTA (E) or IP (J). Conditioned locomotor responding to SAL and dendritic morphology were assessed 2-3 weeks following exposure. Data are shown as means (±SEM). Numbers at the base of each bar indicate hemispheres/group. *, p<0.05, **, p<0.01, ***, p<0.001, significantly different from two other groups for locomotor conditioning test and the SAL exposure condition for dendritic morphology.
In contrast to these findings, Paired rats that received VTA-AMPH in the activity boxes showed no evidence of conditioned locomotion 2-3 weeks later (Figure 2A). The ANOVA revealed only a significant effect of time. These findings are consistent with previous reports (15-17) showing that exposure to either IP or VTA AMPH produces sensitization, but that unlike with IP AMPH, rats exposed to VTA AMPH do not form associations linking the drug and the environment in which it was administered.
Dendritic Morphology
Experiments were then conducted to determine what changes in dendritic morphology were associated with exposure to IP or VTA AMPH 2-3 weeks earlier. Consistent with previous reports (7-9), exposure to IP AMPH produced significant changes in the NAcc, increasing spine density [F(1,22)=7.2, p<0.01], dendritic length [F(1,31)=4.83, p<0.05] and branching [F(1,31)=12.97, p<0.01] relative to SAL controls (Figure 2G-I). In contrast, exposure to VTA AMPH produced significant but opposite effects: decreased spine density [F(1,18)=31.82, p<0.001], dendritic length [F(1,20)=34.48, p<0.001] and branching [F(1,20)=20.35, p<0.001] relative to controls (Figure 2B-D). The differential change in spine density produced by the two routes of AMPH exposure is illustrated in the camera lucida drawings in Figure 2E and J. The cells illustrated and described above were selected from the NAcc shell. Additional analyses of cells in the NAcc core revealed identical effects for all measures (Supplement 6).
Exposure to IP AMPH produces long-lasting increases in spine density in striatum as well (21). Consistent with the lack of effect on DA release observed in the striatum (Figure 1C), exposure to VTA AMPH did not significantly alter spine density in this site (Supplement 7), again supporting the integrity and anatomical specificity of the VTA microinjections.
In cortical regions, a similar differentiation between the long-lasting effects of IP and VTA AMPH exposure was observed (for statistical analyses of all cortical effects, see Supplement 8). In prefrontal cortex, exposure to systemic AMPH produces increases in spine density, dendritic length and branching. These effects are restricted to apical dendrites (7-9). Exposure to VTA AMPH 2-3 weeks earlier produced significant but opposite effects: decreased spine density, dendritic length and branching relative to controls (Figure 3A). In addition, these effects were statistically significant in both basilar and apical dendrites. In orbital cortex, decreases in spine density in both apical and basilar dendrites are observed following systemic AMPH (22; effects on dendritic length and branches have not yet been reported). Again, exposure to VTA AMPH produced the opposite effect: significant increases in spine density in both aspects of these neurons relative to controls (Figure 3B). Basilar, but not apical, dendrites also showed a significant increase in branching and a trend for increased length in this region. In parietal cortex, exposure to systemic AMPH decreases spine density in apical and basilar dendrites with no effects observed in dendritic length and branching (7,9,23). Unlike with the prefrontal and orbital cortex, exposure to VTA AMPH produced the same effects in spine density in this region as exposure to systemic AMPH. Basilar, but not apical, dendrites also showed a significant decrease in branching and a trend for decreased length in this region (Supplement 9).
Figure 3.

Dendritic morphology was altered in cortex 2-3 weeks following exposure to VTA AMPH. Note the different effects observed in the prefrontal (A) and orbital (B) cortices and that both are opposite to those reported following IP AMPH (see text). The Camera Lucida drawings are of representative pyramidal cells from rats exposed to SAL or AMPH in the VTA. Data are shown as means (+SEM). Numbers at the base of each bar indicate hemispheres/group. *, p<0.05, **, p<0.01, ***, p<0.001, significantly greater than the SAL exposure condition.
Discussion
A number of reports have proposed a link between the expression of psychostimulant sensitization and increased spine density, dendritic length and branching in the NAcc. The present findings suggest that such a link may not be justified. Exposure to VTA AMPH, which like IP AMPH led to sensitized locomotor activity and DA release in the NAcc, failed to increase and in fact significantly decreased all three measures of dendritic morphology in this site. This finding indicates that psychostimulant sensitization can in fact be expressed even when spine density, dendritic length and branching are reduced in the NAcc. The additional finding that rats exposed to IP AMPH, but not VTA AMPH, exhibited conditioned locomotion suggests that the increases in dendritic morphology observed in the NAcc following systemic exposure to psychostimulants may support the expression of associative drug conditioning rather than non-associative drug sensitization.
The ubiquity of environmental stimuli surrounding the systemic administration of drugs ensures the formation of associations between the drug and any number of these stimuli. These associations are likely mediated at least in part by DA-glutamate interactions in VTA innervated forebrain areas like the NAcc as well as the prefrontal and orbital cortices (29). Because the effects of VTA AMPH are restricted to the midbrain where it locally increases extracellular levels of DA and initiates the neuroadaptations that underlie sensitization, it does not acutely stimulate locomotion or increase NAcc DA overflow (30,31) and likely for these reasons does not produce conditioned effects. Thus, the increase in spine density, dendritic length and branching observed in the NAcc following exposure to IP AMPH may reflect the formation of new drug-stimulus associations while the decrease observed following exposure to VTA AMPH may reflect the inability of these infusions to support conditioning. The idea that changes in dendritic morphology may underlie learning and memory is not new (12) and recently, changes in spine morphology and density in hippocampus have been linked to the formation of learned associations (13,14). The present results suggest that this kind of structural plasticity may be linked to associative conditioning in the NAcc as well. Of course, how specific morphological changes are produced and how they might affect the generation of drug- and drug cue-induced behaviors remains to be determined (see below).
The convergence of DA and glutamate inputs onto the dendritic spines of medium spiny neurons in the NAcc (6) supports a role for these neurotransmitters in the formation of drug-sensory stimulus associations in this site. Indeed, the increases in spine density and dendritic branching observed after exposure to AMPH are confined to the distal dendrites of medium spiny neurons, the primary locus of DA and glutamate inputs to these cells (7-9). It is difficult, without ultrastructural studies, to establish with certainty whether the increased spine density observed following psychostimulant exposure is necessarily accompanied by an increase in synaptic contacts, although electron microscopic analyses have shown that the increase in dendritic surface produced by various learning experiences is accompanied by increases in the number of synapses per neuron (32). Thus, it is conceivable that an increased number of synaptic contacts in the NAcc mediates information about conditioned associations and that this information is used to direct the expression of sensitization (29,33). Interestingly, exposure to experimenter- or self-administered psychostimulants leads to similar increases in spine density in the NAcc (7, 26) suggesting that this structural change is not related to instrumental conditioning. However, in light of the possibility that the increase in spine density observed in both conditions reflects classically conditioned associations, it is difficult to conceive that this measure of dendritic morphology could distinguish between these two forms of conditioning.
Changes in dendritic morphology are also observed in cortical areas following exposure to either systemic or VTA AMPH. Because a similar decrease in spine density was observed in parietal cortex following IP and VTA AMPH, it is unlikely that this change is related to conditioning. However, different effects following systemic and VTA AMPH were observed in the prefrontal and orbital cortices, suggesting that structural changes in these sites following exposure to systemic AMPH may contribute to conditioned effects. Both of these sites are known to participate in various forms of conditioning (29). While it remains unknown why different changes are observed in spine density in the prefrontal and orbital cortices following exposure to AMPH (increase and decrease, respectively, following systemic AMPH; decrease and increase, respectively, following VTA AMPH), both of these regions constitute primary midbrain DA cortical projection fields and they are will positioned to influence information processing in subcortical areas like the NAcc and, as a result, to impact the generation of appetitive behaviors directed at drug conditioned stimuli (4,29). Changes in dendritic morphology in these sites may thus lead to the deficits in reversal learning and reinforcer devaluation and the loss of executive control of behavior observed following psychostimulant exposure (22). Interestingly, exposure to IP AMPH has been reported to inhibit neuronal activity in the prefrontal cortex and excite activity in the orbital cortex (34). It is possible that these effects reflect compensatory attempts in these sites to regain executive control over behavior.
The release by psychostimulants of DA and glutamate onto the processes of medium spiny neurons in the NAcc and pyramidal cells in cortical regions activates extracellular signal-regulated kinase (ERK) and this kinase is required for the formation of associations between drugs and environmental stimuli (35,36). ERK activity is also essential to the ability of brain-derived neurotrophic factor to increase dendritic spine density and support the formation of long-term memories (37,38). Thus, systemically administered psychostimulants may initiate morphological changes necessary in forebrain sites to mediate conditioned associations via the coincident activation of neighboring DA and glutamate receptors. VTA AMPH exposure may be unable to produce these effects because it lacks at least one of the necessary components (DA release in forebrain). Recently, a role for the myocyte enhancer factor 2 (MEF2) family of transcription factors was also proposed (11). Psychostimulant suppression of MEF2 in the NAcc is required to increase spine density in this site. Interestingly, this effect is mediated in part via a D1 DA receptor initiated pathway.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (DA09397, PV) and the Canadian Institutes for Health Research (108712, BK).
Footnotes
The authors reported no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available on line.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vezina P, Lorrain DS, Arnold GM, Austin JD, Suto N. Sensitization of midbrain dopamine neuron reactivity promotes the pursuit of amphetamine. J Neurosci. 2002;22:4654–4662. doi: 10.1523/JNEUROSCI.22-11-04654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 4.Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- 5.Kim JH, Austin JD, Tanabe L, Creekmore E, Vezina P. Activation of group II mGlu receptors blocks the enhanced drug taking induced by previous exposure to amphetamine. Eur J Neurosci. 2005;21:295–300. doi: 10.1111/j.1460-9568.2004.03822.x. [DOI] [PubMed] [Google Scholar]
- 6.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 7.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci. 1997;17:8491–8497. doi: 10.1523/JNEUROSCI.17-21-08491.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- 9.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47:33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 10.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cyctine-glutamate exchange underlie cocaine relapse. Nature Neurosci. 2003;6:743–749. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 11.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, et al. Cocaine Regulates MEF2 to Control Synaptic and Behavioral Plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamprecht R, LeDoux J. Structural plasticity and memory. Nature Rev Neurosci. 2004;5:45–54. doi: 10.1038/nrn1301. [DOI] [PubMed] [Google Scholar]
- 13.Geinisman Y, Berry RW, Disterhoft JF, Power JM, Van der Zee EA. Associative learning elicits the formation of multiple-synapse boutons. J Neurosci. 2001;21:5568–5573. doi: 10.1523/JNEUROSCI.21-15-05568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leuner B, Falduto J, Shors TJ. Associative memory formation increases the observation of dendritic spines in the hippocampus. J Neurosci. 2003;23:659–665. doi: 10.1523/JNEUROSCI.23-02-00659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stewart J, Vezina P. Conditioning and behavioral sensitization. In: Kalivas PW, Barnes CD, editors. Sensitization in the Nervous System. Caldwell, NJ: Telford Press; 1988. pp. 207–224. [Google Scholar]
- 16.Vezina P, Stewart J. Amphetamine administered to the ventral tegmental area but not to the nucleus accumbens sensitizes rats to systemic morphine - Lack of conditioned effects. Brain Res. 1990;516:99–106. doi: 10.1016/0006-8993(90)90902-n. [DOI] [PubMed] [Google Scholar]
- 17.Stewart J, Vezina P. Extinction procedures abolish conditioned stimulus control but spare sensitized responding to amphetamine. Behav Pharmacol. 1991;2:65–71. [PubMed] [Google Scholar]
- 18.Vezina P. D1 dopamine receptor activation is necessary for the induction of sensitization by amphetamine in the ventral tegmental area. J Neurosci. 1996;16:2411–2420. doi: 10.1523/JNEUROSCI.16-07-02411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pellegrino LJ, Pellegrino AS, Cushman AJ. A Stereotaxic Atlas of the Rat Brain. 2nd. New York, NY: Plenum Press; 1979. [Google Scholar]
- 20.Gibb R, Kolb B. A method for vibratome sectioning of Gogi-Cox stained whole rat brain. J Neurosci Meth. 1998;79:1–4. doi: 10.1016/s0165-0270(97)00163-5. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Kolb B, Robinson TE. The location of persistent amphetamine-induced changes in the density of dendritic spines on medium spiny neurons in the nucleus accumbens and caudate-putamen. Neuropsychopharmacology. 2003;28:1082–1085. doi: 10.1038/sj.npp.1300115. [DOI] [PubMed] [Google Scholar]
- 22.Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- 23.Kolb B, Gorny G, Li Y, Samaha AN, Robinson TE. Amphetamine or cocaine limits the ability of later experience to promote structural plasticity in the neocortex and nucleus accumbens. Proc Natl Acad Sci. 2003;91:12673–12675. doi: 10.1073/pnas.1834271100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman PD, Riesen AH. Environmental effects on cortical dendritic fields. I. Rearing in the dark. J Anat. 1968;102:363–374. [PMC free article] [PubMed] [Google Scholar]
- 25.Kirk RE. Experimental Design: Procedures for the Behavioral Sciences. Pacific Grove: Brooks/Cole; 1968. [Google Scholar]
- 26.Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- 27.Kolta MG, Shreve P, Desouza V, Uretsky NJ. Time course of the development of the enhanced behavioral and biochemical responses to amphetamine after pretreatment with amphetamine. Neuropharmacology. 1985;24:823–829. doi: 10.1016/0028-3908(85)90032-2. [DOI] [PubMed] [Google Scholar]
- 28.Castaneda E, Becker JB, Robinson TE. The long-term effects of repeated amphetamine treatment in vitro. Life Sci. 1988;42:2447–2456. doi: 10.1016/0024-3205(88)90343-8. [DOI] [PubMed] [Google Scholar]
- 29.Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Ann NY Acad Sci. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- 30.Kalivas PW, Weber B. Amphetamine injected into the ventral mesencephalon sensitizes rats to peripheral amphetamine and cocaine. J Pharmacol Exp Ther. 1988;245:1095–1102. [PubMed] [Google Scholar]
- 31.Hu XT, Koeltzow TE, Cooper DC, Robertson GS, White FJ, Vezina P. Repeated ventral tegmental area amphetamine administration alters dopamine D1 receptor signaling i the nucleus accumbens. Synapse. 2002;45:159–170. doi: 10.1002/syn.10095. [DOI] [PubMed] [Google Scholar]
- 32.Greenough WT, Withers GS, Wallace CS. Morphological changes in the nervous system arising from behavioral experience: what is the evidence that they are involved in learning and memory? In: Squire LR, Lindenlaub E, editors. The Biology of Memory. New York, NY: Schattauder; 1990. pp. 159–185. [Google Scholar]
- 33.Vezina P, Leyton M. Conditioned cues and the expression of stimulant sensitization in animals and humans. Neuropharmacology. 2009;56:160–168. doi: 10.1016/j.neuropharm.2008.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Homayoun H, Maghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity? Curr Op Pharmacology. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 Activation Is Necessary for BDNF to Increase Dendritic Spine Density in Hippocampal CA1 Pyramidal Neurons. Learn Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bekinschtein P, Cammarota M, Katche C, Slipczuk L, Rossato JI, Goldin A, et al. BDNF is essential to promote persistence of long-term memory storage. Proc Natl Acad Sci. 2008;105:2711–2716. doi: 10.1073/pnas.0711863105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Compact 3rd. San Diego, CA: Academic Press; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


