Abstract
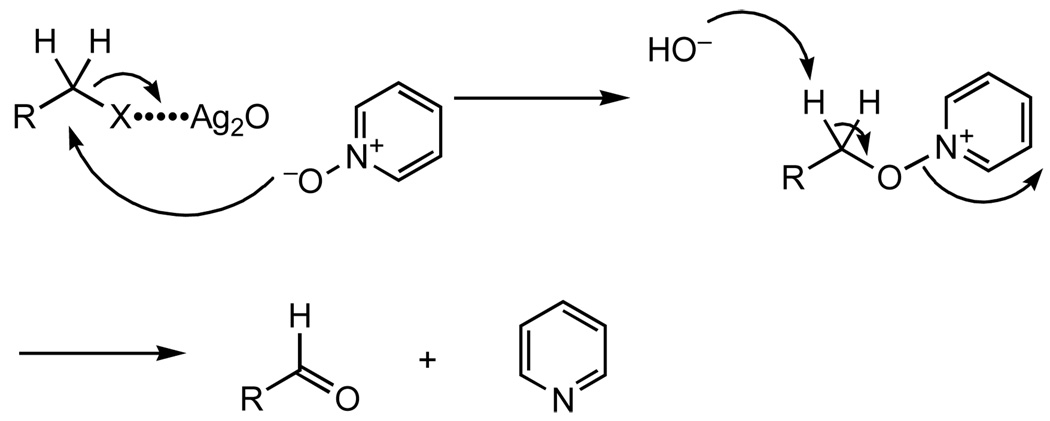
Benzylic and allylic halides were conveniently oxidized to aldehydes and ketones by pyridine N-oxide in the presence of silver oxide under mild conditions.
Aldehydes and ketones, especially aromatic and α,β-unsaturated carbonyl compounds, are important classes of chemicals. Methods for direct conversion of halides to carbonyl compounds have been reviewed.1,2 Dimethyl sulfoxide (DMSO) is often employed as the oxygen donor.3,4 However, high temperature is usually required. 3,4 Oxidations involving amine oxides have also been reported.5–7 In our attempt to modify carbohydrates with benzylic halides, we have found that pyridine N-oxide can effectively and conveniently oxidize benzylic and allylic halides to aromatic and α,β-unsaturated carbonyl compounds, respectively, in the presence of silver oxide (Ag2O) under mild conditions.8,9
The reactions were carried out in acetonitrile (toluene and tetrahydrofuran gave much lower yield). The reaction mechanism should be identical to that proposed for the reaction with DMSO and base, as shown in Scheme 1.1,8 Half equivalent of silver oxide was utilized to facilitate the reaction. Silver oxide assists the heterolysis of the carbon-halogen bond in the substitution reaction with pyridine N-oxide. The resulting hydroxide ion from the reaction between silver oxide and halogen then functions as the base in the elimination reaction to produce the carbonyl group. When DMSO was employed instead of pyridine N-oxide as the source of oxygen, the reaction did not go to completion while giving a mixture of products.
SCHEME 1.
For most bromides, the reactions were conveniently carried out at room temperature. The reaction was complete in a few hours but was stirred overnight for convenience. For chlorides and some benzyl bromides with very strong electron-withdrawing substituents, slightly elevated temperature (50 °C) was required (Entries 4–7 and 12–15 in Table 1). For some chlorides, only partial conversion was observed (Entries 13 and 14). The substituent effects indicate a SN1-like mechanism for the first step in the reaction pathway.
Table 1.
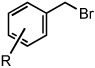
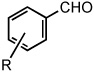
Reduction of Benzylic and Allylic Halides with Pyridine N-Oxide in CH3CN
| Entry | Substrate | Product | Temperature | Yield (isolated) |
|---|---|---|---|---|
 |
 |
|||
| 1 | R = H | 25 °C | 82% | |
| 2 | R = p-Br | 25 °C | 80% | |
| 3 | R = p-CO2CH3 | 25 °C | 81% | |
| 4 | R = m-CN | 50 °C | 84% | |
| 5 | R = p-CN | 50 °C | 80% | |
| 6 | R = m-NO2 | 50 °C | 86% | |
| 7 | R = p-NO2 | 50 °C | 86% | |
| 8 | 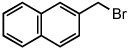 |
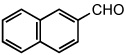 |
25 °C | |
| 9 | 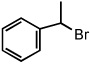 |
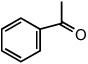 |
25 °C | 84% |
| 10 | 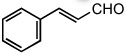 |
25 °C | 85% | |
| 11 | 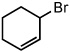 |
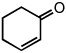 |
25 °C | 85% |
| 12 | 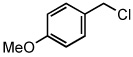 |
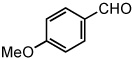 |
50 °C | 92% |
| 13 | 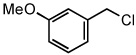 |
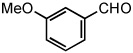 |
50 °C | 74%a |
| 14 | 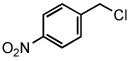 |
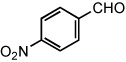 |
50 °C | 75%b |
| 15 | 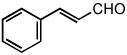 |
50 °C | 95% | |
The yield is based on 71% converted starting material.
The yield is based on 50% converted starting material.
The reaction workup was very simple. Upon completion of the reaction, sodium sulfate or magnesium sulfate was added and the resulting mixture was filtered through a thin layer of Celite. Concentration of the filtrate gave essentially pure product in quantitative yield.10 Further purification, when desired, consisted of filtering the crude product through a short column of silica gel with methylene chloride as the solvent. Pyridine by-product was removed by initial washing of the loaded column with hexane. The percent yields shown in Table 1 refer to the isolated yields of the purified products and are generally good.
The above results have demonstrated that pyridine N-oxide is an efficient oxidizing agent for the conversion of benzylic and allylic halides to aromatic and α,β-unsaturated aldehydes and ketones, respectively. The reaction can be applied to benzylic halides with both electron- donating and withdrawing substitutents. The reaction described here is thus a useful and convenient alternative to existing methods for oxidation of benzylic and allylic halides to conjugated carbonyl compounds.
Experimental Details
All reagents were obtained from commercial sources and used without further purification. The reactions were run in a nitrogen atmosphere. Typical experimental procedure: Silver oxide (0.68 g, 2.93 mmol) was added to a solution of benzyl bromide (1.00 g, 5.85 mmol) and pyridine N-oxide (0.56 g, 5.85 mmol) in acetonitrile (10 mL) in a round-bottom flask. The resulting mixture was stirred overnight under nitrogen. Sodium sulfate or magnesium sulfate was added to the reaction and the resulting mixture was filtered through a thin layer of Celite. The flask and funnel were rinsed with ethyl acetate. Concentration of the combined filtrate gave a slightly yellow oil. The crude product was loaded on a short column of silica gel, which was eluted with hexane to remove pyridine by-product and then with methylene chloride. The methylene chloride solution was concentrated to yield a clear oil (0.51 g, 82%). The product was identified by comparison of its NMR spectrum to that of an authentic sample.10
Acknowledgments
This investigation was supported by the National Institutes of Health, MBRS SCORE Program – Grant #5 S06 GM52588 and by a grant from the Center for Computing for Life Sciences at SFSU. Mr. Q. Y. R. Wu was supported by Project SEED of the American Chemical Society. We are indebted to Mr. Wee Tam at SFSU for obtaining the NMR spectra. The NMR facility was funded by the National Science Foundation (DUE-9451624 and DBI 0521342). We thank Dr. Ihsan Erden (SFSU) for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.For a review on direct conversion of halides to aldehydes and ketones, see: Smith MB, March J. March’s Advanced Organic Chemistry. Reactions, Mechanisms, and Structure. 5th ed. New York: Wiley; 2001. pp. 1535–1536.
- 2.For another review on direct conversion of halides to aldehydes and ketones see: Larock RC. Comprehensive Organic Transformations. New York: Wiley; 1999. pp. 1222–1223.
- 3.Kornblum N, Jones WJ, Anderson GJ. J. Am. Chem. Soc. 1959;81:4113–4114. [Google Scholar]
- 4.Nace HR, Monagle JJ. J. Org. Chem. 1959;24:1792–1793. [Google Scholar]
- 5.(a) Franzen V, Otto S. Chem Ber. 1961;94:1360–1363. [Google Scholar]; (b) Franzen V. Org. Synth. Coll. Vol. V. 1955:872–874. [Google Scholar]
- 6.Suzuki S, Onoshi T, Fujita Y, Misawa H, Otera J. Bull. Chem. Soc. Jpn. 1986;59:3287–3288. [Google Scholar]
- 7.Mukaiyama S, Inanaga J, Yamagauchi M. Bull. Chem. Soc. Jpn. 1981;54:2221–2222. [Google Scholar]
- 8.Alkaline decomposition of pyridinimium salts have been discussed: Ochiai E, Katada M, Naito T. J. Pham. Soc. Jpn. 1944;64:210–214. Katritsky AR. J. Chem. Soc. 1956:2404–2408. Feely W, Lehn WL, Boekelheide V. J. Org. Chem. 1957;22:1135–1135. Silwa H, Tartar A. J. Org. Chem. 1976;41:160–163.
- 9.Microwave-assisted reactions of substituted benzyl bromide with pyridine N-oxide have been reported, but the yields were poor for some bromides: Barbry D, Champagne P. Tetrahedron Lett. 1996;37:7725–7726.
- 10.All products have NMR spectra identical to those of authentic samples. They can be found in: Pouchert CJ, Behnke J. The Aldrich Library of 13C and 1H FT-NMR Spectra. Milwaukee: Aldrich; 1992.