Abstract
The brain is a specialized immune site representing a unique tumor microenvironment. The availability of fresh brain tumor material for ex vivo analysis is often limited because large parts of many brain tumors are resected using ultrasonic aspiration. We analyzed ultrasonic tumor aspirates as a biosource to study immune suppressive mechanisms in 83 human brain tumors. Lymphocyte infiltrates in brain tumor tissues and ultrasonic aspirates were comparable with respect to lymphocyte content and viability. Applying ultrasonic aspirates, we detected massive infiltration of CD4+FoxP3+CD25high CD127low regulatory T cells (Tregs) in glioblastomas (n = 29) and metastatic brain tumors (n = 20). No Treg accumulation was observed in benign tumors such as meningiomas (n = 10) and pituitary adenomas (n = 5). A significant Treg increase in blood was seen only in patients with metastatic brain tumors. Tregs in high-grade tumors exhibited an activated phenotype as indicated by decreased proliferation and elevated CTLA-4 and FoxP3 expression relative to blood Tregs. Functional analysis showed that the tumor-derived Tregs efficiently suppressed cytokine secretion and proliferation of autologous intratumoral lymphocytes. Most tumor-infiltrating Tregs were localized in close proximity to effector T cells, as visualized by immunohistochemistry. Furthermore, 61% of the malignant brain tumors expressed programmed death ligand-1 (PD-L1), while the inhibitory PD-1 receptor was expressed on CD4+ effector cells present in 26% of tumors. In conclusion, using ultrasonic tumor aspirates as a biosource we identified Tregs and the PD-L1/PD-1 pathway as immune suppressive mechanisms in malignant but not benign human brain tumors.
Keywords: brain tumor, immunotherapy, PD-L1, regulatory T cell, tumor-infiltrating lymphocyte
Brain tumors account for 2 percent of all cancers and result in a disproportionately high share of cancer morbidity and mortality. WHO published a grading system that is used, in combination with clinical findings, to predict a response to therapy and outcome.1 The most frequent primary brain tumors are meningiomas (WHO grade I) and glioblastomas (WHO grade IV and associated with a poor prognosis).2 Brain metastases are tumors that originate elsewhere in the body and metastasize to the brain. Brain metastases account for more than 50% of all brain tumors in adults. The median survival of patients with brain metastases ranges from 2 to 8 months, despite maximal treatment.3
The idea that the brain is “immunologically privileged” is being replaced with appreciation that the brain is an immune specialized site under tight regulatory control.4–6 Brain tumor–infiltrating lymphocytes (TILs) provide evidence that the immune system is naturally involved in the immunosurveillance of brain tumors.7,8 Tumors, on the other hand, create an immunosuppressive network by which they can escape from this immune attack.9 Glioblastomas can escape immune regulation by secretion of immunosuppressive cytokines,10 activation of negative regulatory pathways in lymphocytes,11,12 evasion of immune recognition,13 and CCL2-dependent recruitment of CCR4-positive regulatory T cells (Tregs).14
The relative importance of Tregs in this immunosuppressive network is demonstrated in experimental mouse glioma models that show a dramatic influx of Tregs and that temporal Treg depletion markedly augmented anti-tumor immunity.15–17 These data strengthen the view that Tregs create a tolerogenic environment that hampers antitumor immunity.
We examined the presence of CD4+FoxP3+CD25high-CD127low Tregs in patients with brain tumors. Large volumes of fresh brain tumor are needed to analyze the quantity, morphology, and function of intratumoral Tregs. For the surgery of most brain tumors, ultrasonic aspiration is a widely used technique next to normal resection.18 The availability of fresh brain tumor for clinical research is limited as the resected tumor fragments are often small and needed for diagnostic purposes. The integration of a suction adapter into ultrasonic aspirators has made it possible to collect ultrasonic aspirated brain tumor material.19 So far, tumor fragments removed by ultrasonic aspiration are used relatively infrequently in daily diagnostics20 and, to our knowledge, not at all in clinical research.
In this study, we show that the lymphocyte fractions in ultrasonic aspirated material are similar to those in resected brain tumor tissue. Our data demonstrate that it is possible to isolate and study large numbers of viable Tregs from brain tumors that are surgically removed with ultrasonic aspiration. Using ultrasonic aspirated brain tumor as a new source for clinical research, we detected that Tregs accumulate specifically in high-grade brain tumors. The Tregs in the tumor microenvironment are fully activated and strongly suppress TIL proliferation and cytokine production. We further show that a second important immune-regulatory pathway in brain tumors occurs via the PD-L1/PD-1 pathway. These immunomodulating mechanisms may hamper spontaneous in vivo immune responses and thereby contribute to the aggressive clinical behavior of high-grade brain tumors.
Materials and Methods
Patients
We collected blood and freshly resected brain tumor from 83 brain tumor patients treated at the Radboud University Nijmegen Medical Centre (RUNMC) or Canisius Wilhelmina Hospital (The Netherlands). All patients had histologically proven brain tumors, classified according to the WHO 2007 classification.1 The primary brain tumors consisted of 21 WHO grade I tumors (meningioma, n = 10; pituitary adenoma, n = 5; subependymal giant-cell astrocytoma (SEGA), n = 2; hemangioblastoma, n = 1; craniopharyngioma, n = 1; gangliocytoma, n = 1; schwannoma, n = 1), 6 WHO grade II tumors (atypical meningioma, n = 3; hemangio-pericytoma, n = 2; oligodendroglioma, n = 1), 6 WHO grade III tumors (anaplastic oligodendroglioma, n = 4; anaplastic astrocytoma, n = 1; anaplastic ependymoma, n = 1), and 31 WHO grade IV tumors (glioblastoma, n = 29; gliosarcoma, n = 1). We further included 20 patients with a solitary metastatic brain tumor derived from lung (n = 7), breast (n = 3), kidney (n = 2), colon (n = 1), esophagus (n = 1), melanoma (n = 1), rectum (n = 1), lymphoma (n = 1), or unknown origin (n = 3). Informed consent was obtained from all participants, and the study was approved by our institutional review board.
Preparation of Brain Tumor Cell Suspensions
Fresh brain tumor material was obtained by resection of the tumor tissue and/or ultrasonic aspiration with a Sonoca® 300 ultrasonic dissector/aspirator (Söring, Quickborn, Germany). Ultrasonic aspirates were collected in a closed system disposable suction bag (Serres, Kurikantie, Finland). Tumor fragments were filtered and washed to discard blood and suction fluid. Cell suspensions were made as previously described.16 Briefly, mechanically disrupted brain tumor was incubated with collagenase type-IA (50 mg/ml), DNAse type-I (10 μg/ml), and trypsin inhibitor (1 μg/ml) in Hanks Balanced Salt Solution (Invitrogen, Leek, The Netherlands) at 37°C and put on a Ficoll gradient (Axis-Shield PoC AS, Oslo, Norway).
Antibodies, Flow Cytometry, and Cell Sorting
Flow cytometric analysis was performed with a FACS-Calibur (BD Biosciences, San Jose, CA, USA) using directly labeled monoclonal antibodies (mAbs) against CD4, CD8, CD25, CD127, CTLA-4, PD-1, PD-L1, PD-L2 (BD Pharmingen, San Diego, CA, USA), FoxP3 (eBioscience, San Diego, CA, USA), and Ki-67 (Dako, Glostrup, Denmark), all according to the manufacturer’s protocol. Tregs were defined as CD4+FoxP-3+CD25highCD127low cells (Fig. 1A); percentage of Tregs was defined as the number of CD4+FoxP3+ CD25highCD127low cells divided by the total number of CD4+ cells × 100. To compare the levels of FoxP3 and CTLA-4 expression between blood-derived Tregs and Tregs in tumor tissue, mean fluorescence intensity of these markers was measured on both Treg subpopulations. Expression of Tregs in tumor tissue was calculated relative to the expression of blood-derived Tregs, which was set to 1.
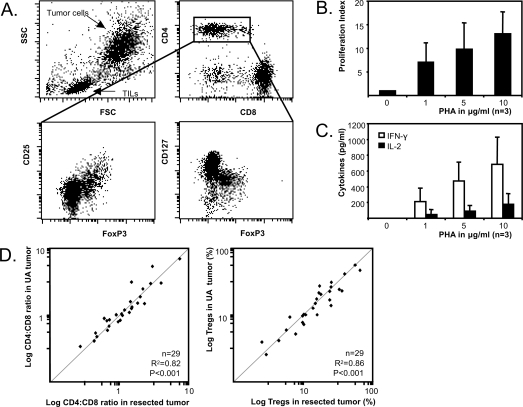
Fig. 1.
Flow cytometry of ultrasonic aspirated (UA) glioblastoma material (A). Lymphocytes can be differentiated into CD4+ cells (25%), CD8+ cells (65%), and a population mainly consisting of B cells (data not shown). CD4+ cells can further be characterized into CD4+FoxP3+ CD25highCD127low cells and CD4+FoxP3−CD25lowCD127high cells. Glioblastoma brain tumor–infiltrating lymphocytes (TILs) proliferate (B) and produce IFN-γ and IL-2 (C) upon increasing phytohemagglutinin (PHA) stimulation (the average of 3 glioblastoma patients is shown). (D) CD4:CD8 ratio (left scatterplot) and percentage of Tregs (right scatterplot) in ultrasonic aspirated brain tumor and resected brain tumor are strongly correlated (n = 29 tumors).
Following CD4 magnetic microbead selection (Miltenyi, Bergisch Gladbach, Germany), CD4+ positive cells were sorted into CD25+CD127− Tregs and CD25−CD127+ effector T cells using the Elite flow cytometric cell sorter (Beckman-coulter, Fullerton, CA, USA).
Functional Analysis of TILs in Ultrasonic Aspirated Tumor
TILs isolated from ultrasonic aspirated material were cultured for 3 days with increasing concentrations of phytohemagglutinin (PHA). Supernatants were taken after 2 days and IFN-γ and IL-2 were measured by a cytometric bead array (Th1/Th2 Cytokine CBA 1; BD Pharmingen) according to the manufacturer's instructions. To measure proliferation, incorporation of 3[H] thymidine was measured in duplicate in a ß-counter.
Suppression Assay
The suppressive capacity of the Tregs was determined by addition of increasing numbers of freshly sorted brain tumor Tregs to 2.5 × 103 brain tumor effector T cells (n = 3). Cells were cultured for 4 days in the presence of 2.5 × 103 CD3/CD28-beads (Invitrogen, Leek, The Netherlands). Cytokines in the supernatants were measured after 24 h as mentioned above. Cell proliferation was monitored by 3[H]thymidine incorporation.
Immunohistochemistry
Immunohistochemistry was performed on 4-μm tissue sections of formalin-fixed paraffin-embedded tissue blocks. Sections were boiled for 20 min in citrate buffer (10 mM, pH 6.0). The following mAbs were used: 236A/E7 (eBioscience) against FoxP3 and SP7 (Labvision, Fremont, CA, USA) against CD3 according to the manufacturer’s protocol. Primary antibodies were detected with biotinylated secondary antibodies (Vector Laboratories, Burlingame, CA, USA) followed by an avidin–biotin complex system (ABC Elite, Vector Laboratories). Diaminobenzidine tetrachloride served as a chromogen.
Results
Ultrasonic Aspirated Brain Tumor as a Source to Study Tumor-Infiltrating Lymphocytes
The effectiveness of the antitumor immune response is strongly influenced by the tumor microenvironment that can only be studied in fresh tumor material. We investigated whether ultrasonic aspirated brain tumor material can be used to study the tumor microenvironment. Heretofore, ultrasonic tumor aspirates were enzymatically digested and characterized by flow cytometry. Lymphocytes could be readily distinguished from tumor cells and were further subdivided into CD8+ T cells, CD4+ T cells, and CD4+FoxP3+CD25highCD127low Tregs (Fig. 1A). Isolated lymphocytes were still functional since they proliferated and secreted IFN-γ and IL-2 in response to increasing concentrations of PHA (Fig. 1B,C). In 29 patients we were able to collect both tumor aspirates and resected brain tumor tissue, allowing a direct comparison of lymphocyte content in both tumor sources. The CD4+:CD8+ T cell ratio and the percentage of tumor-infiltrating Tregs found in aspirated brain tumor fully correlated with the resected tumor tissue (Fig. 1D). These data indicate that ultrasonic aspirated tumor material provides an efficient and readily available source to study the brain tumor microenvironment.
Tregs Specifically Accumulate in High-Grade Brain Tumors
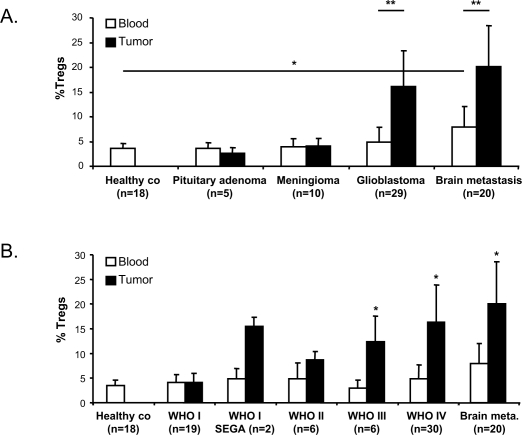
Having shown that TILs from ultrasonic aspirated brain tumor faithfully represent resected tumor tissue, we compared Tregs in tumor tissues and peripheral blood in 83 patients. The group was subdivided into patients with a primary brain tumor and patients with solitary metastatic brain tumors derived from different primary tumors (hereafter called brain metastasis). The percentage of Tregs in the blood of patients with a primary brain tumor was not significantly increased compared to that in healthy volunteers. The amount of Tregs in brain tumors is strongly associated with the tumor type (Fig. 2A). Low percentages of Tregs were detected in benign tumors such as pituitary adenomas (average 3.0%) and meningiomas (average 4.4%). Significantly higher percentages of Tregs accumulate in glioblastomas (average 16.5%, p < 0.003). Moreover, Treg accumulation in brain tumors is positively correlated with the WHO malignancy grade of the tumor (Fig. 2B, p < 0.01). The only exceptions are WHO grade I SEGAs, which also show an increase in the percentages of Tregs relative to blood.
Fig. 2.
Tregs accumulate in high-grade brain tumors. (A) Percentage of Tregs in blood and tumor of patients with brain tumors compared to healthy controls (co) (*p < 0.01, Student’s t-test, **p < 0.003, paired Student’s t-test). (B) Percentage of Tregs in brain tumors positively correlates with the WHO malignancy grade (*p < 0.01, Student’s t-test). WHO grade I subependymal giant-cell astrocytomas (SEGA) are represented as a separate group to illustrate the relatively high percentages of Tregs in this tumor. Abbreviation: co, control.
The highest level of intratumoral Treg recruitment was seen in patients with brain metastases, irrespective of the type of primary tumor, which suggests that Treg recruitment of metastatic brain tumors is a general phenomenon (Fig. 2). Interestingly, the percentage of Tregs in peripheral blood was also significantly increased in these patients with systemic disease. While on average 3.5% Tregs were detected in the blood of healthy controls, the blood of patients with brain metastases contained on average 7.9% Tregs (p = 0.003).
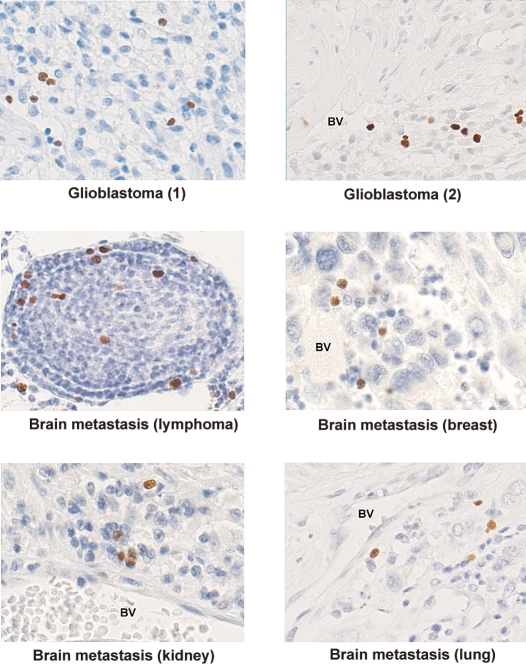
To visualize Tregs in situ, we performed immunohistochemistry on paraffin-embedded tumor tissue. The nuclear staining of FoxP3 in the Tregs could be clearly distinguished from the tumor cells and other TILs. Intratumoral Tregs were mainly localized perivascularly in close proximity to other lymphocytes. A smaller percentage of the Tregs infiltrated deep into the tumor tissue (Fig. 3).
Fig. 3.
FoxP3 positive lymphocytes in paraffin-embedded brain tumors (236A/E7 antibody). Abbreviation: BV, blood vessel.
Tregs Infiltrated into Malignant Tumors Are Highly Activated
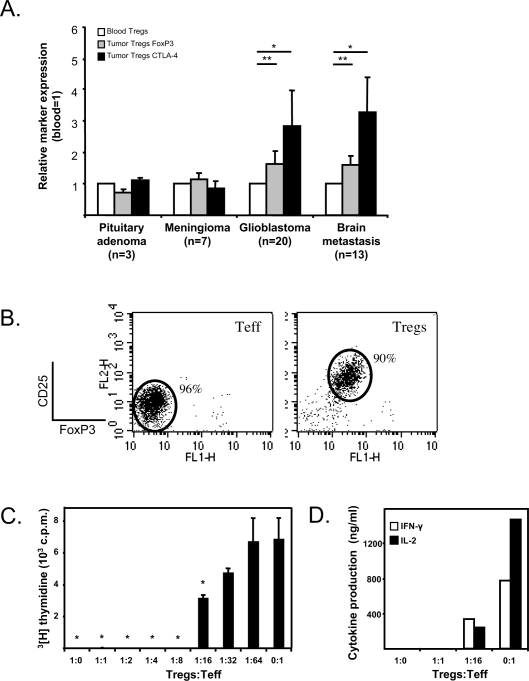
Tregs infiltrated into high-grade brain tumors expressed significantly higher levels of the Treg activation markers FoxP3 and CTLA-4 (Fig. 4A). On average, Tregs in glioblastomas expressed 1.7 times as much FoxP3 and 2.8 times as much CTLA-4 as Tregs in the blood of these patients (p < 0.01). Tregs in brain metastases expressed 1.6 times as much FoxP3 and 3.3 times as much CTLA-4 as Tregs in the blood of these patients (p < 0.001). Tregs in the WHO grade I brain tumors did not express increased levels of either FoxP3 or CTLA-4. The increase of CTLA-4 expression was caused by an increase in both intracellular and cell surface CTLA-4 expression (data not shown). These data indicate that Tregs present in high-grade gliomas and brain metastases are highly activated.
Fig. 4.
Tregs in high-grade brain tumors have an activated phenotype and strongly suppress proliferation of brain tumor–infiltrating lymphocytes (TILs). (A) FoxP3 and CTLA-4 expression are significantly increased in tumor-infiltrating Tregs compared to peripheral blood Tregs in patients with glioblastomas and patients with brain metastases (*p < 0.01; **p < 0.001, paired Student’s t-test). (B) Purified brain tumor–derived CD4+FoxP3−CD25lowCD127high (effector T cells, or Teff) and CD4+FoxP3+CD25highCD127low (Tregs) were titrated in a suppression assay. (C) Proliferation was assessed by 3[H]thymidine incorporation (*p < 0.001, Student’s t-test) and (D) cytokines were analyzed in supernatants. One representative experiment out of three is shown. Abbreviation: c.p.m., count per minute.
Intratumoral Tregs Suppress Lymphocyte Proliferation
To test the suppressive capacity of intratumoral Tregs, TILs were sorted to obtain a CD4+CD25highCD127low fraction (>90% Tregs) and a CD4+CD25lowCD127high fraction (>95% effector T cells) (Fig. 4B). The suppressive capacity of the intratumoral Tregs was analyzed in a titration experiment by incubating the cells with autologous intratumoral effector T cells, directly ex vivo (n = 3). As shown in Fig. 4C, the brain Tregs strongly suppress the proliferation of the autologous TILs (p < 0.001). Even 1 Treg had a suppressive effect when incubated with 16 effector T cells. The decrease of effector T cell proliferation coincides with a strong decrease in the pro-inflammatory cytokines IFN-γ and IL-2 (Fig. 4D).
Proliferation of Tregs in Vivo
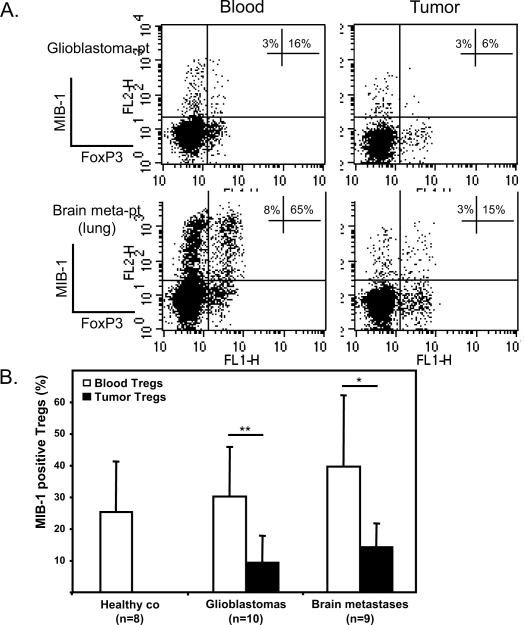
A key question is whether the Treg accumulation is due to specific tumor recruitment or to Treg expansion in the tumor microenvironment. Animal models have shown that Tregs can proliferate fast and that Treg turnover in vivo is short.21,22 We analyzed Treg proliferation in vivo by measuring the expression of proliferation marker MIB-1 (Molecular Immune Borstel-1, Dako, Glostrup, Denmark) using the Ki-67 antibody.23 In both patients with glioblastomas and patients with brain metastases we detected a strong and significant decrease of MIB-1–expressing Tregs in the tumor compared to Tregs in the blood (Fig. 5A,B, p < 0.05). These data imply that the capacity of Tregs to proliferate declines as they infiltrate the tumor. The high percentage of MIB-1–positive Tregs in the blood of healthy controls and patients (range 11%–65%) suggests a high turnover of these cells in vivo.
Fig. 5.
Treg proliferation in vivo. (A) The percentages of MIB-1 (Ki-67 antibody)–positive Tregs (of total CD4+FoxP3+) and MIB-1– positive CD4+ cells (of total CD4+FoxP3−) are indicated in the top right quadrants of the dot plots. Two representative patients are shown (Glioblastoma-pt and Brain meta-pt). (B) Overall MIB-1 expression on blood and intratumoral Tregs of healthy controls (co), patients with glioblastomas, and patients with brain metastases. The percentage of MIB-1–positive Tregs significantly decreases in the tumor (*p < 0.05; **p < 0.01, paired Student’s t-test). Abbreviations: glioblastoma-pt, patient with a glioblastoma; brain meta-pt, patient with a brain metastasis; co, control.
Contribution of PD-L1/PD-1 Pathway in Brain Tumor Immune Evasion
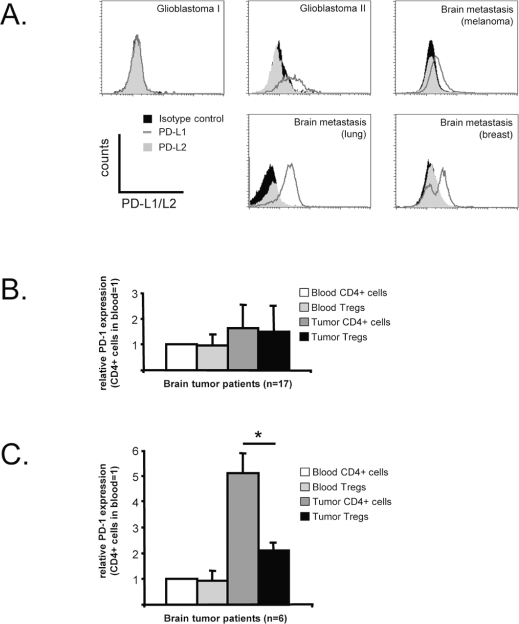
Expression of programmed death ligand-1 (PD-L1) on tumors plays an important role in immune evasion due to interaction of PD-L1 with the inhibitory receptor PD-1 that is expressed on activated lymphocytes.24 Sixty-one percent of the brain tumor samples in our cohort expressed PD-L1; the expression varied strongly between the tumors (Fig. 6A). None of the tested WHO grade I brain tumors expressed PD-L1. In 17 of 23 patients analyzed, PD-1 expression on both tumor-infiltrating CD4+ cells and Tregs was low or negative (Fig. 6B). In six patients, PD-1 expression was detected on intratumoral CD4+-effector T cells. The expression was significantly higher than that of PD-1 on the intratumoral Tregs of these patients (Fig. 6C, p = 0.005). PD-1 cell surface expression in blood lymphocytes was low or absent in all patients tested. These data indicate that the negative survival signal of PD-L1/PD-1 pathway may represent another immune suppression mechanism employed by malignant brain tumors.
Fig. 6.
PD-L1/PD-1 expression in brain tumors. (A) PD-L1 and PD-L2 expression in five representative brain tumors. Sixty-one percent of the tumor samples expressed PD-L1 (dark gray line), which varied strongly between samples. PD-L2 expression (gray shaded histogram) was negative in all tested tumors (n = 23). (B) Blood lymphocytes do not express PD-1 on their cell surface. In 17 of 23 tested tumors, PD-1 expression in brain tumor–infiltrating lymphocytes (TILs) was not significantly upregulated. (C) In 6 of 23 tested tumors, PD-1 cell surface expression was specifically upregulated on tumor-infiltrating CD4+FoxP3− cells (*p = 0.005, paired Student’s t-test).
Discussion
The brain is a specialized immune site and represents a unique microenvironment in which to study immune cell/tumor interactions. Using ultrasonic aspirated brain tumor material as a biosource we show that activated Tregs accumulate specifically in high-grade human brain tumors and brain metastases. These intratumoral Tregs acquire a fully activated phenotype and strongly suppress antitumor immune responses. A second important immune regulatory pathway in brain tumors occurs via the PD-L1/PD-1 pathway.
Studying the tumor microenvironment is often hampered by the limited availability of fresh tumor material. We demonstrate that tumor cells and lymphocytes in ultrasonic aspirated material from brain tumors are viable and functional. Moreover, we provide direct evidence that this material resembles tumor biopsies in multiple aspects. In addition to its use in studying the interplay between tumor cells and immune cells, ultrasonic aspirated brain tumor may represent a valuable biosource for glioma cancer stem cells. Furthermore, the tumor cells could be applied in the clinic to generate tumor lysate for immunotherapeutic interventions.25,26
In peripheral blood, Tregs represent 5%–10% of the CD4+ T cell population. Tregs induce immune tolerance by suppressing host immune responses and thus play a critical role in preventing autoimmune disease.27 However, Tregs also inhibit effective antitumor immune responses.28 In a mouse glioma model, reversible specific depletion of Tregs significantly increased survival, indicating the relevance of Tregs in glioblastomas.15,16 Recent studies demonstrate that the presence of Tregs in various cancer types is correlated with a poor prognosis.29 Glioblastoma is the only human brain tumor in which Tregs have been studied. Two independent studies confirm that Tregs can infiltrate malignant gliomas. However, the data on the Treg fractions in the blood and tumor of these patients are contradictory.30,31
Studying Tregs in humans is complicated by the discovery that human-activated nonregulatory CD4+ T cells transiently express both CD25 and FoxP3.32,33 Recent studies have demonstrated that downregulation of the IL-7 receptor (CD127) on Tregs distinguishes Tregs from activated T cells.34,35 We therefore defined the Tregs in our study as CD4+FoxP3+CD25highCD-127low cells. Massive Treg infiltration in aggressive human primary brain tumors such as glioblastomas and in metastatic brain tumors was readily observed. In contrast, Tregs did not accumulate in WHO grade I meningiomas and pituitary adenomas. Overall, accumulation of Tregs in brain tumors is positively correlated with the WHO malignancy grade of the tumor, except for grade I SEGAs. The reason for this is unclear and warrants further research. Currently, we determine the expression profiles of SEGAs and other WHO grade I brain tumors using microarrays to define the genes responsible for Treg recruitment. Our immunohistochemical data show that brain tumor–infiltrating Tregs are mainly localized perivascularly, and that a smaller fraction of Tregs infiltrate deep into the tumor, always in close proximity to other lymphocytes. This allows the Tregs to exert their contact-dependent suppression in vivo.36 Compared to healthy controls, no increase of Tregs in peripheral blood of patients with primary brain tumors was found. Only in case of brain metastases could a systemic increase in Tregs be demonstrated in the blood.
Relative to blood-derived Tregs, Tregs isolated from malignant brain tumors had a significantly increased expression of CTLA-4 and FoxP3, both of which are considered activation markers that correlate with active immune suppression.37,38 Indeed, the intratumoral Tregs strongly suppress the proliferation of autologous tumor-derived lymphocytes directly ex vivo, which suggests that patients can benefit from tumor-specific elimination of Tregs. We recently reported that in a murine glioma model, the immunosuppressive tumor environment can most efficiently be counteracted with Treg depletion in combination with active immunotherapy.17 Depletion of human Tregs by targeting CD25 or FoxP3 might be an option,39–41 although it should be noted that activated human effector T cells express significant amounts of CD25 and FoxP3.
To address the key question of whether the accumulation of Tregs is caused by an increased recruitment of Tregs to high-grade brain tumors or an increased proliferation of Tregs in the tumor, we measured the proliferation marker MIB-1. MIB-1 expression in peripheral blood Tregs of both patients and healthy controls is very high compared to CD4+FoxP3− T cells, as previously reported for mice.22 In all tested patients, a sharp decrease of MIB-1 expression by intratumoral Tregs relative to peripheral blood Tregs was observed. Overall, these data imply that the specific accumulation of Tregs in high-grade brain tumors is not primarily due to Treg proliferation at the tumor site; they are also in line with recent mouse studies showing that actively proliferating Tregs do not suppress42,43 and that tumors, including gliomas,14 can secrete chemokines to recruit Tregs.44–46 Initial MRI data imply that Treg accumulation cannot be simply explained by the size of the brain tumor; most WHO grade I tumors are large and harbor few Tregs. Possibly, structural and functional abnormalities in the vascular microenvironment in high-grade brain tumors, causing loss of blood-brain barrier function,47 do influence Treg infiltration. Blood-brain barrier integrity can be imaged using [18F]-fluoro-deoxy-L-fluorothymidine PET scans. We currently analyze these scans to correlate lymphocyte infiltration with blood-brain barrier integrity. In addition, the brain tumor microenvironment may particularly favor Treg survival and function, as glioblastomas can produce high levels of TGF-β, which is known to be beneficial for Treg survival and the maintenance of its immune suppressive function.48,49
Recently, it was shown that PD-L1 is expressed on glial tumor cells.12 We confirmed this observation and now show that PD-L1 is also expressed in various brain metastases. Interestingly, we observed in six high-grade brain tumors that the inhibitory receptor PD-1 is expressed several times higher on the cell surface of tumor-infiltrating effector lymphocytes compared to intratumoral Tregs. The PD-L1 expressed on brain tumor cells will therefore preferentially interact with the effector T cell population and may provide another selective survival advantage for Tregs in the tumor microenvironment.24 Our data show that the PD-L1/PD-1 pathway appears to be important in a subset of brain tumor patients. Further research is needed to determine the basis of the variable expression of PD-L1 on tumor cells and the PD-1 expression on intratumoral Tregs between different patients.
In conclusion, we show that viable cell suspensions can be made from fresh ultrasonic aspirated tumor material. Using ultrasonic aspirated brain tumor material as a biosource we show that activated Tregs specifically accumulate in high-grade human brain tumors. The tumor-infiltrating Tregs are potent suppressors directly ex vivo and thus hamper immune responses against the brain tumor. Abundant expression of PD-L1/PD-1 within the microenvironment of a selection of the brain tumors implies that this pathway represents a second immune-regulatory circuit active in brain tumors. These findings underscore the importance of controlling immune suppressive mechanisms as part of active immunotherapy protocols for the treatment of high-grade human brain tumors.
Acknowledgments
The authors thank Rob Woestenenk for excellent cell sorting (Cytometry Facility at the Central Hematology Lab, RUNMC), Jorieke Peters for her expertise on the suppression assays (Department of Blood Transfusion and Transplantation Immunology, RUNMC), and Kwinten Sliepen for technical assistance (Department of Tumor Immunology, Nijmegen Centre for Molecular Life Sciences). This study is financially supported by the Dutch Cancer Society (grant no. KUN2003-2893) and the eTumour Sixth Framework (grant no. LSH-2002-2.2.0-5).
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. Tumours of the Central Nervous System. Lyon: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Legler JM, Ries LA, Smith MA, et al. Cancer surveillance series [corrected]: brain and other central nervous system cancers: recent trends in incidence and mortality. J Natl Cancer Inst. 1999;91:1382–1390. doi: 10.1093/jnci/91.16.1382. [DOI] [PubMed] [Google Scholar]
- 3.Sawaya R, Ligon BL, Bindal RK. Management of metastatic brain tumors. Ann Surg Oncol. 1994;1:169–178. doi: 10.1007/BF02303562. [DOI] [PubMed] [Google Scholar]
- 4.Galea I, Bernardes-Silva M, Forse PA, van Rooijen N, Liblau RS, Perry VH. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med. 2007;204:2023–2030. doi: 10.1084/jem.20070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ransohoff RM, Kivisakk P, Kidd G. Three or more routes for leukocyte migration into the central nervous system. Nat Rev Immunol. 2003;3:569–581. doi: 10.1038/nri1130. [DOI] [PubMed] [Google Scholar]
- 6.Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 7.Dunn GP, Dunn IF, Curry WT. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immun. 2007;7:12. [PMC free article] [PubMed] [Google Scholar]
- 8.Lampson LA. Brain tumor immunotherapy: an immunologist’s perspective. J Neurooncol. 2003;64:3–11. doi: 10.1007/BF02700015. [DOI] [PubMed] [Google Scholar]
- 9.Croci DO, Zacarias Fluck MF, Rico MJ, Matar P, Rabinovich GA, Scharovsky OG. Dynamic cross-talk between tumor and immune cells in orchestrating the immunosuppressive network at the tumor microenvironment. Cancer Immunol Immunother. 2007;56:1687–700. doi: 10.1007/s00262-007-0343-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou JP, Morford LA, Chougnet C, et al. Human glioma-induced immunosuppression involves soluble factor(s) that alters monocyte cytokine profile and surface markers. J Immunol. 1999;162:4882–4892. [PubMed] [Google Scholar]
- 11.Strege RJ, Godt C, Stark AM, Hugo HH, Mehdorn HM. Protein expression of Fas, Fas ligand, Bcl-2 and TGFbeta2 and correlation with survival in initial and recurrent human gliomas. J Neurooncol. 2004;67:29–39. doi: 10.1023/b:neon.0000021739.34343.75. [DOI] [PubMed] [Google Scholar]
- 12.Parsa AT, Waldron JS, Panner A, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13:84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 13.Zagzag D, Salnikow K, Chiriboga L, et al. Downregulation of major histocompatibility complex antigens in invading glioma cells: stealth invasion of the brain. Lab Invest. 2005;85:328–341. doi: 10.1038/labinvest.3700233. [DOI] [PubMed] [Google Scholar]
- 14.Jordan JT, Sun W, Hussain SF, Deangulo G, Prabhu SS, Heimberger AB. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57:123–131. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006;105:430–437. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- 16.Grauer OM, Nierkens S, Bennink E, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121:95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- 17.Grauer OM, Sutmuller RP, van Maren W, et al. Elimination of regulatory T cells is essential for an effective vaccination with tumor lysate-pulsed dendritic cells in a murine glioma model. Int J Cancer. 2007;122:1794–1802. doi: 10.1002/ijc.23284. [DOI] [PubMed] [Google Scholar]
- 18.Brock M, Ingwersen I, Roggendorf W. Ultrasonic aspiration in neurosurgery. Neurosurg Rev. 1984;7:173–177. doi: 10.1007/BF01780701. [DOI] [PubMed] [Google Scholar]
- 19.Levy ML, McComb JG. Integration of a variable action suction adapter into ultrasonic aspirators. Neurosurgery. 1999;45:893–895. doi: 10.1097/00006123-199910000-00033. [DOI] [PubMed] [Google Scholar]
- 20.Neckelmann K, Kristensen BW, Schroder HD. Improved histopathological evaluation of gliomas using tissue fragments obtained by ultrasonic aspiration. Clin Neuropathol. 2004;23:47–52. [PubMed] [Google Scholar]
- 21.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruinsma M, van Soest PL, Leenen PJ, et al. Keratinocyte growth factor induces expansion of murine peripheral CD4+Foxp3+ regulatory T cells and increases their thymic output. J Immunol. 2007;179:7424–7430. doi: 10.4049/jimmunol.179.11.7424. [DOI] [PubMed] [Google Scholar]
- 23.Brown DC, Gatter KC. Ki67 protein: the immaculate deception? Histopathology. 2002;40:2–11. doi: 10.1046/j.1365-2559.2002.01343.x. [DOI] [PubMed] [Google Scholar]
- 24.Blank C, Gajewski TF, Mackensen A. Interaction of PD-L1 on tumor cells with PD-1 on tumor-specific T cells as a mechanism of immune evasion: implications for tumor immunotherapy. Cancer Immunol Immunother. 2005;54:307–314. doi: 10.1007/s00262-004-0593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Vleeschouwer S, Rapp M, Sorg RV, et al. Dendritic cell vaccination in patients with malignant gliomas: current status and future directions Neurosurgery 200659988–999.; discussion 999–1000. [DOI] [PubMed] [Google Scholar]
- 26.Okada H, Lieberman FS, Walter KA, et al. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of patients with malignant gliomas. J Transl Med. 2007;5:67. doi: 10.1186/1479-5876-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Ann Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 28.Wang RF. Regulatory T cells and innate immune regulation in tumor immunity. Springer Semin Immunopathol. 2006;28:17–23. doi: 10.1007/s00281-006-0022-7. [DOI] [PubMed] [Google Scholar]
- 29.Knutson KL, Disis ML, Salazar LG. CD4 regulatory T cells in human cancer pathogenesis. Cancer Immunol Immunother. 2007;56:271–285. doi: 10.1007/s00262-006-0194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro-Oncology. 2006;8:234–243. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 32.Pillai V, Ortega SB, Wang CK, Karandikar NJ. Transient regulatory T-cells: a state attained by all activated human T-cells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang J, Ioan-Facsinay A, van der Voort EI, Huizinga TW, Toes RE. Transient expression of FOXP3 in human activated nonregulatory CD4+ T cells. Eur J Immunol. 2007;37:129–138. doi: 10.1002/eji.200636435. [DOI] [PubMed] [Google Scholar]
- 34.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banham AH. Cell-surface IL-7 receptor expression facilitates the purification of FOXP3(+) regulatory T cells. Trends Immunol. 2006;27:541–544. doi: 10.1016/j.it.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med. 2007;13:108–116. doi: 10.1016/j.molmed.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 37.Sansom DM, Walker LS. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 38.Gavin MA, Rasmussen JP, Fontenot JD, et al. Foxp3–dependent programme of regulatory T-cell differentiation. Nature. 2007;445:771–775. doi: 10.1038/nature05543. [DOI] [PubMed] [Google Scholar]
- 39.Powell DJ, Jr, Felipe-Silva A, Merino MJ, et al. Administration of a CD25–directed immunotoxin, LMB-2, to patients with metastatic melanoma induces a selective partial reduction in regulatory T cells in vivo. J Immunol. 2007;179:4919–4928. doi: 10.4049/jimmunol.179.7.4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–3133. [PubMed] [Google Scholar]
- 41.Nair S, Boczkowski D, Fassnacht M, Pisetsky D, Gilboa E. Vaccination against the forkhead family transcription factor Foxp3 enhances tumor immunity. Cancer Res. 2007;67:371–380. doi: 10.1158/0008-5472.CAN-06-2903. [DOI] [PubMed] [Google Scholar]
- 42.Sutmuller RP, den Brok MH, Kramer M, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. J Clin Invest. 2006;116:485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sutmuller RP, Morgan ME, Netea MG, Grauer O, Adema GJ. Toll-like receptors on regulatory T cells: expanding immune regulation. Trends Immunol. 2006;27:387–393. doi: 10.1016/j.it.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Wald O, Izhar U, Amir G, et al. CD4+CXCR4highCD69+ T cells accumulate in lung adenocarcinoma. J Immunol. 2006;177:6983–6990. doi: 10.4049/jimmunol.177.10.6983. [DOI] [PubMed] [Google Scholar]
- 45.Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 46.Iellem A, Mariani M, Lang R, et al. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vajkoczy P, Menger MD. Vascular microenvironment in gliomas. Cancer Treat Res. 2004;117:249–262. doi: 10.1007/978-1-4419-8871-3_15. [DOI] [PubMed] [Google Scholar]
- 48.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-beta1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J Exp Med. 2005;201:1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci USA. 2004;101:4572–4577. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]