Abstract
Background
Heart disease is a leading cause of mortality throughout the world. Tissue damage from vascular occlusive events results in the replacement of contractile myocardium by nonfunctional scar tissue. The potential of new technologies to regenerate damaged myocardium is significant, although cell-based therapies must overcome several technical barriers. One possible cell-independent alternative is the direct administration of small proteins to damaged myocardium.
Methods and Results
Here we show that the secreted signaling protein stromal cell-derived factor-1 alpha (SDF-1α), which activates the cell-survival factor protein kinase B (PKB/Akt) via the G-protein-coupled receptor CXCR4, protected tissue after an acute ischemic event in mice and activated Akt within endothelial cells and myocytes of the heart. Significantly better cardiac function than in control mice was evident as early as 24 hours post-infarction as well as at 3, 14 and 28 days post-infarction. Prolonged survival of hypoxic myocardium was followed by an increase in levels of vascular endothelial growth factor (VEGF) protein and neo-angiogenesis. Consistent with improved cardiac function, mice exposed to SDF-1α demonstrated significantly decreased scar formation than control mice.
Conclusions
These findings suggest that SDF-1α may serve a tissue-protective and regenerative role for solid organs suffering a hypoxic insult.
Keywords: myocardial infarction, ischemia, apoptosis, angiogenesis
Introduction
Heart disease is the number one killer of adults in the industrialized world. The majority of acquired heart disease is due to coronary artery disease (CAD), in which blood flow to an area of the heart is reduced or eliminated, resulting in death of myocardium and replacement with nonfunctional scar tissue1, 2. Fatal outcomes are common for individuals suffering acute occlusion of a coronary artery, typically within the first 24 hours.
Hypoxic cardiac tissue post-infarction can be broadly divided into three distinct zones. The direct area of ischemia that has total loss of blood supply sustains largely irreversible cell death and scar tissue formation. The myocardium immediately surrounding the infarct zone is less severely affected but remains hypoxic. In some cases, cellular changes occur in this area that decrease energy utilization and promote cell survival. This “hibernating myocardium” may eventually recover if neo-angiogenesis or redirection of blood flow restores supply of oxygen and energy substrates3, 4. Finally, the remaining myocardium typically remains well oxygenated and initially free of damage. The expansion of cell death is a key feature of myocardial infarction as partially ischemic regions of the heart ultimately succumb to hypoxia and are also replaced by scar tissue.
Efficient methods to limit initial loss of myocardium and subsequent expansion of the infarct in the acute period could be of significant value. In fact, overexpression of the survival kinase Akt (protein kinase B) in mesenchymal stem cells injected into mouse hearts post-infarction resulted in a decrease in infarct size5, possibly as a result of secreted factors from the cells introduced into the heart. Subsequently, work from our laboratory demonstrated that the 43—amino acid protein thymosin β4 activates Akt via integrin linked kinase (ILK) and dramatically protects bordering myocardium from cell death in the first 24 hours after coronary occlusion6. Given the efficacy of this small protein in our experimental model and the possibility of bypassing hurdles associated with stem cell administration, we investigated the potential for other proteins that activate Akt and have angiogenic properties similar to thymosin β4 to provide beneficial effects post-infarction.
The secreted chemokine stromal cell-derived factor-1 alpha (SDF-1α) and its G-protein-coupled receptor CXCR4 have been implicated in cardiogenesis. Signaling downstream of CXCR4 can trigger a chemotactic response resulting in migration towards an increasing SDF-1α gradient7-10. In addition, in some cell types, CXCR4 signaling can result in activation of Akt and stimulation of cell proliferation, survival, and angiogenesis11-17.
Based on these characteristics of SDF-1α and the observation that this chemokine is upregulated post-infarction18, we investigated the potential of SDF-1α to improve cardiac function and damage in murine hearts immediately after coronary occlusion. We found that SDF-1α protected cardiomyocytes from cell death within the first 72 hours after hypoxic insult and also resulted in increased angiogenesis in the area of risk within 72 hours. SDF-1α induced Akt phosphorylation and upregulated VEGF protein after coronary occlusion in vivo. In vitro, SDF-1 activated Akt in cardiomyocytes and endothelial cells, and upregulated VEGF only in endothelial cells. These findings indicate that SDF-1α may affect multiple cell types in vivo to protect and repair the heart.
Methods
Animals and Surgical Procedures
Myocardial infarction was produced in a total of 49 male C57BL/6J mice at 16 weeks of age (25–30 g) by ligation of the left anterior descending coronary artery with a 8-0 prolene suture, similar to that described19. The anesthetic agent used was 5% isoflurane with 2.5 l/m O2 for 20 seconds followed by 2% isoflurane and O2. Half were treated with PBS, and half with SDF-1α by intracardiac injection. Subsequent echocardiography and other experimental analysis were conducted at various combinations of 1, 3, 14, and 28 days post-infarction. Of 49 mice, 45 survived the surgery and PBS/SDF-1α administration. Four mice received both SDF-1α and thymosin β4. For all mice, two intracardiac injections were administered into myocardium adjacent to the ischemic zone at the time of ligation. Each injection was 5 μl of one of the following in a collagen base: PBS; recombinant (amino acids 22–89) mature mouse SDF-1α protein (R & D Systems, catalog # 460-SD-010) at 60 ng/μl; synthetic thymosin β4 at 40 ng/μl; and SDF-1α and thymosin β4 at 60 ng/μl and 40 ng/μl, respectively.
Analysis of Cardiac Function by Echocardiography
Echocardiograms to assess systolic function were performed using M-mode and two-dimensional measurements as described19 and were analyzed for FS and EF, respectively. The measurements represented the average of six selected cardiac cycles from at least two separate scans performed in random-blind fashion with papillary muscles as a point of reference for consistency in level of scan. End diastole was defined as the maximal left ventricle (LV) diastolic dimension, and end systole was defined as the peak of posterior wall motion. FS, a surrogate of systolic function, was calculated from LV dimensions as follows: FS = EDD - ESD / EDD × 100%. EF was calculated from two-dimensional images. Data were analyzed by repeated measure procedures. All means and standard deviations represent 95% confidence intervals.
Protein Isolation and Analysis
Protein was isolated from homogenized heart tissue with Trizol Reagent (Invitrogen # 15596-018) and standard Invitrogen protocols. The Bradford assay (Bio-Rad) was used to quantitate protein concentrations. Protein was then used for western blotting with primary antibodies against ILK, Akt1, Akt-P, VEGF, and GAPDH (all from Santa Cruz Biotechnology).
Primary Cell Culture and Protein Analysis
Ventricular cardiomyocytes were isolated from adult male C57Bl/6 mice (19 to 25 g; Charles River) by collagenase type II (Worthington) dissociation as previously described20. Human cardiac fibroblasts were purchased from ScienCell Research Laboratories. Human cardiac-derived microvascular endothelial cells (MVEC) were purchased from Lonza. HL-1 atrial cardiomyocytes were a gift from Dr. W.C. Claycomb, Louisiana State University Medical Center. All cell types were serum and growth factor starved for 16 hours and then treated with 500 ng/ml SDF-1α for 30 min. Cell lysates were collected for western blotting with primary antibodies against Akt1 (Santa Cruz Biotechnology) and Akt1/2/3-P (Cell Signaling). For VEGF expression analysis, serum-starved MVECs or HL-1 cells were treated with 50 ng/ml of SDF-1α for varying times indicated and western blotting was performed with primary antibodies against VEGF and GAPDH (Santa Cruz Biotechnology).
Calculation of Scar Circumference
Scar circumference was calculated from six trichrome-stained sections through the heart of each mouse using Openlab 3.03 software (Improvision) similar to described21. Percent circumference of collagen deposition was measured on each section in blinded fashion and averaged for each mouse. Analyses were done at 42 and 63 days for PBS- or SDF-1α-treated hearts.
Immunohistochemistry
Adult cardiac tissue was processed for immunolabeling experiments by one of three different methods. Frozen tissue after fixation with 4% paraformaldehyde or 10% formalin followed by 10% and 30% sucrose gradients was used for cryosectioning. Other hearts were embedded in paraffin after fixation in 4% paraformaldehyde or 10% formalin. A third group of hearts were fixed in methyl carnoy (10% glacial acetic acid, 60% methanol, 30% chloroform) followed by 70% ethanol storage until embedding. Adult hearts were sectioned through at least 10 equivalent levels from the base of the heart to the apex. Serial sections were used for trichrome staining and reaction with various antibodies, and cryosections were used for immunofluorescence. Apoptosis in adult hearts was assayed using ProMega’s DeadEnd Fluorometric TUNEL System (#G3250), cell proliferation with antibody against phosphohistone H3B (Upstate Biotechnology, # 06-570), angiogenesis with vWF (Dako # A 0082), hematopoietic stem cells with c-kit (Santa Cruz, # sc-168), and muscle actin with HHF35 (Dako # M0635). Paraffin-embedded sections were used for hematoxylin & eosin (H&E), trichrome staining for scar tissue, and PECAM staining (BD Biosciences, # 550274) for angiogenesis. Methyl carnoy fixed sections were used for staining with isolectin B4 (Vector Laboratories, # B-1205) for angiogenesis. Quantitation for apoptosis and angiogenesis was done by counting the number of cells and vessels, respectively, per high magnification field of view with six random fields near the area of infarction assayed for each mouse.
Statistical Analyses
Statistical calculations were performed using t-test of variables (two-sample t-test assuming unequal variances) with 95% confidence intervals.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
SDF-1α Preserves Cardiac Function After Infarction
To test the hypothesis that SDF-1α protein could improve cardiac function post-infarction, we created ligations of the left anterior descending coronary artery in adult male mice to prevent blood flow to a portion of the left ventricle, creating a zone of injury. Injections of PBS or SDF-1α were administered into myocardium at two sites near the infarct zone. Mice were then subjected to echocardiography at various time points to measure cardiac function by assessment of fractional shortening (FS) and ejection fraction (EF). All studies and analyses of data were performed in blinded fashion.
We created myocardial infarctions in adult male mice and treated half with SDF-1α and half with PBS. At 14 days post-infarction, left ventricles of PBS-treated mice had a mean FS of 27.9 +/– 1.5% (n=9). SDF-1α treatment resulted in a mean FS of 38.1 +/– 1.5% (n=11; P < .0001). As a second measure of ventricular function, two-dimensional echocardiographic measurements revealed that the mean fraction of blood ejected from the left ventricle (EF) in PBS-treated mice was 35.0 +/– 7.9%, compared to a mean of 61.9 +/– 3.7% (P < .0001) in SDF-1α-treated mice. (Figure 1A and B). At 28 days after infarction, when additional ventricular remodeling has occurred and the scar is typically well formed, we observed a similar trend in cardiac function of SDF-1α-treated mice. FS was 26.8 +/– 1.2% (n=9) for the PBS group and 39.2 +/– 2.9% (n=11; P < .0001) for the SDF-1α group, while EF was 31.5 +/– 3.5% and 48.8 +/– 2.4% (P < .0001) for PBS and SDF-1α groups, respectively (Figure 1A and B). Cardiac function remained depressed relative to sham-operated animals (∼60% FS; ∼75% EF). The improvement at 28 days in FS or EF (46% and 55%, respectively) upon SDF-1α treatment corresponded to echocardiographic findings that the end diastolic dimensions (EDD) and end systolic dimensions (ESD) were both significantly smaller in the SDF-1α group, indicating that SDF-1α treatment had resulted in increased cardiac function and decreased cardiac dilation after infarction (Figure 1C). Finally, we found that SDF-1α administration in the absence of infarction did not lead to an increase in cardiac function (data not shown).
Figure 1.
SDF-1α treatment after coronary ligation improves myocardial function in vivo. (A) Distribution of left ventricular fractional shortening at 1, 3, 14, and 28 days after coronary ligation with or without SDF-1α treatment. Means and 95% confidence limits are shown for PBS- or SDF-1α-treated animals at each time point. *P < 0.0001 (B) Distribution of left ventricular ejection fraction at 1, 3, 14, and 28 days after coronary ligation with or without SDF-1α treatment. Means and 95% confidence limits are shown for PBS and SDF-1α-treated animals at each time point. *P < 0.0001 except at 3 days (P < 0.005). (C) Echocardiographic measurements at 28 days for administration of PBS (Control) or SDF-1α. Means and 95% confidence limits are shown. *P < 0.0001. EDD, end diastolic dimension; ESD, end systolic dimension.
Histological analysis revealed a marked reduction in the size of the scar tissue area and therefore a thicker functional anterior wall of the heart upon SDF-1α treatment (Figure 2). By 6 weeks post-infarction, the ratio of scar tissue circumferential length to left ventricle circumferential length in SDF-1α-treated animals was reduced by 56% from that seen in PBS-treated controls (P < .001). At 9 weeks post-infarction, the reduction of scar circumference in SDF-1α-treated hearts was 43% relative to controls (P < .001; Figure 2E). The functional improvement persisted in these animals corresponding to the scar improvement.
Figure 2.
SDF-1α reduces levels of scar tissue post-infarction. Representative trichrome staining of transverse heart sections 42 days after coronary ligation and PBS (A, B) or SDF-1α (C, D) treatment. Collagen in scar is indicated in blue. Higher magnifications of boxed areas illustrate that more underlying myocardium is present within scar tissue in SDF-1α-treated hearts (D) than in PBS-treated hearts. (B). (E) Quantification of scar circumference of hearts after coronary ligation taken from six sections per mouse given PBS (n=4) or given SDF-1α (n=5). Bars indicate 95% confidence limits. *P < 0.001. LV, left ventricle; RV, right ventricle.
The functional and histologic improvements observed with the single administration of SDF-1α immediately after coronary ligation suggested that the beneficial effects of SDF-1α may occur in the early stages following infarction. We therefore sought to determine the timeframe of functional improvement by performing echocardiography at numerous time points within days of the coronary ligation. Remarkably, as early as 1 day after infarction, we found that FS was 32.2 +/– 1.6% (n=8) with PBS treatment compared to 40.2 +/– 1.6% (n=8, P < 0.0001) with SDF-1α treatment; correspondingly, EF was 40.7 +/– 2.7% (n=8) or 56.6 +/– 3.7% (n=8, P < 0.0001), respectively. This pattern continued 3 days post-infarction as SDF-1α treated mice again demonstrated significant improvement in FS and EF (Figure 1A and B).
SDF-1α-mediated functional improvement occurred as early as 24 hours post-infarction and continued 3, 14, and 28 days post-infarction. We performed parallel experiments with thymosin β4 to investigate the comparative efficacy of SDF-1α and found that improvement of cardiac function after coronary ligation was similar with SDF-1α or thymosin β4. Interestingly, the combination of SDF-1α and thymosin β4 appeared to have no greater effect than either one alone, suggesting a lack of synergy (Supp. Figure 1). One potential explanation for this observation is that the beneficial effects may occur through similar downstream pathways or mechanisms that are already maximized.
SDF-1α Promotes Survival of Ischemic Myocardium
Our previous data with thymosin β4 suggest that it functions in a cardioprotective fashion within 24 hours after infarction, possibly followed by neoangiogenesis, rather than through recruitment or promotion of stem cell differentiation into cardiomyocytes. However, there are reports suggesting that SDF-1α can attract CXCR4-expressing hematopoietic stem cells to the heart, where they are assumed to take up residence and improve cardiac function22, 23. The mechanism by which the stem cells might improve function remains unclear. Whether stem cells differentiate into functional cardiomyocytes has been controversial, but recent studies have suggested that secreted signals arising from stem cells may somehow potentiate cardiac regeneration or repair24-26.
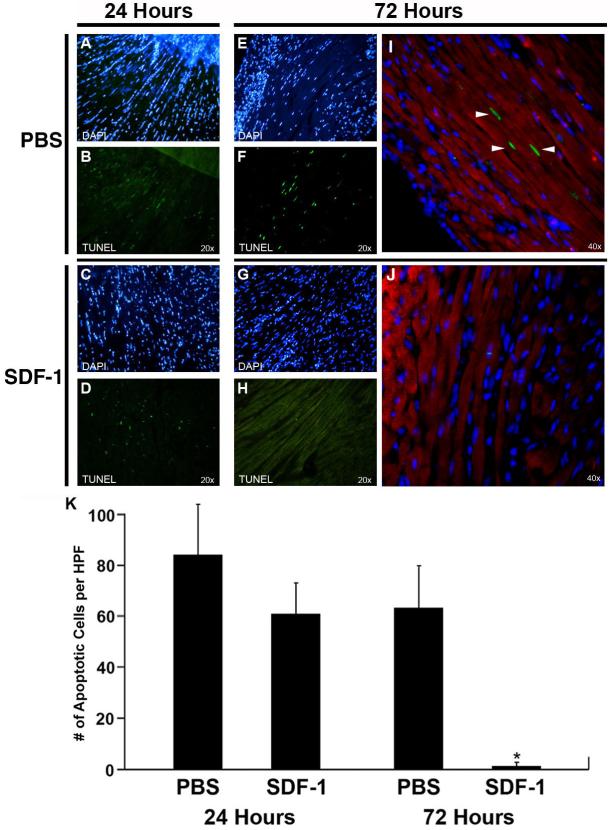
To investigate the mechanism by which SDF-1α induces cardiac repair, we examined the degree of cell death in the direct area of infarction and the neighboring area of hypoxic myocardium. Apoptotic cells marked by TUNEL assay were observed in both control and SDF-1α-treated hearts during the first 24 hours, and were largely isolated to the border zone located immediately adjacent to the area of infarct (Figure 3A–D). However, by 72 hours post-infarction, the apoptosis had spread outside of the immediate area of infarction to surrounding myocardial tissue in all directions in the control PBS-treated hearts. In contrast, the SDF-1α-treated hearts showed little or no apoptosis outside of the area of infarct (Figure 3E–H). Remote myocardium in both groups remained free of any significant apoptosis at these early time points. Co-staining with muscle actin confirmed that cells undergoing apoptosis were indeed myocytes (Figure 3I–J). Quantification revealed that at 24 hours post-infarction, 84.0 +/– 19.6 or 60.7 +/– 11.8 apoptotic myocytes per field of view were present in the immediately adjacent border myocardium of PBS- or SDF-1α-treated hearts, respectively; however, this difference was not statistically significant. But by 72 hours, a highly significant difference was observed with 63.3 +/– 16.4 or 1.3 +/– 1.5 apoptotic myocytes per field of view in the border myocardium of PBS-or SDF-1α-treated hearts, respectively (P < .0001; Figure 3K). Thus, bordering myocardium that is normally irreparably damaged post-infarction is protected by SDF-1α-directed cell survival.
Figure 3.
SDF-1α promotes cell survival post-infarction. TUNEL-positive cells (bright green) in bordering myocardium adjacent to the zone of infarct 24 hours after coronary ligation and PBS (A, B) or SDF-1 (C, D) treatment. TUNEL-positive cells in bordering myocardium were also evident 72 hours after coronary ligation in PBS (E, F) but not SDF-1α (G, H) treatment groups. The right column (I, J) illustrates higher magnification confocal microscopy of TUNEL-positive cells triple-labeled with anti-muscle actin antibody (red striations) to mark cardiomyocytes and DAPI (blue) to mark nuclei. Cardiomyocytes undergoing apoptosis (arrowheads) are present after PBS treatment (I) but absent after SDF-1α treatment (J). Note the reduced DAPI staining in the PBS-treated damaged tissue (I). (K) Quantitation of myocyte apoptosis from six sections per mouse 24 and 72 hours after coronary ligation and administration of PBS or SDF-1α. Bars indicate 95% confidence limits. *P < 0.0001.
SDF-1α Treatment After Myocardial Infarction Results in Increased Angiogenesis
While the cardioprotective effects of SDF-1α may aid in survival of hypoxic myocardium, the myocytes ultimately would need to be vascularized to achieve long-term survival. Hence, we investigated the degree of neo-angiogenesis in the presence of SDF-1α. An antibody to isolectin B4, a known marker of endothelial cells in the microvasculature, demonstrated a significant increase in the number of capillaries in the area of injury (border zone) in SDF-1α-treated hearts compared to PBS-treated hearts within 72 hours (Figure 4A–D). Quantification of the isolectin B4-positive capillaries revealed an approximately 93% increase in microvasculature over controls (Figure 4E). This observation was validated with two other endothelial markers, PECAM and vWF (data not shown). Increased capillary density was not observed in unaffected regions of the myocardium. It is difficult to establish, however, if the increased amount of vasculature bordering the area of infarction is a direct effect of SDF-1α administration, an indirect effect of greater initial cell survival leading to recruitment of new vasculature, or a combination of the two.
Figure 4.
SDF-1α treatment induces increased angiogenesis 72 hours post-infarction. Immunohistochemistry using isolectin B4 antibody staining to mark endothelial cells demonstrated a higher density of microvasculature in bordering myocardium close to the zone of injury after SDF-1α treatment (C, D) than after PBS treatment (A, B). B and D represent high magnification of A and C, respectively. (E) Quantitation of vessel density gleaned from six sections per mouse 72 hours after coronary ligation and subsequent administration of PBS or SDF-1α. Bars indicate 95% confidence limits. *P < 0.005.
SDF-1α Treatment Increases Akt Phosphorylation and Levels of VEGF Protein
Our previous observations of ILK and Akt activation upon thymosin β4 treatment as well as SDF-1α’s known effects on Akt led us to investigate the response of this pathway in infarcted hearts exposed to SDF-1α. In harvested heart cell lysates from the infarct area, we observed increased levels of ILK protein and increased phosphorylation of its downstream kinase Akt upon SDF-1α treatment, while no difference was seen in the amount of total Akt protein. These changes were observed within 24 hours after coronary ligation (data not shown) and were more prominent at 72 hours (Figure 5A). Vascular endothelial growth factor (VEGF), a known regulator of angiogenesis, was similarly upregulated at 72 hours in response to SDF-1α, consistent with the increase in capillary density described in Figure 4.
Figure 5.
SDF-1α induces Akt activation and upregulation of VEGF. (A) Western blots with heart lysates 72 hours after coronary ligation and treatment with PBS or SDF-1α. ILK, upstream of Akt, was upregulated. Levels of Akt overall did not change while antibody specific to phosphorylated, activated Akt at serine-473 revealed increased phosphorylation (Akt-P). VEGF was upregulated as well. (B) Cell-specific responses to SDF-1α treatment. Cultured human cardiac MVECs, human cardiac fibroblasts, HL-1 cells or primary adult mouse cardiomyocytes were serum-starved followed by a 30 min treatment with 500 ng/mL of SDF-1α. Western blotting was performed using antibodies to Akt-P and Akt. Densitometry analysis of Akt-P is indicated. To increase Akt-P signal, 40 ug total protein was loaded per well for Akt-P as compared to 15 ug total protein for the Akt lanes. (C) VEGF protein levels increased in cardiac MVECs within hours of SDF-1α treatment, but did not increase in HL-1 cardiomyocytes. GAPDH was used as a loading control.
To determine which cell types are direct targets of SDF-1α treatment, we treated primary cardiac-derived human microvascular endothelial cells (MVECs), primary human cardiac fibroblasts, a cardiac myocyte cell line (HL-1) and primary adult mouse cardiomyocytes in culture with SDF-1α. We found a modest but reproducible upregulation of phosphorylated Akt in MVECs and cardiomyocytes (HL-1 or primary adult cardiomyocytes), but not in fibroblasts (Figure 5B). These data suggest that SDF-1α may be acting on cardiomyocytes directly, but also on endothelial cells that may potentially signal to myocytes in a paracrine fashion. Consistent with this, we found that while CXCR4 protein was expressed in all of the cell types examined, it was most highly expressed in MVECs (data not shown). VEGF is one of the putative paracrine factors secreted from endothelial cells that functions in an angiogenic and cardioprotective manner. We observed an upregulation of VEGF protein in response to SDF-1α in MVECs, but not in cardiomyocytes. Thus, SDF-1α may function in a cardioprotective manner directly on cardiomyocytes and in a paracrine fashion through endothelial cells, in addition to stimulating new recruitment or expansion of the capillary bed.
Discussion
In this report, we propose that SDF-1α, a secreted chemokine that activates Akt, has cell-protective and angiogenic properties under conditions of cardiac tissue hypoxia. After acute myocardial infarction in mice, SDF-1α treatment resulted in decreased cell death and increased angiogenesis within the hypoxic tissue, ultimately leading to reduced scarring and improved cardiac function. Phosphorylation of Akt was increased in vivo as was upregulation of VEGF in response to SDF-1α, providing potential mechanisms for the observed effects of SDF-1α.
Published reports have alluded to the potential role of SDF-1 in cardiac regeneration and have focused on attraction of bone marrow–derived somatic stem cells to the heart post-infarction22, 23. Recent studies, however, have raised considerable doubt regarding the potential of bone marrow–derived stem cells to transdifferentiate into cardiomyocytes21, 27, 28, although they may provide a non-cell autonomous benefit via secreted factors that are angiogenic. Whether or not SDF-1α protein alone induces regeneration and thereby improves cardiac function after an acute insult had not been previously addressed, but the notion of SDF-1α-induced recruitment of stem cells into an infarcted tissue has been suspected. Our studies suggest the possibility that SDF-1α may be among the beneficial secreted proteins from noncardiomyocytes that can limit hypoxic damage to the heart.
Our observed short-term improvement data suggest that the initial conservation of function upon SDF-1α treatment is due primarily to preservation of myocardial tissue bordering the immediate area of infarct. This preservation may occur through an Akt-mediated pathway or other SDF-1α dependent mechanisms. In addition, the observed upregulation of VEGF and the concurrent increase in microvasculature after SDF-1α-treatment may be involved in the prolonged recovery of initially protected “hibernating” tissue. Of particular interest is a recent report demonstrating that Akt1 is essential for proper angiogenesis both post-ischemia and post-VEGF activation29, lending support to the possibility of SDF-1α-mediated Akt activation having a direct effect on this process. However, the relative contribution of Akt activation is difficult to discern in vivo and has not been tested here. Given our observed upregulation of VEGF in SDF-1α-treated hearts and in cardiac endothelial cells, it is of interest that VEGF can have a direct effect in promoting cardiomyocyte survival30-32. Finally, it is also possible that SDF-1α stimulates recruitment of circulating progenitor cells that promote angiogenesis. Thus, long-term cardiac functional improvement may be multifactorial, involving tissue protection and survival, angiogenesis and progenitor cell recruitment.
In sum, we have presented data here demonstrating a role for SDF-1α in improving cardiac function predominantly by affecting myocyte survival and angiogenesis at an early time point. Future studies will attempt to address what intracellular changes SDF-1α may be inducing in cardiomyocytes and if these changes mirror the known metabolic and energy usage adaptations typical of hibernating myocardium.
Supplementary Material
Acknowledgements
The authors thank James Richardson, John Shelton and the histopathology core for assistance in tissue processing and analysis; Arwyn Hood and Glenn Adams for technical assistance; Stephen Ordway and Gary Howard for editorial assistance.
Funding Sources D.S. was supported by grants from the National Heart, Lung, and Blood Institute/National Institutes of Health, American Heart Association, and March of Dimes Birth Defects Foundation.
Footnotes
Publisher's Disclaimer: This is an un-copyedited author manuscript that was accepted for publication in Circulation, copyright The American Heart Association. This may not be duplicated or reproduced, other than for personal use or within the “Fair Use of Copyrighted Materials” (section 107, title 17, U.S. Code) without prior permission of the copyright owner, The American Heart Association. The final copyedited article, which is the version of record, can be found at http://circ.ahajournals.org. The American Heart Association disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties.
Disclosures
None.
References
- 1.Braunwald E. Shattuck lecture--cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337(19):1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348(20):2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia G, Sosin M, Leahy JF, Connolly DL, Davis RC, Lip GY. Hibernating myocardium in heart failure. Expert Rev Cardiovasc Ther. 2005;3(1):111–122. doi: 10.1586/14779072.3.1.111. [DOI] [PubMed] [Google Scholar]
- 4.Depre C, Vatner SF. Mechanisms of cell survival in myocardial hibernation. Trends Cardiovasc Med. 2005;15(3):101–110. doi: 10.1016/j.tcm.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9(9):1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 6.Bock-Marquette I, Saxena A, White MD, Dimaio JM, Srivastava D. Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature. 2004;432(7016):466–472. doi: 10.1038/nature03000. [DOI] [PubMed] [Google Scholar]
- 7.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184(3):1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aiuti A, Webb IJ, Bleul C, Springer T, Gutierrez-Ramos JC. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J Exp Med. 1997;185(1):111–120. doi: 10.1084/jem.185.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corcione A, Ottonello L, Tortolina G, Facchetti P, Airoldi I, Guglielmino R, Dadati P, Truini M, Sozzani S, Dallegri F, Pistoia V. Stromal cell-derived factor-1 as a chemoattractant for follicular center lymphoma B cells. J Natl Cancer Inst. 2000;92(8):628–635. doi: 10.1093/jnci/92.8.628. [DOI] [PubMed] [Google Scholar]
- 10.Doitsidou M, Reichman-Fried M, Stebler J, Koprunner M, Dorries J, Meyer D, Esguerra CV, Leung T, Raz E. Guidance of primordial germ cell migration by the chemokine SDF-1. Cell. 2002;111(5):647–659. doi: 10.1016/s0092-8674(02)01135-2. [DOI] [PubMed] [Google Scholar]
- 11.Bajetto A, Barbero S, Bonavia R, Piccioli P, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1alpha induces astrocyte proliferation through the activation of extracellular signal-regulated kinases 1/2 pathway. J Neurochem. 2001;77(5):1226–1236. doi: 10.1046/j.1471-4159.2001.00350.x. [DOI] [PubMed] [Google Scholar]
- 12.Kortesidis A, Zannettino A, Isenmann S, Shi S, Lapidot T, Gronthos S. Stromal-derived factor-1 promotes the growth, survival, and development of human bone marrow stromal stem cells. Blood. 2005;105(10):3793–3801. doi: 10.1182/blood-2004-11-4349. [DOI] [PubMed] [Google Scholar]
- 13.Mirshahi F, Pourtau J, Li H, Muraine M, Trochon V, Legrand E, Vannier J, Soria J, Vasse M, Soria C. SDF-1 activity on microvascular endothelial cells: consequences on angiogenesis in in vitro and in vivo models. Thromb Res. 2000;99(6):587–594. doi: 10.1016/s0049-3848(00)00292-9. [DOI] [PubMed] [Google Scholar]
- 14.Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 15.Porcile C, Bajetto A, Barbieri F, Barbero S, Bonavia R, Biglieri M, Pirani P, Florio T, Schettini G. Stromal cell-derived factor-1alpha (SDF-1alpha/CXCL12) stimulates ovarian cancer cell growth through the EGF receptor transactivation. Exp Cell Res. 2005;308(2):241–253. doi: 10.1016/j.yexcr.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 16.Vlahakis SR, Villasis-Keever A, Gomez T, Vanegas M, Vlahakis N, Paya CV. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J Immunol. 2002;169(10):5546–5554. doi: 10.4049/jimmunol.169.10.5546. [DOI] [PubMed] [Google Scholar]
- 17.Zou W, Machelon V, Coulomb-L’Hermin A, Borvak J, Nome F, Isaeva T, Wei S, Krzysiek R, Durand-Gasselin I, Gordon A, Pustilnik T, Curiel DT, Galanaud P, Capron F, Emilie D, Curiel TJ. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat Med. 2001;7(12):1339–1346. doi: 10.1038/nm1201-1339. [DOI] [PubMed] [Google Scholar]
- 18.Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25(5):293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- 19.Garner LB, Willis MS, Carlson DL, DiMaio JM, White MD, White DJ, Adams GAt, Horton JW, Giroir BP. Macrophage migration inhibitory factor is a cardiac-derived myocardial depressant factor. Am J Physiol Heart Circ Physiol. 2003;285(6):H2500–2509. doi: 10.1152/ajpheart.00432.2003. [DOI] [PubMed] [Google Scholar]
- 20.Chatterjee K, Zhang J, Honbo N, Simonis U, Shaw R, Karliner JS. Acute vincristine pretreatment protects adult mouse cardiac myocytes from oxidative stress. J Mol Cell Cardiol. 2007;43(3):327–336. doi: 10.1016/j.yjmcc.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 21.Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Haematopoietic stem cells adopt mature haematopoietic fates in ischaemic myocardium. Nature. 2004;428(6983):668–673. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- 22.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110(21):3300–3305. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 23.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 24.Fraidenraich D, Stillwell E, Romero E, Wilkes D, Manova K, Basson CT, Benezra R. Rescue of cardiac defects in id knockout embryos by injection of embryonic stem cells. Science. 2004;306(5694):247–252. doi: 10.1126/science.1102612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshioka T, Ageyama N, Shibata H, Yasu T, Misawa Y, Takeuchi K, Matsui K, Yamamoto K, Terao K, Shimada K, Ikeda U, Ozawa K, Hanazono Y. Repair of infarcted myocardium mediated by transplanted bone marrow-derived CD34+ stem cells in a nonhuman primate model. Stem Cells. 2005;23(3):355–364. doi: 10.1634/stemcells.2004-0200. [DOI] [PubMed] [Google Scholar]
- 26.Gnecchi M, He H, Noiseux N, Liang OD, Zhang L, Morello F, Mu H, Melo LG, Pratt RE, Ingwall JS, Dzau VJ. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. Faseb J. 2006;20(6):661–669. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 27.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428(6983):664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 28.Nygren JM, Jovinge S, Breitbach M, Sawen P, Roll W, Hescheler J, Taneera J, Fleischmann BK, Jacobsen SE. Bone marrow-derived hematopoietic cells generate cardiomyocytes at a low frequency through cell fusion, but not transdifferentiation. Nat Med. 2004;10(5):494–501. doi: 10.1038/nm1040. [DOI] [PubMed] [Google Scholar]
- 29.Ackah E, Yu J, Zoellner S, Iwakiri Y, Skurk C, Shibata R, Ouchi N, Easton RM, Galasso G, Birnbaum MJ, Walsh K, Sessa WC. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115(8):2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hiasa K, Egashira K, Kitamoto S, Ishibashi M, Inoue S, Ni W, Zhao Q, Nagata S, Katoh M, Sata M, Takeshita A. Bone marrow mononuclear cell therapy limits myocardial infarct size through vascular endothelial growth factor. Basic research in cardiology. 2004;99(3):165–172. doi: 10.1007/s00395-004-0456-9. [DOI] [PubMed] [Google Scholar]
- 31.Ferrarini M, Arsic N, Recchia FA, Zentilin L, Zacchigna S, Xu X, Linke A, Giacca M, Hintze TH. Adeno-associated virus-mediated transduction of VEGF165 improves cardiac tissue viability and functional recovery after permanent coronary occlusion in conscious dogs. Circulation research. 2006;98(7):954–961. doi: 10.1161/01.RES.0000217342.83731.89. [DOI] [PubMed] [Google Scholar]
- 32.Ruixing Y, Dezhai Y, Hai W, Kai H, Xianghong W, Yuming C. Intramyocardial injection of vascular endothelial growth factor gene improves cardiac performance and inhibits cardiomyocyte apoptosis. Eur J Heart Fail. 2007;9(4):343–351. doi: 10.1016/j.ejheart.2006.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.