Abstract
This series of experiments investigated the effects of dorsal and ventral hippocampal lesions on taste aversion learning. Although damage to the hippocampus did not affect the acquisition of a taste aversion when the conditioning procedure used a relatively standard interval between taste and illness, both types of lesions produced a deficit in taste aversion when a long interval (3 hr) was interposed between taste exposure and induction of illness. In the same subjects, trace fear conditioning was selectively impaired by ventral lesions, whereas water maze performance was selectively impaired by dorsal lesions. The results replicate past dissociations of dorsal and ventral hippocampal function, and also suggest that the hippocampus has a less differentiated role in long-trace taste aversion learning.
Keywords: Delay, Freezing, Memory, Spatial, Trace
1. Introduction
In Pavlovian conditioning, the hippocampus plays a role in bridging the gap between the conditioned stimulus (CS) and the unconditioned stimulus (US) in situations in which the CS and US presentations are separated by an appreciable empty interstimulus interval (i.e., trace conditioning). For instance, lesions of the hippocampus, particularly the ventral aspect of the structure, have been consistently shown to impair trace conditioning in cued fear learning paradigms [e.g., 1]. A similar effect has also been reported in trace eyeblink conditioning, although the dorsal hippocampus may also contribute in that task [2]. In contrast, hippocampectomy does not seem to affect conditioning in these preparations when the CS and US are temporally contiguous [3-4].
Curiously, it has not been reported to our knowledge whether the hippocampus has a similar involvement in mediating trace intervals in conditioned taste aversion (CTA), a Pavlovian conditioning paradigm in which a taste CS is associated with an illness US [5]. CTA has the unusual ability to tolerate lengthy delays of minutes to hours between the CS and the US presentations [6-7]. This remarkable potential dwarfs the ability of other learning paradigms that can support only CS-US interstimulus intervals on the order of seconds [e.g., 8]. Although delays of various lengths are routinely incorporated into CTA experiments investigating the neurobiology of CTA learning, the neural substrate of long-trace conditioning in CTA has not been established (note that the term “long-delay conditioning” is traditionally used in the CTA literature to denote trace conditioning as defined above; we will use long-trace conditioning here to avoid confusion with “trace” and “delay” usage in CTA and fear/eyeblink conditioning literatures).
The primary aim of the present study was to examine the contribution of the hippocampus to long-trace CTA learning in rats by lesioning either the dorsal or the ventral aspect of the hippocampus. Functional distinctions between the dorsal and ventral portions of the structure along its septotemporal axis have been increasingly noted [9-10]. However, as both dorsal and ventral portions have been implicated in trace conditioning in Pavlovian learning paradigms [1-2], we hypothesized that lesions of these structures would disrupt long-trace CTA learning. We first examined long-trace CTA learning using a lengthy 3 hr CS-US trace interval, and found a strong CTA impairment in both lesion groups. We then showed that the lesion groups were not impaired when conditioned under a short CS-US interval. Finally, we confirmed that our lesions produced dissociable deficits consistent with those already reported in dorsal and ventral hippocampal-dependent tasks; dorsal hippocampal function was assessed with a spatial learning task in a water maze, whereas ventral hippocampal function was tested in a trace fear conditioning preparation.
2. Materials and methods
2.1. Subjects and surgery
Twenty male, Long-Evans rats, weighing 330 – 380 g at the beginning of the experiment, were individually housed and maintained on a 12-hr light-dark cycle. Food and water were freely available unless otherwise indicated. Under isoflurane anesthesia, rats received bilateral lesions of the dorsal hippocampus (n = 6 after one died during surgery), the ventral hippocampus (n = 7), or sham surgery (n = 6) prior to training. Lesions were made by injecting ibotenic acid (6 mg/ml, 0.5 μl per side, 0.1 μl/min) through an injector cannula. Coordinates (relative to bregma, midline, and skull surface, respectively) for dorsal hippocampal lesions were (1) -2.8 mm, ±1.8 mm, and -3.6 mm, and (2) -4.0 mm, ±3.2 mm, and -3.6 mm, and those for ventral hippocampal lesions were (1) -4.8 mm, ±5.0 mm, and -7.5 mm, and (2) −5.8 mm, ±5.0 mm, and -6.5 mm [11]. Control rats received sham surgery with saline injected into either the dorsal or ventral hippocampus. The rats were given a week to recuperate before training began. All experiments were conducted in accordance with guidelines of the institutional animal care and use committee and the National Institutes of Health.
Prior to the series of experiments described here, all rats went through a neophobia study with a grape juice solution. The procedures used to assess neophobia were the same as those previously reported [12], and no differences were found among the groups either in terms of the initial neophobic response or the subsequent recovery of neophobia. All the rats were then subjected to behavioral testing as described below sequentially.
2.2. CTA
While under a water restriction schedule (30-min water access in the morning and 30 min in the afternoon), the rats were given a 0.9% sodium chloride (salt) solution to drink for 30 min in the morning, and after a 3-hr delay, injected intraperitoneally with 0.3-M lithium chloride (LiCl; 10 ml/kg) to induce gastrointestinal malaise. This dose of LiCl has been repeatedly found to be highly effective at inducing CTAs in rats [e.g., 13-14]. Water was given for 30 min in the afternoon on conditioning day. Water was made available again the next day for 1 hr in the morning (in which intake was comparable among the groups) and 30 min in the afternoon. Two days after conditioning, a test was given in which one bottle of the salt solution was made available for 1 hr. CTA strength was assessed by comparing fluid consumption during the single-bottle test across groups.
To assess CTA learning without a long CS-US interval, the same procedures were used as described above, except that a citric acid-saccharin solution (0.29 g citric acid and 0.05 g of saccharin in 1 L of water) [15] was given as the CS, and LiCl was administered immediately after the drinking session. Rats were conditioned in this manner a day after the end of the long-trace CTA experiment and tested two days later. Training the same rats on multiple CTAs has been done previously [e.g., 16]. To minimize any potential carryover effect of repeated training and testing, we limited conditioning to one trial in each experiment; one-trial conditioning has been shown to be effective at inducing robust CTAs [e.g., 13-14].
2.3. Trace fear conditioning
Rats were subjected to trace fear conditioning a week after the end of the CTA experiments. The fear conditioning apparatus consisted of four cube-shaped operant chambers. A shock generator was used to deliver a 2-s 0.7-mA scrambled footshock through the steel rods that composed the floor of each chamber. Each chamber was enclosed in a sound-attenuating shell with a speaker that could present a 15-s 1500 Hz tone, a dim red houselight, and a bright white light. In order to avoid context fear during testing, a unique test context was created with the same chambers by manipulating lighting conditions (a white light instead of a dim red light was used), adding a mint odor cue, and introducing a floorplate that covered the grid floor. The procedure occurred over three days. On Day 1, the subjects experienced 5 tone→shock pairings in a single 30-min session. The onset of the shock occurred 30 s after the termination of the tone. On Day 2, all subjects were allowed to acclimate to the test context in a single 20-min session. On Day 3, the subjects received a test session in which the CS was presented 3-min after the subjects were placed in the chambers. Performance was videotaped and later scored by an investigator who judged whether the animals were freezing every 1.25 s during the 30-s period before the CS, the 15-s period during the CS, and the 30-s post-CS period.
2.4. Water maze task
Rats were trained on a water maze task a day after the fear conditioning experiment. The water maze was surrounded by black curtains with white cues. The pool measured 1.83 m in diameter and 0.58 m in height, and was filled with tepid water (27° ± 1°C) made opaque by the addition of non-toxic paint. A white retractable escape platform (height = 34.5 cm) was located 1 cm below the water surface. Rats received 6 training trials per day for 3 days to locate the submerged platform. The training trials for each day were divided into 2 blocks of 3 trials separated by 30 min. On each trial, a rat was released in the maze from 1 of 4 equally spaced starting positions around the perimeter of the pool. The starting position varied from trial to trial. If the rat did not locate the escape platform within 60 s on any trial, the experimenter guided and placed the rat on the platform, where it remained for 30 s. The rat was then removed from the platform and placed in a holding cage for another 30 s before the next trial. Two days after the last training day, the rats were given a 120-s probe test without the escape platform. Performance in the probe test was measured by the time spent in an annulus that was the size of the target platform and located in the area that the target platform occupied during training. Performance in the corresponding left, right, and opposite annuli was averaged and used as a control comparison.
2.5. Histology
The rats were deeply anesthetized and perfused with phosphate-buffered saline and then 4% paraformaldehyde. Blocks of brain containing the hippocampus were cut in 50 μm coronal slices, mounted onto glass slides, and stained with thionin. Sections were examined with a light microscope for location and completeness of the lesions.
2.6 Data analysis
Analysis of variance (ANOVA) and Student's t-tests were used for data analyses. The control group consisted of dorsal and ventral hippocampal sham lesions animals; as these animals showed no discernible differences between their behavioral performances, they were combined for the purpose of data analyses.
3. Results
3.1. CTA
Intake of CS solution during conditioning (30 min) showed no differences among the control (mean = 12.2 ml, SEM = 2.42), dorsal lesion (16.0 ml, SEM = 1.89), and ventral lesion (14.1 ml, SEM = 1.52) groups in the long-trace experiment. However, they differed between the control and the two lesion groups during test as shown in Figure 1A (left panel). The rats in the control group consumed significantly less CS solution during test (60 min) than those in either the dorsal or ventral lesion groups, t(10) = 2.48, p = .032 and t(11) = 2.36, p = .038 respectively, with the two lesion groups not differing from each other (t < 1). Therefore, these results demonstrate that dorsal and ventral hippocampal lesions similarly interfered with long-trace taste aversion learning.
Fig. 1.
Panel A shows the consumption of CS during CTA test under long-trace and standard training protocols. Panel B shows the percent of time that animals froze during the test of trace fear conditioning. Panel C displays the total time spent in the target and control annuli during the water-maze probe test. * p < .05.
Unlike the long-trace CTA, standard CTA learning with minimal CS-US delay was not significantly altered by hippocampal damage. Consumption during conditioning of the lesion groups (dorsal = 14.5 ml; SEM = 0.86, ventral = 11.0 ml, SEM = 0.67) did not differ from the control group (12.8 ml; SEM = 1.19); however, the average intake of the ventral group was less than that of the dorsal group (p = .007). Importantly though, the three groups exhibited no differences in intake at test (ps > .115; right panel on Figure 1A). These results are therefore consistent with the majority of previous reports of intact taste aversion learning after hippocampal lesions [17-19], and instead suggest a more specific role for the hippocampus in bridging a long CS-US trace interval.
3.2. Trace fear conditioning
Figure 1B shows the percent freezing from the pre-CS, CS, and post-CS periods on the test day. There were low levels of freezing in all groups prior to CS onset. All rats showed an increase in freezing during and after the CS. Post-CS freezing, which corresponds to the trace interval during training, appears to be greater in the control and dorsal hippocampus lesioned rats relative to the ventral hippocampus lesioned rats. Planned comparisons of the pre-CS period detected no differences between any of the lesion conditions (ps > .36). In the post-CS period, ventral lesions produced a reduction in freezing relative to dorsal and sham lesions, t(11) = 2.72, p = .020 and t(11) = 3.15, p = .009, respectively. Notably, rats with dorsal hippocampal lesions did not differ from those with sham lesions, t < 1. Hence, the ventral hippocampal lesions selectively reduced trace fear learning.
3.3. Spatial learning and memory
The rats were trained for 3 days in the water maze and showed improved proficiency over days as they took progressively less time to escape. All three groups improved similarly in general, although the rats with dorsal hippocampal lesions were slower to escape on the last training day (mean = 17.2 s, SEM = 3.07) than those with sham (9.9 s, SEM = 1.62) or ventral lesions (8.2 s, SEM = 1.62).
Figure 1C shows the results of the memory probe test given two days after the end of training. The rats in the control and ventral lesion groups spent more time searching in the target escape platform location relative to corresponding control locations in other quadrants of the maze, while rats in the dorsal lesion group did not show such a search pattern. Two-way ANOVA (between Group with repeated measures for Location) yielded a significant interaction of Group X Location, F(2, 16) = 5.47, p = .015, suggesting a difference among the groups in search bias. This was confirmed in the subsequent analysis showing that the rats in both the control and ventral lesion groups differed significantly in the location of search with a significant spatial bias for the target annulus, t(5) = 3.10, p = .027 and t(6) = 3.56, p = .012, respectively. No such bias however was present in the group with dorsal lesions (t < 1). These results are consistent with the findings by others that the dorsal but not ventral hippocampus is critical to spatial learning and memory [e.g., 20-21]. Altogether with the previous experiments, this indicates that the dorsal and ventral hippocampal lesions studied here produced dissociable effects as previously established in the literature, but had a similar effect on long-trace CTA. Representative photomicrographs as well as the largest and smallest extent of dorsal and ventral hippocampal lesions in the present study are shown in Figure 2.
Fig.2.
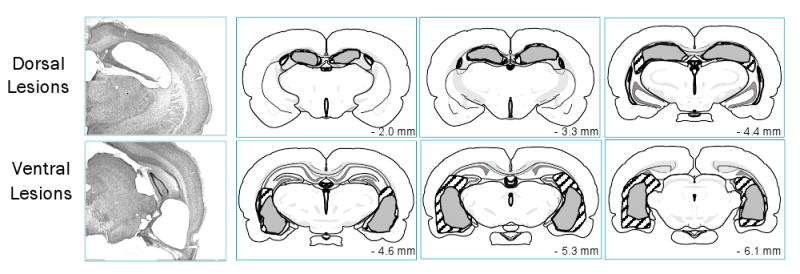
Coronal hemisections from brains representative of the dorsal (top panel) and ventral (bottom panel) hippocampal lesioned groups, and illustrations of the largest (hatched) and smallest (gray) extent of the lesion in each group. In the dorsal group, extensive cell loss in the dorsal hippocampus was evident from approximately 1.8 to 4.5 mm posterior to bregma with minimal damage to the overlaying neocortex and the ventral hippocampus. In the ventral group, the dorsal hippocampus was left completely intact; cell loss was apparent only in the ventral hippocampus from approximately 4.0 to 6.7 mm posterior to bregma, with some damage to the underlaying entorhinal cortex.
4. Discussion
The present series of experiments showed that lesions of either dorsal or ventral divisions of the hippocampus led to impaired long-trace conditioning of a CTA, with no significant disruption to conditioning of a CTA with a short trace. This suggests that both dorsal and ventral hippocampus contribute to some aspect of temporal processing of long CS-US trace intervals in CTAs, a function that is similar to those demonstrated in Pavlovian fear and eyeblink conditioning paradigms. While both dorsal and ventral lesions were effective in disrupting long-trace CTA, lesions of each subdivision selectively interfered with performance in other learning tasks known to be mediated differently by these subdivisions. Consistent with that evidence, ventral but not dorsal lesions interfered with trace fear conditioning, while the opposite effect was seen in the spatial water maze task. This pattern of double dissociation establishes the effectiveness of the current lesions in those settings, underscoring the novel finding here that both the dorsal and the ventral hippocampus are necessary for long-trace conditioning in CTAs.
Limited lesions of the dorsal and ventral hippocampus did not apparently interfere with taste processing as CS intake during conditioning was not different from control levels. Nonetheless, having just the dorsal or ventral hippocampus was clearly not sufficient to support long-trace CTA memory. This appears to contrast with trace fear conditioning in which the ventral but not the dorsal hippocampus is necessary for the acquisition of fear memory in trace conditioning [1, 22]. Others have suggested that stress- and anxiety-related processes in learning are especially dependent on the ventral hippocampus [9, 23]. By this account, and insofar that CTA learning depends on such processes, lesions of the ventral but not dorsal hippocampus would be expected to disrupt CTA learning. The importance of the dorsal hippocampus to trace conditioning of CTA but not conditioned fear may be due to a variety of factors that go beyond differences in affective processes engaged by the type of CS and US involved. Notably the comparatively protracted (3 hr) trace interval used and the remarkably rapid learning from one-trial trace conditioning in the CTA paradigm may engage a more distributed hippocampal system. The selective disruption of long-trace CTA learning by both dorsal and ventral lesions might then be best attributed to the role of the hippocampus in rapidly associating temporally discontiguous events; both dorsal and ventral subregions have been implicated in such a role [1-2].
The present experiments clearly show that the hippocampus influences the acquisition of a long-trace CTA. Further investigations using multiple CS-US pairings could determine whether the impairment caused by hippocampal lesions would be overcome by multiple conditioning trials. If so, then delayed learning after hippocampal damage would be more akin to the deficit observed following lesions of the gustatory cortex [e.g., 24], suggesting a supporting function for the hippocampus in long-trace CTA learning. Conversely, if multiple conditioning trials failed to surmount the impairment, then this complete deficit would suggest an obligatory role for the hippocampus in long-trace CTA association, similar to that required to bridge trace intervals in other paradigms.
The present findings are partially consistent with those reported by Krane, Sinnamon, and Thomas [25] in which non-selective electrolytic lesions of the hippocampus retarded the conditioning of a CTA with a trace interval of 15 min. Here, we showed that selective neurotoxic lesions restricted to either the dorsal or ventral portion of the hippocampus interfered with long-trace CTA learning using a 3 hr delay. However, unlike Krane et al. [25], our lesions did not appear to interfere with neophobia based on consumption during conditioning, suggesting the CTA deficit observed following long CS-US trace interval was likely not due to the disruption of taste processing as concluded by Krane et al. [25], but has a more specific role in bridging trace intervals.
What is the exact function of the hippocampus in trace CTA conditioning? A simple supposition would be to serve as a buffer or intermediate store for the CS taste memory to be associated. This is however not necessarily a role that has been ascribed to the hippocampus in trace conditioning in other learning paradigms. For example, in a neurophysiological study of trace eyeblink conditioning, neurons in the CA1 subregion of the hippocampus first responded to the US before shifting to the predictive CS after multiple training trials [26], suggesting a role in bridging the encoding of CS-US associations for discontiguous events rather than in storing a CS representation over a short period of time. Others have similarly promoted discontiguity theories of hippocampal function, in which the hippocampus is critically involved in associating both temporally and spatially discontiguous events [e.g., 27]. Our data are consistent with the functions ascribed in such theories, and further broaden the role of the hippocampus to exceptionally rapid one-trial learning of discontiguous taste-illness events. Our data also underscore and extend the role of the hippocampus from bridging CS-US trace intervals of a few seconds, as in fear and eyeblink conditioning, to ones of several hours, as seen here in long trace CTA learning. Differences in time scales between these trace conditioning paradigms have been attributed to the nature of the stimuli used (e.g., exteroceptive versus interoceptive cues) [28-29] and their tendency to be affected by other intervening events during trace intervals (e.g., concurrent interference theory) [30]. Our findings suggest that the involvement of hippocampus extends to multiple stimulus dimensions across modalities and time scales.
The determination of the neural network responsible for long-trace CTA learning awaits further experimentation and more sophisticated methodology to probe the functional significance of the structures involved. Here, we have made the important first step of identifying the hippocampus as having a central role in this network.
Acknowledgments
This research was supported by National Institute of Health Grant P01-AG-09973. We thank Professor Peter C. Holland for generously sharing his operant chambers with us.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yoon T, Otto T. Differential contributions of dorsal vs. ventral hippocampus to auditory trace fear conditioning. Neurobiol Learn Mem. 2007;87:464–75. doi: 10.1016/j.nlm.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Weible AP, O'Reilly JA, Weiss C, Disterhoft JF. Comparisons of dorsal and ventral hippocampus cornu ammonis region 1 pyramidal neuron activity during trace eye-blink conditioning in the rabbit. Neuroscience. 2006;141:1123–37. doi: 10.1016/j.neuroscience.2006.04.065. [DOI] [PubMed] [Google Scholar]
- 3.Christian KM, Thompson RF. Neural substrates of eyeblink conditioning: acquisition and retention. Learn Mem. 2003;10:427–55. doi: 10.1101/lm.59603. [DOI] [PubMed] [Google Scholar]
- 4.McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts auditory trace fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–46. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.Garcia J, Kimeldoft DJ, Koelling RA. Conditioned aversion to saccharin resulting from exposure to gamma radiation. Science. 1955;122:157–8. [PubMed] [Google Scholar]
- 6.Revusky SH. Aversion to sucrose produced by contingent x-irradiation: temporal and dosage parameters. J Comp Physiol Psychol. 1968;65:17–22. doi: 10.1037/h0025416. [DOI] [PubMed] [Google Scholar]
- 7.Smith JC, Roll DL. Trace conditioning with X-rays as the aversive stimulus. Psychon Sci. 1967;9:11–12. [Google Scholar]
- 8.Weiss C, Knuttinen MG, Power JM, Patel RI, O'Connor MS, Disterhoft JF. Trace eyeblink conditioning in the freely moving rat: optimizing the conditioning parameters. Behav Neurosci. 1999;113:1100–5. doi: 10.1037//0735-7044.113.5.1100. [DOI] [PubMed] [Google Scholar]
- 9.Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus– memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–83. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3 and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiol Learn Mem. 2008;89:61–9. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 2nd. San Diego, CA: Academic Press; 1986. [Google Scholar]
- 12.Gallagher M, Burwell RD. Relationship of age-related decline across several behavioral domains. Neurobiol Aging. 1989;10:691–708. doi: 10.1016/0197-4580(89)90006-7. [DOI] [PubMed] [Google Scholar]
- 13.Misanin JR, Collins M, Rushanan S, Anderson MJ, Goodhard M, Hinderliter CF. Aging facilitates long-trace taste-aversion conditioning in rats. Physiol Behav. 2002;75:759–64. doi: 10.1016/s0031-9384(02)00671-6. [DOI] [PubMed] [Google Scholar]
- 14.Nachman M, Ashe JH. Learned taste aversions in rats as a function of dosage, concentration, and route of administration of LiCl. Physiol Behav. 1973;10:73–8. doi: 10.1016/0031-9384(73)90089-9. [DOI] [PubMed] [Google Scholar]
- 15.Stolerman IP, Pilcher CWT, D'Mello GD. Stereospecific aversive property of narcotic antagonists in morphine-free rats. Life Sci. 1978;22:1755–62. doi: 10.1016/0024-3205(78)90628-8. [DOI] [PubMed] [Google Scholar]
- 16.Geddes RI, Han L, Baldwin AE, Norgren R, Grigson PS. Gustatory insular cortex lesions disrupt drug-induced, but not lithium chloride-induced, suppression of conditioned stimulus intake. Behav Neurosci. 2008;122:1038–50. doi: 10.1037/a0012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Best PJ, Orr J., Jr Effects of hippocampal lesions on passive avoidance and taste aversion conditioning. Physiol Behav. 1973;10:193–6. doi: 10.1016/0031-9384(73)90296-5. [DOI] [PubMed] [Google Scholar]
- 18.Bures J, Bermúdez-Rattoni F, Yamamoto T. Conditioned taste aversion: Memory of a special kind. Oxford University Press; Oxford, UK: 1998. [Google Scholar]
- 19.McGowan BK, Hankins WG, Garcia J. Limbic lesions and control of the internal and external environment. Behav Biol. 1972;7:841–52. doi: 10.1016/s0091-6773(72)80176-7. [DOI] [PubMed] [Google Scholar]
- 20.Bannerman DM, Yee BK, Good MA, Heupel MJ, Iversen SD, Rawlins JN. Double dissociation of function within the hippocampus: a comparison of dorsal, ventral, and complete hippocampal cytotoxic lesions. Behav Neurosci. 1999;113:1170–88. doi: 10.1037//0735-7044.113.6.1170. [DOI] [PubMed] [Google Scholar]
- 21.Moser MB, Moser EI, Forrest E, Andersen P, Morris RG. Spatial learning with a minislab in the dorsal hippocampus. Proc Natl Acad Sci USA. 1995;92:9697–701. doi: 10.1073/pnas.92.21.9697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rogers JL, Hunsaker MR, Kesner RP. Effects of ventral and dorsal CA1 subregional lesions on trace fear conditioning. Neurobiol Learn Mem. 2006;86:72–81. doi: 10.1016/j.nlm.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Moser MB, Moser EI. Functional differentiation in the hippocampus. Hippocampus. 1998;8:608–19. doi: 10.1002/(SICI)1098-1063(1998)8:6<608::AID-HIPO3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 24.Kiefer SW, Orr MR. Taste avoidance, but not aversion, learning in rats lacking gustatory cortex. Behav Neurosci. 1992;106:140–6. doi: 10.1037//0735-7044.106.1.140. [DOI] [PubMed] [Google Scholar]
- 25.Krane RV, Sinnamon HM, Thomas GJ. Conditioned taste aversions and neophobia in rats with hippocampal lesions. J Comp Physiol Psychol. 1976;90:680–93. doi: 10.1037/h0077236. [DOI] [PubMed] [Google Scholar]
- 26.McEchron MD, Disterhoft JF. Hippocampal encoding of non-spatial trace conditioning. Hippocampus. 1999;9:385–96. doi: 10.1002/(SICI)1098-1063(1999)9:4<385::AID-HIPO5>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;21:317–23. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 28.Garcia J, Ervin FR. Gustatory-visceral and telereceptor-cutaneous conditioning: adaptation in internal and external milieus. Commun Behav Biol. 1968;1:389–415. [Google Scholar]
- 29.Rozin P, Kalat JW. Specific hungers and poison avoidance as adaptive specializations of learning. Psychol Rev. 1971;78:459–86. doi: 10.1037/h0031878. [DOI] [PubMed] [Google Scholar]
- 30.Revusky SH. The role of interference in association over a delay. In: Honig WK, James PHR, editors. Animal memory. New York: Academic Press; 1971. pp. 155–213. [Google Scholar]



