Abstract
Socioemotional selectivity theory suggests that emotion regulation goals motivate older adults to preferentially allocate attention to positive stimuli and away from negative stimuli. This study examined whether anxiety moderates the effect of the positivity bias on attention for threat. We employed the dot probe task to compare subliminal and supraliminal attention for threat in 103 young and 44 older adults. Regardless of anxiety, older but not younger adults demonstrated a vigilant-avoidant response to angry faces. Anxiety influenced older adults’ attention such that anxious individuals demonstrated a vigilant-avoidant reaction to sad faces, but an avoidant-vigilant reaction to negative words.
Keywords: aging, attentional bias, dot probe, anxiety, positivity
Introduction
Mounting research has documented remarkable resiliency in the emotional functioning of older adults. Within the socioemotional selectivity theory (Carstensen, 1993) framework, research supports an age-related positivity bias in attention and memory, such that positively valenced information receives prioritized processing as one ages. In contrast, fewer resources are allocated to process emotionally distressing materials (see reviews by Carstensen, Mikels, & Mather, 2006; Mather & Carstensen, 2005). These adjustments allow older adults to optimize emotional experience as perceived time becomes limited.
A positivity bias refers to changes in cognitive processing motivated by the pursuit of emotional gratification in late life (Mather & Carstensen, 2005), whereas, the positivity effect refers to the observed outcomes of the positivity bias process. Although there is a rich literature detailing the critical impact of emotion on cognition (e.g., Eich, Kihlstrom, Bower, Forgas, & Niedenthal, 2000), little work has been done to address whether the positivity effect is observed under various mood conditions. The goal of this study was to examine whether anxiety moderates the positivity bias in visual attention for threat-related stimuli in older adults. We attempted to capture directional changes in attention over time by including subliminally- and supraliminally-presented stimuli. Attentional bias for threat is defined as a preference to allocate attention toward threat-related stimuli over neutral or positive stimuli.
From an evolutionary perspective, automatic vigilance for threat is an innate mechanism that enhances survival (see work by LeDoux, 1995; Öhman, 1996, 1999; Öhman, Flykt, & Esteves, 2001). Individuals can also develop attentional biases for triggers that threaten their goals based on mood or learning history (Folk, Remington, & Johnston, 1992; Öhman et al., 2001). In the experimental psychology domain, anxiety has been most intimately linked to attention among the basic emotions (see reviews by MacLeod, 1999; Matthews & Wells, 1999). A recent meta-analysis of 172 studies confirmed the presence of an attentional bias for threat of small-to-medium effect size among clinically anxious and non-clinically high anxious individuals (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenberg, & van IJzendoorn, 2007).
Time has been suggested as a moderator in threat-related visual attention, although such studies have focused exclusively on young adults. The vigilance-avoidance hypothesis (Mogg, Bradley, Miles, & Dixon, 2004) postulates that anxious individuals initially orient their attention to threat, but subsequently direct attention away from threat (see Koster, Verschuere, Crombez, & Van Damme, 2005; Rinck & Becker, 2006; Rohner, 2002). Supraliminal avoidance can prevent elaboration of threat meanings (Amir, Foa, & Coles, 1998) and serve as a coping strategy by reducing the negative emotional and physiological consequences of confronting the negative stimuli (Gray, 1976; Luecken, Tartaro, & Appelhans, 2004) in trait anxious individuals.
In the aging literature, research has supported the positivity effect in sustained attention (Mather & Carstensen, 2003; Isaacowitz, Wadlinger, Goren, & Wilson, 2006a; Rösler et al., 2005) and attention disengagement (Hahn, Carlson, Singer, & Gronlund, 2006). Older adults systematically avoided negative faces in negative-neutral face pairs and had an advantage in disengaging from angry faces compared to younger adults. In contrast, no age difference was found in initial orientation to angry faces (Mather & M. Knight, 2006) or valenced photographs (Rösler et al., 2005). Thus older adults manifest the positivity bias in attention mainly during later stages of processing. Interestingly, both non-anxious older adults and anxious young adults seem to adopt supraliminal (strategic) threat avoidance to regulate their emotional experience. Since threat detection is innate, we expected to observe subliminal vigilance for threat regardless of age and anxiety. Based on positivity, we expected non-anxious older adults to direct their attention away from supraliminal threat, so that they can minimize emotional disruption and return to their ongoing activities. Therefore, we hypothesized a vigilant-avoidant response to threat in non-anxious older adults.
Fox and B. Knight (2005) examined the effects of anxiety on selective attention in older adults and observed supraliminal vigilance for negative words in older adults who underwent anxiety induction, but not those in the neutral mood condition. Based on their finding, we expected anxiety to override emotion regulation goals (thereby eliminating the positivity bias) supraliminally, such that anxious older adults would display an attentional preference for supraliminal threat stimuli relative to neutral stimuli. Therefore, we hypothesized that anxious older adults would demonstrate vigilance for threat in both subliminal and supraliminal stages of processing (a vigilant-vigilant response).
We chose to measure trait anxiety rather than state anxiety in our participants. In Bar-Haim et al.’s meta-analysis (2007), trait but not state anxious individuals differed from controls in threat-related attentional bias. Spielberger (1972) conceptualized trait anxious individuals as those who tend to perceive stimuli as dangerous, and as a result experience state anxiety at a high frequency and intensity. We reasoned that high trait anxious individuals are likely to demonstrate threat-related attentional bias due to their ongoing, heightened perception of threat (vigilance) and motivation to reduce aversiveness to threat (avoidance).
Past research on positivity and visual attention have primarily used faces or pictures from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 1995) as stimuli. In the present study, we included an exploratory analysis to examine potential differences in the effects of stimulus modalities on attention trajectories in young and older adults. Our stimuli included: (1) photographs of negative (angry and sad) faces paired with neutral faces; (2) IAPS pictures depicting a range of high-threat scenarios (e.g., mutilation) paired with neutral pictures; and (3) words pertaining to physical and social/evaluative threats. We expected faces to be most powerful in eliciting attentional biases due to their strong ecological relevance.
We used a modified version of the dot probe task (DPT; see Mogg & Bradley, 1999) as a measure of attentional bias in young and older adults. The dot probe paradigm (Macleod, Mathews, & Tata, 1986) has been widely used to study attention allocation to emotionally valenced information. Participants view a valenced-neutral stimulus pair, followed by a dot in the location of the valenced (congruent trials) or the neutral (incongruent trials) stimulus. They press a key upon seeing the dot. A bias score is the mean difference in reaction times (RTs) between congruent and incongruent trials (see Mogg, Bradley, & Williams, 1995). RTs are faster when probes appear in the attended relative to the unattended location. Positive bias scores indicate vigilance for the valenced stimuli and negative bias scores indicate avoidance of the valenced stimuli. Research has supported the DPT as a valid attentional bias measure in young and older adults (Isaacowitz, Wadlinger, Goren, & Wilson, 2006b; Bar-Haim et al., 2007).
Method
Participants
The analysis sample included 103 young (16 men, 87 women; age 18–33, M = 19.95; SD = 2.12) and 44 older adults (17 men, 27 women; age 55–87, M = 71.75; SD = 6.35) 1. Young adults were recruited from the University of Southern California (USC) Psychology Subject Pool and received course credits for participation. Older adults were recruited through the USC Ethel Percy Andrus Gerontology Center and local senior centers. Their participation was voluntary. Exclusion criteria included non-fluency in English and suspected cognitive impairment 2.
Materials & Procedures
Trait anxiety was assessed by the trait scale (STAI-T) of the State Trait Anxiety Inventory-Form Y (Spielberger, 1983), which was extensively used in past studies of anxiety-related selective attention with the dot probe task.
Dot Probe Stimuli
All stimuli were presented in negative-neutral pairs. They consisted of 40 pairs of faces (20 angry-neutral, 20 sad-neutral), 32 pairs of high threat-nonthreat IAPS pictures, and 56 pairs of negative-neutral words 3. Faces within a pair belonged to the same poser and differed only by emotional expression. IAPS pictures within a pair were matched on color, content and arousal ratings. Words within a pair were matched on length and frequency.
For each stimulus modality, each stimulus pair appeared once as a congruent trial and again as an incongruent trial. The threat component appeared on the left/top side of the screen half of the time. Consistent with past studies, words in a pair were presented vertically (top-bottom) whereas faces and IAPS pictures were presented horizontally (left-right). A total of 256 trials were divided into 3 blocks of 85, 85 and 86 trials. Stimulus pairs were presented in a random and unique order for each participant. Stimulus modality was mixed within each block. Participants were allowed to take a 3-minute break between blocks or to continue with the task.
Dot Probe Task (DPT)
The DPT began with a set of on-screen instructions, a step-by-step demonstration and 10 practice trials. Each actual trial began a fixation cross shown in the center of the screen for 500 ms, followed by stimulus presentation (subliminal condition: 20 ms for young adults, 50 ms for older adults 4; supraliminal condition: 1500 ms for both age groups). Each subliminal pair was immediately followed by a mask pair shown for the same duration. Word masks were random letter strings matched for length with the word stimuli that preceded them. Masks for faces and IAPS pictures were constructed with stimuli pictures that were cut up and randomly reassembled (Mogg & Bradley, 2002). A dot probe then replaced one of the components and remained on the screen until the participant responded by pressing an arrow key to indicate the position of the dot probe relative to the center of the screen. Inter-trial intervals were chosen at random to be 500, 750, 1000 or 1500 ms.
Awareness Checks.5
Following Mogg & Bradley (2002), an image discrimination task and a word discrimination task were served as manipulation checks for participants’ awareness of the subliminal stimuli in the DPT. They involved forced-choice discrimination between regular faces/words and jumbled faces/random letter strings that were masked in the same manner as in the DPT. Scoring within the 95% confidence interval of the 50% accuracy mark indicates that one is not consciously aware of the subliminal stimuli (Mogg et al.,1995).
Study Procedures
At the beginning of each experiment, participants completed the STAI–T and a demographics questionnaire, followed by the DPT and discrimination tasks. During debriefing, they were asked to guess the true purpose of the study. They were given a complete description and explanation of the design of the study.
Data Cleaning
Several steps were taken to ensure the validity of the DPT data (see Mogg et al., 1995). Participants were excluded from the analyses if they scored > 5% incorrect across all dot probe trials, if they were systematically responding to the subliminal stimuli in the awareness checks, or if their bias scores were outliers in the sample distribution based on visual inspection. In the final sample, RT data from the first 3 trials at the beginning of each block were excluded to allow subjects to “warm up” to the task. Trials with probe detection latencies over 8s were excluded because they signaled the possibility that a participant was distracted. Following Mogg et al. (1995), trial-level RTs greater than 2 standard deviations from each participant’s mean RT were removed. This resulted in an exclusion of 7.43% and 7.82% of the young adult and older adult RT data, respectively.
Results
The older group had significantly lower STAI–T scores than the young group (young: M = 40.17, SD = 10.95; older: M = 34.17, SD = 9.75; t (145) = 3.22, p = .002). Low, moderate and high trait anxious groups were designated with a tercile split of the recruited sample based on their STAI–T scores (Table 1). In the young group, excluded participants had higher STAI–T scores than the analysis sample (Mexcluded = 46.60, SDexcluded = 10.71; t (114) = −2.00, p = .05).
Table 1.
Mean STAI–T scores and Dot Probe Task RT in Milliseconds (SD) for Each Stimulus by Trait Anxiety and Age
| Low TA | Moderate TA | High TA | |||||
|---|---|---|---|---|---|---|---|
| Young (n=20) |
Older (n=18) |
Young (n=44) |
Older (n=13) |
Young (n=39) |
Older (n=13) |
||
| STAI-T | 27.90 | 24.92 | 35.95 | 34.34 | 51.21 | 46.81 | |
| (3.18) | (3.47) | (2.84) | (2.17) | (7.88) | (4.19) | ||
| FACES | |||||||
| Sub Angry-Neutral | C | 519 (69) | 923 (232) | 518 (91) | 981 (190) | 599 (97) | 843 (248) |
| IC | 531 (91) | 923 (231) | 515 (92) | 990 (185) | 550 (78) | 890 (297) | |
| Supra Angry-Neutral | C | 524 (87) | 924 (206) | 502 (80) | 980 (185) | 535 (81) | 851 (271) |
| IC | 501 (77) | 910 (220) | 506 (67) | 967 (173) | 530 (83) | 820 (260) | |
| Sub Sad-Neutral | C | 512 (74) | 938 (224) | 529 (107) | 983 (184) | 562 (96) | 917 (310) |
| IC | 531 (92) | 923 (226) | 520 (92) | 1002 (201) | 552 (98) | 878 (289) | |
| Supra Sad-Neutral | C | 517 (88) | 905 (215) | 515 (76) | 973 (177) | 540 (95) | 847 (270) |
| IC | 510 (91) | 911 (219) | 522 (98) | 954 (200) | 546 (92) | 847 (285) | |
| WORDS | |||||||
| Sub | C | 570 (85) | 978 (205) | 575 (87) | 1040 (157) | 597 (84) | 954 (222) |
| IC | 570 (77) | 976 (205) | 573 (87) | 1035 (195) | 598 (90) | 932 (218) | |
| Supra | C | 545 (76) | 963 (169) | 551 (88) | 1007 (132) | 578 (86) | 915 (210) |
| IC | 544 (76) | 960 (187) | 547 (79) | 1014 (149) | 575 (97) | 950 (232) | |
| IAPS | |||||||
| Sub | C | 530 (76) | 958 (240) | 532 (104) | 1049 (205) | 569 (109) | 934 (299) |
| IC | 529 (86) | 955 (221) | 528 (96) | 1029 (198) | 559 (87) | 901 (264) | |
| Supra | C | 558 (103) | 931 (219) | 548 (95) | 1025 (210) | 583 (101) | 886 (290) |
| IC | 550 (103) | 946 (219) | 544 (102) | 1009 (205) | 585 (99) | 887 (305) | |
Notes. TA = Trait anxiety. STAI–T = State -Trait Anxiety Inventory (trait form). Sub = subliminal exposure; Supra = supraliminal exposure. C = congruent (dot-at-negative) trials; IC = incongruent (dot-at-neutral) trials.
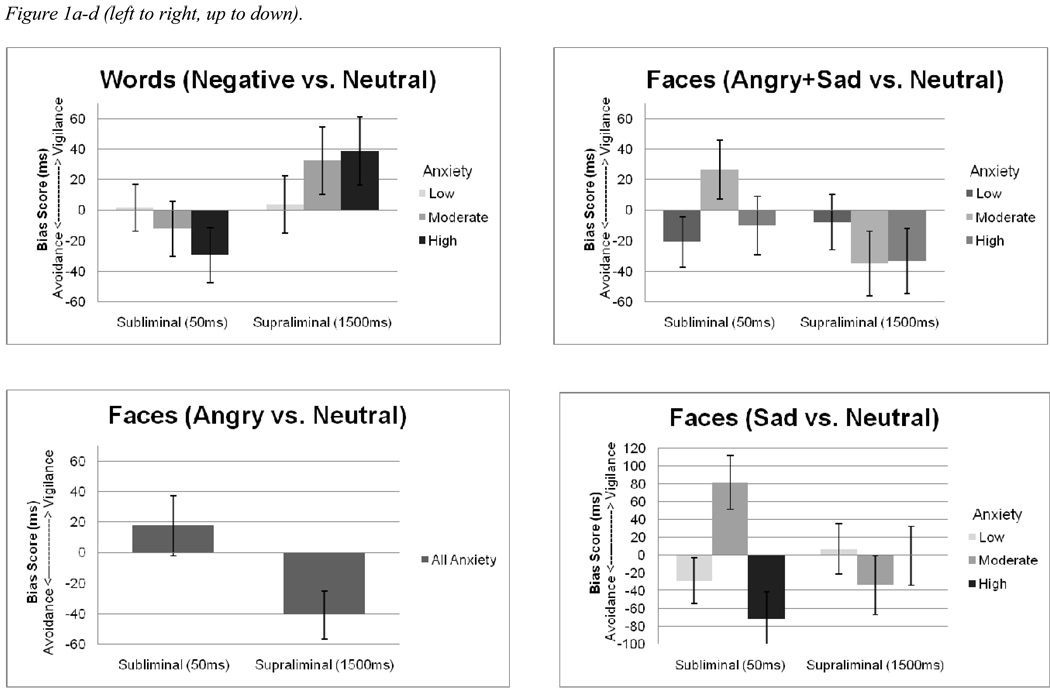
We conducted a repeated measures analysis of variance (ANOVA) of bias scores from the DPT with Age (young, old) and Anxiety (low, moderate, high) as between-subject factors, Stimulus (faces, IAPS pictures, words) and Exposure (subliminal, supraliminal) as within-subject, repeated measures factors. Table 1 shows the raw RT data for the mixed factorial design. Results revealed a significant 4-way Age x Anxiety x Stimulus x Exposure interaction, F (4, 282) = 2.57, p = .04, η2 = .04 and a main effect of Stimulus, F (2, 282) = 3.08, p = .05, η2 = .02. Post-hoc comparisons with Sidak adjustment were performed to decompose the 4-way interaction. Specifically, high anxious older adults had a lower bias score for words in the subliminal condition than the supraliminal condition (i.e., an avoidant-vigilant response to negative words; p = .02, η2 = .04; figure 1a). Moderately anxious older adults displayed a vigilant-avoidant response for negative faces (p = .05, η2 = .03; figure 1b). Despite a significant main effect of stimulus, post-hoc comparisons between bias scores of the stimulus modalities did not reveal any significant contrast. Because no attentional bias involving IAPS stimuli were detected, they were dropped from further analysis.
Figures 1a–d.
Mean attentional bias scores (ms) and standard errors illustrating older adults’ attentional bias for (a) negative-neutral word pairs, (b) negative-neutral face pairs, (c) angry-neutral face pairs, and (d) sad-neutral face pairs.
Based on past research suggesting potential associations between anxiety and attentional bias for sad stimuli (e.g., Mogg, Millar, & Bradley, 2000), we conducted post-hoc analysis separately for angry-neutral and sad-neutral face pairs. In the analysis that included angry-neutral faces and negative-neutral words as stimuli, results revealed a significant Age x Stimulus x Exposure interaction, F (1, 140) = 4.92, p = .03, η2 = .03 and a Stimulus x Exposure interaction, F (1, 140) = 4.91, p = .03, η2 = .03. Regardless of anxiety, older adults displayed a vigilant-avoidant response to angry faces (p = .04, η2 = .03; figure 1c). Results from the lower order interaction were subsumed under the higher order interaction and are not elaborated here.
In the analysis that included sad-neutral faces and negative words as stimuli, results revealed a significant Age x Anxiety x Stimulus x Exposure interaction, F (2, 141) = 5.71, p = .004, η2 = .08, a significant Age x Anxiety x Exposure interaction, F (2, 141) = 3.21, p = .04, η2 = .08, and a significant Anxiety x Exposure interaction, F (2, 141) = 3.40, p = .04, η2 = .05. Specifically, moderately-anxious older adults displayed a vigilant-avoidant response to sad faces (p = .01, η2 = .04; figure 1d); this pattern was not observed in the high-anxious older adults. Again, results from the lower-order interactions are not elaborated.
Discussion
In the aging literature, socioemotional selectivity theory suggests that emotion regulation goals are prioritized in late life, causing a greater emphasis to be placed on processing positive emotional information. The experimental psychology literature suggests that emotion plays a critical role in cognitive styles. Specifically, anxiety has been linked to attentional biases for threat in young adults. The current study bridges the two literatures by investigating whether anxiety moderates the positivity bias in visual attention in older adults. We examined attention using subliminal and supraliminal presentations of faces, pictures and words in a dot probe task.
Our findings revealed that regardless of anxiety, older adults displayed a vigilant-avoidant attentional pattern in response to angry faces. Anxiety moderated older adults’ attention for sad faces, with the vigilant-avoidant reaction showing only at moderate levels of anxiety. Anxiety also affected attentional bias to negative words with an unexpected avoidant-vigilant pattern among high anxiety older participants. Thus the attentional patterns of older adults varied over time by anxiety level and stimulus modality. Overall, the young group did not display attentional biases for threat.
Older adults’ subliminal vigilance to angry faces is consistent with past findings that automatic threat detection is a biological instinct in response to survival threats that can occur independently of anxiety (Morris, Öhman, & Dolan, 1998; Öhman et al., 2001) and remains intact with age (Mather & M. Knight, 2006; LeClerc & Kensinger, 2008). Since subliminal vigilance was only manifested for faces but not for words, it supports the notion that facial stimuli carry unique ecological relevance. While anxiety provides a “cueing effect” to prompt trait anxious young adults to regulate emotions (e.g., by averting gaze from threat), the greater emotional salience in old age removes anxiety as a prerequisite for emotion regulation. The finding that supraliminal avoidance is not related to anxiety in the older participants is therefore consistent with SST’s hypothesized greater focus at advanced age on maintaining a positive emotional state. Regardless of anxiety, older adults prioritize rapid identification of angry faces so that unpleasant social interactions could subsequently be avoided or transformed. Although the psychopathology literature has traditionally conceptualized threat avoidance as a maladaptive mechanism that maintains anxiety through inadequate processing of fear materials (e.g., Foa & Kozak, 1986), some have begun to argue that shifting attention away from threat could be a form of adaptive coping (Derryberry & Reed, 2002), especially in late life. Future work may examine whether this response pattern is maintained or exaggerated in a clinically anxious older sample.
A vigilant-avoidant attentional bias to sad faces was observed in older, moderately anxious participants. The positivity bias appears to generalize the protective vigilance-avoidance effect to sad faces as potential threats to the well being of older adults. Further investigation is needed to disentangle the complexity of this response and the differential patterns across moderate and high trait anxious older adults. Future research may examine attentional biases to threat vs. other negative stimuli, the impact of specific mood on attention (Gotlib, Krasnoperova, Yue, & Joormann, 2004) and the relevance of stimulus contents (e.g., interpersonal vs. physical threat) to specific emotions (e.g., Mathews, Ridgeway, & Williamson, 1996) in old age.
Based on previous studies supporting the ability to perceive meanings of masked words (Diaz & McCarthy, 2007; Van den Hout, Jong, & Kindt, 2000) and preservation of automatic lexical processing from age-related slowing (Stern, Prather, Swinney, & Zurif, 1991; Verhaeghen et al., 2002), we assume that older adults were able to preattentively access the meanings of subliminal words. Anxious older adults’ subliminal avoidance of negative words can be a conditioned response that has become automatic with repeated practice. In Mowrer’s two stage theory of fear (1947), individuals first develop anxiety to situations that are nonthreatening via learning (fear acquisition via classical conditioning). Subsequently, avoidance of the conditioned stimulus is negatively reinforced by removal of anxiety and its consequences. It is possible that high trait anxious individuals develop the tendency to avoid negative elements in their internal lexicon. We propose that a lifetime of practice combined with the age-related positivity bias facilitate the observed automaticity of the verbal threat avoidance response. Note that although threat avoidance in older adults is often interpreted as a positivity bias, it is not the case here because this phenomenon was limited to anxious individuals while the positivity effect generalizes to all older adults.
During supraliminal processing, anxious older adults redirected their attention to negative words. Their attentional dwell on negative words resembles worrying, a cognitive strategy used by anxious individuals to cope with the physiological and emotional arousal caused by negative stimuli (Vasey & Borkovec, 1992). Thus anxiety seems to be a critical determinant of older adults’ supraliminal attention for verbal threat.
We did not find evidence for attentional bias for IAPS pictures. Imaging work comparing facial expressions and IAPS pictures suggest that while both types of stimuli activate similar brain structures, faces are associated with wider neurological activation (Britton, Taylor, Sudheimer, & Liberzon, 2006) despite being less arousing. It is possible IAPS pictures are less powerful in inducing attentional bias because their contents are externally-focused (e.g., warfare, violence) and carry less self-relevance to the concerns of older adults (which are more interpersonally-focused, see Diefenbach, Stanley and Beck, 2001).
No evidence of attentional bias was observed in the young adults. One possibility is that our young sample had relatively low levels of anxiety (see Table 1). The mean STAI score for the high trait anxious group was 51.21 for young adults and 46.81 for older adults , compared to scores in the 55/+ range among clinically anxious samples (Bradley, Mogg, White, Groom, & de Bono, 1999; Mogg et al., 1995; Kabacoff et al., 1997). The literature has documented inconsistencies in observing attentional biases in subclinical populations (e.g., Bradley et al., 1999; Mogg, et al., 2000; Koster, Leyman, de Raedt, & Crombez, 2006; Yovel & Mineka, 2004). It is noteworthy that in the young sample, excluded participants scored higher on the STAI than the analysis sample 6, which might have weakened the effect of anxiety-related attentional bias. It is also possible that young adults have habituated to the threat component early on in the experiment (c.f. Harris & Pashler, 2004).
There are limitations to our study. First, we acknowledge that our age groups were not balanced in size. When we reduced our young sample by randomly deleting half of the cases so that it was of comparable size to the older group, the pattern of findings remained unchanged. Second, inspection of the raw RT data indicates greater variance in older adults’ response. This is consistent with findings on increased intra- and interindividual variability in RT with age (Deary & Der, 2005; Fozard, Vercruyssen, Reynolds, Hancock, & Quilter, 1994). Although this might weaken our statistical power to detect significant effects, we were able to identify attentional biases in the older sample. Moreover, since our dependent variable is a difference score, it removes some variation due to perceptual and motor slowing.
Despite such limitations, our findings show that visual attention in older adults is moderated by anxiety and stimulus modality. Regardless of anxiety, a positivity bias was observed in older adults’ supraliminal attention for angry faces. Moderately anxious older adults responded in a similar, vigilant-avoidant pattern to sad faces. In contrast, high anxious older adults initially avoided but subsequently dwelled on negative words in a worry-like manner.
Acknowledgments
Work on this article was supported by Grant Number F31AG031691 from the National Institute on Aging to the first author. The authors thank Dr. Mara Mather for providing us with photographs of faces. We are grateful to Dr. Tat S. Fu for assistance in programming the dot probe task, to Linda Broder and Nat Kittisarapong for recruitment. Liza Cherney, Brooke Fullmer, Frank Gallop, Helena Geefay, Jennifer Wang, Courtney Wise and Lenore Zion provided invaluable research assistance in this study.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/journals/pag.
A chi-square test of independence revealed that gender was significantly associated with age group in the sample, χ2 (1, N = 47) = 9.45, p = .002. The younger group had a greater proportion of female participants than the older group. Following Isaacowitz et al. (2006a), we tested whether gender influenced attentional bias for threat, we performed a MANOVA with bias scores of each exposure-by-stimulus category (e.g., subliminal angry faces) as the dependent variable. Age group and Gender were fixed variables. We found no main effect of Gender and no Age x Gender interaction for any bias score. We therefore collapsed data for both genders in the main analysis.
Non-fluency in English was determined if subjects could not understand multiple items in the questionnaire packet. Suspected cognitive impairment was determined if subjects could not understand the instructions on the dot probe and manipulation tasks, or made consistent errors in the dot probe practice trials. All older participants were living independently in the community at the time of participation.
We used the same set of facial photographs from the Mather & Carstensen (2003) study. IAPS pictures in our study were derived from the Mogg et al. (2004) study. The pictures were chosen based on established valence and arousal norms, as well as subjective ratings by judges. Readers are referred to the original article for actual valence and arousal ratings. The word pairs were acquired from prior research using the dot-probe and Stroop tasks (Fox & B. Knight, 2005; MacLeod, Mathews & Tata, 1986; MacLeod & Mathews, 1988).
The longer exposure duration for older adults was intended to adjust for their slower processing speed compared to young adults. Fifty milliseconds were chosen as the subliminal exposure duration for older adults because piloting work indicated that older participants were able to consciously recognize stimuli shown at 75 ms but not at 40 ms.
Full details of the awareness checks are available upon request from the corresponding author. We only briefly describe the tasks here due to space limitations.
Among the excluded young adults, the main reason for exclusion was committing more errors in the discrimination tasks than expected by chance.
References
- Amir N, Foa EB, Coles M. Automatic activation and strategic avoidance of threat-relevant information in social phobia. Journal of Abnormal Psychology. 1998;107:285–290. doi: 10.1037//0021-843x.107.2.285. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mogg K, White J, Groom C, de Bono J. Attentional bias for emotional faces in generalized anxiety disorder. British Journal of Clinical Psychology. 1999;38:267–278. doi: 10.1348/014466599162845. [DOI] [PubMed] [Google Scholar]
- Britton JC, Taylor SF, Sudheimer KD, Liberzon I. Facial expressions and complex IAPS pictures: Common and differential networks. NeuroImage. 2006;31:906–919. doi: 10.1016/j.neuroimage.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Carstensen LL. Motivation for social contact across the life span: A theory of socioemotional selectivity. In: Jacobs J, editor. Nebraska Symposium on Motivation: Vol. 40. Developmental perspectives on motivation. Lincoln: University of Nebraska Press; 1993. pp. 209–254. [PubMed] [Google Scholar]
- Carstensen LL, Mikels JA, Mather M. Aging and the intersection of cognition, motivation, and emotion. In: Birren J, Schaie KW, editors. Handbook of the Psychology of Aging. 6th edition. San Diego: Academic Press; 2006. pp. 343–362. [Google Scholar]
- Deary IJ, Der G. Reaction time, age, and cognitive ability: Longitudinal findings from age 16 to 63 years in representative population samples. Aging, Neuropsychology, and Cognition. 2005;12:187–215. [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Diaz MT, McCarthy G. Unconscious word processing engages a distributed network of brain regions. Journal of Cognitive Neuroscience. 2007;19:1768–1775. doi: 10.1162/jocn.2007.19.11.1768. [DOI] [PubMed] [Google Scholar]
- Diefenbach GJ, Stanley MA, Beck JG. Worry content reported by older adults with and without generalized anxiety disorder. Aging & Mental Health. 2001;5:269–274. doi: 10.1080/13607860120065069. [DOI] [PubMed] [Google Scholar]
- Eich E, Kihlstrom JF, Bower GH, Forgas JP, Niedenthal PM. Cognition and Emotion. London: Oxford University Press; 2000. [Google Scholar]
- Foa E, Kozak MJ. Emotional processing of fear: Exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Fox L, Knight B. The effects of anxiety on attentional processes in older adults. Aging & Mental Health. 2005;9:585–593. doi: 10.1080/13607860500294282. [DOI] [PubMed] [Google Scholar]
- Fozard JL, Vercruyssen M, Reynolds SL, Hancock PA, Quilter RE. Age differences and changes in reaction time: The Baltimore Longitudinal Study of Aging. Journal of Gerontology: Psychological Sciences. 1994;49:P179–P189. doi: 10.1093/geronj/49.4.p179. [DOI] [PubMed] [Google Scholar]
- Gotlib I, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004;113:127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gray JA. The behavioral inhibition system: A possible substrate for anxiety. In: Feldman MP, Broadhurst A, editors. Theoretical and Experimental Bases of the Behavior Therapies. London: John Wiley & Sons; 1976. [Google Scholar]
- Hahn S, Carlson C, Singer S, Gronlund SD. Aging and visual search: Automatic and controlled attentional bias to threat faces. Acta Psychologica. 2006;123:312–336. doi: 10.1016/j.actpsy.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Harris CR, Pashler H. Attention and the processing of emotional words and names: Not so special after all. Psychological Science. 2004;15:171–178. doi: 10.1111/j.0956-7976.2004.01503005.x. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Selective preference in visual fixation away from negative images in old age? An eye-tracking study. Psychology & Aging. 2006a;21:40–48. doi: 10.1037/0882-7974.21.1.40. [DOI] [PubMed] [Google Scholar]
- Isaacowitz DM, Wadlinger HA, Goren D, Wilson HR. Is there an age-related positivity effect in visual attention? A comparison of two methodologies. Emotion. 2006b;6:511–516. doi: 10.1037/1528-3542.6.3.511. [DOI] [PubMed] [Google Scholar]
- Kabacoff RI, Segal DL, Hersen M, Van Hasselt VB. Psychometric properties and diagnostic utility of the Beck Anxiety Inventory and the State-Trait Anxiety Inventory with older adult psychiatric outpatients. Journal of Anxiety Disorders. 1997;11:33–47. doi: 10.1016/s0887-6185(96)00033-3. [DOI] [PubMed] [Google Scholar]
- Koster EHW, Leyman L, De Raedt R, Crombez G. Cueing of visual attention by emotional facial expressions: The influence of individual differences in anxiety and depression. Personality and Individual Differences. 2006;41:329–339. [Google Scholar]
- Koster EHW, Verschuere B, Crombez G, Van Damme S. Time course of attention for threatening pictures in high and low trait anxiety. Behaviour Research and Therapy. 2005;43:1087–1098. doi: 10.1016/j.brat.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System: Technical manual and affective ratings. Gainesville: FL: University of Florida; 1995. [Google Scholar]
- Leclerc CM, Kensinger EA. Effects of age on detection of emotional information. Psychology and Aging. 2008;23:209–215. doi: 10.1037/0882-7974.23.1.209. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: Clues from the brain. Annual Review of Psychology. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Luecken LJ, Tartaro J, Appelhans B. Strategic coping responses and attentional biases. Cognitive Therapy and Research. 2004;28:23–37. [Google Scholar]
- MacLeod C. Anxiety and anxiety disorders. In: Dalgleish T, Power MJ, editors. Handbook of cognition and emotion. New York: Wiley; 1999. pp. 447–477. [Google Scholar]
- MacLeod C, Mathews A. Anxiety and the allocation of attention to threat. The Quarterly Journal of Experimental Psychology, 40A. 1988:653–670. doi: 10.1080/14640748808402292. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, Tata P. Attentional bias in emotional disorders. Journal of Abnormal Psychology. 1986;95:15–20. doi: 10.1037//0021-843x.95.1.15. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14:409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight MR. Angry faces get noticed quickly: Threat detection is not impaired among older adults. Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2006;61:P54–P57. doi: 10.1093/geronb/61.1.p54. [DOI] [PubMed] [Google Scholar]
- Mathews A, Ridgeway V, Williamson DA. Evidence for attention to threatening stimuli in depression. Behaviour Research and Therapy. 1996;9:695–705. doi: 10.1016/0005-7967(96)00046-0. [DOI] [PubMed] [Google Scholar]
- Matthews G, Wells A. The cognitive science of attention and emotion. In: Dalgleish T, Power M, editors. Handbook of cognition and emotion. New York: Wiley; 1999. pp. 171–192. [Google Scholar]
- Mogg K, Bradley BP. Some methodological issues in assessing attentional biases for threatening faces in anxiety: a replication study using a modified version of the probe detection task. Behaviour Research & Therapy. 1999;37:595–604. doi: 10.1016/s0005-7967(98)00158-2. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective orienting of attention to masked threat faces in social anxiety. Behaviour Research & Therapy. 2002;40:1403–1414. doi: 10.1016/s0005-7967(02)00017-7. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Miles F, Dixon R. Time course of attentional bias for threat scenes: Testing the vigilance-avoidance hypothesis. Cognition & Emotion. 2004;18:689–700. [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: The role of awareness. British Journal of Clinical Psychology. 1995;34:17–36. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Millar N, Bradley PB. Biases in eye movements to threatening facial expressions in Generalized Anxiety Disorder and Depressive Disorder. Journal of Abnormal Psychology. 2000;109:695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Morris JS, Öhman A, Dolan RJ. Conscious and unconscious emotional learning in the human amygdala. Nature. 1998;393:467–470. doi: 10.1038/30976. [DOI] [PubMed] [Google Scholar]
- Mowrer OH. On the dual nature of learning. A re-interpretation of “conditioning” and “problem solving.” Harvard Educational Review. 1947;17:102–148. [Google Scholar]
- Öhman A. Preferential preattentive processing of threat in anxiety: preparedness and attentional biases. In: Rapee RM, editor. Current Controversies in the Anxiety Disorders. Chichester, England: Wiley & Sons Ltd; 1996. pp. 253–290. [Google Scholar]
- Öhman A. Distinguishing unconscious from conscious emotional processes: Methodological considerations and theoretical implications. In: Dagleish T, Power M, editors. Handbook of Cognition and Emotion. New York: Wiley; 1999. pp. 321–352. [Google Scholar]
- Öhman A, Flykt A, Esteves F. Emotion drives attention: Detecting the snake in the grass. Journal of Experimental Psychology: General. 2001;130:466–522. doi: 10.1037//0096-3445.130.3.466. [DOI] [PubMed] [Google Scholar]
- Rinck M, Becker ES. Spider fearful individuals attend to threat, then quickly avoid it: Evidence from eye movements. Journal of Abnormal Psychology. 2006;115:231–238. doi: 10.1037/0021-843X.115.2.231. [DOI] [PubMed] [Google Scholar]
- Rohner J-C. The time course of visual threat processing: High trait anxious individuals eventually avert their gaze from angry faces. Cognition and Emotion. 2002;16:837–844. [Google Scholar]
- Rösler A, Ulrich C, Billino J, Sterzere P, Weidauer S, Bernhardt T, et al. Effects of arousing emotional scenes on the distribution of visuospatial attention: changes with aging and early subcortical vascular dementia. Journal of the Neurological Sciences. 2005;229–230:109–116. doi: 10.1016/j.jns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Anxiety as an emotional state. In: Spielberger CD, editor. Anxiety: Current Trends in Theory and Research. New York: Academic Press; 1972. pp. 24–54. [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory: STAI (Form Y) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stern C, Prather P, Swinney D, Zurif E. The time-course of automatic lexical access and aging. Brain and Language. 1991;40:359–372. doi: 10.1016/0093-934x(91)90135-n. [DOI] [PubMed] [Google Scholar]
- Vasey MW, Borkovec TD. A catastrophizing assessment of worrisome thoughts. Cognitive Therapy & Research. 1992;16:505–520. [Google Scholar]
- Verhaeghen P, Cerella J, Semenec SC, Leo MA, Bopp KL, Steiz DW. Cognitive efficiency models in old age: Performance on sequential and coordinative verbal and visuospatial tasks. Psychology & Aging. 2002;17:558–570. doi: 10.1037//0882-7974.17.4.558. [DOI] [PubMed] [Google Scholar]
- Van Den Hout MA, De Jong P, Kindt M. Masked fear words produce increased SCRs: An anomaly for Öhman’s theory of pre-attentive processing in anxiety. Psychophysiology. 2000;37:283–288. doi: 10.1017/s0048577200980673. [DOI] [PubMed] [Google Scholar]
- Yovel I, Mineka S. Hierarchical models of emotional disorders and emotion-congruent cognitive biases. Personality and Individual Differences. 2004;36:679–694. [Google Scholar]