Abstract
Advances in modern neuroscience require the identification of principles that connect different levels of experimental analysis, from molecular mechanisms to explanations of cellular functions, then to circuits, and, ultimately, to systems and behavior. Here, we examine how synaptic organization of the sympathetic ganglia may enable them to function as use-dependent amplifiers of preganglionic activity and how the gain of this amplification may be modulated by metabotropic signaling mechanisms. The approach combines a general computational model of ganglionic integration together with experimental tests of the model using the dynamic clamp method. In these experiments, we recorded intracellularly from dissociated bullfrog sympathetic neurons and then mimicked physiological synapses with virtual computer-generated synapses. It thus became possible to analyze the synaptic gain by recording cellular responses to complex patterns of synaptic activity that normally arise in vivo from convergent nicotinic and muscarinic synapses. The results of these studies are significant because they illustrate how gain generated through ganglionic integration may contribute to the feedback control of important autonomic behaviors, in particular to the control of the blood pressure. We dedicate this paper to the memory of Professor Vladimir Skok, whose rich legacy in synaptic physiology helped establish the modern paradigm for connecting multiple levels of analysis in studies of the nervous system.
Keywords: nicotinic synapses, muscarinic receptors, neuropeptides, bullfrog sympathetic ganglia, conductance-based models, baroreceptor reflex
INTRODUCTION
A paradox emerges when comparing the synaptic organization of autonomic ganglia from the cellular and system perspectives. Cellular studies have established that parasympathetic and sympathetic ganglia are characterized by pre-ganglionic anatomical divergence, by post-ganglionic synaptic convergence of ionotropic nicotinic synapses, and by the expression of varied metabotropic synaptic events [1–5]. However, from the system perspective, understanding the integrative significance of these cellular and molecular details remains problematic [6]. Consequently, parasympathetic and sympathetic ganglia are often described simply as synaptic relays that convey neural activity from spinal preganglionic neurons to peripheral end-organs. Excitatory muscarinic synapses provide a good example of this paradox. Although intracellular studies dating back to the 1960s show that pre-synaptic stimulation of sympathetic neurons can generate a two-component excitatory post-synaptic potential (EPSP) consisting of a fast nicotinic EPSP followed by a slow muscarinic EPSP; the functional role of the muscarinic response has remained elusive [1, 3, 6]. The problem arises in part because a pharmacological block of muscarinic receptors has multiple central and peripheral effects, which obscure the consequences of the ganglionic muscarinic receptors for the blood pressure control and other autonomic behaviors. In order to better understand this class of problems, we have recently developed dynamic clamp experiments that permit us to simulate patterns of synaptic activity that occur in vivo, while, at the same time, having the experimental control that is possible in vitro with perforated-patch whole-cell intracellular recordings. This paper summarizes the rationale for this approach and the underlying assumptions, what we have learned to this point and emerging new questions.
ADVANTAGES AND LIMITATIONS OF AMPHIBIAN SYMPATHETIC GANGLIA AS A MODEL SYSTEM
The most important reason why paravertebral sympathetic ganglia 9 and 10 in frogs and toads are a powerful experimental system for the cellular analysis of synaptic mechanisms is the following: These ganglia contain two major cell types that can be identified in isolated preparations [3]. Such cells, B and C neurons, have distinct axonal conduction velocities [7] and are selectively innervated by preganglionic neurons that enter the sympathetic chain at different segmental levels [8–10]. This feature allows researchers to independently stimulate the preganglionic B and C pathways, which has proven very useful in experimental studies of slow synaptic potentials and target specificity. In B cells, nicotinic receptors mediate fast EPSPs, while muscarinic receptors mediate presynaptic inhibition and slow EPSPs [1, 11–13]. Presynaptic stimulation of C cells evokes a fast nicotinic EPSP followed by a slow muscarinic inhibitory postsynaptic potential (IPSP) [13, 14]. Stimulation of the preganglionic C pathway also evokes co-release of a neuropeptide, luteinizing hormone-releasing hormone (LHRH), which produces slow EPSPs in both B and C cells [15, 16]. With regard to the target specificity and function, B cells selectively innervate cutaneous glands that are important for thermoregulation and defensive behaviors, while C cells selectively innervate arteries that regulate the blood flow and pressure [17, 18]. The analysis of synaptic mechanisms in identified cells has thus revealed many interesting features of this system, including clear differences in the cellular expression of muscarinic responses.
Additional advantages of amphibian ganglia for cellular studies of synaptic function arise from their cellular and ganglionic morphology. Neurons in amphibian ganglia are larger than in homologous mammalian ganglia (diameters of 30–70 µm vs 15–30 µm), and the amphibian ganglia are relatively thin. One can, therefore, visualize the cells in great detail under Nomarski optics and obtain high-quality microelectrode recordings from cells in the intact ganglia [19–22]. Amphibian autonomic neurons are also distinguished by their monopolar cell bodies. The absence of dendrites, which are present in mammalian autonomic neurons, indicates that amphibian autonomic neurons are electrotonically compact, thus simplifying the interpretation of electrophysiological data.
Finally, it is relatively easy to dissociate adult ganglia from amphibians and study the properties of fully differentiated sympathetic neurons in tissue culture using high-resolution patch-clamp recordings [23–26]. The most significant limitation in working with amphibian models is the difficulty in applying molecular tools for manipulating gene expression. Additionally, the bullfrogs used in our experiments (Rana catesbiana, 7–18 cm in length) are generally 4–8 years old when collected in the wild. Although different amphibian and mammalian animal models have their respective advantages and limitations, it remains important to compare them and identify those cellular properties and organizational features that are conserved and those that mediate specialized forms of behavior.
KEY EXPERIMENTS THAT SHIFTED THE ANALYSIS OF MODULATION AWAY FROM AFTERDISCHARGES
One of our long-term goals has been to understand the consequences of slow synaptic potentials for integration in sympathetic ganglia. In thinking about this problem, we were strongly influenced by early observations showing that slow EPSPs in extracellular recordings were associated with afterdischarges of postsynaptic action potentials [27, 28]. Intracellular recordings subsequently demonstrated that muscarinic and peptidergic excitation tended to promote repetitive firing, which, in some cases, could be blocked by muscarinic inhibition [14, 29, 30]. Based on these findings, we developed isolated preparations of the bullfrog lumbar ganglia together with the abdominal aorta [18] and cutaneous glands [17]. These experiments demonstrated that peptidergic afterdischarges recorded in the presence of nicotinic blockers could indeed activate responses in the end-organs. However, because very strong presynaptic stimuli were required to see these effects, and the nicotinic blockers that were employed had nonspecific actions, we concluded that metabotropically mediated afterdischarges were unlikely to play a major role under physiological conditions.
THE n + 1 RULE FOR NICOTINIC CONVERGENCE
The experiments examining the effects of afterdischarges upon end-organs [17, 18] were perplexing. If afterdischarges were not of great importance, then how might slow potentials be acting, especially in B neurons where it was widely accepted that each cell received only one very strong nicotinic synapse [7] nearly insensitive to modulation because of its strength.
At that time, Ivanoff (Ivanov) and Smith [31] published an important experiment that completely changed our thinking on the problem and sent us in a new direction. They recorded intracellularly in vivo from bullfrog sympathetic neurons using methods that were originally developed at the Bogomolets Institute of Physiology in the laboratory of Vladimir Skok [2, 32]. Ivanoff and Smith’s data contained substantial numbers of subthreshold nicotinic EPSPs in bullfrog B neurons. This meant that the prevailing view supposing B neurons to be singly innervated should be incorrect. We, therefore, went back to re-examine nicotinic convergence in B neurons and consider the consequences for ganglionic integration and its modulation.
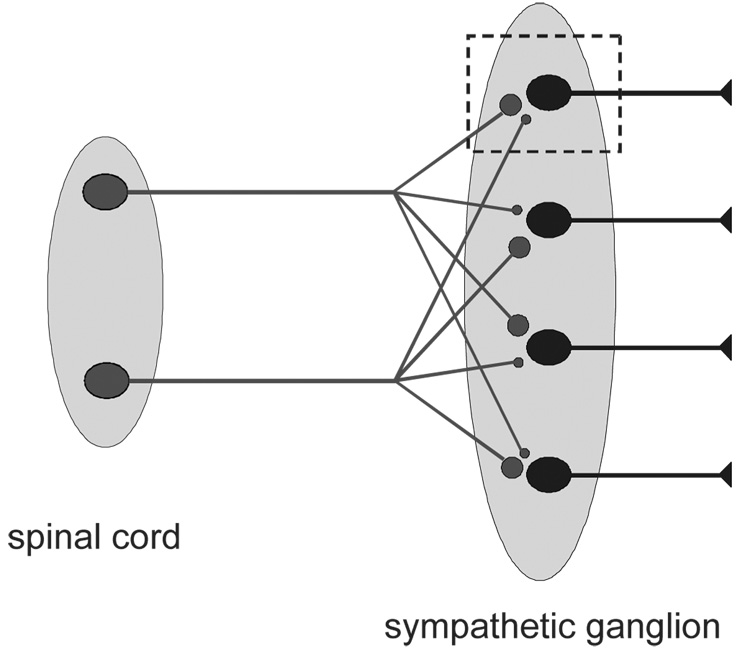
Karila and Horn [33] found that 93% of B neurons receive at least two nicotinic synapses with distinct thresholds for presynaptic stimulation. One input produces EPSPs that are always strong enough to elicit action potentials, and other inputs produce EPSPs that are generally subthreshold in strength. Although all of the cells we studied had a single strong input, the number of weak inputs varied from 0 to 3. We, therefore, called the strong synapses primary and the weak synapses secondary. From the literature, it was also evident that a similar pattern of nicotinic convergence was present in bullfrog C neurons [14] and in mammalian paravertebral sympathetic neurons [32, 34–37]. Papers from the Skok laboratory referred to the secondary synapses as accessory, and those from the McLachlan laboratory simply described two types of synapses as strong and weak. It was also known from systematic studies on the superior cervical ganglion (SCG) in several different mammalian species that the number of synapses converging on individual neurons scaled with animal size [38]. With these observations in mind, Karila and Horn [33] proposed that nicotinic convergence in paravertebral sympathetic ganglia follows an n + 1 rule, which states that each cell receives a variable number (n) of weak secondary synapses and one strong primary synapse (Fig. 1).
Fig. 1.
Nicotinic synaptic connections between spinal pre- and post-ganglionic neurons in the paravertebral sympathetic ganglia are characterized by pre-ganglionic divergence and post-ganglionic convergence, which follows the n + 1 rule. Given the lack of connections between ganglion cells and the uniformity of preganglionic synapses onto ganglion cells, one can predict how the entire population will behave by studying one cell (dashed rectangle). In this schematic, each ganglion cell receives one primary synapse (large bouton) and one secondary synapse (small bouton).
Р и с. 1. Синаптичні зв’язки з нікотиновою рецепцією між спінальними пре- та постгангліонарними нейронами в паравертебральних симпатичних гангліях характеризуються прегангіонарною дивергенцією та постгангліонарною конвергенцією, що відповідає правилу n + 1.
COINCIDENCE DETECTION THEORY PROVIDES A UNIFIED THEORY OF GANGLIONIC INTEGRATION
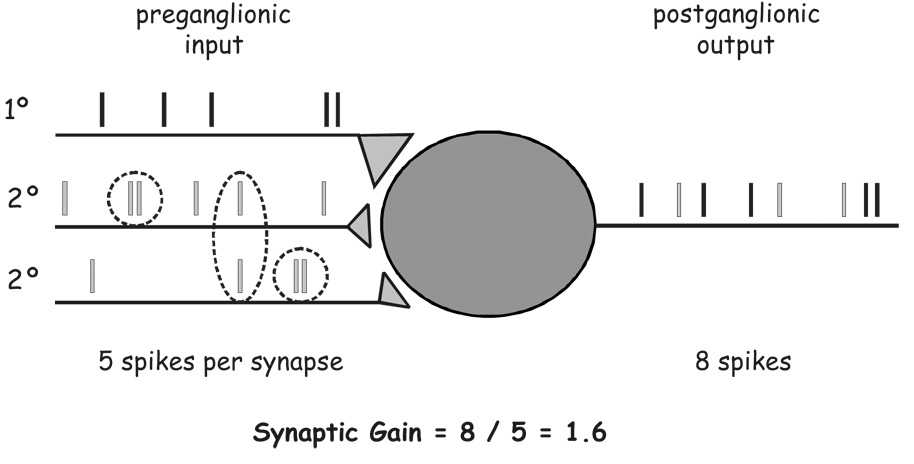
Finding that convergence was minimal in bullfrog B neurons (most cells receive only a total of two inputs) suggested that the weak input could not have much physiological impact, but also provided the simplest possible experimental system for isolating and studying the weak input. Karila and Horn [33], thus, demonstrated that (i) simple trial-to-trial fluctuations in the secondary EPSP amplitude sometimes exceeded the threshold, (ii) pairs of secondary EPSPs can summate to exceed the threshold, (iii) the temporal window for such interaction is lengthened by presynaptic facilitation, and (iv) slow peptidergic EPSPs can summate with secondary EPSPs to reach the threshold. The summation of secondary EPSPs suggested that B neurons might behave as coincidence detectors for pairs of secondary EPSPs (Fig. 2), and that all the various forms of synaptic plasticity in the ganglia might act through a common mechanism to modulate the effective strength of secondary EPSPs.
Fig. 2.
The coincidence detector model of synaptic gain. Coincidences between subthreshold secondary nicotinic EPSPs can produce synaptic amplification by eliciting action potentials in addition to the ones invariably evoked by the strong primary synapse. Bars above the presynaptic axons denote the timing of presynaptic spikes and the EPSPs they evoke. Bars above the postsynaptic axon denote the timing of action potentials triggered by primary EPSPs (black) and coincidences of secondary EPSPs (gray). Note that, for the time epoch shown, the post-synaptic cell fires at a higher rate than the average pre-synaptic rate.
Р и с. 2. Модель синаптичної передачі із задіянням принципу детектора збігів.
УДК 612.8.01
Синаптическая интеграция в симпатических ганглиях (анализ с использованием метода фиксации динамики синаптических влияний) / Хорн Дж. П., Куллманн П. Х. М. // Нейрофизиология / Neurophysiology. –2007. –39, № 6. –С.
Успехи в современной области нейронаук требуют идентификации принципов, объединяющих разные уровни экспериментального анализа, начиная с выяснения молекулярных механизмов и клеточных функций, затем – нейронных цепей и, наконец, – систем и поведенческих феноменов в целом. Наша лекция посвящена обзору известных данных о специфике синаптической организации симпатических ганглиев, которая позволяет им функционировать как зависимые от использования усилители преганглионарной активности. В связи с этим мы исследовали, как коэффициент передачи такой активности может модулироваться механизмами метаботропной сигнализации. Наш подход объединяет обычное расчетное моделирование ганглионарной интеграции с экспериментальным тестированием этой модели с использованием метода фиксации динамики синаптических влияний. В наших экспериментах мы сначала осуществляли внутриклеточные отведения от диссоциированных симпатических нейронов лягушки-быка, а потом имитировали физиологические синапсы (паттерны синаптической активности in vivo), используя действие виртуальных синапсов, генерированное с помощью компьютера. Следовательно, такие процедуры позволяли нам анализировать коэффициент синаптической передачи с использованием регистраций клеточных ответов, обусловленных сложными паттернами синаптической активности, которые обычно возникают в условиях in vivo в конвергентных никотиновых и мускариновых синапсах. Результаты таких исследований достаточно весомы, поскольку они иллюстрируют, как характеристики передачи, зависимые от ганглионарной интеграции, могут вносить вклад в контроль важных вегетативных поведенческих феноменов, осуществляемый по принципу обратной связи, в частности в контроль давления крови. Мы посвящаем эту публикацию памяти проф. Владимира Скока, ценное наследие которого в области физиологии синапсов способствовало созданию современной парадигмы в отношении объединения многочисленных уровней анализа в исследованиях нервной системы. Ил. 2. Библиогр. 44.
To explore the consequences of this simple idea, Karila and Horn derived a model of ganglionic integration by calculating the probabilities of synaptic coincidences. Constructing the model required three assumptions that are consistent with the anatomy and physiology of the sympathetic system. The first assumption is that within a given subclass of sympathetic neurons (e.g., vasoconstrictors) the connections between pre-ganglionic and post-ganglionic neurons are uniform (Fig. 1). Each presynaptic cell forms a certain number of primary synapses and a certain number of secondary synapses, whose strength varies little from one cell to another. Because there are no lateral synaptic connections between the ganglionic neurons, one can see from Fig. 1 that the synaptic properties of each ganglion cell are the same. One can, therefore, model the properties of the entire population of ganglionic neurons by modeling one cell! The second key assumption in Karila and Horn’s model is that the time intervals between synaptic events are exponentially distributed, which is consistent with in vivo intracellular recordings of synaptic activity [37]. The third assumption is that coincidences between pairs of secondary EPSPs can trigger action potentials, but that there is some upper boundary to this firing rate. After making these assumptions and then choosing physiologically realistic presynaptic mean firing rates and temporal windows for summation of fast EPSPs, it was possible to show that n + 1 convergence in the ganglia would result in synaptic amplification of activity, in which post-ganglionic neurons would, on average, fire at a higher rate than pre-ganglionic neurons [33]. Figure 2 illustrates a simple example of how this model works.
Interestingly, Skok, Ivanoff, and Maslov explored a similar mathematical model to describe the rabbit SCG but drew different conclusions [39]. In their analysis, they argued that pairs of EPSPs would not be sufficient to generate spikes and that the probability of higher-order coincidences was too low to have much impact at physiological firing rates, unless some form of preganglionic synchronization came into play. We agree that these are very important considerations and that further investigation is needed to assess the strength of secondary EPSPs in mammalian ganglia and the role of rhythmic presynaptic activity in controlling ganglionic integration.
THE DYNAMIC CLAMP METHOD PERMITS EXPERIMENTAL TESTING OF COMPUTATIONAL PREDICTIONS FROM CONDUCTANCE-BASED MODELS
Although appealing in its simplicity, the coincidence detection model of Karila and Horn is difficult to test experimentally because it does not contain biophysical details. For this purpose, we developed a conductance-based model that accurately describes the resting properties and basic excitability of B neurons and contains the conductances mediating metabotropic excitation by muscarinic and LHRH receptors [26, 40, 41]. With this model, it becomes possible to simulate the data from in vivo intracellular recordings of the membrane potential. Such simulations of physiological activity confirm that coincidences between secondary nicotinic EPSPs can lead to the amplification of preganglionic activity, and they show how these effects depend on the average presynaptic firing rate, the number and the strength of secondary synapses, and the expression of metabotropic excitation and presynaptic facilitation.
Although computational simulations with conductance-based models provide insight into how a cell might behave, they are inadequate unless one can use the insight to devise experimental tests of the computational predictions. The key problem arises from the fact that one cannot stimulate independently all of the inputs to a cell with the classical methods of cellular electrophysiology. With classical methods, one can stimulate simultaneously all of the presynaptic inputs to a cell or isolate one of them by carefully varying the strength of presynaptic stimuli. However, it is impractical to activate five or ten inputs independently, as might occur in vivo. The dynamic clamp method solves this experimental problem and provides a direct link to computational models.
Dynamic clamp is a current-clamp technique that uses feedback to mimic the presence of ion conductances. To implement the dynamic clamp, one must have a mathematical model that describes the gating of the conductance over time and its reversal potential. A rapid feedback loop then measures the membrane potential, determines the magnitude of the virtual conductance at that point in time, calculates, from Ohm’s law, how much current should be flowing through the virtual conductance, and sends an appropriate command signal to the current-clamp circuitry of the recording amplifier. In this way, one can create virtual leak conductances, synaptic conductances, and voltage-dependent conductances, provided that the feedback cycle of the dynamic clamp operates more rapidly than the kinetics of the virtual conductances. To achieve this performance, we built a system that can implement virtual conductances at 20,000 sec−1 and stimulate living neurons with patterns of virtual synaptic activity for periods up to several minutes without interruption [42]. Taking this approach, the strength of virtual nicotinic synapses can be normalized relative to that required to evoke a spike (threshold synaptic conductance). Patterns of virtual synaptic activity (synaptic templates) are then defined by the number of virtual synapses, their strength, and their average rate of firing. Identical synaptic templates can then be used for numerical simulations and for dynamic clamp experiments. In this way, one can determine how well a computational model predicts the behavior of living neurons and determine where and why the model breaks down.
Using this approach, we have begun to explore synaptic integration in bullfrog B and C neurons dissociated from adult ganglia and identifiable by their expression of different muscarinic responses and by the cell size. Several interesting results have already emerged from this approach.
In accordance with predictions of our models, we have observed that synaptic gain increases with larger numbers of secondary synapses [26]. When muscarine is applied to B neurons, suppression of the M-type potassium conductance has little effect on the amplitudes of fast EPSPs but enhances their excitatory efficacy, and this leads to an increase in the synaptic gain [43]. In other words, it now appears that metabotropic excitation of sympathetic B neurons modulates synaptic integration by increasing the ability of secondary EPSPs to generate single action potentials and not by induction of repetitive firing. We have also studied muscarinic activation of a small leak conductance, which sometimes occurs together with M-current suppression. These experiments showed that the leak conductance alone enhances the strength of fast EPSPs, and this effect synergizes with M-current suppression [43]. In other words, muscarinic activation of a branched signaling pathway in B neurons can produce a two-component effect that is greater than the sum of its parts.
FUTURE DIRECTIONS
The development of dynamic clamp methodology to study ganglionic integration has provided the opportunity to address several long-standing questions. We are now developing new experiments to examine the integrative properties of bullfrog C neurons, which appear different than those of B cells. We have also begun to examine the impact of preganglionic rhythmic activity, a prominent feature of baroreceptor-sensitive sympathetic neurons. Our preliminary observations suggest that oscillatory preganglionic activity limited to a part of the cardiac cycle may serve as another mechanism for enhancing synaptic gain in sympathetic ganglia, and that this could be relevant to the overall gain of baroreceptor reflexes that regulate blood pressure. Finally, we have begun to develop criteria for identifying functional cell types in the mammalian SCG [44]. With this information, it should become possible to explore how ideas developed in studies on bullfrog ganglia extend to the mammalian autonomic system. The ultimate tests of such work will be to devise methods for manipulating ganglionic gain, either through pharmacological or genetic methods, and observing the consequences for behavioral phenomena, such as blood pressure control, thermoregulation, and athletic performance.
Дж. П. Хорн1, П. Х. М. Куллманн1
СИНАПТИЧНА ІНТЕГРАЦІЯ В СИМПАТИЧНИХ ГАНГЛІЯХ (АНАЛІЗ З ВИКОРИСТАННЯМ МЕТОДА ФІКСАЦІЇ ДИНАМІКИ СИНАПТИЧНИХ ВПЛИВІВ)
1Університет Піттсбурзької медичної школи, Піттсбург (США).
Р е з ю м е
Поступ у сучасній царині нейронаук потребує ідентифікації принципів, що поєднують різні рівні експериментального аналізу, починаючи з висвітлення молекулярних механізмів і клітинних функцій, потім – нейронних ланцюгів і, нарешті, – систем і поведінкових феноменів загалом. Наша лекція присвячена огляду відомих даних щодо специфіки синаптичної організації симпатичних гангліїв, котра дозволяє їм функціонувати як залежні від використання підсилювачі прегангліонарної активності. В зв’язку з цим ми досліджували, як коефіцієнт передачі такої активності може модулюватися механізмами метаботропної сигналізації. Наш підхід поєдную звичайне розрахункове моделювання гангліонарної інтеграції з експериментальним тестуванням цієї моделі з використанням метода фіксації динаміки синаптичних впливів. У наших експериментах ми спочатку здійснювали внутрішньоклітинні відведення від дисоційованих симпатичних нейронів жаби-бика, а потім імітували фізіологічні синапси (патерни синаптичної активності in vivo), використовуючи дію віртуальних синапсів, генеровану за допомогою комп’ютера. Отже, такі процеси дозволяли нам аналізувати коефіцієнт синаптичної передачі з використанням реєстрацій клітинних відповідей, зумовлених складними патернами синаптичної активності, що звичайно виникають в умовах in vivo в конвергентних нікотинових і мускаринових синапсах. Результати таких досліджень є досить вагомими, оскільки вони ілюструють, як характеристики передачі, залежні від гангіонарної інтеграції, можуть забезпечувати внесок у контроль важливих вегетативних поведінкових феноменів реалізований за принципом зворотного зв’яку, зокрема в контроль тиску крові. Ми присвячуємо цю публікацію пам’яті проф. Володимира Скока, чий цінний спадок у галузі фізіології синапсів сприяв створенню сучасної парадигми щодо поєднання численних рівнів аналізу в дослідженнях нервової системи.
Acknowledgments
Continuing support for this work has been provided through grant NS 21065 from the National Institutes of Health, USA. We also thank Drs. Katrina Rimmer, Ilva Putzier, and Chen Li for many helpful discussions.
REFERENCES
- 1.Kuba K, Koketsu K. Synaptic events in sympathetic ganglia. Prog. Neurobiol. 1978;11:77–169. doi: 10.1016/0301-0082(78)90010-2. [DOI] [PubMed] [Google Scholar]
- 2.Skok V. Physiology of Autonomic Ganglia. Tokyo: Igaku Shoin; 1973. [Google Scholar]
- 3.Smith PA. Amphibian sympathetic ganglia: an owner's and operator's manual. Prog. Neurobiol. 1994;43:439–510. doi: 10.1016/0301-0082(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 4.Gabella G. Structure of the Autonomic Nervous System. London: Chapman and Hall; 1976. [Google Scholar]
- 5.Wang FB, Holst MC, Powley TL. The ratio of pre- to postganglionic neurons and related issues in the autonomic nervous system. Brain Res. Rev. 1995;21:93–115. doi: 10.1016/0165-0173(95)00006-o. [DOI] [PubMed] [Google Scholar]
- 6.Jänig W. Neurobiology of Homeostasis. Cambridge: Cambridge Univ. Press; 2006. The Integrative Action of the Autonomic Nervous System. [Google Scholar]
- 7.Nishi S, Soeda H, Koketsu K. Studies on sympathetic B and C neurons and patterns of preganglionic innervation. J. Cell. Physiol. 1965;66:19–32. doi: 10.1002/jcp.1030660103. [DOI] [PubMed] [Google Scholar]
- 8.Horn JP, Stofer WD. Spinal origins of preganglionic B and C neurons that innervate paravertebral sympathetic ganglia nine and ten of the bullfrog. J. Comp. Neurol. 1988;268:71–83. doi: 10.1002/cne.902680108. [DOI] [PubMed] [Google Scholar]
- 9.Libet B, Chichibu S, Tosaka T. Slow synaptic responses and excitability in sympathetic ganglia of the bullfrog. J. Neurophysiol. 1968;31:383–395. doi: 10.1152/jn.1968.31.3.383. [DOI] [PubMed] [Google Scholar]
- 10.Skok VI. Conduction in tenth ganglion of the frog sympathetic trunk. Fed. Proc. Transl. Suppl. 1965;24:363–367. [PubMed] [Google Scholar]
- 11.Adams PR, Brown DA. Synaptic inhibition of the M-current: slow excitatory post-synaptic potential mechanism in bullfrog sympathetic neurons. J. Physiol. 1982;332:263–272. doi: 10.1113/jphysiol.1982.sp014412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shen WX, Horn JP. Presynaptic muscarinic inhibition in bullfrog sympathetic ganglia. J. Physiol. 1996;491:413–421. doi: 10.1113/jphysiol.1996.sp021225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tosaka T, Chichibu S, Libet B. Intracellular analysis of slow inhibitors and excitatory postsynaptic potentials in sympathetic ganglia of the frog. J Neurophysiol. 1968;31:396–409. doi: 10.1152/jn.1968.31.3.396. [DOI] [PubMed] [Google Scholar]
- 14.Dodd J, Horn JP. Muscarinic inhibition of sympathetic C neurons in the bullfrog. J. Physiol. 1983;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jan LY, Jan YN. Peptidergic transmission in sympathetic ganglia of the frog. J. Physiol. 1982;327:219–246. doi: 10.1113/jphysiol.1982.sp014228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jan YN, Jan LY, Kuffler SW. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc. Natl. Acad. Sci. USA. 1979;76:1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jobling P, Horn JP. In vitro relation between preganglionic sympathetic stimulation and activity of cutaneous glands in the bullfrog. J. Physiol. 1996;494:287–296. doi: 10.1113/jphysiol.1996.sp021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorne R, Horn JP. Role of ganglionic cotransmission in sympathetic control of the isolated bullfrog aorta. J. Physiol. 1997;498:201–214. doi: 10.1113/jphysiol.1997.sp021851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dennis MJ, Harris AJ, Kuffler SW. Synaptic transmission and its duplication by focally applied acetylcholine in parasympathetic neurons in the heart of the frog. Proc. Roy. Soc. London, Ser. B, Biol. Sci. 1971;177:509–539. doi: 10.1098/rspb.1971.0045. [DOI] [PubMed] [Google Scholar]
- 20.Dodd J, Horn JP. A reclassification of B and C neurons in the ninth and tenth paravertebral sympathetic ganglia of the bullfrog. J. Physiol. 1983;334:255–269. doi: 10.1113/jphysiol.1983.sp014493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hartzell HC, Kuffler SW, Stickgold R, Yoshikami D. Synaptic excitation inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurons. J. Physiol. 1977;271:817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahan UJ, Kuffler SW. Visual identification of synaptic boutons on living ganglion cells and of varicosities in postganglionic axons in the heart of the frog. Proc. Roy. Soc. London, Ser. B, Biol. Sci. 1971;177:485–508. doi: 10.1098/rspb.1971.0044. [DOI] [PubMed] [Google Scholar]
- 23.Jones SW. Sodium currents in dissociated bull-frog sympathetic neurons. J. Physiol. 1987;389:605–627. doi: 10.1113/jphysiol.1987.sp016674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuffler SW, Sejnowski TJ. Peptidergic and muscarinic excitation at amphibian sympathetic synapses. J. Physiol. 1983;341:257–278. doi: 10.1113/jphysiol.1983.sp014805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lei S, Dryden WF, Smith PA. Regulation of N- and L-type Ca+2 channels in adult frog sympathetic ganglion B cells by nerve growth factor in vitro and in vivo. J. Neurophysiol. 1997;78:3359–3370. doi: 10.1152/jn.1997.78.6.3359. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler DW, Kullmann PH, Horn JP. Estimating use-dependent synaptic gain in autonomic ganglia by computational simulation and dynamic-clamp analysis. J. Neurophysiol. 2004;92:2659–2671. doi: 10.1152/jn.00470.2004. [DOI] [PubMed] [Google Scholar]
- 27.Nishi S, Koketsu K. Early and late after discharges of amphibian sympathetic ganglion cells. J. Neurophysiol. 1968;31:109–121. doi: 10.1152/jn.1968.31.1.109. [DOI] [PubMed] [Google Scholar]
- 28.Koketsu K. Cholinergic synaptic potentials and the underlying ionic mechanisms. Fed. Proc. 1969;28:101–112. [PubMed] [Google Scholar]
- 29.Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neuron. Nature. 1980;283:673–676. doi: 10.1038/283673a0. [DOI] [PubMed] [Google Scholar]
- 30.Brown DA, Hughes SA, Marsh SJ, Tinker A. Regulation of M(Kv7.2/7.3) channels in neurons by PIP2 and products of PIP2 hydrolysis: significance for receptor-mediated inhibition. J. Physiol. 2007;582:917–925. doi: 10.1113/jphysiol.2007.132498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ivanoff AY, Smith PA. In vivo activity of B- and C-neurons in the paravertebral sympathetic ganglia of the bullfrog. J. Physiol. 1995;485:797–815. doi: 10.1113/jphysiol.1995.sp020770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skok VI, Ivanov AY. What is the ongoing activity of sympathetic neurons? J. Auton. Nerv. Syst. 1983;7:263–270. doi: 10.1016/0165-1838(83)90079-6. [DOI] [PubMed] [Google Scholar]
- 33.Karila P, Horn JP. Secondary nicotinic synapses on sympathetic B neurons and their putative role in ganglionic amplification of activity. J. Neurosci. 2000;20:908–918. doi: 10.1523/JNEUROSCI.20-03-00908.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skok V, Ivanov A, Tatarchenko L, Maslov V. Ganglionic neuronal mechanisms involved in circulatory control system. Acta Neurobiol. Exp. (Wars.) 1996;56:107–115. doi: 10.55782/ane-1996-1110. [DOI] [PubMed] [Google Scholar]
- 35.Tatarchenko LA, Ivanov A, Skok VI. Organization of the tonically active pathways through the superior cervical ganglion of the rabbit. J. Auton. Nerv. Syst. 1990;(30 Suppl):S163–S168. doi: 10.1016/0165-1838(90)90124-2. [DOI] [PubMed] [Google Scholar]
- 36.McLachlan EM, Davies PJ, Habler HJ, Jamieson J. On-going and reflex synaptic events in rat superior cervical ganglion cells. J. Physiol. 1997;501:165–181. doi: 10.1111/j.1469-7793.1997.165bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLachlan EM, Habler HJ, Jamieson J, Davies PJ. Analysis of the periodicity of synaptic events in neurons in the superior cervical ganglion of anaesthetized rats. J. Physiol. 1998;511:461–478. doi: 10.1111/j.1469-7793.1998.461bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Purves D, Rubin E, Snider WD, Lichtman J. Relation of animal size to convergence, divergence, and neuronal number in peripheral sympathetic pathways. J. Neurosci. 1986;6:158–163. doi: 10.1523/JNEUROSCI.06-01-00158.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maslov V, Ivanoff AY, Skok V. Background activity of the rabbit superior cervical ganglion neurons: Soundness of random hypothesis of its formation. Neurophysiology. 1996;28:36–40. [Google Scholar]
- 40.Schobesberger H, Gutkin BS, Horn JP. A minimal model for metabotropic modulation of fast synaptic transmission and firing properties in bullfrog sympathetic B neurons. Neurocomputing. 1998;26/27:255–262. [Google Scholar]
- 41.Schobesberger H, Wheeler DW, Horn JP. A model for pleiotropic muscarinic potentiation of fast synaptic transmission. J. Neurophysiol. 2000;83:1912–1923. doi: 10.1152/jn.2000.83.4.1912. [DOI] [PubMed] [Google Scholar]
- 42.Kullmann PH, Wheeler DW, Beacom J, Horn JP. Implementation of a fast 16-bit dynamic clamp using LabVIEW-RT. J. Neurophysiol. 2004;91:542–554. doi: 10.1152/jn.00559.2003. [DOI] [PubMed] [Google Scholar]
- 43.Kullmann PH, Horn JP. Excitatory muscarinic modulation strengthens virtual nicotinic synapses on sympathetic neurons and thereby enhances synaptic gain. J. Neurophysiol. 2006;96:3104–3113. doi: 10.1152/jn.00589.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C, Horn JP. Physiological classification of sympathetic neurons in the rat superior cervical ganglion. J. Neurophysiol. 2006;95:187–195. doi: 10.1152/jn.00779.2005. [DOI] [PubMed] [Google Scholar]