Abstract
Periprosthetic osteolysis is the most common cause of aseptic loosening in total joint arthroplasty. The role of inflammatory mediators such as prostaglandin E2 (PGE2) and osteoclast promoting factors including RANKL in the pathogenesis of osteolysis has been well characterized. However, the PGE2 receptor (EP1, EP2, or EP4), and cell type in which it is expressed, which is responsible for PGE2 induction of RANKL during wear debris–induced osteolysis, has yet to be elucidated. To address this, we used mice genetically deficient in these EP receptors to assess PGE2 and wear debris responses in vitro and in vivo. Wear debris–induced osteolysis and RANKL expression were observed at similar levels in WT, EP1−/−, and EP2−/− mice, indicating that these receptors do not mediate PGE2 signals in this process. A conditional knockout approach was used to eliminate EP4 expression in FSP1+ fibroblasts that are the predominant source of RANKL. In the absence of EP4, fibroblasts do not express RANKL after stimulation with particles or PGE2, nor do they exhibit high levels of osteoclasts and osteolysis. These results show that periprosthetic fibroblasts are important mediators of osteolysis through the expression of RANKL, which is induced after PGE2 signaling through the EP4 receptor.
Key words: prostaglandin, fibroblast, RANKL, EP4, inflammatory bone loss
INTRODUCTION
Aseptic loosening secondary to periprosthetic osteolysis remains a serious orthopedic problem and the greatest limitation of total joint replacement.(1) This process is initiated by wear debris that accumulates at the tissue/implant interface(2–4) and stimulates the production of proinflammatory cytokines and mediators of osteoclastic bone resorption.(5–7) Among these factors, prostaglandin E2 (PGE2) has been identified as a critical proximal signaling molecule,(8) whereas RANKL has been identified as the final effector of osteoclastogenesis and bone resorption.(9,10)
Several groups have shown that wear debris stimulates the production of PGE2.(11–13) This requires upregulation of at least two inducible synthase enzymes: cyclooxygenase 2 (COX-2) and prostaglandin E synthase 1 (PGES-1). COX-2 reduces arachidonic acid (released from the plasma membrane by phospholipase A) to PGH2, which is converted to PGE2 by PGES-1.(14–16) The resulting PGE2 is secreted where it can act in an autocrine or paracrine manner to stimulate inflammatory and wound healing responses. Because animals genetically deficient in COX-2 or treated with COX-2 inhibitors have no osteolytic response to titanium,(17,18) it is believed that PGE2 (a product of COX-2 activity) stimulates bone resorption. One mechanism by which this may occur is the PGE2-mediated upregulation of RANKL.
Prostaglandin E2 can bind to any of four G protein–coupled receptors (EP1, EP2, EP3, or EP4) with varying downstream effects.(19–21) EP1 is linked to IP3 signaling and the activation of phospholipase C through Gq/p, resulting in mobilization of intracellular calcium and subsequent exocytosis.(22,23) EP2 and EP4 both stimulate adenylyl cyclase and cAMP/PKA signaling,(22,24,25) whereas EP3 activates Gi to inhibit adenylyl cyclase and oppose the effects of EP2 and/or EP4.(20) However, it is unclear which of the PGE2 receptors (EP1, EP2, or EP4) is responsible for the PGE2-mediated induction of RANKL. EP4 is the most highly expressed PGE2 receptor on synovial fibroblasts, followed by EP2 and EP1.(8) EP4−/− mice have reduced inflammatory responses in a collagen-induced arthritis model,(26) fewer osteoclasts, and a dramatic reduction in RANKL expression by osteoblast cells.(27–29) Deletion of the EP2 receptor has similar but less dramatic effects on osteoblast gene expression and function.(27,28,30) Together, these studies suggest that EP4 is the critical receptor involved in the stimulation of osteoclast formation and activity.
Also of interest is the cell type that responds to PGE2 and stimulates wear debris-induced osteolysis through the upregulation of RANKL. The accumulation of wear debris particles stimulates the formation of a fibrovascular inflammatory membrane, which is composed of a variety of cell types including fibroblasts, macrophages, and lymphocytes.(1) To date, the majority of research has focused on the role of the macrophage in promoting osteolysis through proinflammatory cytokine secretion and formation of TRACP+ osteoclasts despite the macrophage population being only 15% of the inflammatory membrane.(1,6,31–33) In contrast, fibroblasts comprise ∼70% of the inflammatory membrane(6,32) and are increasingly noted for their role in the pathogenesis of inflammatory bone disease.(34–36) Synovial fibroblasts are capable of producing proinflammatory cytokines, matrix-degrading enzymes, and osteoclast-promoting factors including RANKL.(36–41)
We hypothesize that particulate wear debris stimulates the production of PGE2 at the bone/implant interface; PGE2 initiates signaling through the EP4 receptor and upregulates the expression of RANKL on the surface of the dominant cell population (fibroblast), leading to osteoclast formation, activation, and survival.
MATERIALS AND METHODS
Particles
The ultra-high molecular weight polyethylene (PE) particles (Ceridust 130) were obtained as a gift from Dr. E. M. Greenfield and were prepared as previously described.(42) The mean size of the particles was 7.1 μm in diameter, and ∼75% of the particles were <9.1 μm in diameter as determined by Coulter counter analysis. They were within the critical size range (0.2–10 μm) for phagocytosis-induced macrophage activation by wear particles.(43) Pure titanium (Ti) particles were obtained from Johnson Matthey Chemicals and resuspended in PBS at a concentration of 1 × 108 particles/ml as previously described.(44) More than 90% of the Ti particles were determined to be <20 μm in diameter by Coulter Channelizer analysis. In this study, PE particles were used in vivo because they have been shown to induce a significant amount of osteolysis, and the polymer material does not interfere with the μCT analysis.(45) Ti particles were used in vitro because of the buoyancy of the PE particles, which prevents them from associating with the cell monolayer.
Experimental animals and surgery
All animal studies were conducted in accordance with principles and procedures approved by the University of Rochester Committee on Animal Resources. Male and female mice, 8–10 wk old, were used for the experiments. EP1−/−, EP2−/−, EP4f/f, and FSP1cre(+) mice were originally obtained from Dr. Matthew Breyer, Dr. Richard Breyer, and Dr. Eric Neilson; C57BL/6 wildtype mice are maintained in this laboratory. The genetic background of EP1−/−, EP2−/−, and EP4f/f was C57BL/6. FSP1cre(+) mice, on a Balb/c genetic background, were backcrossed onto a C57BL/6 background for five generations. The in vivo murine calvaria experiments were performed as previously described.(46) Briefly, eight healthy 8- to 10-wk-old mice were used in each group (n = 8). Mice were anesthetized with 70–80 mg/kg of ketamine and 5–7 mg/kg of xylazine by intraperitoneal injection. Calvarial bone was exposed by making a midline sagittal incision over the head, and the periosteum was removed. PE particles (5 mg) or no particles for sham surgery were spread directly over the surface of calvaria, and the skin was closed with sutures. Mice were killed after 10 days, and tissues were examined using μCT and histological analysis.
In vivo μCT imaging and volumetric osteolysis analysis
High-resolution in vivo μCT (VivaCT40; ScanCo Medical, Basserdorf, Switzerland) of calvarial bone structure was performed on days 0 (before surgery) and 10, and the bone resorption volume was quantified as previously described.(45) Calvaria were scanned at a spatial resolution of 35.8 μm with an X-ray source set to 45 kV and 155 μA using an integration time of 300 ms. The VivaCT40 software was used to create DICOM files, which were transferred to Amira 3.1 (TGS; Mercury Computer Systems) for quantitative analysis. Quantification of the osteolytic volume was performed by subtracting a registered 3D day 10 image from its baseline counterpart. Next, a region of interest defined by the operator as the largest osteolytic volume that fits in a 125 × 125 × 125 voxel template (89.6 mm3) on the subtracted image was manually isolated using the Crop Editor. Finally, the TissueStatistics module was used to quantify this as the osteolytic volume of the calvaria.
Histologic evaluation of osteolysis
Calvaria were harvested 10 days after surgery, fixed in 10% neutral buffered formalin, and decalcified in 10% EDTA for 3 wk. Calvaria samples for immunohistochemistry were fixed, decalcified, and embedded in paraffin. Sections (3 μm) were deparaffinized, rehydrated, and blocked with a 1:20 dilution of normal goat serum and incubated with primary antibody overnight at 4°C. Primary antibodies were obtained from Santa Cruz (anti-RANKL), Cayman Chemicals (anti-EP4), and Abcam [anti-S100a4 (FSP1)]. The sections were washed with PBS and incubated with a 1:200 dilution of secondary antibody for 1 h at room temperature, followed by streptavidin-peroxidase complex, and the binding of antibody was visualized with aminoethyl carbazole (AEC; Biocare Medical) according to the supplier instructions. Osteolysis was examined in serial sections stained with orange G, and osteoclasts were examined using the Diagnostics Acid Phosphatase Kit (Sigma) to detect TRACP. Osteoclasts numbers were quantified as previously described,(46) using three TRACP-stained sections 500 μm apart.
Cell culture
Fibroblast-like cells were isolated from 3-day-old neonatal mouse calvaria. The calvaria were chopped and digested with collagenase A (Roche) for 40 min and centrifuged for 3 min at 1500 rpm to collect cells (fraction 1). The supernatant collagenase solution was reapplied to the calvaria fragments for another 20 min (fraction 2), and cells were collected as described above. Subsequent 20-min digestions were performed and labeled fractions 3–5. Fraction 1 and 2 cells were used as fibroblasts, because normally, fraction 3–5 cells are treated as osteoblasts. Fibroblasts were washed twice and plated in 6-well plates at a density of 1 × 105 cells/well in DMEM medium in the presence of 10% heat-inactivated FBS, 1% penicillin, and 5% CO2 at 37°C. Cells were fed with fresh medium every day; subconfluent cultures (n = 3) were treated with PBS, Ti (5 × 106/ml), or PGE2 (10−6 M; Cayman Chemicals).
In some experiments, fibroblasts from EP2−/− mice were treated with siRNA to knockdown expression of EP1. Briefly, fibroblasts were isolated and plated as described above then transfected with EP1 siRNA (Santa Cruz Biotechnology) according to the manufacturer's instructions. Twenty-four hours after transfection, cells were treated with Ti or PGE2 as described above, and RANKL expression was detected by real-time PCR and Western blot.
Quantitative real-time PCR
Total RNA was extracted for real-time PCR 5 h after Ti or PGE2 treatment using the RNeasy kit (Qiagen) and was reverse transcribed to cDNAs using iScript cDNA synthesis kit (BIO-RAD) according to the manufacturer's instructions. RANKL expression was examined compared with GAPDH. Primers specific for murine RANKL and GAPDH were used (RANKL: 5′-CACCATCAGCTGAAGATAGT-3′, 5′-CCAAGATCTCTAACATGACG-3′; GAPDH: 5′-AACGACCCCTTCATTGAC-3′, 5′-TCCACGACATACTCAGCAC-3′). Triplicate PCR reactions were carried out in the Rotor-Gene 3000 (Corbett Research). SYBR green dye was used for detection of the product using the SYBR Green PCR Master Mix assay (Applied Biosystems). The amplification reaction was performed for 45 cycles with denaturation at 95°C for 30 s, followed by annealing at 58°C for 30 s and extension and detection at 72°C for 30 s.
Western blotting
Protein extracts were obtained from whole cell lysates collected after 24 h of treatment with Ti or PGE2. Fifty micrograms of the protein extract was separated by SDS-PAGE. After transfer to a polyvinylidene fluoride (PVDF) membrane, the blots were probed overnight with antibodies specific for murine RANKL (Calbiochem) or β-actin (Sigma). After washing, horseradish peroxidase (HRP)-conjugated secondary antibodies were used to detect the specific proteins using SuperSignal West Femto Maximum (Pierce).
Statistical analysis
Data are expressed as means ± SD. Statistical significance was determined by one-way ANOVA with multiple comparison of Tukey, where p < 0.05 was considered statistically significant.
RESULTS
To determine whether the EP1 or EP2 receptor is involved in the osteolytic response to particulate wear debris, we tested the knockout mouse strains in our in vivo calvaria model and quantified the induction of RANKL in fibroblasts in vitro. μCT analysis showed a dramatic increase in bone resorption after PE treatment for 10 days in the wildtype, EP1−/−, and EP2−/− knockout mouse strains (Fig. 1). The volume of bone resorbed was 3- to 5-fold higher compared with the sham control group (p < 0.05), and there was no statistical difference between the three strains (Fig. 1B). Fibroblasts isolated from the calvaria of wildtype or knockout mice were stimulated with vehicle control, Ti particles, or PGE2 for 5 h, and RANKL expression was measured by quantitative real-time PCR and Western blot. All fibroblasts responded similarly by upregulating RANKL mRNA production after Ti or PGE2 treatment (Figs. 2A, 2C, 2E, and 2G). mRNA results for the PGE2-treated fibroblasts from wildtype, EP1−/−, and EP2−/− mice all showed an increase in RANKL protein expression compared with the PBS control (Figs. 2B, 2D, 2F, and 2H). Cells lacking both EP1 and EP2 receptors as a result of siRNA knockdown and genetic deletion, respectively, showed even more dramatic increases in RANKL mRNA expression with both Ti and PGE2 treatment (Fig. 2G).
FIG. 1.
PE-induced osteolysis is independent of EP1 and EP2 signaling. WT, EP1−/−, and EP2−/− mice (n = 8) received in vivo μCT scans before and 10 days after sham or PE surgeries to induce osteolysis. (A) Representative reconstructed 3D images of day 10 calvaria and osteolytic volume from each group are shown. (B) The osteolytic volumes from each group are presented as mean ± SD (*p < 0.05 vs. sham group).
FIG. 2.
Particle and PGE2-induced RANKL expression in fibroblasts is independent of EP1 and EP2 signaling in vitro. Fibroblasts were isolated from fractions 1 and 2 of calvaria digestion from WT, EP1−/−, and EP2−/− mice and treated with PBS (control, “C”), Ti (5 × 106 particles/ml) or PGE2 (10−6 M). Total RNA was extracted for real-time PCR after 5 h, and the data are presented as the mean (n = 3) ± SD (A, C, and E). Total protein extracts were obtained from cell lysates after 24 h, and RANKL expression was examined compared with β-actin loading control by Western blot. Representative Western blots from two independent experiments are shown (B, D, and F). siRNA was used to specifically knockdown expression of EP1 in EP2−/− fibroblasts, and cells were examined for RANKL mRNA (G) and protein expression (H). (*p < 0.05 vs. PBS control)
Similar experiments with EP4−/− fibroblasts were not performed because the homozygous knockout mice are not viable.(47) Instead we used a conditional knockout approach using a double transgenic strain that expresses the Cre recombinase gene under the control of the fibroblast-specific protein 1 (FSP1) promoter and contains loxP sites within the EP4 gene [FSP1cre(+); EP4f/f]. To validate this approach, we examined FSP1 expression in the fibroblast and osteoblast fractions of calvaria cells by Western blot; Fig. 3A shows that FSP1 is much more highly expressed in fibroblast cells. Using the FSP1 promoter to drive Cre recombinase expression in EP4 floxed animals resulted in nearly 50% reduction in EP4 mRNA expression by fibroblasts with no change in expression levels in osteoblast cells (Figs. 3B and 3C). EP4 receptor levels were also reduced as shown by immunohistochemistry (IHC) (Figs. 3D–3G). Interestingly, in this model, osteoblasts do not seem to express high levels of EP4 receptor even in the absence of Cre recombinase.
FIG. 3.
FSP1 is a specific marker for fibroblasts and drives efficient Cre recombinase expression and activity. Fibroblast (1 and 2) or osteoblast (3–5) fractions from neonatal calvaria were examined for FSP1 expression by Western blot (A). EP4 mRNA expression was examined by real-time PCR in fibroblasts (B) and osteoblasts (C) [*p < 0.05 vs. FSP1cre(−) control]. Histologic sections from FSP1cre(−) (D and F) or FSP1cre(+); EP4f/f mice (E and G) treated with PE particles for 10 days were stained immunohistochemically for EP4. Representative images are shown at ×100 (D and E) and ×400 (F and G).
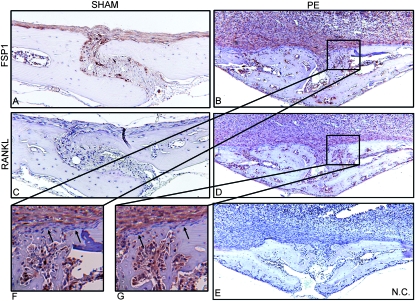
The large number of EP4+ fibroblasts in the PE-treated wildtype mice suggests that these may play more than just a bystander role in the pathogenesis of osteolysis. Using FSP1 as a fibroblast-specific marker, Fig. 4 shows that only a handful of fibroblasts are present in the sham animals and that these do not express RANKL as detected by IHC (Figs. 4A and 4C). However, 10 days after PE particle implantation, the FSP1+ and RANKL+ staining was dramatically increased and co-localized within the bone marrow and inflammatory tissue layers (Figs. 4B and 4D). At higher magnification, it was noted that the osteoblasts and bone-lining cells were unstained (Figs. 4F and 4G).
FIG. 4.
FSP1 and RANKL co-localize in particle-induced inflammation tissues. Calvaria tissues of wildtype mice were isolated 10 days after PE or sham surgeries and used for immunohistochemical staining with FSP1 (A, B, and F), RANKL antibody (C, D, and G), or secondary antibody alone (E). Representative photographs (n = 8) shown at ×100 (A–E) and ×400 (F and G). Arrows indicate unstained osteoblasts/bone-lining cells.
Next, the EP4 conditional knockout mice were examined in vivo and in vitro for their ability to support osteoclastogenesis. When the volume of the midline sagittal suture was determined by μCT, it was seen that animals without the Cre recombinase (with functional EP4 receptor) had nine times the bone resorption level of the sham animals after PE treatment (Fig. 5). Mice lacking EP4 showed a 3-fold increase in bone resorption volume compared with the sham controls, but this was significantly less than the FSP1cre(−); EP4f/f control (Fig. 5). Histological analysis confirmed these results (Figs. 6A, 6D, 6G, and 6J). Cytochemical staining for TRACP+ osteoclasts showed a greater number of osteoclasts in the midline sagittal suture area of mice capable of responding to PGE2; mice lacking EP4 had significantly fewer osteoclasts (a 50% reduction; Fig. 6).
FIG. 5.
EP4 signaling in fibroblasts is required for particle-induced osteolysis. FSP1cre(+) or (−) × EP4f/f mice (n = 8) received sham and PE surgeries with in vivo μCT scans and volumetric analyses as described in Fig. 1. Representative reconstructed 3D images of day 10 calvaria and osteolytic volume from each group are shown (A), with corresponding statistical analyses (B). Quantitative results are shown as mean ± SD [*p < 0.05 vs. sham; # p < 0.05 vs. FSP1cre(−)].
FIG. 6.
Histological evidence of decreased PE-induced bone resorption in FSP1cre(+) × EP4f/f mice. The calvaria tissues of the mice described in Fig. 5 were sectioned and stained with orange G (A, D, G, and J), TRACP (B, E, H, and K), or RANKL IHC (C, F, I, and L). Representative stained sections are presented at ×100 (A–L). The boxed regions in F and L are enlarged to ×400 original magnification (N and O, respectively). Black arrows indicate RANKL+ fibroblasts, and green arrows indicate RANKL+ osteoblasts/bone-lining cells. The numbers of osteoclasts as determined by histomorphometry are shown in M as mean ± SD (n = 8, *p < 0.05).
In an effort to determine whether this reduction in osteoclast numbers and activity correlates to RANKL expression, fibroblasts from the EP4 conditional knockout mice were cultured, stimulated, and analyzed as described for Fig. 2. Unlike results previously for the EP1−/− and EP2−/− knockout strains, Ti particles or PGE2 did not stimulate any increase in RANKL mRNA expression in the fibroblasts lacking EP4 (Figs. 2 and 7A). Significantly more RANKL mRNA was found in fibroblasts that did not express Cre and therefore still contained the EP4 receptor (Fig. 7A). RANKL protein expression was noticeably reduced in all samples lacking EP4 compared with EP4+ cells (Fig. 7B). However, osteoblasts were still capable of inducing RANKL mRNA expression after treatment (Fig. 7C). Correspondingly, conditional deletion of EP4 resulted in dramatically fewer RANKL+ cells in the inflammatory tissue over the calvaria, whereas a small proportion of osteoblast-like cells retained expression of RANKL as determined by IHC (Figs. 6F, 6L, 6N, and 6O).
FIG. 7.
EP4 expression in fibroblasts is required for wear debris and PGE2-induced expression of RANKL in vitro. Total RNA and protein extract of cultured fibroblasts (A) or osteoblasts (C) from FSP1cre(+) or (−) × EP4f/f mice were isolated after the indicated treatments as described in Fig. 2. Quantitative real-time PCR data are presented as the mean (n = 3) ± SD [*p < 0.05 vs. Cre(−) control]. (B) Representative Western blots from two independent experiments are shown.
DISCUSSION
Similar levels of wear debris–induced osteolysis were observed in WT, EP1−/−, and EP2−/− mice, indicating that these receptors do not mediate PGE2 signals in this process. RANKL mRNA and protein expression in fibroblast cultures were equivalent in all three genotypes. This was consistent with our hypothesis that EP4 is the critical receptor for PGE2-mediated inflammatory bone loss. However, the ability of any of the prostaglandin receptors to compensate for each other in this system was not evaluated. Because double knockout mice are not available, we attempted to address this question by using siRNA to knockdown the expression of EP1 in EP2-deficient cells. Loss of both EP1 and EP2 did not abrogate the effects of Ti or PGE2, suggesting that these two receptors are not involved and that EP4 is the primary prostaglandin receptor mediating the effects of Ti and PGE2.
Because it is difficult to study the role of EP4 using knockout mice because of the early lethality of the mutation,(47) we instead used a conditional knockout approach involving the Cre-LoxP system. In these mice, EP4 expression is specifically eliminated from the fibroblast population, leaving the fibroblasts otherwise intact and maintaining EP4 expression in other cell types; IHC showed that this approach was successful. There is no skeletal phenotype in these mice as would be expected because the global COX-2−/− mouse has no observed skeletal abnormalities in the complete absence of PGE2 signaling.(18) In wildtype mice, the inflammatory membrane produced in the calvaria model was composed primarily of fibroblasts that expressed FSP1 and also RANKL afer stimulation with particulate wear debris. Interestingly, the osteoblasts and bone-lining cells in the PE-treated mice did not seem to express RANKL, making the fibroblast the dominant stimulator of osteoclastogenesis (Fig. 4).
To assess the role of EP4 in fibroblastic cells during wear debris–induced osteolysis, FSP1cre(−); EP4f/f and FSP1cre(+); EP4f/f mice received sham and PE surgeries, followed by μCT and histology. FSP1cre(+); EP4f/f mice showed significantly reduced osteolysis, osteoclast numbers, RANKL mRNA, and RANKL protein compared with FSP1cre(−); EP4f/f mice. These results were also consistent with our hypothesis, and we can conclude that, in this fibroblast population, the effects of PGE2 are mediated by signals downstream of EP4 and not the other prostaglandin receptors.
However, it should be noted that the absence of the EP4 receptor did not completely block osteolysis, because a 3-fold increase (p < 0.05 compared with sham controls) in the sagittal suture volume was measured using μCT (Fig. 5). This small increase may be PGE2 dependent through EP2 (absence of EP2 produced a slight reduction in resorbed volume compared with wildtype; Fig. 1B) or PGE2/fibroblast independent. Several studies have identified proinflammatory cytokines as pro-osteoclastogenic factors, including TNFα, IL-1β, and IL-6, and various studies have shown the production of these cytokines by fibroblast populations stimulated with particulate debris.(35,48,49) Because mice deficient in COX-2 do not resorb bone after particle stimulation,(18) it would seem that the process is indeed dependent on PGE2 (or another product of the COX-2 enzyme).
Others have also observed a partial inhibitory effect on osteoclastogenesis and RANKL expression when deleting either EP2 or EP4 alone.(27,28,50,51) Suzawa et al.(52) could only obtain a level of osteoclastogenesis equivalent to that induced by exogenous PGE2 when using both an EP2 receptor agonist and an EP4 receptor agonist. The redundancy of EP4 and EP2 in this system could be examined by crossing the EP4 conditional knockout mice with the EP2−/− to create double knockouts, provided the offspring are viable. Alternatively, antagonists could be used to treat single receptor knockout animals to therapeutically inhibit multiple receptors; however, this approach could be problematic because of specificity and efficacy of the available antagonists.
Other explanations of the partial effect of EP4 deficiency also exist. The partial inhibition of osteolysis in the EP4 conditional knockout may actually be caused by a partial knockdown of EP4 expression. Indeed, some fibroblast cells expressing EP4 were still detected in the inflammatory tissue adjacent to the calvaria using IHC, and the relative proportion of these cells varied between animals, suggesting that the effect was caused by incomplete Cre recombinase activity. It is also possible that osteoclastogenesis and osteoclast activity is supported by osteoblasts or stromal cells that express RANKL as a result of particle stimulation. Unlike the global COX-2 or EP2 knockout mice, the FSP1cre(+); EP4f/f mice would retain intact PGE2/EP4 signaling in osteoblasts. Indeed, we show that RANKL is upregulated on osteoblast cells in vitro after Ti or PGE2 treatment (Fig. 7C). Liu et al.(53) also showed upregulation of RANKL on osteoblastic cells after PGE2 signaling, but the specific EP receptor responsible for the effect was not examined. However, our in vivo IHC suggests that RANKL is not expressed on the osteoblasts and bone-lining cells in this model (Fig. 4). Together, these results suggest that signaling through other EP receptors on fibroblasts and osteoblast involvement through EP4 and RANKL play only minor roles (if any) in the process of particle-mediated osteolysis.
Interestingly, we also observed that, despite the reduction in osteolysis, there was no effect on fibroblast proliferation and formation of the inflammatory membrane as indicated by the similar thickness of the fibrovascular tissue on the superior surface of the calvaria (Fig. 6). This suggests that macrophage-mediated phagocytosis of the polyethylene particles stimulates release of proinflammatory cytokines(54) and that the inflammation/proliferation of fibroblasts is not dependent on the action of PGE2 on EP4 in the fibroblasts.
Our results do, however, support a critical role for the fibroblast population in the overall pathogenesis of osteolysis, because EP4 was only deleted from the fibroblast cell type, and resident or infiltrating macrophages expressing EP4(55,56) did not compensate for the activities of the modified fibroblasts. Previous work in our laboratory has also shown that these RANKL-expressing fibroblasts are capable of inducing osteoclastogenesis in vitro from murine splenocytes.(8) In addition, the fibroblast represents a long-lived cell type and may be an attractive target for gene therapy to reduce EP4 expression.
ACKNOWLEDGMENTS
This study was supported by NIH R01 AR46545 and the DePuy J&J Fellowship. We thank Minjie Zhang and Ryan Tierney for technical and histological support, respectively.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Schwarz EM, Looney RJ, O'Keefe RJ. Anti-TNF-alpha therapy as a clinical intervention for periprosthetic osteolysis. Arthritis Res. 2000;2:165–168. doi: 10.1186/ar81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goldring SR, Jasty M, Roelke MS, Rourke CM, Bringhurst FR, Harris WH. Formation of a synovial-like membrane at the bone-cement interface. Its role in bone resorption and implant loosening after total hip replacement. Arthritis Rheum. 1986;29:836–842. doi: 10.1002/art.1780290704. [DOI] [PubMed] [Google Scholar]
- 3.Hirakawa K, Bauer TW, Stulberg BN, Wilde AH, Secic M. Characterization and comparison of wear debris from failed total hip implants of different types. J Bone Joint Surg Am. 1996;78:1235–1243. doi: 10.2106/00004623-199608000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Margevicius KJ, Bauer TW, McMahon JT, Brown SA, Merritt K. Isolation and characterization of debris in membranes around total joint prostheses. J Bone Joint Surg Am. 1994;76:1664–1675. doi: 10.2106/00004623-199411000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Childs LM, Goater JJ, O'Keefe RJ, Schwarz EM. Effect of anti-tumor necrosis factor-alpha gene therapy on wear debris-induced osteolysis. J Bone Joint Surg Am. 2001;83-A:1789–1797. doi: 10.2106/00004623-200112000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Jiranek WA, Machado M, Jasty M, Jevsevar D, Wolfe HJ, Goldring SR, Goldberg MJ, Harris WH. Production of cytokines around loosened cemented acetabular components. Analysis with immunohistochemical techniques and in situ hybridization. J Bone Joint Surg Am. 1993;75:863–879. doi: 10.2106/00004623-199306000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Sieving A, Wu B, Mayton L, Nasser S, Wooley PH. Morphological characteristics of total joint arthroplasty-derived ultra-high molecular weight polyethylene (UHMWPE) wear debris that provoke inflammation in a murine model of inflammation. J Biomed Mater Res A. 2003;64:457–464. doi: 10.1002/jbm.a.10368. [DOI] [PubMed] [Google Scholar]
- 8.Wei X, Zhang X, Zuscik MJ, Drissi MH, Schwarz EM, O'Keefe RJ. Fibroblasts express RANKL and support osteoclastogenesis in a COX-2-dependent manner after stimulation with titanium particles. J Bone Miner Res. 2005;20:1136–1148. doi: 10.1359/JBMR.050206. [DOI] [PubMed] [Google Scholar]
- 9.Hofbauer LC, Heufelder AE. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J Mol Med. 2001;79:243–253. doi: 10.1007/s001090100226. [DOI] [PubMed] [Google Scholar]
- 10.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujishiro T, Nishikawa T, Shibanuma N, Akisue T, Takikawa S, Yamamoto T, Yoshiya S, Kurosaka M. Effect of cyclic mechanical stretch and titanium particles on prostaglandin E2 production by human macrophages in vitro. J Biomed Mater Res A. 2004;68:531–536. doi: 10.1002/jbm.a.20098. [DOI] [PubMed] [Google Scholar]
- 12.Schwab LP, Xing Z, Hasty KA, Smith RA. Titanium particles and surface-bound LPS activate different pathways in IC-21 macrophages. J Biomed Mater Res B Appl Biomater. 2006;79:66–73. doi: 10.1002/jbm.b.30512. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki Y, Nishiyama T, Hasuda K, Fujishiro T, Niikura T, Hayashi S, Hashimoto S, Kurosaka M. Effect of etidronate on COX-2 expression and PGE(2) production in macrophage-like RAW 264.7 cells stimulated by titanium particles. J Orthop Sci. 2007;12:568–577. doi: 10.1007/s00776-007-1180-8. [DOI] [PubMed] [Google Scholar]
- 14.Caughey GE, Cleland LG, Penglis PS, Gamble JR, James MJ. Roles of cyclooxygenase (COX)-1 and COX-2 in prostanoid production by human endothelial cells: Selective up-regulation of prostacyclin synthesis by COX-2. J Immunol. 2001;167:2831–2838. doi: 10.4049/jimmunol.167.5.2831. [DOI] [PubMed] [Google Scholar]
- 15.Shinji Y, Tsukui T, Tatsuguchi A, Shinoki K, Kusunoki M, Suzuki K, Hiratsuka T, Wada K, Futagami S, Miyake K, Gudis K, Sakamoto C. Induced microsomal PGE synthase-1 is involved in cyclooxygenase-2-dependent PGE2 production in gastric fibroblasts. Am J Physiol Gastrointest Liver Physiol. 2005;288:G308–G315. doi: 10.1152/ajpgi.00313.2004. [DOI] [PubMed] [Google Scholar]
- 16.Takano T, Panesar M, Papillon J, Cybulsky AV. Cyclooxygenases-1 and 2 couple to cytosolic but not group IIA phospholipase A2 in COS-1 cells. Prostaglandins Other Lipid Mediat. 2000;60:15–26. doi: 10.1016/s0090-6980(99)00033-7. [DOI] [PubMed] [Google Scholar]
- 17.Im GI, Kwon BC, Lee KB. The effect of COX-2 inhibitors on periprosthetic osteolysis. Biomaterials. 2004;25:269–275. doi: 10.1016/s0142-9612(03)00523-4. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Morham SG, Langenbach R, Young DA, Xing L, Boyce BF, Puzas EJ, Rosier RN, O'Keefe RJ, Schwarz EM. Evidence for a direct role of cyclo-oxygenase 2 in implant wear debris-induced osteolysis. J Bone Miner Res. 2001;16:660–670. doi: 10.1359/jbmr.2001.16.4.660. [DOI] [PubMed] [Google Scholar]
- 19.Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278:12151–12156. doi: 10.1074/jbc.M212665200. [DOI] [PubMed] [Google Scholar]
- 20.Hatae N, Sugimoto Y, Ichikawa A. Prostaglandin receptors: Advances in the study of EP3 receptor signaling. J Biochem. 2002;131:781–784. doi: 10.1093/oxfordjournals.jbchem.a003165. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen M, Solle M, Audoly LP, Tilley SL, Stock JL, McNeish JD, Coffman TM, Dombrowicz D, Koller BH. Receptors and signaling mechanisms required for prostaglandin E2-mediated regulation of mast cell degranulation and IL-6 production. J Immunol. 2002;169:4586–4593. doi: 10.4049/jimmunol.169.8.4586. [DOI] [PubMed] [Google Scholar]
- 22.Breyer RM, Bagdassarian CK, Myers SA, Breyer MD. Prostanoid receptors: Subtypes and signaling. Annu Rev Pharmacol Toxicol. 2001;41:661–690. doi: 10.1146/annurev.pharmtox.41.1.661. [DOI] [PubMed] [Google Scholar]
- 23.Ohnishi A, Shimamoto C, Katsu K, Ito S, Imai Y, Nakahari T. EP1 and EP4 receptors mediate exocytosis evoked by prostaglandin E(2) in guinea-pig antral mucous cells. Exp Physiol. 2001;86:451–460. doi: 10.1113/eph8602160. [DOI] [PubMed] [Google Scholar]
- 24.Fedyk ER, Phipps RP. Prostaglandin E2 receptors of the EP2 and EP4 subtypes regulate activation and differentiation of mouse B lymphocytes to IgE-secreting cells. Proc Natl Acad Sci USA. 1996;93:10978–10983. doi: 10.1073/pnas.93.20.10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regan JW. EP2 and EP4 prostanoid receptor signaling. Life Sci. 2003;74:143–153. doi: 10.1016/j.lfs.2003.09.031. [DOI] [PubMed] [Google Scholar]
- 26.McCoy JM, Wicks JR, Audoly LP. The role of prostaglandin E2 receptors in the pathogenesis of rheumatoid arthritis. J Clin Invest. 2002;110:651–658. doi: 10.1172/JCI15528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Okada Y, Pilbeam CC, Lorenzo JA, Kennedy CR, Breyer RM, Raisz LG. Knockout of the murine prostaglandin EP2 receptor impairs osteoclastogenesis in vitro. Endocrinology. 2000;141:2054–2061. doi: 10.1210/endo.141.6.7518. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Pilbeam CC, Pan L, Breyer RM, Raisz LG. Effects of prostaglandin E2 on gene expression in primary osteoblastic cells from prostaglandin receptor knockout mice. Bone. 2002;30:567–573. doi: 10.1016/s8756-3282(02)00683-x. [DOI] [PubMed] [Google Scholar]
- 29.Miyaura C, Inada M, Suzawa T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T. Impaired bone resorption to prostaglandin E2 in prostaglandin E receptor EP4-knockout mice. J Biol Chem. 2000;275:19819–19823. doi: 10.1074/jbc.M002079200. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Tomita M, Pilbeam CC, Breyer RM, Raisz LG. Prostaglandin receptor EP2 mediates PGE2 stimulated hypercalcemia in mice in vivo. Prostaglandins Other Lipid Mediat. 2002;67:173–180. doi: 10.1016/s0090-6980(01)00186-1. [DOI] [PubMed] [Google Scholar]
- 31.Nakashima Y, Sun DH, Trindade MC, Maloney WJ, Goodman SB, Schurman DJ, Smith RL. Signaling pathways for tumor necrosis factor-alpha and interleukin-6 expression in human macrophages exposed to titanium-alloy particulate debris in vitro. J Bone Joint Surg Am. 1999;81:603–615. doi: 10.2106/00004623-199905000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Perry MJ, Mortuza FY, Ponsford FM, Elson CJ, Atkins RM. Analysis of cell types and mediator production from tissues around loosening joint implants. Br J Rheumatol. 1995;34:1127–1134. doi: 10.1093/rheumatology/34.12.1127. [DOI] [PubMed] [Google Scholar]
- 33.Sethi RK, Neavyn MJ, Rubash HE, Shanbhag AS. Macrophage response to cross-linked and conventional UHMWPE. Biomaterials. 2003;24:2561–2573. doi: 10.1016/s0142-9612(03)00056-5. [DOI] [PubMed] [Google Scholar]
- 34.Bradfield PF, Amft N, Vernon-Wilson E, Exley AE, Parsonage G, Rainger GE, Nash GB, Thomas AM, Simmons DL, Salmon M, Buckley CD. Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue. Arthritis Rheum. 2003;48:2472–2482. doi: 10.1002/art.11219. [DOI] [PubMed] [Google Scholar]
- 35.Ninomiya JT, Struve JA, Stelloh CT, Toth JM, Crosby KE. Effects of hydroxyapatite particulate debris on the production of cytokines and proteases in human fibroblasts. J Orthop Res. 2001;19:621–628. doi: 10.1016/S0736-0266(00)00061-9. [DOI] [PubMed] [Google Scholar]
- 36.Ritchlin C. Fibroblast biology. Effector signals released by the synovial fibroblast in arthritis. Arthritis Res. 2000;2:356–360. doi: 10.1186/ar112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perlman H, Bradley K, Liu H, Cole S, Shamiyeh E, Smith RC, Walsh K, Fiore S, Koch AE, Firestein GS, Haines GK, III, Pope RM. IL-6 and matrix metalloproteinase-1 are regulated by the cyclin-dependent kinase inhibitor p21 in synovial fibroblasts. J Immunol. 2003;170:838–845. doi: 10.4049/jimmunol.170.2.838. [DOI] [PubMed] [Google Scholar]
- 38.Ren W, Yang SY, Fang HW, Hsu S, Wooley PH. Distinct gene expression of receptor activator of nuclear factor-kappaB and rank ligand in the inflammatory response to variant morphologies of UHMWPE particles. Biomaterials. 2003;24:4819–4826. doi: 10.1016/s0142-9612(03)00384-3. [DOI] [PubMed] [Google Scholar]
- 39.Sakai H, Jingushi S, Shuto T, Urabe K, Ikenoue T, Okazaki K, Kukita T, Kukita A, Iwamoto Y. Fibroblasts from the inner granulation tissue of the pseudocapsule in hips at revision arthroplasty induce osteoclast differentiation, as do stromal cells. Ann Rheum Dis. 2002;61:103–109. doi: 10.1136/ard.61.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, Koshihara Y, Oda H, Nakamura K, Tanaka S. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–269. doi: 10.1002/1529-0131(200002)43:2<259::AID-ANR4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 41.van der Laan WH, Quax PH, Seemayer CA, Huisman LG, Pieterman EJ, Grimbergen JM, Verheijen JH, Breedveld FC, Gay RE, Gay S, Huizinga TW, Pap T. Cartilage degradation and invasion by rheumatoid synovial fibroblasts is inhibited by gene transfer of TIMP-1 and TIMP-3. Gene Ther. 2003;10:234–242. doi: 10.1038/sj.gt.3301871. [DOI] [PubMed] [Google Scholar]
- 42.Taki N, Tatro JM, Nalepka JL, Togawa D, Goldberg VM, Rimnac CM, Greenfield EM. Polyethylene and titanium particles induce osteolysis by similar, lymphocyte-independent, mechanisms. J Orthop Res. 2005;23:376–383. doi: 10.1016/j.orthres.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 43.Green TR, Fisher J, Matthews JB, Stone MH, Ingham E. Effect of size and dose on bone resorption activity of macrophages by in vitro clinically relevant ultra high molecular weight polyethylene particles. J Biomed Mater Res. 2000;53:490–497. doi: 10.1002/1097-4636(200009)53:5<490::aid-jbm7>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Bi Y, Van De Motter RR, Ragab AA, Goldberg VM, Anderson JM, Greenfield EM. Titanium particles stimulate bone resorption by inducing differentiation of murine osteoclasts. J Bone Joint Surg Am. 2001;83-A:501–508. doi: 10.2106/00004623-200104000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Tsutsumi R, Hock C, Bechtold CD, Proulx ST, Bukata SV, Ito H, Awad HA, Nakamura T, O'Keefe RJ, Schwarz EM. Differential effects of biologic versus bisphosphonate inhibition of wear debris-induced osteolysis assessed by longitudinal micro-CT. J Orthop Res. 2008;26:1340–1346. doi: 10.1002/jor.20620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwarz EM, Benz EB, Lu AP, Goater JJ, Mollano AV, Rosier RN, Puzas JE, Okeefe RJ. Quantitative small-animal surrogate to evaluate drug efficacy in preventing wear debris-induced osteolysis. J Orthop Res. 2000;18:849–855. doi: 10.1002/jor.1100180602. [DOI] [PubMed] [Google Scholar]
- 47.Schneider A, Guan Y, Zhang Y, Magnuson MA, Pettepher C, Loftin CD, Langenbach R, Breyer RM, Breyer MD. Generation of a conditional allele of the mouse prostaglandin EP4 receptor. Genesis. 2004;40:7–14. doi: 10.1002/gene.20048. [DOI] [PubMed] [Google Scholar]
- 48.Koreny T, Tunyogi-Csapo M, Gal I, Vermes C, Jacobs JJ, Glant TT. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis Rheum. 2006;54:3221–3232. doi: 10.1002/art.22134. [DOI] [PubMed] [Google Scholar]
- 49.Sabokbar A, Itonaga I, Sun SG, Kudo O, Athanasou NA. Arthroplasty membrane-derived fibroblasts directly induce osteoclast formation and osteolysis in aseptic loosening. J Orthop Res. 2005;23:511–519. doi: 10.1016/j.orthres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 50.Ono K, Akatsu T, Kugai N, Pilbeam CC, Raisz LG. The effect of deletion of cyclooxygenase-2, prostaglandin receptor EP2, or EP4 in bone marrow cells on osteoclasts induced by mouse mammary cancer cell lines. Bone. 2003;33:798–804. doi: 10.1016/s8756-3282(03)00264-3. [DOI] [PubMed] [Google Scholar]
- 51.Tomita M, Li X, Okada Y, Woodiel FN, Young RN, Pilbeam CC, Raisz LG. Effects of selective prostaglandin EP4 receptor antagonist on osteoclast formation and bone resorption in vitro. Bone. 2002;30:159–163. doi: 10.1016/s8756-3282(01)00688-3. [DOI] [PubMed] [Google Scholar]
- 52.Suzawa T, Miyaura C, Inada M, Maruyama T, Sugimoto Y, Ushikubi F, Ichikawa A, Narumiya S, Suda T. The role of prostaglandin E receptor subtypes (EP1, EP2, EP3, and EP4) in bone resorption: An analysis using specific agonists for the respective EPs. Endocrinology. 2000;141:1554–1559. doi: 10.1210/endo.141.4.7405. [DOI] [PubMed] [Google Scholar]
- 53.Liu XH, Kirschenbaum A, Yao S, Levine AC. Interactive effect of interleukin-6 and prostaglandin E2 on osteoclastogenesis via the OPG/RANKL/RANK system. Ann NY Acad Sci. 2006;1068:225–233. doi: 10.1196/annals.1346.047. [DOI] [PubMed] [Google Scholar]
- 54.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Effects of particles on fibroblast proliferation and bone resorption in vitro. Clin Orthop Relat Res. 1997:205–217. [PubMed] [Google Scholar]
- 55.Akaogi J, Yamada H, Kuroda Y, Nacionales DC, Reeves WH, Satoh M. Prostaglandin E2 receptors EP2 and EP4 are up-regulated in peritoneal macrophages and joints of pristane-treated mice and modulate TNF-alpha and IL-6 production. J Leukoc Biol. 2004;76:227–236. doi: 10.1189/jlb.1203627. [DOI] [PubMed] [Google Scholar]
- 56.Pavlovic S, Du B, Sakamoto K, Khan KM, Natarajan C, Breyer RM, Dannenberg AJ, Falcone DJ. Targeting prostaglandin E2 receptors as an alternative strategy to block cyclooxygenase-2-dependent extracellular matrix-induced matrix metalloproteinase-9 expression by macrophages. J Biol Chem. 2006;281:3321–3328. doi: 10.1074/jbc.M506846200. [DOI] [PubMed] [Google Scholar]