Abstract
Cell-based therapy for neuropathic pain could provide analgesics to local pain modulatory regions in a sustained, renewable fashion. In order to provide enhanced analgesic efficacy, transplantable cells may be engineered to produce complementary or increased levels of analgesic peptides. In addition, genetic labeling of modified cells is desirable for identification and tracking, but it should be retained intracellularly as desired analgesic peptides are secreted. Usually constructs encode proteins destined for either extra- or intra-cellular compartments, as these pathways do not cross. However, interactions between intracellular destinations provide a window of opportunity to overcome this limitation. In this report, we have explored this approach using a potential supplementary analgesic peptide, [Ser1]-histogranin (SHG), the stable synthetic derivative of a naturally occurring peptide with N-methyl D-aspartate (NMDA) antagonistic properties. A synthetic SHG gene was combined with (i) nerve growth factor-β (NGF-β) amino-terminal signal peptide to enable secretion, and (ii) a fluorescent cellular label (mRFP) with intervening cathepsin L cleavage site, and subcloned into a lentiviral vector. In addition, an endoplasmic retention signal, KDEL, was added to enable retrieval of mRFP. Using immunocytochemistry and confocal microscopic profile analysis, cells transduced by such lentiviruses were shown to synthesize a single SHG-mRFP polypeptide that was processed, targeted to expected subcellular destinations in several cell types. Dot blot and Western analysis revealed stable transduction and long-term secretion of SHG from PC12 cells in vitro. Transplantation of such cells provided modest analgesia in a rodent pain model consistent with low levels of SHG peptide in the cerebrospinal fluid (CSF). These results suggest that it is possible to deliver proteins with different final destinations from a single construct, such as pharmacologically active peptide for secretion and intracellular label for identifying transplantable cells.
Keywords: Pain, Transplantation, Lentiviral, NMDA antagonist, PC12 cells, Histogranin
INTRODUCTION
Transplantation of cells into the CNS can provide a constant and replenishable source of analgesic substances for alleviation of chronic pain. This cellular minipump approach has several potential benefits, such as long-term delivery, reduced systemic side effects, and the ability to provide agents that would otherwise have limited penetration past the blood–brain barrier. Further, cells can be combined or genetically manipulated to deliver a therapeutic cocktail of multiple desired agents for synergistic effects or for treatment of chronic pain of different etiologies. Cell therapy using primary adrenal chromaffin cells from a variety of sources, as well as immortalized and tumor cell lines, has been attempted in several pain models with varying degrees of success (32). In addition, reports from experimental clinical studies using bovine and human chromaffin cells have been promising, but variable (32). It is likely that improved analgesic efficacy could be achieved by engineering transplantable cells to secrete novel and/or increased levels of synergistic analgesic peptides.
A potentially promising target for enhancing analgesic activity is the NMDA receptor. Histogranin (HG) is a naturally occurring peptide with NMDA-antagonistic activity (23). Its stable synthetic analog, [Ser1]-histogranin (SHG), when administered intrathecally, blocks the hyperalgesia and allodynia produced in rats by intrathecal NMDA (18). The SHG-mediated analgesia may in part be mediated through its noncompetitive inhibition of NMDA/kainate subtype glutamate receptors as well as tachykinin receptors (21,34). Furthermore, unlike prototypic NMDA antagonists, SHG does not have attendant motor side effects (18,21). SHG was identified in a screen for compounds that enhanced adrenal transplant-mediated analgesia in rat pain models (29). SHG has the potential to be delivered recombinantly on a long-term basis using emerging lentiviral technologies.
In order to identify cells containing a recombinant gene, typically a reporter gene such as enhanced green fluorescent protein (EGFP) or “myc,” can be inserted downstream and in frame with the gene of interest. Transcription of the recombinant DNA results in unique mRNA species that when translated results in a tagged or fluorescent fusion protein that can later be identified by immunofluorescence and staining with antibodies against either the protein itself of its “tag.” This method can also be used to enrich transduced cell populations for transplantation using FACS. However, fusion of SHG with bulky EGFP would likely render it biologically inactive due to its oligomerization. Thus, we have devised an approach that would enable unimpeded secretion of the antinociceptive peptides while also genetically tagging the secreting cells by creating a construct in which our gene of interest, SHG, and a modified mRFP (monomeric red fluorescent protein), which does not oligomerize (8), are separated by a dibasic residue (KR) that is the substrate for cathepsin L (43). In addition, an endoplasmic retention/retrieval signal (ER) KDEL was added to enable retrieval of the mRFP. The goal of this study was to develop and evaluate this strategy for use in potential transplantable cell sources.
MATERIALS AND METHODS
Cell Lines
The cell lines utilized were 293T cells, PC12 cells, human bone marrow cells (41), and cadaver-derived marrow-isolated adult multilineage inducible (MIAMI) cells (10).
Generation of Red SHG Construct
The SHG protein sequence was reverse translated and the resulting DNA sequences were used to synthesize oligos. Oligos were annealed in the presence of ligation buffer; the double-stranded DNA (dsDNA) thus generated was flanked by BglII and XbaI to enable subcloning into ppNGF-β signal sequence containing vector digested with the same enzymes. The ppNGF-β signal peptide enables production of secretable peptides and has been described elsewhere (13). A PCR-based strategy was used to fuse the ppNGF-β-SHG cDNA in frame with mRFP cDNA (8). First, the ppNGF-SHG construct flanked by the BamHI site at the 5′ end was amplified with primers that remove the stop codon and introduce an XbaI site at the 3′ end. Second, the mRFP cDNA was amplified using primers that introduce an XbaI site at the 5′ end, a KDEL coding sequence, and a SalI site at the 3′ end. Both the PCR products were purified, digested with XbaI, subcloned, and sequenced. The BamHI-SalI fragment containing ppNGF-SHG-mRFPKDEL was subcloned into lentiviral vector pRRL.
Generation of Lentiviruses and Infection of Cells
The SHG construct in lentiviral vector pRRL and lentiviral packaging vectors were cotransfected into 293T cells. The next day when the expression of the mRFP could be seen, the culture was washed and supplemented with fresh media. The p24 titer was assessed by p24 ELISA; the viral titer was determined by infecting cells. Routinely titers of 107–108 transducing agents/ml were obtained. Cells that were to be infected with the virus were plated a day before the infection. After washing the cells, high titer purified lentivirus was added to cells in fresh culture media at an MOI of 5 for 4 h.
Immunocytochemistry and Antibodies
Cells were fixed with 4% paraformaldehyde, permeabilized with 0.1% Triton X-100, blocked with 10% normal goat serum, followed by primary antibodies: rabbit anti-Giantin (1:500, Covance), rabbit anti-mRFP (1:1000, Chemicon), rabbit anti-SHG (1:1000, 21st Century Biochemicals), mouse anti-β-actin (1:100000) and mouse anti-cytochrome c (1:1000, Sigma), mouse anti-human mitochondria (1:500), with mouse anti-Erd2 (1:500, Abcam and Stressgen). The Erd2 antibody staining was done in the presence of 0.2% Saponin (Sigma) as described previously (39). Appropriate fluorescent secondary antibodies (1:200) were used (Molecular Probes). Cells were stained with 5 nM DAPI in the final wash to identify the nuclei. For dot blots and Western analysis appropriate HRP conjugated secondary antibodies [1: 10000 goat anti-rabbit HRP (Santa Cruz); 1:5000 goat anti-mouse IgG-HRP (31)] were used.
Culture Supernatants, Dot Blots, and Western Blots
Ten million naive or red SHG transduced PC12 cells were incubated in serum-free medium overnight (16 h). Cell culture supernatant was harvested by centrifugation and filtered through a 22-μm syringe; protein was estimated and equal amounts of protein were used in blots. The dot blot protocol was adopted from Martin-Schild et al. (25). Briefly, a pair of PVDF membranes was wetted in methanol for 5 s, water for 2 min, and transfer buffer for 5 min. The membranes are placed on two sheets of blotting paper presoaked in transfer buffer. Serial dilutions of the sample were prepared in a 96-well plate and placed in the wells. Vacuum was applied briefly and the topmost membrane was blocked overnight in 2% BSA, washed three times with TBST for 15 min each, probed with appropriate dilution of the anti-SHG antibody for 45 min are room temperature, washed, and probed with secondary antibody. The blots are washed again and developed with chemiluminiscent reagents (Perkin-Elmer).
Commercial gradient gels (4–20% acrylamide, Lifegels) were used with SeaBlue II protein ladder (Invitrogen) and electroblotted onto a PVDF membrane (Immobilon PSQ; Millipore). Blots were processed using standard manufacturer’s protocol and bands were detected with chemiluminiscence (Perkin-Elmer).
For densitometry, X-ray films with subsaturating chemiluminiscent signals were scanned using the BioRad FluorS scanner equipped with Quantity One software. A circle/band tool was used to draw contours around the signal; background was traced and their density determined and plotted.
Confocal Microscopy
Images were acquired using an LSM510 equipped Zeiss Confocal microscope at the Image Core. The “Profile” tool of LSM510 was used to quantitate fluorescence along a particular section of the image. A table generated by the same tool was used to estimate the dimensions of SHG puncta.
Formalin Testing and Behavior
In order to conduct an initial screen on the ability of transduced cells to release recombinant SHG and alter pain sensitivity, a small group of animals was transplanted with either red SHG PC12 cells or control PC12 cells (n = 6 per group). Cell suspensions were transplanted into the spinal subarachnoid space via laminectomy and a slit in the dura at the level of the lumbar enlargement (10 μl of 50,000 cells/μl) according to routine procedures in our laboratory. Animals received cyclosporine A (10 mg/kg, IP daily, beginning the day before transplantation surgery). At 1 week following transplantation, 50 μl of 5% formalin was injected in the plantar surface of one hind paw to elicit the formalin pain response. This results in typical biphasic formalin flinching response: phase 1, thought to be indicative of acute pain, occurring during the first minute postformalin; and phase 2, thought to result from central sensitization and indicative of tonic, more prolonged pain, separated by a quiescent interphase between phase 1 and phase 2. The numbers of flinches that occurred during the first minute of each 5-min interval up to 70 min were measured.
In order to determine whether transplanted cells can produce detectable levels of engineered peptides in the spinal CSF, we initiated evaluation of spinal CSF samples following cell transplantation. In preliminary studies, 100–150 μl of CSF was collected by aspiration from the subarachnoid cavity of the spinal cord at the level of the foramen magnum 2 weeks following transplantation. The CSF was centrifuged at 1500 rpm for 5 min to pellet any red blood cells and stored at −20°C until assayed by dot blot or Western blot for SHG.
RESULTS
SHG-mRFPKDEL Construct
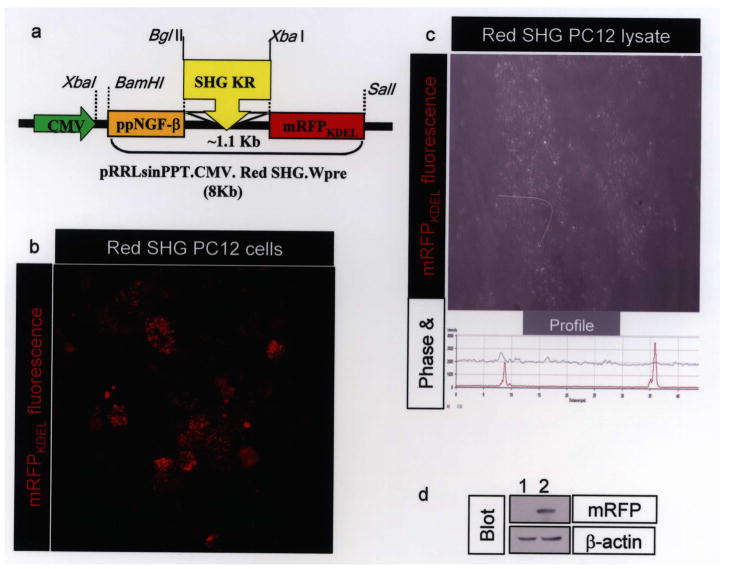
A schematic in Figure 1a shows the design of the SHG-mRFPKDEL lentiviral construct. The cytomegalovirus (CMV) promoter drives expression of cDNA with prepro-nerve growth factor (ppNGF) signal peptide for secretion; the interchangeable peptide cDNA is followed by mRFP with KDEL at the carboxy-terminus. Introduction of KDEL at the carboxy-terminus is known to be sufficient for retrieval of proteins to endoplasmic reticulum (ER). Interaction of KDEL receptor is optimal at acidic pH [e.g., trans-golgi network (TGN)] (16,28,33). Furin, a prohormone convertase responsible for cleavage of the ppNGF signal peptide, is also active only in TGN at acidic pH. KDEL-tagged monomeric red fluorescent protein (mRFPKDEL) with a signal peptide has been used extensively and found to be localized to “vesicular” ER in neurons and cell lines (1,5,24). To test if the mRFPKDEL generated for this study was also similarly distributed, cells transduced with lentivirus and cell lysates prepared using nitrogen cavitation were imaged by confocal microscopy. Several red puncta with sizes ranging from 0.2 to > 0.9 μm in diameter (n = 28) were observed (Fig. 1b). These puncta were intact following nitrogen cavitation (Fig. 1c), a procedure routinely used to prepare intact vesicles and organelles (11,15,40). To confirm the red puncta were indeed mRFP, lysates were analyzed by immunoblotting. Using anti-mRFP antibody, a band corresponding to 26 kDa (Fig. 1d, upper panel) was detected only in the red SHG PC12 lysate (Fig. 1d, lane 2) but not in naive PC12 cell lysates (Fig. 1d, lane 1). The blot was stripped and reprobed with anti-β-actin to normalize protein loading (Fig. 1d, lower panel).
Figure 1.
(a) Schematic of interchangeable lentiviral construct. Antinociceptive peptide cDNAs can be cloned between BglII and XbaI sites. (b) Punctate red fluorescence from mRFPKDEL vesicles ranging from 0.2 to >0.9 μm in diameter (n = 28) were observed. (c) Cells were lysed by nitrogen cavitation; an aliquot was fixed and imaged by confocal microcopy. A projection of confocal images acquired in red and phase channels shows several fluorescent red puncta in a background of a cellular ghost. Using the profile, a white arrow drawn over the field identifies two of the red fluorescent puncta shown as peaks of red fluorescence (profile below the image). (d) An aliquot of lysate produced by cavitation was analyzed by mRFP immunoblotting. The mRFP band was observed in the “red SHG lentivirus” transduced lysate (lane 2) but not naive cell lysate (lane 1). The same blot was reprobed with β-actin antibody to visualize β-actin, a control for protein loading.
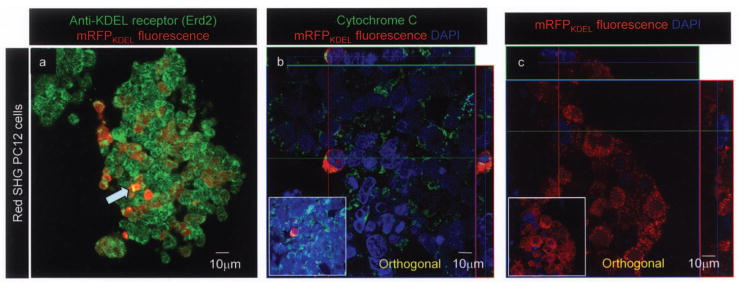
To further assess localization of the mRFPKDEL, cells were stained with Erd2 (endoplasmic retrieval defective 2), a receptor for KDEL-tagged proteins, cytochrome c, a marker for mitochondria, and DAPI to visualize nuclei. Using Erd2 antibody, yellow bands suggesting points of interaction between mRFPKDEL and Erd2 were observed (Fig. 2a, arrow). KDEL receptor interaction with KDEL-tagged proteins is optimal at acidic pH; therefore, the localization of KDEL receptor staining and mRFPKDEL is limited. The ERD2 staining pattern was similar to that described previously (39). In Figure 2b, the red signal from mRFPKDEL and green signal from cytochrome c (long green line in the x-axis) did not overlap, suggesting that the mRFPKDEL was absent in mitochondria. Nuclei visualized with DAPI suggested that mRFPKDEL was also not translocated into the nucleus (Fig. 2c).
Figure 2.
mRFPKDEL is recognized by Erd2, excluded from the nucleus and mitochondria. Anti-Erd2 antibody staining confirms that the red fluorescence puncta (a) and the punctate green fluorescence from KDEL receptor Erd2 overlap; yellow fluorescence is due to combined fluorescence from Erd2 and mRFPKDEL (a prominent example is indicated by blue arrow). (b) ‘Red SHG mRFPKDEL’ lentivirus infected PC12 cells were stained for cytochrome c; mitochondria appear green, DAPI-stained nuclei are blue. Orthogonal analysis along cytochrome c-stained mitochondria (green on x-axis) does not overlap or include any red puncta (mRFP) that surrounds DAPI-stained blue nuclei. The inset shows normal view of the orthogonal projection. (c) Orthogonal projection shows punctate red fluorescence (red) and the DAPI-stained nucleus (blue) do not overlap. Inset shows the normal view of the field. Original magnification 400×.
Anti-SHG Immunostaining of Cells Transduced With SHG Lentivirus
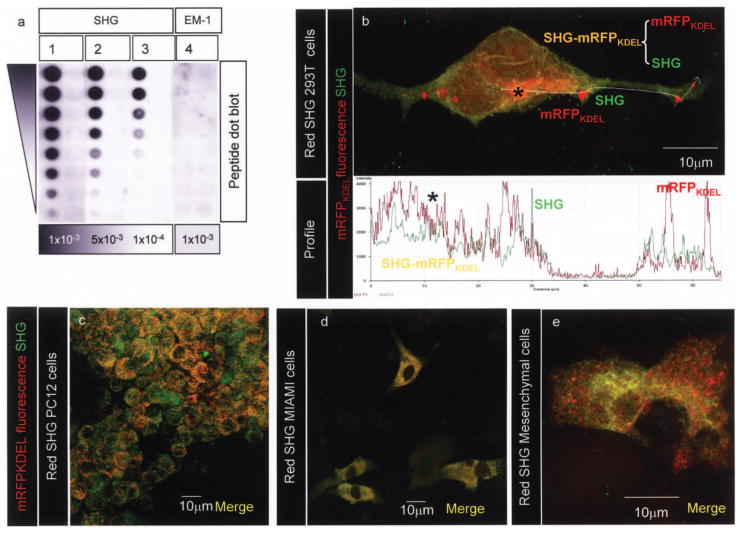
There is no commercial source for anti-histogranin or anti-serine histogranin antibodies; therefore, a custom antibody for SHG was generated for us with 21st Century Biochemicals. A synthetic SHG peptide was used to immunize rabbits. Anti-SHG antibody showed a dose-dependent reaction with the synthetic SHG peptide (Fig. 3a). Densitometric scanning of the blots showed that anti-SHG signal fades proportionately with twofold decreasing concentration of the SHG peptide (y-axis) and with the anti-SHG antibody dilution (x-axis). The specificity of the reaction is supported by the lack of reaction of anti-SHG antibody (high titers) with an irrelevant peptide, endomorphin-1 (EM-1) (Fig. 3a, lane 4).
Figure 3.
(a) Twofold serial dilutions of SHG peptide (lanes 1–3) and a control peptide (endomorphin-1; EM-1; lane 4) were blotted onto PDVF membrane. Anti-SHG antibody concentration used is indicated below the lane. Films were analyzed with a Biorad densitometer FluorS equipped with Quantity One software to deduce density. (b) Representative section from a projection of 48 confocal images of phoenix cells transfected with ppNGF-β-SHG-mRFPKDEL lentiviral construct. The colocalization and quantitation of fluorescent intensities along defined space (white arrow left to right) were measured and analyzed using the “Profile” tool in LSM510 software. Freshly synthesized unprocessed polypeptide is perinuclear, indicated by an asterisk; the ppNGF-β-mRFPKDEL-SHG appears yellow due to combined red (mRFPKDEL) and green fluorescence (anti-SHG antibody). The presence of several distinct red fluorescent vesicular aggregates containing only mRFPKDEL and green puncta containing only SHG indicates processed and sorted polypeptide. Original magnification 1000×. The lower panel shows overlapping red and green fluorescence (asterisk); sorted mRFPKDEL and SHG vesicles appear as single red and green peaks, respectively. Immunostaining with anti-SHG antibody (43) in red SHG-expressing PC12 cells (c), MIAMI cells (d), and bone marrow stromal cells (e) showing the presence of several yellow puncta (unprocessed polypeptide), red (mRFP) or green puncta (processed secretable SHG). Original magnification 400×.
Evidence for SHG Synthesis and Processing by Confocal Microscopy
Based on published literature, the NGF-β signal sequence directs the polypeptide into the regulated secretory pathway (RSP). In TGN the polypeptide would be cleaved by furin, cathepsin L, and carboxypeptidases to release SHG but retain mRFPKDEL, via interaction with the KDEL receptors (3,43).
Confocal imaging was used to visualize the separation of the SHG-mRFPKDEL polypeptide into SHG and mRFPKDEL. The 293T cells, which have flat morphology, were used for this evaluation. Following transduction with “red SHG lentivirus,” cells were fixed and stained for SHG (43). Upon imaging by confocal microscopy diffuse perinuclear yellow fluorescence and punctate red and green fluorescence could be observed (Fig. 3b). Based on previous experiments the sole source of red fluorescence is mRFPKDEL, while that for green fluorescence is Alexa 488 anti-rabbit antibody that is bound to anti-SHG antibody. The yellow fluorescence results from combined red and green fluorescent signals. The presence of diffuse yellow-orange fluorescence indicates freshly synthesized, as yet unprocessed, SHG-mRFPKDEL polypeptide (asterisk). The location of diffuse yellow-orange fluorescence is consistent with the location of endoplasmic reticulum and Golgi at the base of the nucleus. A graphic representation of fluorescence intensities along a line (white line drawn left to right) across the image was used to visualize processing of “SHG-mRFPKDEL” polypeptide. Care was taken to avoid imaging artifacts: (i) the fluorescence signals were acquired by setting exposure at subsaturating levels, (ii) as an internal control, the line was drawn outside the cell (between 35 and 50 μm from start) and the fluorescence signals in that region are very low and correspond to background. An asterisk in the image marks the diffuse yellow fluorescence region that arises due to overlapping red (mRFPKDEL) and green (SHG) signals (0–20 μm from start in the profile, lower panel). The cluster of red puncta (at ~25 μm) corresponds to multiple mRFPKDEL “red-only” peaks. A single green peak (at 30 μm from start) corresponds to “SHG-only” signal. These distinct red and green peaks correspond to processed peptide (i.e., “cellular label” mRFPKDEL and SHG). Similar results were obtained in PC12 cells (Fig. 3c) and two different types of human bone marrow stromal cells (Fig. 3d, e).
Secretion of SHG From Transduced Cells
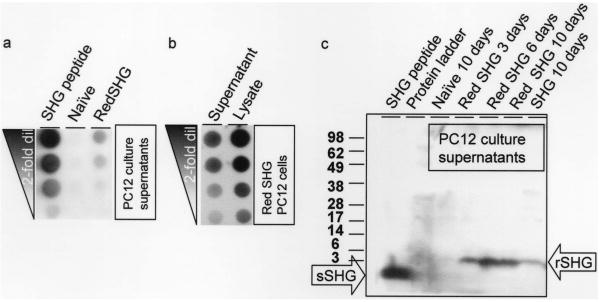
The above data suggest that SHG-mRFPKDEL was properly processed and mRFPKDEL appropriately localized; the following experiments address the fate of secretable peptide SHG. To assay secretion of SHG, culture supernatants of naive PC12 cells and “red SHG” lentivirus transduced PC12 cells were analyzed using dot blotting. Assays are shown from PC12 cells that had been maintained for 17 months in vitro in order to confirm stable expression of the transgene. The dot-blotted membranes were probed with anti-SHG antibody and developed using standard protocols. Only the “red SHG” lentivirus transduced PC12 supernatants contained SHG (Fig. 4a). Further, to test how much of total cellular encoded SHG is secreted, we compared the SHG content released into the supernatants with cell lysates. Densitometric scanning of dot blots revealed that ~42% of the intracellular SHG is secreted into the supernatant over a 3-day period (Fig. 4b). In order to verify that dot blotting was detecting authentic SHG peptides, the supernatants were collected, analyzed on a gradient Tris-tricine SDS-PAGE, and blotted (Fig. 4c). The blots probed with anti-SHG antibody showed a specific band in the expected range. The synthetic peptide SHG (~2 kDa) can be seen in first lane. The SHG band is absent in lanes containing the protein ladder (second lane) and naive PC12 culture supernatants (third lane). The SHG band of slightly higher molecular weight (~3 kDa) can be seen to increase in the “red SHG” PC12 culture supernatants over various collection times (3, 6, and 10 days). A band of similar size was seen from PC12 cell cultures that had been transduced to express SHG alone (Fig. 4c, last lane). Blots were stripped and reprobed with anti-mRFP antibody. No signal corresponding to mRFPKDEL could be detected (data not shown).
Figure 4.
(a) Dot blot analysis of culture supernatants indicates that SHG can be detected specifically in the red SHG transduced PC12 culture supernatants but not naive PC12 culture supernatants. SHG peptide (SHG) is the positive control. (b) Comparison of SHG content in red SHG transduced PC12 culture supernatant cell lysate (7) suggests that 42% of the total SHG synthesized in PC12 is secreted. (c) Immunoblot of culture supernatants collected from cells as indicated above the image were probed with anti-SHG antibody. Molecular weights are shown in kDa next to the blot. A strong band (~2 kDa) is seen in the chemically synthetsized SHG peptide lane (sSHG arrow). No bands are seen in the negative controls (lanes containing protein ladder and culture supernatant from naive PC12 cells). A recombinant SHG of apparent molecular weight ~3 kDa (rSHG arrow) is seen in red SHG-expressing cells at 3–10 days. A band of similar size is seen PC12 cells that express only SHG.
Biological Activity of Recombinant SHG From Transplanted Cells
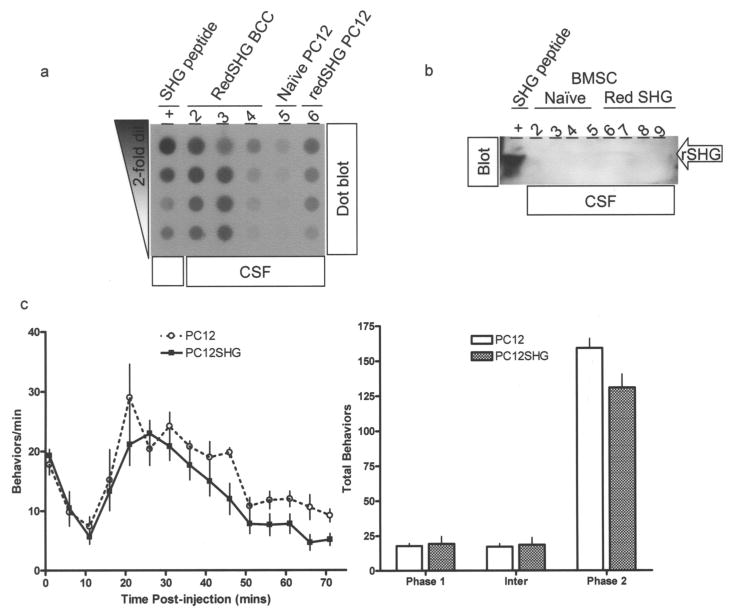
Cerebrospinal fluids from rats that received SHG secreting or control PC12 cells (500,000/rat) were analyzed by dot blots and Western blots. SHG was detected in the CSF from animals that received SHG-secreting cells (Fig. 5, upper panel lane 6), compared with control PC12 cell-transplanted animals (lane 5). Examples from similarly transduced bovine chromaffin cells (RedSHG BCC) are also shown (lanes 2–4). These have not been evaluated yet in behavioral models due to insufficient quantities obtained thus far. Western blots confirmed the ~2 kDa SHG band as the source of the signal in dot blots (Fig. 5a, lower panel). Taken together these results suggest that the recombinant DNA encoded SHG is secreted specifically by cells transduced with either SHG or “red SHG” lentiviruses. The recombinant SHG secreted by PC12 cells seems to be of slightly higher molecular weight than the synthetic SHG.
Figure 5.
Cerebrospinal fluid (CSF) samples collected from rats that received transplants as indicated were analyzed by dot blot (a) or Western blot (b). Samples are indicated above the image; in (b) arrow indicates the position of the SHG in CSF. (c) Effects of intrathecal (IT) transplants of PC12 cells on formalin-induced pain behaviors over time (left) and on total behaviors during phase 1 (0–1 min); interphase (6–11 min), and phase 2 (16–70 min) (right). Modest suppression of formalin-induced behaviors during phase 2 by red SHG PC12 cells compared to naive PC12 cells can be seen. No behavioral effect is seen in phase 1 or interphase. Data are expressed as mean ±SEM (n = 5–6/treatment group). p < 0.05.
To test if the secreted recombinant SHG possessed biological activity from transplanted cells, rats with either naive PC12 cells or “red SHG” transduced PC12 cell transplants were tested using a standard formalin test. Briefly, the animals receive a formalin injection in the hind paw, and rate of paw flinching indicate extent and duration of pain (12). The tonic phase (phase 2) is thought to be initiated by activation of NMDA receptors as it is suppressed by NMDA antagonists including chemically synthesized SHG (17,35). Compared to the naive group, the “red SHG” group showed a modest re- duction in hind paw flinching in response to formalin injection during phase 2 (p < 0.05) (Fig. 5b, c).
DISCUSSION
The study was an initial attempt to produce transplantable cells that can retain genetic labeling and the ability to secrete novel potential analgesic peptides such as SHG, a peptide that is not naturally occurring. Several recombinant DNA strategies are available to produce expression of two transgenes. Among these, dual promoter-based vectors, bicistronic vectors with viral internal ribosomal entry sites (IRES), and self-cleaving peptide-based vectors have been used (2,22,27,38). Due to the limited capacity of lentiviral vectors, use of dual promoter vectors is a viable option only when transgenes are short. The IRES-based system has many drawbacks when used in a lentiviral context. IRES-driven second gene expression in a lentiviral construct often fails or is poor; variables such as gene composition and arrangement need to be optimized (6,20,37,44,45). The self-cleaving peptide mechanism is not well understood, and it is not possible to predict whether certain post-translational modifications required for analgesic peptide biological activity would be negatively impacted (14,38).
In initial studies in our laboratory, we found a significant reduction of IRES-driven EGFP expression within weeks of lentiviral transduction (not shown), rendering this approach unusable for long-term expression studies. Fluorescent protein fusions are often used to monitor gene expression. However, tagging SHG (a 14-amino acid peptide) with a bulky fluorescent protein (~300 amino acids), or even the smallest labels such as hexahistidine tag (19), could interfere with SHG biological function and activity. Thus, we sought a novel approach to overcome these drawbacks, and SHG and the “cellular label” were designed to be synthesized as a single polypeptide, in a similar fashion to endogenous preproenkephalin, which is synthesized as a single polypeptide and enzymatically processed into bioactive peptide enkephalins. Our construct contains a ppNGF-β signal sequence followed by a furin cleavage site RSKR, SHG cDNA, a cathepsin L cleavage site (dipeptide lysine and arginine, KR), and KDEL-tagged mRFP. The recombinant protein would be cotranslationally inserted into ER via ppNGF-β signal sequence. Despite being KDEL tagged, it is not expected to be retrieved by Erd2 until it reaches the acidic environment in trans-Golgi. Our data indicate that in such acidic environments, the three activities of furin, cathepsin L, and Erd2 process polypeptide into secretable SHG and intracellular mRFPKDEL (30). Furin and KDEL receptors are known to colocalize (9), and KDEL receptors retrieve KDEL-terminated proteins in acidic pH all the way from TGN (16,26). Furin cleaves only in the acidic environment of TGN. Following cleavage from signal peptide in TGN, mRFPKDEL is retrieved from TGN by the KDEL receptors.
Some KDEL-terminated proteins are found on the cell surface also (4,42). KDEL-terminated proteins such as viral, bacterial, and plant toxins enter cells in a two-step process: (a) endocytosis followed by (b) KDEL receptor-mediated shuttling from TGN to ER is well documented (36). This route has been exploited to transport even KDEL-tagged oligonucleotides into the ER (31). This may be a mechanism by which cells could take up any escaped mRFPKDEL from extracellular space. The addition of KDEL at the carboxy-terminus of several soluble proteins, including GFP and mRFP, has been shown to be sufficient for their retention in the endoplasmic reticulum (1,5,28).
Consistent with previous work showing that the CMV promoter-driven signal peptide containing RFPKDEL was not localized to endosomes, lysosomes, or mitochondria (1,5), our results indicated mRFPKDEL in small structures scattered throughout the cytoplasm: vesicular subcompartment of ER, but not in the nucleus or mitochondria. The nitrogen cavitation data unequivocally showed that mRFPKDEL was vesicular. Confocal and biochemical analysis of a variety of red SHG-expressing cells suggested that the polypeptide was synthesized initially as a fusion protein as designed, and subsequently processed into cellular label and secretable SHG.
Findings from the current study indicated that the SHG peptide made by PC12 cells was of higher molecular weight than synthetic SHG. The increase could not be due to unprocessed fusion protein ppNGF-β-SHG-mRFPLKDEL (~40 kDa) or SUMOylation (~15 kDa), but could be due to persistence of residual amino acids or other posttranslational modifications of the peptide, and will be addressed in future studies.
Regardless of possible modifications, preliminary findings suggest that the secreted transgene can produce biological activity from cells transplanted into the spinal subarachnoid space, similar to intrathecally injected SHG. The formalin test phase 2 is thought to be mediated by NMDA-dependent mechanisms, and is reduced in response to administration of NMDA antagonists including synthetic SHG. Consistent with those results, the recombinant SHG-producing PC12 transplants produced a modest reduction in phase 2 flinching compared with naive PC12 cells. These modest effects may be due to incomplete processing or insufficient levels of other potentially synergistic antinociceptive agents produced by this cell type compared with normal chromaffin cells. Compared to the culture supernatants, the amount of peptide SHG in CSF is low due to lower number of transplanted cells.
In summary, these findings are an initial step in the generation of genetically labeled transplantable cells for delivery of novel analgesic molecules. Taken together, these data suggest that genetic modification of cells to secrete biologically active analgesic peptides, while retaining identification markers, is a viable approach for transplantation. In addition, the simplicity of the design of the interchangeable construct should allow for substitution and insertion of other potent natural or synthetic analgesic peptides or combinations that can be tailored for the treatment of chronic pain of different etiologies.
Acknowledgments
The authors would like to thank Beata Frydel, Imaging Core, Miami Project to Cure Paralysis, Jian Huang, Lyudmila Rusakova, Linda White, Diaz Francisca, and Vempati Uma, Miami Project to Cure Paralysis, and David Vasquez of Miami Veterans Affairs. Supported by NS51669 and Paralyzed Veterans of America #2340.
References
- 1.Altan-Bonnet N, Sougrat R, Liu W, Snapp EL, Ward T, Lippincott-Schwartz J. Golgi inheritance in mammalian cells is mediated through endoplasmic reticulum export activities. Mol Biol Cell. 2006;17(2):990–1005. doi: 10.1091/mbc.E05-02-0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amendola M, Venneri MA, Biffi A, Vigna E, Naldini L. Coordinate dual-gene transgenesis by lentiviral vectors carrying synthetic bidirectional promoters. Nat Biotechnol. 2005;23(1):108–116. doi: 10.1038/nbt1049. [DOI] [PubMed] [Google Scholar]
- 3.Anderson ED, VanSlyke JK, Thulin CD, Jean F, Thomas G. Activation of the furin endoprotease is a multiple-step process: Requirements for acidification and internal propeptide cleavage. EMBO J. 1997;16(7):1508–1518. doi: 10.1093/emboj/16.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arosa FA, de Jesus O, Porto G, Carmo AM, de Sousa M. Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex class I molecules. J Biol Chem. 1999;274(24):16917–16922. doi: 10.1074/jbc.274.24.16917. [DOI] [PubMed] [Google Scholar]
- 5.Bannai H, Inoue T, Nakayama T, Hattori M, Mikoshiba K. Kinesin dependent, rapid, bi-directional transport of ER sub-compartment in dendrites of hippocampal neurons. J Cell Sci. 2004;117(Pt 2):163–175. doi: 10.1242/jcs.00854. [DOI] [PubMed] [Google Scholar]
- 6.Bochkov YA, Palmenberg AC. Translational efficiency of EMCV IRES in bicistronic vectors is dependent upon IRES sequence and gene location. Biotechniques. 2006;41(3):283–284. 286–288. doi: 10.2144/000112243. [DOI] [PubMed] [Google Scholar]
- 7.Buchser E, Goddard M, Heyd B, Joseph JM, Favre J, de Tribolet N, Lysaght M, Aebischer P. Immunoisolated xenogenic chromaffin cell therapy for chronic pain. Initial clinical experience. Anesthesiology. 85(5):1005–1012. doi: 10.1097/00000542-199611000-00007. discussion 1029A–1030A; 1996. [DOI] [PubMed] [Google Scholar]
- 8.Campbell RE, Tour O, Palmer AE, Steinbach PA, Baird GS, Zacharias DA, Tsien RY. A monomeric red fluorescent protein. Proc Natl Acad Sci USA. 2002;99(12):7877–7882. doi: 10.1073/pnas.082243699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole NB, Ellenberg J, Song J, DiEuliis D, Lippincott-Schwartz J. Retrograde transport of Golgi-localized proteins to the ER. J Cell Biol. 1998;140(1):1–15. doi: 10.1083/jcb.140.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’Ippolito G, Diabira S, Howard GA, Menei P, Roos BA, Schiller PC. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117(Pt 14):2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 11.Dowben RM, Gaffey A, Lynch PM. Isolation of liver and muscle polyribosomes in high yield after cell disruption by nitrogen cavitation. FEBS Lett. 1968;2(1):1–3. doi: 10.1016/0014-5793(68)80083-3. [DOI] [PubMed] [Google Scholar]
- 12.Dubuisson D, Dennis SG. The formalin test: A quantitative study of the analgesic effects of morphine, meperidine, and brain stem stimulation in rats and cats. Pain. 1977;4(2):161–174. doi: 10.1016/0304-3959(77)90130-0. [DOI] [PubMed] [Google Scholar]
- 13.Duplan H, Li RY, Vue C, Zhou H, Emorine L, Herman JP, Tafani M, Lazorthes Y, Eaton MJ. Grafts of immortalized chromaffin cells bio-engineered to improve met-enkephalin release also reduce formalin-evoked c-fos expression in rat spinal cord. Neurosci Lett. 2004;370(1):1–6. doi: 10.1016/j.neulet.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 14.Furler S, Paterna JC, Weibel M, Bueler H. Recombinant AAV vectors containing the foot and mouth disease virus 2A sequence confer efficient bicistronic gene expression in cultured cells and rat substantia nigra neurons. Gene Ther. 2001;8(11):864–873. doi: 10.1038/sj.gt.3301469. [DOI] [PubMed] [Google Scholar]
- 15.Gottlieb RA, Adachi S. Nitrogen cavitation for cell disruption to obtain mitochondria from cultured cells. Methods Enzymol. 2000;322:213–221. doi: 10.1016/s0076-6879(00)22022-3. [DOI] [PubMed] [Google Scholar]
- 16.Griffiths G, Ericsson M, Krijnse-Locker J, Nilsson T, Goud B, Soling HD, Tang BL, Wong SH, Hong W. Localization of the Lys, Asp, Glu, Leu tetrapeptide receptor to the Golgi complex and the intermediate compartment in mammalian cells. J Cell Biol. 1994;127(6 Pt 1):1557–1574. doi: 10.1083/jcb.127.6.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hama A, Basler A, Sagen J. Enhancement of morphine antinociception with the peptide N-methyl-D-aspartate receptor antagonist [Ser1]-histogranin in the rat formalin test. Brain Res. 2006;1095(1):59–64. doi: 10.1016/j.brainres.2006.04.012. [DOI] [PubMed] [Google Scholar]
- 18.Hama AT, Siegan JB, Herzberg U, Sagen J. NMDA-induced spinal hypersensitivity is reduced by naturally derived peptide analog [Ser1]histogranin. Pharmacol Biochem Behav. 1999;62(1):67–74. doi: 10.1016/s0091-3057(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 19.Hauser CT, Tsien RY. A hexahistidine-Zn2+-dye label reveals STIM1 surface exposure. Proc Natl Acad Sci USA. 2007;104(10):3693–3697. doi: 10.1073/pnas.0611713104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hennecke M, Kwissa M, Metzger K, Oumard A, Kroger A, Schirmbeck R, Reimann J, Hauser H. Composition and arrangement of genes define the strength of IRES-driven translation in bicistronic mRNAs. Nucleic Acids Res. 2001;29(16):3327–3334. doi: 10.1093/nar/29.16.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hentall ID, Hargraves WA, Sagen J. Inhibition by the chromaffin cell-derived peptide serine-histogranin in the rat’s dorsal horn. Neurosci Lett. 2007;419:88–92. doi: 10.1016/j.neulet.2007.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Houdebine LM, Attal J. Internal ribosome entry sites (IRESs): Reality and use. Transgenic Res. 1999;8(3):157–177. doi: 10.1023/a:1008909908180. [DOI] [PubMed] [Google Scholar]
- 23.Lemaire S, Shukla VK, Rogers C, Ibrahim IH, Lapierre C, Parent P, Dumont M. Isolation and characterization of histogranin, a natural peptide with NMDA receptor antagonist activity. Eur J Pharmacol. 1993;245(3):247–256. doi: 10.1016/0922-4106(93)90104-h. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz H, Hailey DW, Lippincott-Schwartz J. Fluorescence protease protection of GFP chimeras to reveal protein topology and subcellular localization. Nat Methods. 2006;3(3):205–210. doi: 10.1038/nmeth857. [DOI] [PubMed] [Google Scholar]
- 25.Martin-Schild S, Zadina JE, Gerall AA, Vigh S, Kastin AJ. Localization of endomorphin-2-like immuno-reactivity in the rat medulla and spinal cord. Peptides. 1997;18(10):1641–1649. doi: 10.1016/s0196-9781(97)00320-3. [DOI] [PubMed] [Google Scholar]
- 26.Miesenbock G, Rothman JE. The capacity to retrieve escaped ER proteins extends to the trans-most cisterna of the Golgi stack. J Cell Biol. 1995;129(2):309–319. doi: 10.1083/jcb.129.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizuguchi H, Xu Z, Ishii-Watabe A, Uchida E, Hayakawa T. IRES-dependent second gene expression is significantly lower than cap-dependent first gene expression in a bicistronic vector. Mol Ther. 2000;1(4):376–382. doi: 10.1006/mthe.2000.0050. [DOI] [PubMed] [Google Scholar]
- 28.Munro S, Pelham HR. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- 29.NasiriNezhad F, Sagen J. NMDA antagonist peptide supplementation enhances pain alleviation by adrenal medullary transplants. Cell Transplant. 2005;14(4):203–211. doi: 10.3727/000000005783983115. [DOI] [PubMed] [Google Scholar]
- 30.Njus D, Kelley PM, Harnadek GJ. Bioenergetics of secretory vesicles. Biochim Biophys Acta. 1986;853(3–4):237–265. doi: 10.1016/0304-4173(87)90003-6. [DOI] [PubMed] [Google Scholar]
- 31.Pichon C, Arar K, Stewart AJ, Dodon MD, Gazzolo L, Courtoy PJ, Mayer R, Monsigny M, Roche AC. Intracellular routing and inhibitory activity of oligo-nucleopeptides containing a KDEL motif. Mol Pharmacol. 1997;51(3):431–438. [PubMed] [Google Scholar]
- 32.Sagen J, Castellanos DA, Gajavelli S. Transplants for chronic pain. In: Halberstadt C, Emerich D, editors. Cellular transplantation from laboratory to clinic. New York: Elsevier; 2007. pp. 455–475. [Google Scholar]
- 33.Schroers R, Davis CM, Wagner HJ, Chen SY. Lentiviral transduction of human T-lymphocytes with a RANTES intrakine inhibits human immunodeficiency virus type 1 infection. Gene Ther. 2002;9(13):889–897. doi: 10.1038/sj.gt.3301711. [DOI] [PubMed] [Google Scholar]
- 34.Shukla VK, Lemaire S, Dumont M, Merali Z. N-methyl-D-aspartate receptor antagonist activity and phencyclidine-like behavioral effects of the pentadecapeptide, [Ser1]histogranin. Pharmacol Biochem Behav. 1995;50(1):49–54. doi: 10.1016/0091-3057(94)00247-g. [DOI] [PubMed] [Google Scholar]
- 35.Siegan JB, Sagen J. A natural peptide with NMDA inhibitory activity reduces tonic pain in the formalin model. Neuroreport. 1997;8(6):1379–1381. doi: 10.1097/00001756-199704140-00012. [DOI] [PubMed] [Google Scholar]
- 36.Spooner RA, Smith DC, Easton AJ, Roberts LM, Lord JM. Retrograde transport pathways utilised by viruses and protein toxins. Virol J. 2006;3:26. doi: 10.1186/1743-422X-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugaya I, Qu T, Sugaya K, Pappas GD. Genetically engineered human mesenchymal stem cells produce met-enkephalin at augmented higher levels in vitro. Cell Transplant. 2006;15(3):225–230. doi: 10.3727/000000006783981981. [DOI] [PubMed] [Google Scholar]
- 38.Szymczak AL, Workman CJ, Wang Y, Vignali KM, Dilioglou S, Vanin EF, Vignali DA. Correction of multigene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat Biotechnol. 2004;22(5):589–594. doi: 10.1038/nbt957. [DOI] [PubMed] [Google Scholar]
- 39.Tang BL, Wong SH, Qi XL, Low SH, Hong W. Molecular cloning, characterization, subcellular localization and dynamics of p23, the mammalian KDEL receptor. J Cell Biol. 1993;120(2):325–338. doi: 10.1083/jcb.120.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vempati UD, Diaz F, Barrientos A, Narisawa S, Mian AM, Millan JL, Boise LH, Moraes CT. Role of cytochrome C in apoptosis: Increased sensitivity to tumor necrosis factor alpha is associated with respiratory defects but not with lack of cytochrome C release. Mol Cell Biol. 2007;27(5):1771–1783. doi: 10.1128/MCB.00287-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Huso DL, Harrington J, Kellner J, Jeong DK, Turney J, McNiece IK. Outgrowth of a transformed cell population derived from normal human BM mesenchymal stem cell culture. Cytotherapy. 2005;7(6):509–519. doi: 10.1080/14653240500363216. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K, Fujii R, Toyofuku Y, Saito T, Koseki H, Hsu VW, Aoe T. The KDEL receptor mediates a retrieval mechanism that contributes to quality control at the endoplasmic reticulum. EMBO J. 2001;20(12):3082–3091. doi: 10.1093/emboj/20.12.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yasothornsrikul S, Greenbaum D, Medzihradszky KF, Toneff T, Bundey R, Miller R, Schilling B, Petermann I, Dehnert J, Logvinova A, Goldsmith P, Neveu JM, Lane WS, Gibson B, Reinheckel T, Peters C, Bogyo M, Hook V. Cathepsin L in secretory vesicles functions as a prohormone-processing enzyme for production of the enkephalin peptide neurotransmitter. Proc Natl Acad Sci USA. 2003;100(16):9590–9595. doi: 10.1073/pnas.1531542100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu X, Zhan X, D’Costa J, Tanavde VM, Ye Z, Peng T, Malehorn MT, Yang X, Civin CI, Cheng L. Lentiviral vectors with two independent internal promoters transfer high-level expression of multiple transgenes to human hematopoietic stem-progenitor cells. Mol Ther. 2003;7(6):827–838. doi: 10.1016/s1525-0016(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 45.Zhu Y, Feuer G, Day SL, Wrzesinski S, Planelles V. Multigene lentiviral vectors based on differential splicing and translational control. Mol Ther. 2001;4(4):375–382. doi: 10.1006/mthe.2001.0469. [DOI] [PubMed] [Google Scholar]