Abstract
Molecular chaperones including the small heat shock protein, αB crystallin and sHSP27 participate in the assembly, disassembly, and reorganization of the cytoskeleton during cell development and differentiation. While αB crystallin and sHSP27 stabilize and modulate filament assembly and re-organization, the sequences and structural domains mediating interactions between these proteins and filaments are unknown. It is important to define these interactive domains in order to understand differential interactions between chaperones and stable or unfolding filaments and their function in the cellular stress response. Protein pin arrays identified sequences in human αB crystallin that selectively interacted with native or partially unfolded filament proteins desmin, glial-fibrillary acidic protein, and actin. Circular dichroism spectroscopy determined the differences in structure of these filaments at 23°C and 45°C. Seven αB crystallin sequences had stronger interactions with desmin and six sequences had stronger interactions with glial-fibrillary acidic protein at 23°C than at 45°C. The αB crystalline sequences 33LESDLFPTSTSLSPFYLRPPSFLR56 and 129DPLTITSSLSSDGV145 had the strongest interactions with actin at 23°C, while 57APSWFDTG64, 111HGFISREF118, 145VNGPRKQVSG154, and 155PERTIPITREEK165 had the strongest interactions with actin at 45°C. The actin interactive sequences of αB crystallin overlapped with previously identified αB crystallin chaperone sequences and were synthesized to evaluate their effect on the assembly and aggregation of actin. Full-length αB crystallin and the core domain chaperone sequence 131LTITSSLSSDGV143 promoted actin polymerization at 37°C and inhibited depolymerization and aggregation at 50°C. The results support the hypothesis that interactive domains in αB crystallin have multiple functions in stabilizing the cytoskeleton and protecting cytosolic proteins from unfolding.
Keywords: molecular chaperone, small heat shock protein, α crystallin, actin, intermediate filament, desmin-related myopathy (DRM), glial-fibrillary acidic protein
Introduction
Interactions between small heat shock proteins (sHSPs) and filaments are important during normal cell differentiation and in protein aggregation diseases (Iwaki et al., 1989; Kato et al., 1992; Prescott et al., 1996; van Rijk and Bloemendal, 2000). An association between α crystallin, the archetype of sHSPs, and the cytoskeletal network has been recognized since the earliest observations of beaded filaments in extracted lens cells (Bagchi, Katar, & Maisel, 2002; Carter, Hutcheson, & Quinlan, 1995; FitzGerald & Graham, 1991; Ireland & Maisel, 1989; R. A. Quinlan, Carte, Sandilands, & Prescott, 1996). The lens fiber cytoplasm contains a dense network of filaments, which are required for normal transparency and lens refraction and the lens cytoskeleton is intimately associated with α crystallin (Liang & MacRae, 1997; MacRae, 2000; Nicholl & Quinlan, 1994; Perng, Cairns et al., 1999; Sandilands et al., 1995). It is believed that sHSPs participate in the dynamic assembly and stabilization of the filamentous cytoskeleton (Melkani, Cammarato, & Bernstein, 2006; Singh, Rao, Ramakrishna, Rangaraj, & Rao, 2006). In normal tissues, αB crystallin interacts directly with a number of filament proteins including phakinin (CP49), filensin (CP119), desmin, glial fibrillary acidic protein (GFAP), vimentin, and actin where it functions in the organization and stabilization of the filament networks formed by these proteins (Bennardini, Wrzosek, & Chiesi, 1992; Djabali, Piron, de Nechaud, & Portier, 1999; Gopalakrishnan, Boyle, & Takemoto, 1993; Head, Hurwitz, Kegel, & Goldman, 2000; Launay, Goudeau, Kato, Vicart, & Lilienbaum, 2006; Liang & MacRae, 1997; Lieska, Chen, Maisel, & Romero-Herrera, 1980; Maglara, Vasilaki, Jackson, & McArdle, 2003; Muchowski, Valdez, & Clark, 1999; Prescott, Sandilands, Hutcheson, Carter, & Quinlan, 1996; R. Quinlan, 2002; Tomokane, Iwaki, Tateishi, Iwaki, & Goldman, 1991; Wisniewski & Goldman, 1998). Mutations in αB crystallin including the R120G mutation that alters the selective association between αB crystallin and filaments cause cataracts, myofibrillar myopathy and desmin-related cardiomyopathy (DRM) apparently through the defective assembly of intracellular filament networks (Bova et al., 1999; Litt et al., 1998; Selcen & Engel, 2003; Vicart et al., 1998).
Identification and characterization of the filament interactive sites in human αB crystallin will provide a structural basis for understanding the function of sHSPs in the recognition, solubilization, assembly, and stabilization of filament networks. In the current study, human αB crystallin sequences that interacted with cytoskeletal filaments in their native or partially unfolded states were identified using protein pin arrays that were used previously to identify interactive sequences for chaperone activity and subunit-subunit interactions in αB crystallin (Ghosh & Clark, 2005; Ghosh, Estrada, & Clark, 2005). The αB crystallin filament interactive sequences identified by the pin arrays selectively recognized and interacted with partially unfolded desmin and GFAP, and native and partially unfolded actin. In addition, these sequences overlapped with previously identified subunit-subunit and chaperone sequences of αB crystallin suggesting a novel mechanism for sHSP functions in vivo that depends on the selective recognition of the unfolding state of filament and chaperone target proteins.
Materials and Methods
Synthesis of αB crystallin peptides
The αB crystallin peptides 41STSLSPFYLRPPSFLRAP58 (ST), 113FISREFHR120 (FI), 131LTITSSLSSDGV142 (LT), and 156ERTIPITRE164 (ER) were synthesized by Genscript Corporation (Piscataway, NJ).
Ultra-violet circular dichroism spectroscopy
The secondary and tertiary structure of each filament protein was determined using ultra-violet circular dichroism (UVCD) at 23°C and after heating at 45°C for 15mins. A concentration of 0.02mg/ml protein was used for far UVCD measurements (200-249nm) and a concentration of 0.1mg/ml was used for near UVCD measurements (250-320nm). The UVCD spectrum of each protein was adjusted to the baseline using the UVCD spectrum of PBS buffer (5mM phosphate buffer, 1.3mM potassium chloride and 137mM sodium chloride, pH 7.0). The far UVCD spectra of the filament proteins were deconvoluted using Dichroweb (Lobley, Whitmore, & Wallace, 2002; Whitmore & Wallace, 2004) to calculate the secondary structure composition of the filaments.
Protein pin array assay
A protein pin array (Mimotopes, San Diego, CA) consisting of eighty-four eight amino acid long peptides corresponding to the primary sequence of human αB crystallin was synthesized and used to measure interactions with desmin, glial fibrillary acid protein (GFAP), and actin as described previously (Geysen, 1990; Ghosh & Clark, 2005). Proteins and antibodies purchased from suppliers are listed in Table 1. The purity of the filament proteins, desmin, GFAP and actin used in the pin array assays were determined to be >95% by SDS-PAGE analysis (data not shown). Primary antibodies used in the pin array assays were specific to each target protein and minor contaminating proteins that bound to the αB crystallin peptides were not detected. Positive interactions resulted in blue color and were plotted as absorbance at λ = 450nm and plotted on the Y-axis. The sequences of the αB crystallin peptides were plotted on the X-axis. The pin array assays were conducted in duplicate with filament proteins at 23°C or with proteins heated at 45°C for 15mins. Human myoglobin did not interact with any of the αB crystallin peptides and was the negative control for the protein pin array assay (Ghosh & Clark, 2005). A single pin array was used for all experiments and no significant decrease in efficiency was observed even after repeated use up to thirty times. The last three peptides of the protein pin array correspond to the epitope, 163REEKPAVTAAPKK175, recognized by the primary antibody for αB crystallin and were used to measure the efficiency of the pin array as described previously (Ghosh, Estrada, & Clark, 2005). The loss of efficiency for the pin array was determined to be <5% by after more than thirty assays. Average absorbances were calculated by averaging the measured absorbances of all eighty-four αB crystallin peptides at each temperature. The percentage change in absorbance was calculated as follows:
Table 1.
List of proteins and antibodies used in the protein pin array assays.
| Proteins | Catalogue No. | Supplier | Molecular weight | Amount used/well |
|---|---|---|---|---|
| Human desmin | RDI-PRO62016 | Research Diagnostics, NJ | 55kDa | 2.5μg |
| Human glial fibrillary acidic protein | GFAP1 | Advanced Immunochemical, CA | 52kDa | 2.0μg |
| Rabbit muscle actin | A2522 | Sigma-Aldrich, MO | 43kDa | 1.8μg |
| Antibodies | Catalogue No. | Supplier | Type | Dilution used |
| Anti-human desmin | RDI-PRO10519 | Research Diagnostics, NJ | Mouse | 1:100 |
| Anti-human glial fibrillary acidic protein | G9269 | Sigma-Aldrich, MO | Rabbit | 1:1,000 |
| Anti-chicken skeletal actin | 10601 | ICN Pharmaceuticals, OH | Mouse | 1:100 |
| Anti-Mouse IgG-HRP | A9044 | Sigma-Aldrich, MO | Rabbit | 1:40,000 |
| Anti-Rabbit IgG-HRP | SAB-300 | Stressgen, MI | Goat | 1:10,000 |
Actin polymerization and thermal aggregation assay
The effects of the αB crystallin peptides that had strong interactions with actin in the pin arrays on actin polymerization were evaluated using an Actin Polymerization Kit using the protocol supplied by the manufacturer (Cytoskeleton; Denver, CO)(Kumar, Tomar, Parrill, & Khurana, 2004). Polymerization of globular actin (G-actin) to filamentous actin (F-actin) results in incorporation of pyrene which increases pyrene fluorescence at λ = 460nm. 4.5ml of 5mM Tris-HCl, 0.2mM CaCl2, 0.2mM ATP, pH 8.0 was mixed with 100μl of 10mg/ml pyrene-labeled actin and incubated at 25°C for 60mins. 80μl of the sample was added to a well containing 8μl of 250μM peptide in 0.31% DMSO. Fluorescence of the samples were recorded at room temperature for 20mins prior to the addition of Mg++ using a Victor3 V fluorescence plate reader (Excitation λ = 355nm, Emission λ = 460nm). 10μl of 50mM KCl, 2mM MgCl2, 1mM ATP was then added to each sample and fluorescence was recorded continuously for 125mins. Subsequently, the plate was heated at 50°C to initiate thermal aggregation and the fluorescence was recorded for 60mins to evaluate the effect of the αB crystallin peptides on the aggregation of F-actin.
Note: Previous studies reported the critical concentrations for the assembly of desmin and GFAP filaments were ~0.08μg/ml and ~0.12μg/ml respectively (Chou, Stromer, Robson, & Huiatt, 1990; Orban, Halasi, Papp, Barko, & Bugyi, 2005; Pan & Ware, 1988; Papp, Bugyi, Ujfalusi, Halasi, & Orban, 2005; F. Wang, Sampogna, & Ware, 1989; Yang & Babitch, 1988). UVCD measurements were conducted at protein concentrations of 2mg/ml, while pin array assays were conducted at protein concentrations of 25μg/ml, both of which are well above the critical concentration for filament assembly.
Results
The secondary and tertiary structure of the desmin, GFAP, and actin were determined at 23°C and 45°C by UVCD spectroscopy (Fig. 1 and Table 2). The deconvoluted far UVCD spectrum for desmin at 23°C corresponded to 16% helix, 47% coil, and 37% β sheet secondary structure. When desmin was heated at 45°C for 15mins, the helical content in the secondary structure of desmin increased from 16% to 80% helix, random coil decreased from 47% to 19% coil, and β sheet decreased from 37% to 1%. The deconvoluted far UVCD spectrum of GFAP at 23°C corresponded to a secondary structure of 28% helix, 53% coil, and 19% β sheet. At 45°C for 15mins, the helical content of GFAP increased from 28% to 40% helix, coil decreased from 53% to 44% and β sheet decreased from 19% to 16%. The deconvoluted far UVCD spectrum of actin at 23°C corresponded to a secondary structure of 23% helix, 52% coil, and 25% β sheet. At 45°C, there was a small decrease in helical content from 23% to 18%, a decrease from 52% coil to 48% and an increase from 25% to 34% β sheet. Desmin and GFAP had higher helical content at 45°C than at 23°C, while actin had slightly lower helical content at 45°C than at 23°C.
Fig. 1.

UVCD spectroscopic analysis of the effect of heat on the structure of human desmin (A), human GFAP (B), and rabbit muscle actin (C). The far UVCD spectra of desmin, GFAP, and actin were measured at 23°C and after heating at 45°C for 15mins, which was identical to the conditions used in the pin array assays. In the desmin spectrum, the negative ellipticity at 222nm increased from -4467 deg.cm2.dmol-1 at 23°C to -24677 deg.cm2.dmol-1 at 45°C indicating a gain of helical secondary structure with temperature which was consistent with previous reports (Fuchs & Weber, 1994). In the GFAP spectrum, the negative ellipticity at 222nm increased from -8596 deg.cm2.dmol-1 at 23°C to -11230 deg.cm2.dmol-1 at 45°C indicating a gain in helical secondary structure with temperature which was consistent with previous reports (Fuchs & Weber, 1994). Actin was thermostable up to 45°C, and the negative ellipticity at 222nm decreased from -2787 deg.cm2.dmol-1 at 23°C to -2753 deg.cm2.dmol-1 at 45°C, which was consistent with previous reports (Bertazzon, Tian, Lamblin, & Tsong, 1990; Bertazzon & Tsong, 1990). Based on UVCD, desmin and GFAP were determined to have more helical content at 45°C than at 23°C, while actin had slightly lower helical content at 45°C than at 23°C.
Table 2.
Secondary structure composition of desmin, GFAP, and actin at 23°C and 45°C. The ultra-violet circular dichroism spectra of desmin, GFAP, and actin were deconvoluted using Dichroweb (Lobley, Whitmore, & Wallace, 2002; Whitmore & Wallace, 2004) to determine the secondary structure composition of these proteins at 23°C and after heating at 45°C for fifteen minutes. Column 1 lists the elements of secondary structure of the proteins. Columns 3 and 4 list the secondary structure of human desmin at 23°C and 45°C respectively. Columns 5 and 6 list the secondary structure of human GFAP at 23°C and 45°C respectively. Columns 3 and 4 list the secondary structure of rabbit muscle actin at 23°C and 45°C respectively. Desmin and GFAP had more helical content at 45°C than at 23°C, while the secondary structure of actin was similar at both 23°C and 45°C.
| Proteins | Desmin | GFAP | Actin | |||
|---|---|---|---|---|---|---|
| 23°C | 45°C | 23°C | 45°C | 23°C | 45°C | |
| % helix | 16% | 80% | 28% | 40% | 23% | 18% |
| % random coil | 47% | 19% | 53% | 44% | 52% | 48% |
| % sheet | 37% | 1% | 19% | 16% | 25% | 34% |
Interactions between the intermediate filament protein desmin and the eighty-four αB crystallin peptides were measured using a protein pin array (Fig. 2 and Table 3). When the pin array assay was conducted with desmin at 23°C, absorbances were observed in the wells corresponding to eighty-three of the eighty-four peptides. The highest absorbances were observed for peptides corresponding to the N-terminal sequences 15FPFHSPSRLF24 and 41STSLSPFYLRPPSFLRAPSWFD62, the α crystalline core domain sequences, 75FSVNLDVKHFSPEELK90, 113FISREFHRKYRIPADV128, 131LTITSSLS138, and 145VNGPRKQV152, and the C-terminal sequence, 163REEKPAVTAAPK174. When the pin array assay was conducted with desmin at 45°C, the magnitude of the absorbances of eighty-three αB crystallin peptides was approximately 45% of the magnitude at 23°C. The αB crystallin peptides had stronger interactions with desmin at 23°C than at 45°C the temperature corresponding to high helical content characteristic of structured intermediate filaments (Herrmann & Aebi, 2004).
Fig. 2.

Pattern of interaction between human αB crystallin peptides and human desmin. The amino acid sequence of each αB crystallin eight-mer peptide is listed on the X-axis. The Y-axis plots the absorbances measured at 450nm for the αB crystallin peptides in the presence of desmin at 23°C (clear bars) and 45°C (solid black bars). Grey bars represent the difference in the recorded absorbance of each peptide with desmin at 23°C and 45°C. Positive grey bars represent peptides that had a stronger interaction at 23°C than at 45°C. The heights of the bars are a measure of the strength of the interactions between αB crystallin peptides and desmin. Peptides that had the strongest interactions with desmin were flanked on either side by peptides that had weak or no interactions with desmin resulting in a pattern of high and low absorbances. All eighty-four αB crystallin peptides interacted with desmin at 23°C and eighty-three of eighty-four peptides interacted with desmin at 45°C. At 45°C, the average absorbance of the αB crystallin pin array was 45% of the average absorbance at 23°C. The seven αB crystallin sequences that had the strongest interactions were labeled and identified as desmin interactive sequences of human αB crystallin.
Table 3.
List of the interactive sequences in human αB crystallin involved in chaperone activity and filament interactions. Column 1 lists the structural domain in human αB crystallin where each interactive sequence is located. Column 2 lists the chaperone sequences identified previously (Ghosh, Estrada, & Clark, 2005). Columns 3-5 list the sequences in human αB crystallin for interactions with desmin, GFAP, and actin respectively. Sequences that interacted with actin at 23°C are in BOLD. Sequences that interacted with partially unfolded actin at 45°C are in regular font. Common αB crystallin sequences interacted with filaments and unfolded cytosolic proteins (Ghosh, Estrada, & Clark, 2005).
| Location | Interactive sequences | Desmin sequences | GFAP sequences | Actin sequences |
|---|---|---|---|---|
| N-terminus | 9WIRRPFFPFHSP20 | 15FPFHSPSRLF24 | 9WIRRPFFP16 | 15FPFHSPSRLFDQ26 |
| N-terminus | 43SLSPFYLRPPSFLRAP58 | 41STSLSPFYLRPPSFLRAPSWFD62 | 45SPFYLRPPSFLRAPSWFD62 | 33LESDLFPTSTSLSPFYLRPPSFLR56 |
| N-terminus | - | - | - | 57APSWFDTG64 |
| α crystallin core domain | 75FSVNLDVK82 | 75FSVNLDVKHFSPEELK90 | 89LKVKVLGDVI98 | - |
| α crystallin core domain | 113FISREFHR120 | 113FISREFHRKYRIPADV128 | 113FISREFHRKYRIPADV128 | 111HGFISREF118 |
| α crystallin core domain | 131LTITSSLS138 | 131LTITSSLS138 | - | 129DPLTITSSLSSDGV145 |
| α crystallin core domain | 141GVLTVNGP148 | 145VNGPRKQV152 | 143LTVNGPRK150 | 145VNGPRKQVSG154 |
| C-terminus | 157ERTIPITRE164 | 163REEKPAVTAAPK174 | 155PERTIPITREEKPAVTAA172 | 155PERTIPITREEK165 |
Interactions between the intermediate filament protein GFAP and eighty-four αB crystallin peptides were measured using a protein pin array (Fig. 3 and Table 3). When the pin array assay was conducted with GFAP at 23°C, absorbances were observed in the wells corresponding to eighty-two of eighty-four αB crystallin sequences. Peptides that had the highest absorbances with GFAP at 23°C were 9WIRRPFFP16 and 45SPFYLRPPSFLRAPSWFD62 in the αB crystallin N-terminus, 89LKVKVLGDVI98, 113FISREFHRKYRIPADV128, and 143LTVNGPRK150 in the α crystallin core domain and, 155PERTIPITREEKPAVTAA172 in the C-terminus. When the pin array assay was conducted with GFAP at 45°C, the magnitude of the absorbances of was approximately 23% of the magnitude at 23°C. While 82/84 αB crystallin peptides are observed to interact with GFAP at 23°C, only 33/84 peptides had signals stronger than the baseline value of 0.1. At 45°C, 40/84 αB crystallin peptides interacted with GFAP and only 22/84 peptides had signals greater than 0.1. The αB crystallin peptides had stronger interactions with GFAP at 23°C than at 45°C the temperature corresponding to high helical content characteristic of structured intermediate filaments (Herrmann & Aebi, 2004).
Fig. 3.

Pattern of interaction between human αB crystallin peptides and human GFAP. The amino acid sequence of each αB crystallin eight-mer peptide is listed on the X-axis. The Y-axis plots the absorbances measured at 450nm for the αB crystallin peptides in the presence of GFAP at 23°C (clear bars) and 45°C (solid black bars). Grey bars represent the difference in the recorded absorbance of each peptide at 23°C and 45°C. Positive grey bars represent peptides that had a stronger interaction with GFAP at 23°C than at 45°C. The heights of the bars are a measure of the strength of the interactions between αB crystallin peptides and GFAP. Eighty-two of eighty-four αB crystallin peptides interacted with GFAP at 23°C and thirty-seven of eighty-four peptides interacted with GFAP at 45°C. At 45°C, the average absorbance of the αB crystallin pin array was 23% of the average absorbance at 23°C. The six αB crystallin sequences that had the strongest interactions with unheated GFAP are labeled and identified as GFAP interactive sequences.
Interactions between the cytoskeletal microfilament protein actin and eighty-four αB crystallin peptides were measured using a protein pin array (Fig. 4 and Table 3). When the pin array assay was conducted with actin at 23°C, absorbances were observed in the wells corresponding to all eighty-four αB crystallin sequences. When the pin array assay was conducted with actin at 45°C, the magnitude of absorbances of the αB crystallin peptides was approximately 91% of the magnitude at 23°C. Peptides corresponding to the N-terminal sequence 33LESDLFPTSTSLSPFYLRPPSFLR56 and the C-terminal sequence 129DPLTITSSLSSDGV142 had the highest absorbances with actin at 23°C, while peptides corresponding to the N-terminal sequences 15FPFHSPSRLFDQ26 and 57APSWFDTG64, the α crystallin core domain sequence 111HGFISREF118, and the C-terminal sequences 145VNGPRKQVSG154 and 155PERTIPITREEK165 had the highest absorbances with actin at 45°C.
Fig. 4.

Pattern of interaction between human αB crystallin peptides and rabbit muscle actin. The amino acid sequence of each αB crystallin eight-mer peptide is listed on the X-axis. The Y-axis plots the absorbances measured at 450nm for the αB crystallin peptides in the presence of actin at 23°C (clear bars) and 45°C (solid black bars). Grey bars represent the difference in the recorded absorbance for each peptide at 23°C and 45°C. Positive grey bars represent peptides that had a stronger interaction with actin at 23°C than with actin at 45°C. Negative grey bars represent peptides that had stronger interactions with actin at 45°C than with actin at 23°C. Thirty-seven of eighty-four αB crystallin peptides had stronger interactions with actin at 23°C than with actin at 45°C (positive grey bars), thirty-four peptides had stronger interactions with actin at 45°C than actin at 23°C (negative grey bars), and thirteen peptides had similar interactions with both actin at 23°C and 45°C. The two αB crystallin sequences that had the strongest interactions with actin at 23°C, and the four αB crystallin sequences that had the strongest interactions with actin at 45°C are labeled and identified as actin interactive sequences of human αB crystallin. At 45°C, the average absorbance of the αB crystallin pin array was 91% of the average absorbance at 23°C.
The four αB crystallin sequences 41STSLSPFYLRPPSFLRAP58, 113FISREFHF120, 131LTITSSLSSDGV142, and 156ERTIPITRE164 that had strong interactions with actin in the pin array assay overlapped with previously identified chaperone sequences of αB crystallin (Ghosh, Estrada, & Clark, 2005, 2006; Ghosh, Estrada, Houck, & Clark, 2006). Consequently, these four peptides were synthesized to determine their individual effects on the polymerization and thermal aggregation of unassembled globular actin (G-actin) and assembled filamentous actin (F-actin) using pyrene-labeled actin (Fig. 5). When 4μM pyrene-labeled actin alone was incubated in the polymerization buffer at 37°C, the pyrene fluorescence at λ = 460nm increased to a maximum value of 8187 RFU after 125mins. The increased fluorescence was due to incorporation of pyrene-labeled actin into assembled actin filaments. In contrast, incubation of 4μM pyrene-labeled actin at 37°C in the absence of polymerization buffer resulted in a decrease in fluorescence at λ = 460nm to a minimum value of -8792 RFU due to quenching of pyrene-fluorescence by the solvent. When 4μM pyrene-labeled actin was incubated with 4μM full-length wt human αB crystallin, the fluorescence at λ = 460nm increased rapidly and reached a maximum value of 19276 RFU. When 4μM pyrene-labeled actin was incubated with 20μM of the synthetic peptides ST and ER in the polymerization buffer at 37°C, the fluorescence at λ = 460nm increased rapidly and reached maximum values of 9533 RFU and 10,000 RFU respectively in approximately 125mins. The fluorescence maxima of the samples containing the ST and ER synthetic peptides were not significantly different from the sample containing actin alone. In contrast, incubation of 4μM pyrene-labeled actin with 20μM FI increased the fluorescence at λ = 460nm to a maximum of 12407 RFU without an effect on the rate of polymerization of G-actin to F-actin. Incubation of 4μM pyrene-labeled actin with 20μM LT increased the rate of polymerization and the fluorescence maximum to 12790 RFU after 125mins. Full-length wt human αB crystallin and the synthetic peptides FI and LT had a positive effect on actin polymerization, while the ST and ER synthetic peptides had little to no effect on actin polymerization.
Fig. 5.
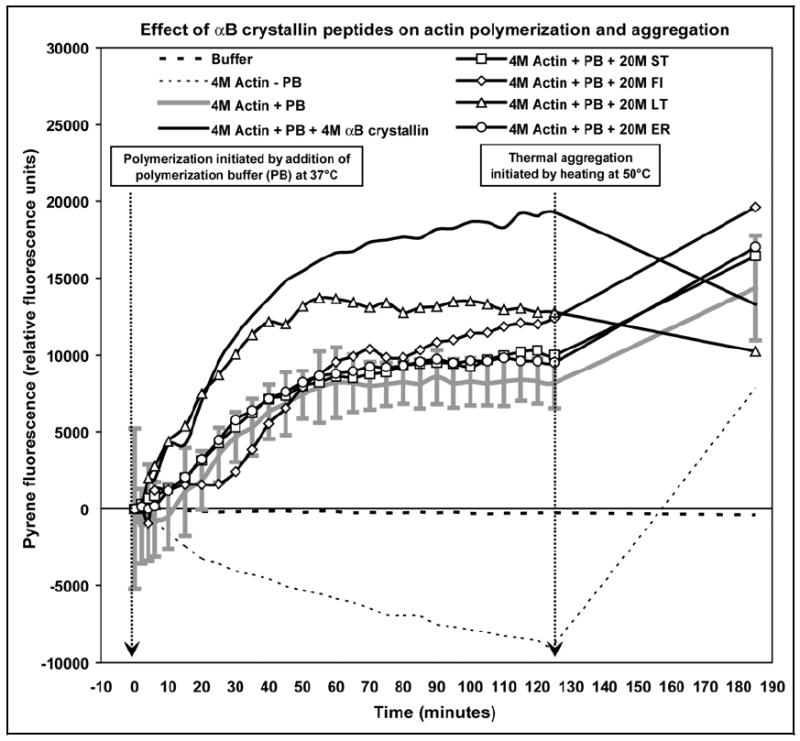
Comparison of select αB crystallin peptides with full-length wt human αB crystallin on the polymerization and thermal aggregation of actin. In the presence of polymerization buffer (PB) only (solid grey line), G-actin polymerization to F-actin was measured as an increase in fluorescence at λ = 460nm at 37°C in approximately 125mins. In the absence of polymerization buffer, no polymerization was observed and the fluorescence at λ = 460nm decreased (thin broken line). Wt human αB crystallin (solid black line) increased both the rate and the total amount of polymerization of G-actin to F-actin. The synthetic αB crystallin peptides ST (-□-) and ER (-○-) had no effect on the polymerization of G-actin to F-actin, while the FI peptide (-◊-) increased the amount of polymerization but had no effect on the rate of polymerization of G-actin to F-actin. The LT peptide (-Δ-) increased both the rate and the total amount of polymerization of G-actin to F-actin similar to wt αB crystallin. When unpolymerized G-actin (thin broken line) was heated to 50°C for 60mins increased aggregation of G-actin was measured as increased fluorescence at λ = 460nm. Similarly, the fluorescence at λ = 460nm of the sample containing F-actin alone (solid grey line) increased upon heating at 50°C for 60mins indicating aggregation of F-actin. Wt human αB crystallin (solid black line) protected against the aggregation of F-actin at 50°C. No protection against thermal aggregation of F-actin was observed in the presence of the synthetic peptides ST (-□-), FI (-◊-), and ER (-○-). In contrast, protection against thermal aggregation of F-actin was observed in the presence of the peptide LT (-Δ-). The action of the peptide, LT, resembled the action of full-length wt human αB crystallin which was the strongest promoter of actin polymerization and the strongest inhibitor of F-actin aggregation. Errors bars are shown for only the actin-alone sample to prevent over-crowding of the figure. The data shown are an average of three experiments performed in triplicate.
After complete polymerization was achieved at 125mins, the samples were heated to 50°C for 60mins to evaluate the effect of the synthetic peptides on the thermal stability of F-actin. After 60mins of incubation at 50°C, the fluorescence of unpolymerized G-actin incubated without polymerization buffer increased dramatically from -8792 to 7835 RFU indicating thermal aggregation of G-actin. Similarly, after 60mins of incubation at 50°C, the fluorescence of F-actin increased from -8187 to 14370 RFU indicating thermal aggregation of F-actin. Full-length wt human αB crystallin protected against the thermal aggregation of F-actin and the fluorescence of the sample containing full-length wt human αB crystallin decreased from 19276 to 13308 RFU after 60mins at 50°C. The αB crystallin peptides ST, FI and ER had no significant effect on the thermal stability of F-actin and the fluorescence of all three samples increased after heating at 50°C for 60mins. In contrast, the fluorescence of the sample containing the LT peptide decreased from 12790 RFU at 37°C to 10247 RFU after heating at 50°C for 60mins indicating protection against the thermal aggregation of F-actin. Full-length wt human αB crystallin and the chaperone peptide LT protected against the thermal aggregation of actin, while the ST, FI and ER synthetic peptides had no effect on the thermal aggregation of actin.
Discussion
Protein pin arrays identified sequences in αB crystallin used for interactions with the intermediate filaments desmin and GFAP, and the microfilament actin. The results were consistent with previous reports of interaction between αB crystallin and the filaments desmin, GFAP, and actin (Launay, Goudeau, Kato, Vicart, & Lilienbaum, 2006; Muchowski, Valdez, & Clark, 1999; Nicholl & Quinlan, 1994; Perng, Cairns et al., 1999; R. Quinlan, 2002; R. A. Quinlan, Carte, Sandilands, & Prescott, 1996). Pin array analysis of the αB crystallin-desmin interactions determined that the αB crystallin peptide 113FISREFHR120 had strong interactions with human desmin. The result confirmed the importance of the R120G mutation in human αB crystallin which is responsible for abnormal interactions between human αB crystallin and desmin filaments leading to cataracts in the filament cardiomyopathy, desmin-related myopathy (DRM) (Bova et al., 1999; Inagaki et al., 2006; Litt et al., 1998; Perng, Muchowski et al., 1999; Perng, Wen, van den, Prescott, & Quinlan, 2004; Selcen & Engel, 2003; Sun & Macrae, 2005; Vicart et al., 1998). Similarly, pin array analysis determined that the sequence, 57APSWFDTG64 had strong interactions with partially unfolded actin. This interactive sequence contains Ser-59, a key residue in human αB crystallin that modulates interactions with actin filaments in response to cardiac stress (Ecroyd et al., 2006; Kato et al., 2001; Morrison, Hoover, Thuerauf, & Glembotski, 2003; Shu et al., 2005; Webster, 2003). Phosphorylation of Ser-59 results in increased interactions between αB crystallin and actin that is believed to protect actin filaments from unfolding and aggregation (Launay, Goudeau, Kato, Vicart, & Lilienbaum, 2006). The result confirmed the importance of the Ser-59 residue in interactions between αB crystallin and actin filaments under stress.
Synthesis of the individual αB crystallin peptides permitted an evaluation of the function of each interactive domain on actin polymerization and aggregation. The αB crystallin core domain sequence 131LTITSSLSSDGV142 that was previously shown to have chaperone activity in vitro (Ghosh & Clark, 2005) promoted the polymerization of G-actin into F-actin and protected against the aggregation of actin filaments. In contrast, the α crystallin core domain sequence 113FISREFHR120 promoted actin polymerization without a measurable effect on the aggregation of F-actin. The N-terminal sequence 41STSLSPFYLRPPSFLRAP58 and the C-terminal sequence 156ERTIPITRE164 had no measurable effect on the polymerization and aggregation of actin, which was consistent with recent studies in which these two sequences were observed to have no intrinsic chaperone activity but were shown to play a critical role in the recognition, selection, and solubilization of substrate proteins (Ghosh, Shenoy, & Clark, 2006). The results suggested that the sequences 41STSLSPFYLRPPSFLRAP58 and 156ERTIPITRE164 facilitate the recognition and binding of native and partially unfolded actin by human αB crystallin under normal and stress conditions. The results support the hypothesis that under physiological conditions, αB crystallin facilitates the assembly of actin filaments, while under thermal and oxidative stress, αB crystallin protects actin filaments from unfolding and aggregation (Benndorf et al., 1994; Gopalakrishnan & Takemoto, 1992; Liang & MacRae, 1997; Longoni, Lattonen, Bullock, & Chiesi, 1990; Mounier & Arrigo, 2002; K. Wang & Spector, 1996). Pin array analysis, actin polymerization, and thermal aggregation assays indicated that αB crystallin employs common interactive sequences to promote the assembly of actin filaments and stabilize actin filaments.
The presence of common αB crystallin interactive sequences for cytoskeletal filament proteins and cytosolic lens crystallins (Table 3 and Fig. 6) suggested a novel mechanism for the chaperone activity of αB crystallin in filament assembly and chaperone activity. Differences in the UVCD spectra for desmin and GFAP at 23°C and 45°C determined that the filaments were partially unfolded and contained low helical secondary structure at 23°C but not at 45°C. In contrast, the cytosolic lens crystallins β/γ crystallins were stable at 23°C and begin unfolding at temperatures >45°C (Ghosh, Estrada, & Clark, 2005). The same αB crystallin sequences that had weak or no interactions with β/γ crystallins at 23°C (Ghosh, Estrada, & Clark, 2005) had strong interactions with desmin and GFAP at 23°C. The results suggest a preferential interaction with filaments relative to β/γ crystallins at 23°C and a preferential interaction with unfolding β/γ crystallins at 45°C relative to the filaments (Ghosh, Estrada, & Clark, 2005). In the absence of stress, αB crystallin interacts with and stabilizes filaments. Under conditions of stress, αB crystallin dissociates from the filaments to interact with unfolding β/γ crystallins using the same interactive surface domains to protect against aggregation and loss of transparency. The R120G mutation is an example of a post-translational modification that modifies the interactive sites of αB crystallin in a key surface domain to disrupt both αB crystallin-filament interactions and the αB crystallin response to unfolding proteins (Bloemendal et al., 2004; Fu & Liang, 2003; Perng, Wen, van den, Prescott, & Quinlan, 2004). The results are consistent with the hypothesis that filaments play an important role in the dynamic subunit model for chaperone activity of sHSPs (Launay, Goudeau, Kato, Vicart, & Lilienbaum, 2006; Liu, Ghosh, Clark, & Jiang, 2006).
Fig. 6.

Interactive surfaces for chaperone activity and filament interactions. Interactive sequences in human αB crystallin that mediate interactions with chaperone target proteins (blue), desmin (pink), GFAP (orange), and actin (green) were mapped to the surface of the 3-D homology model of human αB crystallin described previously (Ghosh & Clark, 2005; Ghosh, Estrada, & Clark, 2005). While the interactions with filaments occur in similar domains on αB crystallin, there appear to be slight differences in the surface areas used for these interactions. The interactive domains for filaments correspond to the interactive domains for unfolded chaperone target proteins.
In summary, pin arrays identified interactive domains on the molecular chaperone, human αB crystallin that mediated interactions with the filament proteins desmin, GFAP, and actin. The presence of common functional surface domains that mediate interactions with both filaments and cytosolic lens crystallins suggested that differential affinities between αB crystallin and substrate proteins may be the basis for the recognition, selection, and binding steps of sHSP chaperone function. Further experiments are underway using surface plasmon resonance to quantify the functional significance of differences in affinities between αB crystallin subunits, filaments and unfolded proteins in a mechanism involving dynamic subunit exchange in the unfolding protein response.
Acknowledgments
This work was supported by EY04542 from the NEI.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bagchi M, Katar M, Maisel H. Heat shock proteins of adult and embryonic human ocular lenses. J Cell Biochem. 2002;84(2):278–284. [PubMed] [Google Scholar]
- Bennardini F, Wrzosek A, Chiesi M. Alpha B-crystallin in cardiac tissue. Association with actin and desmin filaments. Circ Res. 1992;71(2):288–294. doi: 10.1161/01.res.71.2.288. [DOI] [PubMed] [Google Scholar]
- Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J Biol Chem. 1994;269(32):20780–20784. [PubMed] [Google Scholar]
- Bertazzon A, Tian GH, Lamblin A, Tsong TY. Enthalpic and entropic contributions to actin stability: calorimetry, circular dichroism, and fluorescence study and effects of calcium. Biochemistry. 1990;29(1):291–298. doi: 10.1021/bi00453a040. [DOI] [PubMed] [Google Scholar]
- Bertazzon A, Tsong TY. Effects of ions and pH on the thermal stability of thin and thick filaments of skeletal muscle: high-sensitivity differential scanning calorimetric study. Biochemistry. 1990;29(27):6447–6452. doi: 10.1021/bi00479a016. [DOI] [PubMed] [Google Scholar]
- Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86(3):407–485. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Bova MP, Yaron O, Huang Q, Ding L, Haley DA, Stewart PL, et al. Mutation R120G in alphaB-crystallin, which is linked to a desmin-related myopathy, results in an irregular structure and defective chaperone-like function. Proc Natl Acad Sci U S A. 1999;96(11):6137–6142. doi: 10.1073/pnas.96.11.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JM, Hutcheson AM, Quinlan RA. In vitro studies on the assembly properties of the lens proteins CP49, CP115: coassembly with alpha-crystallin but not with vimentin. Exp Eye Res. 1995;60(2):181–192. doi: 10.1016/s0014-4835(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Chou RG, Stromer MH, Robson RM, Huiatt TW. Determination of the critical concentration required for desmin assembly. Biochem J. 1990;272(1):139–145. doi: 10.1042/bj2720139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djabali K, Piron G, de Nechaud B, Portier MM. alphaB-crystallin interacts with cytoplasmic intermediate filament bundles during mitosis. Exp Cell Res. 1999;253(2):649–662. doi: 10.1006/excr.1999.4679. [DOI] [PubMed] [Google Scholar]
- Ecroyd H, Meehan S, Horwitz J, Aquilina JA, Benesch JL, Robinson CV, et al. Mimicking phosphorylation of alphaB-crystallin affects its chaperone activity. Biochem J. 2006 doi: 10.1042/BJ20060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FitzGerald PG, Graham D. Ultrastructural localization of alpha A-crystallin to the bovine lens fiber cell cytoskeleton. Curr Eye Res. 1991;10(5):417–436. doi: 10.3109/02713689109001750. [DOI] [PubMed] [Google Scholar]
- Fu L, Liang JJ. Alteration of protein-protein interactions of congenital cataract crystallin mutants. Invest Ophthalmol Vis Sci. 2003;44(3):1155–1159. doi: 10.1167/iovs.02-0950. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Geysen HM. Molecular technology: peptide epitope mapping and the pin technology. Southeast Asian J Trop Med Public Health. 1990;21(4):523–533. [PubMed] [Google Scholar]
- Ghosh JG, Clark JI. Insights into the domains required for dimerization and assembly of human alphaB crystallin. Protein Sci. 2005;14(3):684–695. doi: 10.1110/ps.041152805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Estrada MR, Clark JI. Interactive Domains for Chaperone Activity in the Small Heat Shock Protein, Human alphaB Crystallin. Biochemistry. 2005;44(45):14854–14869. doi: 10.1021/bi0503910. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Estrada MR, Clark JI. Structure-based analysis of the beta8 interactive sequence of human alphaB crystallin. Biochemistry. 2006;45(32):9878–9886. doi: 10.1021/bi060970k. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Estrada MR, Houck SA, Clark JI. The function of the beta3 interactive domain in the small heat shock protein and molecular chaperone, human alphaB crystallin. Cell Stress Chaperones. 2006;11(2):187–197. doi: 10.1379/CSC-186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JG, Shenoy AK, Jr, Clark JI. N- and C-Terminal Motifs in Human alphaB Crystallin Play an Important Role in the Recognition, Selection, and Solubilization of Substrates. Biochemistry. 2006;45(46):13847–13854. doi: 10.1021/bi061471m. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Boyle D, Takemoto L. Association of actin with alpha crystallins. Trans Kans Acad Sci. 1993;96(12):7–12. [PubMed] [Google Scholar]
- Gopalakrishnan S, Takemoto L. Binding of actin to lens alpha crystallins. Curr Eye Res. 1992;11(9):929–933. doi: 10.3109/02713689209033490. [DOI] [PubMed] [Google Scholar]
- Head MW, Hurwitz L, Kegel K, Goldman JE. AlphaB-crystallin regulates intermediate filament organization in situ. Neuroreport. 2000;11(2):361–365. doi: 10.1097/00001756-200002070-00028. [DOI] [PubMed] [Google Scholar]
- Herrmann H, Aebi U. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular Scaffolds. Annu Rev Biochem. 2004;73:749–789. doi: 10.1146/annurev.biochem.73.011303.073823. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Hayashi T, Arimura T, Koga Y, Takahashi M, Shibata H, et al. AlphaB-crystallin mutation in dilated cardiomyopathy. Biochem Biophys Res Commun. 2006;342(2):379–386. doi: 10.1016/j.bbrc.2006.01.154. [DOI] [PubMed] [Google Scholar]
- Ireland M, Maisel H. A family of lens fiber cell specific proteins. Lens Eye Toxic Res. 1989;6(4):623–638. [PubMed] [Google Scholar]
- Kato K, Inaguma Y, Ito H, Iida K, Iwamoto I, Kamei K, et al. Ser-59 is the major phosphorylation site in alphaB-crystallin accumulated in the brains of patients with Alexander’s disease. J Neurochem. 2001;76(3):730–736. doi: 10.1046/j.1471-4159.2001.00038.x. [DOI] [PubMed] [Google Scholar]
- Kumar N, Tomar A, Parrill AL, Khurana S. Functional dissection and molecular characterization of calcium-sensitive actin-capping and actin-depolymerizing sites in villin. J Biol Chem. 2004;279(43):45036–45046. doi: 10.1074/jbc.M405424200. [DOI] [PubMed] [Google Scholar]
- Launay N, Goudeau B, Kato K, Vicart P, Lilienbaum A. Cell signaling pathways to alphaB-crystallin following stresses of the cytoskeleton. Exp Cell Res. 2006 doi: 10.1016/j.yexcr.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. J Cell Sci. 1997;110(Pt 13):1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- Lieska N, Chen J, Maisel H, Romero-Herrera AE. Subunit characterization of lens intermediate filaments. Biochim Biophys Acta. 1980;626(1):136–153. doi: 10.1016/0005-2795(80)90205-6. [DOI] [PubMed] [Google Scholar]
- Litt M, Kramer P, LaMorticella DM, Murphey W, Lovrien EW, Weleber RG. Autosomal dominant congenital cataract associated with a missense mutation in the human alpha crystallin gene CRYAA. Hum Mol Genet. 1998;7(3):471–474. doi: 10.1093/hmg/7.3.471. [DOI] [PubMed] [Google Scholar]
- Liu L, Ghosh JG, Clark JI, Jiang S. Studies of alphaB crystallin subunit dynamics by surface plasmon resonance. Anal Biochem. 2006;350(2):186–195. doi: 10.1016/j.ab.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Lobley A, Whitmore L, Wallace BA. DICHROWEB: an interactive website for the analysis of protein secondary structure from circular dichroism spectra. Bioinformatics. 2002;18(1):211–212. doi: 10.1093/bioinformatics/18.1.211. [DOI] [PubMed] [Google Scholar]
- Longoni S, Lattonen S, Bullock G, Chiesi M. Cardiac alpha-crystallin. II. Intracellular localization. Mol Cell Biochem. 1990;97(2):121–128. doi: 10.1007/BF00221053. [DOI] [PubMed] [Google Scholar]
- MacRae TH. Structure and function of small heat shock/alpha-crystallin proteins: established concepts and emerging ideas. Cell Mol Life Sci. 2000;57(6):899–913. doi: 10.1007/PL00000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglara AA, Vasilaki A, Jackson MJ, McArdle A. Damage to developing mouse skeletal muscle myotubes in culture: protective effect of heat shock proteins. J Physiol. 2003;548(Pt 3):837–846. doi: 10.1113/jphysiol.2002.034520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melkani GC, Cammarato A, Bernstein SI. alphaB-Crystallin Maintains Skeletal Muscle Myosin Enzymatic Activity and Prevents its Aggregation under Heat-shock Stress. J Mol Biol. 2006 doi: 10.1016/j.jmb.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Morrison LE, Hoover HE, Thuerauf DJ, Glembotski CC. Mimicking phosphorylation of alphaB-crystallin on serine-59 is necessary and sufficient to provide maximal protection of cardiac myocytes from apoptosis. Circ Res. 2003;92(2):203–211. doi: 10.1161/01.res.0000052989.83995.a5. [DOI] [PubMed] [Google Scholar]
- Mounier N, Arrigo AP. Actin cytoskeleton and small heat shock proteins: how do they interact? Cell Stress Chaperones. 2002;7(2):167–176. doi: 10.1379/1466-1268(2002)007<0167:acashs>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchowski PJ, Valdez MM, Clark JI. AlphaB-crystallin selectively targets intermediate filament proteins during thermal stress. Invest Ophthalmol Vis Sci. 1999;40(5):951–958. [PubMed] [Google Scholar]
- Nicholl ID, Quinlan RA. Chaperone activity of alpha-crystallins modulates intermediate filament assembly. Embo J. 1994;13(4):945–953. doi: 10.1002/j.1460-2075.1994.tb06339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban J, Halasi S, Papp G, Barko S, Bugyi B. Thermodynamic characterization of different actin isoforms. Journal of Thermal Analysis and Calorimetry. 2005;82(1):287–290. [Google Scholar]
- Pan XX, Ware BR. Actin assembly by lithium ions. Biophys J. 1988;53(1):11–16. doi: 10.1016/S0006-3495(88)83060-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papp G, Bugyi B, Ujfalusi Z, Halasi S, Orban J. The effect of pH on the thermal stability of a-actin isoforms. Journal of Thermal Analysis and Calorimetry. 2005;82(1):281–285. [Google Scholar]
- Perng MD, Cairns L, van den IP, Prescott A, Hutcheson AM, Quinlan RA. Intermediate filament interactions can be altered by HSP27 and alphaB-crystallin. J Cell Sci. 1999;112(Pt 13):2099–2112. doi: 10.1242/jcs.112.13.2099. [DOI] [PubMed] [Google Scholar]
- Perng MD, Muchowski PJ, van Den IP, Wu GJ, Hutcheson AM, Clark JI, et al. The cardiomyopathy and lens cataract mutation in alphaB-crystallin alters its protein structure, chaperone activity, and interaction with intermediate filaments in vitro. J Biol Chem. 1999;274(47):33235–33243. doi: 10.1074/jbc.274.47.33235. [DOI] [PubMed] [Google Scholar]
- Perng MD, Wen SF, van den IP, Prescott AR, Quinlan RA. Desmin aggregate formation by R120G alphaB-crystallin is caused by altered filament interactions and is dependent upon network status in cells. Mol Biol Cell. 2004;15(5):2335–2346. doi: 10.1091/mbc.E03-12-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott AR, Sandilands A, Hutcheson AM, Carter JM, Quinlan RA. The intermediate filament cytoskeleton of the lens: an ever changing network through development and differentiation. A minireview. Ophthalmic Res. 1996;28(Suppl 1):58–61. doi: 10.1159/000267946. [DOI] [PubMed] [Google Scholar]
- Quinlan R. Cytoskeletal competence requires protein chaperones. Prog Mol Subcell Biol. 2002;28:219–233. doi: 10.1007/978-3-642-56348-5_12. [DOI] [PubMed] [Google Scholar]
- Quinlan RA, Carte JM, Sandilands A, Prescott AR. The beaded filament of the eye lens: an unexpected key to intermediate filament structure and function. Trends Cell Biol. 1996;6(4):123–126. doi: 10.1016/0962-8924(96)20001-7. [DOI] [PubMed] [Google Scholar]
- Sandilands A, Prescott AR, Carter JM, Hutcheson AM, Quinlan RA, Richards J, et al. Vimentin and CP49/filensin form distinct networks in the lens which are independently modulated during lens fibre cell differentiation. J Cell Sci. 1995;108(Pt 4):1397–1406. doi: 10.1242/jcs.108.4.1397. [DOI] [PubMed] [Google Scholar]
- Selcen D, Engel AG. Myofibrillar myopathy caused by novel dominant negative alpha B-crystallin mutations. Ann Neurol. 2003;54(6):804–810. doi: 10.1002/ana.10767. [DOI] [PubMed] [Google Scholar]
- Shu E, Matsuno H, Akamastu S, Kanno Y, Suga H, Nakajima K, et al. alphaB-crystallin is phosphorylated during myocardial infarction: Involvement of platelet-derived growth factor-BB. Arch Biochem Biophys. 2005;438(2):111–118. doi: 10.1016/j.abb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Singh BN, Rao KS, Ramakrishna T, Rangaraj N, Rao CM. Association of alphaB-Crystallin, a Small Heat Shock Protein, with Actin: Role in Modulating Actin Filament Dynamics in Vivo. J Mol Biol. 2006 doi: 10.1016/j.jmb.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Sun Y, Macrae TH. The small heat shock proteins and their role in human disease. Febs J. 2005;272(11):2613–2627. doi: 10.1111/j.1742-4658.2005.04708.x. [DOI] [PubMed] [Google Scholar]
- Tomokane N, Iwaki T, Tateishi J, Iwaki A, Goldman JE. Rosenthal fibers share epitopes with alpha B-crystallin, glial fibrillary acidic protein, and ubiquitin, but not with vimentin. Immunoelectron microscopy with colloidal gold. Am J Pathol. 1991;138(4):875–885. [PMC free article] [PubMed] [Google Scholar]
- Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, et al. A missense mutation in the alphaB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet. 1998;20(1):92–95. doi: 10.1038/1765. [DOI] [PubMed] [Google Scholar]
- Wang F, Sampogna RV, Ware BR. pH dependence of actin self-assembly. Biophys J. 1989;55(2):293–298. doi: 10.1016/S0006-3495(89)82804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Spector A. alpha-crystallin stabilizes actin filaments and prevents cytochalasin-induced depolymerization in a phosphorylation-dependent manner. Eur J Biochem. 1996;242(1):56–66. doi: 10.1111/j.1432-1033.1996.0056r.x. [DOI] [PubMed] [Google Scholar]
- Webster KA. Serine phosphorylation and suppression of apoptosis by the small heat shock protein alphaB-crystallin. Circ Res. 2003;92(2):130–132. doi: 10.1161/01.res.0000056967.51841.21. [DOI] [PubMed] [Google Scholar]
- Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32(Web Server issue):W668–673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski T, Goldman JE. Alpha B-crystallin is associated with intermediate filaments in astrocytoma cells. Neurochem Res. 1998;23(3):385–392. doi: 10.1023/a:1022465702518. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Babitch JA. Factors modulating filament formation by bovine glial fibrillary acidic protein, the intermediate filament component of astroglial cells. Biochemistry. 1988;27(18):7038–7045. doi: 10.1021/bi00418a055. [DOI] [PubMed] [Google Scholar]


