Abstract
Fibroblast growth factor 23 (FGF23) promotes phosphaturia and suppresses 1,25-dihydroxyvitamin D [1,25(OH)2D] production. PTH also promotes phosphaturia, but, in contrast, stimulates 1,25(OH)2D production. The relationship between FGF23 and PTH is unclear, and the acute effect of pharmacologically dosed PTH on FGF23 secretion is unknown. Twenty healthy men were infused with human PTH(1-34) [hPTH(1-34)] at 44 ng/kg/h for 24 h. Compared with baseline, FGF23, 1,25(OH)2D, ionized calcium (iCa), and serum N-telopeptide (NTX) increased significantly over the 18-h hPTH(1-34) infusion (p < 0.0001), whereas serum phosphate (PO4) transiently increased and then returned to baseline. FGF23 increased from 35 ± 10 pg/ml at baseline to 53 ± 20 pg/ml at 18 h (p = 0.0002); 1,25(OH)2D increased from 36 ± 16 pg/ml at baseline to 80 ± 33 pg/ml at 18 h (p < 0.0001); iCa increased from 1.23 ± 0.03 mM at baseline to 1.46 ± 0.05 mM at hour 18 (p < 0.0001); and NTX increased from 17 ± 4 nM BCE at baseline to 28 ± 8 nM BCE at peak (p < 0.0001). PO4 was 3.3 ± 0.6 mg/dl at baseline, transiently rose to 3.7 ± 0.4 mg/dl at hour 6 (p = 0.016), and then returned to 3.4 ± 0.5 mg/dl at hour 12 (p = 0.651). hPTH(1-34) infusion increases endogenous 1,25(OH)2D and FGF23 within 18 h in healthy men. Whereas it is possible that the rise in PO4 contributed to the observed increase in FGF23, the increase in 1,25(OH)2D was more substantial and longer sustained than the change in serum phosphate. Given prior data that suggest that neither PTH nor calcium stimulate FGF23 secretion, these data support the assertion that 1,25(OH)2D is a potent physiologic stimulator of FGF23 secretion.
Key words: fibroblast growth factor 23; phosphate; 1,25-dihydroxyvitamin D; PTH
INTRODUCTION
Fibroblast growth factor 23 (FGF23) is a phosphate-regulating hormone that was identified through the study of acquired and inherited forms of rickets/osteomalacia, characterized by renal phosphate wasting, hypophosphatemia, and inappropriately low 1,25-dihydroxyvitamin D [1,25(OH)2D] levels.(1–4) The regulation of FGF23 in vivo, and how FGF23 fits in the physiological regulation of phosphate metabolism, is currently of great interest. Whereas human studies have yielded sometimes conflicting results, 1,25(OH)2D, dietary phosphate, and serum phosphate seem to stimulate FGF23 release.(5–10) The relationship between FGF23 and PTH, however, remains unclear.(11–14) We thus sought to address this question by assessing the impact of infusing pharmacologically dosed human PTH(1-34) [hPTH(1-34)] in young healthy men. By assessing the response of circulating FGF23 levels, as well as other potential mediators of FGF23 such as 1,25(OH)2D and serum phosphate levels, we hoped to gain a better understanding of how FGF23 fits into the overall regulation of mineral metabolism.
MATERIALS AND METHODS
Study subjects
Twenty subjects (20–45 yr of age) were randomly selected from a cohort of healthy volunteers that had completed a larger study investigating the effects of androgens and estrogens on bone and mineral metabolism in men.(15) Subjects were required to have normal serum testosterone, serum calcium and renal and hepatic function. Subjects were excluded if they had congenital or acquired bone disease (e.g., osteomalacia, hyperparathyroidism, or Paget's disease); a disorder known to affect bone metabolism (e.g., hyperthyroidism, Cushing's disease or hyperprolactinemia); recent fracture or immobilization; a history of significant cardiopulmonary, oncologic, prostatic, or psychiatric disease; or a history of significant drug or alcohol abuse or use of any drug known to interact with the study drugs or to alter bone turnover markers (e.g., anticoagulants, sex steroids, glucocorticoids, anticonvulsants, suppressive doses of thyroxine, lithium, bisphosphonates, calcitonin, sodium fluoride, or statins). The study was approved by the Human Research Committee of Partners HealthCare Systems, and all subjects provided written consent.
Study protocol
Subjects were admitted to the Mallinckrodt General Clinical Research Center (GCRC) of Massachusetts General Hospital (a tertiary academic center in Boston, MA, USA) where they underwent a 24-h intravenous infusion of hPTH(1-34) (Bachem, Torrance, CA, USA) at a dose of 44 ng/kg/h (10.7 pmol/kg/h). In a 24-h infusion, a 70-kg subject would have thus received ∼3.5 times the currently marketed dose of subcutaneous hPTH(1-34). This dose of PTH was chosen because we had previously shown that it reliably stimulates bone resorption, increases ionized calcium, and clearly overwhelms any endogenous variation in PTH.(16) Whereas these subjects were enrolled in a protocol assessing the effects of androgens and estrogens on skeletal sensitivity to hPTH(1-34),(15) the hPTH(1-34) infusions described here were all performed at baseline (before any gonadal steroid hormonal manipulation).
Blood ionized calcium levels were measured every 6 h until the 18-h time point (0, 6, 12, and 18 h) and then every 2 h (20, 22, and 24 h). The infusions were discontinued if the ionized calcium exceeded 1.5 mM. Subjects were fasting for the baseline blood draw but were allowed to consume an ad libitum nonstandardized GCRC diet.
Laboratory methods
All testing was performed on previously unthawed samples that were stored at −80°C. Serum intact FGF23 was measured with a commercial kit that detects only the intact FGF23 peptide (Kainos, Tokyo, Japan). This immunometric assay has a sensitivity of 3 pg/ml. The intra- and interassay CVs are ≤3% and ≤4%, respectively. Serum phosphate was measured by colorimetric method (Roche Diagnostics, Indianapolis, IN, USA) with intra- and interassay CVs of <2% and <4%, respectively. Serum PTH was measured by a two-site immunoradiometric assay (Nichols Institute) with a sensitivity of 1 pg/ml and intra- and interassay CVs of 2–3% and 6%, respectively. Serum 25-hydroxyvitamin D [25(OH)D] was measured using an extraction double-antibody RIA (DiaSorin, Stillwater, MN, USA) with a sensitivity of 1.5 ng/ml and intra- and interassay CVs of 9–13% and 8–11%, respectively. Serum 1,25(OH)2D was measured by RIA (DiaSorin) with intra- and interassay CVs of 7–11% and 11–15%, respectively. Serum N-telopeptide (NTX) was measured using an automated chemiluminescent immunoassay (Ortho-Clinical Diagnostics, Rochester, NY, USA) with an interassay CV of 7%.
Study endpoints
The primary study endpoints were change in serum phosphate and FGF23 levels. Secondary endpoints included change in 1,25(OH)2D, ionized calcium, and serum NTX.
Statistical analysis
All data are expressed as the mean ± SD unless specified otherwise. For all variables, the 0-, 6-, 12-, and 18-h values were compared by repeated-measures ANOVA to assess whether there was a statistically significant change with time. If a statistically significant difference was detected by ANOVA, paired t-tests were performed to compare the 6-, 12-, and 18-h time points to baseline. p ≤ 0.05 was considered statistically significant.
RESULTS
The baseline characteristics of the 20 subjects are described in Table 1. The mean serum intact FGF23 level was consistent with normative data from our group and others.(6,9,17) Six of the 20 subjects had 25(OH)D levels <20 ng/ml; however, only 2 subjects had PTH levels >60 pg/ml.
Table 1.
Baseline Characteristics
| Mean ± SD | Normal range | |
| Age (yr) | 30 ± 8 | |
| Weight (kg) | 75 ± 9 | |
| Ionized calcium (mM) | 1.23 ± 0.03 | 1.14–1.30 |
| Serum phosphate (mg/dl) | 3.3 ± 0.6 | 2.6–4.5 |
| Creatinine (mg/dl) | 1.0 ± 0.1 | 0.6–1.5 |
| FGF23 (pg/ml) | 35 ± 10 | * |
| 25-hydroxyvitamin D (ng/ml) | 32 ± 17 | † |
| 1,25-dihydroxyvitamin D (pg/ml) | 36 ± 16 | 22–67 |
| PTH (pg/ml) | 41 ± 26 | 10–60 |
Systeme international conversion factors: serum phosphate (mM), 0.2495; creatinine (μM), 88.4; 25-hydroxyvitamin D (nM), 2.496; 1,25-dihydroxyvitamin D (pM), 2.6; and parathyroid hormone (ng/liter), 1.
† The normal range for 25-hydroxyvitamin D increased from >15 ng/ml at the time of subject recruitment to >30 ng/ml currently.
Serum ionized calcium, NTX, 1,25(OH)2D, phosphate, and FGF23
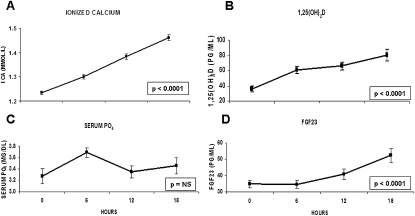
Figure 1 shows the change in serum ionized calcium, 1,25(OH)2D, phosphate, and FGF23 with hPTH(1-34) infusion. Consistent with the known effects of PTH infusion on bone and renal calcium metabolism, mean ionized calcium increased steadily over 18 h (p < 0.0001; Fig. 1A), and serum NTX increased from 17 ± 4 nM BCE at baseline to a peak level of 28 ± 8 nM (p < 0.0001). Similarly, consistent with the known PTH stimulation of 1α-hydroxylation, 1,25(OH)2D levels increased significantly over the 18-h infusion (p < 0.0001; Fig. 1B). Serum phosphate levels, however, were stable over the 18 h, with the exception of a transient rise at 6 h (p = 0.016 versus baseline; Fig. 1C). In contrast to the observed changes in serum phosphate and similar to the observed changes with ionized calcium and 1,25(OH)2D, FGF23 levels increased significantly over 18 h (p < 0.0001; Fig. 1D). FGF23 was 35 ± 10 pg/ml at baseline and stable at 6 h (p = 0.9), but increased to 41 ± 14 pg/ml at 12 h (p = 0.023) and 53 ± 20 pg/ml at 18 h (p = 0.0002).
FIG. 1.
Mean (±SE) (A) ionized calcium, (B) 1,25-dihydroxyvitamin D, (C) serum phosphate, and (D) FGF23 at 0, 6, 12, and 18 h with hPTH(1-34) infusion. *p value assesses change over time by repeated-measures ANOVA compared with baseline. ns, not significant. Systeme International conversion factors: 1,25-dihydroxyvitamin D (pM), 2.6; phosphate (mM), 0.2495; and parathyroid hormone (ng/liter), 1.
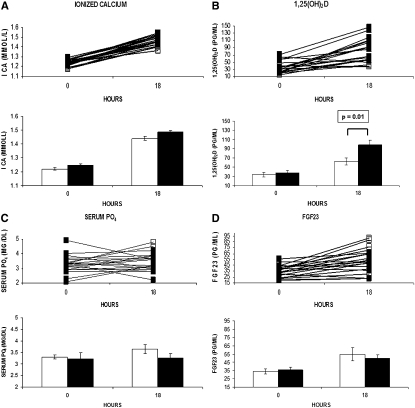
As a secondary analysis, to determine whether there was an effect of hyperparathyroidism and/or vitamin D deficiency [defined as 25(OH)D < 30 ng/ml] on the response to PTH infusion, we assessed the baseline and 18-h ionized calcium, 1,25(OH)2D, serum phosphate, and FGF23 levels in subjects with or without hyperparathyroidism/vitamin D deficiency at baseline [defined as PTH > 60 pg/ml or 25(OH)D < 30 ng/ml, n = 10; Fig. 2]. The only between-group differences identified were in the change in 1,25(OH)2D and consequently the 18-h value, with both being larger in the men with normal values (Fig. 2B).
FIG. 2.
Individual data for (A) ionized calcium, (B) 1,25-dihydroxyvitamin D, (C) serum phosphate, and (D) FGF23 at 0 and 18 h with hPTH(1-34) infusion for subjects with hyperparathyroidism/vitamin D deficiency [PTH > 60 pg/ml and 25(OH)D < 30 ng/ml (n = 2) or PTH ≤ 60 pg/ml and 25(OH)D < 30 ng/ml (n = 8)] (□) vs. those with normal PTH and vitamin D levels [PTH < 60 pg/ml and 25(OH)D ≥ 30 ng/ml (n = 10)] (■). For each variable, the top panel shows the individual data and the bottom panel shows the mean (±SE) for the two groups. *p value assesses a between-group difference at either hour 0 or 18. Systeme International conversion factors: 25-hydroxyvitamin D (nM), 2.496, 1,25-dihydroxyvitamin D (pM), 2.6; phosphate (mM), 0.2495; and parathyroid hormone (ng/liter), 1.
DISCUSSION
In this study, we showed that, within 18 h, infusion of pharmacologic levels of PTH first increases endogenous 1,25(OH)2D and then FGF23 levels. Additionally, consistent with the known effects of PTH to stimulate bone resorption and increase urinary calcium reabsorption, PTH infusion induced hypercalcemia and increased serum NTX. In contrast to some prior studies,(18,19) however, significant hypophosphatemia did not develop. The reason for this difference is not clear, because we used a dose of hPTH(1-34) that was similar to the dose used in one study(18) and much lower than the dose used in another.(19) It is unlikely that our sampling times contributed to us missing a decrease in serum phosphate because, in prior studies, such a decrease was observed from hour 11 or 12 onward of the infusion.(18,19) Instead, we observed a transient rise in serum phosphate that might have been secondary to the influence of meals.(18,20) Finally, we observed similar changes in ionized calcium, serum phosphate, and FGF23 with hPTH(1-34) infusion in the subjects with normal 25(OH)D and PTH levels compared with those with either hyperparathyroidism or vitamin D deficiency.
The relationship between PTH and FGF23 is complex. Prior studies have shown that FGF23 induces renal phosphate wasting and suppression of the 1α-hydroxylase enzyme in the absence of PTH,(21) and recent studies suggested that FGF23 suppresses PTH mRNA and protein both in vitro and in vivo.(22,23) Conversely, animal and human studies differ in how elevated PTH levels affect FGF23 when renal function is normal. In an animal model of primary hyperparathyroidism, FGF23 levels are elevated and decrease after parathyroidectomy.(14) In human studies, there is not a consensus regarding the relationship between elevated PTH levels and FGF23.(11–13) Thus, the effect of PTH on circulating FGF23 remains unclear. In contrast to these prior studies where PTH is chronically elevated, this protocol assessed the effects of an acute increase in circulating PTH likely before a new steady state was achieved.
Dietary phosphate, serum phosphate, and 1,25(OH)2D have all been proposed as physiologic regulators of circulating FGF23. In the majority of animal and human studies, dietary phosphate has been shown to stimulate FGF23 secretion.(8,9,24,25) Discordance exists in the literature, however, with respect to whether serum phosphate stimulates FGF23 secretion. Most human studies,(6,8,24) but not all,(10) show no association between serum phosphate and circulating FGF23, whereas animal and in vitro studies show the converse.(26,27) In contrast, in both animal and human studies, 1,25(OH)2D has been found to stimulate FGF23 secretion or be associated with higher serum FGF23 levels.(6,8,24,26,27) Our observation that 1,25(OH)2D and FGF23 levels increased, whereas serum phosphate levels remained essentially stable, is consistent with the prior findings, by us and others, that 1,25(OH)2D stimulates FGF23 secretion but that, in the physiologic range, serum phosphate does not seem to do the same. This study also provides key insight into how quickly an increase in endogenous 1,25(OH)2D may increase FGF23. Our study confirms the findings of Collins et al.(5) regarding the apparent stimulation of FGF23 by 1,25(OH)2D in patients with hypoparathyroidism or pseudohypoparathyroidism and extends the findings to healthy subjects with increased endogenous 1,25(OH)2D.
The following limitations deserve mention. We are unable to exclude the possibility that the increase in circulating FGF23 is caused by the effects of calcium or PTH. However, in prior in vitro studies, calcium did not stimulate FGF23 promoter activity, whereas 1,25(OH)2D did,(26) arguing against calcium being a physiologic regulator of FGF23. In vivo data argue that PTH is not critical for the stimulation of FGF23 because FGF23 levels actually increase in parathyroidectomized rats administered phosphate or 1,25(OH)2D.(21,27) Furthermore, FGF23 levels are elevated in patients with hypoparathyroidism, arguing that physiologic levels of PTH are not required for FGF23 stimulation.(10) In this study, whereas the initial serum measurements were performed on fasting subjects, subsequent measurements were nonfasting. Additionally, the timing and content of meals were not standardized. Importantly, prior data suggest that eating does not acutely affect circulating FGF23,(20) and a recent report suggests that both serum phosphate and FGF23 are stable for 6 h after a meal of varying phosphate content.(28) Nonetheless, despite not controlling dietary phosphate, which would bias our findings toward the null, we observed a dramatic increase in endogenous FGF23. Another limitation is the lack of a control group of normal volunteers who did not receive PTH infusion. Notably, however, previous data suggested that FGF23 levels in healthy volunteers do not change in a diurnal pattern, and thus, our main finding cannot be explained by such a mechanism.(4)
In summary, in this study, we showed that hPTH(1-34) infusion stimulates a 3-fold increase in endogenous 1,25(OH)2D levels and a 2-fold increase in circulating FGF23 levels. Whereas we cannot exclude the possibility that the increase in FGF23 may also be influenced by transient changes in serum phosphate, these results clearly support the role of 1,25(OH)2D as a potent stimulator of FGF23 in human physiology.
ACKNOWLEDGMENTS
We are grateful to the staff of the MGH Mallinckrodt GCRC for the implementation of the study protocol. This work was supported by National Institute of Health Grants K23-RR-161310 (to B.Z.L.), K23-DK-073356-01 (to S.M.B.), and M01-RR-01066 (to the Mallinckrodt GCRC) and MGH Physician-Scientist Development Award (to S.M.B.).
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Shimada T. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quarles LD. Evidence for a bone-kidney axis regulating phosphate homeostasis. J Clin Invest. 2003;112:642–646. doi: 10.1172/JCI19687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.ADHR Consortium. Autosomal dominant hypophosphatemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- 4.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Juppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- 5.Collins MT, Lindsay JR, Jain A, Kelly MH, Cutler CM, Weinstein LS, Liu J, Fedarko NS, Winer KK. Fibroblast growth factor-23 is regulated by 1alpha,25-dihydroxyvitamin D. J Bone Miner Res. 2005;20:1944–1950. doi: 10.1359/JBMR.050718. [DOI] [PubMed] [Google Scholar]
- 6.Burnett-Bowie SM, Mendoza N, Leder BZ. Effects of gonadal steroid withdrawal on serum phosphate and FGF-23 levels in men. Bone. 2007;40:913–918. doi: 10.1016/j.bone.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari SL, Bonjour JP, Rizzoli R. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab. 2005;90:1519–1524. doi: 10.1210/jc.2004-1039. [DOI] [PubMed] [Google Scholar]
- 9.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21:1187–1196. doi: 10.1359/jbmr.060507. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Winer K, Econs MJ, Marx SJ, Collins MT. FGF-23 is elevated by chronic hyperphosphatemia. J Clin Endocrinol Metab. 2004;89:4489–4492. doi: 10.1210/jc.2004-0724. [DOI] [PubMed] [Google Scholar]
- 11.Yamashita H, Yamashita T, Miyamoto M, Shigematsu T, Kazama JJ, Shimada T, Yamazaki Y, Fukumoto S, Fukagaw M, Noguchi S. Fibroblast growth factor (FGF)-23 in patients with primary hyperparathyroidism. Eur J Endocrinol. 2004;151:55–60. doi: 10.1530/eje.0.1510055. [DOI] [PubMed] [Google Scholar]
- 12.Tebben PJ, Singh RJ, Clarke BL, Kumar R. Fibroblast growth factor 23, parathyroid hormone, and 1alpha,25-dihydroxyvitamin D in surgically treated primary hyperparathyroidism. Mayo Clin Proc. 2004;79:1508–1513. doi: 10.4065/79.12.1508. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi K, Imanishi Y, Miyauchi A, Onoda N, Kawata T, Tahara H, Goto H, Miki T, Ishimura E, Sugimoto T, Ishikawa T, Inaba M, Nishizawa Y. Regulation of plasma fibroblast growth factor 23 by calcium in primary hyperparathyroidism. Eur J Endocrinol. 2006;154:93–99. doi: 10.1530/eje.1.02053. [DOI] [PubMed] [Google Scholar]
- 14.Kawata T, Imanishi Y, Kobayashi K, Miki T, Arnold A, Inaba M, Nishizawa Y. Parathyroid hormone regulates fibroblast growth factor-23 in a mouse model of primary hyperparathyroidism. J Am Soc Nephrol. 2007;18:2683–2688. doi: 10.1681/ASN.2006070783. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, Finkelstein JS, Miller M, Comeaux SJ, Cohen RI, Leder BZ. Effects of selective testosterone and estradiol withdrawal on skeletal sensitivity to parathyroid hormone in men. J Clin Endocrinol Metab. 2006;91:1069–1075. doi: 10.1210/jc.2005-2495. [DOI] [PubMed] [Google Scholar]
- 16.Leder BZ, Smith MR, Fallon MA, Lee ML, Finkelstein JS. Effects of gonadal steroid suppression on skeletal sensitivity to parathyroid hormone in men. J Clin Endocrinol Metab. 2001;86:511–516. doi: 10.1210/jcem.86.2.7177. [DOI] [PubMed] [Google Scholar]
- 17.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz MJ, Tedesco MB, Sereika SM, Hollis BW, Garcia-Ocana A, Stewart AF. Direct comparison of sustained infusion of human parathyroid hormone-related protein-(1-36) [hPTHrP-(1-36)] versus hPTH-(1-34) on serum calcium, plasma 1,25-dihydroxyvitamin D concentrations, and fractional calcium excretion in healthy human volunteers. J Clin Endocrinol Metab. 2003;88:1603–1609. doi: 10.1210/jc.2002-020773. [DOI] [PubMed] [Google Scholar]
- 19.Slovik DM, Adams JS, Neer RM, Holick MF, Potts JT., Jr Deficient production of 1,25-dihydroxyvitamin D in elderly osteoporotic patients. N Engl J Med. 1981;305:372–374. doi: 10.1056/NEJM198108133050704. [DOI] [PubMed] [Google Scholar]
- 20.Nishida Y, Taketani Y, Yamanaka-Okumura H, Imamura F, Taniguchi A, Sato T, Shuto E, Nashiki K, Arai H, Yamamoto H, Takeda E. Acute effect of oral phosphate loading on serum fibroblast growth factor 23 levels in healthy men. Kidney Int. 2006;70:2141–2147. doi: 10.1038/sj.ki.5002000. [DOI] [PubMed] [Google Scholar]
- 21.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 22.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro OM, Mohammadi M, Sirkis R, Naveh-Many T, Silver J. The parathyroid is a target organ for FGF23 in rats. J Clin Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE. Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol. 2007;195:125–131. doi: 10.1677/JOE-07-0267. [DOI] [PubMed] [Google Scholar]
- 24.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. J Clin Endocrinol Metab. 2006;91:3144–3149. doi: 10.1210/jc.2006-0021. [DOI] [PubMed] [Google Scholar]
- 25.Perwad F, Azam N, Zhang MY, Yamashita T, Tenenhouse HS, Portale AA. Dietary and serum phosphorus regulate fibroblast growth factor 23 expression and 1,25-dihydroxyvitamin d metabolism in mice. Endocrinology. 2005;146:5358–5364. doi: 10.1210/en.2005-0777. [DOI] [PubMed] [Google Scholar]
- 26.Ito M, Sakai Y, Furumoto M, Segawa H, Haito S, Yamanaka S, Nakamura R, Kuwahata M, Miyamoto K. Vitamin D and phosphate regulate fibroblast growth factor-23 in K-562 cells. Am J Physiol Endocrinol Metab. 2005;288:E1101–E1109. doi: 10.1152/ajpendo.00502.2004. [DOI] [PubMed] [Google Scholar]
- 27.Saito H, Maeda A, Ohtomo S, Hirata M, Kusano K, Kato S, Ogata E, Segawa H, Miyamoto K, Fukushima N. Circulating FGF-23 is regulated by 1alpha,25-dihydroxyvitamin D3 and phosphorus in vivo. J Biol Chem. 2005;280:2543–2549. doi: 10.1074/jbc.M408903200. [DOI] [PubMed] [Google Scholar]
- 28.Isakova T, Gutierrez O, Shah A, Castaldo L, Holmes J, Lee H, Wolf M. Postprandial mineral metabolism and secondary hyperparathyroidism in early CKD. J Am Soc Nephrol. 2008;19:615–623. doi: 10.1681/ASN.2007060673. [DOI] [PMC free article] [PubMed] [Google Scholar]