Abstract
Background and Objective
A retrospective cross-sectional study was conducted to demonstrate an analysis of an outer retinal layer reconstructed by the three-dimensional and high-speed spectral domain optical coherence tomography (SD-OCT) instrument.
Patients and Methods
New measurement protocols for SD-OCT and methods of analysis and visualization of the individual segmented retinal layer reconstructed by SD-OCT were proposed. Three contour maps representing mutual distances between the basal part of the retinal pigment epithelium, the junction between the inner and outer segments of photoreceptors, and a reference contour representing the shape of a healthy retina were introduced.
Results
The analysis of the outer retina was performed on pathological eyes. Three cases of central serous chorioretinopathy, age-related macular degeneration, and acute zonal occult outer retinopathy are demonstrated.
Conclusion
Three contour maps reconstructed for clinical cases demonstrate high variability of observed patterns depending on analyzed pathology. The authors believe this can help to present OCT data simultaneously in a more comprehensive and convenient way to assist in everyday clinical diagnosis.
Introduction
Optical coherence tomography (OCT) is one of the modern optical imaging technologies useful in ophthalmology when used in addition to standard fundus photography and fluorescein angiography, which are currently accepted as the “gold standard” of diagnostic ophthalmic imaging methods.
Spectral domain OCT (SD-OCT) is a novel variant that enables collecting three-dimensional data within seconds and increases the effective coverage of the retina, revealing small and focal pathological changes.1 A primary purpose of our study was the creation of a tool based on OCT technology that can be easily compared with fundus photography and fluorescein angiography images. A unique feature of the SD-OCT technology, and one of the most promising, is the possibility of quantitative analysis of photoreceptor layers. It has been demonstrated that increased axial resolution significantly improves visualization of photoreceptor morphology.2-4 Using the combined high axial resolution and high speed offered by SD-OCT instruments, it is possible to create maps of the thickness of outer retinal layers. Preliminary results of using this technology in healthy eyes were published in 2005.1
Three-dimensional imaging performed by the SD-OCT instrument provides a significant amount of data. Precise analysis of 200 or more B-scans is often difficult and time consuming. Thickness maps enable simultaneous access to all B-scans. Unlike a single B-scan, the thickness maps reveal the exact location and dimensions of pathologies in respect to the retinal vasculature in a way that is easy to compare with fluorescein angiography and retinal photography. Pathological changes present in the OCT images of outer retinal regions demonstrate high variability of reconstructed patterns. Therefore, we assume that thickness maps of this region can also help to perform detailed analysis, providing better diagnosis and understanding of pathogenesis. Another important issue is that thickness maps enable obtaining objective, quantitative data corresponding to the extent of elevations and depressions present in different pathologies.
In this article, we present for the first time maps of outer retinal layer thickness in pathological eyes using a new method of numerical analysis of SD-OCT data. Exhaustive, technical description of this new algorithm, including a layer segmentation procedure, has been recently presented by our group.5 Additionally, new, specialized measurement protocols have been applied to obtain SD-OCT data in the ophthalmology clinic. We use these new methods of analysis and visualization of the individual segmented layer representing the complex of the retinal pigment epithelium (RPE) and outer segments of photoreceptors, which we believe can provide a better understanding of disease pathogenesis, more sensitive diagnostic indicators of early disease, and better ways to follow the progress of and recovery from disease in the future.
Patients and Methods
Measurement System
In our experiments, we used a prototype SD-OCT instrument, which has been optimized for use in clinical conditions. The instrument was constructed at Nicolaus Copernicus University and is described in detail elsewhere.5 Two scanning protocols were implemented. To collect general information about pathological changes in retinal structure, 35 cross-sectional images were taken, each consisting of 3,000 lines (optical A-scans) covering an area of X = 6 mm/Y = 3 mm within less than 4 seconds. For quantitative analysis, the second protocol was applied: 200 cross-sectional images, each consisting of 400 A-scans, covering an area of X = 6 mm/Y = 6 mm within 3 seconds.
Data Analysis Tool
OCT provides information on retinal structure based on the intensity and distribution of back-scattered or back-reflected light. We implemented a method of retinal layer segmentation that delineates areas characterized by similar distribution of back-scattered intensity. A detailed description of the segmentation algorithm is published elsewhere.5 We will briefly describe a numerical procedure that is optimized for analysis of the outer retina, the merged layers of the RPE, and the outer segments of the photoreceptors.
Figure 1 shows the data processing scheme. In the first step, the operator chooses one image from the set of 200 cross-sectional images and defines the rectangular region of interest inside the layer of interest (Fig. 1A, ROI 1). This region enables the operator to calculate parameters, which will be used for the further automated analysis of the entire 3D set of data.
Figure 1.
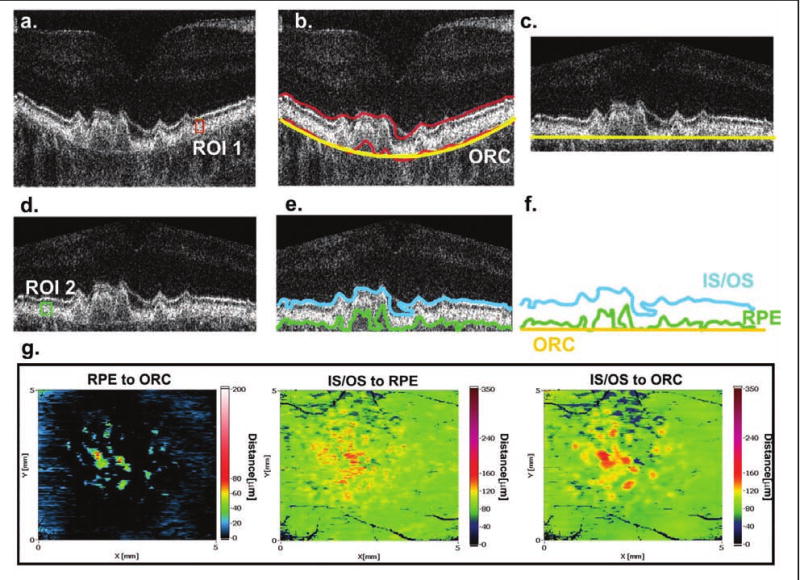
Retinal layer segmentation procedure. (A) The operator defines the region of interest (ROI 1) inside the required layer. (B) The contour delineating the posterior retina based on ROI 1 is coarsely created and the reference surface, the outer retinal contour (ORC), is calculated. (C) Each cross-sectional image is flattened and cropped with respect to the ORC. (D) The operator defines a new ROI (ROI 2) to accurately segment the posterior retina. (E) Lines representing the basal part of the retinal pigment epithelium (RPE) and the IS/OS junction are automatically identified and three contours corresponding to the RPE, IS/OS, and ORC are calculated. (F) Finally, 200 sets of three contours (the RPE, IS/OS, and ORC) can be analyzed with respect to each other. (G) Contour maps representing the thickness between the RPE and ORC, the RPE and IS/OS, and the ORC and IS/OS.
In the second step, our algorithm enables the operator to create a reference plane corresponding to the shape of the posterior retina. The natural architecture of the eye and the properties of the OCT scanning system usually result in an approximately parabolic shape for the reconstructed retina. Therefore, our software uses a simple parabolic fit procedure applied to the contour delineating the posterior retina for each cross-sectional image. The set of such parabolic curves creates the reference surface outer retinal contour (Fig. 1B, ORC).
In the third step, each OCT scan is realigned to flatten the ORC while preserving the original radial dimensions (Fig. 1C). Once the cross-sectional images are flattened and cropped, one can perform a detailed analysis of the outer retina. The parameters previously set for segmentation are no longer valid, a new ROI can be chosen manually by the operator (Fig. 1D, ROI 2), and the automated processing of the entire 3D set of data is repeated, this time providing exact delineation of OCT layers corresponding to the RPE and the inner segment/outer segment (IS/OS) junction.
In the final step, our software automatically provides two contour lines most likely representing the basal part of the RPE and the IS/OS junction, respectively (Fig. 1E). As a result of the entire processing procedure, one obtains 200 sets of three contours, the RPE, the IS/OS, and the ORC (Fig. 1F), which can be analyzed with respect to each other and the results displayed as contour maps in a false color scale (Fig. 1G). We think that the simultaneous analysis of the three maps representing separations between the RPE and IS/OS, the IS/OS and ORC, and the RPE and ORC may provide additional diagnostic information significant from the clinical perspective.
Additionally, it is possible to construct the SD-OCT fundus view based on cropped and flattened SD-OCT cross-sectional images (Fig.1C).1,6 This procedure augments pathological changes present in the posterior retina. All images of the SD-OCT fundus view presented in Results are created in this way.
Patients and Examination
The prototype SD-OCT device operates on an everyday basis at the ophthalmology clinic. The quantitative analysis of the outer retina was performed on 34 eyes from 20 patients and an additional 15 healthy eyes from volunteers with normal vision (20–76 years of age). In this article, we present the most representative cases demonstrating various patterns of contour maps depending on the group of pathological changes.
Patient age ranged from 18 to 81 years. After complete ophthalmologic examination, including fluorescein angiography and color fundus photography (TRC-50 EX; Topcon, Tokyo, Japan), early age-related macular degeneration in the form of soft drusen was diagnosed in 24 eyes. Central serous chorioretinopathy was discovered in seven eyes. Features of photoreceptor atrophies or dysfunctions were found in three eyes.
The optical power incident on the eye was 750 μW, which is consistent with Polish norms and American National Standards Institute safety standards.7,8 The examination was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all subjects after all consequences of the study had been explained. The study protocol was approved by the ethics committee of the Collegium Medicum Nicolaus Copernicus University, Bydgoszcz, Poland.
SD-OCT examination was performed in each eye according to the measurement protocols described above. The SD-OCT measurements were made after pupil dilation (0.5% tropicamide) with the patient in the sitting position with the head placed on a chin rest.
Results
Simultaneous analysis of the RPE-ORC, IS/OS-RPE, and IS/OS-ORC maps calculated separately for each of the eyes examined revealed high variability depending on the type of lesion. In healthy eyes, all three thickness maps showed almost uniform distribution of colors representing constant thicknesses of analyzed layers.5 Contour maps of different pathological eyes demonstrate patterns composed from areas of increased (yellow-red) and decreased (blue-black) distances between segmented layers. To demonstrate high variability of thickness maps, we describe three cases of retinal diseases.
Elevations of RPE
The common feature of RPE elevations, independent of their origin, is an increased distance between the ORC and RPE. This can be observed as changes in shape, size, and location of elevations in the RPE-ORC map. Theoretically, the IS/OS junction should be intact, and no significant changes should be visible in the IS/OS-RPE map.
Figure 2 demonstrates multifocal detachment of the RPE in a patient with chronic central serous chorioretinopathy. Cross-sectional SD-OCT scan through the central macula shows two areas of fluid under the RPE. The most prominent RPE elevation on the RPE-ORC map (Fig. 2D) has an irregular shape and is located temporal to the foveal center. Two smaller areas of RPE elevation are visible superiorly to the fovea and a third, smaller one directly in the foveola.
Figure 2.
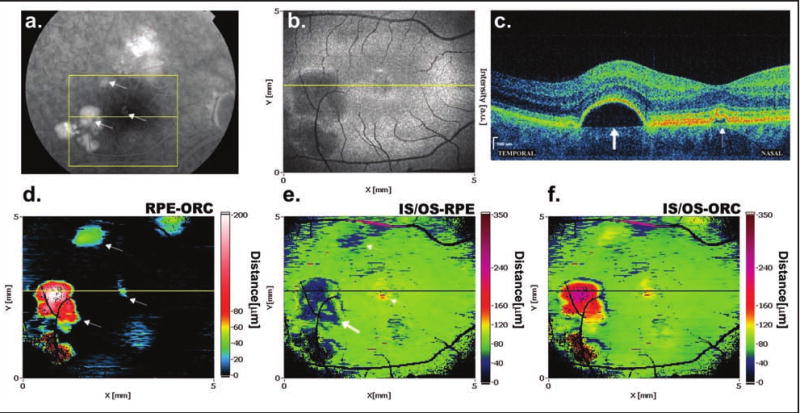
Fluid under the RPE in a patient with multifocal RPE detachments in chronic central serous chorioretinopathy. (A) Fluorescein angiography reveals three hyperfluorescent areas of RPE detachments (indicated by arrows) within the square area limited by yellow lines representing the retina scanned by SD-OCT. (B) Cropped and flattened 3D OCT data can be displayed as a SD-OCT fundus view representing the sum of RPE and IS/OS reflectivity. (C) SD-OCT cross-sectional image scanned along the yellow line reveals two areas of fluid under the RPE, the first larger one located temporal to the fovea (thick arrow) and the second smaller one located in the foveolar center (thin arrow). (D) The RPE-ORC map shows the same areas of RPE detachments as fluorescein angiography: significant RPE elevation temporal to the macula together with a second smaller elevation in the foveal center and a third elevation localized close to the superior margins of the fovea. (E) The IS/OS-RPE map indicates areas of thinning of this layer above the pigment epithelial detachments (arrows) and increased distance over the smaller detachment in the foveal center (thick arrow). (F) Elevations in the IS/OS-ORC map correspond to areas of RPE detachment also visible on the RPE-ORC map.
The IS/OS-ORC map (Fig. 2F) shows the same areas of the RPE detachment as the RPE-ORC map, but the elevations are displayed as yellow-red islands surrounded by green areas where the 80-μm distance between the RPE and IS/OS junction, which is characteristic for a healthy retina, is maintained. The loss of reflectivity from the IS/OS junction above the highest RPE elevation, especially in areas not perpendicular to the direction of illuminating light, is probably the reason for the decrease in separation observed in the IS/OS-RPE map (Fig. 2E). However, some photoreceptor damage cannot be excluded. This loss of reflectivity is not observed in the case of small RPE elevations, as can be seen in the foveal center.
Elevation of RPE and Neurosensory Retina
Figure 3 shows soft drusen in an eye with age-related macular degeneration. The drusen visible on the RPE-ORC map have the form of oval elevations of up to 200 μm, with a relatively smooth regular surface. We observed that the dimensions and localization of drusen differ significantly from eye to eye. The common feature is an increased separation between the RPE and IS/OS junction around the drusen that strains the overlying neurosensory retina. As a result, concave elevations (red) surrounding the oval area of deposits (green) under the RPE may be visible on the IS/OS-RPE maps (Fig. 3E). Photoreceptor atrophy, which sometimes appears anteriorly to the drusen, may be responsible for the small localized depressions (blue dots) located centrally in the drusen areas (green).
Figure 3.
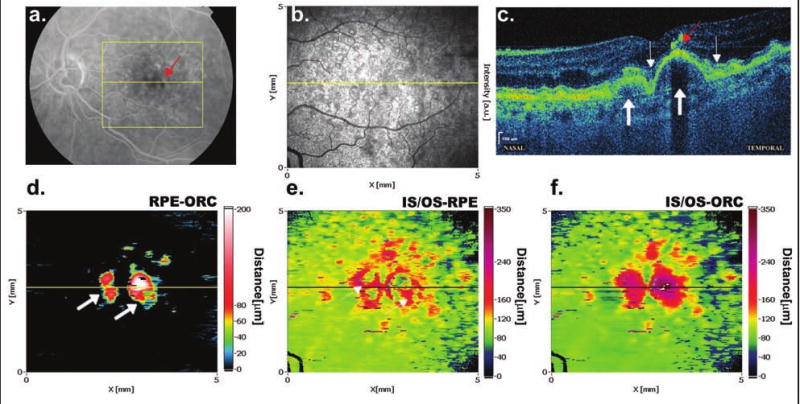
Deposits under the RPE in the form of soft drusen in age-related macular degeneration. (A) The late phase of fluorescein angiography demonstrates large soft drusen in the center and small hard drusen in the periphery of the examined area (yellow lines). (B) Scattered hard drusen in the periphery are also visible on the SD-OCT fundus view. (C) Regular elevations of the RPE by homogenous material of low reflectivity visible in the spectral domain OCT image correspond to drusen (thick arrows). The places of increased separation between the IS/OS junction and RPE around drusen are marked (thin arrows). A small, highly reflective structure indicated by the red arrow is probably related to a pigment deposit visible on fluorescein angiography as a hypofluorescent spot (red arrow). (D) Regular, high elevations of the RPE due to drusen are visible on the RPE-ORC map (thick arrows). (E) The IS/OS-RPE map reveals concave areas of increased distance between the RPE and IS/OS junction around drusen (thin arrows). (F) The IS/OS-ORC map helps to assess the distribution of RPE elevations in respect to the IS/OS elevations.
The IS/OS-ORC map (Fig. 3F) can be understood as a combination of the IS/OS-RPE and the RPE-ORC map. It helps to evaluate the distribution and height of the RPE elevations with respect to areas of increased separation between the RPE and IS/OS junction. The highest elevations in this map (up to 240 μm) are localized exactly in the areas of RPE elevations corresponding to the drusen in the RPE-ORC map (Fig. 3D).
Atrophy of Photoreceptors
Figure 4 illustrates a case of acute zonal occult outer retinopathy. The characteristic feature of OCT cross-sectional images of retinal photoreceptor cell atrophy is a loss in reflectivity of the IS/OS junction. The IS/OS junction is defined as the border between the inner and outer photoreceptor segments. Therefore, it is natural to expect that this layer will disappear together with the photoreceptor cell bodies. In areas of atrophy the contour line, which in other cases corresponds to the IS/OS junction, now appears almost at the level of the ORC. This results in effective areas of depression (blue) in both the IS/OS-RPE and IS/OS-ORC maps (Figs. 4E and 4F). A similar situation will be present if the reflectivity of the IS/OS junction is much smaller than that observed in healthy eyes.
Figure 4.
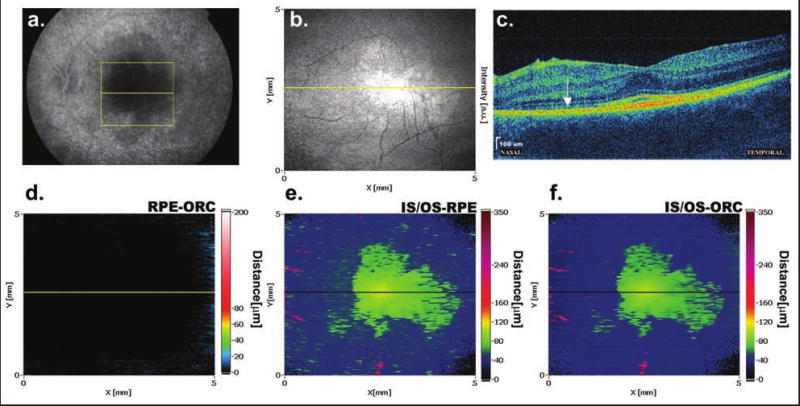
Photoreceptor dysfunction in a patient with acute zonal occult outer retinopathy. (A) Fluorescein angiography shows hypopigmentation of the RPE in the perifoveal and peripheral retina. (B) SD-OCT fundus view helps to identify the position of the OCT scan in respect to retinal vasculature. (C) SD-OCT image reveals a loss of IS/OS junction reflectivity nasally to the fovea (arrow). (D) The uniform black color in the RPE-ORC map indicates a complete lack of RPE elevations. (E) An irregular region of unaffected photoreceptors in the fovea, surrounded by a blue area of lower IS/OS reflectivity in the periphery, is visible on the IS/OS-RPE map. (F) A similar pattern is visible on the IS/OS-ORC map. Due to the greater separation between the ORC and IS/OS junction, green is dominant in the areas that are free from the atrophy.
In this patient, broad zones of photoreceptor dysfunction confirmed by electrophysiological examination can be seen in the IS/OS-RPE and IS/OS-ORC maps as a blue depression. The yellow-green irregular island in the center is the area of highest IS/OS reflectivity. The separation between the RPE and the IS/OS junction, as well as between the ORC and the IS/OS junction, is normal in this region (60-80 μm) because the photoreceptors in this area are unaffected. In the peripheral part of the fovea, the blue color in the IS/OS-RPE map and the blue-green color in the IS/OS-ORC map are dominating. In these regions, the contour lines run close to each other, indicating zones of lower reflectivity of the IS/OS junction. Due to lack of significant abnormalities in the RPE, the RPE-ORC map (Fig. 4D) does not demonstrate any elevations or depressions and is uniformly black.
Discussion
Based on our preliminary results, we observed that specific patterns visible on the contour thickness maps can be attributed to different groups of RPE elevations, depending on their origin. There are also specific patterns of the contour maps associated with different origin of detachments of neurosensory retina and dysfunctions of photoreceptors. However, it is important to note that the correct interpretation of observed morphological changes should be based on all three maps and spectral domain OCT cross-sectional images analyzed together. This is caused by limited information provided by the measurement and the analysis based on the distribution of back-reflected intensity The proposed contour maps can be also treated as a convenient way of displaying 3D data, enhancing morphological changes and helping an ophthalmologist in the timely and reliable evaluation of the disease. Moreover, the contour maps provide additional information of morphological features such as shape and dimensions.
Despite many advantages, thickness maps also have some limitations. They can be generated only if the OCT signal is strong enough to reconstruct easily recognizable hyperreflective lines corresponding to the RPE and IS/OS junction. This condition is usually fulfilled in early stages of macular diseases. Another source of artifacts is a decrease of the IS/OS reflectivity within elevated retina in the areas of the RPE detachment. As a result, the reference line running along the IS/OS junction jumps to the level of the RPE. Due to the decreased reflectivity of the IS/OS junction, the depression is visible (Fig. 2E) instead of normal-constant distance.
Our newly developed algorithm enables segmentation of highly reflective OCT signals from posterior retina, including the RPE and outer segments of photoreceptors, in healthy and pathological cases. After calculation of an additional reference line called the ORC, three types of thickness maps can be generated. These contour maps reconstructed for clinical cases demonstrate high variability of observed patterns depending on analyzed pathology. Despite the presence of artifacts in more advanced cases, we believe that simultaneous analysis of SD-OCT cross-sectional images together with all three proposed contour maps can bring additional valuable and clinically significant information. In the future, this also can help to find new, more sensitive diagnostic markers for early detection of retinal diseases and can provide a better understanding of disease pathogenesis and progression.
Acknowledgments
Supported by Polish Ministry of Science, grants for years 2006 to 2009. Dr. Wojtkowski receives additional support of Foundation for Polish Science (Homing 34 project) and Rector of NCU for the scientific grant 504-F.
References
- 1.Wojtkowski M, Srinivasan V, Fujimoto JG, Schuman JS, Kowalczyk A, Duker JS. Three-dimensional retinal imaging with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2005;112:1734–1746. doi: 10.1016/j.ophtha.2005.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ko TH, Fujimoto JG, Duker JS, et al. Comparison of ultrahigh- and standard-resolution optical coherence tomography for imaging macular hole pathology and repair. Ophthalmology. 2004;111:2033–2043. doi: 10.1016/j.ophtha.2004.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko TH, Adler DC, Fujimoto JG, et al. Ultrahigh resolution optical coherence tomography imaging with a broadband superluminescent diode light source. Optics Express. 2004;12:2112–2119. doi: 10.1364/opex.12.002112. [DOI] [PubMed] [Google Scholar]
- 4.Srinivasan VJ, Wojtkowski M, Witkin AJ, et al. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2006;113:e1–e14. doi: 10.1016/j.ophtha.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Szkulmowski M, Wojtkowski M, Sikorski B, et al. Analysis of posterior retinal layers in spectral optical coherence tomography images of the normal retina and retinal pathologies. J Biomed Optics. 2007;12:041207, 1–11. doi: 10.1117/1.2771569. [DOI] [PubMed] [Google Scholar]
- 6.Jiao SL, Knighton R, Huang XR, Gregori G, Puliafito CA. Simultaneous acquisition of sectional and fundus ophthalmic images with spectral-domain optical coherence tomography. Optics Express. 2005;13:444–452. doi: 10.1364/opex.13.000444. [DOI] [PubMed] [Google Scholar]
- 7.Polskie N. Bezpieczenstwo przy promieniowaniu emitowanym przez urzadzenia laserowe. Klasyfikacja sprzetu. Wymagania i wytyczne dla uzytkownika. Warsaw, Poland: Wydawnictwo Normalizacyjne Alfa-Wero; 1992. PN-91/T-06700. [Google Scholar]
- 8.American National Standards Institute. American National Standard for Safe Use of Lasers. Orlando, FL: Laser Institute of America; 2000. ANSI Z136.1-2000. [Google Scholar]


