Abstract
Lactate dehydrogenase type A (LDH-A) is a key metabolic enzyme catalyzing pyruvate into lactate and is excessively expressed by tumor cells. Transforming growth factor-β2 (TGF-β2) is a key regulator of invasion in high-grade gliomas, partially by inducing a mesenchymal phenotype and by remodeling the extracellular matrix. In this study, we tested the hypothesis that lactate metabolism regulates TGF-β2–mediated migration of glioma cells. Small interfering RNA directed against LDH-A (siLDH-A) suppresses, and lactate induces, TGF-β2 expression, suggesting that lactate metabolism is strongly associated with TGF-β2 in glioma cells. Here we demonstrate that TGF-β2 enhances expression, secretion, and activation of matrix metalloproteinase-2 (MMP-2) and induces the cell surface expression of integrin αvβ3 receptors. In spheroid and Boyden chamber migration assays, inhibition of MMP-2 activity using a specific MMP-2 inhibitor and blocking of integrin αvβ3 abrogated glioma cell migration stimulated by TGF-β2. Furthermore, siLDH-A inhibited MMP2 activity, leading to inhibition of glioma migration. Taken together, we define an LDH-A–induced and TGF-β2–coordinated regulatory cascade of transcriptional regulation of MMP-2 and integrin αvβ3. This novel interaction between lactate metabolism and TGF-β2 might constitute a crucial mechanism for glioma migration.
Keywords: glioma, lactate, LDH-A, MMP-2, TGF-β2
Transforming growth factor-β (TGF-β) is a key player of glioma carcinogenesis.1 Its isoform TGF-β2 plays a pivotal role as an autocrine stimulus of growth and dedifferentiation.2 Besides autocrine effects, various other—mainly paracrine—functions emphasize the role of TGF-β as a highly potent suppressor of immune reactions, inductor of angiogenesis, and promoter of cell motility and malignant invasive capacity.3–6 TGF-β is induced by several mechanisms; however, a potential regulation by metabolic events has not been investigated so far.
Changes in tumor metabolism are known to contribute significantly to the malignant course of solid tumors. It is well known that tumor cells use aerobic glycolysis despite sufficient oxygen levels, producing high amounts of lactate.7,8 As a consequence, the extracellular pH decreases significantly,9 leading to apoptosis of nontumor cells10,11 and invasion of malignant cells into the parenchyma following a front of acidic microenvironment.12 In some solid tumors, certain extracellular matrix (ECM) proteins are induced by lactate. An altered extracellular environment may therefore permit enhanced migration of tumor cells.13,14 Lactate dehydrogenase (LDH) is a key metabolic enzyme catalyzing the transition of pyruvate to lactate in glycolysis. Tumor cells express enhanced levels of LDH, and LDH serves as tumor marker in some entities.15–18 Because TGF-β is a known stimulator of glioma invasion, and because metabolic events trigger migration of solid tumor cells, there might be a regulatory cascade that starts with LDH-mediated regulation of TGF-β, leading to molecular events downstream of TGF-β, that explain the enhanced migratory capacity of gliomas.
Matrix metalloproteinases (MMPs) are a growing family of zinc-dependent endopeptidases that are capable of degrading various components of the ECM. The proteolytic cleavage of ECM governed by cell surface and soluble MMPs is critically involved in many physiological and pathophysiological processes, including tumor cell growth, proliferation, migration, and invasion.19 Among the MMPs, MMP-2 and MMP-9, also named gelatinase A and gelatinase B, are strongly associated with malignant progression and matrix remodeling. MMP-2 is expressed in vivo in normal neurons and glia and in malignant glioma cells and blood vessels, and in vitro in glioma cell lines. The expression of MMP-2 is dramatically upregulated in high-grade gliomas compared with low-grade gliomas and normal brain tissue, correlating with the malignant progression of human gliomas in vivo.20 In contrast, the expression of MMP-9 is more restricted and is preferentially found in blood vessels at proliferating margins, as well as tumor cells in some cases in vivo.21–24 MMP-9 expression has also been demonstrated to correlate with increasing malignancy in glial tumors but is closely linked to angiogenesis, demonstrated by immunohistological and in situ hybridization histochemical localization of MMP-9 within and around the vasculature.22,23
Several in vitro studies showed that MMP-2 activation modulates glioma cell migration and invasion.25,26 The transcription of MMP genes is likely to be mediated by intracellular signals in response to impinging growth factors and cytokines, ECM composition, and likely other unidentified factors that compose the tumor microenvironment.27
In previous studies, increasing concentrations of recombinant human TGF-β2 (rhTGF-β2) revealed induction of MMP-2 protein levels in human glioma cell lines and in primary cell cultures of human brain tumors.28,29 It is unclear at this point how MMP-2 transcription is induced by TGF-β2 in gliomas.
Integrins are cell surface receptor proteins that bind to the ECM and mediate signal transduction. An integrin molecule consists of two noncovalently associated transmembrane glycoprotein subunits, α and β. They act as specific adhesion receptors active in glioma–ECM adhesion and play a major role in glioma cell–matrix interactions.14 Integrin αvβ3 has been found to play a particular role in gliomas. Both integrin αvβ3 and its ligand vitronectin are specifically expressed at the advancing margin of high-grade gliomas, and inhibition of integrin αvβ3 ligation reduces glioma cell invasion.30,31 MMP-2 has been shown to complex with integrin αvβ3, and disruption of MMP-2 binding to integrin αvβ3 inhibits angiogenesis and tumor growth in vivo.32 Furthermore, integrin αv antagonists can inhibit orthotopic brain tumor growth and lead to tumor regression in animal models of high-grade gliomas.33 These observations have led to the suggestion that integrin αvβ3 may play a role in the growth, invasion, and angiogenesis of glioblastoma. More recent studies have challenged the role of integrins as tumor malignancy promoters. The over-expression of integrin β3 in orthotopic glioblastoma has been shown to suppress both tumor oxygenation and growth.34
In the present study, we investigated the role of LDH-A–induced TGF-β2 in glioma migration with a focus on the involvement of MMP-2 in these processes and further related molecular mechanisms. Because metabolic events triggered by LDH-A and TGF-β2 expression have significant impacts on tumor invasion in high-grade gliomas, our studies aimed to provide a basis for further characterization of the molecular network involved in TGF-β2–coordinated invasion, which might yield promising insights for the development of new diagnostic and therapeutic applications for invasive tumors.
Materials and Methods
Cell Culture
Different glioma cell lines and primary cultures were used for in vitro experiments. Human high-grade glioma cell lines U87MG and A172 were obtained from American Type Culture Collection (Manassas, VA, USA). The gliomas designated “HTZ” were primary tumor cell cultures derived from surgical specimens of human glioblastomas as previously described.6 Tumor cells were maintained as monolayer cultures in Dulbecco’s modified Eagle’s medium (DMEM; Invitrogen, Carlsbad, CA, USA), supplemented with 5% fetal calf serum (PAA Laboratories, Pasching, Austria) at 37°C, 5% CO2, 95% humidity in a standard tissue culture incubator.
TGF-β2, MMP-2, Integrin αvβ3, and Lactic Acid Stimulation Assays
To elucidate the effect of exogenous TGF-β2 on the regulation of MMP-2, we performed stimulation assays with different concentrations of TGF-β2. We seeded 8 × 105 glioma cells in medium cell culture flasks containing growth medium as described above. After 24 h, triplicates of subconfluent cell layers were treated with four different concentrations (1, 5, 10, and 50 ng/ml) of activated rhTGF-β2 protein (R&D Systems, Minneapolis, MN, USA) and incubated for 72 h. Cells and supernatants were harvested to prepare total RNA or protein as described below. In time-point assays, cells were treated with 10–50 ng/ml TGF-β2, and supernatants were harvested at four different time points. Cell lysates and supernatants of untreated cells were used as controls in both assays.
Similar approaches were used for the downregulation of MMP-2 with 20 μM of a specific MMP-2 inhibitor (Calbiochem, Darmstadt, Germany), integrin αvβ3 with 20 ng/ml of an integrin αvβ3 antibody (Chemicon, Schwalbach, Germany), LDH-A with 200 pmol/μl of a small interfering RNA (siRNA) specific against LDH-A (siLDH-A; 5′-AGG TTC ACA AGC AGG TGG-3′), and induction of TGF-β2 with 10 and 20 mM lactic acid (Fluka, Buchs, Switzerland). Concentrations and time points for optimal regulation were defined in preassays (data not shown).
Reverse Transcriptase PCR
Total RNA was extracted from tumor cells with the RNA purification system RNeasy Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. RNA concentration and purity were determined by measuring optical density at wavelengths of 260 and 280 nm using a standard spectrophotometer. First-strand gene-specific cDNAs from expressed genes were generated from 1 μg of total RNA samples by using a reverse transcription kit (Promega, Madison, WI, USA). Appropriate forward and reverse primers to detect transcripts of interest were used in PCR reactions for cDNA amplification. The primers used were as follows: TGF-β2 (forward: 5′-GCA GGT ATT GAT GGC ACC TCC-3′; reverse: 5′-GGC ATG CTC CAG CAC AGA AG-3′), resulting in a 301-bp fragment (Genebank accession no. NM003238); MMP-2 (forward: 5′-AAC CCT CAG AGC CAC CCC TA-3′; reverse: 5′-GTG CAT ACA AAG CAA ACT GC-3′), resulting in a 286-bp fragment (Genebank accession no. NM004530); integrin β3 (forward: 5′-ACA CTG GCA AGG ATG CAG TGA ATT GTA C-3′; reverse: 5′-CGT GAT ATT GGT GAA GGT AGA CGT GGC-3′), resulting in a 308-bp fragment (Genebank accession no. NM000212); and integrin αv (forward: 5′-GAT GTT GGG CCA GTT GTT CAG CAC ATC TAT G-3′; reverse: 5′-CAG ACG ACT TCA GAG AAT AGG AAT GAT TCT G-3′), resulting in a 429-bp fragment (Genebank accession no. NM002210). Annealing temperatures were optimized for each primer pair using the following program: 95°C for 5 min; 30 cycles × (95°C for 45 sec, 57–60°C for 1 min, 72°C for 45 sec); 72°C for 5 min.
PCR products were analyzed on a 1% agarose gel and visualized with ethidium bromide staining. The housekeeping gene β-actin was used as a positive control to assess cDNA quality.
Quantitative PCR
Quantification of mRNA expression was performed by real-time PCR (Mx3000P Quantitative PCR [qPCR] System, Stratagene, CA, USA) based on SYBR-Green I fluorescence. Target-cDNA–specific primers as described above were established. Briefly, five serial twofold dilutions of cDNA were amplified in triplicates to construct standard curves for both the target gene and the endogenous reference (18s or β-actin). Standard curves generated by the software were used for extrapolation of expression levels for the unknown samples based on their threshold cycle (Ct) values. All amplifications of unknown samples were in the linear range. For each reaction, melting curves and agarose gel electrophoresis of PCR products were used to verify the identity of the amplification products. Each probe was run in parallel with primers specific for 18s as standard for quantification of target cDNA. The target gene amount was divided by the housekeeping gene (β-actin or 18s) amount to obtain a normalized target value. Each of the experimental normalized values was divided by the normalized control (untreated) sample value to generate the relative expression levels in folds.
Gas Chromatography/Mass Spectrometry Analysis of Glucose and Lactate
Glucose and lactate concentrations in the cell culture medium were analyzed by gas chromatography/mass spectrometry (6890 GC-5975 Inert XL MS; Agilent Technologies Inc., Palo Alto, CA, USA). A 10-μl aliquot of the cell culture medium was spiked with 10 μl of an internal standard solution containing [U-13C] glucose and [U-13C]lactate (1 mM each). The samples were dried using a vacuum evaporator and derivatized prior to injection. For derivatization, 50 μl of 10 mg/ml methoxylamine hydrochloride in pyridine were added and incubated at 60°C for 60 min, followed by 50 μl N-methyl-N-(trimethylsilyl)trifluoroacetamide for 60 min at 60°C. Sample injection was performed in split-less mode at 280°C using an injection volume of 1 μl. Separation was carried out on an RXI-5MS column (30 m × 0.25 mm inner diameter × 0.25 μm film thickness; Restek GmbH, Bad Homburg, Germany). The initial oven temperature was set at 50°C, ramped at 8°C/min to 300°C, and held for 10 min. Helium was used as carrier gas at a flow rate of 0.6 ml/min. The mass spectrometer was operated in full-scan mode from 50 to 600 m/z with a scan time of 0.5 s. Quantification was performed with a dilution series of glucose and lactate standards. Calibration curves were generated by normalizing the peak areas of standard to the area of the internal standard, and concentrations in the samples were then inferred from the calibration curves.
TGF-β2 Enzyme-Linked Immunosorbent Assay
For the quantitative determination of activated human TGF-β2 concentrations in cell culture supernatants, the quantitative sandwich enzyme immunoassay technique was used with a commercially available human TGF-β2–specific immunoassay kit (R&D Systems, Minneapolis, MN, USA). The minimum detectable dose of TGF-β2 was less than 7.0 pg/ml. The assay was performed in triplicate according to the manufacturer’s instructions.
MMP-2 Enzyme-Linked Immunosorbent Assay
For the quantitative determination of total MMP-2 concentrations in cell culture supernatants, the quantitative sandwich enzyme immunoassay technique was used with a commercially available human MMP-2–specific immunoassay kit (R&D Systems, Abingdon, UK). The minimum detectable dose of MMP-2 was less than 0.8 ng/ml. The assay was performed in triplicate according to the manufacturer’s instructions.
Flow Cytometry
To determine whether the cell surface expression of integrin αvβ3 was regulated by exogenous TGF-β2 and MMP-2 inhibitor, HTZ-349 cells were treated either with 0, 1, 5, 10, and 50 ng/ml TGF-β2 on days 1 and 4 (5-day assay), or with 50 ng/ml TGF-β2 with and without 20 nM MMP-2 inhibitor on day 1 (3-day assay). At day 3 or 5, cells were trypsinized and washed twice in 100 μl phosphate-buffered saline. We resuspended 0.5 × 106 cells per probe in 100 μl fluorescence-activated cell sorting (FACS) buffer, and added 1 μl integrin αvβ3 mouse antihuman Alexa-coupled antibody (Chemicon, Temecula, CA, USA) or mouse IgG antihuman Alexa-coupled antibody (Upstate, Lake Placid, NY, USA) to the suspension as isotype control. After an incubation of 30 min at 4°C, the cells were washed twice in 2.5 ml FACS buffer, resuspended in 300 μl FACS buffer, and analyzed by flow cytometry (FACScan, BD, Franklin Lakes, NY, USA). All steps after cell detachment were performed at 4°C to avoid internalization of antibody–receptor complexes. The mean fluorescence intensity per cell was recorded as expression of the relative antigen density. The antibody-induced fluorescent shift was compared and normalized to the shift induced by the isotype control and blotted using the software Win MDI version 2.9 (freeware available from trotter@scripps.edu).
Gelatin Zymography
In concentration assays, supernatants from glioma cells that were stimulated by culture medium of siLDH-A–treated cells, by TGF-β2 protein in different concentrations (5, 10, and 50 ng/ml), by TGF-β2 plus integrin αvβ3 antibody (20 ng/ml), or by TGF-β2 plus MMP-2 inhibitor (20 nM) were collected after 72 h of cell culture. Supernatants from untreated glioma cells were used as control in these assays. To analyze the proteolytic activities of MMP-2 toward gelatin, supernatants containing 20 μg of total protein quantified in a bicinchoninic acid assay (Uptima, Montpellier, France) were separated at 4°C in a 7.5% sodium dodecyl sulfate polyacrylamide gel containing 0.1% gelatin (BioRad Laboratories, Palo Alto, CA, USA). The gel was washed in a substrate buffer containing 2% of Triton X-100 and developed in a buffer containing 50 mM Tris, 0.02% Brij 35 nonionic surfactant, and 5 mM CaCl2 at 37°C for 16 h. The gel was stained with 0.5% (wt/vol) Coomassie blue R-250 for 30 min and destained in 10% acetic acid solution. Gels were photographed, and areas of protease activity where the protease had digested the substrate appeared as clear bands against a dark blue background.
Single-Cell Attachment Assay
Noncoated 96-well cell culture plates (BD Biosciences, San Jose, CA, USA) were seeded with 3,000 vital cells each, using wells with no cells as a negative control and nonwashed seeded cells as a positive control. The optimal seeding time was defined in preassays for each cell line (data not shown). HTZ-349 cells were pretreated with 0, 1, 5, 10, and 20 ng/ml integrin αvβ3 antibody, seeded, and allowed to attach for 30 min. After three washing steps with each 100 μl phosphate-buffered saline, plates were incubated for 4 h to allow complete attachment. The number of attached cells was measured using a 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) assay (Roche, Penzberg, Germany). Assays were performed in triplicate and repeated once.
Spheroid Migration Assay
Tumor spheroids were initiated by seeding 3–8 × 103 cells in agar-coated wells. Mature spheroids with a mean diameter of 200–250 μm were explanted to uncoated 96-well plates containing the corresponding protein (TGF-β2, 20 ng/ml), 20 nM MMP-2 inhibitor (Calbiochem, Laeufelfingen, Germany), specific integrin αvβ3 function-blocking antibody (Chemicon, Temecula, CA, USA), and combinations of each. Spheroids were allowed to migrate for 1–5 days using the earliest time point where migration was visible to prevent a dilution of the effect by enhanced proliferation of cells. The diameter of the area covered by cells migrating away from a spheroid was photographed and measured manually by a blinded investigator using the greatest diameter. Bovine serum albumin was used as a control protein. Assays were performed in triplicates and repeated twice.
Boyden Chamber Migration Assay
A suspension of 200,000 tumor cells/ml (200 μl total volume) were pipetted in the upper chamber of the Boy-den device (BD). The lower chamber was loaded with 210 μl of a chemoattractant consisting of cell culture medium that had been harvested after a 24-h incubation of fibroblasts grown in DMEM. The chambers are divided by an uncoated membrane with pores of 8 μm diameter. After 4 h of incubation, the number of cells that had migrated to the lower side of the membrane was counted after staining with hematoxylin and eosin. Five visual fields were counted by a blinded investigator on each filter of a triplet and were evaluated calculating means of migrated cells and standard deviation.
Statistics
The Student’s t-test was used to compare the results (mean values and SDs) of control versus treated cell samples in the investigator-sensitive assays (zymography, attachment, and migration assays). Significance was set at p < 0.05.
Results
Regulation of TGF-β2 by siLDH-A and Lactic Acid
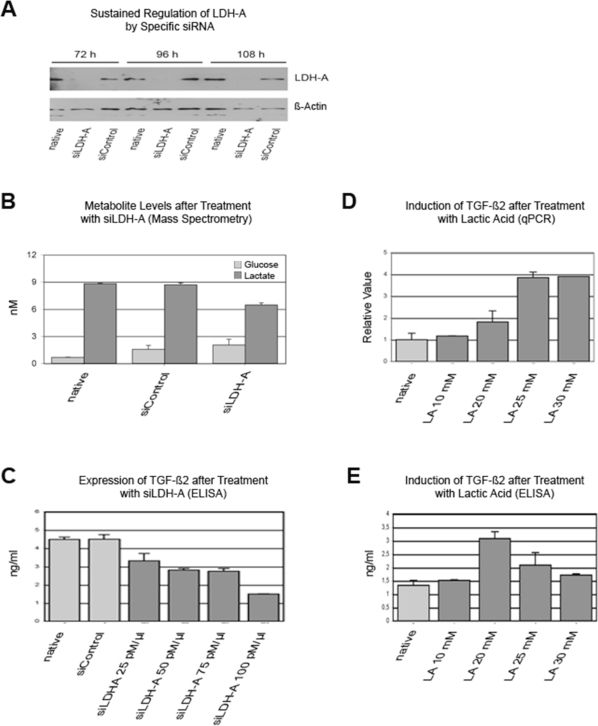
To evaluate a possible interaction of lactate metabolism and TGF-β2, we designed siLDH-A, which was able to inhibit LDH-A transcription for at least 108 h (Fig. 1A). In HTZ-349 cells, downregulation of LDH-A resulted in decreased extracellular lactate levels and reduced glucose uptake over 96 h (Fig. 1B). In the same assay, siLDH-A downregulated TGF-β2 protein secretion in a concentration-dependent manner (Fig. 1C). Conversely, lactic acid induced both TGF-β2 gene and protein expression (Fig. 1D,E). The decrease of protein levels in contrast to RNA levels may be explained by decreasing cell numbers due to toxic effects of lactic acid in high concentrations.
Fig. 1.
Lactic acid regulates transforming growth factor-β2 (TGF-β2). (A) A small interfering RNA (siRNA) specific against lactate dehydrogenase type A (siLDH-A; 200 pmol/μl) downregulated LDH-A, but not LDH-B (data not shown), in a time-dependent manner at the protein level (Western blot with LDH-A–specific antibody) in HTZ-349 cells. An siRNA control (siControl) did not regulate LDH-A. (B) siRNA–mediated downregulation of LDH-A reduced extracellular lactate level and glucose uptake. siLDH-A and siControl were applied at 200 pmol/μl. (C) Treatment with siLDH-A for 72 h inhibited TGF-β2 protein expression in HTZ-349 cells in a dose-dependent manner; controls were supernatants of untreated and control siRNA-treated cells. The optimal time point was defined by preassays before the time assays were performed (data not shown). ELISA, enzyme-linked immunosorbent assay. (D and E) Treatment with 10, 20, 25, and 30 mM of lactic acid (LA) for 48 h induced TGF-β2 expression as detected by quantitative PCR (qPCR; D) and ELISA (E).
Expression of TGF-β2 and MMP-2 in Human High-Grade Glioma Cells
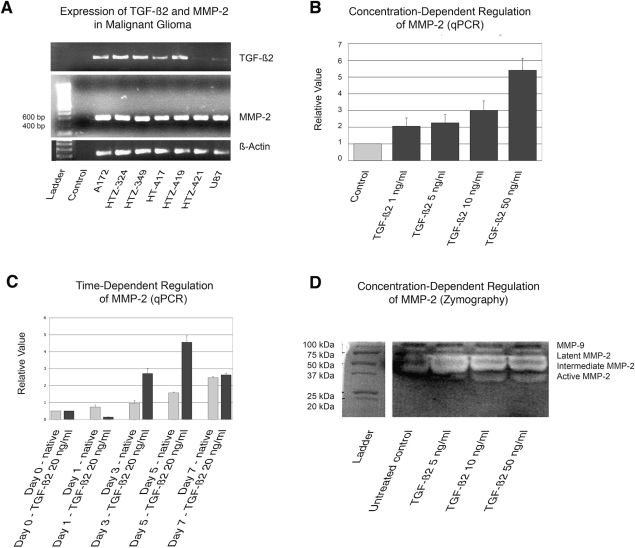
To evaluate the expression profiles of TGF-β2 and MMP-2 in two human high-grade glioma cell lines (U87, A172) and five primary cell cultures (HTZ-324, HTZ-349, HTZ-417, HTZ-419, HTZ-421), expression levels of TGF-β2 and MMP-2 were measured by reverse transcriptase PCR using β-actin as a control gene (Fig. 2A). All cell lines and primary cultures expressed MMP-2 mRNA, while the cell line U87 and the primary culture HTZ-421 showed significantly decreased TGF-β2 expression.
Fig. 2.
Transforming growth factor-β2 (TGF-β2) induces the expression and activation of matrix metalloproteinase-2 (MMP-2). (A) Expression of TGF-β2 and MMP-2 in two human glioma cell lines (U87, A172) and five primary cell cultures (HTZ-324, HTZ-349, HTZ-417, HTZ-419, HTZ-421) as shown by semiquantitative PCR analysis. β-Actin was used to adjust for cDNA quantity. (B) mRNA expression of MMP-2 was induced by TGF-β2 in a concentration-dependent manner as analyzed by quantitative PCR (qPCR) in HTZ-349. (C) MMP-2 expression in TGF-β2 (20 ng/ml)–treated HTZ-349 cells at days 1, 3, 5, and 7, respectively. Data are mean fold changes ±S D, using β-actin for normalization. (D) MMP-2 activity was assessed using gelatin zymography. Supernatants from HTZ-349 glioma cells treated with TGF-β2 (5, 10, and 50 ng/ml) were subjected to gelatin zymography. In TGF-β2–treated cells, the endogenous pro-MMP-2 (latent form, 68 kDa) was efficiently converted to the 64-kDa intermediate MMP-2, which was then processed further to the 62-kDa active form in a dose-dependent manner. Gelatinolytic activity of MMP-9 (gelatinase B) was also detected around 100 kDa.
Regulation of MMP-2 Expression and Activation by Exogenous TGF-β2
To investigate whether TGF-β2 modulates MMP-2 expression in high-grade gliomas, we assessed the regulation of MMP2 mRNA by qPCR in HTZ-349 cells in two different experiments. For the concentration-dependent assay, cells were treated with four different concentrations (1, 5, 10, and 50 ng/ml) of activated rhTGF-β2 protein. After 72 h of incubation, we analyzed MMP-2 mRNA expression by qPCR. Exogenous TGF-β2 dose dependently increased MMP-2 mRNA expression up to 5.4-fold after incubation with 50 ng/ml TGF-β2 compared with untreated cells (Fig. 2B). For the time-point assay, cells were treated with TGF-β2 for 1, 3, 5, and 7 days. After 5 days, the TGF-β2–mediated induction of MMP-2 mRNA expression peaked and subsequently disappeared until day 7 (Fig. 2C).
The effect of TGF-β2–induced MMP-2 expression on enzymatic activity was analyzed by gelatin zymography using supernatants of HTZ-349 treated with increasing amounts of TGF-β2 (5, 10, and 50 ng/ml). Only in TGF-β2–treated cells, endogenous pro-MMP-2 (68 kDa) was efficiently converted to the 64-kDa intermediate and 62-kDa active form, suggesting that TGF-β2 mediates pro-MMP2 expression and activation (Fig. 2D).
Regulation of Integrin αv and β3 Expression by Exogenous TGF-β2
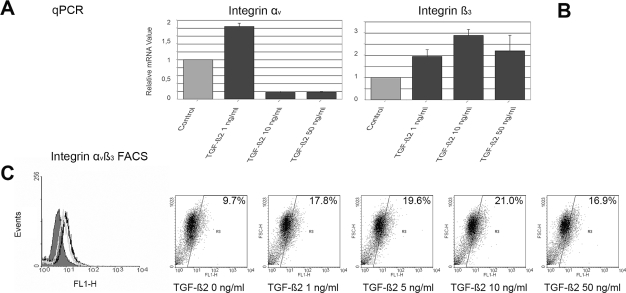
Integrin αvβ3 is a TGF-β2–induced mediator of glioma migration and forms complexes with MMP-2.5,32 We therefore investigated the regulation of integrin αv and β3 expression by exogenous TGF-β2 in the cell line HTZ-349. Low concentrations of TGF-β2 upregulated mRNA expression of integrin αv up to twofold. In contrast, higher doses of TGF-β2 (10 and 50 ng/ml) significantly inhibited the expression of integrin αv (Fig. 3A). Similarly, HTZ-349 cells treated with TGF-β2 had significantly higher integrin β3 expression levels with a 10 ng/ml dose of TGF-β2 compared with untreated cells but showed decreasing levels with higher TGF-β2 concentrations (Fig. 3B).
Fig. 3.
Transforming growth factor-β2 (TGF-β2) induces integrin αvβ3 expression in low concentrations. Expression of integrin αv and β3 at the mRNA level was analyzed separately by quantitative PCR (qPCR) in HTZ-349 cells treated with different concentrations of TGF-β2 (1, 10, and 50 ng/ml) and counted by fluorescence-activated cell sorting (FACS) because the integrin antibody used recognizes integrin αvβ3. (A) mRNA expression of integrin αv was significantly (p < 0.001) upregulated with 1 ng/ml of TGF-β2 but inhibited with 10 and 50 ng/ml compared with untreated controls. (B) TGF-β2 significantly (p < 0.001) upregulated expression of integrin β3. Normalized values with the housekeeping gene 18s are reported as relative values in fold change. Mean values ± SD are representative of triplicates. Mean values for the untreated (control) group are set to a value of 1. (C) Exogenous TGF-β2 (0, 1, 5, 10, and 50 ng/ml) induced expression of integrin αvβ3 on the cell surface of HTZ-349 cells as measured by FACS analysis. The FACS histogram (left) shows a shift from the mouse immunoglobulin G antihuman isotype control (gray-shaded curve) to the specifically stained untreated control cells (light gray curve) and the TGF-β2 (50 ng/ml)–treated cells (black curve) FL1-H, Alexa-coupled integrin αvβ1 antibody. FACS blots (right five panels) show a TGF-β2 concentration-dependent shift toward a higher relative antigen density of integrin αvβ3 compared with an isotype control in cells treated with high amounts of TGF-β2. The expression of integrin αvβ3 decreased with 50 ng/ml TGF-β2, corresponding to the qPCR results shown in A and B.
TGF-β2 also enhanced the cell surface expression of the adhesion receptors integrin αvβ3 as determined by flow cytometry (Fig. 3C). Similar to qPCR results, high concentrations of TGF-β2 (50 ng/ml) resulted in reduced surface expression of integrin αvβ3 compared with lower doses.
Role of Integrin αvβ3 in Glioma Attachment
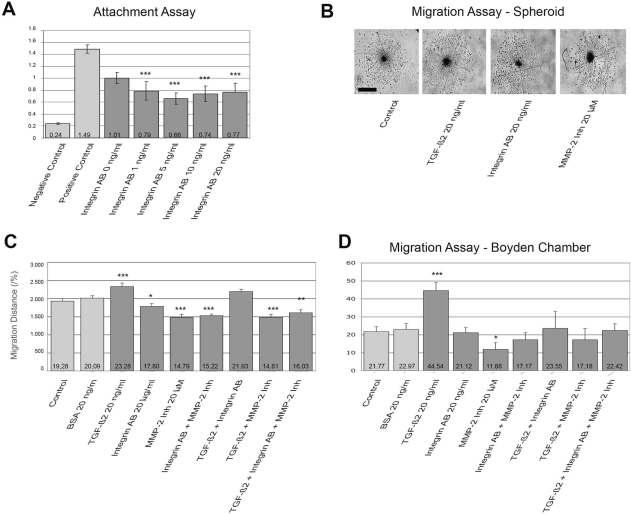
To demonstrate the functional relevance of integrin αvβ3 expression on the glioma cell line HTZ-349, we blocked integrin αvβ3 using a specific antibody directed against integrin αvβ3 (Fig. 4A). In the cell attachment assay, 5 ng/ml antibody significantly impaired the adhesion of tumor cells, suggesting that integrin αvβ3 mediates cellular attachment.
Fig. 4.
Matrix metalloproteinase-2 (MMP-2) and integrin αvβ3 modulate glioma migration mediated by transforming growth factor-β2 (TGF-β2). The effects of MMP-2 inhibitor (Inh) and integrin αvβ3 on glioma HTZ-349 attachment and migration were analyzed in attachment, spheroid, and Boyden chamber migration assays. MMP-2 inhibitor (20 μM) was used to block protease activity, while a specific antibody (20 ng/ml) was used to block integrin αvβ3, in TGF-β2–treated or untreated cells. (A) Attachment of HTZ-349 to a noncoated surface was inhibited significantly under treatment with integrin αvβ3 antibody. Y-axis indicates relative values. Treated samples are compared with controls: *p < 0.05, **p < 0.01, ***p < 0.001. (B) Spheroid migration assay. Representative photographs of cells migrating from spheroids under different treatment conditions were taken at day 3. Scale bar, 200 μm. (C) Spheroid migration assay. The migration rate was significantly higher in TGF-β2 (20 ng/ml)–treated spheroids compared with untreated controls (***p < 0.001). MMP-2 inhibitor decreased the migration distance with or without exogenous TGF-β2 protein (***p < 0.001). The effect of an antibody against integrin αvβ3 (*p < 0.05) was still significant, though not as pronounced, but the effects of MMP-2 inhibitor and antiintegrin antibody were not additive. Experiments were performed in triplicate and repeated twice. BSA, bovine serum albumin (negative control). (D) Boyden chamber assay without Matrigel showed similar effects as in A and B. Y-axis indicates cell number. Migration of HTZ-349 cells was increased after treatment with TGF-β2 (20 ng/ml) compared with untreated control (***p < 0.001; B). Specific MMP-2 inhibitor (20 μM) plus TGF-β2 led to a trend of migration inhibition (p = 0.269), but MMP-2 inhibitor alone inhibited migration significantly (*p = 0.048). Functional blockage of integrin αvβ3 had no significant effect on reversing the migration-promoting effect of TGF-β2 on glioma cell migration (p = 0.435). A coincubation with MMP-2 inhibitor and integrin αvβ3 antibody inhibited migration, but not significantly. All assays were duplicated.
Role of MMP-2 in TGF-β2–Mediated Glioma Migration
To further elucidate how TGF-β2 enhances glioma migration TGF-β2, we examined whether the upregulation of MMP-2 and cell adhesion receptor integrin αvβ3 by TGF-β2 might be involved. As previously described, TGF-β2 (20 ng/ml) significantly increased the migration rate and the migration distance of HTZ-349 cells compared with untreated controls (p < 0.001; Fig. 4B,C). This effect was completely abolished by a specific MMP-2 inhibitor, confirming a strong dependence of TGF-β2 on MMP-2 in glioma migration in vitro. Of note, incubation with the MMP-2 inhibitor alone significantly reduced glioma cell migration (p < 0.001). This suggests a relevant TGF-β2–independent activation of MMP-2 in glioma cells. Functional blockage of integrin αvβ3 only marginally reversed the migration-promoting effect of TGF-β2 on glioma migration (p < 0.05).
The results were confirmed in Boyden chamber migration assays. Migration of HTZ-349 cells was increased after treatment with TGF-β2 (20 ng/ml) compared with untreated control (p < 0.001; Fig. 4D). Again, a 20-nM concentration of the specific MMP-2 inhibitor inhibited TGF-β2–induced tumor cell migration. Functional blockage of integrin αvβ3 did not significantly reverse the migration-promoting effect of TGF-β2 on glioma cell migration.
Coregulation of MMP-2 and Integrin αvβ3
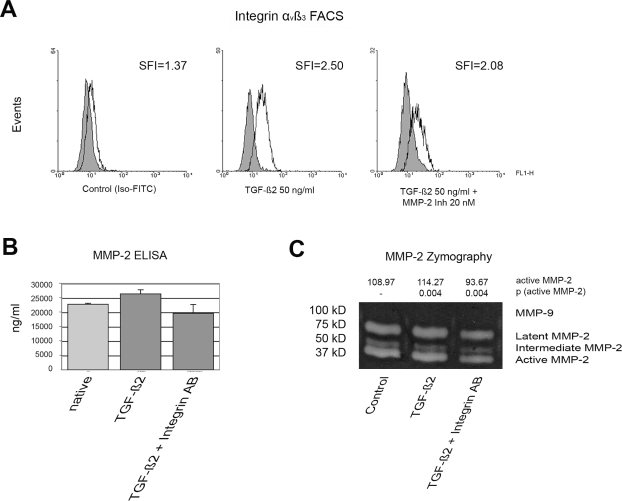
To assess possible interactions of MMP-2 and integrin αvβ3, we treated HTZ-349 cells with TGF-β2 (50 ng/ml) alone and in combination with 20 nM MMP-2 inhibitor. Cytometric determination of the cell surface expression of integrin αvβ3 confirmed that TGF-β2 induced integrin αvβ3 expression (Fig. 5A). However, this effect was reversed upon the combined treatment of cell with TGF-β2 and MMP-2 inhibitor. Correspondingly, MMP-2 was downregulated after cotreatment with TGF-β2 and integrin αvβ3 in MMP-2 enzyme-linked immunosorbent assays as well as zymography assays (Fig. 5B,C).
Fig. 5.
Matrix metalloproteinase-2 (MMP-2) and integrin αvβ3 regulate each other. (A) Fluorescence-activated cell sorting (FACS) analysis of integrin αvβ3 surface expression after treatment with transforming growth factor-β2 (TGF-β2) as indicated. TGF-β2 increased the relative level of integrin αvβ3 on the cell surface, and the level was further decreased after treatment with TGF-β2 and MMP-2 inhibitor (Inh). Shift in fluorescence intensity (SFI) is shown for each panel. Iso-FITC, fluorescein isothiocyanate–labeled immunoglobulin G–isotype control antibody. (B) Extracellular MMP-2 protein levels increase under TGF-β2 (50 ng/ml) treatment for 72 h. After treatment with TGF-β2 plus integrin antibody (20 ng/ml) for 72 h, the level of MMP-2 decreased significantly. ELISA, enzyme-linked immunosorbent assay. (C) Similarly, TGF-β2 plus integrin αvβ3 abrogated the induction effect of TGF-β2 on MMP2 activity in gelatin gel zymography. The induction of MMP-2 after TGF-β2 and the decrease after cotreatment with TGF-β2 and integrin αvβ3 are significant (**p < 0.02).
Regulation of MMP-2 and Migration by siLDH-A
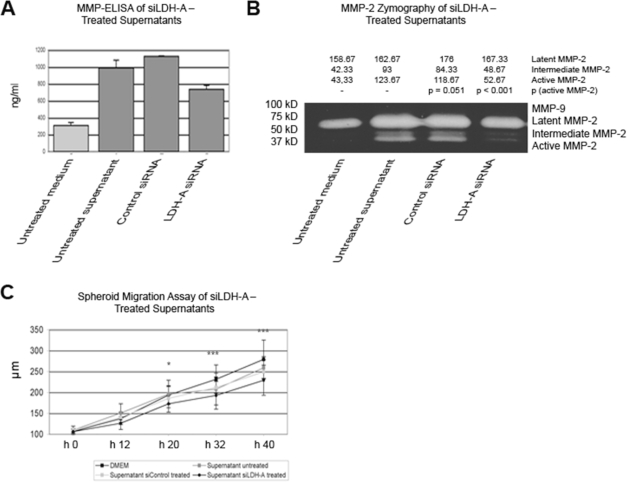
We have shown that regulation of small interfering RNA directed against LDH-A (siLDH-A) and treatment with lactic acid affect levels of TGF-β2. On the other hand, TGF-β2 regulated the expression of MMP-2 and integrin αvβ3, with remarkable functional effects in vitro. To further substantiate the link between LDH-A and MMP-2, we used supernatants of the siLDH-A–treated HTZ-349 cell line. siLDH-A–pretreated supernatants were able to reduce the total and active MMP-2 significantly (Fig. 6A,B) suggesting that impaired LDH-A activity results in the downregulation of TGF-β2 (Fig. 1B), followed by a decreased induction of MMP-2. This hypothesis was further substantiated by performing a spheroid migration assay using the same supernatants as in the zymography assay. In fact, the migration distance of siLDH-A–treated supernatants was significantly decreased (Fig. 6C). We conclude that siLDH-A inhibited glioma migration by downregulation of TGF-β2 expression followed by decreased MMP-2 activation (Fig. 4B–D).
Fig. 6.
Lactate dehydrogenase type A (LDH-A) downregulation leads to decreased matrix metalloproteinase-2 (MMP-2) expression and activity and to reduced migration distance in glioma spheroids. (A) To substantiate the results on downregulation of TGF-β2 by siLDH-A and its possible impact on MMP-2 and migration, HTZ-349 cells were treated with supernatants from siLDH-A–treated HTZ-349. The cells were pretreated for 72 h before the supernatants were harvested for MMP-2–specific enzyme-linked immunosorbent assay (ELISA). Untreated supernatants induced MMP-2 compared with fresh Dulbecco’s modified Eagle’s medium (DMEM), which resulted from the release of growth factors by cells into the supernatants. Pretreating cells with control siRNA (siControl) made no meaningful difference to untreated supernatant. However, under treatment with 200 pmol/μl siLDH-A, MMP-2 was significantly less induced than in the controls. (B) Supernatants of HTZ-349 cells treated with siLDH-A for 72 h were able to significantly (p = 0.005) reduce the levels of active MMP-2 as confirmed by gelatin zymography. (C) In a spheroid migration assay using the same supernatants as in the ELISA and gelatin zymography assays, the migration distance of siLDH-A–treated supernatants was significantly decreased compared with all controls. The difference between untreated supernatant and siLDH-A–treated supernatant was significant after 20 h (p < 0.05) and highly significant (p < 0.001) after 32 and 40 h.
Discussion
Several pathophysiological mechanisms of TGF-β induction have been described, for example, carcinogenesis, trauma, and irradiation,1,35 that are mediated by several known transcription factors.36,37 However, the effect of tumor cell metabolism on glioma cell migration has not yet been elucidated. Here, we show for the first time that LDH-A and lactate regulate TGF-β2 expression in glioblastoma cells. By mediating TGF-β2–dependent MMP-2 expression and activity, LDH-A activity regulates the migration capacity of human glioblastoma in vitro. The demonstrated effects speak for a lactate-mediated regulation of migration in high-grade gliomas: First, lactate induces TGF-β2, which leads to enhanced levels of MMP-2 and enhanced glioma cell migration. Second, a downregulation of LDH-A by siLDH-A (which leads to lowered levels of lactate) is followed by lowered levels of TGF-β2, leading to lower MMP-2 levels and a reduced migration of glioma cells.
TGF-β2 has been implicated in glioma cell motility and migration via several mechanisms that involve cell adhesion factors (e.g., integrins),5 MMPs (e.g., MMP-2),38,39 and ECM proteins such as versican.6 In the present study, we confirmed that TGF-β2 upregulates not only MMP-2 mRNA and protein expression but also its activation.
In previous studies, increasing concentrations of rhTGF-β2 increased MMP-2 protein levels in human glioma cell lines and in primary cell cultures of human brain tumors.28,29 However, it was not clear whether TGF-β2 leads to MMP mRNA stabilization or enhanced transcriptional activity.27
To become functionally active, TGF-β2–induced MMP-2 has to interact with other proteins in the ECM or at cell surfaces. We found that TGF-β2 induces the expression of integrin αvβ3, which is known to interact with MMP-2.32 Interestingly, we observed that higher doses of TGF-β2 inhibited rather than induced the expression of integrin αv. Previously, extensive MMP-2 activation was reported to result in decreased cell surface expression of integrin αvβ3 and decreased migration of glioma cells.25 Because we have demonstrated dose-dependent activation of MMP-2 by exogenous TGF-β2, extensive MMP-2 activity with high doses of TGF-β2 might explain lower cell surface expression levels of integrin αvβ3. In addition, we found that expression of human glioma integrin αvβ3 facilitated activation of MMP-2 in glioma cells. Our results correlate well with the earlier finding that integrin αvβ3 interaction is a prerequisite for efficient activation and maturation of MMP-2 in tumor cells.39 Our results further substantiate a potential cross talk between MMP-2 and integrin αvβ3 that could be intimately involved in regulating tumor invasion and metastasis. However, the detailed mechanisms involved in this process await further clarification.
To further evaluate the functional role of MMP-2 in glioma migration mediated by TGF-β2, we used two-dimensional spheroid and Boyden migration assays. In these assays, TGF-β2 enhanced glioma migration, and inhibition of MMP-2 activity with a specific inhibitor led to abrogation of this enhancement, which confirms that MMP-2 activity is an important modulator of glioma migration mediated by TGF-β2. In addition, a functional blockage of integrins αvβ3 expressed on the cell surface only slightly inhibited the effects of TGF-β2 on glioma migration. Therefore, we conclude that TGF-β2–mediated glioma migration is strongly dependent on MMP-2 activity but only marginally on integrin αvβ3 ligation. However, because there was no synergistic or cumulative effect if both proteins were blocked, we think that MMP-2 interacts with integrin αvβ3 if available but might also exert its functional effects using alternative targets.
Because we detected that inhibition of integrin αvβ3 reduced glioma attachment, we hypothesize that reduced attachment might decrease migration because cells have to attach to other cells and the ECM during migration. However, because our in vitro model is highly artificial, this hypothesis will have to be proven in an in vivo model.
The fact that the efficacy of MMP inhibitors in high-grade glioma patients has been so uniformly disappointing despite the compelling preclinical data may also reflect the underlying complexity of the metastatic process, such that multiple steps in multiple biological pathways, and not just MMPs, may need to be targeted for therapy to be effective.40 In this study, we show that the migration-promoting effect of TGF-β2 is strongly mediated by MMP-2 and integrin αv and β3 receptors.
In the clinical setting, glioblastomas have increased lactate peaks as detected by magnetic resonance spectroscopy.41 It is as yet not entirely clear why pyruvate is not primarily utilized for oxidative phosphorylation in these tumors, even under normoxic conditions. The reduction of pyruvate to lactate via LDH (aerobic glycolysis)7,8 deteriorates the energy balance significantly, but enables the tumor cells to migrate after shuttling of lactate to the microenvironment,13,14 to invade,12,42 and to induce apoptosis in normal cells of the surrounding parenchyma.10,11 After knockdown of LDH-A, the ability of tumor cells to proliferate is massively decreased, and the tumorigenicity of LDH-A–deficient cells is severely diminished.43 Lactate has long been regarded as an end product of anaerobic (and, in tumor cells, aerobic) glycolysis, and its fate in the normal and pathological metabolism of the brain has not been precisely delineated. However, astrocytes generate lactate, which can be used as an energy source for neurons, which substantiates the role of lactate even in the normal brain.44 Recent publications further report on the ability of a human astrocytic cell line to consume lactate and to generate ATP via oxidative phosphorylation after LDH-mediated transition of lactate to pyruvate.45 Whether the same mechanism is effective in glioblastoma cells is unknown.
Together with our previous studies, we have established a system that might help in deciphering the pathophysiological mechanisms underlying the invasive behavior of glioma cells in vitro. We show for the first time that a cascade triggered by changes in LDH-A expression, most likely mediated by changes in the extracellular lactate levels and promoted by TGF-β2, leads to significant changes in glioblastoma cell migration that are mediated by MMP-2 and integrin αvβ3. In addition, a direct nonmetabolic pathway seems to trigger a cascade, which leads to downregulation of TGF-β2 after downregulation of LDH-A by means of a specific siRNA. Defining the pathways by which TGF-β2 induces tumor migration and invasion might therefore provide critical information regarding the potential of TGF-β2 and its effectors as new therapeutic targets in glioma treatment.
Acknowledgments
F.B and P.L. contributed equally to this article. This work was supported in part by the intramural ReForM program of the University Hospital of Regensburg and BayGene. We thank Nadine Nürnberger for technical assistance.
References
- 1.Wick W, Naumann U, Weller M. Transforming growth factor-beta: a molecular target for the future therapy of glioblastoma. Curr Pharm Des. 2006;12:341–349. doi: 10.2174/138161206775201901. [DOI] [PubMed] [Google Scholar]
- 2.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 3.Wick W, Grimmel C, Wild-Bode C, Platten M, Arpin M, Weller M. Ezrin-dependent promotion of glioma cell clonogenicity, motility, and invasion mediated by BCL-2 and transforming growth factor-beta2. J Neurosci. 2001;21:3360–3368. doi: 10.1523/JNEUROSCI.21-10-03360.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stiles JD, Ostrow PT, Balos LL, et al. Correlation of endothelin-1 and transforming growth factor beta 1 with malignancy and vascularity in human gliomas. J Neuropathol Exp Neurol. 1997;56:435–439. doi: 10.1097/00005072-199704000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Platten M, Wick W, Wild-Bode C, Aulwurm S, Dichgans J, Weller M. Transforming growth factors beta(1) and TGF-beta(2) promote glioma cell migration via up-regulation of alpha(V)beta(3) integrin expression. Biochem Biophys Res Commun. 2000;268:607–611. doi: 10.1006/bbrc.2000.2176. [DOI] [PubMed] [Google Scholar]
- 6.Arslan F, Bosserhoff AK, Nickl-Jockschat T, Doerfelt A, Bogdahn U, Hau P. The role of versican isoforms V0/V1 in glioma migration mediated by transforming growth factor-beta2. Br J Cancer. 2007;96:1560–1568. doi: 10.1038/sj.bjc.6603766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Warburg O. London: Constable Press; 1930. The Metabolism of Tumors. [Google Scholar]
- 8.Walenta S, Schroeder T, Mueller-Klieser W. Lactate in solid malignant tumors: Potential basis of a metabolic classification in clinical oncology. Curr Med Chem. 2004;11:2195–2204. doi: 10.2174/0929867043364711. [DOI] [PubMed] [Google Scholar]
- 9.Stubbs M, Rodrigues L, Howe FA, et al. Metabolic consequences of a reversed pH gradient in rat tumors. Cancer Res. 1994;54:4011–4016. [PubMed] [Google Scholar]
- 10.Park HJ, Lyons JC, Ohtsubo T, Song CW. Acidic environment causes apoptosis by increasing caspase activity. Br J Cancer. 1999;80:1892–1897. doi: 10.1038/sj.bjc.6690617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams AC, Collard TJ, Paraskeva C. An acidic environment leads to p53 dependent induction of apoptosis. Oncogene. 1999;18:3199–3204. doi: 10.1038/sj.onc.1202660. [DOI] [PubMed] [Google Scholar]
- 12.Gatenby RA, Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion: Insights through mathematical models. Cancer Res. 2003;633:3847–3854. [PubMed] [Google Scholar]
- 13.Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibro-blast expression of hyaluronan and CD44: The Warburg effect revisited. Exp Cell Res. 2002;276:24–31. doi: 10.1006/excr.2002.5508. [DOI] [PubMed] [Google Scholar]
- 14.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36:1046–1469. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Koukourakis MI, Giatromanolaki A, Simopoulos C, Polychronidis A, Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin Exp Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- 16.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Tumour and Angiogenesis Research Group Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89:877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung KC, Huang D, Chen Y, et al. Identification of a silencer module which selectively represses cyclic AMP-responsive element-dependent gene expression. Mol Cell Biol. 1995;15:6139–6149. doi: 10.1128/mcb.15.11.6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Short ML, Huang D, Milkowski DM, et al. Analysis of the rat lactate dehydrogenase A subunit gene promoter/regulatory region. Biochem J. 1994;304:391–398. doi: 10.1042/bj3040391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deryugina EI, Quigley JP. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006;25:9–34. doi: 10.1007/s10555-006-7886-9. [DOI] [PubMed] [Google Scholar]
- 20.Sawaya RE, Yamamoto M, Gokaslan ZL, et al. Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis. 1996;14:35–42. doi: 10.1007/BF00157684. [DOI] [PubMed] [Google Scholar]
- 21.Forsyth PA, Wong H, Laing TD, et al. Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer. 1999;79:1828–1835. doi: 10.1038/sj.bjc.6990291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rooprai HK, Van Meter T, Rucklidge GJ, Hudson L, Everall IP, Pilkington GJ. Comparative analysis of matrix metalloproteinases by immunocytochemistry, immunohistochemistry and zymography in human primary brain tumours. Int J Oncol. 1998;13:1153–1157. doi: 10.3892/ijo.13.6.1153. [DOI] [PubMed] [Google Scholar]
- 23.Raithatha SA, Muzik H, Muzik H, et al. Localization of gelatinase-A and gelatinase-B mRNA and protein in human gliomas. Neuro-Oncology. 2000;2:145–150. doi: 10.1093/neuonc/2.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakano A, Tani E, Miyazaki K, Yamamoto Y, Furuyama J. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in human gliomas. J Neurosurg. 1995;83:298–307. doi: 10.3171/jns.1995.83.2.0298. [DOI] [PubMed] [Google Scholar]
- 25.Deryugina EI, Luo GX, Reisfeld RA, Bourdon MA, Strongin A. Tumor cell invasion through Matrigel is regulated by activated matrix metalloproteinase-2. Anticancer Res. 1997;17:3201–3210. [PubMed] [Google Scholar]
- 26.Deryugina EI, Bourdon MA, Jungwirth K, Smith JW, Strongin AY. Functional activation of integrin alpha V beta 3 in tumor cells expressing membrane-type 1 matrix metalloproteinase. Int J Cancer. 2000;86:15–23. doi: 10.1002/(sici)1097-0215(20000401)86:1<15::aid-ijc3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 27.VanMeter TE, Rooprai HK, Kibble MM, Fillmore HL, Broaddus WC, Pilkington GJ. The role of matrix metalloproteinase genes in glioma invasion: Co-dependent and interactive proteolysis. J Neurooncol. 2001;53:213–235. doi: 10.1023/a:1012280925031. [DOI] [PubMed] [Google Scholar]
- 28.Wick W, Platten M, Weller M. Glioma cell invasion: Regulation of met-alloproteinase activity by TGF-β. J Neurooncol. 2001;53:177–185. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- 29.Rooprai HK, Rucklidge GJ, Panou C, Pilkington GJ. The effects of exogenous growth factors on matrix metalloproteinase secretion by human brain tumour cells. Br J Cancer. 2000;82:52–55. doi: 10.1054/bjoc.1999.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gladson CL, Wilcox JN, Sanders L, Gillespie GY, Cheresh DA. Cerebral microenvironment influences expression of the vitronectin gene in astrocytic tumors. J Cell Sci. 1995;108:947–956. doi: 10.1242/jcs.108.3.947. [DOI] [PubMed] [Google Scholar]
- 31.Paulus W, Tonn JC. Basement membrane invasion of glioma cells mediated by integrin receptors. J Neurosurg. 1994;80:515–519. doi: 10.3171/jns.1994.80.3.0515. [DOI] [PubMed] [Google Scholar]
- 32.Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin alpha vbeta 3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci USA. 2001;98:119–124. doi: 10.1073/pnas.011343298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taga T, Suzuki A, Gonzalez-Gomez I, et al. alpha v-Integrin antagonist EMD 121974 induces apoptosis in brain tumor cells growing on vitronectin and tenascin. Int J Cancer. 2002;98:690–697. doi: 10.1002/ijc.10265. [DOI] [PubMed] [Google Scholar]
- 34.Kanamori M, Kawaguchi T, Berger MS, Pieper RO. Intracranial microenvironment reveals independent opposing functions of host alphaVbeta3 expression on glioma growth and angiogenesis. J Biol Chem. 2006;281:37256–37264. doi: 10.1074/jbc.M605344200. [DOI] [PubMed] [Google Scholar]
- 35.Piccolo S. p53 regulation orchestrates the TGF-beta response. Cell. 2008;133:767–769. doi: 10.1016/j.cell.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 36.Weigert C, Brodbeck K, Sawadogo M, Häring HU, Schleicher ED. Upstream stimulatory factor (USF) proteins induce human TGF-beta1 gene activation via the glucose-response element-1013/-1002 in mesangial cells: Up-regulation of USF activity by the hexosamine biosynthetic pathway. J Biol Chem. 2004;279:15908–15915. doi: 10.1074/jbc.M313524200. [DOI] [PubMed] [Google Scholar]
- 37.Kingsley-Kallesen M, Luster TA, Rizzino A. Transcriptional regulation of the transforming growth factor-beta2 gene in glioblastoma cells. In Vitro Cell Dev Biol Anim. 2001;37:684–690. doi: 10.1290/1071-2690(2001)037<0684:TROTTG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 38.Deryugina EI, Bourdon MA, Luo GX, Reisfeld RA, Strongin A. Matrix metalloproteinase-2 activation modulates glioma cell migration. J Cell Sci. 1997;110:2473–2482. doi: 10.1242/jcs.110.19.2473. [DOI] [PubMed] [Google Scholar]
- 39.Deryugina EI, Ratnikov B, Monosov E, et al. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Exp Cell Res. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 40.Overall CM, Kleifeld O. Tumour microenvironment—opinion: Validating matrix metalloproteinases as drug targets and anti-targets for cancer therapy. Nat Rev Cancer. 2006;6:227–239. doi: 10.1038/nrc1821. [DOI] [PubMed] [Google Scholar]
- 41.McKnight TR. Proton magnetic resonance spectroscopic evaluation of brain tumor metabolism. Semin Oncol. 2004;31(5):605–617. doi: 10.1053/j.seminoncol.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 42.Gatenby RA, Gawlinski ET, Gmitro AF, et al. Acid-mediated tumor invasion: A multidisciplinary study. Cancer Res. 2006;66(10):5216–5223. doi: 10.1158/0008-5472.CAN-05-4193. [DOI] [PubMed] [Google Scholar]
- 43.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9(6):425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 44.Pellerin L. Lactate as a pivotal element in neuron-glia metabolic cooperation. Neurochem Int. 2003;43(4–5):331–338. doi: 10.1016/s0197-0186(03)00020-2. [DOI] [PubMed] [Google Scholar]
- 45.Lemire J, Mailloux RJ, Appanna VD. Mitochondrial lactate dehydrogenase is involved in oxidative-energy metabolism in human astrocytoma cells (CCF-STTG1) PLoS ONE. 2008;3(2):e1550. doi: 10.1371/journal.pone.0001550. [DOI] [PMC free article] [PubMed] [Google Scholar]