Abstract
Background
Many general anesthetics are thought to produce a loss of wakefulness, in part, by enhancing gamma-aminobutyric acid (GABA) neurotransmission. However, GABAergic neurotransmission in the pontine reticular formation promotes wakefulness. This study tested the hypotheses that: 1) relative to wakefulness, isoflurane decreases GABA levels in the pontine reticular formation; and 2) pontine reticular formation administration of drugs that increase or decrease GABA levels increases or decreases, respectively, isoflurane induction time.
Methods
To test hypothesis 1, cats (n = 5) received a craniotomy and permanent electrodes for recording the electroencephalogram and electromyogram. Dialysis samples were collected from the pontine reticular formation during isoflurane anesthesia and wakefulness. GABA levels were quantified using high performance liquid chromatography. For hypothesis 2, rats (n = 10) were implanted with a guide cannula aimed for the pontine reticular formation. Each rat received microinjections of Ringer’s (vehicle control), the GABA uptake inhibitor nipecotic acid, and the GABA synthesis inhibitor 3-mercaptopropionic acid. Rats were then anesthetized with isoflurane and induction time was quantified as loss of righting reflex. Breathing rate was also measured.
Results
Relative to wakefulness, GABA levels were significantly decreased by isoflurane. Increased power in the electroencephalogram and decreased activity in the electromyogram caused by isoflurane co-varied with pontine reticular formation GABA levels. Nipecotic acid and 3-mercaptopropionic acid significantly increased and decreased, respectively, isoflurane induction time. Nipecotic acid also increased breathing rate.
Conclusion
Decreasing pontine reticular formation GABA levels comprises one mechanism by which isoflurane causes loss of consciousness, altered cortical excitability, muscular hypotonia, and decreased respiratory rate.
Introduction
The pontine reticular formation is part of the ascending reticular activating system, contributes to the electroencephalographic activation characteristic of wakefulness,1 and is involved in generating rapid eye movement (REM) sleep2 and some traits of general anesthesia.3 Synaptic terminals from local and distant gamma-aminobutyric acid (GABA) containing neurons4,5 as well as GABA type A (GABAA) receptors6-8 are present in the pontine reticular formation and modulate behavioral arousal. For example, microinjecting the GABAA receptor agonist muscimol into the pontine reticular formation increases wakefulness.9,10 Conversely, microinjecting the GABAA receptor antagonist bicuculline into the pontine reticular formation increases REM sleep and decreases wakefulness.10,11 Dialysis delivery of bicuculline to cat pontine reticular formation increases local acetylcholine release and also triggers REM sleep.12 Furthermore, administering drugs that selectively increase or decrease GABA levels into rat pontine reticular formation causes an increase or decrease, respectively, in wakefulness.13 The foregoing data suggest that GABAergic transmission in the pontine reticular formation suppresses sleep and promotes wakefulness.
Most anesthetic drugs potentiate GABAA receptor-mediated inhibition, and this potentiation is thought to comprise part of the mechanism by which general anesthetics produce hypnosis.14,15 Given the role of the pontine reticular formation in promoting wakefulness and causing activation of the cortical electroencephalogram,3 anesthetics are likely to alter GABAergic neurotransmission within the pontine reticular formation in order to produce a loss of wakefulness. No previous studies have quantified the effect of isoflurane anesthesia on endogenous GABA levels within the pontine reticular formation. The present study used in vivo microdialysis to test the hypothesis that, relative to wakefulness, isoflurane decreases extracellular GABA levels in the pontine reticular formation. The effects of isoflurane on electroencephalographic power and skeletal muscle tone also were quantified. To further explore the relationship between GABA levels in the pontine reticular formation and isoflurane-induced loss of wakefulness, this study next examined the effects of pharmacologically altering endogenous GABA levels in the pontine reticular formation on isoflurane induction time and rate of breathing. Intracranial microinjection of a GABA uptake inhibitor and a GABA synthesis inhibitor was used to test the hypothesis that isoflurane induction time is increased by increasing pontine reticular formation GABA levels, and decreased by decreasing pontine reticular formation GABA levels.
Materials and Methods
Animals and Chemicals
All animal procedures were approved by the University of Michigan Committee on Use and Care of Animals and conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press, Washington D.C., 1996*) and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Academies Press, Washington, D.C., 2003#). In vivo microdialysis experiments designed to measure GABA levels, activation of the cortical electroencephalogram, and skeletal muscle tone during wakefulness and isoflurane anesthesia were conducted in cat. Adult male cats (n=5) were purchased from Harlan (Indianapolis, IN). Several lines of evidence support the use of cat as an experimental animal for these preclinical studies. First, most of what is currently known about brain stem neurophysiologic mechanisms regulating arousal state is derived from studies using cat.2 Second, the large size of the cat brain stem16 provides optimal spatial resolution for accurate stereotaxic placement of microdialysis probes. Third, numerous studies have shown that neurotransmitter systems within the pontine reticular formation of cat are altered by anesthetic17-19 and analgesic20,21 drugs currently used in clinical practice. Finally, pontine GABAergic transmission in cat modulates arousal state.10 Therefore, in the present study, cat provided an ideal model for gaining insights into mechanisms of anesthetic action and arousal state control.
Intracranial microinjection studies designed to determine the effects on isoflurane induction time of drugs that increase or decrease pontine reticular formation GABA levels were performed using rat. Adult male Sprague-Dawley rats (n=10) were purchased from Charles River Laboratories (Wilmington, MA). Rats were used for this portion of the study for multiple reasons. First, loss of righting reflex studies are commonly performed using rat, not cat. Second, similar to cat, GABAergic mechanisms in rat pontine reticular formation have been shown to promote wakefulness and inhibit sleep.9,11,13 Third, the relatively large sample size required for these experiments made use of cat economically unfeasible.
Chemicals for Ringer’s solution, o-phosphoric acid, sodium phosphate dibasic, sucrose, and formalin were purchased from Fischer Scientific (Pittsburgh, PA). High performance liquid chromatography (HPLC) grade methanol, acetonitrile, sodium tetraborate decahydrate, ß-mercaptoethanol, nipecotic acid, 3-mercaptopropionic acid (3-MPA), and GABA were obtained from Sigma-Aldrich (St. Louis, MO). O-phthaldialdehyde was purchased from Mallinckrodt (St. Louis, MO).
Surgical Preparation of Cats for In Vivo Microdialysis Studies
Surgery to prepare cats for subsequent microdialysis studies was performed under sterile conditions, as described previously.12 Briefly, cats were anesthetized by mask induction with isoflurane and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, CA) equipped with a Kopf 880 semi-chronic head holder. Standard recording electrodes were implanted for objective assessment of arousal state.22 A permanent craniotomy was created over the cerebellum and sealed with sterile Bone Wax (Ethicon, Somerville, NJ). The craniotomy provided subsequent access for a microdialysis probe to be placed in the pontine reticular formation. The recording electrodes and two parallel stainless steel tubes that attach to the Kopf 880 holder were secured to the skull with dental acrylic. The Kopf 880 holder and these tubes allowed placement of the animal in stereotaxic position during dialysis experiments without using ear bars. Cats were allowed to recover from surgery and conditioned to the experimental setting for three weeks before starting experiments.
In Vivo Microdialysis: Study Design and Procedures
All experiments were conducted at the same time of day. Sample collection began between 10:30 and 12:30 h and experiments lasted 3.5 to 4 h. Two groups of microdialysis experiments were performed. Group I experiments aimed to 1) determine the time needed to achieve stable GABA levels after a dialysis probe is inserted into the pontine reticular formation during general anesthesia, and 2) test the hypothesis that GABA levels in cat pontine reticular formation are greater during post-anesthesia wakefulness than during anesthesia. Group II experiments aimed to determine 1) the amount of time required to achieve stable GABA levels following dialysis probe placement into the pontine reticular formation during wakefulness, and 2) whether GABA levels in the pontine reticular formation are greater during wakefulness than during anesthesia when wakefulness precedes anesthesia. Thus, the second set of experiments served to ensure that an order effect did not account for differences in GABA levels during anesthesia and wakefulness.
Each group I experiment began by mask induction with isoflurane. The vocal cords were sprayed with 1% lidocaine, the trachea was intubated with a pediatric endotracheal tube, and the cat was mechanically ventilated using a SAV 2500 Anesthesia Ventilator (SurgiVet, Waukesha, WI). The animal was placed in stereotaxic position and end tidal isoflurane and carbon dioxide concentrations, core body temperature, heart rate, and blood oxygen saturation were continuously monitored (Cardiocap/5 Datex-Ohmeda, Louisville, MO and Ohmeda Biox 3700 Pulse Oximeter, Boulder, CO). Core body temperature was maintained at 37.5°C using a waterfilled heating pad connected to a T500 T/Pump Heat Therapy System (Gaymar Industries, Inc., Orchard Park, NY). End tidal concentrations of carbon dioxide and isoflurane were maintained throughout the experiment at 30-33 mmHg and 2.1-2.2% (1.1-1.2 MAC23), respectively (Cardiocap/5 Datex-Ohmeda).
Once all monitors were in place, a stainless steel guide tube was stereotaxically aimed for the pontine reticular formation. Selected stereotaxic coordinates16 for dialysis aim sites ranged from: anterior 1.0 to 3.0 mm, lateral 1.0 to 2.5 mm, and horizontal -4 to -5 mm. Each dialysis site was used for only one experiment and was separated from other aim sites by at least 1 mm in the anterior-posterior and medio-lateral planes. A custom-made microdialysis probe (CMA/10 or 12, CMA Microdialysis, North Chelmsford, MA) with a shaft length of 70 mm and polycarbonate membrane (20 kDa cutoff, 2 mm length, 0.5 mm diameter) was inserted into the guide tube. The probe was continuously perfused with Ringer’s solution (147.0 mM NaCl, 2.4 mM CaCl2, 4.0 mM KCl, 1.0 mM MgSO4, pH 6.0) at a flow rate of 3.0 μl/min using a CMA/400 syringe pump.
Thirty sequential dialysis samples (15 μl each, 5 min/sample) were collected during isoflurane anesthesia. Sample collection started 20 min after probe insertion. Following the first 30 samples, anesthesia was discontinued and additional samples were collected during wakefulness following recovery from anesthesia. All dialysis samples were collected on ice for subsequent analysis.
For group II experiments, a dialysis probe was stereotaxically aimed for the pontine reticular formation in the awake cat. Animals were permitted to sleep ad libitum during the first 120 min of dialysis during which time GABA levels stabilized. Thereafter, using mild auditory and non-nociceptive stimulation, cats were kept awake (min 125 to 170) during sample collection (n = 10 samples per experiment). After the last dialysis sample was collected during wakefulness, cats were anesthetized, intubated, and ventilated until stable anesthesia was achieved, and 10 additional dialysis samples were collected during anesthesia.
For all experiments, after collecting the last dialysis sample the dialysis probe and guide tube were removed from the brain. The craniotomy was sealed with bone wax, and the animal was observed until it had fully recovered. The animal was then returned to its home cage in the Unit for Laboratory Animal Medicine.
Quantification of GABA Using HPLC and Electrochemical Detection
Quantification of GABA in microdialysis samples has been described in detail.13,24 Briefly, dialysis samples were analyzed using an ESA HPLC system (Chelmsford, MA). Each dialysis sample (12.5 μl) was loaded into an autosampler, mixed with 6.0 μl of derivatization solution (5.0 mM o-phthaldialdehyde, 1.8 mM ß-mercaptoethanol, 97.1 mM borate buffer, 2.5% (v/v) methanol, pH 9.3) for 1 min, and injected (10 μl) into a Shiseido CAPCELL PAK C-18 separation column (JM Science Inc., Grand Island, NY). Samples were carried through the system (0.6 μl/min) by a mobile phase consisting of 100 mM sodium phosphate buffer, 25% (v/v) methanol, and 3 % (v/v) acetonitrile, pH 6.75. The potentials on the electrochemical detector were set at 750 mV for the 5020 guard cell, and 200 mV and 600 or 650 mV for channels 1 and 2 of the 5011A analytical cell, respectively. Automation of the HPLC system and analysis of GABA peaks were performed using EZChrom Elite chromatography data system (Scientific Software, Inc., Pleasanton, CA). Nine known concentrations of GABA were used to generate standard curves before and after each experiment. Standard curves were used to calculate the amount of GABA in each dialysis sample, and to assure that the sensitivity of the HPLC instrument did not change during the analysis.
The amount of GABA recovered by the dialysis probe in vitro was calculated before and after each experiment by placing the dialysis probe in a vial containing a known concentration of GABA. Data from experiments in which probe recovery significantly changed in the same direction as the hypothesized change in brain GABA levels were eliminated from further analysis. Mean ± standard deviation (SD) recovery for all the probes used in the present study was 7.5 ± 1.9%.
Polygraphic Recording and Objective Assessment of Post-Anesthesia Wakefulness
During the microdialysis experiments a Grass Model 7D polygraph (Grass Instruments, Quincy, MA) was used to record the cortical electroencephalogram and neck muscle electromyogram. These physiological signals were acquired and analyzed to assess arousal state using a Power CED1401 data acquisition board and Spike2 software (Cambridge Electronic Design, Cambridge, England). Electroencephalographic and electromyographic power were obtained using fast Fourier transform. Analyses were performed in 10 s epochs and in 1 Hz increments ranging from 0.5 to 60 Hz for electroencephalographic frequencies, and 10 to 75 Hz for electromyographic frequencies. The mean electroencephalographic and electromyographic power were obtained by averaging 10 sec epochs over a 20 min period during isoflurane anesthesia and during wakefulness.
Wakefulness is the most heterogeneous arousal state and a sedative recovery scale developed for human patients25 was modified to objectively score post-anesthesia wakefulness. When isoflurane delivery was stopped, arousal state was scored as wakefulness when animals exhibited the following traits. 1) Eye appearance: bright eyes; eyelids and nictitating membranes spontaneously open or open in response to mild auditory-tactile stimulation; pupils accommodate; eyes exhibit purposeful and spontaneous movements. 2) Motor activity and coordination: presence of spontaneous limb movements; high neck muscle tone with periodic bursts of motor activity; ability to drink water. 3) Electroencephalogram: low amplitude (<50 μV), high frequency (>20 Hz) activity. 4) Breathing: deep and regular.
Intracranial Microinjections: Study Design and Procedures
Rats were implanted as previously described13,24 with a guide cannula (Plastics One, Roanoke, VA) aimed 3 mm above the pontine reticular nucleus, oral part (AP: -8.4 mm from bregma, lateral: 1.0 mm and height: -6.2 mm),26 which is the rodent homologue of the pontine reticular formation. Recovery from surgery was followed by one week of conditioning to being handled and placed in the anesthesia chambers, after which time the microinjection experiments began. All microinjections were performed between 12:30 and 15:30 using a within-subjects design. In separate experiments, each rat received microinjections (100 nl) of Ringers (vehicle control), the selective GABA uptake inhibitor nipecotic acid27-29 (1.29 μg; 100 mM), and the selective GABA synthesis inhibitor 3-MPA28 (1.06 μg; 100 mM). Rats received Ringer’s and drug microinjections in varying order, and microinjections into the same rat were separated by one week. Fifteen min post-microinjection, anesthesia was induced in an acrylic chamber prefilled with isoflurane. Delivered isoflurane concentrations were 1.5% (1.1 MAC30) for testing responses to nipecotic acid, and 1% (0.7 MAC30) for testing responses to 3-MPA. Delivered isoflurane concentration and temperature in the anesthesia chamber (38-39 °C) were monitored continuously and held constant throughout the experiment. Time to loss of wakefulness (min) was objectively evaluated by measuring loss of righting reflex.14 Anesthesia was maintained for 30 min after loss of wakefulness. Breathing rate (breaths/min) was assessed at 5, 15, and 25 min after the onset of anesthesia.
Histological Localization of Microdialysis and Microinjection Sites
Microdialysis sites
Following the last dialysis experiment, cats were deeply anesthetized with pentobarbital (35-40 mg/kg, i.v.) and perfused transcardially with cold saline (0.9%) followed by 10% formalin. Brains were removed and fixed in 10% formalin for three to four weeks. For cryoprotection, the brain stem block was transferred to 30% sucrose in 10% formalin for seven days. Serial sagittal brain stem sections (40 μm thick) were cut on a sliding microtome, float mounted onto chrom-alum coated glass slides, dried overnight, stained with cresyl violet, and coverslipped using Gel/Mount (Biomeda Corp., Foster City, CA). All sections containing microdialysis probe sites were digitized and the stereotaxic coordinates of the dialysis sites were defined by comparison with a cat brain stem atlas.16
Microinjection sites
After the final microinjection experiment, rats were deeply anesthetized with isoflurane and immediately decapitated. Brains were removed, frozen, and serial coronal brainstem sections were cut (40 μm) using a cryostat (Leica Microsystems, Nussloch, Germany). Sections corresponding to the pons were slide-mounted and stained with cresyl violet for histological confirmation of microinjection sites. All sections containing microinjection sites were digitized and the stereotaxic coordinates were defined by comparison with a rat brain atlas.26
Statistical Analyses
All data were tested for normality, and statistical tests were performed in consultation with the University of Michigan Center for Statistical Consultation and Research. Statistical analyses were performed using programs developed by Statistical Analysis System v9.1.3, (SAS Institute, Inc., Cary, NC) and GBStat (Dynamic Microsystems, Inc., Silver Spring, MD). Data that met assumptions of the underlying general linear model were analyzed by inferential statistics and plotted as mean + SD. Data for which normality was rejected were plotted to show median and interquartile range, and were analyzed using non-parametric statistics.
The effect of nipecotic acid on pontine reticular formation GABA levels was evaluated using nonparametric Friedman’s ANOVA and Neuman-Keuls post hoc test. The stability of GABA levels during anesthesia and wakefulness following microdialysis probe insertion was assessed by linear regression analysis. Effects of isoflurane anesthesia on pontine reticular formation GABA levels, and electromyogram amplitude, were evaluated by nonparametric Mann-Whitney U test. Electroencephalographic power during anesthesia and wakefulness was quantitatively evaluated by t-test with Bonferroni correction. Drug effects on induction time and breathing rate were assessed by Wilcoxon Matched Pairs Sign Ranks test and paired t-test, respectively. A P value < 0.05 was considered statistically significant.
Results
Chromatographic and In Vivo Pharmacologic Confirmation of GABA in Cat Pontine Reticular Formation
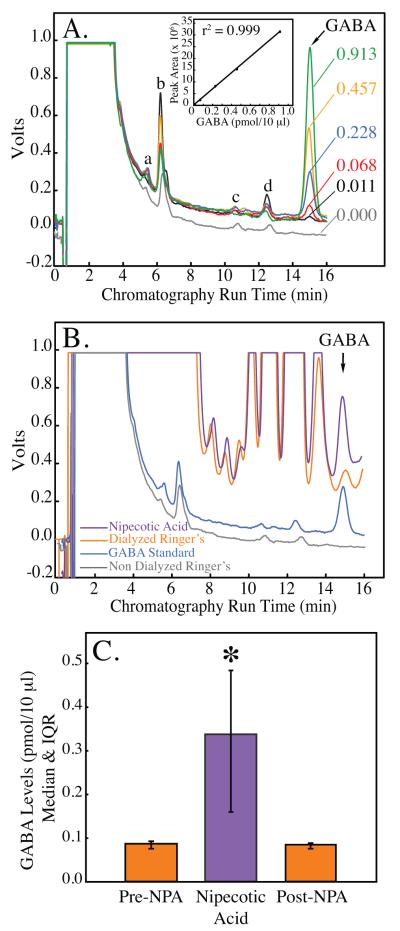
Figures 1A and 1B demonstrate the ability to identify the chromatographic peak corresponding to GABA. Figure 1A presents representative chromatograms produced by injecting five standardized concentrations of GABA (ranging from 0.011-0.913 pmol/10 μl) into the HPLC system. Only one set of peaks generated by the GABA standards systematically increased in area when increasing concentrations of GABA were injected. These peaks all showed the same retention time (15 min), and, when plotted as peak area versus GABA concentration (inset), produced a linear standard curve. Note the absence of a peak at 15 min in the non-dialyzed Ringer’s sample (fig. 1A, gray trace labeled 0.000), which contained no GABA. Other peaks produced in the same chromatograms (indicated as “a to d”) revealed no correlation between peak area and concentration of GABA. In addition, peaks “a to d” also appeared following injection of non-dialyzed Ringer’s (0.000 pmol/10 μl GABA).
Figure 1.
Gamma aminobutyric acid (GABA) levels in cat pontine reticular formation are reliably quantified using in vivo microdialysis and HPLC. A. Known amounts of GABA were used to create a standard curve for each microdialysis experiment. Numbers to the right of each chromatogram indicate GABA concentration (pmol/10 μl). Peaks labeled “a to d” did not increase with increasing concentrations of GABA. B. The four traces show representative chromatograms obtained from non-dialyzed Ringer’s (gray trace), a GABA standard (0.228 pmol/10 μl; blue trace), and samples collected from the pontine reticular formation during in vivo microdialysis with Ringer’s (orange trace) and Ringer’s containing nipecotic acid (violet trace). The increase in peak area produced by nipecotic acid confirmed the presence of GABA in cat pontine reticular formation. C. GABA levels were significantly increased (290%) during dialysis delivery of the GABA uptake inhibitor nipecotic acid (NPA) to the pontine reticular formation, providing additional confirmation that the peak at 15 min represents GABA.
The present study also used nipecotic acid to confirm the GABA peak in the chromatograms derived from brain samples. Dialysis administration of nipecotic acid, a GABA uptake inhibitor, selectively increases GABA levels in rat sriatum31 and pontine reticular formation.24 Figure 1B confirms the presence of GABA in dialysis samples obtained from the pontine reticular formation. First, there is a peak in the control biological sample (dialyzed Ringers, orange trace) with the same retention time (15 min) as the GABA standard (blue trace). Second, the size of this peak increased during dialysis administration of the GABA uptake inhibitor nipecotic acid (violet trace). Third, there is no peak with a 15 min retention time in the non-dialyzed Ringer’s sample (gray trace). Therefore, nipecotic acid delivery to the pontine reticular formation increased the peak area corresponding to GABA, confirming the presence of endogenous GABA in cat pontine reticular formation.
Figure 1C plots median GABA levels during sequential dialysis with Ringer’s (pre-NPA bar), Ringer’s containing nipecotic acid (middle bar), and Ringer’s (post-NPA bar). Friedman’s ANOVA revealed a significant (P = 0.03) treatment effect on GABA levels. Neuman-Keuls multiple comparisons test showed that nipecotic acid significantly (P < 0.05) increased GABA levels, and that this increase was reversed after dialysis administration of nipecotic acid was discontinued. For all subsequent experiments, identification of GABA peaks in chromatograms from brain dialysis samples was based on the retention time of GABA standards, as shown in Figure 1B.13,24
Pontine Reticular Formation GABA Levels during Wakefulness and Isoflurane Anesthesia were not Confounded by the High GABA Levels Observed Immediately Following Microdialysis Probe Insertion
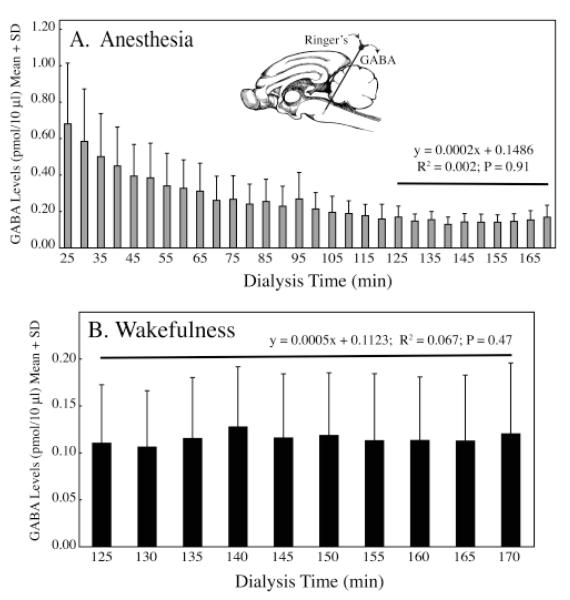
This study next sought to determine the time needed to achieve stable GABA levels after placement of a microdialysis probe in the pontine reticular formation.32,33 Figure 2A plots the time course of pontine reticular formation GABA levels following dialysis probe insertion into cat pontine reticular formation during anesthesia. The graph shows the characteristically high initial GABA levels, followed by a slow decay until stability was achieved. Regression analysis34 was used to objectively identify when the decline in GABA levels ended. Thus, the goal of these methodological experiments was to determine when the slope of the regression line was no longer changing. Figure 2A demonstrates that the slope of the linear regression function for the last 10 samples collected during anesthesia (min 125 to 170) was not significantly different from zero. Therefore, stability of GABA levels was confirmed after 125 min of dialysis, and the last 10 samples provided stable, control GABA levels during anesthesia. Figure 2B illustrates GABA levels in the pontine reticular formation obtained during spontaneous wakefulness. Regression analysis demonstrated that GABA levels during wakefulness were stable after 125 min of dialysis, similar to findings in anesthetized cat (fig. 2A) and consistent with previous studies in rat.35,36
Figure 2.
Time course for achieving stable GABA levels in cat pontine reticular formation. A. Regression analysis (horizontal line) demonstrated that stability of GABA levels was achieved after 125 min of dialysis. The inset shows a schematic sagittal view of the cat brain with a microdialysis probe placed in the pontine reticular formation. B. Regression analysis (horizontal line) indicated that GABA levels in 10 sequential samples collected during wakefulness were stable between 125 and 170 min of dialysis.
Isoflurane Decreased Pontine Reticular Formation GABA Levels, Increased Cortical Electroencephaolgraphic Power, and Decreased Neck Muscle Tone
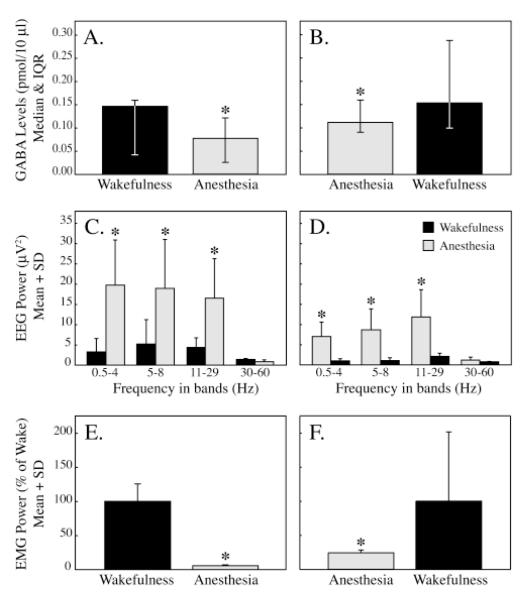
Figure 3 summarizes three traits that characterized the state of isoflurane anesthesia. For data shown in figures 3A, 3C, and 3E sample collection started during wakefulness and continued during anesthesia. For the data reported in figures 3B, 3D, and 3F samples were collected during anesthesia followed by post-anesthesia wakefulness. Figures 3A and 3B plot median GABA levels in the pontine reticular formation during states of anesthesia and wakefulness. Relative to wakefulness, GABA levels were significantly decreased by clinically relevant concentrations of isoflurane (2.1 to 2.2%; 1.1 to 1.2 MAC23). GABA levels were significantly decreased whether isoflurane administration followed (fig. 3A) or preceded (fig. 3B) wakefulness. Figure 3C shows that compared to spontaneous wakefulness, frontal cortex electroencephalographic power was significantly increased during isoflurane anesthesia in all bands except 30 to 60 Hz. Significant increases in electroencephalographic power were also observed during isoflurane anesthesia compared to post-anesthesia wakefulness (fig. 3D). Figures 3E and 3F show that neck muscle electromyographic power was significantly reduced by isoflurane.
Figure 3.
GABA levels, electroencephalographic (EEG) power, and neck electromyographic (EMG) power during wakefulness and anesthesia. GABA levels were decreased by isoflurane (A and B): A. Isoflurane significantly (P < 0.001, Mann-Whitney U test) decreased GABA levels when administered following wakefulness. B. GABA levels also were significantly (P < 0.02, Mann-Whitney U test) decreased relative to wakefulness when isoflurane delivery preceded wakefulness. Electroencephalographic power was increased by isoflurane (C and D): Compared to spontaneous wakefulness (C) or post-anesthesia wakefulness (D), power of the frontal cortex electroencephalogram in the 0.5 to 4 Hz (delta), 5 to 8 Hz (theta), and 11 to 29 Hz (sigma & beta) bands was significantly (P < 0.01, t-test with Bonferroni correction) increased during isoflurane anesthesia. Neck muscle tone was decreased by isoflurane (E and F): Expressed as percent of electromyographic power during wakefulness, neck muscle activity was significantly (P < 0.001) reduced by isoflurane when anesthesia followed (E) or preceded (F) wakefulness.
Isoflurane Induction Time was Decreased by a GABA Synthesis Inhibitor and Increased by a GABA Uptake Blocker Administered to the Pontine Reticular Formation
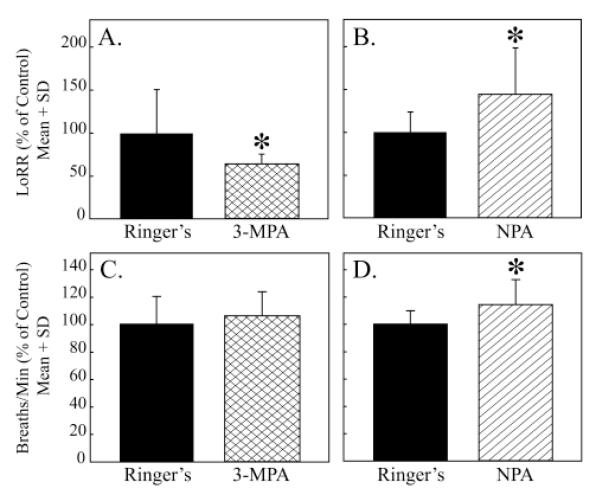
Figure 4A shows that microinjection of the GABA synthesis inhibitor 3-MPA into rat pontine reticular formation significantly decreased isoflurane induction time. Consistent with the effect of 3-MPA, pontine reticular formation administration of the GABA uptake inhibitor nipecotic acid caused a significant increase in isoflurane induction time (fig. 4B). Figures 4C and 4D illustrate the effects of 3-MPA and nipecotic acid on rate of breathing. Relative to control (Ringer’s), nipecotic acid significantly increased breathing rate during anesthesia (fig. 3D).
Figure 4.
Pontine reticular formation microinjection of drugs that alter GABA levels significantly changed isoflurane induction time and breathing rate. A. The GABA synthesis inhibitor 3-mercaptopropionic acid (3-MPA) significantly (P < 0.02) decreased time to loss of righting response (LoRR). B. Pontine reticular formation microinjection of the GABA uptake inhibitor nipecotic acid (NPA) significantly (P < 0.01) increased the amount of time required for isoflurane to cause a loss of the righting response. C. Rate of breathing was not significantly altered by 3-MPA. D. Nipecotic acid significantly (P < 0.002) increased rate of breathing.
Discussion
Deconstructing the state of isoflurane anesthesia into component traits led to four novel findings. First, GABA levels in cat pontine reticular formation were decreased by isoflurane. Second, the traits of increased cortical electroencephalographic power and decreased skeletal muscle tone induced by isoflurane co-varied with pontine reticular formation GABA levels. Third, the time to loss of righting reflex in response to isoflurane was decreased by a GABA synthesis inhibitor and increased by a GABA uptake inhibitor when these drugs were microinjected directly into the pontine reticular formation. Fourth, rate of breathing during isoflurane anesthesia was increased by pontine reticular formation administration of a GABA uptake blocker. These results suggest that one mechanism by which isoflurane causes the traits of loss of wakefulness, altered cortical excitability, diminished muscle tone, and decreased breathing rate is by reducing GABA levels in the pontine reticular formation.
GABAergic Transmission in the Pontine Reticular Formation Promotes Wakefulness
Several studies performed in the context of sleep neurobiology suggest that GABAergic neurotransmission13 at GABAA receptors in the pontine reticular formation9-11 promotes wakefulness. The concept of a wakefulness promoting role for pontine reticular formation GABA is also supported by the finding that GABA levels in the pontine reticular formation are decreased during the loss of wakefulness caused by isoflurane (fig. 3), and by the data showing that pontine reticular formation administration of drugs that decrease or increase GABA levels decrease or increase, respectively, the time need for isoflurane to cause a loss of wakefulness (fig. 4).
The mechanisms by which GABA in the pontine reticular formation promotes wakefulness remain to be elucidated. GABA originating in the basal forebrain causes cortical excitation by inhibiting cortical GABAergic interneurons, resulting in disinhibition.37 A similar mechanism may operate in the pontine reticular formation. The recent finding that GABA is directly excitatory to a subpopulation of neurons in the adult suprachiasmatic nucleus38 indicates the need for synaptic level studies in the pontine reticular formation. The present data do not address the role of pontine reticular formation GABA receptor subtypes mediating the isoflurane induced loss of wakefulness. The results reported here encourage additional pharmacological studies using GABA receptor subtype selective agonists and antagonists to determine the extent to which the isoflurane induced loss of wakefulness is mediated by GABAA and/or GABAB receptors in the pontine reticular formation.
The functional significance of the finding that GABA levels in the pontine reticular formation decrease during isoflurane anesthesia is illustrated by the fact that isoflurane does not homogenously decrease GABA levels throughout the brain. Dong et al.,35 showed that isoflurane anesthesia caused a significant decrease in GABA levels in the cortex and basal forebrain, and no change in GABA levels in the posterior hypothalamus. Moreover, these authors35 also demonstrated that isoflurane anesthesia caused an increase in glutamate levels in the cortex and basal forebrain, but no change in hypothalamic glutamate levels. These data showing brain-region-specific changes in neurotransmitter levels induced by isoflurane,35 together with the present results demonstrating that drugs known to alter GABA levels change isoflurane induction time (Fig. 4) support the interpretation that decreases in pontine reticular formation GABA levels causally contribute to the loss of wakefulness.
Cortical Excitability and Muscle Tone Characteristic of Isoflurane Anesthesia Co-Vary with GABA Levels in the Pontine Reticular Formation
Diverse molecular and cellular targets, including multiple neurotransmitter systems in different brain regions, are involved in generating the neurobehavioral effects of general anesthetics.14,15,39,40 The fact that different brain regions regulate different neurobehavioral functions means that insights into the mechanisms of anesthetic action can be achieved by deconstructing anesthetic states into their component traits.3,41,42 The present study applied this deconstruction approach by relating measures of pontine reticular formation GABA to the traits of cortical electroencephalogram and muscle hypotonia (fig. 3). Wakefulness preceding anesthesia was characterized by greater GABA levels, lower electroencephalographic power, and greater muscle tone. When anesthesia preceded wakefulness, the relationship between all three measures was reversed such that GABA levels and electromyographic power were increased and electroencephalographic power was decreased during wakefulness. Thus, the figure 3 data show that GABA levels in the pontine reticular formation vary consistently with physiological traits known to define the state of isoflurane anesthesia. The isoflurane-induced increase in electroencephalographic power agrees with human data demonstrating that increases in electroencephalographic power caused by propofol and sevoflurane are associated with anesthetically induced loss of arousal.43
Supraspinal Modulation of Immobility by GABA
The spinal cord is recognized as one site of action for anesthetic-induced immobility, and it is also appreciated that supraspinal sites contribute to motor hypotonia and immobility.44 Motor control is hierarchically organized in the central nervous system and considerable data demonstrate that the pontine reticular formation plays a major role in the supraspinal regulation of motor tone. During the progression from wakefulness to NREM sleep and REM sleep, somatic motor tone decreases. The hypotonia and immobility that occur during the loss of wakefulness are known to be regulated by descending projections arising from the same pontine reticular formation areas from which the GABA measures were obtained in the present study. The activity of these descending projections is modulated by multiple neurotransmitter systems in the pontine reticular formation.45-49 The present results support the interpretation that GABAergic transmission in the pontine reticular formation contributes to isoflurane-induced muscular hypotonia (figs. 3E and 3F) and immobility (figs. 4A and 4B). These are new findings that contribute to understanding the mechanisms by which isoflurane causes the trait of immobility.
Manipulating GABA Synthesis and Uptake Altered the Loss of Righting Response and Rate of Breathing
The results indicating a faithful relationship between pontine reticular formation GABA levels, cortical excitability, and muscle tone (fig. 3) are limited by their correlational nature. This limitation was addressed by experiments designed to determine whether microinjecting the pontine reticular formation with a GABA synthesis inhibitor and a GABA uptake blocker caused changes in isoflurane induction time. Rather than using only a global assessment of anesthetic state, these experiments quantified two anesthetic traits (fig. 4). The first trait, loss of consciousness, is reliably assessed in pre-clinical studies by measuring time to loss of righting response.50,51 The second trait quantified was rate of breathing. The GABA synthesis inhibitor 3-MPA significantly decreased the time to loss of righting caused by isoflurane. The 3-MPA results are consistent with previous data showing that pontine reticular formation microinjection of 3-MPA in the awake rat increases sleep.13 The present respiratory measures indicate that 3-MPA had no effect on rate of breathing during isoflurane anesthesia. The lack of effect on breathing rate by 3-MPA may reflect a “floor effect” caused by the rapid and powerful depressant action of isoflurane on respiratory rate. The selective GABA uptake inhibitor nipecotic acid increased the time needed for isoflurane to cause a loss of wakefulness. Compared to control, nipecotic acid significantly increased rate of breathing during anesthesia, consistent with Fink’s52 concept of a “wakefulness stimulus for breathing”. Together, these data permit the inference that levels of GABA in the pontine reticular formation causally contribute to the regulation of traits characterizing isoflurane anesthesia.
Measures of GABA in the Pontine Reticular Formation are Valid, Stable, and Functionally Significant
Brain GABA levels are relatively low compared to other amino acid neurotransmitters27 and it is important to unambiguously identify the chromatographic peak corresponding to GABA. The present study employed three methods to confirm that the peak in the chromatograms (fig. 1) represents GABA. First, in the chromatograms generated by GABA standards, only the peak with a retention time of 15 min systematically increased in area with increasing concentrations of GABA. Second, the Ringer’s solution used for standards and for brain dialysis did not contain a peak with a 15 min retention time. Third, microdialysis delivery of nipecotic acid, which selectively blocks GABA uptake, caused a significant increase in GABA, thus confirming the presence of pontine reticular formation GABA.13,24 Considered together, the three approaches illustrated by figure 1 demonstrate reliable and functionally significant measurement of GABA in cat pontine reticular formation.
A second gap in knowledge addressed by the present results concerns the fact that no prior data existed quantifying the amount of time required for GABA levels to stabilize following microdialysis probe placement in cat pontine reticular formation. Previous in vivo microdialysis studies measuring GABA levels in cat dorsal raphe nucleus and locus coeruleus did not quantify stabilization time, but reported that a minimum of 17 h followed probe insertion into the brain before onset of dialysis sample collection.53,54 GABA levels are well known to be high immediately following probe insertion, and to decline, then stabilize with time.32,33 In the absence of the figure 2 data quantifying GABA stabilization time in the pontine reticular formation, it is not be possible to attribute changes in GABA to isoflurane anesthesia. Time course studies (fig. 2) conducted during anesthesia and during wakefulness demonstrate that initially high GABA levels decrease and become stable 2 h after dialysis probe insertion. Interestingly, in the pontine reticular formation of halothane anesthetized rat less time (42 min) was required to achieve stable GABA levels.13 The reasons for these different stabilization times are unknown but may be due to the use of different dialysis probe membranes and flow rates, species, or inhalation anesthetics. Comparison of these two studies further emphasizes the necessity of empirically demonstrating the time course for achieving stable GABA levels.
The origin of extracellular GABA levels is complex (reviewed in24,33,55) and this complexity restricts the ability of the present study to identify the sources of GABA in the pontine reticular formation. Synaptic GABA mediates transient, postsynaptic inhibitory currents (phasic inhibition) whereas extrasynaptic GABA released from neurons and glia produces a persistent tonic inhibition.56 In addition to postsynaptic GABA receptors, extrasynaptic GABAA receptors are highly expressed in many brain regions including those involved in generating sleep, and extrasynaptic GABAA receptors are likely targets for sedative-hypnotic and anesthetic drugs.40,57-61 The present report is conservative in referring to GABA levels rather than GABA release because the cellular sources of GABA are unidentified.
Conclusion and Interpretation
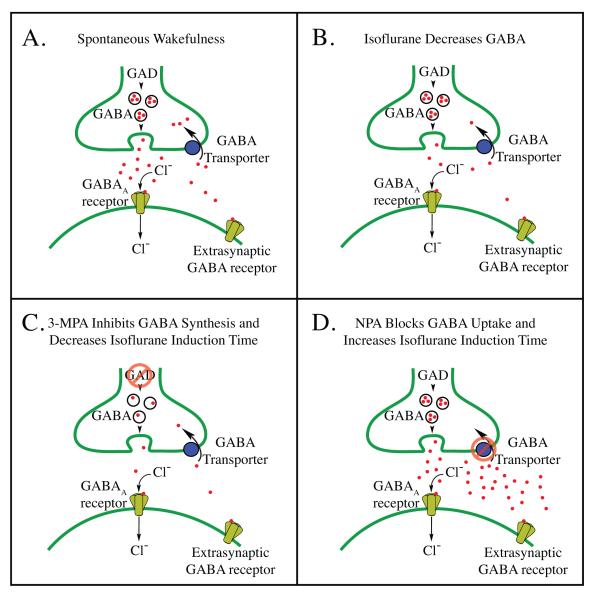
The present results support the conclusion that directly or indirectly decreasing GABA levels in the pontine reticular formation comprises one mechanism by which isoflurane causes loss of consciousness, altered cortical excitability, muscular hypotonia, and decreased respiratory rate. Figure 5 provides a schematic model that interprets the present findings concerning traits of isoflurane anesthesia relative to mechanisms regulating GABA levels. Compared to levels of GABA during wakefulness (fig. 5A), isoflurane caused a decrease in pontine reticular formation GABA levels (fig. 5B). The finding that microinjecting 3-MPA into the pontine reticular formation shortened the time required for isoflurane to cause loss of righting reflex (fig. 4A) fits with the interpretation that 3-MPA decreases GABA levels in the pontine reticular formation by inhibiting glutamic acid decarboxylase, resulting in decreased GABA synthesis (fig. 5C). This finding is also consistent with data showing that microinjecting 3-MPA into rat pontine reticular formation decreases wakefulness and increases sleep.13 Previous studies demonstrated that microinjecting the GABA uptake inhibitor nipecotic acid into the pontine reticular formation of intact, unanesthetized rat increases wakefulness.13 Thus, the present data showing that nipecotic acid administered to the pontine reticular formation significantly increased time required for isoflurane to cause loss of righting (fig. 4B) and increased rate of breathing (fig. 4D) are consistent with an increase in GABA (fig. 1C) resulting from GABA transporter blockade (fig. 5D).
Figure 5.
Schematic illustrating mechanisms of action of isoflurane, 3-mercaptopropionic acid (3-MPA), and nipecotic acid (NPA) within the pontine reticular formation. A. During wakefulness, pontine reticular formation GABA (red dots) is released from vesicles contained in pre-synaptic terminals, increasing chloride ion (Cl-) conductance by activation of post-synaptic and extra-synaptic GABAA receptors. GABA is then actively removed from the synaptic cleft by uptake mechanisms (GABA transporters). B. Isoflurane decreases pontine reticular formation GABA levels, in part, by inhibiting action potential dependent neurotransmitter release,62 by potentiation of GABAA receptor mediated inhibition of GABAergic neurons,15 and/or by pre-synaptic inhibition (disfacilitation) of excitatory inputs acting on GABAergic neurons.35 These three actions of isoflurane on GABAergic transmission likely occur in combinations that vary as a function of brain region. C. Inhibition of glutamic acid decarboxylase (GAD) by 3-MPA decreases GABA levels in the pontine reticular formation, decreases arousal, and facilitates isoflurane-induced loss of wakefulness. D. Inhibition of pontine reticular formation GABA uptake mechanisms by NPA increases GABA levels, increases arousal, and prolongs isoflurane induction time. Parts A — D include extrasynaptic GABA receptors because these receptors are targets for anesthetic drugs,61 and because GABA measured by in vivo microdialysis is thought to act at these extrasynaptic receptors.33
Acknowledgments
For expert assistance the authors thank Lindsay A. Bouchard, B.S. (Research Assistant), Sha Jiang, B.S. (Research Associate), Mary A. Norat, B.S. (Senior Research Associate), Haideliza Soto-Calderon, B.S. (Research Assistant), and Bradley L. Wathen, M.S. (Research Assistant) from the Department of Anesthesiology, University of Michigan. Kathy Welch, M.S., M.P.H., (Computer Systems Consultant, Department of Biostatistics, University of Michigan Center for Statistical Consultation and Research) provided input on statistical analyses.
Support: Supported by grants MH45361, HL57120, HL40881, and HL65272 from the National Institutes of Health, Bethesda, Maryland, and by the Department of Anesthesiology, University of Michigan, Ann Arbor, Michigan
Footnotes
Meeting at which work was presented: Presented in part at the Annual Meeting of the Society for Neuroscience, Atlanta, Georgia, October 16, 2006
www.nap.edu/readingroom/books/labrats, accessed July 18, 2008
www.national-academies.org/ilar, accessed July 18, 2008
References
- 1.Moruzzi G, Magoun HW. Brain stem reticular formation and activation of the EEG. Electroencephalog Clin Neurophysiol. 1949;1:455–73. [PubMed] [Google Scholar]
- 2.Steriade M, McCarley RW. Brain Control of Wakefulness and Sleep. Kluwer Academic/Plenum Press; New York: 2005. [Google Scholar]
- 3.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–95. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 4.de la Roza C, Reinoso-Suarez F. GABAergic structures in the ventral part of the oral pontine reticular nucleus: An ultrastructural immunogold analysis. Neuroscience. 2006;142:1183–93. doi: 10.1016/j.neuroscience.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigo-Angulo ML, Heredero S, Rodriguez-Veiga E, Reinoso-Suarez F. GABAergic and non-GABAergic thalamic, hypothalamic and basal forebrain projections to the ventral oral pontine reticular nucleus: Their implication in REM sleep modulation. Brain Res. 2008;1210:116–25. doi: 10.1016/j.brainres.2008.02.095. [DOI] [PubMed] [Google Scholar]
- 6.Mohler H. GABAA receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–16. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 7.Nutt D. GABAA receptors: subtypes, regional distribution, and function. J Clin Sleep Med. 2006;2:S7–11. [PubMed] [Google Scholar]
- 8.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–50. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 9.Camacho-Arroyo I, Alvarado R, Manjarrez J, Tapia R. Microinjections of muscimol and bicuculline into the pontine reticular formation modify the sleep-waking cycle in the rat. Neurosci Lett. 1991;129:95–7. doi: 10.1016/0304-3940(91)90728-c. [DOI] [PubMed] [Google Scholar]
- 10.Xi MC, Morales FR, Chase MH. Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism. J Neurophysiol. 1999;82:2015–9. doi: 10.1152/jn.1999.82.4.2015. [DOI] [PubMed] [Google Scholar]
- 11.Sanford LD, Tang X, Xiao J, Ross RJ, Morrison AR. GABAergic regulation of REM sleep in reticularis pontis oralis and caudalis in rats. J Neurophysiol. 2003;90:938–45. doi: 10.1152/jn.00993.2002. [DOI] [PubMed] [Google Scholar]
- 12.Vazquez J, Baghdoyan HA. GABAA receptors inhibit acetylcholine release in cat pontine reticular formation: implications for REM sleep regulation. J Neurophysiol. 2004;92:2198–206. doi: 10.1152/jn.00099.2004. [DOI] [PubMed] [Google Scholar]
- 13.Watson CJ, Soto-Calderon H, Lydic R, Baghdoyan HA. Pontine reticular formation (PnO) administration of hypocretin-1 increases PnO GABA levels and wakefulness. Sleep. 2008;31:453–64. doi: 10.1093/sleep/31.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 15.Mashour GA, Forman SA, Campagna JA. Mechanisms of general anesthesia: from molecules to mind. Best Pract Res Clin Anaesthesiol. 2005;19:349–64. doi: 10.1016/j.bpa.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Berman AL. A Cytoarchitectonic Atlas with Stereotaxic Coordinates. University of Wisconsin; Madison: 1968. The Brain Stem of the Cat. [Google Scholar]
- 17.Keifer JC, Baghdoyan HA, Becker L, Lydic R. Halothane decreases pontine acetylcholine release and increases EEG spindles. Neuroreport. 1994;5:577–80. doi: 10.1097/00001756-199401000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Keifer JC, Baghdoyan HA, Lydic R. Pontine cholinergic mechanisms modulate the cortical electroencephalographic spindles of halothane anesthesia. Anesthesiology. 1996;84:945–54. doi: 10.1097/00000542-199604000-00023. [DOI] [PubMed] [Google Scholar]
- 19.Lydic R, Baghdoyan HA. Ketamine and MK-801 decrease acetylcholine release in the pontine reticular formation, slow breathing, and disrupt sleep. Sleep. 2002;25:617–22. [PubMed] [Google Scholar]
- 20.Lydic R, Keifer JC, Baghdoyan HA, Becker L. Microdialysis of the pontine reticular formation reveals inhibition of acetylcholine release by morphine. Anesthesiology. 1993;79:1003–12. doi: 10.1097/00000542-199311000-00019. [DOI] [PubMed] [Google Scholar]
- 21.Mortazavi S, Thompson J, Baghdoyan HA, Lydic R. Fentanyl and morphine, but not remifentanil, inhibit acetylcholine release in pontine regions modulating arousal. Anesthesiology. 1999;90:1070–7. doi: 10.1097/00000542-199904000-00021. [DOI] [PubMed] [Google Scholar]
- 22.Ursin R, Sterman M. Manual for Standardized Scoring of Sleep and Waking States in Adult Cats. BIS/BRI, University of California; Los Angeles: 1981. [Google Scholar]
- 23.Barter LS, Ilkiw JE, Steffey EP, Pypendop BH, Imai A. Animal dependence of inhaled anaesthetic requirements in cats. Br J Anaesth. 2004;92:275–7. doi: 10.1093/bja/aeh047. [DOI] [PubMed] [Google Scholar]
- 24.Watson CJ, Lydic R, Baghdoyan HA. Sleep and GABA levels in the oral part of rat pontine reticular formation are decreased by local and systemic administration of morphine. Neuroscience. 2007;144:375–86. doi: 10.1016/j.neuroscience.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macnab AJ, Levine M, Glick N, Phillips N, Susak L, Elliott M. The Vancouver sedative recovery scale for children: validation and reliability of scoring based on videotaped instruction. Can J Anaesth. 1994;41:913–8. doi: 10.1007/BF03010934. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos G, Watson C. The Rat Brain. Sixth Edition Academic Press; 2007. [Google Scholar]
- 27.Boyd BW, Witowski SR, Kennedy RT. Trace-level amino acid analysis by capillary liquid chromatography and application to in vivo microdialysis sampling with 10-s temporal resolution. Anal Chem. 2000;72:865–71. doi: 10.1021/ac990872n. [DOI] [PubMed] [Google Scholar]
- 28.Krogsgaard-Larsen P, Frolund B, Frydenvang K. GABA uptake inhibitors. Design, molecular pharmacology, and therapeutic aspects. Curr Pharm Des. 2000;6:1193–209. doi: 10.2174/1381612003399608. [DOI] [PubMed] [Google Scholar]
- 29.Juhász G, Kékesi KA, Nyitrai G, Dobolyi A, Krogsgaard-Larsen P, Schousboe A. Differential effects of nipecotic acid and 4,5,6-7 tetrahydroisoxazolo[4,5-c]pyridin-3-ol on extracellular γ-aminobutyrate levels in rat thalamus. Eur J Pharmacol. 1997;331:139–44. doi: 10.1016/s0014-2999(97)01044-3. [DOI] [PubMed] [Google Scholar]
- 30.White PF, Johnston RR, Eger EI. Determination of anesthetic requirement in rats. Anesthesiology. 1974;40:52–7. doi: 10.1097/00000542-197401000-00012. [DOI] [PubMed] [Google Scholar]
- 31.Kehr J, Ungerstedt U. Fast HPLC estimation of gamma-aminobutyric acid in microdialysis perfusates: effect of nipecotic and 3-mercaptopropionic acids. J Neurochem. 1988;51:1308–10. doi: 10.1111/j.1471-4159.1988.tb03101.x. [DOI] [PubMed] [Google Scholar]
- 32.Benveniste H, Huttemeier PC. Microdialysis--theory and application. Prog Neurobiol. 1990;35:195–215. doi: 10.1016/0301-0082(90)90027-e. [DOI] [PubMed] [Google Scholar]
- 33.Watson CJ, Venton BJ, Kennedy RT. In vivo measurements of neurotransmitters using microdialysis sampling. Anal Chem. 2006;78:1391–9. doi: 10.1021/ac0693722. [DOI] [PubMed] [Google Scholar]
- 34.Osman NI, Baghdoyan HA, Lydic R. Morphine inhibits acetylcholine release in rat prefrontal cortex when delivered systemically or by microdialysis to basal forebrain. Anesthesiology. 2005;103:779–87. doi: 10.1097/00000542-200510000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Dong HL, Fukuda S, Murata E, Higuchi T. Excitatory and inhibitory actions of isoflurane on the cholinergic ascending arousal system of the rat. Anesthesiology. 2006;104:122–33. doi: 10.1097/00000542-200601000-00018. [DOI] [PubMed] [Google Scholar]
- 36.Smith A, Watson CJ, Frantz KJ, Eppler B, Kennedy RT, Peris J. Differential increase in taurine levels by low-dose ethanol in the dorsal and ventral striatum revealed by microdialysis with on-line capillary electrophoresis. Alcohol Clin Exp Res. 2004;28:1028–38. doi: 10.1097/01.alc.0000131979.78003.34. [DOI] [PubMed] [Google Scholar]
- 37.Zaborszky L, Duque A. Local synaptic connections of basal forebrain neurons. Behav Brain Res. 2000;115:143–58. doi: 10.1016/s0166-4328(00)00255-2. [DOI] [PubMed] [Google Scholar]
- 38.Choi HJ, Lee CJ, Scroeder A, Kim YS, Jung SH, Kim JS, Kim DY, Son EJ, Han HC, Hong SK, Colwell CS, Kim YI. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;28:5450–9. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grasshoff C, Drexler B, Rudolph U, Antkowiak B. Anaesthetic drugs: linking molecular actions to clinical effects. Curr Pharm Des. 2006;12:3665–79. doi: 10.2174/138161206778522038. [DOI] [PubMed] [Google Scholar]
- 40.Hemmings HC, Jr., Akabas MH, Goldstein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 41.Lydic R, Biebuyck JF. Sleep neurobiology: relevance for mechanistic studies of anaesthesia. Br J Anaesth. 1994;72:506–8. doi: 10.1093/bja/72.5.506. [DOI] [PubMed] [Google Scholar]
- 42.Van Dort CJ, Baghdoyan HA, Lydic R. Neurochemical modulators of sleep and anesthetic states. Int Anesthesiol Clin. 2008;46:1–30. doi: 10.1097/AIA.0b013e318181a8ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gugino LD, Chabot RJ, Prichep LS, John ER, Formanek V, Aglio LS. Quantitative EEG changes associated with loss and return of consciousness in healthy adult volunteers anaesthetized with propofol or sevoflurane. Br J Anaesth. 2001;87:421–8. doi: 10.1093/bja/87.3.421. [DOI] [PubMed] [Google Scholar]
- 44.Antognini JF, Carstens E. Neural Mechanisms of Anesthesia. Humana Press; New Jersey: 2003. [Google Scholar]
- 45.Baghdoyan HA, Lydic R, Callaway CW, Hobson JA. The carbachol-induced enhancement of desynchronized sleep signs is dose dependent and antagonized by centrally administered atropine. Neuropsychopharmacology. 1989;2:67–79. doi: 10.1016/0893-133x(89)90009-2. [DOI] [PubMed] [Google Scholar]
- 46.Lai YY, Siegel JM. Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression. J Neurosci. 1991;11:2931–7. doi: 10.1523/JNEUROSCI.11-09-02931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morales FR, Engelhardt JK, Soja PJ, Pereda AE, Chase MH. Motoneuron properties during motor inhibition produced by microinjection of carbachol into the pontine reticular formation of the decerebrate cat. J Neurophysiol. 1987;57:1118–29. doi: 10.1152/jn.1987.57.4.1118. [DOI] [PubMed] [Google Scholar]
- 48.Xi MC, Morales FR, Chase MH. The motor inhibitory system operating during active sleep is tonically suppressed by GABAergic mechanisms during other states. J Neurophysiol. 2001;86:1908–15. doi: 10.1152/jn.2001.86.4.1908. [DOI] [PubMed] [Google Scholar]
- 49.Wills N, Chase MH. Brain stem control of masseteric reflex activity during sleep and wakefulness: mesencephalon and pons. Exp Neurol. 1979;64:98–117. doi: 10.1016/0014-4886(79)90008-6. [DOI] [PubMed] [Google Scholar]
- 50.Tung A, Szafran MJ, Bluhm B, Mendelson WB. Sleep deprivation potentiates the onset and duration of loss of righting reflex induced by propofol and isoflurane. Anesthesiology. 2002;97:906–11. doi: 10.1097/00000542-200210000-00024. [DOI] [PubMed] [Google Scholar]
- 51.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–72. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 52.Fink BR. Influence of cerebral activity in wakefulness on regulation of breathing. J Appl Physiol. 1961;16:15–20. doi: 10.1152/jappl.1961.16.1.15. [DOI] [PubMed] [Google Scholar]
- 53.Nitz D, Siegel J. GABA release in the dorsal raphe nucleus: role in the control of REM sleep. Am J Physiol. 1997;273:R451–5. doi: 10.1152/ajpregu.1997.273.1.R451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nitz D, Siegel JM. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience. 1997;78:795–801. doi: 10.1016/s0306-4522(96)00549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westerink BHC, Rea K, Oldenziel WH, Cremers TIFH. Microdialysis of glutamate and GABA in the brain: analysis and interpretation. In: Westerink BHC, Cremers TIFH, editors. Handbook of Microdialysis. Elsevier; The Netherlands: 2007. pp. 17–31. [Google Scholar]
- 56.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 57.Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic GABAA receptors of thalamocortical neurons: a molecular target for hypnotics. J Neurosci. 2005;25:11513–20. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. GABAA receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci USA. 2006;103:15230–5. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic GABAA receptor mediates tonic inhibition in thalamic VB neurons. J Neurophysiol. 2005;94:4491–501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 60.Jia F, Yue M, Chandra D, Homanics GE, Goldstein PA, Harrison NL. Isoflurane is a potent modulator of extrasynaptic GABAA receptors in the thalamus. J Pharmacol Exp Ther. 2008;324:1127–35. doi: 10.1124/jpet.107.134569. [DOI] [PubMed] [Google Scholar]
- 61.Orser BA. Extrasynaptic GABAA receptors are critical targets for sedative-hypnotic drugs. J Clin Sleep Med. 2006;2:S12–8. [PubMed] [Google Scholar]
- 62.Hemmings HC, Jr., Yan W, Westphalen RI, Ryan TA. The general anesthetic isoflurane depresses synaptic vesicle exocytosis. Mol Pharmacol. 2005;67:1591–9. doi: 10.1124/mol.104.003210. [DOI] [PubMed] [Google Scholar]