Abstract
Classical immunotherapeutic approaches to glioblastoma multiforme (GBM) have shown mixed results, and therapies focused on innate lymphocyte activity against GBM have not been rigorously evaluated. We examined peripheral blood lymphocyte phenotype, γδ T-cell number, mitogenic response, and cytotoxicity against GBM cell lines and primary tumor explants from GBM patients at selected time points prior to and during GBM therapy. Healthy volunteers served as controls and were grouped by age. T-cell infiltration of tumors from these patients was assessed by staining for CD3 and T-cell receptor γδ. Our findings revealed no differences in counts of mean absolute T-cells, T-cell subsets CD3+CD4+ and CD3+CD8+, and natural killer cells from healthy volunteers and patients prior to and immediately after GBM resection. In contrast, γδ T-cell counts and mitogen-stimulated proliferative response of γδ T-cells were markedly decreased prior to GBM resection and throughout therapy. Expanded/activated γδ T-cells from both patients and healthy volunteers kill GBM cell lines D54, U373, and U251, as well as primary GBM, without cytotoxicity to primary astrocyte cultures. Perivascular T-cell accumulation was noted in paraffin sections, but no organized T-cell invasion of the tumor parenchyma was seen. Taken together, these data suggest that γδ T-cell depletion and impaired function occur prior to or concurrent with the growth of the tumor. The significant cytotoxicity of expanded/activated γδ T-cells from both healthy controls and selected patients against primary GBM explants may open a previously unexplored approach to cellular immunotherapy of GBM.
Keywords: γδ T-cells, glioblastoma multiforme, immunotherapy, innate immunity
Glioblastoma multiforme (GBM) remains impervious to both traditional and nontraditional therapeutic approaches, stubbornly persisting with a dismal median survival of 12 months.1 Classical approaches to immunotherapy for GBM have shown promise in the laboratory but have had little success in vivo, principally due to the brain’s protected environment and the immunosuppressive effects of the tumor itself.2–16 Indeed, tumor-derived immunosuppressive cytokines such as transforming growth factor β (TGF-β) can suppress the immune response through multiple pathways such as induction of CD4+CD25+FoxP3+ regulatory T-cells and inhibition of dendritic cell (DC) maturation and antigen presentation.17,18 Attempts to overcome systemic immunosuppression by infusion of ex vivo activated cytotoxic T-lymphocytes (CTLs)3,16 have shown little effect on prevention of tumor recurrence. Placement of autologous lymphokine-activated killer (LAK) cells, a mixed population of interleukin-2 (IL-2)-activated T-cells and natural killer (NK) cells, into the postresection tumor cavity has not shown a clear survival benefit, and several reports of IL-2–related toxicity have discouraged widespread use of this therapy.2,19–22 An alternative approach using intracavitary placement of ex vivo–expanded tumor-infiltrating CTLs (TILs) resulted in a longer remission for a few patients but with no evidence that the TILs were specifically cytotoxic to the tumor.10 Placement of allogeneic T-lymphocytes into the tumor bed has also produced isolated cases of prolonged remission. This approach is based on the fact that normal neuroglial cells do not express class I major histocompatibility complex (MHC) antigens, whereas gliomas do. However, a significant survival benefit has not been shown, and the success of therapy has been found to be highly dependent on the degree of MHC class I expression of the individual tumor.11,12 Other barriers to these therapies include the short in vivo life of LAK cell preparations coupled with their failure to migrate throughout the tumor,13 GBM-associated NK inhibitory molecules,23,24 and the lack of clearly defined GBM-associated antigens to provoke a significant adaptive immune response.
Recent studies have shown that GBM may be vulnerable to elements of the innate immune system through its expression of several MHC class I-like stress-associated molecules, such as MHC class I chain-related proteins A and B (MIC-A/B) and human cytomegalovirus membrane glycoprotein (UL-16)-binding proteins.25 These antigens are recognized by NK cells via the stimulatory receptor NK group 2 member D (NKG2D) and by γδ T-cells by both NKG2D and T-cell receptors (TCRs)26–30 using innate mechanisms that are MHC independent and require no prior antigen exposure or priming.27,31,32 Indeed, early preclinical work has shown γδ T-cells to be cytotoxic against GBM cells, but these early findings did not progress beyond the conceptual stage,32–34 likely due to the lack of a robust procedure to expand the numbers of γδ T-cells to produce sufficient cells for therapy.
We report initial studies that examine the potential for use of γδ T-cells as a therapeutic agent for GBM. We first determined the frequency, phenotype, and mitogenstimulated proliferative activity of γδ T-cells in patients with GBM at various stages of the disease process, from diagnosis through standard chemoradiotherapy. These data were analyzed in the context of overall immune function by parallel assessment of lymphocyte and subset phenotype and relevant serum cytokines content in order to evaluate the effect of the disease and therapeutic regimen on the potential immunogenicity of these cells. The mitogenic assays used in this study were also designed to assess the potential for use of autologous or allogeneic γδ T-cells for cellular therapy by using a procedure that can easily be translated for clinical expansion of γδ T-cells. Finally, we examined the cytolytic activity of γδ T-cells from both GBM patients and healthy volunteers against GBM cell lines and primary GBM cultures. These studies provide information essential to determine the optimal timing and donor source for γδ T-cell therapy.
Materials and Methods
Patients and Controls
Patients presenting with CT or MRI evidence of probable GBM were accrued for this study and enrolled following histological diagnosis. Patients and controls were excluded if they had been diagnosed with a coexisting immune system disorder; active viral, bacterial, or parasitic infection; or prior organ or bone marrow transplant. This study was approved by the University of Alabama (UAB) Institutional Review Board (IRB; Study Approval no. X070508001). Informed consent was obtained from each patient or designated family member and from healthy volunteers, following an explanation of the research studies.
All patients who elected to receive their treatment at UAB were treated with standard chemoradiation following the Stupp regimen.35 Patients received fractionated external beam radiation therapy to a prescription dose of 5,940 Gy and concurrent daily chemotherapy with temozolomide at 75 mg/m2. Following the completion of radiation therapy, patients entered a maintenance phase where temozolomide was administered at 150–200 mg/m2 for 5 consecutive days followed by 23 days off. These cycles were repeated until tumor progression. A subset of patients reported here also participated in a therapeutic trial of the thrombospondin fragment ABT-510, which was administered daily subcutaneously until tumor progression.
Cell Lines and Tissues
U251MG, D54MG, and U373MG glioma cell lines were a gift of Darell D. Bigner, Duke Comprehensive Cancer Center, Duke University (Durham, NC, USA). The U87MG glioma cell line was purchased from American Type Culture Collection (Rockville, MD, USA). All GBM cell lines were maintained in a 50:50 mixture of Dulbecco’s minimum Eagle’s medium (DMEM) and Ham’s nutrient mixture F12 enriched with 7% heat-inactivated pooled human current good manufacturing practices (cGMP)-grade serum (Labquip, Woodbridge, ON, Canada), 2 mM l-glutamine, 100 μg/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin (Mediatech, Herndon, VA, USA). Normal human astrocytes were established in culture by disaggregating human cortex removed as a consequence of surgery for intractable epilepsy. Tissue was obtained and processed by the Brain Tumor Tissue Core of the UAB Brain Tumor Specialized Program of Research Excellence (CA-097247) as approved by the UAB IRB (Study Approval no. X0504015007). Normal human astrocyte cultures were maintained in DMEM/F12 medium supplemented as indicated above. Spent culture medium was exchanged for fresh, and cells were passaged (0.5% trypsin-0.53 mM EDTA, GIBCO, InVitrogen, Carlsbad, CA, USA) using a 1:10 split, as needed according to growth. Tumor specimens were obtained at the time of surgical resection as approved by the IRB and were processed in the Brain Tumor Tissue Core to generate formalin-fixed, paraffin-embedded (FFPE) specimens, flash-frozen specimens stored in liquid nitrogen, and explant cultures.
Immunohistochemistry
FFPE sections of primary GBM were used for immunohistochemistry. Sections of normal spleen, provided by the Tissue Procurement Facility of the UAB Comprehensive Cancer Center, served as the antibody control. Sections not reacted with primary antibody served as controls for background staining. Tissue sections were blocked with avidin for 15 min, rinsed, blocked with biotin for 15 min, drained, and finally blocked for 20 min with 3% normal goat serum. Primary antibody (CD3 ready to use [Neomarkers, Fremont, CA, USA] or TCR γδ [BD Pharmingen, San Jose, CA, USA]) was applied for 1 h. Biotinylated goat antimouse or antirabbit antibody (Biogenex, San Ramon, CA, USA) was applied for 20 min, followed by avidin-horseradish peroxidase (HRP) for 20 min. Diaminobenzidine tetrahydrochloride (DAB; Biogenex) was then applied for approximately 8 min. After DAB staining, slides were rinsed with dionized water and counterstained with pure hematoxylin for 45 s. Tissue sections were dehydrated with graded ethanols and xylene, and coverslips were mounted.
Primary Tumor Explant Cultures
Short-passage (<5) primary GBM cultures were initiated from operative specimens obtained from accrued patients. Tissues were minced finely with #11 scalpel blades and transferred aseptically into T-75 flasks in about 15 ml of complete culture medium (DMEM/F12 50:50 [Mediatech] supplemented to 7% with heat-inactivated fetal bovine serum and 2.6 mM l-glutamine). Medium was exchanged after 5–7 days, and any cells growing out from the explants were maintained in primary culture. The cultures were monitored for growth and confluence, and the media were changed weekly. Once cells on the flask were confluent, the cells were detached using trypsin and passaged and/or cryopreserved.
Lymphocyte Phenotyping
Lymphocyte phenotyping was performed on patients and healthy volunteers. Patients were evaluated at four different time points: prior to tumor resection, following tumor resection but prior to initiation of cytotoxic therapy, following initial chemoradiotherapy (7–13 weeks), and later in the treatment course (6–10 months). Peripheral blood was collected in evacuated tubes containing sodium heparin anticoagulant. Flow cytometric lymphocyte immunophenotyping was performed for each specimen using whole blood lysis technique after labeling with fluorochrome-conjugated monoclonal antibodies (mAbs) to CD3, CD4, CD8, CD19, CD45, CD16/56, TCR γδ (BD Biosciences, San Jose, CA), Vδ1 (Endogen, Rockford, IL, USA), and Vδ2 (BD Pharmingen).
Cytokine Assays
Plasma samples were obtained from patients at the time points described above and from healthy controls and analyzed by the multiplex suspension array system using Luminex beads (Bio-Rad Laboratories, Hercules, CA, USA), which included the following cytokines: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, IL-17, granulocyte/macrophage colony-stimulating factor, interferon γ (IFN-γ), monocyte chemotactic protein 1 (MCP-1), macrophage inflammatory protein 1β (MIP-1β), tumor necrosis factor α (TNF-α), and granulocyte-colony–stimulating factor. Plasma samples were thawed and diluted 1:1, and 100-μl aliquots were analyzed according to the manufacturer’s protocol. All samples were run in duplicate, and analytes were measured in picograms per milliliter of plasma. TGF-β1 was measured using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Bender Medsystems, Burlingame, CA, USA). Plasma specimens were diluted 1:10 using the assay buffer; 100-μl aliquots placed in duplicate wells of the ELISA plate, incubated (4 h, room temperature) on a microplate shaker, washed, and developed using the included HRP substrate. Absorbance was read at a wavelength of 450 nm.
Evaluation of γδ T-Cell Proliferative Capacity
Proliferative capacity of γδ T-cells was evaluated for both patients and healthy volunteers using previously described methods36 modified for use with clinically approvable reagents. Studies were performed at three different time points: prior to tumor resection, following tumor resection but prior to initiation of cytotoxic therapy, and following initial chemoradiotherapy (7–13 weeks). Peripheral blood mononuclear cells (PBMCs) were obtained by density-gradient centrifugation on Ficoll (Histopaque 1077; Sigma-Aldrich, St. Louis, MO, USA). After cell count, viability assessment (trypan blue dye exclusion), and flow cytometric immunophenotyping, cultures were initiated at a cell density of 1 × 106 cells/ml in RPMI-1640 with l-glutamine, enriched with 9% pooled human cGMP-grade serum (Labquip, Wood-bridge, ON, Canada), 10% 1 M HEPES (Mediatech), 1,000 U/ml human recombinant IFN-γ (Roche, Indianapolis, IN, USA), 10 U/ml human recombinant IL-12 (R&D Systems, Minneapolis, MN, USA), and 1 μg/ml mouse antihuman CD2 mAb clone CLB-CD2 6G4 (Baxter Oncology, Deerfield, IL, USA) at 5% CO2, 37°C. Twenty-four hours later, 10 ng/ml anti-CD3 mAb OKT3 (muromonab) (Ortho Biotech, Bridgewater, NJ, USA) and 300 U/ml human recombinant IL-2 (Roche) were added to the culture media. Cultures were maintained for 17–23 days with the addition of fresh complete RPMI as needed; human recombinant IL-2 was added weekly at a concentration of 10 U/ml. On the day of the assay, CD4+ and CD8+ T-cells were depleted from the PBMC cultures using immunomagnetic microspheres directly conjugated with the respective mAbs (Dynal InVitrogen, Carlsbad, CA, USA). This procedure routinely results in a cell product consisting of >70% expanded/activated γδ T-cells with the balance of the cells comprising a mixture of monocytes and CD8− NK cells.
Cytotoxicity Assays
Three methods were used to determine cytotoxicity based on the target cell. Initially, cytotoxicity of γδ T-cells against GBM cell lines in suspension was evaluated using a standard [51Cr] release assay. Cytotoxicity against GBM cells obtained from primary explant cultures was analyzed by flow cytometry adapted from Gomez-Roman et al.37 Target cells labeled with the membrane dye PKH26 (Sigma, St. Louis, MO, USA) were placed in aliquots of 1 × 105 in 3 ml polypropylene tubes. The required numbers of γδ T-cells were then added to the targets and incubated for 4 h at 37°C and 5% CO2. To-Pro iodide solution (20 μl; InVitrogen, Grand Island, NY, USA) was added to each tube immediately prior to acquisition on the flow cytometer. Cytotoxicity was calculated as the ratio of PKH26+To-Pro+ cells to total PKH26+ cells. We determined γδ T-cell cytotoxicity to adherent GBM cells using ATPlite luminescence assay system (PerkinElmer, Boston, MA, USA), with modifications to the manufacturer’s procedure to accommodate cell-based killing. Target cells (GBM cell lines) were washed, trypsinized, counted, plated in 96-well plates (1,000 cells/well), and incubated overnight. Effectors (enriched γδ T-cells) were then added to the wells in a constant volume. Wells containing only effector cells were included as an internal control. After a 4-h incubation, 50 μl ATP lysis solution was added to each well, followed by 50 μl ATP Lite reagent after 5 min. Plates were read on a luminometer (TopCount NXT, Packard, Meriden, CT, USA), and data were reported as counts per second (CPS). The mean CPS value of effectors was subtracted from that of the respective targets and divided by the mean control CPS value to give a percentage of viability for each group. In assays requiring both GBM cell lines and primary GBM explant cultures as targets, the same assay was used for both.
Statistical Methods
Matched data from patients and controls were analyzed using standard descriptive statistics and paired t-tests for evaluation of proliferative and cytotoxic functions. Relations among cytokines were analyzed using Pearson’s correlation for markers that are normally distributed (with variable transformation, if needed) and Spearman’s correlation for those that were nonparametric.
Results
Patients and Controls
A total of 18 patients and 18 healthy volunteers were initially enrolled in this study. Three patients were excluded for a diagnosis of WHO grade III glioma (n = 2) or comorbid conditions that did not allow for tumor resection (n = 1). Evaluable patient characteristics are detailed in Table 1. Nine patients had partial resection, three patients had gross total resection, and three patients received surgical intervention before being seen at UAB. Controls were obtained from a heterogeneous group of healthy volunteers. An older group of volunteers (41–78, n = 7) was enrolled to match the age range of the patients. A younger adult control group was also included (20–40, n = 11) to determine if there was an age-related effect on γδ T-cell absolute count and function.
Table 1.
Evaluable patient characteristics
| UPN | Age (Years) | Race/Sex | Surgery | Chemotherapy/Radiation | Time to Progression |
|---|---|---|---|---|---|
| 1 | 60 | W/F | Partial resection | TMZ + ABT-510/XRT | 6 months |
| 2 | 47 | W/F | Gross total resection | TMZ/XRT | 11 months |
| 3 | 77 | W/F | Partial resection | None | 0 months |
| 4 | 53 | W/F | Partial resection | TMZ/XRT | 13 months |
| 5 | 40 | W/F | Partial resection | TMZ/XRT | 10 months NEDa |
| 6 | 67 | W/F | Gross total resection | TMZ + ABT-510/XRT | 8 months out with NED |
| 7 | 58 | W/M | Gross total resection | None | Hospice post-op |
| 8 | 67 | W/M | Partial resection | TMZ/XRT | 5 months |
| 9 | 64 | AA/F | Partial resection | TMZ/XRT | 7 months out with SD |
| 10 | 79 | W/F | Partial resection; GW | Lost to F/U | Lost to F/U |
| 11 | 61 | W/M | Resection at OSH; GW | TMZ/XRT | 15 months |
| 12 | 50 | W/M | Resection at OSH | TMZ/XRT | 11 months |
| 13 | 71 | W/F | Partial resection | TMZ + ABT-510/XRT | 9 months |
| 14 | 74 | W/F | Resection at OSH | TMZ/XRT | Stage IV lung cancer→hospice |
| 15 | 53 | W/F | Partial resection | Lost to F/U | Lost to F/U |
Abbreviations: UPN, unique patient number; W, white; F, female; TMZ, temozolomide; XRT, radiation therapy; NED, no evidence of disease; M, male; AA, African American; SD, stable disease; GW, Gliadel wafers; F/U, follow-up; OSH, outside hospital.
But with radiation necrosis.
T-Cell Infiltration of GBM Prior to Resection
Immunohistochemical analysis of T-cell infiltration of GBM was performed on paraffin-embedded sections from five patients (patients 3, 4, 5, 8, 9 listed in Table 1). Aside from occasional observations of perivascular infiltration or “cuffing” of CD3+ cells, there was no evidence for infiltration of CD3+ cells or TCR-γδ+ cells deep within the tumor parenchyma (Fig. 1).
Fig. 1.
Immunohistochemical staining for CD3 in tumors from two GBM patients. Perivascular accumulation of T-cells is noted with little to no invasion of the tumor parenchyma.
T-Cell, NK Cell, and γδ T-Cell Subsets in Healthy Controls and GBM Patients
Lymphocyte phenotypes and absolute lymphocyte and subset counts were obtained as described above once for peripheral blood from healthy volunteers and at four specific intervals on peripheral blood from patients: prior to tumor resection (n = 9), following tumor resection but prior to initiation of cytotoxic therapy (3–23 days, n = 5), following initial chemoradiotherapy (7–13 weeks, n = 10), and later in the treatment course (6–10 months, n = 5). Data are shown in Fig. 2. The total T-cell count as well as CD3+CD4+ and CD3+CD8+ subsets did not differ between younger and older controls. There also were no differences in T-lymphocyte counts between older controls and patients prior to and immediately after resection. However, significant deterioration of T-cell numbers occurred in the interval following initial chemotherapy and radiotherapy and persisted throughout the course of treatment (Fig. 2A).
Fig. 2.
Absolute lymphocyte counts for younger (<45 years) and older (>45 years) healthy controls and for preresection GBM patients, early (3–23 days) postoperative patients prior to initiation of cytoreductive therapy, patients following completion of the first series of chemoradiotherapy, and patients very late (VL; 7–13 weeks) into the treatment course. The average age of patients shown is 59 years. (A) Absolute CD3, CD4, and CD8 counts. T-lymphocyte and CD4 and CD8 subset counts were maintained until completion of the first round of cytoreductive therapy, after which time they did not recover significantly. (B) Total absolute γδ T-cell counts did not differ between younger and older populations of healthy volunteers (p = 0.29), but a trend toward significance was seen in patients at both the preoperative and early postoperative stages compared with older controls (p = 0.07 and p = 0.08, respectively), and counts significantly decreased after cytoreductive therapy (p = 0.02 and p = 0.01). (C) NK counts, which fell slightly but not significantly after resection and throughout the course of therapy.
Total absolute γδ T-cell counts did not differ between younger and older populations of healthy volunteers (p = 0.29). We observed a trend that approached significance for a decrease of total γδ T-cell in patients at both the preoperative and early postoperative stages compared with older controls (p = 0.07 and p = 0.08, respectively) as shown in Fig. 2B. Compared with healthy volunteers, there was a significant decrease in the number of γδ T-cells in patients after cytoreductive therapy (p = 0.02 and p = 0.01). There was no significant change in the ratio of Vδ1 to Vδ2 cells at any time point, but a trend showing a greater decrease in the Vδ2 population in the patient groups was noted (data not shown). NK cell numbers were significantly higher in the older controls than in the younger healthy controls (p = 0.02). NK cell counts also fell following tumor resection and throughout the course of therapy but were not significantly reduced from NK counts obtained from older control volunteers at any point (Fig. 2C).
Cytokines
We assessed specific immunoregulatory cytokines and soluble factors from plasma obtained from healthy controls and patients at the same time intervals that lymphocyte phenotyping and functional studies were performed. This was done to obtain a clearer understanding of factors that could influence lymphocyte function at each sampling interval. These data are shown in Table 2. There were no differences between controls and preresection patients for cytokines IL-1β, IL-2, IL-4, IL-10, IL-12, IL-15, eotaxin, RANTES, IFN-γ, TNF-α, platelet-derived growth factor, fibroblast growth factor, MCP-1, TGF-β, vascular epithelial growth factor (VEGF), interferon-γ–inducible protein (IP-10), and IL-1 receptor antagonist (IL-1rα). Five cytokines were increased in plasma samples taken at one or more time points during diagnosis and treatment. TGF-β was increased after resection and therapy. VEGF was increased prior to and immediately following tumor resection but returned to control levels following radiation and chemotherapy. MIP-1β was increased over control levels at all sampling times, whereas IP-10 and IL-1rα increased following radiation and chemotherapy.
Table 2.
Plasma immunoregulatory cytokines and soluble factors for healthy controls and patients
| Cytokine | Source/Interval | Concentration (pg/ml Plasma) | p-Value |
|---|---|---|---|
| TGF-β | Control | 0.02 ± 0.01 | |
| Patient preresection | 0.04 ± 0.01 | 0.092 | |
| Patient postresection | 0.06 ± 0.02 | 0.047* | |
| Patient posttherapy | 0.04 ± 0.01 | 0.015* | |
| VEGF | Control | 29.71 ± 8.16 | |
| Patient preresection | 95.09 ± 40.19 | 0.172 | |
| Patient postresection | 95.63 ± 23.48 | 0.038* | |
| Patient posttherapy | 68.95 ± 18.22 | 0.750 | |
| IP-10 | Control | 350.32 ± 133.11 | |
| Patient preresection | 213.05 ± 50.34 | 0.379 | |
| Patient postresection | 450.16 ± 57.51 | 0.522 | |
| Patient posttherapy | 1,036.31 ± 250.92 | 0.034* | |
| MIP-1β | Control | 17.14 ± 2.94 | |
| Patient preresection | 29.68 ± 3.14 | 0.017* | |
| Patient postresection | 32.19 ± 2.85 | 0.005* | |
| Patient posttherapy | 45.27 ± 9.48 | 0.020* | |
| IL-1rα | Control | 33.53 ± 9.68 | |
| Patient preresection | 93.26 ± 25.07 | 0.067 | |
| Patient postresection | 104.96 ± 35.56 | 0.101 | |
| Patient posttherapy | 112.42 ± 22.49 | 0.008* |
Abbreviations: TGF-β, transforming growth factor β; VEGF, vascular epithelial growth factor; IP-10, interferon-γ–inducible protein; MIP-1β, microphage inflammatory protein 1β; IL-1rα, interleukin-1 receptor antagonist.
Significant at p < 0.05.
γδ T-Cell Function in Healthy Controls and GBM Patients
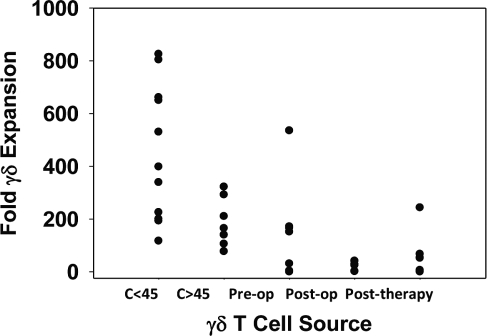
Proliferative and cytotoxic functions of γδ T-cells were independently assessed using a modified mitogen proliferation assay and by a cytotoxicity assay for γδ T-cells against immortalized GBM cell lines and primary GBM cultures. Assays were performed once for both groups of healthy volunteers and at four specific intervals for patients prior to tumor resection (n = 7), following tumor resection but prior to initiation of cytotoxic therapy (3–23 days postoperative; n = 5), following initial chemoradiotherapy (7–13 weeks postoperative; n = 10), and later in the treatment course (6–10 months; n = 5). Fig. 3 shows the mean fold expansion of γδ T-cells from healthy controls and patients in response to mitogenic stimulation. Younger healthy controls tended to show more robust proliferation compared with older healthy controls from the patients’ age range (p = 0.057). Although there was no statistical difference between older healthy controls and GBM patients prior to resection with regard to proliferative response (p = 0.46), we did note that γδ T-cells from two GBM patients showed essentially no response to mitogenic stimulation. Proliferative responses fell significantly after resection, with some evidence of only a slight recovery after cytoreductive therapy (p = 0.001 and p = 0.004, respectively).
Fig. 3.
Proliferation assay for γδ T-cells. γδ T-cells from younger (<45 years; C<45) donors show a trend toward more robust expansion compared with older (>45 years; C>45) donors (p = 0.57). In addition, γδ T-cells from some newly diagnosed GBM patients (pre-op) still retain the ability to proliferate as well as older controls (p = 0.65), but this function drops off rapidly after resection (post-op; p = 0.001) and following the first series of cytoreductive therapy (posttherapy; p = 0.004).
Mitogen-stimulated expanded/activated γδ T-cells showed lytic activity against standard GBM cell lines and against primary short-term cultures from two GBM tumor explants but did not kill normal astrocytes (Fig. 4). This activity was immediate and was more effective than that seen with mitogen-activated αβ T-cells (Fig. 4A). Expanded/activated γδ T-cells from healthy controls killed GBM cells from both established GBM cell lines and cultured primary tumor explants. This was shown in both suspension (Fig. 4B) and adherent cytotoxicity (Fig. 4C) assays.
Fig. 4.
Antitumor activity of expanded/activated γδ T-cells demonstrated against human glioma cell line U251MG. (A) Expanded γδ T-cells (G/D T-cells) and expanded αβ T-cells (A/B T-cells) from three separate donors were sorted to >95% purity. U251MG glioma cells were labeled with [51Cr] and incubated with γδ T-cells (open circles) or αβ T-cells (solid circles) for 4 h at the indicated effector to target (E:T) ratios. Supernatants were removed to determine [51Cr] release in counts per minute. Data are presented at the mean ± SD percent target lysis from triplicate determinations. (B) Antitumor activity of expanded γδ T-cells against cultured primary human astrocytes, selected GBM cell lines, and primary GBM explant cultures (GBM 1 and 2) at an effector to target ratio of 16:1. Targets were labeled with PKH26 and incubated with γδ T-cells for 4 h at the indicated effector to target ratios. Live:dead ratios were calculated from the percentage of target cells that incorporated the DNA stain To-Pro iodide as determined by flow cytometry. (C) Killing of adherent primary GBM explant cultures at incubation with expanded/activated γδ T-cells as determined by a commercial ATP release assay.
Most important, when we were able to successfully activate and expand γδ T-cells from a GBM patient, these cells were also cytotoxic. Fig. 5 shows cytotoxicity to the immortalized GBM cell line U251 of frozen/thawed γδ T-cells from patient 6 obtained prior to and after resection. These data suggest that activated γδ T-cells from GBM patients will also lyse tumor cells.
Fig. 5.
Expanded/activated γδ T-cells from patient 6 obtained prior to resection (preoperative) and after resection but prior to cytoreductive therapy (postoperative). The γδ T-cells were cryopreserved, stored, and thawed, and cytotoxicity was determined against U251MG cells.
Discussion
The principal objective of this study was to examine whether γδ T-cells could play a role in the immune response to GBM and, by extension, to assess the potential value of γδ T-cell–based therapy against GBM. We sought to place these findings in the context of total peripheral blood lymphocyte composition and immune function at several time points over the patients’ course from diagnosis through therapy. We compared immune status and function in both younger and older patients to identify a group of healthy older individuals that are more closely matched to the age of our GBM patient population while also evaluating younger individuals that approach the age range of the patients’ children who would be the likely haploidentical donors for innate lymphocyte cell therapy clinical trials.
Lymphocyte subsets did not differ between younger and older controls with the exception of a significantly increased NK cell count in older individuals (Fig. 2B), a finding that is consistent with previously published reports.38 We also noted lower absolute γδ T-cell counts and decreased proliferative response to mitogen-stimulation function in older volunteers, although not to the point of statistical significance. These findings are consistent with a larger previous study of healthy individuals younger than 50 and older than 60 years of age that showed decreasing γδ T-cell counts with age, principally due to a declining Vδ2 population.39
Generally, absolute counts of αβ T-cell and CD4/CD8 αβ T-cell subsets did not significantly change in GBM patients prior to cytoreductive therapy. This is in contrast with γδ T-cell absolute counts, which were reduced in patients at initial presentation. Decreased γδ T-cell count has been previously reported in other malignancies,40–42 has generally been associated with activation-induced cell death (AICD) resulting from γδ T-cell–tumor contact, and is principally due to selective depletion of the Vδ2 population. We could not document significant infiltration of the tumors by γδ T-cells (Fig. 1) or a significant depletion of any particular Vδ subtype in GBM patients. The combined effect of AICD, tumor-derived cytokines, and postresection dexamethasone therapy could all contribute to a global decrease in circulating γδ T-cells.
We also showed that ex vivo activated/expanded γδ T-cells from healthy controls can effectively kill GBM cell lines and cultured cells from primary GBM tumor explants while sparing cultured astrocytes, and that patient-derived γδ T-cells can also recognize and kill immortalized GBM cell lines. These findings expand previous reports that described cytotoxicity of ex vivo activated γδ tumor-infiltrating lymphocytes against GBM cell lines.43,44 Moreover, these data suggest that GBM tumors express specific ligands that are innately recognized by γδ T-cells ligands and are not expressed by normal cultured astrocytes. Indeed, Friese et al.25 showed that many immortalized GBM lines express mRNA for known stress-associated antigens such as MIC-A/B and the UL-16–binding proteins, known ligands for γδ T-cells.27,32,45–47 To this point, quantitative measurement of stable surface expression of these ligands in freshly resected glioma tissues or in a broader panel of human malignant glioma cell lines has not been systematically evaluated.
Mitogen-stimulated proliferation of γδ T-cells is impaired in patients with GBM prior to and following resection, although not completely abolished. Several factors that are known to suppress the general antitumor immune response could contribute to this finding, including perioperative dexamethasone therapy, the recruitment of immunosuppressive mesenchymal stromal cells into the tumor parenchyma, and the effects of tumor-derived cytokines. TGF-β can effectively paralyze the immune response to the tumor via multiple mechanisms, such as inhibition of DC maturation, antigen presentation, inhibition of T-cell activation, and expansion of CD3+CD4+FoxP3+ regulatory T-cells.48–50 Indeed, a significant progressive increase in plasma TGF-β was seen in this group of patients compared with healthy controls (Table 2).
Cellular immune responses are influenced by a variety of factors, including multiple cell types, the local tumor environment, circulating cytokines and soluble factors, and the impact of surgery, chemotherapy, and radiation. We examined plasma cytokine concentrations at the defined testing intervals specified above to have a more complete understanding of immune status and for factors that could impact γδ T-cell and general immune function. In addition, the release of inflammatory cytokines during radiation and chemotherapy can substantially alter immune function and increase the sensitivity of tumors to a cellular immune response.51 The increased expression of inflammatory cytokines such as MIP-1β, IL-1rα, and IP-10, although from a small sample of patients, is consistent with an ongoing inflammatory response. MIP-1β is one of a family of macrophage inflammatory proteins that mediate acute and chronic inflammatory host responses. These proteins are crucial for T-cell chemotaxis to inflamed tissue and for transendothelial migration of monocytes, DCs, and NK cells.52 MIP-1β is also expressed within tumors and by activated tumor-infiltrating lymphocytes.53 GBM-derived autocrine production of IL-1rα can block growth-inhibiting IL-1, resulting in aggressive tumor growth,54 another of the many escape mechanisms employed by this tumor. IP-10 plays a central role in recruiting effector T-cells to sites of CNS inflammation55 and, more specific to this study, has been shown to facilitate the migration of adoptively transferred T-cells to GBM tumors through brain tissue and to increase T-cell cytotoxic activity against GBM,56,57 findings that could ultimately help to guide the timing of T-cell–based immunotherapies.
As discussed above, plasma TGF-β is known to be produced by the tumor and is increased in GBM patients in relation to tumor burden.58 Increased VEGF production is also a known mechanism of GBM growth,59 but the timing of our observation (e.g., following resection) may be more likely due to postsurgical wound repair. These findings provide a framework for future studies that will determine their impact on the innate immune response to GBM and the potential efficacy of donor innate lymphocyte immunotherapy.
Pending the results of further studies, the possibility for translation of these findings into phase I/II safety/efficacy trials is enhanced by the recent publication of several methods for the large-scale expansion of γδ T-cells for immunotherapeutic applications. These include clinically approved nitrogen-containing bisphosphonates (NBPs) such as zoledronate60,61 and bromohydrin pyrophosphate62 in combination with IL-2, as well as the investigational two-stage expansion method using CD2, IFN-γ, IL-12, OKT3, and IL-236,63 described above. Effector cells generated ex vivo by these methods have shown potent innate antitumor activity against a wide variety of human tumor cell lines.36,64 Systemic injection of NBPs has also resulted in expansion of circulating γδ T-cells in patients with lymphoma65 and renal cell carcinoma,66 although the degree of tumor reduction has been modest.
In summary, these data strongly support a potential role for donor innate lymphocyte immunotherapy with γδ T-cells as an approach to the management of GBM. The absolute γδ T-cell count is reduced and γδ T-cell proliferative function is impaired in GBM patients, but ex vivo expanded/activated apoptosis-resistant γδ T-cells from healthy donors are highly cytotoxic to GBM cell lines and short-passage primary tumor explants. Moreover, these γδ T-cells are not cytotoxic to cultured astrocytes, a finding that suggests that γδ T-cells could be safely used as local intracranial therapy. Studies currently under way in our laboratory and others should begin to clarify the role of GBM-derived immunosuppressive factors on γδ T-cell survival, migration, and function.
Acknowledgments
We gratefully acknowledge financial support from the National Institutes of Health NCI grant P50 CA 097247-06A1 (J.M.M. and G.Y.G.), National Institute of Neurological Disorders and Stroke grant R21 NS057431-01A1 (L.S.L.), and the Brain Tumor Society Samuel Gershon Leadership Chair (L.S.L.).
References
- 1.Castro MG, Cowen R, Williamson IK, et al. Current and future strategies for the treatment of malignant brain tumors. Pharmacol Ther. 2003;98:71–108. doi: 10.1016/s0163-7258(03)00014-7. [DOI] [PubMed] [Google Scholar]
- 2.Merchant RE, Ellison MD, Young HF. Immunotherapy for malignant glioma using human recombinant interleukin-2 and activated autologous lymphocytes. A review of pre-clinical and clinical investigations. J Neurooncol. 1990;8:173–188. doi: 10.1007/BF00177842. [DOI] [PubMed] [Google Scholar]
- 3.Merchant RE, Baldwin NG, Rice CD, Bear HD. Adoptive immunotherapy of malignant glioma using tumor-sensitized T lymphocytes. Neurol Res. 1997;19:145–152. doi: 10.1080/01616412.1997.11740788. [DOI] [PubMed] [Google Scholar]
- 4.Farkkila M, Jaaskelainen J, Kallio M, et al. Randomised, controlled study of intratumoral recombinant gamma-interferon treatment in newly diagnosed glioblastoma. Br J Cancer. 1994;70:138–141. doi: 10.1038/bjc.1994.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahaley MS, Jr, Bertsch L, Cush S, Gillespie GY. Systemic gamma-interferon therapy for recurrent gliomas. J Neurosurg. 1988;69:826–829. doi: 10.3171/jns.1988.69.6.0826. [DOI] [PubMed] [Google Scholar]
- 6.Boiardi A, Silvani A, Ruffini PA, et al. Loco-regional immunotherapy with recombinant interleukin-2 and adherent lymphokine-activated killer cells (A-LAK) in recurrent glioblastoma patients. Cancer Immunol Immunother. 1994;39:193–197. doi: 10.1007/BF01533386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rainov NG, Kramm CM, Banning U, et al. Immune response induced by retrovirus-mediated HSV-tk/GCV pharmacogene therapy in patients with glioblastoma multiforme. Gene Ther. 2000;7:1853–1858. doi: 10.1038/sj.gt.3301311. [DOI] [PubMed] [Google Scholar]
- 8.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen-specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 9.Plautz G, Barnett G, Miller D, et al. Systemic T cell adoptive immunotherapy of malignant gliomas. J Neurosurg. 1998;89:42–51. doi: 10.3171/jns.1998.89.1.0042. [DOI] [PubMed] [Google Scholar]
- 10.Quattrocchi KB, Miller CH, Cush S, et al. Pilot study of local autologous tumor infiltrating lymphocytes for the treatment of recurrent malignant gliomas. J Neurooncol. 1999;45:141–157. doi: 10.1023/a:1006293606710. [DOI] [PubMed] [Google Scholar]
- 11.Kruse CA, Cepeda L, Owens B, Johnson SD, Stears J, Lillehei KO. Treatment of recurrent glioma with intracavitary alloreactive cytotoxic T lymphocytes and interleukin-2. Cancer Immunol Immunother. 1997;45:77–87. doi: 10.1007/s002620050405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Read SB, Kulprathipanja NV, Gomez GG, et al. Human alloreactive CTL interactions with gliomas and with those having upregulated HLA expression from exogenous IFN-gamma or IFN-gamma gene modification. J Interferon Cytokine Res. 2003;23:379–393. doi: 10.1089/107999003322226032. [DOI] [PubMed] [Google Scholar]
- 13.Komatsu F, Kajiwara M. CD18/CD54(+CD102), CD2/CD58 pathway-independent killing of lymphokine-activated killer (LAK) cells against glioblastoma cell lines T98G and U373MG. Oncol Res. 2000;12:17–24. doi: 10.3727/000000001108747408. [DOI] [PubMed] [Google Scholar]
- 14.Wiendl H, Mitsdoerffer M, Hofmeister V, et al. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol. 2002;168:4772–4780. doi: 10.4049/jimmunol.168.9.4772. [DOI] [PubMed] [Google Scholar]
- 15.Wischhusen J, Friese MA, Mittelbronn M, Meyermann R, Weller M. HLA-E protects glioma cells from NKG2D-mediated immune responses in vitro: implications for immune escape in vivo. J Neuropathol Exp Neurol. 2005;64:523–528. doi: 10.1093/jnen/64.6.523. [DOI] [PubMed] [Google Scholar]
- 16.Plautz GE, Barnett GH, Miller DW, et al. Systemic T cell adoptive immunotherapy of malignant gliomas. J Neurosurg. 1998;89:42–51. doi: 10.3171/jns.1998.89.1.0042. [DOI] [PubMed] [Google Scholar]
- 17.Smyth M, Strobl S, Young H, Ortaldo J, Ochoa A. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8+ T lymphocytes. Inhibition by transforming growth factor-beta. J Immunol. 1991;146:3289–3297. [PubMed] [Google Scholar]
- 18.Inge T, McCoy K, Susskind B, Barrett S, Zhao G, Bear H. Immunomodulatory effects of transforming growth factor-beta on T lymphocytes. Induction of CD8 expression in the CTLL-2 cell line and in normal thymocytes. J Immunol. 1992;148:3847–3856. [PubMed] [Google Scholar]
- 19.Barba D, Saris SC, Holder C, Rosenberg SA, Oldfield EH. Intratumoral LAK cell and interleukin-2 therapy of human gliomas. J Neurosurg. 1989;70:175–182. doi: 10.3171/jns.1989.70.2.0175. [DOI] [PubMed] [Google Scholar]
- 20.Saris SC, Patronas NJ, Rosenberg SA, et al. The effect of intravenous interleukin-2 on brain water content. J Neurosurg. 1989;71:169–174. doi: 10.3171/jns.1989.71.2.0169. [DOI] [PubMed] [Google Scholar]
- 21.Hayes RL, Koslow M, Hiesiger EM, et al. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995;76:840–852. doi: 10.1002/1097-0142(19950901)76:5<840::aid-cncr2820760519>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 22.Dillman RO, Duma CM, Schiltz PM, et al. Intracavitary placement of autologous lymphokine-activated killer (LAK) cells after resection of recurrent glioblastoma. J Immunother. 2004;27:398–404. doi: 10.1097/00002371-200409000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Wischhusen J, Friese MA, Mittelbronn M, Meyermann R, Weller M. HLA-E protects glioma cells from NKG2D-mediated immune responses in vitro: implications for immune escape in vivo. J Neuropathol Exp Neurol. 2005;64:523–528. doi: 10.1093/jnen/64.6.523. [DOI] [PubMed] [Google Scholar]
- 24.Wiendl H, Mitsdoerffer M, Hofmeister V, et al. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol. 2002;168:4772–4780. doi: 10.4049/jimmunol.168.9.4772. [DOI] [PubMed] [Google Scholar]
- 25.Friese MA, Platten M, Lutz SZ, et al. MICA/NKG2D-mediated immunogene therapy of experimental gliomas. Cancer Res. 2003;63:8996–9006. [PubMed] [Google Scholar]
- 26.Girardi M, Oppenheim DE, Steele CR, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science. 2001;294:605–609. doi: 10.1126/science.1063916. [DOI] [PubMed] [Google Scholar]
- 27.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial gammadelta T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 28.Kaminski MJ, Cruz PD, Jr, Bergstresser PR, Takashima A. Killing of skin-derived tumor cells by mouse dendritic epidermal T-cells. Cancer Res. 1993;53:4014–4019. [PubMed] [Google Scholar]
- 29.Morita C, Mariuzza R, Brenner M. Antigen recognition by human gamma delta T cells: pattern recognition by the adaptive immune system. Springer Semin Immunopathol. 2000;22:191–217. doi: 10.1007/s002810000042. [DOI] [PubMed] [Google Scholar]
- 30.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 31.Bauer S, Groh V, Wu J, et al. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727–729. doi: 10.1126/science.285.5428.727. [DOI] [PubMed] [Google Scholar]
- 32.Groh V, Rhinehart R, Secrist H, Bauer S, Grabstein KH, Spies T. Broad tumor-associated expression and recognition by tumor-derived gamma delta T cells of MICA and MICB. Proc Natl Acad Sci U S A. 1999;96:6879–6884. doi: 10.1073/pnas.96.12.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujimiya Y, Suzuki Y, Katakura R, et al. In vitro interleukin 12 activation of peripheral blood CD3(+)CD56(+) and CD3(+)CD56(–) gammadelta T cells from glioblastoma patients. Clin Cancer Res. 1997;3:633–643. [PubMed] [Google Scholar]
- 34.Yamaguchi T, Fujimiya Y, Suzuki Y, Katakura R, Ebina T. A simple method for the propagation and purification of gamma delta T cells from the peripheral blood of glioblastoma patients using solid-phase anti-CD3 antibody and soluble IL-2. J Immunol Methods. 1997;205:19–28. doi: 10.1016/s0022-1759(97)00062-8. [DOI] [PubMed] [Google Scholar]
- 35.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 36.Lopez RD, Xu S, Guo B, Negrin RS, Waller EK. CD2-mediated IL-12-dependent signals render human gamma-delta T cells resistant to mitogen-induced apoptosis, permitting the large-scale ex vivo expansion of functionally distinct lymphocytes: implications for the development of adoptive immunotherapy strategies. Blood. 2000;96:3827–3837. [PubMed] [Google Scholar]
- 37.Gomez-Roman VR, Florese RH, Patterson LJ, et al. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods. 2006;308:53–67. doi: 10.1016/j.jim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 38.Argentati K, Re F, Donnini A, et al. Numerical and functional alterations of circulating gammadelta T lymphocytes in aged people and centenarians. J Leukoc Biol. 2002;72:65–71. [PubMed] [Google Scholar]
- 39.Argentati K, Re F, Serresi S, et al. Reduced number and impaired function of circulating gamma delta T cells in patients with cutaneous primary melanoma. J Invest Dermatol. 2003;120:829–834. doi: 10.1046/j.1523-1747.2003.12141.x. [DOI] [PubMed] [Google Scholar]
- 40.Ferrarini M, Heltai S, Toninelli E, et al. Daudi lymphoma killing triggers the programmed death of cytotoxic V gamma 9/V delta 2 T lymphocytes. J Immunol. 1995;154:3704–3712. [PubMed] [Google Scholar]
- 41.Meeh PF, King M, O’Brien RL, et al. Characterization of the gamma-delta T cell response to acute leukemia. Cancer Immunol Immunother. 2006;55:1072–1080. doi: 10.1007/s00262-005-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamaguchi T, Fujimiya Y, Suzuki Y, Katakura R, Ebina T. A simple method for the propagation and purification of gamma delta T cells from the peripheral blood of glioblastoma patients using solid-phase anti-CD3 antibody and soluble IL-2. J Immunol Methods. 1997;205:19–28. doi: 10.1016/s0022-1759(97)00062-8. [DOI] [PubMed] [Google Scholar]
- 43.Yamaguchi T, Suzuki Y, Katakura R, Ebina T, Yokoyama J, Fujimiya Y. Interleukin-15 effectively potentiates the in vitro tumor-specific activity and proliferation of peripheral blood gammadeltaT cells isolated from glioblastoma patients. Cancer Immunol Immunother. 1998;47:97–103. doi: 10.1007/s002620050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Catellani S, Poggi A, Bruzzone A, et al. Expansion of Vdelta1 T lymphocytes producing IL-4 in low-grade non-Hodgkin lymphomas expressing UL-16-binding proteins. Blood. 2007;109:2078–2085. doi: 10.1182/blood-2006-06-028985. [DOI] [PubMed] [Google Scholar]
- 45.Poggi A, Venturino C, Catellani S, et al. Vdelta1 T lymphocytes from B-CLL patients recognize ULBP3 expressed on leukemic B cells and up-regulated by trans-retinoic acid. Cancer Res. 2004;64:9172–9179. doi: 10.1158/0008-5472.CAN-04-2417. [DOI] [PubMed] [Google Scholar]
- 46.Wu J, Groh V, Spies T. T cell antigen receptor engagement and specificity in the recognition of stress-inducible MHC class I-related chains by human epithelial gamma delta T cells. J Immunol. 2002;169:1236–1240. doi: 10.4049/jimmunol.169.3.1236. [DOI] [PubMed] [Google Scholar]
- 47.Smyth MJ, Strobl SL, Young HA, Ortaldo JR, Ochoa AC. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8+ T lymphocytes. Inhibition by transforming growth factor-beta. J Immunol. 1991;146:3289–3297. [PubMed] [Google Scholar]
- 48.Inge TH, McCoy KM, Susskind BM, Barrett SK, Zhao G, Bear HD. Immunomodulatory effects of transforming growth factor-beta on T lymphocytes. Induction of CD8 expression in the CTLL-2 cell line and in normal thymocytes. J Immunol. 1992;148:3847–3856. [PubMed] [Google Scholar]
- 49.Jachimczak P, Bogdahn U, Schneider J, et al. The effect of transforming growth factor-beta 2-specific phosphorothioate-anti-sense oligodeoxynucleotides in reversing cellular immunosuppression in malignant glioma. J Neurosurg. 1993;78:944–951. doi: 10.3171/jns.1993.78.6.0944. [DOI] [PubMed] [Google Scholar]
- 50.Zitvogel L, Apetoh L, Ghiringhelli F, Kroemer G. Immunological aspects of cancer chemotherapy. Nat Rev Immunol. 2008;8:59–73. doi: 10.1038/nri2216. [DOI] [PubMed] [Google Scholar]
- 51.Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 52.Gough M, Crittenden M, Thanarajasingam U, et al. Gene therapy to manipulate effector T cell trafficking to tumors for immunotherapy. J Immunol. 2005;174:5766–5773. doi: 10.4049/jimmunol.174.9.5766. [DOI] [PubMed] [Google Scholar]
- 53.Oelmann E, Kraemer A, Serve H, et al. Autocrine interleukin-1 receptor antagonist can support malignant growth of glioblastoma by blocking growth-inhibiting autocrine loop of interleukin-1. Int J Cancer. 1997;71:1066–1076. doi: 10.1002/(sici)1097-0215(19970611)71:6<1066::aid-ijc25>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 54.Christensen JE, de Lemos C, Moos T, Christensen JP, Thomsen AR. CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system. J Immunol. 2006;176:4235–4243. doi: 10.4049/jimmunol.176.7.4235. [DOI] [PubMed] [Google Scholar]
- 55.Honeth G, Staflin K, Kalliomaki S, Lindvall M, Kjellman C. Chemokine-directed migration of tumor-inhibitory neural progenitor cells towards an intracranially growing glioma. Exp Cell Res. 2006;312:1265–1276. doi: 10.1016/j.yexcr.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 56.Nishimura F, Dusak JE, Eguchi J, et al. Adoptive transfer of type 1 CTL mediates effective anti-central nervous system tumor response: critical roles of IFN-inducible protein-10. Cancer Res. 2006;66:4478–4487. doi: 10.1158/0008-5472.CAN-05-3825. [DOI] [PubMed] [Google Scholar]
- 57.Walker PR, Calzascia T, Dietrich PY. All in the head: obstacles for immune rejection of brain tumours. Immunology. 2002;107:28–38. doi: 10.1046/j.1365-2567.2002.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunn IF, Heese O, Black PM. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol. 2000;50:121–137. doi: 10.1023/a:1006436624862. [DOI] [PubMed] [Google Scholar]
- 59.Sato K, Kimura S, Segawa H, et al. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116:94–99. doi: 10.1002/ijc.20987. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe N, Narita M, Yokoyama A, et al. Type I IFN-mediated enhancement of anti-leukemic cytotoxicity of gammadelta T cells expanded from peripheral blood cells by stimulation with zoledronate. Cytotherapy. 2006;8:118–129. doi: 10.1080/14653240600620200. [DOI] [PubMed] [Google Scholar]
- 61.Salot S, Laplace C, Saiagh S, et al. Large scale expansion of gamma 9 delta 2 T lymphocytes: Innacell gamma delta cell therapy product. J Immunol Methods. 2007;326:63–75. doi: 10.1016/j.jim.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 62.Guo B, Hollmig K, Lopez RD. Down-regulation of IL-2 receptor a (CD25) characterizes human gd-T cells rendered resistant to apoptosis after CD2 engagement in the presence of IL-12. Cancer Immunol Immunother. 2002;50:625–637. doi: 10.1007/s00262-001-0244-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo B, Hollmig K, Lopez RD. In vitro activity of apoptosis-resistant human gd-T cells against solid malignances [abstract] J Clin Oncol. 2001;20:267. [Google Scholar]
- 64.Wilhelm M, Kunzmann V, Eckstein S, et al. Gammadelta T cells for immune therapy of patients with lymphoid malignancies. Blood. 2003;102:200–206. doi: 10.1182/blood-2002-12-3665. [DOI] [PubMed] [Google Scholar]
- 65.Bennouna J, Bompas E, Neidhardt EM, et al. Phase-I study of Innacell gammadeltatrade mark, an autologous cell-therapy product highly enriched in gamma9delta2 T lymphocytes, in combination with IL-2, in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1599–1609. doi: 10.1007/s00262-008-0491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]